Summary
Extensive metabolic changes accompany T cell activation including a switch to glycolytic energy production and increased biosynthesis. Recent studies suggest that subsequent return to reliance on oxidative phosphorylation and increasing spare respiratory capacity are essential for the differentiation of memory CD8+ T cells. In contrast, we found that constitutive glycolytic metabolism and suppression of oxidative phosphorylation in CD8+ T cells, achieved by conditional deletion of hypoxia inducible factor regulator Vhl, accelerated CD8+ memory cell differentiation during viral infection. Despite sustained glycolysis, CD8+ memory cells emerged that upregulated key memory-associated cytokine receptors and transcription factors, and showed a heightened response to secondary challenge. In addition, increased glycolysis not only permitted memory formation, but it also favored the formation of long-lived effector-memory CD8+ T cells. These data redefine the role of cellular metabolism in memory cell differentiation, showing that reliance on glycolytic metabolism does not hinder formation of a protective memory population.
eTOC
Whether alterations in cellular metabolism correlate with or drive CD8+ T cell differentiation is unclear. Phan and colleagues demonstrate that memory T cell differentiation does not require generation of SRC or a switch to reliance on OXPHOS. Furthermore, glycolytic metabolism not only supports memory differentiation, but may promote Tem cells.
Introduction
Memory CD8+ T cells can provide enduring protection against intracellular pathogens and tumors (Kaech and Wherry, 2007). As such, eliciting memory T cells is a primary objective of vaccination strategies. Memory CD8+ T cells are a heterogeneous population consisting of multiple subsets: central memory (Tcm) cells, that reside in secondary lymphoid tissues and have high proliferative potential upon secondary infection; effector memory (Tem) cells, that patrol peripheral tissues and provide rapid effector responses; and tissue resident memory (Trm) cells, that permanently reside in non-lymphoid tissues, provide localized defense, and aid in rapid recruitment of adaptive and innate immune cells to sites of infection (Chang et al., 2014; Jameson and Masopust, 2009; Kaech and Wherry, 2007; Mueller et al., 2013).
Rapamycin-mediated inhibition of mechanistic target of rapamycin (mTOR) signaling (Araki et al., 2009) or metformin-induced activation of adenosine monophosphate-activated protein kinase (AMPK) signaling (Pearce et al., 2009) can enhance the production of memory CD8+ T cells, suggesting that CD8+ T cell differentiation can be manipulated by altering cellular metabolism (O’Sullivan et al., 2014; Pearce et al., 2013; Rao et al., 2010; Sukumar et al., 2013; van der Windt et al., 2012). It has been hypothesized that reliance on fatty acid oxidation (FAO) and a concomitant increase in spare respiratory capacity (SRC) support both memory cell survival and the ability of these cells to respond rapidly to reinfection (O’Sullivan et al., 2014; van der Windt et al., 2012; van der Windt et al., 2013). These data demonstrate that metabolic pathway usage can be correlated with fate determination in CD8+ T cells, leading to the suggestion that metabolic pathway choice drives memory CD8+ T cell differentiation (Chang et al., 2014; MacIver et al., 2013; Pearce et al., 2013). However, the degree to which metabolism controls memory formation and how metabolic flux integrates with transcriptional control of effector function and differentiation is currently unknown. The differential metabolic states of in vivo differentiated memory CD8+ T cell subsets have not been determined either in terms of the requirement for SRC or the role of oxidative phosphorylation in generation of protective cells.
A number of transcription factors have been implicated in the regulation of T cell metabolism following activation such as c-myc, mTOR, FOXO1, and the Hypoxia-Inducible Factor (HIF) (Chang et al., 2014; Pearce et al., 2013). The HIF family of transcription factors (HIFs) serves as the central sensor of oxygen tension and adaptation to low oxygen tensions in all cells, including T cells (Haase et al., 2001; McNamee et al., 2013; Nizet and Johnson, 2009; Phan and Goldrath, 2015). Post-translational regulation by the von Hippel Lindau tumor suppressor protein (VHL), an E3 ubiquitin ligase, drives degradation of HIFα subunits in normal oxygen tensions (McNamee et al., 2013; Nizet and Johnson, 2009; Phan and Goldrath, 2015). HIF drives oxygen conservation through the upregulation of glycolytic metabolism and direct suppression of oxygen consumption by mitochondria (Nizet and Johnson, 2009). Suppression of oxygen consuming mitochondrial respiration is the result of HIF-dependent increased expression of nearly all glycolytic enzymes. In particular, HIF drives expression of lactate dehydrogenase a (LDHA), which potentiates increased glycolytic throughput, and simultaneously suppresses mitochondrial respiration by preventing the shunting of pyruvate into the citric acid cycle through inhibition of pyruvate dehydrogenase by also increasing expression of pyruvate dehydrogenase kinase 1 (PDK1) (Kim et al., 2006; Phan and Goldrath, 2015). Thus, HIF-dependent enhancement of glycolytic metabolism and suppression of cellular respiration presents a unique model by which to interrogate the relationship between metabolic pathway choice and CD8+ T cell differentiation.
To determine the necessity of enhanced SRC and oxidative phosphorylation in memory CD8+ T cell formation, we altered the source of cellular energy production during CD8+ T cell differentiation in vivo. This was accomplished through conditional deletion of Vhl in mature T cells by expression of the Cre recombinase driven by the distal Lck promoter (dLck-cre), resulting in constitutive stabilization of HIF transcription factors (Haase et al., 2001). Previously, we demonstrated that deletion of Vhl leading to constitutive HIF activity drives a differentiation program resistant to T cell exhaustion following chronic viral infection (Doedens et al., 2013). Constitutive HIF activity additionally alters the cellular metabolism of CD8+ T cells in vitro and pharmacological inhibition of glycolytic metabolism following in vitro activation and culture suggests that heightened glycolytic metabolism impacts effector function and co-stimulatory and inhibitory receptor expression (Doedens et al., 2013). Therefore, we reasoned that modulation of glycolysis and oxidative phosphorylation by HIF provides a powerful in vivo model for assessing the role of cellular metabolism on CD8+ memory T cell differentiation and function without eliminating critical mitochondrial transporters or enzymes. Using this model, we tested the impact of constitutive glycolytic metabolism on CD8+ T cell differentiation to the memory state during the response to acute infection and found that generation of increased SRC and reliance on oxidative phosphorylation were not essential for the generation of long-lived CD8+ T cells. Vhl-deficient CD8+ T cells formed fully functional long-lived memory cells that maintained reliance on glycolytic metabolism. These cells responded with improved kinetics to secondary challenge compared to primary challenge despite their altered cellular metabolism. Furthermore, ex vivo measurement of metabolism of wildtype memory cell subsets showed that Tcm cells exhibited greater SRC than Tem cells, mirroring the transcriptional heterogeneity found in memory CD8+ T cell subsets, suggesting a link between metabolic pathway usage and memory T cell subset heterogeneity.
