Abstract
Context:
Hand, foot and mouth disease (HFMD) is a widespread pediatric disease caused primarily by human enterovirus 71 (EV-A71) and Coxsackievirus A16 (CV-A16).
Objective:
This study reports a systematic review of the epidemiology of HFMD in Asia.
Data Sources:
PubMed, Web of Science and Google Scholar were searched up to December 2014.
Study Selection:
Two reviewers independently assessed studies for epidemiologic and serologic information about prevalence and incidence of HFMD against predetermined inclusion/exclusion criteria.
Data Extraction:
Two reviewers extracted answers for 8 specific research questions on HFMD epidemiology. The results are checked by 3 others.
Results:
HFMD is found to be seasonal in temperate Asia with a summer peak and in subtropical Asia with spring and fall peaks, but not in tropical Asia; evidence of a climatic role was identified for temperate Japan. Risk factors for HFMD include hygiene, age, gender and social contacts, but most studies were underpowered to adjust rigorously for confounding variables. Both community-level and school-level transmission have been implicated, but their relative importance for HFMD is inconclusive. Epidemiologic indices are poorly understood: No supporting quantitative evidence was found for the incubation period of EV-A71; the symptomatic rate of EV-A71/Coxsackievirus A16 infection was from 10% to 71% in 4 studies; while the basic reproduction number was between 1.1 and 5.5 in 3 studies. The uncertainty in these estimates inhibits their use for further analysis.
Limitations:
Diversity of study designs complicates attempts to identify features of HFMD epidemiology.
Conclusions:
Knowledge on HFMD remains insufficient to guide interventions such as the incorporation of an EV-A71 vaccine in pediatric vaccination schedules. Research is urgently needed to fill these gaps.
Keywords: hand, foot and mouth disease, EV-A71, CV-A16, epidemiology
Hand, foot and mouth disease (HFMD) has become an endemic childhood disease in East and Southeast Asia. Its main etiologic agents are human enterovirus 71 (EV-A71) and Coxsackievirus 16 (CV-A16). Although usually mild—with symptoms limited to >38°C fever, malaise, rashes on the volar regions of the hands and feet, herpangina and difficulty eating and drinking—more rarely, infection can lead to complications of the nervous or cardiopulmonary systems. Such cases can result in long-term sequelae such as cognitive and motor disorders1,2 or death, usually from pulmonary edema or brainstem encephalitis.3 Although complications are rare, the number of children being infected in high-incidence countries such as China (≈2.7 M cases in 20143) means the death toll can be substantial (384 deaths in China in 20143). The EV-A71 virus seems to be responsible for more severe outcomes, while CV-A16 and other Coxsackieviruses, such as CV-A2, CV-A6 and CV-A10, usually present milder symptoms that resolve within a few weeks.4–6
There are nearly 25 years of literature from Asia that describes the epidemiology of HFMD, drawing on pediatric cohorts, national surveillance systems, outbreak investigations and clinical data, and from disparate countries that span stages of economic development and with climates that range from tropical to temperate. This diversity complicates attempts to identify general features of HFMD epidemiology and conceals gaps in the body of knowledge of this important pediatric disease.
The objective of this paper is to provide a robust systematic review of the epidemiology of HFMD that informs public health policy making about HFMD epidemics. The review covers 3 major areas: (1) history and seasonality of HFMD, and the efforts in predictive modeling; (2) risk factors for infection, to guide control and (3) global epidemiologic parameters, such as the incubation period and basic reproduction number, which may determine the effectiveness of control policies.
Methods
Search Strategy and Selection Criteria
Using a combination of search terms, including “Hand foot and mouth disease,” “Hand foot and mouth,” “HFMD,” “Enterovirus,” “Enterovirus 71,” “EV-A71,” “Coxsackie A16,” “CV-A16,” “CVA16,” we searched PubMed, Thomson Reuters Web of Science and Google Scholar to identify 1305, 1255 and 100 articles, respectively.
Eligibility criteria were articles that: (1) were published in peer-reviewed journals from January 1957 to December 2014; (2) were studies with epidemiologic and/or serologic information (quantitative/qualitative) about incidence and prevalence of HFMD; and/or (3) contained information about factors associated with prevalence and incidence and/or (4) employed statistical models to derive the above. Articles not in English, not related to HFMD, or HFMD articles that did not cover epidemiologic or clinical factors were excluded.
Two independent readers examined each of the 407 abstracts to determine if specific research questions were answered. The 8 specific research questions were as follows: (1) What time of the year do HFMD outbreaks occur, and with what seasonal factors are outbreaks associated? (2) How long have EV-A71 and CV-A16 been circulating in Asia? (3) What age groups are at higher risk of infection? (4) What risk factors are associated with infection and severe outcomes? (5) Where do infections predominantly occur (home or school)? (6) What is the incubation period? (7) What proportion of infections are symptomatic? and (8) What is the basic reproduction number for HFMD by virus? An article was retained as long as both readers indicated that it answered at least 1 specific research question and was discarded if both readers agreed that no questions were answered. A third independent reader arbitrated when there was a disparity between the original readers.
The 2 original readers each read the full text of half of the articles to identify answers to the questions. A second pair of independent readers read the articles again. Finally, the first author compiled all answers to the specific questions and compared the extracted answers to the original text. Relevant references from these papers were included in the analysis, in particular to identify non-English and early references. In total, information from 242 papers was compiled and 108 papers were used in data synthesis.
Hourly weather data were downloaded from the Weather Underground and aggregated at a weekly scale. Incidence data from Tokyo, Hong Kong, Taiwan and Singapore were extracted from routine surveillance data published by government agencies (the National Institute of Infectious Diseases, Japan7; the Department of Health, Hong Kong8; the Taiwan National Infectious Disease Statistics System9 and the Ministry of Health, Singapore).
Nontabular data were extracted from figures using Plot Digitizer.10 Data on weather and incidence were analyzed using a time series model. Symptomatic proportions were pooled by aggregating denominators and numerators. Other analyses used standard statistical methods and were conducted using R.11
RESULTS
Timing and Seasonality of HFMD Outbreaks
Outbreaks of HFMD do not occur uniformly throughout the year across Asia. In Fukuoka, Japan, for example, weekly numbers of HFMD cases have been found to increase with average temperature and humidity, especially among younger children.12 By digitizing the incidence data from publications on Japan5,12–14 and North China15–20 (Fig. 1), we observe that May through July are the months with highest incidence in temperate regions of Asia. However, this relationship is less clear for tropical and subtropical Asia. The extracted data on Southwest China,15,21 South China,2,15,22,23 Hong Kong24,25 and Taiwan26–28 show that outbreaks typically happen in late spring and fall. No distinct pattern is obvious for tropical regions as seen from data in Thailand,29–31 Vietnam,32,33 Malaysia34 and Singapore,35–38 where outbreaks occur sporadically throughout the year, although models have been developed for Singapore (≈1° north) that show a positive statistical relationship between maximum daily temperature above 32°C with HFMD incidence in the subsequent 1–2 weeks.37
FIGURE 1.
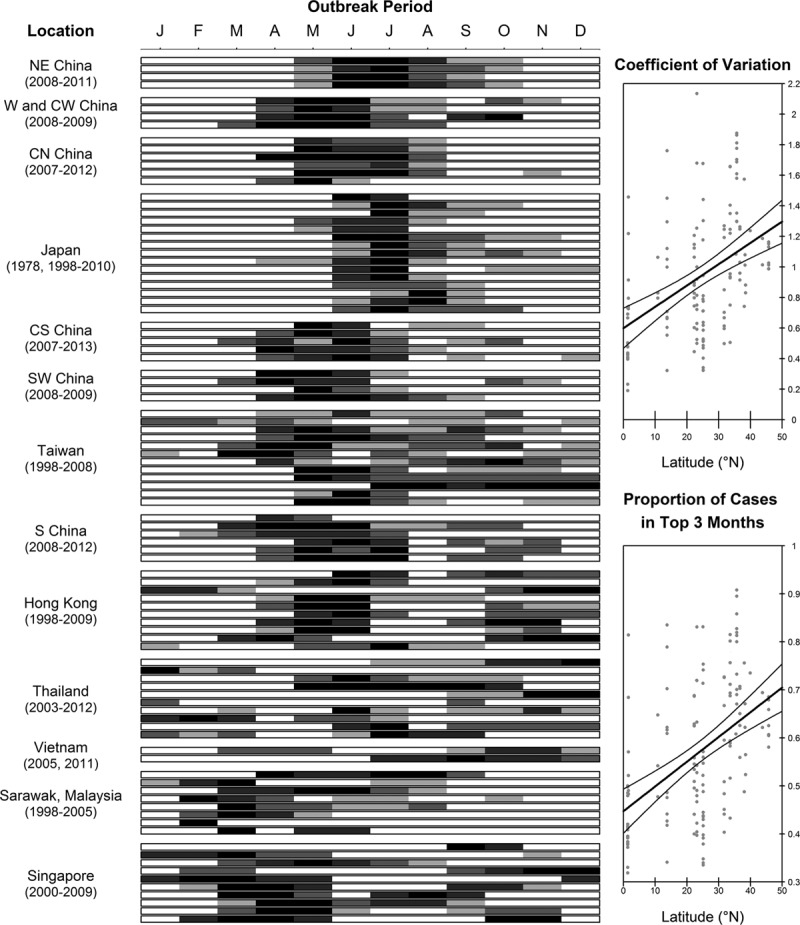
Temporal patterns of HFMD outbreaks in Asia, by latitude. Left: Plot Digitizer is used to convert charts into numbers. White boxes are the months where HFMD cases fall below the year’s median. The remaining cells are then shaded into 4 darker shades by octiles. The regions of China were based on Wang et al’s classification101(C standing for central). The regions are arranged by latitude. South China, Hong Kong and Taiwan have subtropical climates. Areas further north are temperate, while the Southeast Asian regions are tropical. Right: The coefficient of variation is the ratio of the standard deviation to its mean, and the proportion of cases in top 3 months is the proportion of cases of the 3 months with highest incidence to the annual incidence. Points represent 1 year per region. The lines are obtained from ordinary least squares regression with latitude as the independent variable and show how clearly defined epidemics become the further north from the equator.
To assess how general the relationship between climate and transmissibility of HFMD was, we took incidence data from Tokyo, Hong Kong, Taiwan and Singapore (Fig. 2, Appendix 1), that is, spanning temperate, subtropical and tropical latitudes, and fitted time series models to them. After controlling for contagion via autoregression terms, the effect of meteorologic factors was weak: a small positive increase in transmissibility with rising absolute humidity/temperature during the current week in Tokyo and Singapore. There was no evidence for temperature and humidity in having the same effect in Hong Kong or Taiwan, although rising relative humidity seems to decrease transmissibility in Singapore.
