ABSTRACT
During early stages of neural development, neuroepithelial cells translocate their nuclei along the apicobasal axis in a harmonized manner with the cell cycle. How cell cycle progression and neuroepithelium polarity are coordinated remains unclear. It has been proposed that developmental cues, epigenetic mechanisms and cell cycle regulators must be linked in order to orchestrate these processes. We have recently discovered that a master epigenetic factor, EZH2 is essential to coordinate these events. EZH2 directly represses the cell cycle regulator p21WAF1/CIP in the chicken spinal cord. By doing so, EZH2 controls neural progenitor cell renewal and fine-tunes Rho signaling pathway, which is essential to maintain neuroepithelial structure. Our findings point to a new role of EZH2 during development that could have potential implication in other areas as cancer.
KEYWORDS: apicobasal polarity, EZH2, gene silencing, histone methylation, neuroblast proliferation, neurogenesis
The development of the nervous system is a highly organized process where bipolar neuroepithelial cells play a key role. During neurogenesis these cells divide to expand the neural progenitor pool and generate differentiated neural cells. Their cell cycle is coordinated with the apicobasal interkinetic nuclear migration in such a way that mitosis occurs in the apical membrane.1,2 It is believed that external developmental cues, epigenetic mechanisms and cell cycle regulators might be linked in order to coordinate these processes. Although apicobasal polarity is known to influence self-renewal at early neurogenesis,3 the exact molecular mechanisms underlying this coordination program remain unclear.
During the last decade, great efforts have been made to elucidate the role of epigenetic regulators that govern different aspects of neural development. The epigenetic control, understood as the heritable changes in genome activity that do not involve alteration of the DNA sequence, is mainly mediated by covalent modifications of histones and DNA.4 Recently, histone methylation has received special attention as an essential regulator of gene expression. In particular, methylation of lysine 27 of histone H3 (H3K27me3) has been found to be an important regulator of embryonic development.5 The enzymes responsible for this activity are Enhancer of Zeste Homolog's 1 and 2 (EZH1/2)6 that belong to the Polycomb Repressive Complex2 (PRC2). H3K27me3 is recognized by Polycomb proteins leading to transcriptional repression of many developmental regulators.7-9 This mark is removed by JMJD3 and UTX demethylase activity.10-13 H3K27me3 has shown to play a role in hereditary transmission of chromatin states and structure. Although it is still unclear how H3K27me3 levels are restored after several DNA replication rounds and mitosis, it has been proposed that the unmodified newly synthetized histones incorporated in the daughter strands get the H3K27me3 mark through the recruitment of PRC2 to the older H3K27me3. This mechanism also accounts for mitosis, maintaining H3K27me3 at targets genes through the cell cycle14 and allows to preserve transcriptional programs and cellular identity during development.
PRC2 controls proliferation of progenitors cells, in part by repressing of the Ink4A/Arf locus,15-17 and regulates cellular differentiation.8,16,18,19 In addition to its role as an epigenetic factor, EZH2 cooperates with different signals in the cytoplasm to allow actin reorganization.20 Although the function of EZH2 as a transcriptional repressor is well characterized, its role during vertebrate development, and particularly in neurogenesis, is just emerging.