Results
Deletion of Vhl does not impair formation or survival of memory CD8+ T cells
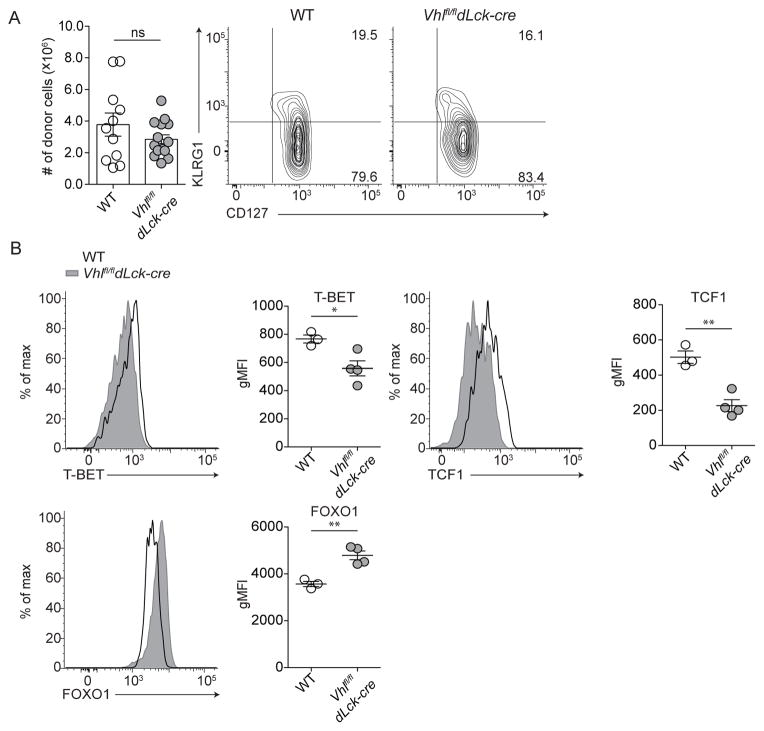
We previously demonstrated that in vitro activation of Vhl-deficient CD8+ T cells results in elevated glycolytic metabolism while suppressing oxidative phosphorylation in comparison to wildtype CD8+ T cells (Doedens et al., 2013). Thus, we asked whether the enhanced glycolytic metabolism that characterizes these cells impaired formation of memory CD8+ T cells in vivo. Vhl-deficient CD8+ T cells (Vhlfl/fldLck-cre) or wildtype CD8+ T cells (WT) expressing a gp33-specific transgenic T cell receptor (P14) were adoptively transferred into naive host mice that were infected one day later with the Lymphocytic Choriomeningitis Virus (LCMV) Armstrong strain, resulting in a rapidly cleared acute viral infection (Figure S1A). Deletion of Vhl and constitutive HIF activity did not impair the generation or survival of Vhlfl/fldLck-cre memory cells in secondary lymphoid tissues, or alter expression of CD127 at memory time points (>60 days post infection, Figure 1A). Long-lived Vhlfl/fldLck-cre cells expressed similar protein levels of key transcription factors relative to WT memory CD8+ T cells as measured by geometric mean fluorescence intensity (gMFI), albeit with subtle differences: lower gMFI of T-bet and TCF1 protein, and higher gMFI of FOXO1 protein (Figure 1B). Thus, long-lived memory CD8+ T cells were formed and maintained at similar numbers compared to WT regardless of constitutive HIF activity (Figure 1).
Figure 1. VHL-deficient CD8+ T cells form long-lived memory CD8+ T cells.
(A) Representative KLRG1 and CD127 surface phenotype of memory WT and Vhlfl/fldLck-cre cells (n = 3–5 per 5 independent experiments) and absolute numbers from spleen of host mice (cumulative from 4 independent experiments, n = 26). (B) Representative flow cytometric quantitation of transcription factors; total donor WT (open black histogram) or Vhlfl/fldLck-cre (filled grey histogram) cells from spleen. gMFI of total donor WT or Vhlfl/fldLck-cre memory CD8+ T cells (n = 3–5 per 5 independent experiments). (A) Numbers represent percentage of cells in respective gates. Data in (A–B) show mean ± SEM with Student’s t test, ns p > 0.05, * p < 0.05, ** p < 0.01.
Memory Vhlfl/fldLck-cre CD8+ T cells maintain reliance on glycolytic metabolism
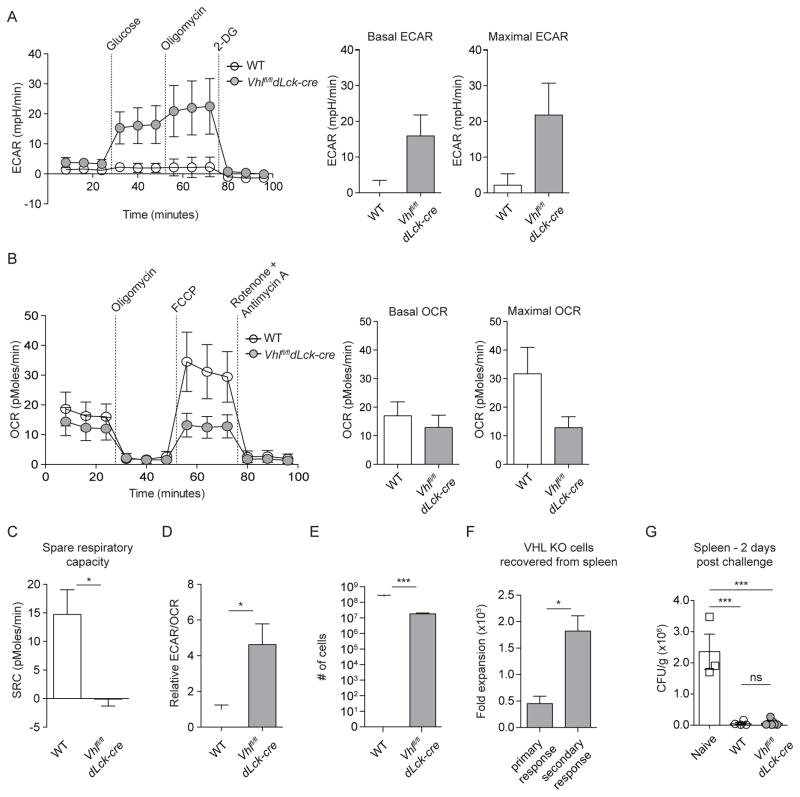
Given that memory cell formation has been closely correlated with reliance on oxidative phosphorylation (O’Sullivan et al., 2014; van der Windt et al., 2012), we investigated the possibility that long-lived Vhlfl/fldLck-cre cells bypassed HIF-mediated suppression of oxidative phosphorylation and generated SRC for differentiation and survival. We measured ex vivo glycolytic and oxidative metabolism of resting long-lived Vhlfl/fldLck-cre and WT cells via extracellular flux analysis of extracellular acidification rate (ECAR) and oxygen consumption rate (OCR), 60+ days following infection (Figure 2). Long-lived Vhlfl/fldLck-cre cells continued to exhibit substantially higher basal and maximal glycolytic rates (>5-fold) and lower basal and maximal oxidative phosphorylation rates compared to their WT counterparts (Figures 2A and 2B). Suppression of cellular respiration in Vhlfl/fldLck-cre responding to infection was consistent with our analysis of in vitro activated cells, and an expected result of HIF-driven induction of PDK1, a canonical HIF target gene (Kim et al., 2006). This was indicative of sustained HIF-transcriptional activity and continued suppression of oxidative phosphorylation in Vhlfl/fldLck-cre cells that resulted in a complete lack of SRC and a dramatic skewing towards glycolytic metabolism (Figures 2C and 2D). To confirm an inability to produce SRC by Vhlfl/fldLck-cre memory CD8+ T cells, we activated and cultured WT and Vhlfl/fldLck-cre cells in vitro in conditions previously shown to generate SRC in WT cells (O’Sullivan et al., 2014; van der Windt et al., 2012) and performed extracellular flux analysis (Figure S1B). Validating our ex vivo extracellular flux analysis, we observed that Vhlfl/fldLck-cre cells cultured with either IL-2 or IL-15 failed to generate SRC (Figure 2C and S1B).
Figure 2. Memory Vhlfl/fldLck-cre CD8+ T cells rely on glycolytic metabolism and are functional secondary effectors.
(A–D) Experimental design as in Figure S1A, (n = 3–5), WT or Vhlfl/fldLck-cre donor cells were sorted from pooled spleens and lymph nodes and assayed directly ex vivo with the Seahorse Extracellular Flux XF-96 analyzer under basal conditions and following addition of indicated metabolic inhibitors. Data from 3 independent experiments with rate measurements normalized to 1.25x105 cells/sample. (A) Extracellular Acidification Rate (ECAR) and (B) Oxygen Consumption Rate (OCR) of WT and Vhlfl/fldLck-cre cells at day >60 following acute viral infection measured over time after addition of metabolic inhibitors (left). Basal and maximal (A) ECAR or (B) OCR (right). (C) SRC of WT and Vhlfl/fldLck-cre cells calculated from (B). (D) Ratio of basal ECAR to basal OCR of WT and Vhlfl/fldLck-cre cells relative to ratio of WT cells from measurements in (B). Long-lived WT or Vhlfl/fldLck-cre donor cells were harvested from secondary lymphoid tissues, sort purified, and 1 x 104 were retransferred into congenically distinct naive host mice followed by infection with LCMV Armstrong one day later. Representative absolute numbers (E) of donor WT or Vhlfl/fldLck-cre cells on day 5 of secondary challenge (n = 4–5 per 2 experiments) and comparative fold expansion (F) of Vhlfl/fldLck-cre effector cells following primary (day 6) versus secondary (day 5) LCMV Armstrong challenge from spleen. (G) Colony forming units (CFU) per gram of spleen 2 days following challenge with Lm-gp33 of naive B6 mice and mice with memory WT or Vhlfl/fldLck-cre cells. Data in (A–G) show mean ± SEM: (A–F) Student’s t test, ns p > 0.15, * p < 0.05, *** p < 0.001; (G) one-way ANOVA followed by Tukey’s Multiple Comparison Test, ns p > 0.05, *** p < 0.001. See also Figure S1.