FIGURE 2.
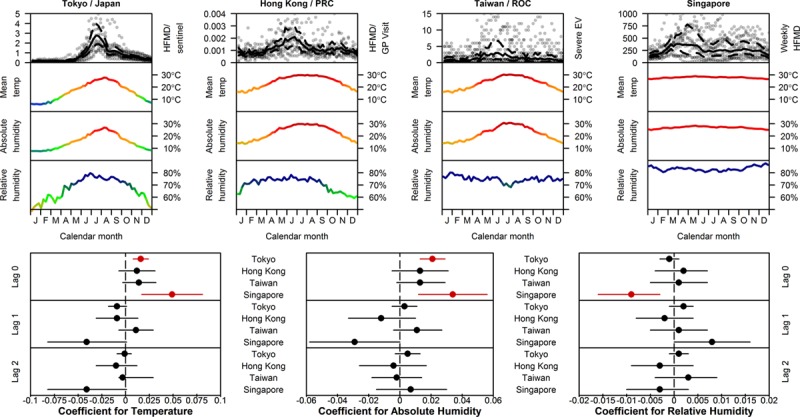
Temporal incidence of HFMD or enterovirus and climatic factors for 4 Asian cities spanning temperate, subtropical and tropical latitudes. Top: incidence, temperature, absolute and relative humidity. Top panels indicate incidence (data in gray, mean and 95% interval in black) for the time period Jan 2001 to Mar 2012 (Tokyo), Jan 2001 to Dec 2009 (Hong Kong, Peoples Republic of China), Jan 2001 to Dec 2011 (Taiwan, Republic of China) and Jan 2001 to Jan 2012 (Singapore). Middle and bottom panels show mean daily temperature, absolute and relative humidity at Tokyo Narita, Hong Kong International, Taipei Taoyuan International and Singapore Changi airports, downloaded from the Weather Underground. Bottom: Coefficients of meteorological variables at 0–2 week time lags in autoregressive models of Z-scored HFMD case counts to facilitate comparison between locations. Each city is analyzed separately using a model in which HFMD incidence in week t is (auto)regressed on incidence in weeks t-1 up to t-3 (using the Akaike information criterion to select the order of the autoregression component) and, independently, on each meteorological variable. Weather parameters are not regressed together in a single model because of collinearity. The effect of weather on HFMD incidence can be seen by coefficient mean (points) and 95% confidence intervals (lines), colored red if statistically significant at the 5% level.
The earliest recorded cases of HFMD in Asia are from Japan (1967),30 Singapore (1970),31 Taiwan (1980)32 and Shanghai, China (1981).33 Since then, outbreaks have been reported in many parts of Asia, including mainland China,12–14,33–52 Korea,53–55 Japan,56–70 Taiwan,6,69,71–74 Hong Kong,17,18,75 India,76–81 Thailand,21,23,82 Vietnam,24 Malaysia,26,69,83–87 Singapore4,88 and Brunei,89 as summarized in Figure 3. These reported outbreaks are unlikely to reflect the true first outbreaks of HFMD, as serologic studies provide evidence that by the time surveillance systems were established, EV-A71 and CV-A16 were already endemic in many of these countries. Early serologic tests conducted in Japan in 1970 show evidence of EV-A71 and CV-A16 circulation.90 Serum taken in the late 1990s in Singapore, before the start of surveillance in 2000, shows that around 50% children and 44% cord blood, indicating maternal infection, had already seroconverted to EV-A71.91 Blood samples from Taiwan (1989–1997) show 3%–11% EV-A71 incidence per year, and up to 68% of children92 had serologic evidence of EV-A71 infection before the large HFMD outbreak of 1997. Similarly, although China has reported millions of HFMD cases since the beginning of the HFMD surveillance program in 2008, evidence from Anhui47 shows high seroprevalence of up to 74.6% in older children before the 2008 outbreaks. Retrospective seroepidemiologic tests from blood serum collected in 200593 also show that China had positive rates of 32.0% to EV-A71 and 43.4% to CV-A16, indicating that outbreaks happened earlier but were simply not reported in the literature.
FIGURE 3.
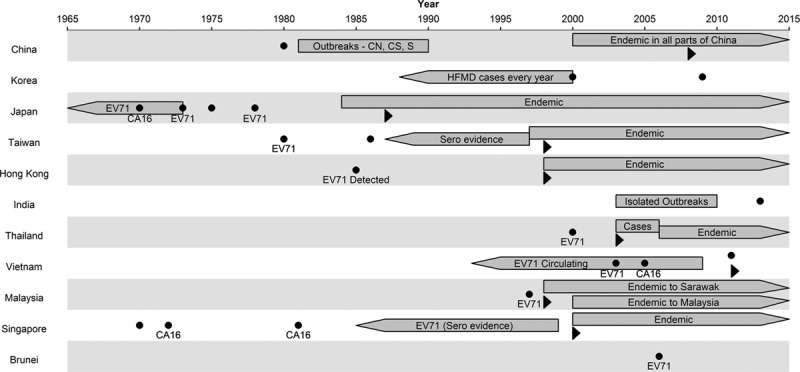
Historical establishment of HFMD in Asia. Dots represent a reported outbreak in that year, with the main causal agent written below. Boxes with right arrow indicate endemicity of HFMD, evidenced by repeated reporting of HFMD. Boxes with left arrow indicate seroepidemiologic evidence that the pathogen is already in existence, even if no significant outbreaks were documented previously. Triangles indicate the point where data on HFMD started to be collected systematically, for example, through government surveillance. The length of the left arrows are arbitrary as there is no way to know how long has HFMD been circulating before the tests.
Risk Factors
Risk factors for infection are depicted in Figure 4 (Appendix 2) and summarized below.
FIGURE 4.
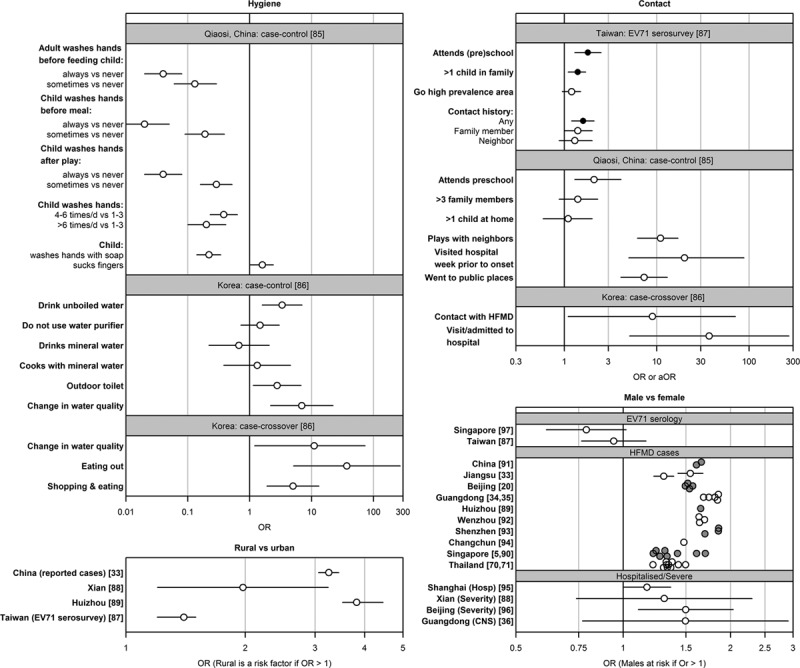
Excerpts from studies on risk factors. Ruan et al case-control study in Qiaosi,94 China, was conducted by taking 273 diagnosed HFMD/herpangina as cases (6 years of age or younger) and 273 stratified random sample as controls. Park et al case-control study uses hospital cases of enteroviral aseptic meningitis (n = 205) and HFMD (n = 116), and nonenteroviral disease controls (n = 170). Their case-crossover design uses only cases. 1–7 days before admission was set as the hazard period, and 22–28 days prior to admission was set as the nonhazard period. Both studies gather data via questionnaires. White points indicate nonadjusted ORs, black points indicate adjusted OR. For the effect of sex (bottom right), confidence intervals are omitted from complete case notifications, and colored gray and white alternately for visual distinction.
Hygiene
Evidence from Qiaosi, China,94 indicates the importance of hygiene for protection against HFMD infection. Children who always wash their hands before meals are about 50 times less likely to contract HFMD, while those whose caregivers wash their hands before feeding are about 25 times less likely. Additional protective habits include washing of hands after play, washing of hands more than 4 times per day, using soap, and not sucking fingers.
A study in Korea95 revealed that drinking unboiled water [odds ratio (OR): 3.34 (1.59–6.99)], a change in water quality such as color, taste, smell, presence of precipitation or floating materials [OR: 6.93 (2.17–22.15)], using communal toilets/toilets outside the house [OR: 2.77 (1.14–6.74)] and eating outside the home [OR: 37.0 (5.1–269.5)] were risk factors for HFMD.
Rural Versus Urban Areas
All papers51,96–98 that compared urban with rural areas agreed that the latter conferred a higher risk for HFMD. However, this might be confounded by socioeconomic status and hygiene practices.
Sex
Although most papers show that being male is a risk factor for both mild4,14,16,23,27,34,37,51,82,98–101 and severe52,97,102,103 HFMD (OR ranges between 1.2 and 2), surprisingly, serologic evidence does not support this finding: A study from Singapore104 shows marginal evidence that females are more likely to have seroconverted to EV-A71 [OR: 0.79 (0.61–1.01)], while a Taiwanese96 study shows no statistically significant differences [OR: 0.94 (0.76–1.16)]. Taken together, these suggest that infection rates are comparable, but that boys are more likely to develop symptoms, more involved in propagation of outbreaks or more likely to be brought for medical care than girls.
Other
A case–control study in Xi’an, China,97 found that breastfeeding may lower the risk of developing severe HFMD [adjusted OR: 0.57 (0.33–0.98)], even though breastfeeding does not apparently lower the chance of being infected by EV-A71 [OR: 1.1 (0.93–1.3)].96 It further found that patients with a history of Epstein–Barr virus are at greater risk of contracting severe, rather than mild, HFMD [adjusted OR: 2.6 (1.5–4.4)]. A spatial-temporal model of Guangdong14 showed that sunshine could be protective against HFMD. This is agreed by a matched case-control study of preschoolers in Beijing,41 which showed that UV radiation in classrooms is associated with lower HFMD attack rate (P value of 0.027), and recommended installing UV lamps to sterilize unoccupied classrooms. These findings are, however, inconsistent with the seasonal nature of HFMD, where outbreaks in temperate countries tend to occur in summer, when sunlight and UV exposure are strongest.
