Abstract
Tissue engineered skeletal muscle holds promise as a source of graft tissue for repair of volumetric muscle loss and as a model system for pharmaceutical testing. To reach this potential, engineered tissues must advance past the neonatal phenotype that characterizes the current state of the art. In this review, we describe native skeletal muscle development and identify important growth factors controlling this process. By comparing in vivo myogenesis to in vitro satellite cell cultures and tissue engineering approaches, several key similarities and differences that may potentially advance tissue engineered skeletal muscle were identified. In particular, the use of HGF and FGF to accelerate satellite cell activation and proliferation, followed by addition of IGF as a potent inducer of differentiation are proven methods for increased myogenesis in engineered muscle. Additionally, we review our recent novel application of dexamethasone (DEX), a glucocorticoid that stimulates myoblast differentiation, in skeletal muscle tissue engineering. Using our established skeletal muscle unit (SMU) fabrication protocol, timing and dose dependent effects of DEX were measured. The supplemented SMUs demonstrated advanced sarcomeric structure and significantly increased myotube diameter and myotube fusion index, compared to untreated controls. Most significantly, these SMUs exhibited a 5-fold rise in force production. Thus, we concluded that DEX may serve to improve myogenesis, advance muscle structure, and increase force production in engineered skeletal muscle.
Keywords: Skeletal Muscle, Tissue Engineering, Growth factors
Introduction
Proposed applications for tissue engineered skeletal muscle include implantation as a graft material for repair of traumatic damage(Bach et al. 2003; Koning et al. 2009), recapitulation in vitro of native development and regeneration for detailed physiological study or pharmaceutical testing(Lee and Vandenburgh. 2013), and use as biomechanical actuators(Neal et al. 2014; Sakar et al. 2012). In all cases, mimicking the complex structure and function of skeletal muscle in vivo is an essential consideration. To date, however, engineered tissues have been characterized by a neonatal phenotype in terms of vascularity, force production, and structural maturity(Juhas et al. 2014; Williams et al. 2013). Without major advances, especially in the area of vascularization, use of engineered muscle as a graft material will be severely limited. Implantation of engineered skeletal muscle into an in vivo regenerative environment, however, has promoted development towards the adult phenotype(Corona et al. 2014; VanDusen et al. 2014), and several recent studies have attempted to utilize key chemical and mechanical stimuli to improve the maturity of these engineered muscles in vitro (Dennis et al. 2009; Martin et al. 2013). This review focuses on important in vitro biochemical stimuli, summarizing the current state of the art in growth factors utilized for skeletal muscle tissue engineering. Additionally, data is presented on the steroid dexamethasone (DEX) and its effects on tissue engineered skeletal muscle units (SMUs) as a novel stimulus for in vitro muscle maturation.
Growth factors in native skeletal muscle development and regeneration
Skeletal Muscle Development During Embryogenesis
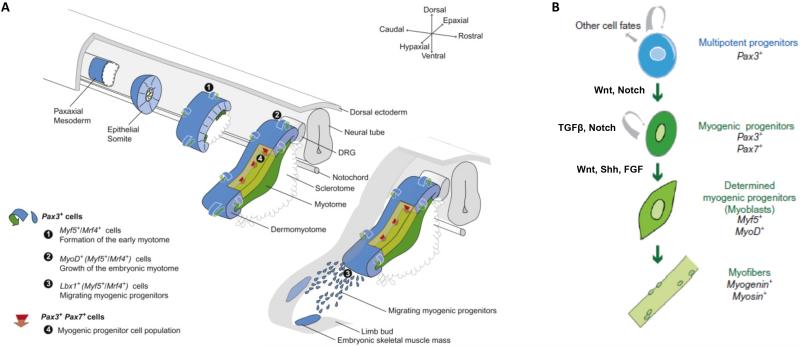
Before attempting to describe the ideal in vitro biochemical environment for engineered skeletal muscle, it is necessary to understand the in vivo environment tissue engineers seek to emulate. During embryogenesis, morphogen gradients control patterning of the developing tissues. Following germ layer formation in the pre-patterned embryo, localized variations in gene expression and signaling gradients prompt condensations of the paraxial mesoderm into somites (Figure 1)(Bentzinger et al. 2012). Genes in the Notch and Wnt pathways prompt somitogenesis in concert with spatiotemporal gradients of fibroblast growth factor (FGF) and Wnt proteins. The most dorsal section of the somite becomes the dermomyotome, from which the majority of skeletal muscles are derived. Myogenesis is initiated as the Pax3+ progenitor cell pool present in the somite delaminates and progressively establishes the primary myotome(Biressi et al. 2007). Members of the Wnt family of proteins again play a central role in this process. Through binding to Frizzled receptors, Wnt signaling activates the β-catenin/TCF complex and induces somite patterning and expression of the myogenic transcription factors Pax3 and Myf5(Bentzinger et al. 2012). A small subset of the progenitor cell pool migrates into the myotome, proliferates, and then terminally differentiates into myoblasts. In turn, the terminally differentiated myoblasts fuse with each other to form the first multinucleated myotubes and primary myofibers(Buckingham and Mayeuf. 2012). This stage of myogenesis is primarily regulated by the canonical myogenic regulatory factors: Myf5, MyoD, and myogenin. Sonic hedgehog (Shh), released from the notochord and floor plate of the neural tube, promotes Myf5 expression and commitment to the myogenic lineage(Bentzinger et al. 2012; Buckingham and Mayeuf. 2012). With the establishment of innervation, the remaining cells from the progenitor pool differentiate into myoblasts and fuse to form secondary myofibers. In addition, a subset of skeletal muscle progenitor cells start to co-express Pax3 and Pax7, becoming post-natal progenitor cells often referred to as satellite cells(Grefte et al. 2007; Mauro. 1961). These satellite cells are not activated during embryonic myogenesis and remain as a reserve pool for post-natal muscle growth and regeneration. Bone morphogenic proteins (BMPs), a sub-class of the transforming growth factor-beta (TGFβ) superfamily, serve to preserve this progenitor pool by inhibiting Myf5 and MyoD expression while upregulating Pax3.