To further assess the continued reliance on glycolytic metabolism by memory Vhlfl/fldLck-cre cells, we sort-purified WT and Vhlfl/fldLck-cre memory P14 CD8+ T cells following acute LCMV infection and assessed the proliferation following restimulation in the presence of oligomycin and 2-deoxyglucose, metabolic inhibitors of mitochondrial respiration and glycolysis respectively (Figure S1C). In agreement with the ex vivo extracellular flux analysis, Vhlfl/fldLck-cre memory cells proliferated even in the presence of oligomycin, while WT cells failed to divide in the presence of oligomycin. Proliferation of both WT and Vhlfl/fldLck-cre memory cells was inhibited by 2-deoxyglucose demonstrating that glycolytic metabolism was necessary for the function of both WT and Vhlfl/fldLck-cre memory CD8+ T cells (Figure S1C). Enhanced glycolytic metabolism, suppression of oxidative phosphorylation, and lack of SRC did not prevent differentiation to a memory phenotype or survival of Vhlfl/fldLck-cre cells in secondary lymphoid tissues (Figures 1 and 2). Moreover, these data demonstrated that Vhlfl/fldLck-cre memory CD8+ T cells differentiated and survived despite reliance on glycolytic metabolism and suppression of oxidative phosphorylation. Thus, we next evaluated the functional capacity of Vhlfl/fldLck-cre memory CD8+ T cells in a secondary response (Figures 1 and 2).
Vhlfl/fldLck-cre memory CD8+ T cells function as bona fide memory CD8+ T cells
A principal characteristic of immunological memory is the capacity to respond with faster kinetics to a pathogen upon rechallenge. To measure the capacity of long-lived Vhlfl/fldLck-cre cells to respond to secondary infection, Vhlfl/fldLck-cre or WT memory CD8+ T cells were harvested from spleen and lymph nodes, and re-transferred into naive hosts that were then infected with LCMV Armstrong. Both WT and Vhlfl/fldLck-cre secondary effector cells responded robustly to infection (Figures 2E–2F). Vhlfl/fldLck-cre secondary effector cells accumulated at lower numbers in the spleen than WT cells (Figure 2E). However, secondary Vhlfl/fldLck-cre effector cells showed significantly higher fold expansion than primary Vhlfl/fldLck-cre effector cells, a hallmark of memory responses (Figure 2F). Additionally, secondary challenge of mice with WT or Vhlfl/fldLck-cre memory CD8+ T cells with Listeria monocytogenes expressing the LCMV peptide gp33 and subsequent culture of bacteria isolated from the spleen two days following secondary challenge demonstrated the ability of Vhlfl/fldLck-cre memory cells to rapidly clear secondary infection, further supporting the conclusion that long-lived Vhlfl/fldLck-cre cells are ‘bona fide’ memory CD8+ T cells in spite of their sustained glycolytic metabolism. (Figures 1 and 2).
Vhlfl/fldLck-cre memory-precursor cells sustain elevated glycolytic metabolism and suppress oxidative phosphorylation
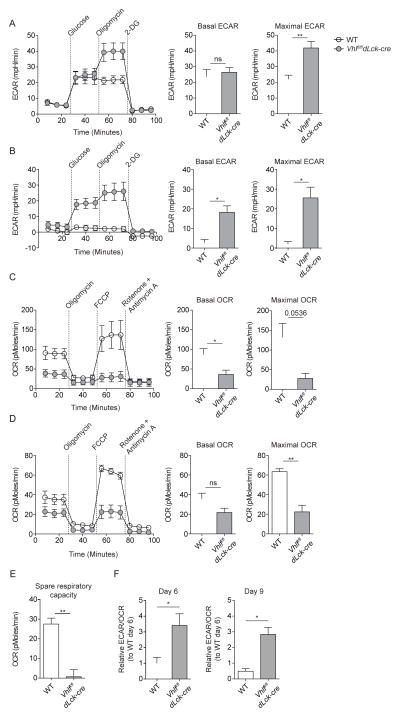
Ex vivo measurement of resting metabolic rates of Vhlfl/fldLck-cre and WT cells demonstrated substantially altered metabolic programming in Vhlfl/fldLck-cre cells that suggested that SRC and reliance on oxidative phosphorylation were dispensable for survival of memory CD8+ T cells. We next examined whether HIF-driven glycolytic metabolism was maintained during expansion and contraction phases of the immune response when CD8+ T cell fate is specified. Therefore, we measured ex vivo glycolytic and oxidative metabolism of Vhlfl/fldLck-cre and WT cells responding to acute viral infection. Effector cells were sort-purified from LCMV infected hosts during the expansion phase (day 6 of infection, Figures 3A, 3C and 3F) and contraction phase (day 9 of infection, Figures 3B, 3D–3F) of LCMV infection, and metabolic activity was measured by extracellular flux analysis (Figure 3). Ex vivo measurement of glycolytic rates revealed that Vhlfl/fldLck-cre cells sustained significantly higher maximal glycolytic rates during expansion (nearly 2-fold) and contraction (>10-fold) in comparison to WT cells. However, basal glycolytic rates were significantly higher only during the contraction phase (>5-fold) when WT cells reduce glycolytic activity as the need for effector function wanes (Figures 3A and 3B). These data correlated well with recent reports that demonstrate the importance of glycolytic metabolism in CD8+ T cell effector function (Chang et al., 2013; Kidani et al., 2013; Sukumar et al., 2013; Wang et al., 2011) and may support the sustained effector capacity of Vhlfl/fldLck-cre CD8+ T cells following chronic viral infection (Doedens et al., 2013). Conversely, the rate of oxidative phosphorylation in Vhlfl/fldLck-cre cells was substantially lower at basal levels during expansion and contraction; it was also lower throughout the response to infection following treatment with the ionophore FCCP, an agent that uncouples mitochondria and induces maximal oxygen consumption (Figures 3C and 3D).
Figure 3. Sustained HIF activity drives glycolysis and suppresses oxidative phosphorylation during the effector response.
(A–F) ECAR and OCR of KLRG1lo CD44hi WT and Vhlfl/fldLck-cre cells on (A,C) day 6 and (B,D) day 9 of infection (left). Summarized metabolic measures of basal and maximal ECAR or OCR of WT and Vhlfl/fldLck-cre cells (right). (E) Spare respiratory capacity (SRC) of WT and Vhlfl/fldLck-cre cells on day 9 of infection. (F) Ratio of basal ECAR to basal OCR of WT and Vhlfl/fldLck-cre cells at indicated day post infection relative to basal ECAR to basal OCR ratio of WT cells at 6 following infection. Data in (A–F) are mean ± SEM with two-tailed Student’s t test. ns p > 0.05, * p < 0.05, ** p < 0.01. See also Figure S1.