Age Distribution of HFMD Cases
The age distribution of HFMD cases in Asia, compiled from a variety of sources including surveillance and cohort data, is summarized in Figure 5. Data from China12–14,34,49–52,94,100–103,105–107 and Taiwan5,6,73,108–111 are particularly abundant. Other sources include Hong Kong,17,18 India,76,80 Japan,56,112 Korea,54,95 Malaysia,84,113 Singapore,4,27,88 Thailand22,23 and Vietnam.24
FIGURE 5.
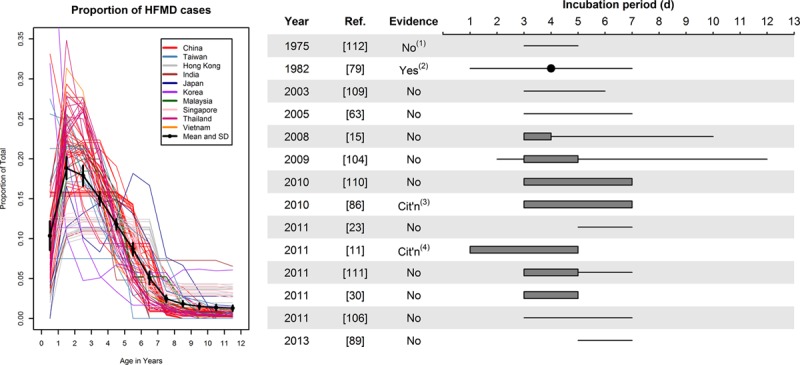
HFMD cases by age and estimates of incubation period. Left: Each line indicates a unique data set (total 79 lines). Distributions within age ranges were assumed to be constant. The black dots are average proportion for that age (with 95% CI). Right: Reported incubation periods for HFMD, by year of publication and provision of evidence to support claimed period. Lines indicate that the incubation period “is X days.” Gray bars indicate that the incubation period is “usually” or “typically” X days. Gray bars with lines shows an extended interval “can be up to X days.” The point indicates a median. Notes: (1) provides information within the paper, which is inconsistent in this estimate; (2) uses generation interval distribution as a proxy for incubation period; (3) cites a US CDC factsheet on aseptic meningitis, which in turn provides no supporting evidence and (4) cites Goh et al.88 CI indicates confidence interval.
The symptomatic HFMD incidence rate varies widely even within the narrow 0- to 6-year age-band. The greatest proportion of cases occur at ages 1 [18.8% (17.4%–20.2%)] and 2 [17.9% (16.6%–19.2%)]. By the age of formal schooling, from 6 years in most Asian countries, the proportion is substantially lower [8.7% (7.9%–9.5%)]. Overall, 82.6% (82.2%–82.9%) of all cases occur before age 6. The lower rate during the first year of life could be because of lack of contact with other children or to presence of maternal antibodies.91
Community Versus School as Medium for Infection
The literature is ambiguous about the importance of locations for transmission. Four studies showed that contact with a case, particularly a household member, is as or more significant a risk factor than preschool attendance.21,88,96,108 An early study in Singapore observed 60 families with secondary cases and found the secondary attack rate amongst children below 12 years old to be 77%.88 Similarly, in a large seroepidemiologic study of EV-A71 in Taiwanese children,96 multivariate analysis showed attendance at a preschool imparted a similar magnitude of risk as contact with a case [adjusted ORs: 1.6 (1.2–2.1) and 1.8 (1.3–2.5), respectively], as well as a strong concordance (84%) between seropositivity in younger and older siblings.
Also, a number of studies showed that a higher percentage of diagnoses occurred among children who did not attend a nursery or preschool.37,51 Liu et al49 note that about half of symptomatic cases in Nanchang, China, are among children under 3 years, the age at which preschooling starts in China.
Conversely, some studies suggest that preschool attendance is a key risk factor.4,114 For example, a seroepidemiologic study in 1996 to 1997 in Singapore showed that seropositivity to EV-A71 increases rapidly from age 2 to 5,91 when attendance at childcare or preschool is the norm. Also, a case-control study in Japan114 showed that preschool attendance was associated with increased risk of severe disease.
Other studies suggest that both locations are important. In Shanghai, China,103 there was a marked shift from 2007 to 2008 in the proportion of cases among children in preschools (from 59% to 37%) with a concurrent shift from local to migrant children, suggesting that the importance of routes of transmission can vary over time within the same locale. A case-control study from Zhejiang94 showed that although attending preschool is a risk factor (OR: 2.1), other factors such as contact with neighbors (OR: 11), going to hospital (OR: 20) and going to parties (OR: 31) impart greater risk. Yet, a Korean case-control study95 found no significant relationship between infection and school attendance or household size.
Overall, the evidence points to both home and school environments contributing to transmission, but the relative importance of these venues remains murky.
Incubation Period
Several papers describe the incubation period (Fig. 5, Appendix 3) though it is striking that the majority do not provide a source to justify the claimed period. These unsupported claims vary substantially from paper to paper, from the incubation period “is” 3–6 days115 or 3–7 days,76 “is usually 3–4 days, but can be ... 10 days or more,”32 or “is usually 3–5 days (range, 2–12 days),”111 is “typically” 3–7 days116 or 3–5 days,49 ranges from 5 to 7 days42,98 or 3 to 7 days113 and the “usual period” is 3–5 days “with longest period of 7 days.”117 Only a few provide evidence to justify the claim: one reports95 that the incubation period is usually 3–7 days, citing a US Centers for Disease Control and Prevention (CDC) factsheet on aseptic meningitis. Another cites118 an early study from Singapore,88 which presented the median and range for the serial interval (3 days [1–7]), not the incubation period. Another early study119 states that the incubation period is “said to be” 3–5 days, but notes that this is inconsistent with the serial interval observed in the study. It appears that there is no empirical support whatsoever for any distribution of incubation periods.
Symptomatic Proportion
Although several studies report that the asymptomatic rate of EV-A71 infection is high, few studies report data (Table 1). Two studies, from Taiwan and Shanghai, tested sera for evidence of EV-A71 infection and asked patients or their families to recall past HFMD infection, deriving estimates of 29%120 and 10%106 of symptomatic infection, respectively. Some HFMD cases may have been caused by other enteroviruses, biasing these estimates upwards, while some may have been diagnosed as another viral illness or forgotten, biasing them downwards.
Table 1.
Estimates of Symptomatic Proportion From the Literature
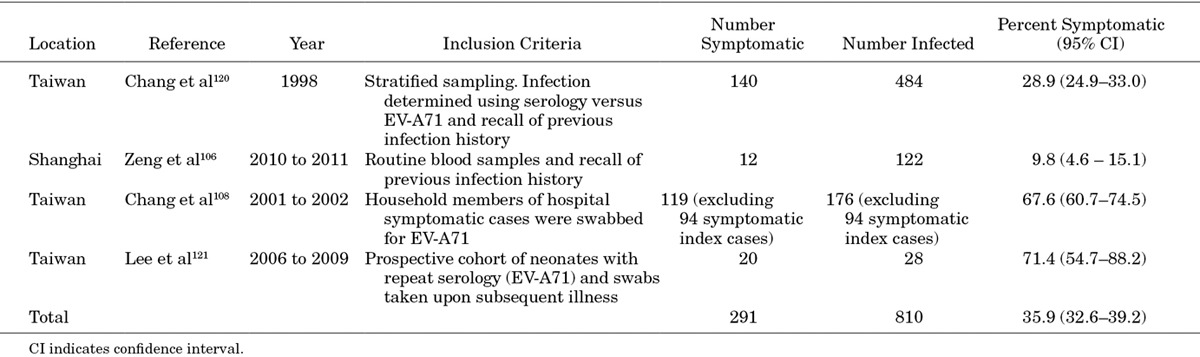
Two additional studies in Taiwan found much higher symptomatic infection rates. The first108 study recruited symptomatic cases suspected of having EV-A71 infection, and took throat and rectal swabs or stool samples, of cases and their household members. Signs and symptoms of the entire household were monitored with follow-up telephone interviews. Excluding the 94 symptomatic index cases, 68% of confirmed infections in the household were symptomatic (88% of infected children and 47% of infected adults). A second study121 prospectively followed a cohort of neonates over 3 years, taking repeat sera, requesting that parents report suspected HFMD and giving reminders during HFMD epidemics. This study found that 71% of serologically confirmed infections were symptomatic, though the sample size is only 28.
The discrepancy between these 2 pairs of papers is substantial, undoubtedly because of differences in methodology. An overall estimate, combining the 4 studies, is 36% (33%–39%), but given the large discrepancy between studies, this estimate does not appear reliable. The latter pair of studies is prospective, thereby circumventing recall bias, and thus appear to provide a more accurate description of the epidemiology of enterovirus infection.
Basic Reproduction Number for HFMD by Virus
Only 3 papers have sought to estimate the reproduction number for HFMD or the viruses that cause it. One paper101 estimates what they call the “local effective reproduction number” in China—meaning using the average number of secondary cases from a randomly selected index to estimate the cases that would be caused in a fully susceptible population (note, this is substantially different from the effective reproduction number122 in a partially susceptible population)—using a sophisticated Poisson regression model that incorporated infection from the environment, the prefecture and neighboring prefectures. This model did not, however, account for the accumulation of herd immunity and required arbitrary assignment of the infectious period, so the estimated local effective reproduction number of 1.1–1.2 during peak periods may be biased.
A second paper117 used a method from Choi and Pak123 to estimate the basic reproduction number to be 5.5 (interquartile range, 4.2–6.5) for EV-A71 and 2.5 (interquartile range, 2.0–3.7) for CV-A16. These estimates are likely inaccurate because the method assumes (i) a known generation time distribution, labeled incubation period in the paper; (ii) a completely immunonaïve population, though applied to groups of individuals for whom past exposure was highly plausible and (iii) an early exponential growth period, despite being applied to complete outbreak data.
The third paper124 attempted to estimate the reproduction number using a SEIQRS (Susceptible, Exposed, Infectious, Quarantined, Recovered) simulation model and obtained an estimate of 1.1 for the years 2009 to 2012 in China. However, the model used 10% of China population as the initial susceptible population, but did not conduct a sensitivity analysis on this vital parameter.