Figure 1. Initial Skeletal Muscle Formation During Embryogenesis.
(A) Development of the somite and subsequent establishment of the myotome. The initial myogenic lineage of the progenitor cells involved is detailed in the bottom left. (B) Regulation of progenitor cell renewal and differentiation by myogenic regulator factors and external signaling. Adapted from (Buckingham and Mayeuf. 2012).
In summary, myogenesis during embryonic development is primarily regulated by Myf5, MyoD, and myogenin(Buckingham and Mayeuf. 2012), but several molecular signals and growth factors interact with these myogenic regulatory factors. Wnt and FGF gradients direct initial somitogenesis. Subsequently, Shh and Wnt signaling lead to specification and expression of Myf5. Additionally, TGFβ is known to act through serine-threonine kinase receptors, activating SMAD proteins inhibiting Myf5 and MyoD induction. As a result, the onset of skeletal muscle formation is delayed and the myogenic progenitor pool is preserved. Finally, FGF acts as an antagonist to TGFβ in regulating the equilibrium between renewal and differentiation of progenitor pool. Specifically, FGF upregulates Myf5 and MyoD, promotes activation of progenitors in the myogenic lineage(Schiaffino and Mammucari. 2011), and begins the transition to post-natal skeletal muscle development.
Post-natal Skeletal Muscle Development
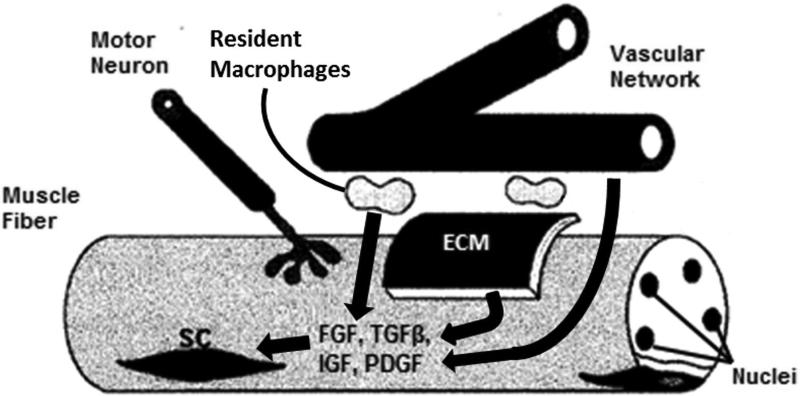
Post-natal skeletal maturation and lengthening of skeletal muscle relies on the contribution of nuclei and contractile proteins by satellite cells to keep pace with the growing skeleton and to mature in structure. This process is characterized by addition of new sarcomeres along the length of each fiber, establishment of myotendinous junctions, and the transition in myosin heavy chain (MHC) expression from embryonic to adult fast and slow isoforms(Biressi et al. 2007; Dhawan and Rando. 2005). Satellite cells provide the nuclei required to regulate this continued growth. Until activated, satellite cells remain quiescent under the basal lamina of skeletal muscle fibers and are characterized by the expression of Pax7(Mauro. 1961). The surrounding stem cell microenvironment or niche, composed of extracellular matrix, vascular and neural networks, neighboring cells, and growth factors, is highly influential on myogenic function (Figure 2)(Cosgrove et al. 2009; Yin et al. 2013). In response to a complex series of signals, satellite cells are activated and progress toward a committed myogenic lineage, with a sub-population returning to quiescence to maintain the progenitor pool. As in embryonic development, Notch signaling and Wnt proteins act as essential regulators of post-natal maturation of the satellite cell. In particular, Wnt3a signaling promotes satellite cell activation and differentiation, whereas Wnt7a induces self-renewal and maintenance of the satellite cell pool(Bentzinger et al. 2012). Mammalian (or mechanistic) target of rapamycin (mTOR) also plays a key role in mediating post-natal satellite cell activation, proliferation, and differentiation. Although more commonly associated with skeletal muscle hypertrophy and homeostasis, mTOR has recently been shown to regulate satellite cell activity and myogenesis by upregulating expression of Pax7, Myf5, MyoD, and myogenin(Zhang et al. 2015). Additionally, several growth factors stimulate quiescent satellite cells. Hepatocyte growth factor (HGF) is present in inactive form in the extracellular matrix adjacent to satellite cells(Dhawan and Rando. 2005). When released due to injury or length damage, HGF is thought to bind c-met receptors present on quiescent satellite cells, leading to their activation. Similarly, members of the FGF family are present in the satellite cell niche and bind to quiescent satellite cell receptors following FGF release(Buckingham and Mayeuf. 2012; Dhawan and Rando. 2005). After activation via growth factors such as HGF or FGF, those satellite cells induced to a myogenic lineage are often referred to as myogenic precursor cells or myoblasts, characterized by their expression of canonical myogenic transcription factors MyoD and Myf5. Following their proliferation and differentiation, the myoblasts fuse with maturing muscle fibers and promote protein synthesis and muscle growth. Connective tissue fibroblasts in the extracellular matrix have been shown to interact with satellite cells throughout this stage, with reciprocal signaling between the two cell types prompting increased proliferation of both(Murphy et al. 2011). Furthermore, expression of the transcription factor Tcf4 by connective tissue fibroblasts intrinsically regulates the maturation of MHC isoforms through β-catenin activation(Buckingham and Mayeuf. 2012; Mathew et al. 2011). In conclusion, post-natal skeletal muscle development depends on activation and myogenic differentiation of satellite cells. Although the satellite cell pool is considered heterogeneous, several common signaling pathways play essential roles in this myogenic lineage, with HGF and FGF as the primary growth factors involved.