During contraction, when WT cells exhibited increased SRC relative to naive and effector CD8+ T cells (proposed as a key feature of memory formation (van der Windt et al., 2012)), Vhlfl/fldLck-cre cells failed to generate substantial SRC (Figure 3E). Furthermore, examining the ratio of glycolytic metabolism to cellular respiration revealed that Vhlfl/fldLck-cre cells skewed towards increased reliance towards glycolytic metabolism during expansion (nearly 4-fold) and contraction (5-fold) phases of the effector response. Identically to memory Vhlfl/fldLck-cre cells, naive Vhlfl/fldLck-cre cells were resistant to suppression of proliferation following inhibition of mitochondrial ATP synthase activity with oligomycin treatment in vitro (Figure S1C and S1D). Moreover, proliferation of naive WT and Vhlfl/fldLck-cre cells was abolished by culture with 2-deoxyglucose further emphasizing the importance of glycolytic metabolism in the function of Vhlfl/fldLck-cre cells (Figure S1C and S1D). Additionally, analysis of relevant metabolites of in vitro cultured WT and Vhlfl/fldLck-cre cells supported ex vivo measurements of extracellular flux as Vhlfl/fldLck-cre cells exhibited significantly higher concentrations of lactate (nearly 2-fold), indicative of high glycolytic activity, as well as a significant buildup of the TCA cycle metabolite citrate (2-fold), representative of reduced TCA cycle flux (Figure S1E). These results demonstrated that elevated HIF activity drove a skewing towards glycolytic metabolism that was sustained throughout the CD8+ T cell effector response to acute infection, which the prevailing model would argue should suppress memory formation (Figure 3).
Sustained HIF activity accelerates memory-precursor cell emergence
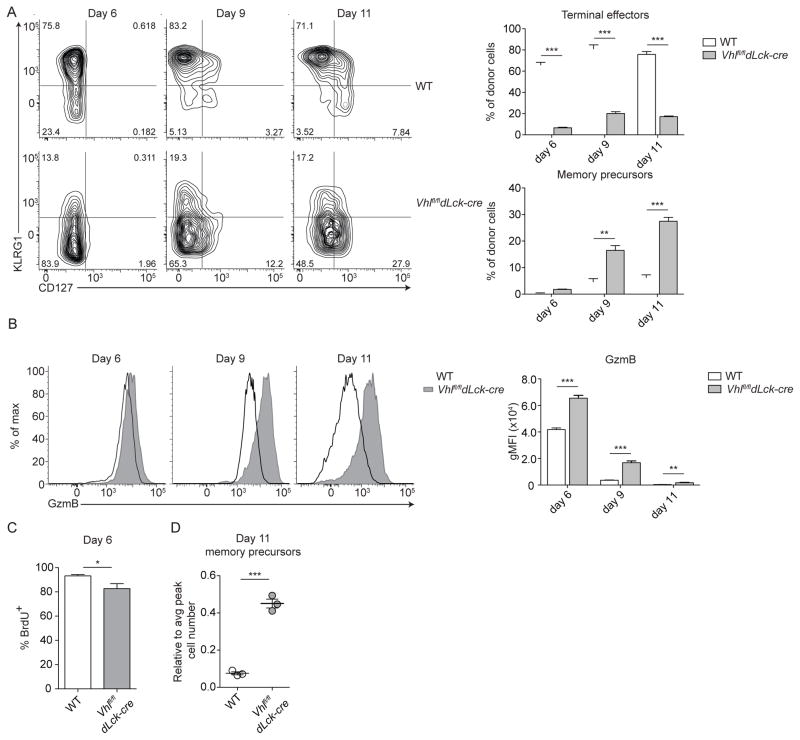
To examine the contraction phase, when memory precursors can be first followed, we examined differentiation of Vhlfl/fldLck-cre and WT effector CD8+ T cell subsets (Figure 4A). We previously reported that Vhlfl/fldLck-cre CD8+ T cells become activated and clear both chronic and acute infections with heightened effector function (Doedens et al., 2013). We observed that Vhlfl/fldLck-cre CD8+ T cells showed significantly impaired formation of terminally-differentiated effector cells (Figure 4A and (Doedens et al., 2013)). Instead, Vhlfl/fldLck-cre cells formed a population of KLRG1loCD127hi memory-precursor cells at a higher frequency than WT cells, which is seen by day 9 of infection (Figure 4A). This coincided with increased expression of Granzyme B (GzmB) throughout the response to acute viral infection (Figure 4B). The accelerated appearance of memory-precursors by Vhlfl/fldLck-cre cells also occurred when Vhlfl/fldLck-cre and WT cells were transferred into the same host, thus confirming constitutive HIF activity alters CD8+ T cell differentiation in a cell intrinsic manner and that is partially independent of alterations in inflammation or antigen load (Figure S2A).
Figure 4. Constitutive HIF activity enhances CD8+ memory cell formation.
(A) Representative surface phenotype of donor WT (top) or Vhlfl/fldLck-cre (bottom) cells at indicated time points following acute viral infection from spleen (n = 3–5 per 3 independent experiments). Numbers represent percentage of cells in respective gates. Bar graphs summarize percentage of terminal effectors and memory precursors of indicated donor cells. (B) Representative intracellular staining of Granzyme B (GzmB) expression of donor WT (open black histogram) or Vhlfl/fldLck-cre (filled grey histogram) cells from spleen at indicated time points. Bar graph shows geometric mean fluorescence intensity (gMFI) of indicated donor populations (n = 3–5 per 3 independent experiments) (C) Percent of indicated donor cells incorporating BrdU on day 6 of LCMV infection. (D) Efficiency of memory cell generation: To normalize Vhlfl/fldLck-cre and WT responses to their absolute number of memory-precursor WT or Vhlfl/fldLck-cre cells on day 11 of infection were divided by their respective peak number of responding donor WT or Vhlfl/fldLck-cre cells from day 9 of infection. (C,D) Representative data, n = 3–4 from 2 independent experiments. Data in (A–D) show mean ± SEM with Student’s t test, * p < 0.05, ** p < 0.01, *** p < 0.001. See also Figure S2 and S3.
Previously, it has been reported that VHL deficiency can lead to suppression of mTOR kinase complex 1 (MTORC1) signaling in renal cell carcinoma. Furthermore, inhibition of mTOR signaling by rapamycin treatment has been shown to improve generation of memory CD8+ T cells. Thus, a potential mechanism by which conditional Vhl-deletion in CD8+ T cells may drive memory differentiation, despite altered cellular metabolism, could be the suppression of mTOR signaling (Araki et al., 2009; Kucejova et al., 2011). Therefore, we assessed the impact of constitutive HIF activation on MTORC1 signaling in effector CD8+ T cells during the expansion phase of the response to infection (Figure S2B). Flow cytometric analysis of phosphorylated ribosomal S6 protein (pS6) in WT and Vhlfl/fldLck-cre cells showed a slight reduction in MTORC1 signaling in Vhlfl/fldLck-cre cells; however, this reflected a substantial increase in signaling when compared to naive host CD8+ T cells that were not responding to the infection (Figure S2B). Furthermore, the reduction in pS6 staining was lost at memory time points as restimulation of memory WT and Vhlfl/fldLck-cre cells with peptide drove equivalent increases in pS6 signal in antigen-specific memory cells (Figure S2C). These data do not rule out a role for MTORC1 inhibition in the accelerated differentiation of memory precursor cells by Vhlfl/fldLck-cre cells. However, the magnitude of the reduction does not reflect the substantial skewing of Vhlfl/fldLck-cre effector cell differentiation, suggesting that additional factors contributed to the altered effector differentiation of Vhlfl/fldLck-cre cells (Figures 4A, S2B and S2C). Furthermore, deficiency of HIF1α and HIF2α along with VHL in P14 CD8+ T cells rescued much of the defect in terminal effector differentiation, reduced GzmB expression, and normalized proliferation during the expansion phase following acute viral infection (Figure S2D–S2F). These data demonstrated that many of the effects of VHL-deficiency on effector differentiation and function were dependent on HIF1α and HIF2α, similar to effects we demonstrate in the context of chronic viral infection (Doedens et al., 2013).
Microarray analysis revealed that while Vhlfl/fldLck-cre cells express genes associated with effector function (Doedens et al., 2013), they also exhibited enriched expression of genes associated with memory differentiation (Figure S3A). This expression pattern is distinct from that seen in WT KLRG1hi effector cells, suggesting Vhlfl/fldLck-cre cells undergo altered differentiation towards the memory fate, rather than a failure to upregulate KLRG1 itself (Figure S3B and (Doedens et al., 2013)).