EZH2 in neurogenesis
Research efforts in the last decade have highlighted the relevance of EZH2 and Polycomb proteins in a wide range of neurogenic processes. During the development of the cerebral cortex, conditional deletion of Ezh2 at early embryonic stages induces premature neuronal differentiation, which leads to a decreased number of neurons at birth.21 At later stages, EZH2 and Polycomb proteins repress Ngn1 expression. By doing so Polycomb proteins regulate the timing of neurogenic to astrogenic fate switch that occurs as the cortex develops.22
In addition to its role in the cerebral cortex EZH2 is a critical player in the development of the cerebellum. Ezh2 is highly expressed in the cerebellar primordium as early as embryonic day E10.5 and regulates transcription of multiple genes involved in proliferation of progenitor cells and differentiation of cerebellar neuronal lineages. Accordingly, deletion of Ezh2 in the dorsal neural tube, leads to underdevelopment of the cerebellum.23 But the function of EZH2 in hindbrain development goes even further. The use of a sophisticated combination of genetic tools has recently revealed a complex role of EZH2 in precerebellar neuron migration, mostly mediated by the transcriptional regulation of the guidance ligand-receptor molecules in migrating cells and their environment. Either the deletion of Ezh2 from the migrating precerebellar neurons or from the surrounding neural tube cells alters their final localization and connectivity.24
More recently, an essential role of EZH2 in adult neurogenesis has been reported. In hippocampal neural progenitor cell EZH2 regulates proliferation by suppressing Pten expression and promoting the activation of Akt-mTOR. As a consequence, conditional deletion of Ezh2 results in defective hippocampal neurogenesis and impaired spatial learning and memory.25 Furthermore, EZH2 is also required for fate determination of adult neural progenitor cells. Although functional relevance in humans is still unknown, EZH2 expression was found in adult neural progenitor cells suggesting that EZH2 might be key for postnatal human neurogenesis as well.26
EZH2 in the spinal cord development
Our recent report provides new insight into the EZH2 function at early neurogenesis in the spinal cord, revealing a new mechanism that coordinates neuroblast proliferation and neuroepithelium (NE) structure.27 We observed that EZH2 is highly expressed in the ventricular zone of the chicken neural tube, where the neuroblasts reside, and dramatically decreased in the mantle zone, where the differentiated neurons accumulate. By in ovo electroporation of specific shRNAs, we depleted EZH2 resulting in a small neural tube. The reduced size was due to the blocking of self-renewal of neuroepithelial cells and cell death by apoptosis. Remarkably, the EZH2 knock down (KD) embryos showed, in addition, a structurally disorganized neural tube: the apical membrane was severely disrupted along the luminal surface, and a subset of cells lost the apical junctions and invaded the neural tube lumen. Moreover, functional, ectopic lumens were observed in the EZH2 KD neural tubes.
It is well established that Rho signaling pathway couples developmental signals downstream cytoskeletal rearrangements and cell proliferation.28 Interestingly, Rho pathway is subjected to a strict spatiotemporal regulation in the neural tube29 and it is repressed at early embryonic neural tubes.30,31 These data led us to test whether EZH2 could affect the NE structure by regulating Rho activity at the early stages of neurogenesis. Our results indicated that the observed structural alterations were concomitant with increased Rho activity in the EZH2 depleted embryos. These data reveal an unexpected new role of EZH2: in addition to allow the self-renewal and survival of neural progenitor cells, EZH2 is required to maintain apicobasal polarity and NE integrity.
EZH2-mediated p21WAF1/CIP1 expression coordinates apicobasal polarity and cell proliferation
To gain insight into the role of EZH2 on progenitor self-renewal and NE apicobasal polarity at a molecular level, we analyzed the EZH2-mediated transcriptional profile by microarray experiments. We found that p21WAF1/CIP1, a well-known tumor suppressor gene, was highly up regulated in EZH2 KD neural tubes. Interestingly, restoration of p21WAF1/CIP1 levels in EZH2 depleted embryos rescued not only proliferation defects but also many of the NE structural alterations and partially restored the Rho activity levels. Moreover, we demonstrated that cytoplasmic p21WAF1/CIP1 (but not the nuclear p21WAF1/CIP1) induced the formation of ectopic and functional lumens, similar but milder to those observed in EZH2 depleted neural tubes. Our data suggested that the increase on Rho activity mediated by EZH2-depletion was partially due to high levels of p21WAF1/CIP1. However, the partial rescue of Rho activity by p21WAF1/CIP1 depletion, and the milder NE structural defects by electroporation of cytoplasmic p21WAF1/CIP1 indicated that additional factors might contribute to Rho activity in EZH2 depleted spinal cord. Consistent with this, the expression arrays showed that some Rho family members, RhoBTB2, and ARHGAP10 were regulated by EZH2. Moreover, EZH2 non transcriptional effects need to be taken into account, since cytoplasmic EZH2 regulates actin polymerization by interacting with vav1, an activator of RhoA GTPase signaling.20
One of the unsolved questions in early neurogenesis is how neural tube structure and cell renewal are coordinated. Our results bring light to this problem. At early stages of development, EZH2 keeps silenced some Rho signaling components and the cell cycle regulator p21WAF1/CIP1. By doing so, EZH2 allows the establishment of apicobasal polarity that ensures proper cell renewal (Fig. 1). Future investigations of this model will provide additional data of the process by which p21WAF1/CIP1 regulates the Rho signaling pathway and the contribution of non transcriptional role of EZH2 on Rho activity regulation as has been previously proposed.20
Figure 1.
