Abstract
Aims:
To systematically review the effectiveness of preventative and therapeutic interventions for respiratory tract infections (RTIs) in people with Down’s syndrome.
Methods:
Databases were searched for any published and ongoing studies of respiratory tract diseases in children and adults with Down’s syndrome. These databases were searched for controlled trials, cohort studies and controlled before–after studies. Trial registries were searched for ongoing studies. Initially, all study types were included to provide a broad overview of the existing evidence base. However, those with a critical risk of bias were excluded using the Cochrane Risk of Bias tool.
Results:
A total of 13,575 records were identified from which 5 studies fulfilled the eligibility criteria and 3 fulfilled our criteria for data extraction. One randomized controlled trial of moderate risk of bias compared zinc therapy with placebo. Outcome data were only reported for 50 (78%) children who presented with extreme symptoms; no benefit of zinc therapy was found. One non-randomized controlled trial with serious risk of bias included 26 children and compared pidotimod (an immunostimulant) with no treatment; pidotimod was associated with fewer upper RTI recurrences compared with no treatment (1.43 vs. 3.82). A prospective cohort study with moderate risk of bias compared 532 palivizumab treated children with 233 untreated children and found that children treated with palivizumab had fewer respiratory syncytial virus-related hospitalization (23 untreated and 8 treated), but the same number of overall RTI-related hospitalizations (73 untreated and 74 treated) in the first 2 years of life.
Conclusions:
The evidence base for the management of RTIs in people with Down’s syndrome is incomplete; current studies included children only and carry a moderate to serious risk of bias. Methodologic rigorous studies are warranted to guide clinicians in how best to prevent and treat RTIs in children with Down’s syndrome.
Keywords: Down’s syndrome, respiratory tract infection, prevention
Down’s syndrome, also known as trisomy 21, is amongst the commonest genetic conditions worldwide, with an incidence of 1 in 1000 live births in the United Kingdom.1 Despite advances in antenatal screening since the 1990s, the number of children born with Down’s syndrome in the United Kingdom has remained stable.2
Discrete immune deficiencies, morphologic variations of the airways, generalized hypotonia and swallowing dysfunction predispose children with Down’s syndrome to frequent and more severe respiratory tract infections (RTIs).3–7 One in 3 of all hospitalization of children with Down’s syndrome less than 3 years of age are caused by RTIs, with 80% occurring before 2 years of age.8,9
Children with Down’s syndrome on average spend 2–3 times more time in hospital than those without Down’s syndrome.4,10 In children with Down’s syndrome, up to the age of 18, RTIs are the second leading cause of death. It is therefore important that effective interventions to prevent and treat these infections are developed. A number of preventive interventions are commonly practiced and believed to be of benefit including use of prophylactic antibiotics, respiratory syncytial virus (RSV) prophylaxis for subgroups (eg, those with cardiac disease), additional immunizations and longer treatment courses. However, no previous systematic review has been undertaken to ascertain the evidence base.
The aim of this study is to systematically review the literature on the management of RTIs in this vulnerable group to identify which strategies work best.
Methods
Search Strategy
We developed a broad search strategy combining the terms Down’s Syndrome, Respiratory Tract Infections and relevant synonyms. To increase the yield of potential relevant articles, management options for Down’s syndrome-related comorbidities such as sleep-disordered breathing, chronic lung disease and congenital heart disease were also included in the syntax. To obtain a broad overview of the existing evidence base, we did not limit our search strategy to specific study types, language or publication date (Appendix 1).
Information Sources
We searched the following electronic databases from their inception up to February 2015: PubMed, EMBASE, CINAHL and Cochrane Library. Trial registries such as WHO ICTRP and clinicaltrials.gov were also searched for completed or ongoing studies. To identify any additional studies, reference lists of all included articles and relevant systematic reviews were screened.