DISCUSSION
Despite the substantial number of papers on HFMD, this systematic review shows that many fundamental questions about EV-A71 and CV-A16 persist. Both viruses occur year round in tropical Asia, but are epidemic in the summer in Northeast Asia. A role for temperature or humidity therefore seems plausible,14,125–128 although given the relative lack of seasonality in equatorial Asia, it is not clear whether prediction of outbreaks is possible there. In Japan, summer temperatures peak after HFMD incidence does, suggesting correlation but not temporality, and that it may not be possible to provide early warning of impending epidemics. This also differs from other human enteric viruses including poliovirus 1 (also an enterovirus), hepatitis A and adenovirus that have been shown to survive longer on colder surfaces.129
Urashima et al125 claimed that enteroviruses experience a more rapid virus decline during dry seasons than during wet seasons, which could explain the seasonality. This result is supported by Wang et al,130 where they showed that precipitation patterns has the most similar structure as HFMD incidence, more so than other meteorologic variables, albeit with only 11 months of data.
While any causal relationship between climate and HFMD is unknown, speculations include a lower HFMD incidence because of decreased social contact during temperate zones’ winter.118,131 In contrast, increased social contacts during winter have been speculated to facilitate spread of other droplet-borne diseases, such as influenza,132 which are epidemic in winter. Given the unknowns surrounding this issue, further research is clearly required to ascertain whether meteorologic factors or seasonal social contact patterns is an adequate explanation for the seasonality of HFMD.
The next step to analyzing the dynamics of HFMD seasonality is likely to involve social and environmental factors, another under-researched area for this pediatric disease. For instance, the literature is unclear on the relative importance of school versus community transmission, with evidence to support both, yet knowledge of where HFMD most often is transmitted is important as school closure policies are employed to control outbreaks in some countries. Further, the environment of schools in Asia may vary widely, and attributes such as hygiene practices should be characterized and quantified to allow more definitive results and conclusions in future studies.
Even without being able to determine the relative importance of school versus community transmission, the effectiveness of school closure to prevent large-scale HFMD outbreaks is questionable, as the interruption to social networks cannot be enforced while children are out of school. Additionally, although we know little about the infectiousness of asymptomatic cases of HFMD, the proportion of infections that are asymptomatic is substantial, and so even quite modest school closure attack rate thresholds, such as Singapore’s 25%,133 corresponds to a possible majority of students being infected before the trigger for closure being met. Further, EV-A71 can be found in fecal samples for up to 54 days after infection,134 and thus continue to be shed after a school is closed, disinfected and reopened.
Studies on risk factors were rare, and we identified only 3 papers that describe risk factors for hygiene and contact patterns, making a meta-analysis of risk factors unfeasible. These typically were only powered to provide unadjusted effect sizes, and so provide evidence of correlation, not causation. One interesting finding was the apparent protective effect of a caregiver “always washing” their hands. This suggests that adult to child transmission might be important, even if adults are mostly asymptomatic with EV-A71 and CV-A16, but may reflect confounding with general hygiene. Future work may elicit hygiene factors at the preschool level and relate these to attack rates.
A recently developed EV-A71 vaccine has undergone phase 3 trials in China.135–137 To determine the cost-effectiveness of incorporating the vaccine in pediatric vaccination schedules, or of other interventions such as school closure or isolation, would require epidemiologic models that account for the protective effects of herd immunity. However, this review indicates that vital parameters for such models remain unknown. The asymptomatic rate and relative infectiousness of asymptomatic cases are both poorly known, while estimates of the incubation period, although commonly cited as 3–5 days, appear to be based solely on expert opinion. Most importantly, estimates of the basic reproduction number range widely from 1.1 to 5.5. This uncertainty prohibits utilitarian estimation of the necessary vaccine coverage to prevent epidemics of EV-A71.
To reconcile the differences between the disparate estimates, the age distributions of the samples need to be considered. As shown in this review, symptomatic HFMD incidence rate differ greatly even between ages 0 and 6, and thus, studies conducted predominantly on preschoolers may derive higher estimates of R0 compared with studies in older children. Accordingly, future studies on HFMD should use narrower age bands and also state the distribution clearly to allow adjustments or standardization.
Two final omissions from the literature are quantitative estimates of the impact of infection on complications, child and caregiver absenteeism and costings of complications, and qualitative evidence on the impact of infection and enforced isolation on families and schools. Given the promising direct effects of the EV-A71 vaccine and the huge public health impacts of HFMD in East and Southeast Asia, research is urgently needed to fill these gaps.
The research questions in this systematic review were generally answered only by a limited number of papers, with substantial differences in their study design, and thus, most data were not synthesized through meta-analysis. More research to assess risk factors and measure key epidemiologic parameters is needed. We were also unable to trace the earliest cases of HFMD in Asia as our scope only covers published material on outbreaks, which leads us back to 1967 in Japan. Finally, we limited the scope of this study to exclude virologic characteristics or molecular epidemiology, which have been well reviewed elsewhere,116,138–141 and clinical manifestations of EV-A71 and CV-A16.28,116,138,141,142 A recent review of the case-fatality rate has recently been published,143 as has a review of the epidemiology in Taiwan.144
Appendix 1. Timing and Seasonality of HFMD Outbreaks
An autoregressive (AR) model was used to investigate the effect of meteorological variables after correcting for contagion via autoregression.
A lag 2 model can be specified as follows:
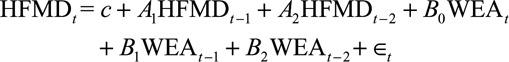 |
The A coefficients are the coefficients for the AR terms, while the B coefficients represent how a change in weather is correlated with changes in HFMD incidence. The number of lag terms is determined by the Akaike information criterion values of the regression models.
This same model was used for 4 countries—Japan (lag 2), Hong Kong (lag 3), Taiwan (lag 3) and Singapore (lag 2)—and for 3 meteorological parameters—temperature, absolute humidity and relative humidity. As this model carries autocorrelated terms, we used generalized least squares for model fitting. Coefficients from the fitted models are presented in Tables A1–A3.
TABLE A1.
Coefficients for Autoregressive Model Using Temperature as Predictor
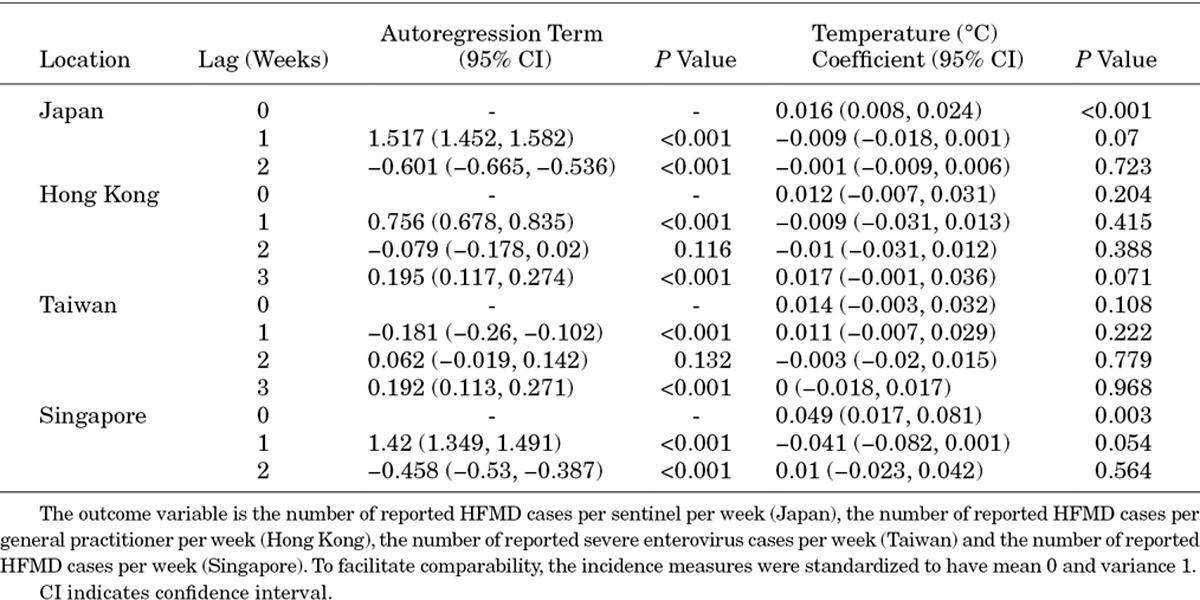
TABLE A2.
Coefficients for Autoregressive Model Using Relative Humidity as Predictor
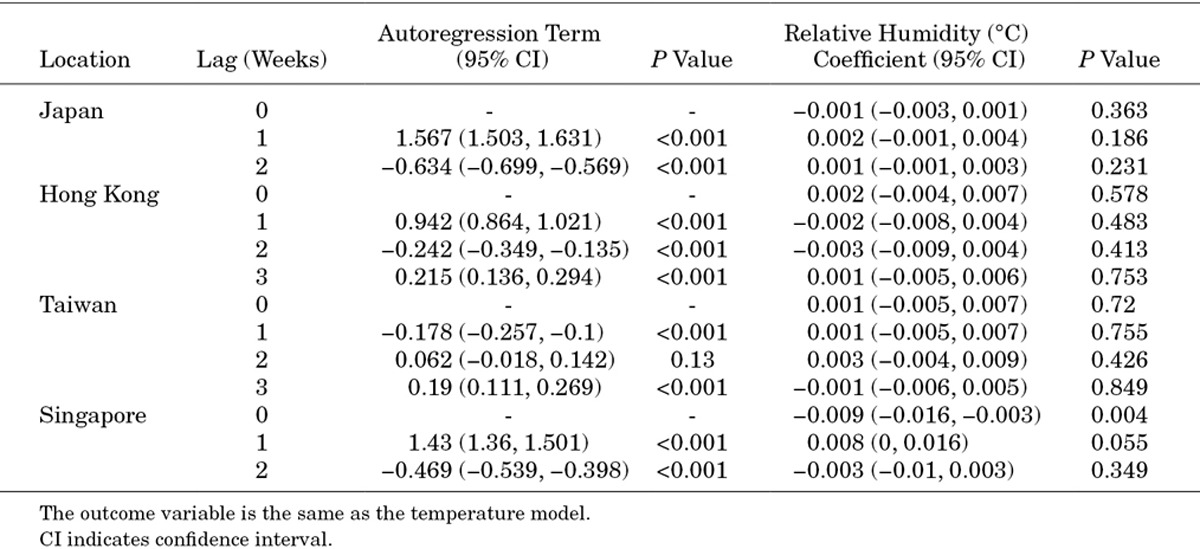
TABLE A3.