Figure 2. Growth Factor Signalling in Skeletal Muscle Regeneration.
The microenvironment surrounding the satellite cell (SC) niche plays a key role in repair of skeletal muscle damage. Resident immune cells, extracellular matrix (ECM), and capillary and neural networks compose this niche. This schematic describes a simplified process by which essential growth factors (FGF, TGFβ, IGF, and PDGF) are supplied to stimulate and regulate SCs.
Skeletal Muscle Homeostasis
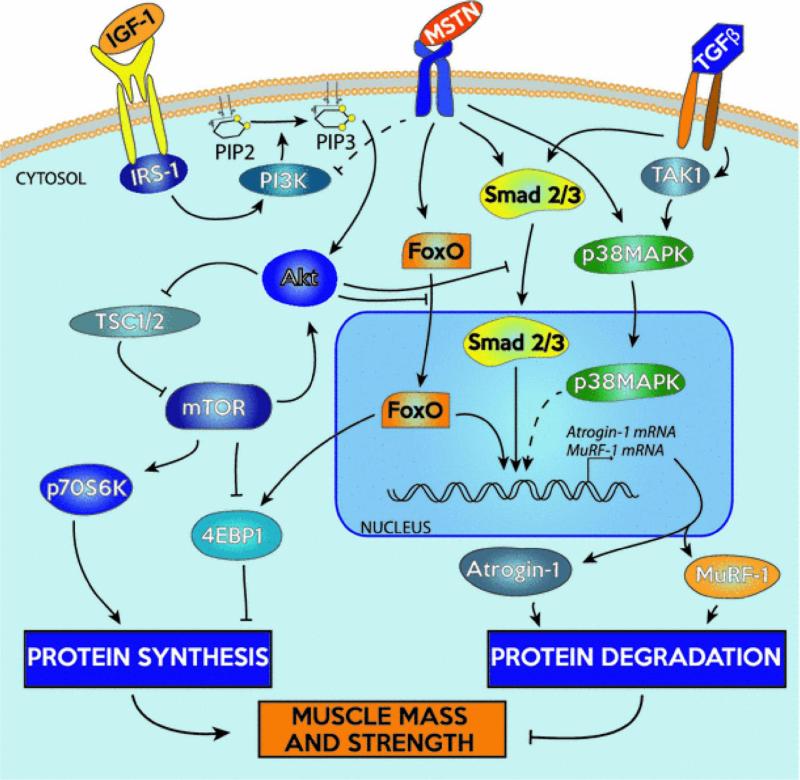
Once skeletal maturation is complete, skeletal muscle homeostasis is maintained through hypertrophy and atrophy. Hypertrophy and regeneration are primarily regulated by a signaling pathway initiated by insulin-like growth factor 1 (IGF1)(Schiaffino and Mammucari. 2011). Whether expressed by muscle cells in response to injury and exercise, secreted by macrophages and endothelial cells with inflammation, or supplied by the circulatory system in the blood, IGF levels increase rapidly in preparation for protein synthesis(Zanou and Gailly. 2013). Following IGF1 binding, an intracellular cascade mediates its effects (Figure 3)(Glass. 2003; Glass. 2005). Among these intracellular signals, Akt, or protein kinase B (PKB), plays an essential role(Jackman and Kandarian. 2004). To promote protein synthesis and hypertrophy, Akt indirectly activates mTOR while simultaneously inhibiting glycogen synthase kinase 3b (GSK3b). At the same time, Akt prevents protein degradation and muscle atrophy by blocking the FoxO family of transcriptions factors(Sandri. 2008). Myostatin, a member of the TGFβ family predominantly expressed in skeletal muscle, conversely functions as a potent cytokine for the inhibition of muscle growth and induction of muscle atrophy. Following activation and release from the extracellular matrix, myostatin activates Smad2/3 and TAK1/P38 MAPK signaling cascades, leading to upregulation of Atrogin-1 and muscle RING-finger protein-1 (MuRF-1) and subsequent proteolysis(Gumucio and Mendias. 2013). Both IGF1 and myostatin, along with their downstream effectors, have been targeted for therapeutic use in vivo(Sandri. 2008; Zanou and Gailly. 2013), and it is expected that similar benefits will translate to engineered skeletal muscle. External to the IGF/Akt/mTOR signaling pathway, several growth factors have been examined due to their effects on myogenic progenitor cells implicated in skeletal muscle growth and development. As in embryonic development, TGFβ inhibits progression of muscle precursor cells(Sandri. 2008; Wagers and Conboy. 2005). In contrast, HGF, FGF, and platelet derived growth factor (PDGF) have all been associated with muscle repair and hypertrophy(Huard et al. 2002; Husmann et al. 1996). As in post-natal development, HGF is released from the extracellular matrix with injury to prompt satellite cell activation and proliferation via p38 MAPK and PI3K(Zanou and Gailly. 2013). FGF serves a similar purpose, mainly stimulating proliferation of satellite cells. After release from activated platelets and macrophages, PDGF again promotes myogenic proliferation, in addition to angiogenesis. Interestingly, these three growth factors (HGF, FGF, and PDGF) all have an inhibitory effect on myogenic differentiation(Husmann et al. 1996; Zanou and Gailly. 2013). Overall, it is evident how various growth factors are intricately involved in these native pathways controlling the development, growth, and regeneration of skeletal muscle. Their interplay with the canonical myogenic regulatory factors is evident in the activation and proliferation of myogenic progenitors, terminal differentiation to myoblasts, and up- or down-regulation of protein synthesis and degradation.