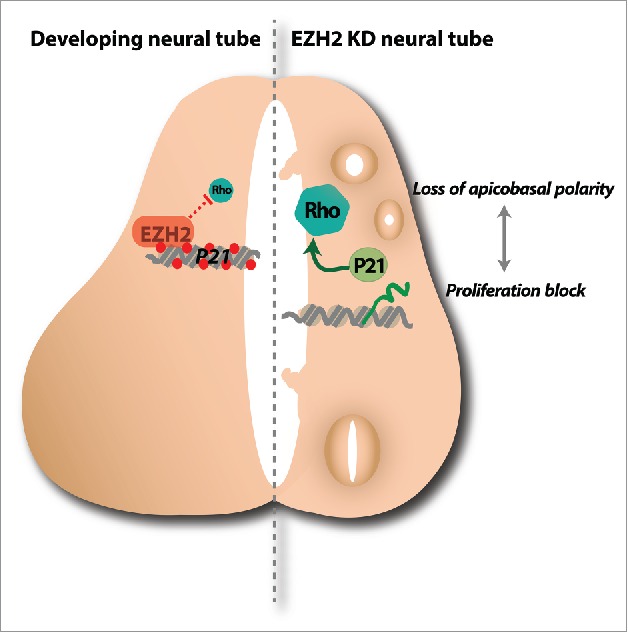
A model for EZH2-mediated regulation of neurogenesis at early stages of development in the spinal cord. EZH2 keeps silenced some Rho signaling components and the cell cycle regulator p21WAF1/CIP1 at the ventricular zone of the neural tube. In this way, EZH2 ensures the correct establishment of apicobasal polarity that allows the neuroepithelial cell self-renewal (left panel). In EZH2 depleted embryos, an increased Rho activity due to low EZH2 activity and increased p21WAF1/CIP1 levels leads to a structurally altered neural tube where the neuroblasts do not proliferate (right panel). Red dots on the chromatin indicate H3K27me3. Darker areas on the neural tube refer to differentiated neurons.
Implications for medicine in central nervous system
EZH2 is well known by its role as an oncogene. More than a decade ago, the overexpression of EZH2 was associated with prostate cancer malignancy.32 Since then, multiple studies have shown that high levels of EZH2 correlate with tumor cell proliferation, aggressive behavior and poor prognosis in a wide range of cancers, including primary brain tumors.33-35 For instance, overexpression of EZH2 correlates with poor prognosis of glioblastoma,35 one of the most aggressive primary brain tumors due to its rapid progression and invasiveness. EZH2 is indeed necessary for maintenance and self-renewal of glioblastoma cancer stem cells, since its pharmacological inhibition or depletion with interference RNA impairs proliferation and tumor initiation capabilities of the glioblastoma cancer stem cells. Additional studies on medulloblastoma, ependidoma and neuroblastoma have revealed that EZH2 overexpression is commonly found in adult and pediatric primary brain tumors as well.33
According to most of the reported studies, molecular mechanism underlying oncogenic activity of EZH2 points toward its methyltransferase activity on H3K27 and consequent target gene repression. Although p16INK4A/p19ARF locus was one of the first Polycomb targets found misregulated in a mouse model of glioma36 most recent transcriptional profiling studies have identified additional groups of EZH2 target genes involved in cell cycle regulation, stem cell differentiation and cellular migration.33 However, just a handful of these genes, have been shown to act as effectors of EZH2 oncogenic activity in brain tumors. Frequently, these effectors are also regulated by EZH2 in neural stem cells during embryonic development. This is the case of BMPR1B, which progressive expression promotes astrocyte differentiation from neural stem cells during development, but persistent repression by EZH2 in glioblastoma stem cells contribute to tumor cell proliferation and maintenance.37
H3K27 methyltransferase activity of EZH2 is also fundamental in the chicken embryo spinal cord. As we observed, EZH2 guarantees neural stem cell self-renewal and proliferation, in part, by repressing p21WAF1/CIP1 expression. In addition to their oncogenic and tumor suppressor activity respectively, EZH2 and p21WAF1/CIP1 have antagonistic roles on senescence.38 Cellular senescence is an irreversible growth arrest induced by cellular damage or stress conditions. Consistent with its role as a tumor suppressor mechanism, senescence in premalignant tumors avoid progression of tumorigenesis, and inactivation of senescence activating proteins, typically p16CDKN2A, is found in full-blown cancers.39-41 However, recent findings indicate that developmental cues also activate senescence during embryogenesis contributing to the development and morphogenesis of multiple embryonic tissues.42,43 Interestingly, one of the major players of developmental senescence is p21WAF1/CIP1. But does this have any relevance for the nervous system development and diseases?