Coefficients for Autoregressive Model Using Absolute Humidity as Predictor
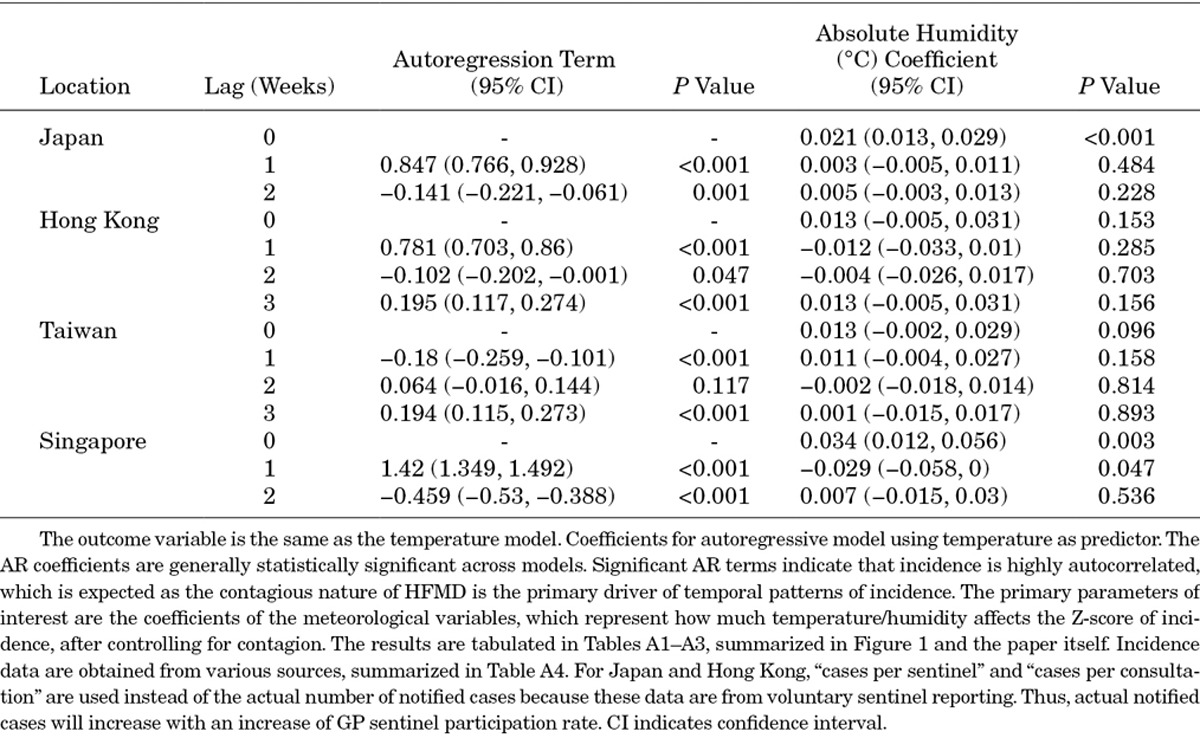
TABLE A4.
Data Source for Incidence
APPENDIX 2. Odds Ratio From Figure 4
APPENDIX 3. Data Source for Figure 5 (Left)
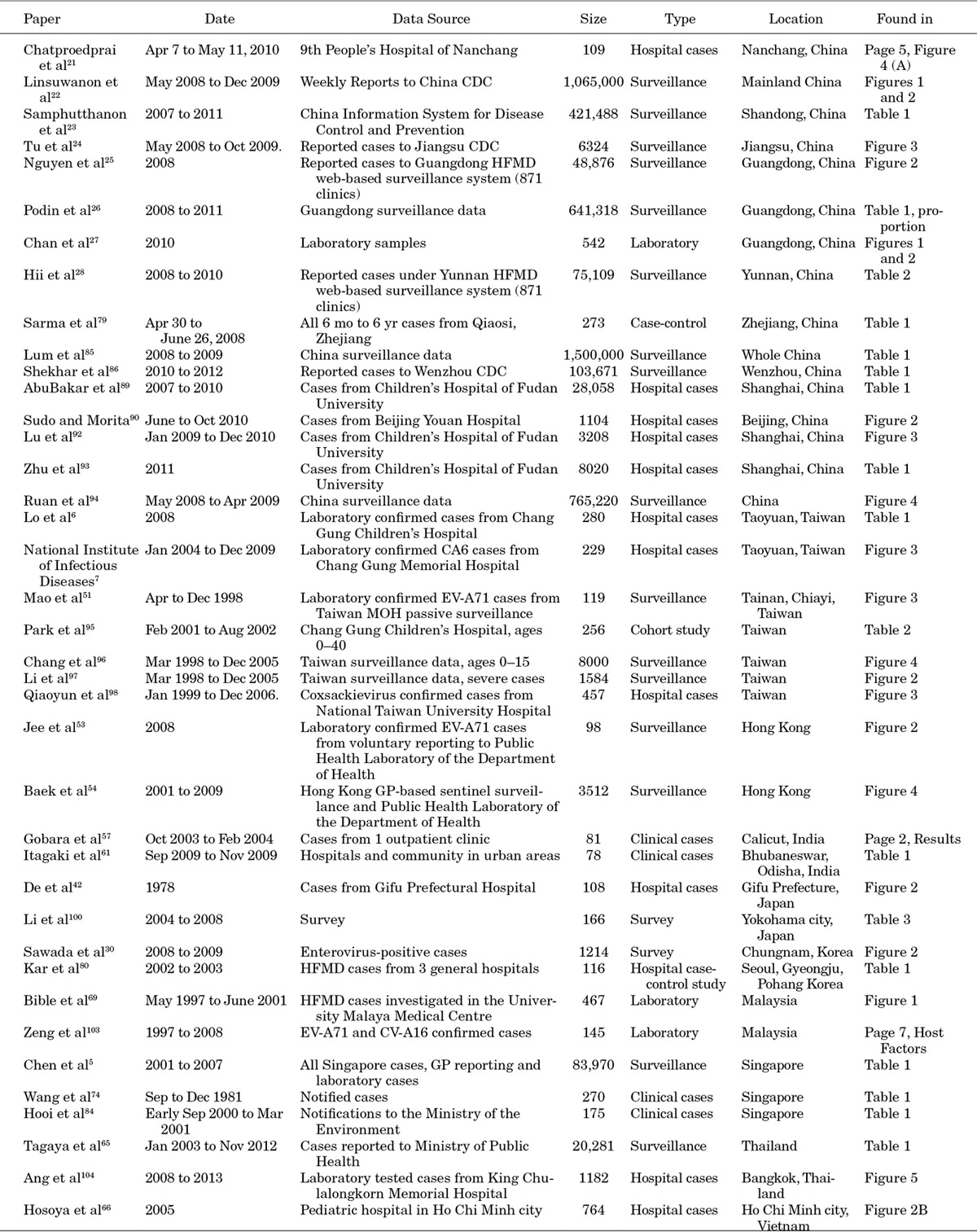
APPENDIX 4. PRISMA 2009 Checklist

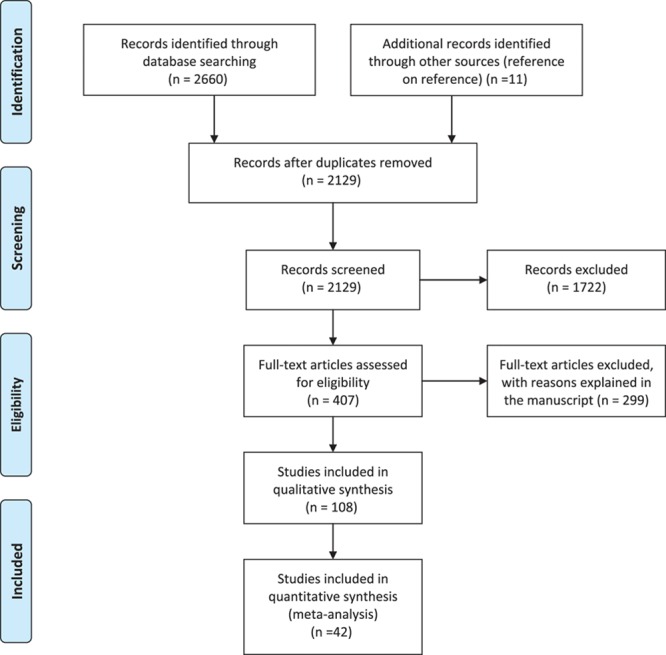
APPENDIX 5. PRISMA 2009 Flow Diagram
ACKNOWLEDGMENTS
We thank Alex S. Leow and Vincent J. Pang for screening some of the papers and Zhao Xiahong for processing the meteorologic data.
Footnotes
Supported by Singapore’s Ministry of Health Services Research (HSRG12MAY023), Communicable Disease Public Health Research (CDPHRG12NOV021), the Centre for Infectious Disease Epidemiology and Research, the Ministry of Education Tier 1 grant and the President’s Graduate Fellowship to W.M.K. The funders had no role in the decision to publish. T.B. is employed by commercial company, Standard Analytics. The remaining authors have no financial relationships relevant to this article to disclose. The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Huang MC, Wang SM, Hsu YW, et al. Long-term cognitive and motor deficits after enterovirus 71 brainstem encephalitis in children. Pediatrics. 2006;118:e1785–e1788. doi: 10.1542/peds.2006-1547. [DOI] [PubMed] [Google Scholar]
- 2.Chang LY, Huang LM, Gau SS, et al. Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med. 2007;356:1226–1234. doi: 10.1056/NEJMoa065954. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Western Pacific Region. Hand, foot, and mouth disease situation update. 2014. Dec 29, Available at: http://www.wpro.who.int/emerging_diseases/hfmd_biweekly_29dec2014.pdf?ua=1. Accessed March 11, 2015.
- 4.Ang LW, Koh BK, Chan KP, et al. Epidemiology and control of hand, foot and mouth disease in Singapore, 2001–2007. Ann Acad Med Singapore. 2009;38:106–112. [PubMed] [Google Scholar]
- 5.Chen SP, Huang YC, Li WC, et al. Comparison of clinical features between coxsackievirus A2 and enterovirus 71 during the enterovirus outbreak in Taiwan, 2008: a children’s hospital experience. J Microbiol Immunol Infect. 2010;43:99–104. doi: 10.1016/S1684-1182(10)60016-3. [DOI] [PubMed] [Google Scholar]
- 6.Lo S-H, Huang Y-C, Huang C-G, et al. Clinical and epidemiologic features of coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J Microbiol Immunol Infect. 2011;44:252–257. doi: 10.1016/j.jmii.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Infectious Diseases, Japan. Infectious disease weekly report, (IDWR), IDWR Surveillance Data Table, Sentinel-reporting Diseases. Available at: http://www.nih.go.jp/niid/en/survaillance-data-table-english.html. Accessed October 30, 2013.
- 8.Centre for Health Protection, Department of Health, Hong Kong. Updated situation of hand, foot and mouth disease (HFMD) and enterovirus infection 2010. 2010. Aug, Available at: http://www.chp.gov.hk/files/pdf/updated_situation_of_hand_foot_and_mouth_disease_hfmd_r.pdf. Accessed November 5, 2015.