Analysis of the number of memory-precursor cells following contraction revealed that while Vhlfl/fldLck-cre cells proliferated at a slightly reduced rate compared to WT cells, the proportion of cells which form memory-precursors compared to the peak number of donor effector cells was 5-fold greater for Vhlfl/fldLck-cre than WT cells (Figures 4C and 4D). Despite a lack of SRC, Vhlfl/fldLck-cre cells both maintained increased effector molecule expression and demonstrated enhanced memory differentiation (Figures 3 and 4). The enhancement in memory differentiation by Vhlfl/fldLck-cre cells demonstrates that a metabolic shift towards reliance on oxidative phosphorylation is not a requirement for differentiation of memory CD8+ T cells (Figures 2–4).
Sustained glycolytic metabolism yields sufficient ATP for memory formation
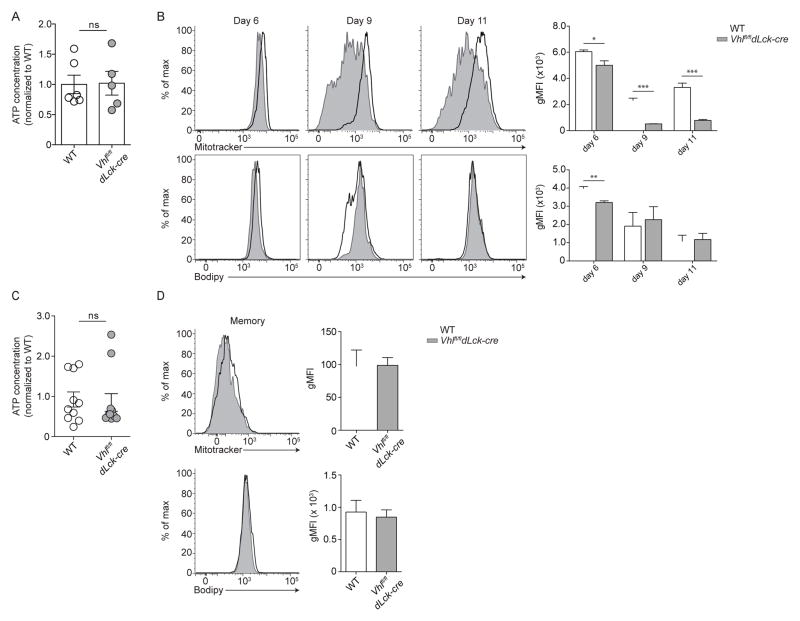
While secondary metabolites may be essential for specific cellular functions or fate decisions, adenosine triphosphate (ATP) produced by both glycolysis and oxidative phosphorylation may be central to survival during contraction and throughout the memory phase (Cui et al., 2015). We examined whether enhanced glycolytic metabolism and suppressed oxidative phosphorylation of Vhlfl/fldLck-cre cells negatively impacts ATP production during contraction. Vhlfl/fldLck-cre or WT memory-precursor cells (KLRG1loCD127hi) from individual mice were sort purified during the contraction phase, cellular ATP was extracted, and ATP concentration was then measured by luciferase assay (Figure 5A). The skewing of Vhlfl/fldLck-cre cell metabolism towards glycolysis during the effector response did not alter the amount of ATP in Vhlfl/fldLck-cre memory-precursor cells when compared to WT memory-precursor cells. This suggested that a primary threshold for CD8+ T cells to differentiate into a memory population was sufficient production of ATP, rather than the usage of specific metabolic pathways (Figure 5A). Further, we found that Vhlfl/fldLck-cre cells did not generate additional ATP through a compensatory increase in mitochondrial abundance or through increased fatty acid stores. Flow cytometric analysis of cells by Mitotracker and Bodipy, stains of mitochondrial content and free fatty acids respectively (O’Sullivan et al., 2014; van der Windt et al., 2012), showed a reduction in mitochondrial content during the effector response, but similar free fatty acid levels in Vhlfl/fldLck-cre cells relative to WT cells (Figure 5B). In addition, Vhlfl/fldLck-cre and WT resting memory cells had similar amounts of ATP (Figure 5C), and also maintained similar mitochondrial content and fatty acid stores, suggesting that any compensatory energy production is generated through the glycolytic pathway (Figure 5D).
Figure 5. Glycolytic metabolism does not impair ATP production and compensates for suppressed oxidative phosphorylation.
(A,C) Cellular ATP extracted from WT and Vhlfl/fldLck-cre donor cells (per 104 cells) sorted from spleen and lymph nodes of individual host mice (A) post peak of CD8+ response and (C) >60 days following infection. Data are relative to average WT cell ATP levels. Cumulative data (n = 6 mice) from two independent experiments. (B,D) Representative flow cytometric analysis of total donor WT (open black histogram) or Vhlfl/fldLck-cre (filled grey histogram) cells for analysis of mitochondrial mass and free fatty acid levels at indicated time point following infection. Bar graphs show Mitotracker and Bodipy gMFI of indicated donor populations. (B, n = 3–4 mice per 2 independent experiments D, n = 3–5 mice per 3 independent experiments). Data in (A,C) show mean ± SEM with two-tailed Student’s t test, ns p > 0.5, * p < 0.05, ** p < 0.01, *** p <0.001.
CD8+ memory cells exhibit heterogeneous transcription factor expression and metabolic activity
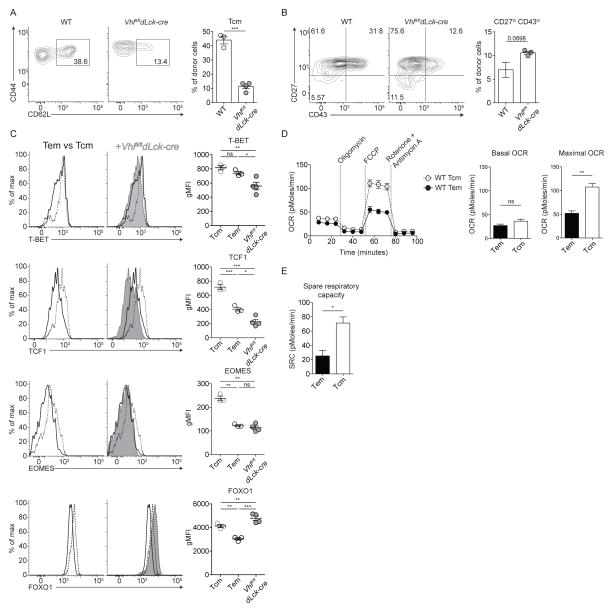
Our data suggested that generation of SRC and reliance on oxidative phosphorylation were not essential for survival or differentiation of memory CD8+ T cells. However, differential metabolic pathway usage could still play a role in specification of memory subset heterogeneity (Kawalekar et al., 2016); thus, we examined memory populations in more detail following contraction (Figure 6). Here, we found that fewer Vhlfl/fldLck-cre cells in secondary lymphoid tissues re-expressed L-selectin (CD62L), a marker of Tcm cells. These cells remained CD62Llo, a characteristic of Tem cells, and formed approximately 4-fold fewer Tcm cells compared to WT cells (Figure 6A). Similarly, the Vhlfl/fldLck-cre memory population showed a higher proportion of CD27loCD43lo cells (1.5-fold), which correlated with an effector-like memory phenotype (Olson et al., 2013) (Figure 6B). A comparison of key transcription factor expression for WT memory subsets by flow cytometry showed that Tem cells displayed significantly lower protein levels of T-BET, TCF1, EOMES and FOXO1 than Tcm cells (Figure 6C). Interestingly, Vhlfl/fldLck-cre memory cells expressed transcription factors at levels similar to WT Tem cells (Figure 6C), with the exception of FOXO1, which is higher than WT Tcm and Tem cells. An elevation in FOXO1, an essential mediator of Tcm cells, (Kim et al., 2013; Michelini et al., 2013; Rao et al., 2012; Tejera et al., 2013) by Vhlfl/fldLck-cre memory cells makes the resemblance to Tem cells by other criteria even more striking. It is unclear why Vhlfl/fldLck-cre memory cells maintained a Tem cell phenotype given their elevated FOXO1 expression. However, it is possible that differential post-translational regulation of FOXO1, such as sequestration to the cytoplasm, occurs in Vhlfl/fldLck-cre cells and could explain the maintenance of an elevated proportion of Tem cells.