Other than the presence of senescent cells that correlates with p21WAF1/CIP1 expression,42 little is known about the role of senescence in the neural tube development. Preliminary data from our lab indicates that p21WAF1/CIP1 is expressed in the mantle zone, where we also observed postmitotic senescent neural cells. Although senescence was not evaluated in EZH2 KD neural tubes, through p21WAF1/CIP1 repression in the ventricular zone, EZH2 might act blocking senescence. Indeed, several aspects of the phenotype we observe knocking down EZH2 could result from the activation of senescence, for example, the non-cell autonomous behavior of certain Tuj1 positive differentiated cells or migration of cells that invade the lumen. One of the hallmarks of senescent cells is the senescence associated secretory phenotype (SASP) that allows signaling from senescent cells to the external environment through the secretion of growth factors, cytokines/chemiokines and extracellular remodeling factors. In some cases SASP has been implicated in epithelial mesenchymal transition (EMT) and invasion of neighboring cells.44
EMT is one of the first steps that a tumor cell undergoes to become metastatic. This process requires loss of epithelial polarity and cell adhesion, typically triggered by repression of cell adhesion molecules like E-cadherin. The repression of E-cadherin by EZH2 is well established in a variety of tumors, including prostate, breast and melanoma.45,46 Likewise, corepression of E-cadherin by Snail and EZH2 is also a key step for migration of neural crest cells during development.47 In the ventricular zone of the developing embryo however, loss of neuroepithelial structure is driven by EZH2 depletion and consequent induction of Rho GTPase activity by cytoplasmic p21WAF1/CIP1 as our work has revealed. Moreover, we also found additional Rho GTPase family members activated by EZH2 KD that could contribute to the loss of neuroepithelial structure and invasive behavior of certain cells in our model. Hence, our work suggests a counter intuitive tumorigenic mechanism of EZH2 depletion. Indeed, homozygous or heterozygous deletions of EZH2 are common in malignant myeloid diseases;48-50 and both activating and inactivating mutations of EZH2 are related to malignancy.51,52 Although inactivating EZH2 mutations are not found in brain tumors yet, lysine to methionine mutations of the residue 27 of the histone H3 tail, are recurrently found in several aggressive pediatric brain tumors, likely resembling consequences of EZH2 depletion.53 In addition, knockout of EZH2 cells with Ink4a/Arf locus deletion do not inhibit cell proliferation and de-represses Olig2, an oncogenic driver of glial tumors.26 Moreover, EZH2 positive tumors, including glioblastoma tumor cells, develop resistance to pharmacological inhibition of EZH2,54 suggesting that persistent repression of EZH2 can also contribute to malignancy. Thus, the observation that EZH2 regulates the activity of Rho GTPase and the tumor suppressor p21WAF1/CIP1 expression opens new avenues to fully understand the role of EZH2 in cancer and metastasis.