- 9.Taiwan Centers for Disease Control, Taipei (Taiwan) Taiwan national infectious disease statistics system. 2014. Apr 15, Available at: http://nidss.cdc.gov.tw/en/. Accessed October 30, 2013.
- 10.Huwaldt J. Plot digitizer (version 2.6.3) 2013. Jul 05, Available at: http://sourceforge.net/projects/plotdigitizer/. Accessed October 29, 2013. [Google Scholar]
- 11.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 12.Liu Y, Wang X, Liu Y, et al. Detecting spatial-temporal clusters of HFMD from 2007 to 2011 in Shandong Province, China. PLoS One. 2013;8:e63447. doi: 10.1371/journal.pone.0063447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu W, Jiang L, Thammawijaya P, et al. Hand, foot and mouth disease in Yunnan Province, China, 2008–2010. Asia Pac J Public Health. 2015;27:769–777. doi: 10.1177/1010539511430523. [DOI] [PubMed] [Google Scholar]
- 14.Deng T, Huang Y, Yu S, et al. Spatial-temporal clusters and risk factors of hand, foot, and mouth disease at the district level in Guangdong Province, China. PLoS One. 2013;8:e56943. doi: 10.1371/journal.pone.0056943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di B, Zhang Y, Xie H, et al. Circulation of coxsackievirus A6 in hand-foot-mouth disease in Guangzhou, 2010-2012. Virol J. 2014;11:157. doi: 10.1186/1743-422X-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang YR, Sun LL, Xiao WL, et al. Epidemiology and clinical characteristics of hand foot, and mouth disease in a Shenzhen sentinel hospital from 2009 to 2011. BMC Infect Dis. 2013;13:539. doi: 10.1186/1471-2334-13-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma E, Chan KC, Cheng P, et al. The enterovirus 71 epidemic in 2008—public health implications for Hong Kong. Int J Infect Dis. 2010;14:e775–e780. doi: 10.1016/j.ijid.2010.02.2265. [DOI] [PubMed] [Google Scholar]
- 18.Ma E, Lam T, Chan KC, et al. Changing epidemiology of hand, foot, and mouth disease in Hong Kong, 2001-2009. Jpn J Infect Dis. 2010;63:422–426. [PubMed] [Google Scholar]
- 19.Lee T-C, Guo H-R, Su H-JJ, et al. Diseases caused by enterovirus 71 infection. Pediatr Infect Dis J. 2009;28:904–910. doi: 10.1097/INF.0b013e3181a41d63. [DOI] [PubMed] [Google Scholar]
- 20.Yang TT, Huang LM, Lu CY, et al. Clinical features and factors of unfavorable outcomes for non-polio enterovirus infection of the central nervous system in northern Taiwan, 1994-2003. J Microbiol Immunol Infect. 2005;38:417–424. [PubMed] [Google Scholar]
- 21.Chatproedprai S, Theanboonlers A, Korkong S, et al. Clinical and molecular characterization of hand-foot-and-mouth disease in Thailand, 2008-2009. Jpn J Infect Dis. 2010;63:229–233. [PubMed] [Google Scholar]
- 22.Linsuwanon P, Puenpa J, Huang SW, et al. Epidemiology and seroepidemiology of human enterovirus 71 among Thai populations. J Biomed Sci. 2014;21:16. doi: 10.1186/1423-0127-21-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samphutthanon R, Tripathi NK, Ninsawat S, et al. Spatio-temporal distribution and hotspots of hand, foot and mouth disease (HFMD) in northern Thailand. Int J Environ Res Public Health. 2014;11:312–336. doi: 10.3390/ijerph110100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu PV, Thao NTT, Perera D, et al. Epidemiologic and virologic investigation of hand, foot, and mouth disease, southern Vietnam, 2005. Emerg Infect Dis. 2007;13:1733–1741. doi: 10.3201/eid1311.070632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen NT, Pham HV, Hoang CQ, et al. Epidemiological and clinical characteristics of children who died from hand, foot and mouth disease in Vietnam, 2011. BMC Infect Dis. 2014;14:341. doi: 10.1186/1471-2334-14-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podin Y, Gias EL, Ong F, et al. Sentinel surveillance for human enterovirus 71 in Sarawak, Malaysia: lessons from the first 7 years. BMC Public Health. 2006;6:180. doi: 10.1186/1471-2458-6-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan KP, Goh KT, Chong CY, et al. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis. 2003;9:78–85. doi: 10.3201/eid1301.020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hii YL, Rocklöv J, Ng N. Short term effects of weather on hand, foot and mouth disease. PLoS One. 2011;6:e16796. doi: 10.1371/journal.pone.0016796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Yeo A, Phoon MC, et al. The largest outbreak of hand, foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:e1076–e1081. doi: 10.1016/j.ijid.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Sawada K, Nakamura Y, Ito S. On the hand, foot and mouth disease prevailed in Ohmiya city in summer, 1967. Nihon Shonika Gakkai Zasshi. 1968;74:1594. [Google Scholar]
- 31.Chan MCK, Wong HB. Hand-foot and mouth disease. J Singapore Paediatr Soc. 1973;15:31–34. [Google Scholar]
- 32.Chang LY. Enterovirus 71 in Taiwan. Pediatr Neonatol. 2008;49:103–112. doi: 10.1016/S1875-9572(08)60023-6. [DOI] [PubMed] [Google Scholar]
- 33.Li C. The prevalence of hand, foot and mouth disease. J Pediatr Pharm. 2008;14:64–66. [Google Scholar]
- 34.Sun LM, Zheng HY, Zheng HZ, et al. An enterovirus 71 epidemic in Guangdong Province of China, 2008: epidemiological, clinical, and virogenic manifestations. Jpn J Infect Dis. 2011;64:13–18. [PubMed] [Google Scholar]
- 35.Zhang Y, Tan XJ, Wang HY, et al. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J Clin Virol. 2009;44:262–267. doi: 10.1016/j.jcv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Ding N-Z, Wang X-M, Sun S-W, et al. Appearance of mosaic enterovirus 71 in the 2008 outbreak of China. Virus Res. 2009;145:157–161. doi: 10.1016/j.virusres.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Cao Z, Zeng DD, et al. Epidemiological analysis, detection, and comparison of space-time patterns of Beijing hand-foot-mouth disease (2008–2012). PLoS One. 2014;9:e92745. doi: 10.1371/journal.pone.0092745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Wu X, Jia L, et al. Estimating the number of hand, foot and mouth disease amongst children aged under-five in Beijing during 2012, based on a telephone survey of healthcare seeking behavior. BMC Infect Dis. 2014;14:437. doi: 10.1186/1471-2334-14-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian H, Zhang Y, Sun Q, et al. Prevalence of multiple enteroviruses associated with hand, foot, and mouth disease in Shijiazhuang city, Hebei Province, China: outbreaks of coxsackieviruses A10 and B3. PLoS One. 2014;9:e84233. doi: 10.1371/journal.pone.0084233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao Z, Zeng D, Wang Q, et al. An epidemiological analysis of the Beijing 2008 hand-foot-mouth epidemic. Chin Sci Bull. 2010;55:1142–1149. [Google Scholar]
- 41.Wu X, Sun Y, Lin C, et al. A case-control study to identify environmental risk factors for hand, foot, and mouth disease outbreaks in Beijing. Jpn J Infect Dis. 2014;67:95–99. doi: 10.7883/yoken.67.95. [DOI] [PubMed] [Google Scholar]
- 42.De W, Changwen K, Wei L, et al. A large outbreak of hand, foot, and mouth disease caused by EV71 and CAV16 in Guangdong, China, 2009. Arch Virol. 2011;156:945–953. doi: 10.1007/s00705-011-0929-8. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Yi L, Su J, et al. Seroprevalence of human enterovirus 71 and coxsackievirus A16 in Guangdong, China, in pre- and post-2010 HFMD epidemic period. PLoS One. 2013;8:e80515. doi: 10.1371/journal.pone.0080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu W, Liu CF, Yan L, et al. Distribution of enteroviruses in hospitalized children with hand, foot and mouth disease and relationship between pathogens and nervous system complications. Virol J. 2012;9:8. doi: 10.1186/1743-422X-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Zhu Z, Yang W, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J. 2010;7:94. doi: 10.1186/1743-422X-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bingjun T, Yoshida H, Yan W, et al. Molecular typing and epidemiology of non-polio enteroviruses isolated from Yunnan Province, the People’s Republic of China. J Med Virol. 2008;80:670–679. doi: 10.1002/jmv.21122. [DOI] [PubMed] [Google Scholar]
- 47.Yu H, Wang M, Chang H, et al. Prevalence of antibodies against enterovirus 71 in children from Lu’an city in Central China. Jpn J Infect Dis. 2011;64:528–532. [PubMed] [Google Scholar]
- 48.Cardosa MJ, Perera D, Brown BA, et al. Molecular epidemiology of human enterovirus 71 strains and recent outbreaks in the Asia-Pacific region: comparative analysis of the VP1 and VP4 genes. Emerg Infect Dis. 2003;9:461–468. doi: 10.3201/eid0904.020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu MY, Liu W, Luo J, et al. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Q, Hao Y, Ma J, et al. Surveillance of hand, foot, and mouth disease in mainland China (2008–2009). Biomed Env Sci. 2011;24:349–356. doi: 10.3967/0895-3988.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Mao LX, Wu B, Bao WX, et al. Epidemiology of hand, foot, and mouth disease and genotype characterization of enterovirus 71 in Jiangsu, China. J Clin Virol. 2010;49:100–104. doi: 10.1016/j.jcv.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 52.He S-J, Han J-F, Ding X-X, et al. Characterization of enterovirus 71 and coxsackievirus A16 isolated in hand, foot, and mouth disease patients in Guangdong, 2010. Int J Infect Dis. 2013;17:e1025–e1030. doi: 10.1016/j.ijid.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Jee YM, Cheon D-S, Kim K, et al. Genetic analysis of the VP1 region of human enterovirus 71 strains isolated in Korea during 2000. Arch Virol. 2003;148:1735–1746. doi: 10.1007/s00705-003-0133-6. [DOI] [PubMed] [Google Scholar]