Figure 3. Central pathways to regulation of skeletal muscle hypertrophy and atrophy.
Adapted from (Gumucio and Mendias. 2013).
Growth factors for skeletal muscle tissue engineering
Tissue engineers have used this understanding of growth factors and their role in myogenesis to direct techniques for the fabrication of skeletal muscle. To date, tissue engineering technologies utilize either scaffold materials ranging from decellularized tissues(Sicari et al. 2014; Wu et al. 2012) to collagen and fibrin hydrogels(Juhas et al. 2014; Lam et al. 2009; Lee and Vandenburgh. 2013) or opt for a scaffold-free approach(Carosio et al. 2013; VanDusen et al. 2014; Williams et al. 2013) to promote the development of an extracellular matrix (ECM) for subsequent muscle tissue. The success of these techniques ultimately depends on the in vitro cultivation of isolated primary muscle precursor cells for the development of mature muscle cells within the ECM. The typical techniques for culture of these myogenic cells involves an initial proliferation phase, to allow cell numbers to expand to a sufficiently large population, followed by differentiation and fusion into myotubes and maturation to myofibers(Clegg et al. 1987). Detailed study of growth factors known to play a role in each of these phases of myogenesis is thus essential to further the understanding of muscle growth in vitro. It is generally accepted that media rich in serum promotes initial proliferation of skeletal muscle stem cells to myoblasts while delaying the onset of differentiation, potentially due to large number of hormones and growth factors of varying concentration and potency found in the serum(Allen et al. 1997). Dramatic changes in function and speed of contraction were recently observed between engineered muscles cultured in serum from the United States and from the European Union(Khodabukus and Baar. 2014). Similarly, environmental factors in the culture media, such as glucose and antibiotic concentration, have been shown to alter engineered muscle phenotype and function(Khodabukus and Baar. 2015). In this study, ideal conditions for maximizing force production in tissue engineered skeletal muscle involved high glucose (25mM) and an absence of streptomycin. A drastic reduction in media serum content following the proliferation phase triggers differentiation of the myoblast to the myotube, possibly due to the absence of key mitogenic components. Due to the lot-to-lot variations in growth factors present in the commercially available serum and the consequent variability in satellite cell induction and proliferation, an optimum serum formulation and the identity of these mitogenic components has yet to be fully defined(Doumit and Merkel. 1992). To date, several growth factors have been implicated as potential serum components with important myogenic effects.
As would be expected based on their in vivo influence on skeletal muscle hypertrophy described above, FGF, PDGF, and HGF promote activation and proliferation of myogenic progenitor cells and delay terminal differentiation(Allen et al. 1995; Husmann et al. 1996; Kuang et al. 2008). The stimulatory effect of FGF on satellite cell proliferation has been shown to produce a two-fold increase in DNA content, relative to untreated cultures(Düsterhöft and Pette. 1999). This enhanced proliferation translated to formation of larger myotubes and increased expression of myogenin and MHC. When added during the differentiation phase of the culture, however, these significant effects were not observed(Düsterhöft and Pette. 1999; Maley et al. 1995). Similarly, the addition of PDGF led to a two-fold increase in DNA synthesis and improved satellite cell proliferation, but did not yield a significant increase in desmin expression and myotube formation(Maley et al. 1995; Yablonka-Reuveni et al. 1990). In the case of HGF, the time between isolation of satellite cells and the onset of the cell cycle decreased from approximately 42-60 hours to less than 24 hours(Allen et al. 1995). These results demonstrate conservation of the signaling pathways involved in native myogenesis in an in vitro setting, with HGF activating quiescent satellite cells to begin progressing down the myogenic lineage. By combining HGF with either FGF or PDGF, tissue engineers may be able to maximize the replicative potential of satellite cells by achieving activation earlier and then increasing subsequent proliferation. Furthermore, HGF and FGF have a tendency to delay terminal differentiation, allowing for extension of the window for satellite cell proliferation. Additionally, IGF plays a key role in all phases of satellite cell myogenesis, from activation and proliferation to induction of the onset of myogenic differentiation(Allen and Boxhorn. 1989; Chakravarthy et al. 2000). In one study, IGF was supplied in the fibrin gel used as a 3D scaffold to support the fabricated muscle tissue(Huang et al. 2004). The beneficial effects of IGF addition were seen in the form of MyoD upregulation during initial proliferation, followed by a 50% increase in force production in the final engineered tissue. The functional improvement with IGF addition illustrates its importance in engineering skeletal muscle. In contrast, TGFβ typically has a negative influence on both myogenic phases in vitro, slightly suppressing proliferation and severely inhibiting differentiation(Allen and Boxhorn. 1989; Husmann et al. 1996; Maley et al. 1995). TGFβ, however, can enhance contractility of engineered muscle by promoting collagen type I synthesis in the extracellular matrix, supporting myofiber development and force transmission(Weist et al. 2013). As a result of this findings, it is common to supplement media supplied during the initial proliferation phase with HGF and FGF, prior to switching to a media supplemented with IGF or insulin for the induction of differentiation(Lee and Vandenburgh. 2013; Williams et al. 2013). By adding these specific growth factors to influence satellite cell proliferation and differentiation, tissue engineers have successfully created skeletal muscle constructs in vitro featuring neonatal functional and structural characteristics(VanDusen et al. 2014; Williams et al. 2013).