Figure 6. Glycolytic metabolism correlates with differentiation of effector-memory CD8+ T cells.
(A–B) Representative flow cytometric analysis of splenic WT or Vhlfl/fldLck-cre cells at memory time points (A) for effector memory and central memory CD8+ T cell subsets (n = 3–5 per 4 independent experiments) with summarized frequency of central memory cells. (B) CD27 and CD43 expression and summarized frequency of CD27loCD43lo memory cells (n = 3 per 2 independent experiments). (C) Representative expression of transcription factors and cytokine receptors of WT central memory (Tcm, dashed line) and effector memory cells (Tem, black line, left histograms) and with total Vhlfl/fldLck-cre memory cell (grey filled) expression overlaid for comparison (right histograms). Representative staining of n = 3–5 per 4 independent experiments. gMFI of transcription factor expression for WT Tcm and Tem compared to total Vhlfl/fldLck-cre memory cells. (D) OCR of WT Tcm (dotted line) and Tem (solid line) measured as in Figure 2 (left) with basal and maximal OCR (right) of 3 independent experiments. (E) SRC of WT Tcm and Tem from (D). Data show mean ± SEM: (A–B, D–E) Student’s t test, ns p > 0.05, *** p < 0.0005; (C) one-way ANOVA followed by Tukey’s Multiple Comparison Test, ns p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001.
Given that WT Tcm and Tem cells expressed key transcription factors at distinct levels, we hypothesized that metabolic pathways may be differentially utilized as well. Thus, we sort-purified WT Tcm and Tem cells (CD44hiCD62Lhi and CD44hiCD62Llo, respectively) from mice previously infected with LCMV at least 60 days prior and measured ECAR and OCR via extracellular flux analysis (Figure 6D). WT Tem cells maintained significantly lower SRC and a reduced basal rate of cellular respiration compared to WT Tcm cells; these levels are similar to those seen in Vhlfl/fldLck-cre memory cells (Figures 1A–1B and 6D–6E). In contrast, WT Tcm cells exhibited a slightly higher basal rate of oxidative phosphorylation, but developed higher SRC (Figures 6D and 6E). These data supported previous studies regarding SRC of WT cells where no discrimination between Tcm and Tem cells was used; thus the predominance of Tcm cells in WT memory cell populations may have biased the metabolic measurements observed (van der Windt et al., 2012). Moreover, these data showed that Vhlfl/fldLck-cre memory cells exhibited a distinct transcription factor expression profile in comparison to WT Tcm and Tem, but maintained a metabolic phenotype most similar to WT Tem, emphasizing that WT memory CD8+ T cell phenotype and function are likely governed by both transcription factors as well as metabolic pathway usage (Figure 6).
Discussion
Recent studies have demonstrated that skewing of metabolic pathways in CD8+ T cells correlates with formation of memory CD8+ T cells and pharmacological inhibition of critical metabolic sensors can perturb CD8+ T cell differentiation (Araki et al., 2009; Pearce et al., 2009; van der Windt et al., 2012). Furthermore, deletion of molecules critical for various metabolic processes can have both deleterious or beneficial effects on effector and memory cell generation and survival (Chaoul et al., 2015; Cui et al., 2015; O’Sullivan et al., 2014; Okoye et al., 2015; Rao et al., 2010; Rolf et al., 2013; Sena et al., 2013; Shrestha et al., 2014). These data support a role for cellular metabolism in CD8+ T cell differentiation and function; however, the critical question of whether metabolic pathway choice is the driving force behind memory cell differentiation is not yet resolved. By enhancing glycolytic metabolism throughout the effector response to acute infection, we demonstrated that the generation of SRC and a shift towards reliance on oxidative phosphorylation were not essential for the generation of functional long-lived memory CD8+ T cells. Our study finds that constitutive HIF-dependent glycolytic metabolism did not hinder differentiation of memory CD8+ T cells, but may have promoted differentiation of Tem cells, skewing the memory pool and impacting functional immunity.
Through manipulation of metabolite transporters (Cui et al., 2015; van der Windt et al., 2012) or enzymes (Blagih et al., 2015; O’Sullivan et al., 2014; Pearce et al., 2009; Rolf et al., 2013), several studies demonstrate that inhibition of FAO and oxidative phosphorylation yield defects in CD8+ memory T cell differentiation or survival. Knockdown of carnitine palmitoyl transferase 1 (CPT1a) or deletion of aquaporin 9 (AQP9) starve memory cells of sufficient fatty acids, and deletion of AMPK prevents induction of oxidative phosphorylation in CD8+ T cells, supporting a model in which provision of cellular energy through mitochondrial respiration is critical for memory survival and function (Cui et al., 2015; Rolf et al., 2013; van der Windt et al., 2012). These findings support early reports where memory cell formation was enhanced by promoting mitochondrial fatty acid oxidation and suppressing glycolytic metabolism (Araki et al., 2009; Pearce et al., 2009) and the strong correlation between memory differentiation and the reliance of memory CD8+ T cells on increased mitochondrial biogenesis and fatty acid fueled oxidative phosphorylation lead to the inference that memory differentiation is driven by mitochondrial respiration.
An alternative explanation of these observations is that promoting or reducing total energy production in CD8+ T cells can improve or inhibit differentiation of memory irrespective of metabolic pathway. We demonstrated that elevated glycolytic metabolism, as a result of VHL deficiency, resulted in production of similar concentrations of ATP in Vhlfl/fldLck-cre cells relative to WT cells, thereby allowing survival of contracting effector cells and potentiating differentiation of memory cells. These results argue that it is “the fuel, not the refinery” which is paramount in memory cell differentiation. Generation of sufficient ATP by CD8+ T cells, regardless of the source, may provide for the basal conditions that then allow for additional cell intrinsic factors (i.e. transcription factors) to drive differentiation of memory cells. This suggests that fate determination in CD8+ T cells can accommodate a range of cellular adaptations as long as basic conditions are met, such as basal energy levels. Further study of the impact of alterations in cellular energy stores on fate determination will be necessary to clearly define how cellular metabolism affects T cell differentiation.
Moreover, while it is clear that cellular metabolism is essential for numerous processes within T cells, our data and those of others do not demonstrate that specific metabolic pathways can inherently drive specific differentiation programs. In light of this, our interpretation of our findings and that of the current literature suggest that T cells are highly flexible in their reliance on metabolic pathways for differentiation. Function, on the other hand, may be uniquely dependent on environmental conditions, nutrients, and metabolic pathways utilized, in a context specific fashion. Additionally, our data fit well with the inherent migratory differences of Tcm and Tem subsets as Tcm cells primarily reside in secondary lymphoid tissues with minimal migration, while Tem cells likely encounter a wide range of environmental conditions, and therefore, nutrient levels which may drive a necessity for metabolic flexibility. Further work carefully dissecting metabolic requirements for differentiating and sustaining all memory subsets (Tcm, Tem, and Trm) and enabling the distinct functional roles of each will be necessary to determine whether manipulating cellular metabolism may sustain or prevent particular cellular fates following T cell activation.