- 54.Baek K, Yeo S, Lee B, et al. Epidemics of enterovirus infection in Chungnam Korea, 2008 and 2009. Virol J. 2011;8:297. doi: 10.1186/1743-422X-8-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim SJ, Kim J-H, Kang J-H, et al. Risk factors for neurologic complications of hand, foot and mouth disease in the Republic of Korea, 2009. J Korean Med Sci. 2013;28:120. doi: 10.3346/jkms.2013.28.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miwa C, Ohtani M, Watanabe H, et al. Epidemic of hand, foot and mouth disease in Gifu Prefecture in 1978. Jpn J Med Sci Biol. 1980;33:167–180. doi: 10.7883/yoken1952.33.167. [DOI] [PubMed] [Google Scholar]
- 57.Gobara F, Itagaki A, Ito Y, et al. Properties of virus isolated from an epidemic of hand-foot-and-mouth disease in 1973 in the city of Matsue. Comparison with coxsackievirus group A type 16 prototype. Microbiol Immunol. 1977;21:207–217. doi: 10.1111/j.1348-0421.1977.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 58.Hagiwara A, Tagaya I, Yoneyama T. Epidemic of hand, foot and mouth disease associated with enterovirus 71 infection. Intervirology. 1978;9:60–63. doi: 10.1159/000148922. [DOI] [PubMed] [Google Scholar]
- 59.Tagaya I, Moritsugu Y. Epidemic of hand, foot and mouth disease in Japan. Jpn J Med Sci Biol. 1973;26:143–147. doi: 10.7883/yoken1952.26.143. [DOI] [PubMed] [Google Scholar]
- 60.Fujimiya Y, Oyama S, Numazaki Y, et al. Serologic relationship of coxsackie A-16 viruses from the epidemic of hand-foot-and mouth disease in Japan, 1970, to the prototype strain. Jpn J Microbiol. 1974;18:379–384. doi: 10.1111/j.1348-0421.1974.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 61.Itagaki A, Gobara F, Ito Y, et al. A survey on enterovirus infections in Matsue city, Shimane Prefecture in the period from May, 1969 to March, 1971. J Jpn Assoc Infect Dis. 1972;46:280–289. doi: 10.11150/kansenshogakuzasshi1970.46.280. [DOI] [PubMed] [Google Scholar]
- 62.Tagaya I, Tachibana K. Epidemic of hand, foot and mouth disease in Japan, 1972–1973: difference in epidemiologic and virologic features from the previous one. Jpn J Med Sci Biol. 1975;28:231–234. doi: 10.7883/yoken1952.28.231. [DOI] [PubMed] [Google Scholar]
- 63.Gobara F, Itagaki A, Ito Y, et al. A survey on enterovirus infection in Matsue city, Shimane Prefecture in the period from April to October, 1973. J Jpn Assoc Infect Dis. 1975;49:282–287. doi: 10.11150/kansenshogakuzasshi1970.49.282. [DOI] [PubMed] [Google Scholar]
- 64.Ishimaru Y, Nakano S, Yamaoka K, et al. Outbreaks of hand, foot, and mouth disease by enterovirus 71. High incidence of complication disorders of central nervous system. Arch Dis Child. 1980;55:583–588. doi: 10.1136/adc.55.8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tagaya I, Takayama R, Hagiwara A. A large-scale epidemic of hand, foot and mouth disease associated with enterovirus 71 infection in Japan in 1978. Jpn J Med Sci Biol. 1981;34:191–196. doi: 10.7883/yoken1952.34.191. [DOI] [PubMed] [Google Scholar]
- 66.Hosoya M, Kawasaki Y, Sato M, et al. Genetic diversity of coxsackievirus A16 associated with hand, foot, and mouth disease epidemics in Japan from 1983 to 2003. J Clin Microbiol. 2007;45:112–120. doi: 10.1128/JCM.00718-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mizuta K, Abiko C, Murata T, et al. Frequent importation of enterovirus 71 from surrounding countries into the local community of Yamagata, Japan, between 1998 and 2003. J Clin Microbiol. 2005;43:6171–6175. doi: 10.1128/JCM.43.12.6171-6175.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Komatsu H, Shimizu Y, Takeuchi Y, et al. Outbreak of severe neurologic involvement associated with enterovirus 71 infection. Pediatr Neurol. 1999;20:17–23. doi: 10.1016/s0887-8994(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 69.Bible JM, Pantelidis P, Chan PK, et al. Genetic evolution of enterovirus 71: epidemiological and pathological implications. Rev Med Virol. 2007;17:371–379. doi: 10.1002/rmv.538. [DOI] [PubMed] [Google Scholar]
- 70.Fujimoto T, Chikahira M, Yoshida S, et al. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol Immunol. 2002;46:621–627. doi: 10.1111/j.1348-0421.2002.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 71.Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. 1999;341:929–935. doi: 10.1056/NEJM199909233411301. [DOI] [PubMed] [Google Scholar]
- 72.Wang J-R, Tuan Y-C, Tsai H-P, et al. Change of major genotype of enterovirus 71 in outbreaks of hand-foot-and-mouth disease in Taiwan between 1998 and 2000. J Clin Microbiol. 2002;40:10–15. doi: 10.1128/JCM.40.1.10-15.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu CC, Tseng HW, Wang SM, et al. An outbreak of enterovirus 71 infection in Taiwan, 1998: epidemiologic and clinical manifestations. J Clin Virol. 2000;17:23–30. doi: 10.1016/s1386-6532(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 74.Wang JR, Tsai HP, Chen PF, et al. An outbreak of enterovirus 71 infection in Taiwan, 1998. II. Laboratory diagnosis and genetic analysis. J Clin Virol. 2000;17:91–99. doi: 10.1016/s1386-6532(00)00079-2. [DOI] [PubMed] [Google Scholar]
- 75.Samuda GM, Chang WK, Yeung CY, et al. Monoplegia caused by enterovirus 71: an outbreak in Hong Kong. Pediatr Infect Dis J. 1987;6:206–208. doi: 10.1097/00006454-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 76.Sasidharan CK, Sugathan P, Agarwal R, et al. Hand-foot-and-mouth disease in Calicut. Indian J Pediatr. 2005;72:17–21. doi: 10.1007/BF02760573. [DOI] [PubMed] [Google Scholar]
- 77.Beig FK, Malik A, Rizvi M, et al. Etiology and clinico-epidemiological profile of acute viral encephalitis in children of western Uttar Pradesh, India. Int J Infect Dis. 2010;14:e141–e146. doi: 10.1016/j.ijid.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 78.Saoji VA. Hand, foot and mouth disease in Nagpur. Indian J Dermatol Venereol Leprol. 2008;74:133–135. doi: 10.4103/0378-6323.39697. [DOI] [PubMed] [Google Scholar]
- 79.Sarma N, Sarkar A, Mukherjee A, et al. Epidemic of hand, foot and mouth disease in West Bengal, India in August, 2007: a multicentric study. Indian J Dermatol. 2009;54:26–30. doi: 10.4103/0019-5154.48982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kar BR, Dwibedi B, Kar SK. An outbreak of hand, foot and mouth disease in Bhubaneswar, Odisha. Indian Pediatr. 2013;50:139–142. doi: 10.1007/s13312-013-0033-0. [DOI] [PubMed] [Google Scholar]
- 81.Kashyap S, Verma GK. Hand-foot-mouth disease: outbreak in Shimla. Indian Pediatr. 2014;51:155. doi: 10.1007/s13312-014-0334-y. [DOI] [PubMed] [Google Scholar]
- 82.Puenpa J, Mauleekoonphairoj J, Linsuwanon P, et al. Prevalence and characterization of enterovirus infections among pediatric patients with hand foot mouth disease, herpangina and influenza like illness in Thailand, 2012. PLoS One. 2014;9:e98888. doi: 10.1371/journal.pone.0098888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cardosa MJ, Krishnan S, Tio PH, et al. Isolation of subgenus B adenovirus during a fatal outbreak of enterovirus 71-associated hand, foot, and mouth disease in Sibu, Sarawak. Lancet. 1999;354:987–991. doi: 10.1016/S0140-6736(98)11032-2. [DOI] [PubMed] [Google Scholar]
- 84.Hooi PS, Chua BH, Lee CS, et al. Hand, foot and mouth disease: university Malaya Medical Centre experience. Med J Malaysia. 2002;57:88–91. [PubMed] [Google Scholar]
- 85.Lum LC, Wong KT, Lam SK, et al. Fatal enterovirus 71 encephalomyelitis. J Pediatr. 1998;133:795–798. doi: 10.1016/s0022-3476(98)70155-6. [DOI] [PubMed] [Google Scholar]
- 86.Shekhar K, Lye MS, Norlijah O, et al. Deaths in children during an outbreak of hand, foot and mouth disease in Peninsular Malaysia–clinical and pathological characteristics. Med J Malaysia. 2005;60:297–304. [PubMed] [Google Scholar]
- 87.Ooi MH, Wong SC, Mohan A, et al. Identification and validation of clinical predictors for the risk of neurological involvement in children with hand, foot, and mouth disease in Sarawak. BMC Infect Dis. 2009;9:3. doi: 10.1186/1471-2334-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goh KT, Doraisingham S, Tan JL, et al. An outbreak of hand, foot, and mouth disease in Singapore. Bull World Health Organ. 1982;60:965–969. [PMC free article] [PubMed] [Google Scholar]
- 89.AbuBakar S, Sam IC, Yusof J, et al. Enterovirus 71 outbreak, Brunei. Emerg Infect Dis. 2009;15:79–82. doi: 10.3201/eid1501.080264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sudo T, Morita M. [Hand, foot and mouth disease]. Nihon Rinsho. 1971;29:1137–1142. [PubMed] [Google Scholar]
- 91.Ooi EE, Phoon MC, Ishak B, et al. Seroepidemiology of human enterovirus 71, Singapore. Emerg Infect Dis. 2002;8:995–997. doi: 10.3201/eid0809.10.3201/eid0809.010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu C-Y, Lee C-Y, Kao C-L, et al. Incidence and case-fatality rates resulting from the 1998 enterovirus 71 outbreak in Taiwan. J Med Virol. 2002;67:217–223. doi: 10.1002/jmv.2210. [DOI] [PubMed] [Google Scholar]
- 93.Zhu Z, Zhu S, Guo X, et al. Retrospective seroepidemiology indicated that human enterovirus 71 and coxsackievirus A16 circulated wildly in central and southern China before large-scale outbreaks from 2008. Virol J. 2010;7:300. doi: 10.1186/1743-422X-7-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127:e898–e904. doi: 10.1542/peds.2010-1497. [DOI] [PubMed] [Google Scholar]
- 95.Park SK, Park B, Ki M, et al. Transmission of seasonal outbreak of childhood enteroviral aseptic meningitis and hand-foot-mouth disease. J Korean Med Sci. 2010;25:677–683. doi: 10.3346/jkms.2010.25.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chang LY, King CC, Hsu KH, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics. 2002;109:e88. doi: 10.1542/peds.109.6.e88. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, Dang S, Deng H, et al. Breastfeeding, previous Epstein-Barr virus infection, enterovirus 71 infection, and rural residence are associated with the severity of hand, foot, and mouth disease. Eur J Pediatr. 2013;172:661–666. doi: 10.1007/s00431-013-1939-1. [DOI] [PubMed] [Google Scholar]