Effects of dexamethasone on satellite cells during skeletal muscle tissue engineering
Dexamethasone has previously been used to influence satellite cell cultures in vitro, but its potential benefits for tissue engineered skeletal muscle have yet to be examined. To expand on the understanding of DEX during fabrication of tissue engineered skeletal muscle, our laboratory has studied the effect of dose and time of administration of DEX on our engineered muscle constructs. Clinically, DEX has anti-inflammatory or immunosuppressant activity and is used in treating several rheumatologic and skin diseases, in addition to severe asthma and allergies. In skeletal muscle, exogenously delivered DEX has profoundly different effects depending on the dosage and timing of the administration. When administered in supraphysiological doses to adult skeletal muscle, DEX leads to atrophy through upregulation of the myostatin promoter and inhibition of IGF-1 expression (Figure 3)(Inder et al. 2010; Qin et al. 2013). These findings make it a potential agent for the induction of adult skeletal muscle myopathy. In contrast, the addition of 5 to 25 nM DEX has been shown to improve myogenesis in vitro by enhancing differentiation and myotube fusion, potentially through its induction of dysferlin, a calcium-binding transmembrane protein thought to play an important role in both myogenesis and membrane repair(Belanto et al. 2010). Other in vitro studies have demonstrated that DEX can inhibit myoblast proliferation and protein synthesis(Desler et al. 1996), however, reinforcing the need for careful timing of addition to culture. The studies referenced above, delineating DEX effects in vitro, primarily experimented with the immortal C2C12 mouse myoblast cell line, rather than the primary cell population used in our tissue engineering methods. Our study exposed the heterogeneous pool of cells obtained from a soleus muscle isolation (primarily satellite cells and fibroblasts, but also containing endothelial cells, perivascular cells, immune cells, and several other cell types) to different doses of DEX. By administering DEX at several time points we found the optimal conditions for improving myogenesis, and ultimately maximizing in vitro structural and functional development of tissue engineered skeletal muscle. These findings are briefly summarized below.
Dexamethasone Induction of Muscle Satellite Cell Differentiation and Myotube Fusion
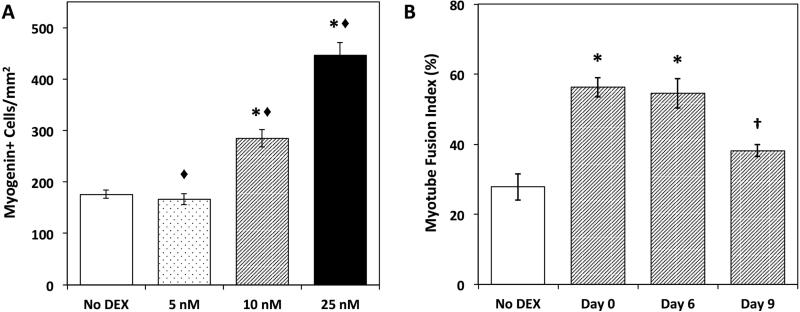
Using our established SMU fabrication protocol, muscle isolates were cultured with four DEX concentrations (0, 5, 10, and 25nM)(Syverud et al. June 2015 (Submitted)). Following seeding onto a laminin-coated Sylgard substrate, the administration of DEX was initiated at seeding (Day 0), during the proliferative stage (Day 6), or in the differentiation phase (Day 9) and was sustained until the completion of SMU fabrication. Immunocytochemical analysis of myogenin expression was used as an indicator of satellite cell differentiation into myoblasts(McFarland et al. 2013). A dose-dependent increase in myogenin-positive cell density was observed in response to the administration of DEX (Figure 4A) regardless of the growth stage at which it was added.
Figure 4. DEX Effects on Myogenic Differentiation.
(A) Myogenin expression indicated increasing terminal differentiation of satellite cells and muscle progenitors into myoblasts. A dose-dependent response to DEX addition was observed for myogenin-positive cell density (B) Myotube fusion index calculated from desmin and DAPI staining quantified the number of nuclei associated with a myotube as a percentage of total nuclei. Fusion index increased significantly with DEX addition on Day 0 and Day 6. Error bars indicate mean ± standard error. * Indicates statistical difference from control, ◆ from other DEX concentrations, † from other DEX timings.
Progressing along the myogenic pathway, myotube fusion index was analyzed to assess the ability of myoblasts to form a robust network of terminally differentiated myotubes in the presence of dexamethasone. All three doses of DEX (5 nM, 10 nM, and 25 nM) significantly improved myotube fusion relative to untreated controls (No DEX). No significant difference was observed between the three doses. Early addition of DEX at either cell seeding on Day 0 or during the proliferative stage on Day 6, resulted in significantly increased myotube fusion (Figure 4B). Treatment with DEX during the subsequent differentiation stage (Day 9), however, exhibited no improvement to myotube fusion, and in fact showed no difference from controls.
to myotube fusion, myotube size and number were affected by administration of DEX at early time points. Again, DEX addition at either cell seeding (Day 0) or during the proliferative stage (Day 6), resulted in significantly increased myotube diameter (Figure 5). Treatment with DEX during the later differentiation stage (Day 9) exhibited no improvement to myotube diameter. In addition, a dose-dependent response to DEX was observed, with myotube diameter consistently increasing as DEX concentration increased. Not surprisingly, the greatest effect of DEX on myotube density occurred when DEX was administered during the proliferative stage (Day 6). Administration of DEX on either Day 0 or during differentiation (Day 9) showed no effect and resulted in cell densities similar to the No DEX controls. Additionally, no differences in myotube densities were observed with respect to DEX concentrations. Together, myotube size and density data suggest that addition of DEX on Day 6 leads to an increased number of more robust myotubes, which may be preferable for engineering skeletal muscle.