In further support of this model, our examination of metabolic activity by memory CD8+ T cell subsets confirms that SRC and oxidative phosphorylation were elevated in Tcm cells similar to the total CD8+ memory T cell pool as previously described (MacIver et al., 2013; O’Sullivan et al., 2014; van der Windt et al., 2012). However, we also found that Tem cells exhibited lower levels of SRC which may reflect the different functional roles and localization of Tcm and Tem cells. These data allow for a context-specific role for specific pathways or metabolites in particular memory CD8+ T cell subsets and suggest a plausible cell-intrinsic role for metabolic pathway choice in promoting heterogeneity in the memory pool. While the apparent metabolic flexibility demonstrated by memory CD8+ T cells following acute viral infection suggests a direct role for metabolic regulation of CD8+ T cell fate, the significant differences in transcription factor expression between Tcm and Tem cells emphasize the need to dissect whether specific metabolic pathways drive differential transcriptional programs or simply correlate with subset diversity. These data parallel studies in CD4+ T cells where particular T helper (Th) subsets, that express distinct master transcription factors, exhibit differential reliance on metabolic pathways for differentiation and function (Dang et al., 2011; De Rosa et al., 2015; Mascanfroni et al., 2015; Michalek et al., 2011; Shi et al., 2011); however, in many cases the contribution of differential metabolic regulation towards Th subset differentiation has yet to be absolutely defined. Our studies and those of others highlight the difficulty and importance of discerning direct impacts of manipulating cellular metabolism in vivo through modulation of critical cellular sensors such as HIF (Doedens et al., 2013; Finlay et al., 2012; Shi et al., 2011), mTOR (Araki et al., 2009; Lee et al., 2010; Rao et al., 2010; Shrestha et al., 2014), and AMPK (Blagih et al., 2015; Pearce et al., 2009; Rolf et al., 2013). For example, in our model conditional deletion of Vhl constitutively stabilizes HIF, results in the upregulation of numerous transcriptional targets, some that promote glycolytic metabolism, and some that may promote memory CD8+ T cell differentiation independent of cellular metabolism (Kim et al., 2006; Phan and Goldrath, 2015). These results further emphasize the need for future studies dissecting whether transcriptional or metabolic requirements are paramount in specifying T cell fate. Additionally, while our study suggests a previously unappreciated metabolic flexibility in differentiating CD8+ T cells, an aspect of our model that remains unexplored is the temporal impact of metabolic adaptations. Conditional deletion of Vhl by dLck-cre results in mature naive Vhlfl/fldLck-cre cells that begin with a reliance on glycolytic metabolism, without any dramatic alterations in activation state or phenotype, which presents an intriguing question as to whether Vhl-deficient CD8+ T cells are uniquely “trained” prior to activation in comparison to WT cells (Doedens et al., 2013). Further work examining when metabolic adaptations can be tolerated by differentiating T cells will be necessary to clarify the impact of cellular metabolism on effector and memory cell differentiation. Our work does not negate a role for cellular metabolism in specifying T cell fate, but demonstrates that CD8+ T cell differentiation makes use of specific pathways in a much more nuanced fashion than previously appreciated.
In light of the relationship between metabolism and memory cell subsets, modulation of metabolic pathways holds promise as a means to increase the efficacy of T-cell mediated clinical therapies, including vaccination and adoptive cell transfers. Alteration of cellular metabolism by T cells is clearly essential for the functional adjustments and environmental adaptations that occur during the response to infection. Our studies suggested that CD8+ T cells can make use of glycolysis and oxidative phosphorylation for the specification of memory fate and raise the total energy supply in a context-dependent fashion. Emphasis on specific metabolic characteristics (e.g., SRC) may drive diversification of the CD8+ T cell memory pool. It remains to be determined whether the metabolic differences between Tcm and Tem are primary or secondary to the unique transcriptional and migratory circumstances of each subset, highlighting important outstanding questions relating to the generation of a population of protective CD8+ memory T cells.
Experimental Procedures
Mice and experimental design
Mice were bred and housed in specific pathogen-free conditions in accordance with the Institutional Animal Care and Use Guidelines of the University of California San Diego. Vhlfl/fl mice have been described (Haase et al., 2001). Deletion of LoxP-flanked Vhl in T cells was achieved by crossing Vhlfl/fl mice to mice hemizygous for dLck-cre (Zhang et al., 2005). P14 mice, which express a transgenic TCR that recognizes an immunodominant epitope of the LCMV glycoprotein expressed by LCMV Armstrong were bred to Vhlfl/fl dLck-cre lines to generate mice with WT or Vhlfl/fldLck-cre P14 CD8+ T cells. All mice were backcrossed over ten generations to the C57BL/6 background.
Infection and cell transfer
Vα2+Vβ8.1.2+ CD8+ cells were injected intravenously with 1x104 per host for all adoptive transfer experiments except for post peak ATP analysis where 1x106 Vα2+ Vβ8.1.2+ CD8+ cells were transferred and mice were infected with LCMV Armstrong (2x105 plaque-forming units, injected intraperitoneally). For secondary rechallenge experiments infections with Lm-gp33 (naive mice 2x104 colony-forming units, mice with memory P14 cells 2x106 colony-forming units) were performed intravenously.
Determination of colony forming units
On day 2 following infection, spleen was isolated, weighed, and placed in 0.2% IGEPAL solution (Sigma-Aldrich) and homogenized. Serial dilutions were plated onto BHI plates and incubated for 24 hrs at 37° C. Bacterial colonies were counted and colony forming units were normalized per gram spleen plated.
Flow cytometry and sorting
Cells were immunostained and analyzed on a BD Fortessa or Fortessa X-20 or sort purified on a BD FACSAria IIu. All antibodies from EBiosciences unless indicated. The following biotin-conjugated antibodies were used for depletion of unwanted cells prior to sorting: anti-B220 (RA3-6B2), anti-CD4 (GK1.5), anti-Ter119 (TER-119), anti-MHCII (M5/114.15.2), anti-NK1.1 (PK136). Fluorophore-conjugated antibodies used for flow cytometry analysis are as follows: anti-CD8α (53–6.7), anti-KLRG1 (2F1), anti-CD127 (A7R34), anti-Vα2 (B20.1), anti-Vβ8.1.2 (KJ16-133), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-CD27 (LG.7F9), anti-CD43 (1B11), anti-T-bet (4B10), anti-TCF1 (Cell Signaling Technology, C63D9), anti-Eomes (Dan11Mag), anti-Foxo1 (Cell Signaling Technology, C29H4), anti-phosphorylated S6 (cupk43k), and anti-S6 ribosomal protein (Cell Signaling Technology, 54D2). Mitotracker Deep Red FM (ThermoFisher, M22426) was used for mitochondrial staining at 25 nM according to manufacturer’s instructions. For staining of neutral lipids Bodipy 493/503 (ThermoFisher, D-2191) was used at 500 ng/mL according to manufacturer’s instructions.
Metabolism assays
Indicated numbers of sort purified cells were plated in buffer free, glucose free media (Seahorse Biosciences or Sigma-Aldrich) with glutamine (2 mM) and ± glucose (11mM) for OCR and ECAR measurements which were made under basal conditions and following addition of oligomycin (1 μM), FCCP (1 μM), Rotenone (1μM) and Antimycin A (1 μM), 2-Deoxyglucose (100 mM) at indicated time points and recorded on a Seahorse XF-96. All compounds from Sigma-Aldrich. Basal ECAR and maximal ECAR calculated from average of three measurements following addition of glucose and oligomycin respectively. Basal OCR and maximal OCR calculated from average of three measurements before addition of oligomycin and following addition of FCCP respectively. Cellular ATP was measured with the ATP Determination Kit (ThermoFisher, A22066) according to manufacturer’s instructions.
Statistical analysis
Two-group comparisons were assessed with an unpaired two-tailed Student’s t test and multi-group comparisons were assessed by one-way analysis of variance followed by Tukey’s Multiple Comparison test.
Supplementary Material
Highlights.
Increased SRC and a reliance on OXPHOS are not essential for memory CD8+ T cells.
Glycolytic metabolism does not hinder differentiation of memory CD8+ T cells.
Provision of ATP is paramount to metabolic pathway usage in effector T cell responses.
Glycolytic metabolism may preferentially promote differentiation of Tem cells.
Acknowledgments
Funding of this work provided by UCSD NIH Cell and Molecular Genetics Training Grant (5T32GM007240-36) for A.T.P, the US NIH and UCSD Cancer Biology Fund for A.L.D, the National Institutes of Health (A1096852, A1072117), the Leukemia and Lymphoma Society, and Pew Scholars Fund for A.W.G. We thank J. J. Milner, J. T. Chang, S. Hedrick, and Goldrath lab members for critical discussions and/review of the manuscript; B. Yu for assistance with bioinformatics analysis and critical discussions; J. V. Nguyen and K. T. Brannan for technical assistance.