Clinical relevance of EZH2, however, goes beyond its oncogenic activity. Recent work identified that high levels of EZH2 and H3K27me3 are present in the cerebellum of Ataxia Telangiectasia patients, a devastating condition caused by ATM protein kinase deficiency. Remarkably, reduction of EZH2 levels rescued Purkinje cell degeneration and behavioral defects of an Ataxia Telangiectasia mouse model, pointing toward a promising future therapeutic strategy for these patients.55
In summary, our work uncovers an intricate crosstalk between factors that modify chromatin, cell cycle and cell polarity factors that fine-tune spinal cord development. We have described a new role for EZH2 regulating cell renewal and neuroepithelial structure in the spinal cord. Underlying molecular mechanisms that our work revealed will hopefully provide new opportunities to target context dependent EZH2 function in different physiological and pathological conditions.
Abbreviations
- EZH1/2
Enhancer of Zeste Homolog's 1 and 2
- H3K27me3
Trimethylation of lysine 27 of histone H3
- KD
Knock down
- NE
Neuroepithelium
- PRC2
Polycomb Repressive Complex2
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We want to thank Dr Xavier de la Cruz for corrections and for critical reading of the manuscript.
Funding
Our study was supported by grants BFU-2012-34261 to MAMB from the Spanish Ministry of Education and Science, 090210 from Fundaciò La Marató de TV3 and Fondation Jérôme Lejeune to MAMB.
References
- [1].Hinds JW, Ruffett TL. Cell proliferation in the neural tube: an electron microscopic and golgi analysis in the mouse cerebral vesicle. Z Zellforsch Mikrosk Anat 1971; 115:226-64; PMID:4102323; http://dx.doi.org/ 10.1007/BF00391127 [DOI] [PubMed] [Google Scholar]
- [2].Das T, Payer B, Cayouette M, Harris WA. In vivo time-lapse imaging of cell divisions during neurogenesis in the developing zebrafish retina. Neuron 2003; 37:597-609; PMID:12597858; http://dx.doi.org/ 10.1016/S0896-6273(03)00066-7 [DOI] [PubMed] [Google Scholar]
- [3].Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development 2008; 135:1575-87; PMID:18356248; http://dx.doi.org/ 10.1242/dev.014977 [DOI] [PubMed] [Google Scholar]
- [4].Kouzarides T. Chromatin modifications and their function. Cell 2007; 128:693-705; PMID:17320507; http://dx.doi.org/ 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- [5].Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature 2011; 469:343-9; PMID:21248841; http://dx.doi.org/ 10.1038/nature09784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev 2004; 14:155-64; PMID:15196462; http://dx.doi.org/ 10.1016/j.gde.2004.02.001 [DOI] [PubMed] [Google Scholar]
- [7].Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002; 298:1039-43; PMID:12351676; http://dx.doi.org/ 10.1126/science.1076997 [DOI] [PubMed] [Google Scholar]
- [8].Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al.. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 2006; 441:349-53; PMID:16625203; http://dx.doi.org/ 10.1038/nature04733 [DOI] [PubMed] [Google Scholar]
- [9].Ringrose L, Paro R. Polycomb/Trithorax response elements and epigenetic memory of cell identity. Development 2007; 134:223-32; PMID:17185323; http://dx.doi.org/ 10.1242/dev.02723 [DOI] [PubMed] [Google Scholar]
- [10].Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 2007; 449:731-4; PMID:17713478; http://dx.doi.org/ 10.1038/nature06145 [DOI] [PubMed] [Google Scholar]
- [11].De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 2007; 130:1083-94; PMID:17825402; http://dx.doi.org/ 10.1016/j.cell.2007.08.019 [DOI] [PubMed] [Google Scholar]
- [12].Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, Iwase S, Alpatov R, Issaeva I, Canaani E, et al.. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature 2007; 449:689-94; PMID:17851529; http://dx.doi.org/ 10.1038/nature06192 [DOI] [PubMed] [Google Scholar]