We searched gray literature through web searches via Google Scholar, SIGLE and relevant research websites [including National Institute for Health Research (NIHR), Wellcome Trust and Medical Research Council]. Contact with research networks and charities were also made (including Trisomy 21 Research Society,11 Down’s Syndrome Association,12 Downs Syndrome International,13 Down’s Heart Group14 and Downs Syndrome Medical Interest Group United Kingdom and Ireland).15
Eligibility Criteria
We included studies of individuals with Down’s syndrome irrespective of age and covering any intervention (ie, medical and/or surgical) for the prevention or treatment of RTIs including watchful waiting and supportive care. We anticipated that the number of randomized controlled trials (RCTs) for this topic would be limited because of the specific study population. Therefore, we included all study types except for case series and case reports.
Study Selection and Inclusion
Two review authors (L.M. and K.R.) independently screened titles and abstracts retrieved from the database searches along with the reference lists of the included studies and relevant systematic reviews. The same authors independently reviewed the full text of potentially relevant studies against the predefined eligibility criteria. A third author (R.V.) reviewed the discrepancies, and differences were resolved by consensus. Data extraction was performed by 1 reviewer (K.R.) and was independently checked by 2 reviewers (L.M. and R.V.). Two reviewers (L.M. and R.V.) independently performed the quality assessment of included studies.
Outcomes of Interest
As our systematic review aimed to identify interventions to either prevent or treat RTIs, identified papers were likely to encompass a broad range of outcome measures. As a consequence, we did not pre-specify any detailed outcome measures. We looked specifically at impact on frequency and recurrence of RTIs and any documented adverse effects.
Data Extraction
Data were extracted using a standardized form including information on study characteristics, setting, design, randomization, inclusion and exclusion criteria, data-analysis methods, interventions, outcomes and results.
Risk of Bias Assessment
We measured risk of bias in RCTs and non-randomized studies using the relevant “Risk of Bias” tools developed by Cochrane.16,17 We excluded studies with a critical risk of bias from our analyses.
Assessment of Heterogeneity
We assessed clinical heterogeneity across the included studies by reviewing differences in populations, interventions and outcomes measured. In view of the marked differences in the interventions used in the individual studies, we did not perform a meta-analysis.
Role of the Funding Source
This article presents independent research funded through a PhD fellowship awarded to the first author by the NIHR. The views expressed are those of the authors and not necessarily those of the NIHR.
RESULTS
Study Selection
Our searches identified 13,575 articles. After screening of titles and abstracts, 157 potentially relevant published articles were identified. After reviewing the full texts, 5 published studies were deemed suitable for inclusion (Fig. 1).18–22 An additional unpublished ongoing study was identified through our searches.23
FIGURE 1.
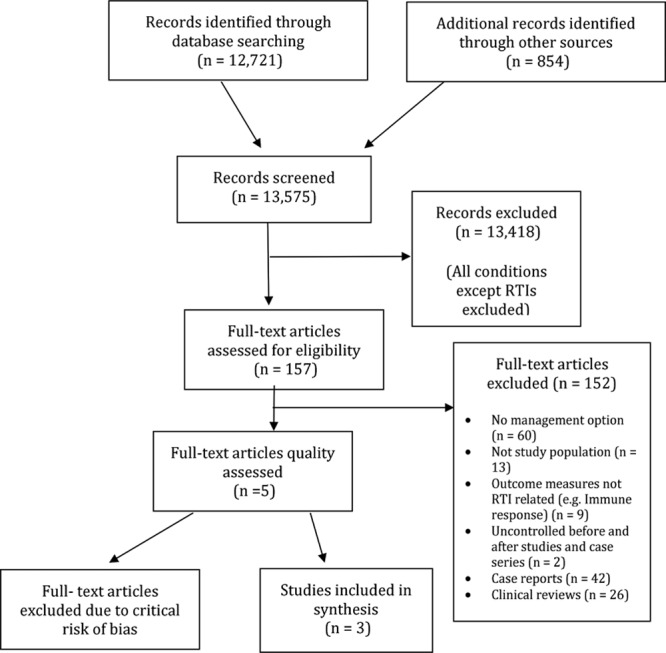
Flow diagram of search results and selecting studies for inclusion.
Study Characteristics
The main characteristics of the 5 published studies are presented in Table 1. All studies evaluated preventative interventions against RTI in children with Down’s syndrome: 2 studies assessed the effectiveness of passive immunotherapy, with palivizumab and pidotimod, respectively18,19; 2 studies looked at prophylactic treatment with oral zinc supplements20,22 and 1 study at the effects of a school-based infection-control program.21
Table 1.
