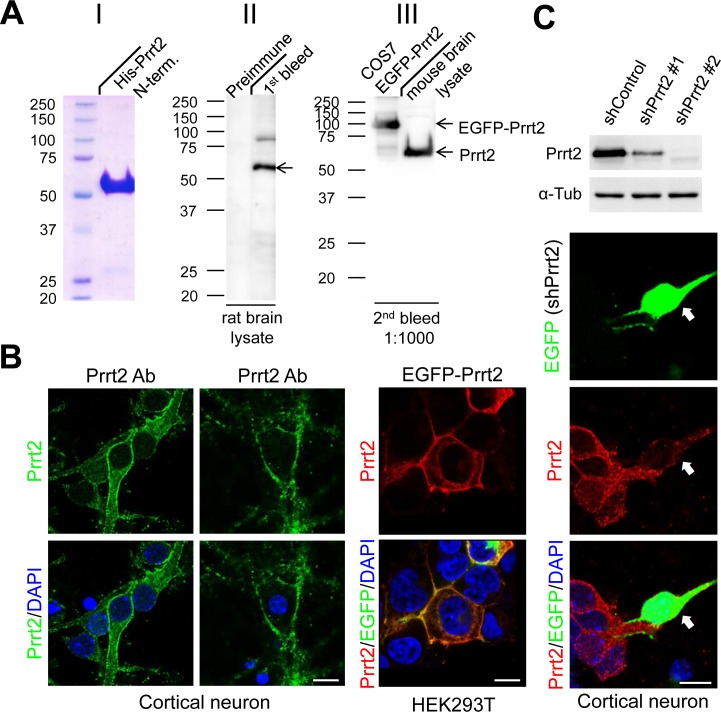
Figure 2. Prrt2 protein recognized by a specific polyclonal antibody.
A. A polyclonal antibody against amino acids 1-268 of the bacterially-expressed Prrt2 protein (I) was generated. Prrt2 protein from adult rat brain lysates was detected using the first bleed of Prrt2 anti-serum obtained from a rabbit (II, right lane). Preimmune serum drawn from the rabbit before antigen injection was the control (II, left lane). The antibody also recognized Prrt2 in mouse brain lysates and EGFP-tagged Prrt2 transfected into COS7 cells (III). B. Subcellular localization of Prrt2 protein by immunoflourescence staining with the polyclonal antibody. Endogenous Prrt2 protein (green) localized predominantly to cell membranes in cultured cortical neurons 3 and 16 days in vitro (DIV) (left panel). The distribution of EGFP-tagged Prrt2 also localized to the cell membrane in HEK293T cells transfected with EGFP-Prrt2, as revealed by the EGFP signal (green) and Prrt2 immuostaining (red). The distribution of Prrt2 based on EGFP signal and antibody staining was highly concordant and resembled the pattern of endogenous Prrt2 in cultured neurons. All cells were counterstained with DAPI staining (blue) to show the nuclei. Scale bar = 10 μm. C. Prrt2 protein level revealed by immunostaining with polyclonal antibody in cultured neurons transfected with Prrt2 shRNA. Upper panel: Prrt2 expression was knocked down by cotransfecting short hairpin RNA (shRNA) with Prrt2 into cultured cells. Two shRNA plasmids targeting different Prrt2 mRNA regions showed 65% (shPrrt2 #1) and 98% (shPrrt2 #2) knockdown efficiency 48 hours after transfection. Lower panel: Immunostaining in cultured neurons shows that endogenous Prrt2 signal (red) was significantly reduced in shPrrt2-transfected neurons (green, arrows) compared with the surrounding untransfected cells. All cells were counterstained with DAPI staining (blue) to show the nuclei. Scale bar = 10 μm.