- 98.Qiaoyun F, Xiongfei J, Lihuan L, et al. Epidemiology and etiological characteristics of hand, foot and mouth disease in Huizhou city between 2008 and 2011. Arch Virol. 2013;158:895–899. doi: 10.1007/s00705-012-1566-6. [DOI] [PubMed] [Google Scholar]
- 99.Yan L, Li X, Yu Y, et al. Distribution and risk factors of hand, foot, and mouth disease in Changchun, northeastern China. Chin Sci Bull. 2014;59:533–538. [Google Scholar]
- 100.Li J, Fu Y, Xu A, et al. A spatial-temporal ARMA model of the incidence of hand, foot, and mouth disease in Wenzhou, China. Abstr Appl Anal. 2014;2014:1–9. [Google Scholar]
- 101.Wang Y, Feng Z, Yang Y, et al. Hand, foot, and mouth disease in China: patterns of spread and transmissibility. Epidemiology. 2011;22:781–792. doi: 10.1097/EDE.0b013e318231d67a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu D, Zhao XY, Yao Y, et al. A new factor influencing pathogen detection by molecular assay in children with both mild and severe hand, foot, and mouth disease. Diagn Microbiol Infect Dis. 2013;76:162–167. doi: 10.1016/j.diagmicrobio.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zeng M, Li Y-F, Wang X-H, et al. Epidemiology of hand, foot, and mouth disease in children in Shanghai 2007–2010. Epidemiol Infect. 2012;140:1122–1130. doi: 10.1017/S0950268811001622. [DOI] [PubMed] [Google Scholar]
- 104.Ang LW, Phoon MC, Wu Y, et al. The changing seroepidemiology of enterovirus 71 infection among children and adolescents in Singapore. BMC Infect Dis. 2011;11:270. doi: 10.1186/1471-2334-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan X-F, Gao S, Xia J-F, et al. Epidemic characteristics of hand, foot, and mouth disease in Shanghai from 2009 to 2010: enterovirus 71 subgenotype C4 as the primary causative agent and a high incidence of mixed infections with coxsackievirus A16. Scand J Infect Dis. 2012;44:297–305. doi: 10.3109/00365548.2011.634433. [DOI] [PubMed] [Google Scholar]
- 106.Zeng M, El Khatib NF, Tu S, et al. Seroepidemiology of enterovirus 71 infection prior to the 2011 season in children in Shanghai. J Clin Virol. 2012;53:285–289. doi: 10.1016/j.jcv.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 107.Zhang J, Sun J, Chang Z, et al. Characterization of hand, foot, and mouth disease in China between 2008 and 2009. Biomed Env Sci. 2011;24:214–221. doi: 10.3967/0895-3988.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 108.Chang LY, Tsao KC, Hsia SH, et al. Transmission and clinical features of enterovirus 71 infections in household contacts in Taiwan. JAMA. 2004;291:222–227. doi: 10.1001/jama.291.2.222. [DOI] [PubMed] [Google Scholar]
- 109.Chen K-T, Chang H-L, Wang S-T, et al. Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998 2005. Pediatrics. 2007;120:e244–e252. doi: 10.1542/peds.2006-3331. [DOI] [PubMed] [Google Scholar]
- 110.Chen SC, Chang HL, Yan TR, et al. An eight-year study of epidemiologic features of enterovirus 71 infection in Taiwan. Am J Trop Med Hyg. 2007;77:188–191. [PubMed] [Google Scholar]
- 111.Yen FB, Chang LY, Kao CL, et al. Coxsackieviruses infection in northern Taiwan–epidemiology and clinical characteristics. J Microbiol Immunol Infect. 2009;42:38–46. [PubMed] [Google Scholar]
- 112.Momoki ST. Surveillance of enterovirus infections in Yokohama city from 2004 to 2008. Jpn J Infect Dis. 2009;62:471–473. [PubMed] [Google Scholar]
- 113.Chan Y-F, Sam I-C, Wee K-L, et al. Enterovirus 71 in Malaysia: a decade later. Neurol Asia. 2011;16:1–15. [Google Scholar]
- 114.Suzuki Y, Taya K, Nakashima K, et al. Risk factors for severe hand foot and mouth disease. Pediatr Int. 2010;52:203–207. doi: 10.1111/j.1442-200X.2009.02937.x. [DOI] [PubMed] [Google Scholar]
- 115.Frydenberg A, Starr M. Hand, foot and mouth disease. Aust Fam Physician. 2003;32:594–595. [PubMed] [Google Scholar]
- 116.Wong SSY, Yip CCY, Lau SKP, et al. Human enterovirus 71 and hand, foot and mouth disease. Epidemiol Infect. 2010;138:1071–1089. doi: 10.1017/S0950268809991555. [DOI] [PubMed] [Google Scholar]
- 117.Ma E, Fung C, Yip SH, et al. Estimation of the basic reproduction number of enterovirus 71 and coxsackievirus A16 in hand, foot, and mouth disease outbreaks. Pediatr Infect Dis J. 2011;30:675–679. doi: 10.1097/INF.0b013e3182116e95. [DOI] [PubMed] [Google Scholar]
- 118.Onozuka D, Hashizume M. The influence of temperature and humidity on the incidence of hand, foot, and mouth disease in Japan. Sci Total Environ. 2011;410–411:119–125. doi: 10.1016/j.scitotenv.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 119.Dale MH. Hand, foot and mouth disease–1973. J R Coll Gen Pract. 1975;25:35–38. [PMC free article] [PubMed] [Google Scholar]
- 120.Chang LY, Lin TY, Hsu KH, et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet. 1999;354:1682–1686. doi: 10.1016/S0140-6736(99)04434-7. [DOI] [PubMed] [Google Scholar]
- 121.Lee M-S, Chiang P-S, Luo S-T, et al. Incidence rates of enterovirus 71 infections in young children during a nationwide epidemic in Taiwan, 2008–09. PLoS Negl Trop Dis. 2012;6:e1476. doi: 10.1371/journal.pntd.0001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wallinga J, Teunis P. Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am J Epidemiol. 2004;160:509–516. doi: 10.1093/aje/kwh255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Choi BC, Pak AW. A simple approximate mathematical model to predict the number of severe acute respiratory syndrome cases and deaths. J Epidemiol Community Health. 2003;57:831–835. doi: 10.1136/jech.57.10.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Y, Zhang J, Zhang X. Modeling and preventive measures of hand, foot and mouth disease (HFMD) in China. Int J Environ Res Public Health. 2014;11:3108–3117. doi: 10.3390/ijerph110303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Urashima M, Shindo N, Okabe N. Seasonal models of herpangina and hand-foot-mouth disease to simulate annual fluctuations in urban warming in Tokyo. Jpn J Infect Dis. 2003;56:48–53. [PubMed] [Google Scholar]
- 126.Chang HL, Chio CP, Su HJ, et al. The association between enterovirus 71 infections and meteorological parameters in Taiwan. PLoS One. 2012;7:e46845. doi: 10.1371/journal.pone.0046845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ma E, Lam T, Wong C, et al. Is hand, foot and mouth disease associated with meteorological parameters? Epidemiol Infect. 2010;138:1779–1788. doi: 10.1017/S0950268810002256. [DOI] [PubMed] [Google Scholar]
- 128.Huang Y, Deng T, Yu S, et al. Effect of meteorological variables on the incidence of hand, foot, and mouth disease in children: a time-series analysis in Guangzhou, China. BMC Infect Dis. 2013;13:134. doi: 10.1186/1471-2334-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abad FX, Pintó RM, Bosch A. Survival of enteric viruses on environmental fomites. Appl Environ Microbiol. 1994;60:3704–3710. doi: 10.1128/aem.60.10.3704-3710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang JF, Guo YS, Christakos G, et al. Hand, foot and mouth disease: spatiotemporal transmission and climate. Int J Health Geogr. 2011;10:25. doi: 10.1186/1476-072X-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bélanger M, Gray-Donald K, O’Loughlin J, et al. Influence of weather conditions and season on physical activity in adolescents. Ann Epidemiol. 2009;19:180–186. doi: 10.1016/j.annepidem.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 132.Lofgren E, Fefferman NH, Naumov YN, et al. Influenza seasonality: underlying causes and modeling theories. J Virol. 2007;81:5429–5436. doi: 10.1128/JVI.01680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singapore Ministry of Health. Hand, foot & mouth disease: updates. https://www.moh.gov.sg/content/moh_web/home/diseases_and_conditions/h/hand_foot_mouth_disease.html.
- 134.Li J, Lin C, Qu M, et al. Excretion of enterovirus 71 in persons infected with hand, foot and mouth disease. Virol J. 2013;10:13. doi: 10.1186/1743-422X-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu FC, Meng FY, Li JX, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381:2024–2032. doi: 10.1016/S0140-6736(13)61049-1. [DOI] [PubMed] [Google Scholar]
- 136.Li R, Liu L, Mo Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370:829–837. doi: 10.1056/NEJMoa1303224. [DOI] [PubMed] [Google Scholar]
- 137.Zhu F, Xu W, Xia J, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370:818–828. doi: 10.1056/NEJMoa1304923. [DOI] [PubMed] [Google Scholar]
- 138.Solomon T, Lewthwaite P, Perera D, et al. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis. 2010;10:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 139.Tong CW, Bible JM. Global epidemiology of enterovirus 71. Future Virol. 2009;4:501–510. [Google Scholar]
- 140.Yip CCY, Lau SKP, Woo PCY, et al. Human enterovirus 71 epidemics: what’s next? Emerg Health Threats J. 2013;6:19780. doi: 10.3402/ehtj.v6i0.19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ooi MH, Wong SC, Lewthwaite P, et al. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9:1097–1105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 142.Fang Y, Wang S, Zhang L, et al. Risk factors of severe hand, foot and mouth disease: a meta-analysis. Scand J Infect Dis. 2014;46:515–522. doi: 10.3109/00365548.2014.907929. [DOI] [PubMed] [Google Scholar]
- 143.Zhao YY, Jin H, Zhang XF, et al. Case-fatality of hand, foot and mouth disease associated with EV71: a systematic review and meta-analysis. Epidemiol Infect. 2015;143:3094–3102. doi: 10.1017/S095026881500028X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chia MY, Chiang PS, Chung WY, et al. Epidemiology of enterovirus 71 infections in Taiwan. Pediatr Neonatol. 2014;55:243–249. doi: 10.1016/j.pedneo.2013.07.007. [DOI] [PubMed] [Google Scholar]