Characteristics of Included Published Studies
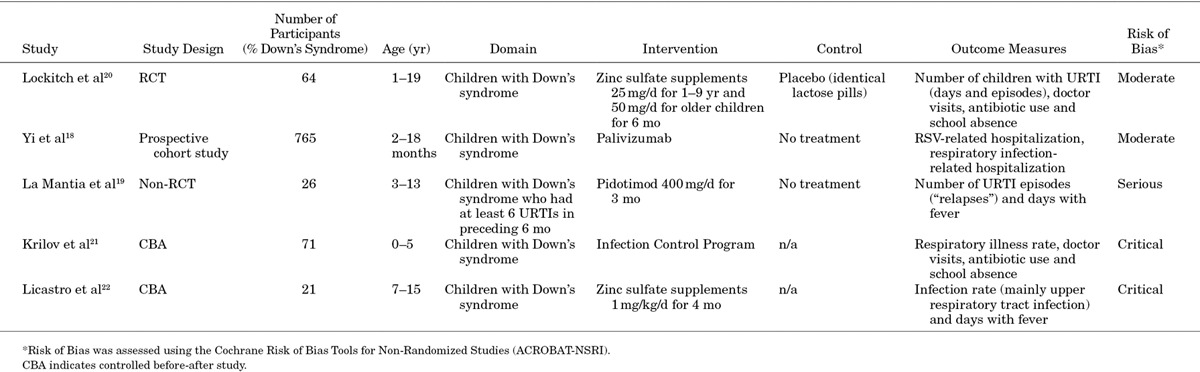
All studies exclusively studied children with Down’s syndrome. The studies varied in terms of design (1 RCT, 1 non-RCT, 1 cohort study and 2 controlled before-after studies), age range of included children and duration of follow-up (Table 1). Two studies were conducted in Italy,19,22 1 in Canada,20 1 in Canada and the Netherlands18 and 1 in the United States.21
We identified 1 postmarketing observational study ongoing in Japan, looking at the effects of palivizumab in preventing lower RTIs caused by RSV in children under the age of 2 who are either immunocompromised or who have Down’s syndrome.23
Risk of Bias Across Studies
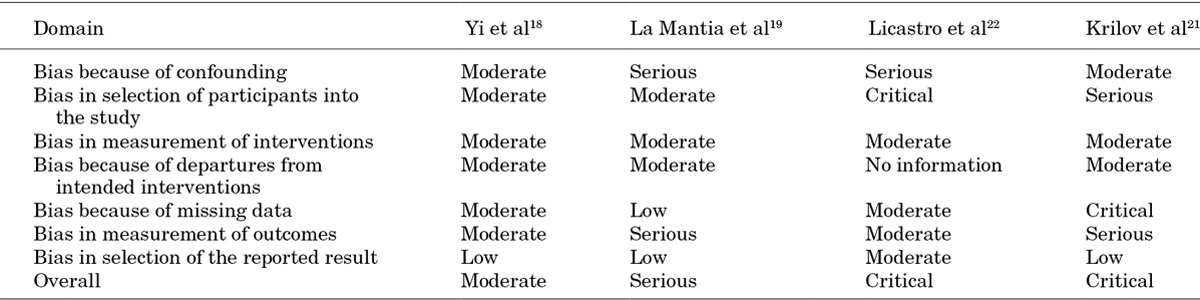
The overall risk of bias of the RCT was moderate (Table 2). The overall risk of bias of the non-randomized studies was moderate for the cohort study20 (although high quality, there was no controlled comparator arm) and serious for the non-RCT.19 For the 2 controlled before-after studies, risk of bias was noted to be critical, and they were therefore excluded from analyses (Table 3).21,22
Table 2.