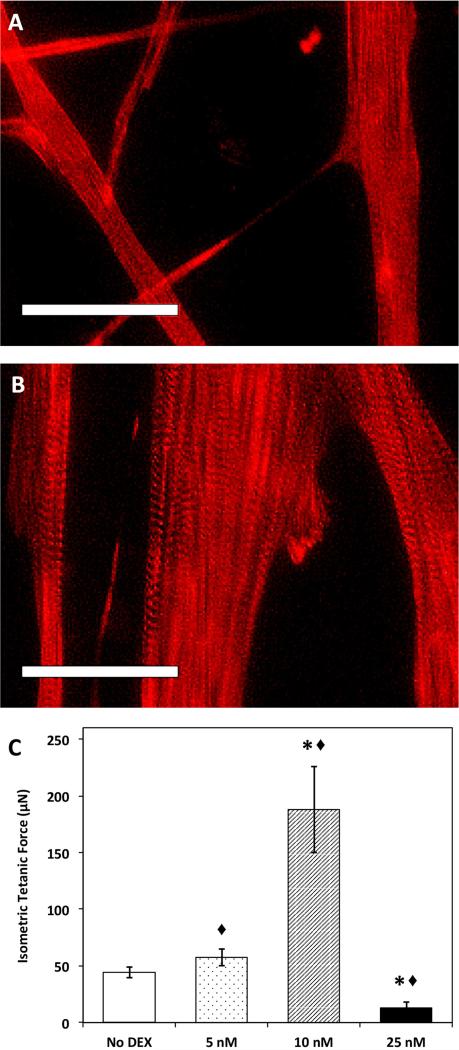
Figure 5. SMU Maturation with DEX addition.
Representative images of engineered skeletal muscle (A) without DEX and (B) following DEX addition. Images show α-actinin in developing muscle monolayers just 10 days post-seeding. Formation of advanced sarcomeric structure and aligned myofibrils was evident in DEX treated plates at this early time point. (C) Based on functional measures of isometric tetanic force in DEX treated SMUs, the addition of 10nM DEX is optimal for SMU fabrication. Interestingly, the addition of 25nM DEX had a consistently detrimental effect on force production. Scale bars = 50 μm. Error bars indicate mean ± standard error. * Indicates statistical difference from control, ◆ from other DEX concentrations.
Structural and Functional Maturation of SMUs with addition of Dexamethasone
Following 3-D formation, our SMUs were evaluated for function by assessing contractile force production. The isometric tetanic forces produced by engineered SMUs are displayed in Figure 5C. Interestingly, only the 10nM DEX concentration led to an improvement in function, characterized by a five-fold increase in maximum isometric force production compared to No DEX controls. The SMUs that received 5nM DEX did not show any improvement in force production relative to control SMUs. Furthermore, the addition of 25nM DEX led to a significant decrease in force production compared to controls. This decrease in function at the 25nM DEX dosage may have resulted from cell injury, indicated by the formation of blebs on the periphery of several myotubes(Wang et al. 2010). Alternatively, since DEX can potentially upregulate fibroblast proliferation and tissue fibrosis (Dammeier et al. 1998), it is possible that the 25nM dosage may have prompted fibroblast overgrowth at the expense of engineered muscle function. Again, the early addition of DEX at either cell seeding (Day 0) or during the proliferative stage (Day 6) resulted in the greatest improvement in force production. In support of the increased force production observed following early phase administration of 10nM DEX, immunocytochemical analysis of α-actinin showed advanced maturation of myotubes demonstrated by the presence of advanced sarcomeric structure within highly aligned myofibrils (Figure 5). In contrast, no sarcomeric structure was observed in the control muscle cultures.
Current Understanding of Growth Factors and DEX in Skeletal Muscle Tissue Engineering
The overall effects of DEX during the SMU fabrication process can be divided into differentiation and maturation phases. In agreement with previous findings in the literature(Belanto et al. 2010), addition of DEX in 10nM and 25nM doses led to increased myogenic differentiation into myoblasts, as shown by expression of the canonical myogenic regulatory factor, myogenin, followed by eventual fusion into myotubes. Generally, the 25nM concentration led to greater differentiation and fusion, but those increases interestingly did not translate to improved force production. Overall, this experiment demonstrated that the addition of exogenous dexamethasone to isolated muscle satellite cells can improve force production and structural characteristics of our tissue engineered skeletal muscle when administered at optimal doses and timings. The most promising results were achieved with the addition of 10nM DEX on either Day 0 or Day 6. In addition to improved myogenic differentiation and myotube fusion, SMUs exposed to this concentration exhibited substantially accelerated structural maturation accompanied by a five-fold increase in force production.
These DEX experiments were conducted as a means of supplementing our existing protocol for engineering skeletal muscle. This established SMU fabrication protocol(VanDusen et al. 2014) has capitalized on the advances in satellite cell cultures and skeletal muscle tissue engineering described in the introduction. Specifically, FGF is supplied in the M-GM as a means of maximizing satellite cell proliferation and delaying terminal differentiation, serving a similar role to either PDGF or HGF during this phase. Insulin is subsequently added to M-DM as an analog of IGF for induction of myogenic differentiation. IGF acts as a potent inducer of myogenesis, both in vivo and in vitro, and is the only growth factor known to promote both satellite cell proliferation and differentiation. By using a novel application of DEX to build on our existing tissue engineering expertise, this article presents a blueprint for advancing tissue engineering of skeletal muscle.
