Abstract
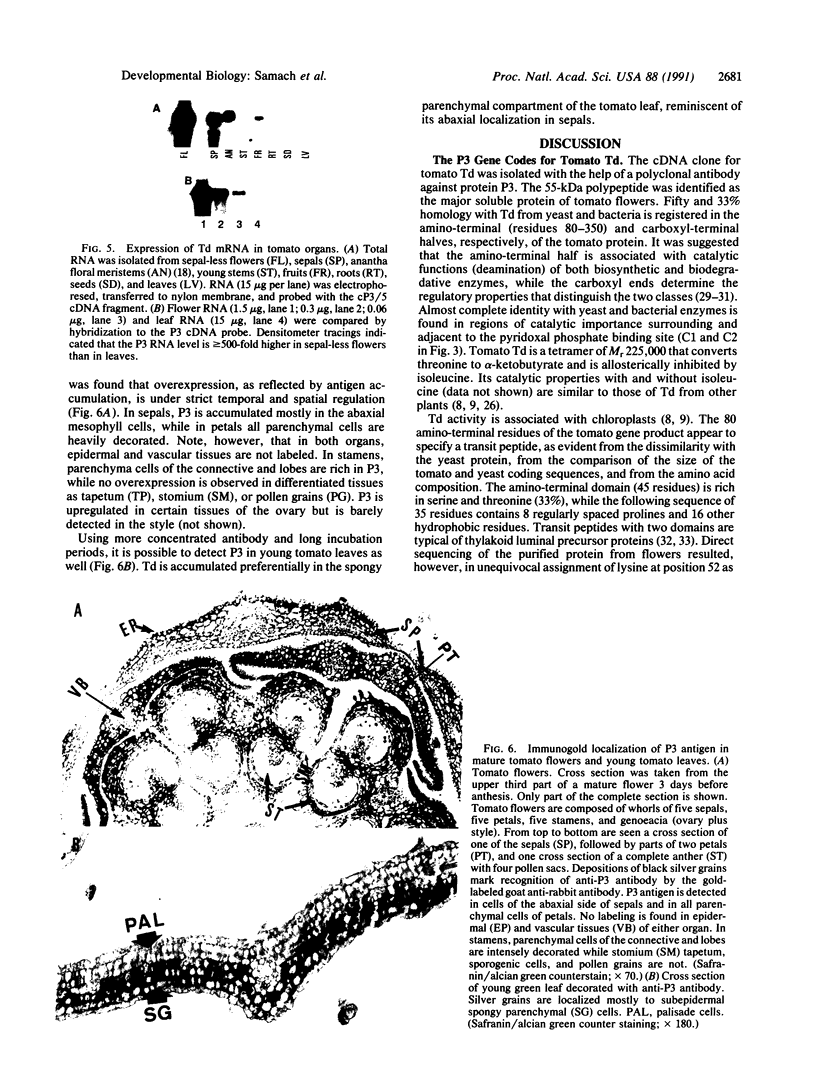
The gene encoding the plant biosynthetic threonine deaminase (Td; EC 4.2.1.16) has been cloned as a result of its unusual upregulation in tomato flowers. The Td gene of tomato encodes a polypeptide of 595 residues, the first 80 of which comprise a putative two-domain transit peptide cleaved at position 51. Comparison of the amino acid sequence with the corresponding enzymes from yeast and bacteria reveals a near identity of the important catalytic regions and greater than 40% overall similarity. The Td gene is unique in the tomato genome and its coding region is interrupted by eight introns. Its expression is greater than 50-fold higher in sepals and greater than 500-fold higher in the rest of the flower than in leaves or roots. Its overexpression, however, is strictly confined to the parenchymal cells of the floral organs. In young tomato leaves, the chloroplast-bound enzyme is found almost exclusively in the subepidermal spongy mesophyll cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlyn M. B., Last R. L., Fink G. R. A gene encoding the tryptophan synthase beta subunit of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4604–4608. doi: 10.1073/pnas.86.12.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colau D., Negrutiu I., Van Montagu M., Hernalsteens J. P. Complementation of a threonine dehydratase-deficient Nicotiana plumbaginifolia mutant after Agrobacterium tumefaciens-mediated transfer of the Saccharomyces cerevisiae ILV1 gene. Mol Cell Biol. 1987 Jul;7(7):2552–2557. doi: 10.1128/mcb.7.7.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. L., Cox B. J., Fidanza V., Calhoun D. H. The complete nucleotide sequence of the ilvGMEDA cluster of Escherichia coli K-12. Gene. 1987;56(2-3):185–198. doi: 10.1016/0378-1119(87)90136-3. [DOI] [PubMed] [Google Scholar]
- Datta P., Goss T. J., Omnaas J. R., Patil R. V. Covalent structure of biodegradative threonine dehydratase of Escherichia coli: homology with other dehydratases. Proc Natl Acad Sci U S A. 1987 Jan;84(2):393–397. doi: 10.1073/pnas.84.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser C. S., Winter J. A., Hironaka C. M., Shah D. M. Structure, expression, and evolution of the 5-enolpyruvylshikimate-3-phosphate synthase genes of petunia and tomato. J Biol Chem. 1988 Mar 25;263(9):4280–4287. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Holmberg S., Petersen J. G. Regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. Curr Genet. 1988 Mar;13(3):207–217. doi: 10.1007/BF00387766. [DOI] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Wek R. C., Lopes J. M., Pereira R., Taillon B. E., Hatfield G. W. The complete nucleotide sequence of the ilvGMEDA operon of Escherichia coli K-12. Nucleic Acids Res. 1987 Mar 11;15(5):2137–2155. doi: 10.1093/nar/15.5.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsot C. Evolution of biosynthetic pathways: a common ancestor for threonine synthase, threonine dehydratase and D-serine dehydratase. EMBO J. 1986 Nov;5(11):3013–3019. doi: 10.1002/j.1460-2075.1986.tb04600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J., Wistow G. J. Enzyme/crystallins: gene sharing as an evolutionary strategy. Cell. 1989 Apr 21;57(2):197–199. doi: 10.1016/0092-8674(89)90956-2. [DOI] [PubMed] [Google Scholar]
- Sharma R. J., Mazumder R. Purification, properties, and feedback control of L-threonine dehydratase from spinach. J Biol Chem. 1970 Jun 10;245(11):3008–3014. [PubMed] [Google Scholar]
- Singh B. K., Stidham M. A., Shaner D. L. Assay of acetohydroxyacid synthase. Anal Biochem. 1988 May 15;171(1):173–179. doi: 10.1016/0003-2697(88)90139-x. [DOI] [PubMed] [Google Scholar]
- Taillon B. E., Little R., Lawther R. P. Analysis of the functional domains of biosynthetic threonine deaminase by comparison of the amino acid sequences of three wild-type alleles to the amino acid sequence of biodegradative threonine deaminase. Gene. 1988 Mar 31;63(2):245–252. doi: 10.1016/0378-1119(88)90528-8. [DOI] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Weisbeek P., Hageman J., de Boer D., Pilon R., Smeekens S. Import of proteins into the chloroplast lumen. J Cell Sci Suppl. 1989;11:199–223. doi: 10.1242/jcs.1989.supplement_11.16. [DOI] [PubMed] [Google Scholar]