Risk of Bias Assessment for Lockitch et al20 Using the Cochrane Risk of Bias Tool 3
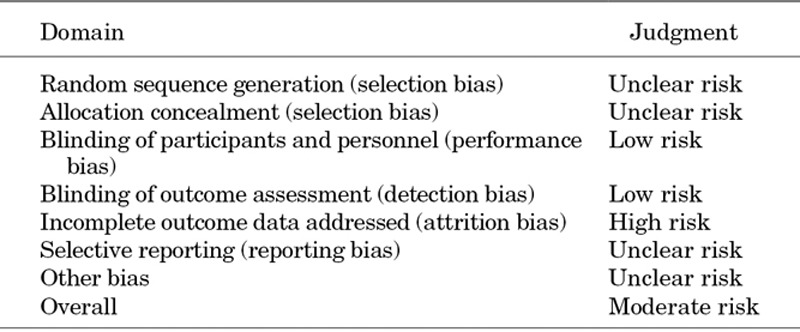
Table 3.
Risk of Bias Assessment for Nonrandomized Studies Using the ACROBAT-NSRI14
Results of Individual Studies
Zinc Therapy
Lockitch et al randomized 64 children with Down’s syndrome to oral zinc therapy for 6 months or placebo, but reported only on the 50 children (23 treated with zinc and 27 with placebo) who had extreme numbers (ie, exceeding the 90th percentile value for siblings and age-matched unrelated children).
In this subset of children with Down’s syndrome, during 6 months of treatment, no significant differences in terms of upper RTI episodes, doctor consultations and antibiotic use were found between children receiving zinc and children receiving placebo.20
Pidotimod
In a non-RCT, La Mantia et al followed 26 children with Down’s syndrome who had experienced at least 6 upper RTIs in the preceding 6 months and who received either the immunostimulant pidotimod for 3 months (14 children) or no treatment (12 children). While on pidotimod treatment, children with Down’s syndrome had fewer parent-reported upper RTI recurrences [mean 2.7, standard deviation (SD) 1.1 vs. mean 6.8, SD 1.3] and days with fever (mean 4.5, SD 3.5 vs. mean 16.9, SD 6.7) compared with those not receiving this treatment.19
Palivizumab
In a prospective cohort study, Yi et al followed 532 Canadian children with Down’s syndrome treated with the palivizumab for 2 RSV seasons and 233 Dutch children with Down’s syndrome who did not receive this immunostimulant. In the first 2 years of life, treatment with palivizumab resulted in a 3.6-fold reduction in the incidence rate ratio (adjusted incidence rate ratio 3.63, 95% confidence interval: 1.52–8.67) of RSV-RTI hospitalizations. Treatment, however, did not reduce overall hospitalizations for RTI (adjusted incidence rate ratio 1.11, 95% confidence interval: 0.80–1.55).18
DISCUSSION
By a broad systematic search and review of the literature, we identified only 5 published studies on the management of RTIs in children with Down’s syndrome. This is remarkable in the light of the large body of literature in this field: Most high-quality studies on the management on RTIs in children have excluded this vulnerable group that are at high risk of these infections.
We found that pidotimod, an immunostimulant, and palivizumab, a human monoclonal antibody, may have a role in preventing RTIs in children with Down’s syndrome with the latter particularly effective in young children (until 2 years of age) against RSV-RTI hospitalizations. Currently, the American Academy of Pediatrics recommends the use of palivizumab in children with Down’s syndrome who are at risk of severe RSV-related infections (eg, congenital heart disease, airway clearance issues, prematurity).24 Once published, the ongoing postmarketing observational study is likely to add to this evidence base. Oral zinc was not noted to be effective in preventing RTIs.
The evidence base for RTI management in people with Down’s syndrome is incomplete, with only a limited number of moderate to serious risk of bias studies available on the prevention of RTIs in children. As such, clinical management of RTIs in this vulnerable group is currently guided by studies including either no or only limited number of children with Down’s syndrome. Methodologically rigorous studies are warranted to guide clinicians on how best to prevent and treat RTIs in children with Down’s syndrome.
APPENDIX 1.