Acknowledgements
The authors would like to acknowledge the support of the NIH's R56 grant: 2-R56-AR-054778-06-A1 and the Microfluidics in Biomedical Sciences Training Program: NIH NIBIB T32 EB005582.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- Syverud B, VanDusen K, Larkin L. Effects of dexamethasone on satellite cells and tissue engineered skeletal muscle units. Tissue Engineering Part A. 2015 doi: 10.1089/ten.tea.2015.0545. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodabukus A, Baar K. Glucose concentration and streptomycin alter in vitro muscle function and metabolism. Journal of Cellular Physiology. 2015;230(6):1226. doi: 10.1002/jcp.24857. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liang X, Shan T, Jiang Q, Deng C, Zheng R, Kuang S. mTOR is necessary for proper satellite cell activity and skeletal muscle regeneration. Biochemical and Biophysical Research Communications. 2015;463(1-2):102. doi: 10.1016/j.bbrc.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Engineering. Part A. 2014;20(3-4):705–715. doi: 10.1089/ten.tea.2012.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhas M, Engelmayr GC, Fontanella AN, Palmer GM, Bursac N. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(15):5508–5513. doi: 10.1073/pnas.1402723111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodabukus A, Baar K. The effect of serum origin on tissue engineered skeletal muscle function. Journal of Cellular Biochemistry. 2014;115(12):2198. doi: 10.1002/jcb.24938. [DOI] [PubMed] [Google Scholar]
- Neal D, Sakar MS, Ong LS, Asada HH. Formation of elongated fascicle-inspired 3D tissues consisting of high-density, aligned cells using sacrificial outer molding. Lab on a Chip. 2014;14(11):1907–1916. doi: 10.1039/c4lc00023d. [DOI] [PubMed] [Google Scholar]
- Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, Turner NJ, Weber DJ, Simpson TW, Wyse A, Brown EHP, Dziki JL, Fisher LE, Brown S, Badylak SF. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Science Translational Medicine. 2014;6(234):1–11. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDusen KW, Syverud BC, Williams ML, Lee JD, Larkin LM. Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Engineering Part A. 2014;20(21-22):2920–2930. doi: 10.1089/ten.tea.2014.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carosio S, Barberi L, Rizzuto E, Nicoletti C, Del Prete Z, Musarò A. Generation of eX vivo-vascularized muscle engineered tissue (X-MET). Scientific Reports. 2013;3:1420–1429. doi: 10.1038/srep01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43(1):12–21. doi: 10.1007/s12020-012-9751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PHU, Vandenburgh HH. Skeletal muscle atrophy in bioengineered skeletal muscle: A new model system. Tissue Engineering. Part A. 2013;19(19-20):2147–2155. doi: 10.1089/ten.TEA.2012.0597. [DOI] [PubMed] [Google Scholar]
- Martin NRW, Passey SL, Player DJ, Khodabukus A, Ferguson RA, Sharples AP, Mudera V, Baar K, Lewis MP. Factors affecting the structure and maturation of human tissue engineered skeletal muscle. Biomaterials. 2013;34(23):5759–5765. doi: 10.1016/j.biomaterials.2013.04.002. [DOI] [PubMed] [Google Scholar]
- McFarland DC, Pesall JE, Coy CS, Velleman SG. Effects of 17ß-estradiol on turkey myogenic satellite cell proliferation, differentiation, and expression of glypican-1, MyoD and myogenin. Comparative Biochemistry and Physiology.Part A, Molecular & Integrative Physiology. 2013;164(4):565–571. doi: 10.1016/j.cbpa.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Qin J, Du R, Yang Y, Zhang H, Li Q, Liu L, Guan H, Hou J, An X. Dexamethasone-induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity. Research in Veterinary Science. 2013;94(1):84–89. doi: 10.1016/j.rvsc.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Weist MR, Wellington MS, Bermudez JE, Kostrominova TY, Mendias CL, Arruda EM, Larkin LM. TGFβ1 enhances contractility in engineered skeletal muscle. Journal of Tissue Engineering and Regenerative Medicine. 2013;7(7):562–571. doi: 10.1002/term.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ML, Kostrominova TY, Arruda EM, Larkin LM. Effect of implantation on engineered skeletal muscle constructs. Journal of Tissue Engineering and Regenerative Medicine. 2013;7(6):434–442. doi: 10.1002/term.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiological Reviews. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanou N, Gailly P. Skeletal muscle hypertrophy and regeneration: Interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cellular and Molecular Life Sciences : CMLS. 2013;70(21):4117. doi: 10.1007/s00018-013-1330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: Molecular regulation of myogenesis. Cold Spring Harbor Perspectives in Biology. 2012;4(2) doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, Mayeuf A. Chapter 52 - skeletal muscle development. In: Olson JAHN, editor. Muscle. Academic Press; Boston/Waltham: 2012. pp. 749–762. [Google Scholar]
- Sakar MS, Neal D, Boudou T, Borochin MA, Li Y, Weiss R, Kamm RD, Chen CS, Asada HH. Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab on a Chip. 2012;12(23):4976. doi: 10.1039/c2lc40338b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Corona BT, Chen X, Walters TJ. A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies. BioResearch Open Access. 2012;1(6):280–290. doi: 10.1089/biores.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138(2):371–384. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138(17):3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-akt/PKB pathway: Insights from genetic models. Skeletal Muscle. 2011;1(1):4. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanto JJ, Diaz-Perez SV, Magyar CE, Maxwell MM, Yilmaz Y, Topp K, Boso G, Jamieson CH, Jamieson CAM, Cacalano NA. Dexamethasone induces dysferlin in myoblasts and enhances their myogenic differentiation. Neuromuscular Disorders. 2010;20(2):111–121. doi: 10.1016/j.nmd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder WJ, Jang C, Obeyesekere VR, Alford FP. Dexamethasone administration inhibits skeletal muscle expression of the androgen receptor and IGF-1 implications for steroid-induced myopathy. Clinical Endocrinology. 2010;73(1):126–132. doi: 10.1111/j.1365-2265.2009.03683.x. [DOI] [PubMed] [Google Scholar]