- [13].Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science 2007; 318:447-50; PMID:17761849; http://dx.doi.org/ 10.1126/science.1149042 [DOI] [PubMed] [Google Scholar]
- [14].Hansen KH, Bracken AP, Pasini D, Dietrich N, Gehani SS, Monrad A, Rappsilber J, Lerdrup M, Helin K. A model for transmission of the H3K27me3 epigenetic mark. Nat Cell Biol 2008; 10:1291-300; PMID:18931660; http://dx.doi.org/ 10.1038/ncb1787 [DOI] [PubMed] [Google Scholar]
- [15].Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, Theilgaard-Mönch K, Minucci S, Porse BT, Marine JC, et al.. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev 2007; 21:525-30; PMID:17344414; http://dx.doi.org/ 10.1101/gad.415507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, Tarakhovsky A, Fuchs E. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell 2009; 136:1122-35; PMID:19303854; http://dx.doi.org/ 10.1016/j.cell.2008.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kheradmand Kia S, Solaimani Kartalaei P, Farahbakhshian E, Pourfarzad F, von Lindern M, Verrijzer CP. EZH2-dependent chromatin looping controls INK4a and INK4b, but not ARF, during human progenitor cell differentiation and cellular senescence. Epigenetics Chromatin 2009; 2:16; PMID:19954516; http://dx.doi.org/ 10.1186/1756-8935-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev 2006; 20:1123-36; PMID:16618801; http://dx.doi.org/ 10.1101/gad.381706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pietersen AM, van Lohuizen M. Stem cell regulation by polycomb repressors: postponing commitment. Curr Opin Cell Biol 2008; 20:201-7; PMID:18291635; http://dx.doi.org/ 10.1016/j.ceb.2008.01.004 [DOI] [PubMed] [Google Scholar]
- [20].Su IH, Dobenecker MW, Dickinson E, Oser M, Basavaraj A, Marqueron R, Viale A, Reinberg D, Wülfing C, Tarakhovsky A. Polycomb group protein ezh2 controls actin polymerization and cell signaling. Cell 2005; 121:425-36; PMID:15882624; http://dx.doi.org/ 10.1016/j.cell.2005.02.029 [DOI] [PubMed] [Google Scholar]
- [21].Pereira JD, Sansom SN, Smith J, Dobenecker MW, Tarakhovsky A, Livesey FJ. Ezh2, the histone methyltransferase of PRC2, regulates the balance between self-renewal and differentiation in the cerebral cortex. Proc Natl Acad Sci U S A 2010; 107:15957-62; PMID:20798045; http://dx.doi.org/ 10.1073/pnas.1002530107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hirabayashi Y, Suzki N, Tsuboi M, Endo TA, Toyoda T, Shinga J, Koseki H, Vidal M, Gotoh Y. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron 2009; 63:600-13; PMID:19755104; http://dx.doi.org/ 10.1016/j.neuron.2009.08.021 [DOI] [PubMed] [Google Scholar]
- [23].Feng X, Juan AH, Wang HA, Ko KD, Zare H, Sartorelli V. Polycomb Ezh2 controls the fate of GABAergic neurons in the embryonic cerebellum. Development 2016; 143:1971-80; PMID:27068104; http://dx.doi.org/ 10.1242/dev.132902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Di Meglio T, Kratochwil CF, Vilain N, Loche A, Vitobello A, Yonehara K, Hrycaj SM, Roska B, Peters AH, Eichmann A, et al.. Ezh2 orchestrates topographic migration and connectivity of mouse precerebellar neurons. Science 2013; 339:204-7; PMID:23307742; http://dx.doi.org/ 10.1126/science.1229326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang J, Ji F, Liu Y, Lei X, Li H, Ji G, Yuan Z, Jiao J. Ezh2 regulates adult hippocampal neurogenesis and memory. J Neurosci 2014; 34:5184-99; PMID:24719098; http://dx.doi.org/ 10.1523/JNEUROSCI.4129-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hwang WW, Salinas RD, Siu JJ, Kelley KW, Delgado RN, Paredes MF, Alvarez-Buylla A, Oldham MC, Lim DA. Distinct and separable roles for EZH2 in neurogenic astroglia. Elife 2014; 3:e02439; PMID:24867641; http://dx.doi.org/ 10.7554/eLife.02439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Akizu N, Garcia MA, Estaras C, Fueyo R, Badosa C, de la Cruz X, Martínez-Balbás MA. EZH2 regulates neuroepithelium structure and neuroblast proliferation by repressing p21. Open Biol 2016; 6; PMID:27248655; http://dx.doi.org/ 10.1098/rsob.150227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629-35; PMID:12478284; http://dx.doi.org/ 10.1038/nature01148 [DOI] [PubMed] [Google Scholar]