Down’s SYNDROME TERMS
MeSH: “Down Syndrome” OR TiAB: Down* syndrome* OR mongolism OR trisomy 21 OR aneuploidy OR down* disease* OR mongoloid idiocy OR Trisomy G AND
INFECTION TERMS
MeSH: Respiratory tract diseases OR Otitis media OR Respiratory syncytial virus, human OR Empyema OR TiAB: respiratory tract infection* OR upper respiratory infection* OR lower respiratory infection* OR RTI OR URTI OR LRTI OR rhinitis OR common cold* OR head cold* OR sinusitis OR rhinosinusitis OR pharyngitis OR laryngitis OR tracheitis OR tonsillitis OR sore throat* OR croup OR epiglottitis OR otitis media OR AOM OR OME OR glue ear OR ear discharge OR otorrhoea OR otorrhea OR bronchitis OR bronchopneumonia OR pneumonia OR cough* OR bronchiolitis OR respiratory syncytial virus OR RSV or empyema OR influenza* OR lung abscess* OR pulmonary tract infection* respiration tract infection* OR human flu OR pulmonary abscess* OR Nasal Catarrh* OR middle ear inflammation* OR bronchial pneumonia* OR lung inflammation* OR
ASSOCIATED CONDITION TERMS
MeSH: cardiovascular diseases OR TiAB: respiratory tract disease* OR lung disease* OR cardiovascular disease* or obstructive sleep apnea* or pleural cyst* or congenital heart disease* or atrioventricular canal defect* or atrial septal defect* or ventricular septal defect* or patent ductus arteriosus or tetralogy of fallot OR double outlet right ventricle or mitral valve prolapse or aortic regurgitation or acquired valve disease* or OSAHS OR fallot* tetralogy or AVC defect* or heart atrium septum defect or heart ventricle septum defect or floppy mitral valve* or aortic incompetence or aortic valve insufficiency or heart valve disease*
Footnotes
We have read and understood the Pediatrics Infectious Diseases Journal policy on declaration of interests and declare that L.M. had financial support from the UK NIHR for the submitted work. There are no financial relationships with any organizations that may have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work exist. The authors have no conflicts of interest to disclose.
L.M., K.R. and R.V. wrote the draft protocol and manuscript. L.M. and K.R. performed systematic literature searches and screened the abstracts. L.M., K.R. and R.V. extracted data and assessed the qualities of the studies. All authors conceived the study, contributed to writing and approved the final manuscript. L.M. and K.R. had full access to all of the data in the study and take responsibility for the integrity and accuracy of the data analysis. L.M. and M.L. are the study guarantors. Supported by the UK NIHR under its Doctoral Research Fellow scheme. M.L. was partly supported by the NIHR Collaboration for Leadership in Applied Health Research and Care North Thames at Bart’s Health NHS Trust, and A.S. was supported by an NIHR Research Professorship in ENT. The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR or the Department of Health. P.L. is supported by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care South London at King’s College Hospital NHS Foundation Trust.
REFERENCES
- 1.Morris JK, Risley L. The National Down Syndrome Cytogenetic Register for England and Wales: 2010 Annual Report. Britain, Ireland: BINOCAR; 2011. [Google Scholar]
- 2.Morris JK, Alberman E. Trends in Down’s syndrome live births and antenatal diagnoses in England and Wales from 1989 to 2008: analysis of data from the National Down Syndrome Cytogenetic Register. BMJ Br Med J. 2009;339:b3794–b3794. doi: 10.1136/bmj.b3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watts R, Vyas H. An overview of respiratory problems in children with Down’s syndrome. Arch Dis Child. 2013;98:812–817. doi: 10.1136/archdischild-2013-304611. [DOI] [PubMed] [Google Scholar]
- 4.McDowell KM, Craven DI. Pulmonary complications of Down syndrome during childhood. J Pediatr. 2011;158:319–325. doi: 10.1016/j.jpeds.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Bloemers BL, Broers CJ, Bont L, et al. Increased risk of respiratory tract infections in children with Down syndrome: the consequence of an altered immune system. Microbes Infect. 2010;12:799–808. doi: 10.1016/j.micinf.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Bloemers BL, van Furth AM, Weijerman ME, et al. Down syndrome: a novel risk factor for respiratory syncytial virus bronchiolitis–a prospective birth-cohort study. Pediatrics. 2007;120:e1076–e1081. doi: 10.1542/peds.2007-0788. [DOI] [PubMed] [Google Scholar]