- Wang B, Yang Z, Brisson BK, Feng H, Zhang Z, Welch EM, Peltz SW, Barton ER, Brown RH, Jr., Sweeney HL. Membrane blebbing as an assessment of functional rescue of dysferlin-deficient human myotubes via nonsense suppression. Journal of Applied Physiology. 2010;109(3):901–905. doi: 10.1152/japplphysiol.01366.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove BD, Sacco A, Gilbert PM, Blau HM. A home away from home: Challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2009;78(2):185–194. doi: 10.1016/j.diff.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis R, Smith B, Philp A, Donnelly K, Baar K. Bioreactors for guiding muscle tissue growth and development. Adv Biochem Engin/Biotechnol. 2009;112:39–79. doi: 10.1007/978-3-540-69357-4_3. [DOI] [PubMed] [Google Scholar]
- Koning M, Harmsen MC, van Luyn MJA, Werker PMN. Current opportunities and challenges in skeletal muscle tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. 2009;3(6):407–415. doi: 10.1002/term.190. [DOI] [PubMed] [Google Scholar]
- Lam MT, Huang Y, Birla RK, Takayama S. Microfeature guided skeletal muscle tissue engineering for highly organized 3-dimensional free-standing constructs. Biomaterials. 2009;30(6):1150–1155. doi: 10.1016/j.biomaterials.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2(1):22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology. 2008;23(3):160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- Biressi S, Molinaro M, Cossu G. Cellular heterogeneity during vertebrate skeletal muscle development. Developmental Biology. 2007;308(2):281–293. doi: 10.1016/j.ydbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Grefte S, Kuijpers-Jagtman AM, Torensma R, Hoff J W V d. Skeletal muscle development and regeneration. Stem Cells and Development. 2007;16(5):857–868. doi: 10.1089/scd.2007.0058. [DOI] [PubMed] [Google Scholar]
- Dhawan J, Rando TA. Stem cells in postnatal myogenesis: Molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends in Cell Biology. 2005;15(12):666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. International Journal of Biochemistry and Cell Biology. 2005;37(10):1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: Current concepts and controversies in adult myogenesis. Cell. 2005;122(5):659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Huang Y, Dennis RG, Larkin LM, Baar K. Rapid formation of functional muscle in vitro using fibrin gels. Journal of Applied Physiology. 2004;98(2):706–713. doi: 10.1152/japplphysiol.00273.2004. [DOI] [PubMed] [Google Scholar]
- Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. American Journal of Physiology - Cell Physiology. 2004;287(4):834–843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- Bach AD, Stern-Straeter J, Beier JP, Bannasch H, Stark GB. Engineering of muscle tissue. Clinics in Plastic Surgery. 2003;30(4):589–599. doi: 10.1016/s0094-1298(03)00077-4. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nature Cell Biology. 2003;5(2):87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- Huard J, Li Y, Fu FH. Muscle injuries and repair: Current trends in research. The Journal of Bone & Joint Surgery. 2002;84(5):822–832. [PubMed] [Google Scholar]
- Chakravarthy MV, Abraha TW, Schwartz RJ, Fiorotto ML, Booth FW. Insulin-like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing G1/S cell cycle progression via the activation of phosphatidylinositol 3′-kinase/akt signaling pathway. The Journal of Biological Chemistry. 2000;275(46):35942–35952. doi: 10.1074/jbc.M005832200. [DOI] [PubMed] [Google Scholar]
- Düsterhöft S, Pette D. Evidence that acidic fibroblast growth factor promotes maturation of rat satellite-cell-derived myotubes in vitro. Differentiation. 1999;65(3):161–169. doi: 10.1046/j.1432-0436.1999.6530161.x. [DOI] [PubMed] [Google Scholar]
- Dammeier J, Beer HD, Brauchle M, Werner S. Dexamethasone is a novel potent inducer of connective tissue growth factor expression - implications for glucocorticoid therapy. The Journal of Biological Chemistry. 1998;273(29):18185–18190. doi: 10.1074/jbc.273.29.18185. [DOI] [PubMed] [Google Scholar]
- Allen RE, Temm-Grove CJ, Sheehan SM, Rice G. Skeletal muscle satellite cell cultures. Methods in Cell Biology. 1997;52:155–176. doi: 10.1016/s0091-679x(08)60378-7. [DOI] [PubMed] [Google Scholar]
- Desler MM, Jones SJ, Smith CW, Woods TL. Effects of dexamethasone and anabolic agents on proliferation and protein synthesis and degradation in C2C12 myogenic cells. Journal of Animal Science. 1996;74(6):1265–1273. doi: 10.2527/1996.7461265x. [DOI] [PubMed] [Google Scholar]
- Husmann I, Soulet L, Gautron J, Martelly I, Barritault D. Growth factors in skeletal muscle regeneration. Cytokine & Growth Factor Reviews. 1996;7(3):249–258. doi: 10.1016/s1359-6101(96)00029-9. [DOI] [PubMed] [Google Scholar]
- Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. Journal of Cellular Physiology. 1995;165(2):307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- Maley MAL, Davies MJ, Grounds MD. Extracellular matrix, growth factors, genetics: Their influence on cell proliferation and myotube formation in primary cultures of adult mouse skeletal muscle. Experimental Cell Research. 1995;219(1):169–179. doi: 10.1006/excr.1995.1217. [DOI] [PubMed] [Google Scholar]
- Doumit ME, Merkel RA. Conditions for isolation and culture of porcine myogenic satellite cells. Tissue and Cell. 1992;24(2):253–262. doi: 10.1016/0040-8166(92)90098-r. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Balestreri TM, Bowen-Pope DF. Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. The Journal of Cell Biology. 1990;111(4):1623–1629. doi: 10.1083/jcb.111.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. Journal of Cellular Physiology. 1989;138(2):311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- Clegg CH, Linkhart TA, Olwin BB, Hauschka SD. Growth factor control of skeletal muscle differentiation: Commitment to terminal differentiation occurs in G1Phase and is repressed by fibroblast growth factor. The Journal of Cell Biology. 1987;105(2):949–956. doi: 10.1083/jcb.105.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. The Journal of Biophysical and Biochemical Cytology. 1961;9(2):493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]