- [29].Kinoshita N, Sasai N, Misaki K, Yonemura S. Apical accumulation of Rho in the neural plate is important for neural plate cell shape change and neural tube formation. Mol Biol Cell 2008; 19:2289-99; PMID:18337466; http://dx.doi.org/ 10.1091/mbc.E07-12-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu JP, Jessell TM. A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development 1998; 125:5055-67; PMID:9811589 [DOI] [PubMed] [Google Scholar]
- [31].Gui H, Li S, Matise MP. A cell-autonomous requirement for Cip/Kip cyclin-kinase inhibitors in regulating neuronal cell cycle exit but not differentiation in the developing spinal cord. Dev Biol 2007; 301:14-26; PMID:17123502; http://dx.doi.org/ 10.1016/j.ydbio.2006.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, et al.. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002; 419:624-9; PMID:12374981; http://dx.doi.org/ 10.1038/nature01075 [DOI] [PubMed] [Google Scholar]
- [33].Crea F, Hurt EM, Farrar WL. Clinical significance of Polycomb gene expression in brain tumors. Mol Cancer 2010; 9:265; PMID:20920292; http://dx.doi.org/ 10.1186/1476-4598-9-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Alimova I, Venkataraman S, Harris P, Marquez VE, Northcott PA, Dubuc A, Taylor MD, Foreman NK, Vibhakar R. Targeting the enhancer of zeste homologue 2 in medulloblastoma. Int J Cancer 2012; 131:1800-9; PMID:22287205; http://dx.doi.org/ 10.1002/ijc.27455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhang J, Chen L, Han L, Shi Z, Pu P, Kang C. EZH2 is a negative prognostic factor and exhibits pro-oncogenic activity in glioblastoma. Cancer Lett 2015; 356:929-36; PMID:25444902; http://dx.doi.org/ 10.1016/j.canlet.2014.11.003 [DOI] [PubMed] [Google Scholar]
- [36].Bruggeman SW, Hulsman D, Tanger E, Buckle T, Blom M, Zevenhoven J, van Tellingen O, van Lohuizen M. Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma. Cancer Cell 2007; 12:328-41; PMID:17936558; http://dx.doi.org/ 10.1016/j.ccr.2007.08.032 [DOI] [PubMed] [Google Scholar]
- [37].Lee J, Son MJ, Woolard K, Donin NM, Li A, Cheng CH, Kotliarova S, Kotliarov Y, Walling J, Ahn S, et al.. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell 2008; 13:69-80; PMID:18167341; http://dx.doi.org/ 10.1016/j.ccr.2007.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fan T, Jiang S, Chung N, Alikhan A, Ni C, Lee CC, Hornyak TJ. EZH2-dependent suppression of a cellular senescence phenotype in melanoma cells by inhibition of p21/CDKN1A expression. Mol Cancer Res 2011; 9:418-29; PMID:21383005; http://dx.doi.org/ 10.1158/1541-7786.MCR-10-0511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chandler H, Peters G. Stressing the cell cycle in senescence and aging. Curr Opin Cell Biol 2013; 25:765-71; PMID:23916530; http://dx.doi.org/ 10.1016/j.ceb.2013.07.005 [DOI] [PubMed] [Google Scholar]
- [40].Bignell GR, Greenman CD, Davies H, Butler AP, Edkins S, Andrews JM, Buck G, Chen L, Beare D, Latimer C, et al.. Signatures of mutation and selection in the cancer genome. Nature 2010; 463:893-8; PMID:20164919; http://dx.doi.org/ 10.1038/nature08768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al.. The landscape of somatic copy-number alteration across human cancers. Nature 2010; 463:899-905; PMID:20164920; http://dx.doi.org/ 10.1038/nature08822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, et al.. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 2013; 155:1119-30; PMID:24238961; http://dx.doi.org/ 10.1016/j.cell.2013.10.041 [DOI] [PubMed] [Google Scholar]