Footnotes
Author contributions
A.T.P. designed and performed experiments, analyzed the data and wrote the paper; A.L.D. designed and performed experiments and assisted in writing the paper; A.P. and P.A.T. contributed to the design and analysis of experiments and assisted in writing the paper; K.P.C. performed experiments, analyzed the data, and assisted in writing the paper; R.S.J. provided reagents, advice for the design of experiments and analysis of experiments and assisted in writing the paper; and A.W.G. supervised the project, designed the experiments, analyzed the data, and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagih J, Coulombe F, Vincent Emma E, Dupuy F, Galicia-Vázquez G, Yurchenko E, Raissi Thomas C, van der Windt Gerritje JW, Viollet B, Pearce Erika L, et al. The Energy Sensor AMPK Regulates T Cell Metabolic Adaptation and Effector Responses In Vivo. Immunity. 2015;42:41–54. doi: 10.1016/j.immuni.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Chang CH, Curtis Jonathan D, Maggi Leonard B, Faubert B, Villarino Alejandro V, O’Sullivan D, Huang Stanley C-C, van der Windt Gerritje JW, Blagih J, Qiu J, et al. Posttranscriptional Control of T Cell Effector Function by Aerobic Glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nature Immunology. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaoul N, Fayolle C, Desrues B, Oberkampf M, Tang A, Ladant D, Leclerc C. Rapamycin Impairs Antitumor CD8+ T-cell Responses and Vaccine-Induced Tumor Eradication. Cancer Research. 2015;75:3279–3291. doi: 10.1158/0008-5472.CAN-15-0454. [DOI] [PubMed] [Google Scholar]
- Cui G, Staron Matthew M, Gray Simon M, Ho PC, Amezquita Robert A, Wu J, Kaech Susan M. IL-7-Induced Glycerol Transport and TAG Synthesis Promotes Memory CD8+ T Cell Longevity. Cell. 2015;161:750–761. doi: 10.1016/j.cell.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al. Control of TH17/Treg Balance by Hypoxia-inducible Factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa V, Galgani M, Porcellini A, Colamatteo A, Santopaolo M, Zuchegna C, Romano A, Simone SD, Procaccini C, Rocca CL, et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nature Immunology. 2015;16:1174–1184. doi: 10.1038/ni.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nature Immunology. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, Cantrell DA. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. The Journal of Experimental Medicine. 2012;209:2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel–Lindau tumor suppressor. Proceedings of the National Academy of Sciences. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, Masopust D. Diversity in T Cell Memory: An Embarrassment of Riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and Cell-Fate Decisions in Effector and Memory CD8+ T Cell Differentiation during Viral Infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawalekar OU, O'Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD, Jr, Patel PR, Guedan S, Scholler J, Keith B, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP, Marbois BN, Komisopoulou E, Wilson EB, Osborne TF, et al. Sterol regulatory element–binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nature Immunology. 2013;14:489–499. doi: 10.1038/ni.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-w, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kim Myoungjoo V, Ouyang W, Liao W, Zhang Michael Q, Li Ming O. The Transcription Factor Foxo1 Controls Central-Memory CD8+ T Cell Responses to Infection. Immunity. 2013;39:286–297. doi: 10.1016/j.immuni.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucejova B, Peña-Llopis S, Yamasaki T, Sivanand S, Tran TAT, Alexander S, Wolff NC, Lotan Y, Xie XJ, Kabbani W, et al. Interplay Between pVHL and mTORC1 Pathways in Clear-Cell Renal Cell Carcinoma. American Association for Cancer Research. 2011;9:1255–1265. doi: 10.1158/1541-7786.MCR-11-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian Target of Rapamycin Protein Complex 2 Regulates Differentiation of Th1 and Th2 Cell Subsets via Distinct Signaling Pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver NJ, Michalek RD, Rathmell JC. Metabolic Regulation of T Lymphocytes. Annual Review of Immunology. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nature Medicine. 2015 doi: 10.1038/nm.3868. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee EN, Korns Johnson D, Homann D, Clambey ET. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol Res. 2013;55:58–70. doi: 10.1007/s12026-012-8349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. The Journal of Immunology. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini RH, Doedens AL, Goldrath AW, Hedrick SM. Differentiation of CD8 memory T cells depends on Foxo1. The Journal of Experimental Medicine. 2013;210:1189–1200. doi: 10.1084/jem.20130392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T Cell Subsets, Migration Patterns, and Tissue Residence. Annual Review of Immunology. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nature Reviews Immunology. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan D, van der Windt Gerritje JW, Huang Stanley C-C, Curtis Jonathan D, Chang CH, Buck Michael D, Qiu J, Smith Amber M, Lam Wing Y, DiPlato Lisa M, et al. Memory CD8+ T Cells Use Cell-Intrinsic Lipolysis to Support the Metabolic Programming Necessary for Development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye I, Wang L, Pallmer K, Richter K, Ichimura T, Haas R, Crouse J, Choi O, Heathcote D, Lovo E, et al. The protein LEM promotes CD8+ T cell immunity through effects on mitochondrial respiration. Science. 2015;348:995–1001. doi: 10.1126/science.aaa7516. [DOI] [PubMed] [Google Scholar]
- Olson Janelle A, McDonald-Hyman C, Jameson Stephen C, Hamilton Sara E. Effector-like CD8+ T Cells in the Memory Population Mediate Potent Protective Immunity. Immunity. 2013;38:1250–1260. doi: 10.1016/j.immuni.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science. 2013;342:1242454–1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan AT, Goldrath AW. Hypoxia-inducible factors regulate T cell metabolism and function. Mol Immunol. 2015;68:527–535. doi: 10.1016/j.molimm.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Rajesh R, Li Q, Bupp Melanie RG, Shrikant Protul A. Transcription Factor Foxo1 Represses T-bet-Mediated Effector Functions and Promotes Memory CD8+ T Cell Differentiation. Immunity. 2012;36:374–387. doi: 10.1016/j.immuni.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR Kinase Determines Effector versus Memory CD8+ T Cell Fate by Regulating the Expression of Transcription Factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf J, Zarrouk M, Finlay DK, Foretz M, Viollet B, Cantrell DA. AMPKα1: A glucose sensor that controls CD8 T-cell memory. European Journal of Immunology. 2013;43:889–896. doi: 10.1002/eji.201243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena Laura A, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman David A, Wang CR, Schumacker Paul T, Licht Jonathan D, Perlman H, et al. Mitochondria Are Required for Antigen-Specific T Cell Activation through Reactive Oxygen Species Signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1 -dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. Journal of Experimental Medicine. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Yang K, Wei J, Karmaus PWF, Neale G, Chi H. Tsc1 promotes the differentiation of memory CD8+ T cells via orchestrating the transcriptional and metabolic programs. Proceedings of the National Academy of Sciences. 2014;111:14858–14863. doi: 10.1073/pnas.1404264111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. The Journal of Clinical Investigation. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejera MM, Kim EH, Sullivan JA, Plisch EH, Suresh M. FoxO1 Controls Effector-to-Memory Transition and Maintenance of Functional CD8 T Cell Memory. The Journal of Immunology. 2013;191:187–199. doi: 10.4049/jimmunol.1300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt Gerritje JW, Everts B, Chang CH, Curtis Jonathan D, Freitas Tori C, Amiel E, Pearce Edward J, Pearce Erika L. Mitochondrial Respiratory Capacity Is a Critical Regulator of CD8+ T Cell Memory Development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJW, O'Sullivan D, Everts B, Huang SCC, Buck MD, Curtis JD, Chang CH, Smith AM, Ai T, Faubert B, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proceedings of the National Academy of Sciences. 2013;110:14336–14341. doi: 10.1073/pnas.1221740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DJ, Wang Q, Wei J, Baimukanova G, Buchholz F, Stewart AF, Mao X, Killeen N. Selective Expression of the Cre Recombinase in Late-Stage Thymocytes Using the Distal Promoter of the Lck Gene. The Journal of Immunology. 2005;174:6725–6731. doi: 10.4049/jimmunol.174.11.6725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.