- 7.Broers CJ, Gemke RJ, Weijerman ME, et al. Frequency of lower respiratory tract infections in relation to adaptive immunity in children with Down syndrome compared to their healthy siblings. Acta Paediatr. 2012;101:862–867. doi: 10.1111/j.1651-2227.2012.02696.x. [DOI] [PubMed] [Google Scholar]
- 8.So SA, Urbano RC, Hodapp RM. Hospitalizations of infants and young children with Down syndrome: evidence from inpatient person-records from a statewide administrative database. J Intellect Disabil Res. 2007;51(pt 12):1030–1038. doi: 10.1111/j.1365-2788.2007.01013.x. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald P, Leonard H, Pikora TJ, et al. Hospital admissions in children with Down syndrome: experience of a population-based cohort followed from birth. PLoS One. 2013;8:e70401. doi: 10.1371/journal.pone.0070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watts R, Vyas H. An overview of respiratory problems in children with Down’s syndrome. Arch Dis Child. 2013;98:812–817. doi: 10.1136/archdischild-2013-304611. [DOI] [PubMed] [Google Scholar]
- 11.Trisomy 21 Research Society. 2016. Feb 04, Available at: http://www.t21rs.org/. Accessed October 2, 2015.
- 12.Down’s Syndrome Association. 2016. Feb 02, Available at: http://www.downs-syndrome.org.uk/. Accessed October 2, 2015.
- 13.Down Syndrome International. Welcome to the Down Syndrome International (DSi) website! February 4, 2016. Available at: https://www.ds-int.org/. Accessed October 2, 2015.
- 14.Down’s Heart Group ... supporting a better life. 2016. Feb 04, Available at: http://www.dhg.org.uk/. Accessed October 2, 2015.
- 15.DSMIG – Down Syndrome Medical Interest Group. 2016. Feb 04, Available at: http://www.dsmig.org.uk/. Accessed October 2, 2015.
- 16.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. Updated March 2011. [Google Scholar]
- 17.Sterne JAC, Higgins JPT, Reeves BC On behalf of the Development Group for ACROBAT-NRSI. A Cochrane Risk of Bias Assessment Tool: For Non-Randomized Studies of Interventions (ACROBAT-NRSI). Version 1.0.0. 2014. [Google Scholar]
- 18.Yi H, Lanctôt KL, Bont L, et al. CARESS Investigators. Respiratory syncytial virus prophylaxis in Down syndrome: a prospective cohort study. Pediatrics. 2014;133:1031–1037. doi: 10.1542/peds.2013-3916. [DOI] [PubMed] [Google Scholar]
- 19.La Mantia I, Grillo C, Mattina T, et al. Prophylaxis with the novel immunomodulator pidotimod reduces the frequency and severity of upper respiratory tract infections in children with Down’s syndrome. J Chemother. 1999;11:126–130. doi: 10.1179/joc.1999.11.2.126. [DOI] [PubMed] [Google Scholar]
- 20.Lockitch G, Puterman M, Godolphin W, et al. Infection and immunity in Down syndrome: a trial of long-term low oral doses of zinc. J Pediatr. 1989;114:781–787. doi: 10.1016/s0022-3476(89)80136-2. [DOI] [PubMed] [Google Scholar]
- 21.Krilov LR, Barone SR, Mandel FS, et al. Impact of an infection control program in a specialized preschool. Am J Infect Control. 1996;24:167–173. doi: 10.1016/s0196-6553(96)90008-5. [DOI] [PubMed] [Google Scholar]
- 22.Licastro F, Chiricolo M, Mocchegiani E, et al. Oral zinc supplementation in Down’s syndrome subjects decreased infections and normalized some humoral and cellular immune parameters. J Intellect Disabil Res. 1994;38(pt 2):149–162. doi: 10.1111/j.1365-2788.1994.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 23.Yasuhiko S. In: clinicaltrials.gov. Bethesda, MD: National Library of Medicine; 2013. Synagis® liquid 50 mg, 100 mg for intramuscular injection special investigation in immunocompromised children with synagis. [Google Scholar]
- 24.Village EG. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–420. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]