- [43].Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, et al.. Programmed cell senescence during mammalian embryonic development. Cell 2013; 155:1104-18; PMID:24238962; http://dx.doi.org/ 10.1016/j.cell.2013.10.019 [DOI] [PubMed] [Google Scholar]
- [44].Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci 2005; 118:485-96; PMID:15657080; http://dx.doi.org/ 10.1242/jcs.01635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zingg D, Debbache J, Schaefer SM, Tuncer E, Frommel SC, Cheng P, Arenas-Ramirez N, Haeusel J, Zhang Y, Bonalli M, et al.. The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors. Nat Commun 2015; 6:6051; PMID:25609585; http://dx.doi.org/ 10.1038/ncomms7051 [DOI] [PubMed] [Google Scholar]
- [46].Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, Mehra R, Laxman B, Cao X, Yu J, et al.. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 2008; 27:7274-84; PMID:18806826; http://dx.doi.org/ 10.1038/onc.2008.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tien CL, Jones A, Wang H, Gerigk M, Nozell S, Chang C. Snail2/Slug cooperates with Polycomb repressive complex 2 (PRC2) to regulate neural crest development. Development 2015; 142:722-31; PMID:25617436; http://dx.doi.org/ 10.1242/dev.111997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ernst T, Chase AJ, Score J, Hidalgo-Curtis CE, Bryant C, Jones AV, Waghorn K, Zoi K, Ross FM, Reiter A, et al.. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet 2010; 42:722-6; PMID:20601953; http://dx.doi.org/ 10.1038/ng.621 [DOI] [PubMed] [Google Scholar]
- [49].Makishima H, Jankowska AM, Tiu RV, Szpurka H, Sugimoto Y, Hu Z, Saunthararajah Y, Guinta K, Keddache MA, Putnam P, et al.. Novel homo- and hemizygous mutations in EZH2 in myeloid malignancies. Leukemia 2010; 24:1799-804; PMID:20724984; http://dx.doi.org/ 10.1038/leu.2010.167 [DOI] [PubMed] [Google Scholar]
- [50].Nikoloski G, Langemeijer SM, Kuiper RP, Knops R, Massop M, Tonnissen ER, van der Heijden A, Scheele TN, Vandenberghe P, de Witte T, et al.. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet 2010; 42:665-7; PMID:20601954; http://dx.doi.org/ 10.1038/ng.620 [DOI] [PubMed] [Google Scholar]
- [51].Sauvageau M, Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell 2010; 7:299-313; PMID:20804967; http://dx.doi.org/ 10.1016/j.stem.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].McCabe MT, Graves AP, Ganji G, Diaz E, Halsey WS, Jiang Y, Smitheman KN, Ott HM, Pappalardi MB, Allen KE, et al.. Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27). Proc Natl Acad Sci U S A 2012; 109:2989-94; PMID:22323599; http://dx.doi.org/ 10.1073/pnas.1116418109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Venneti S, Garimella MT, Sullivan LM, Martinez D, Huse JT, Heguy A, Santi M, Thompson CB, Judkins AR. Evaluation of histone 3 lysine 27 trimethylation (H3K27me3) and enhancer of Zest 2 (EZH2) in pediatric glial and glioneuronal tumors shows decreased H3K27me3 in H3F3A K27M mutant glioblastomas. Brain Pathol 2013; 23:558-64; PMID:23414300; http://dx.doi.org/ 10.1111/bpa.12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fan TY, Wang H, Xiang P, Liu YW, Li HZ, Lei BX, Yu M, Qi ST. Inhibition of EZH2 reverses chemotherapeutic drug TMZ chemosensitivity in glioblastoma. Int J Clin Exp Pathol 2014; 7:6662-70; PMID:25400745 [PMC free article] [PubMed] [Google Scholar]
- [55].Li J, Hart RP, Mallimo EM, Swerdel MR, Kusnecov AW, Herrup K. EZH2-mediated H3K27 trimethylation mediates neurodegeneration in ataxia-telangiectasia. Nat Neurosci 2013; 16:1745-53; PMID:24162653; http://dx.doi.org/ 10.1038/nn.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]


