Summary
Efficient transfection of genes into neurons is a crucial step for the study of neuronal cell biology and functions. These include but are not limited to investigating gene function by overexpression of target proteins via expression plasmids and knocking down the expression levels of neuronal genes by RNA interference (RNAi). In addition, reporter gene constructs are widely used to investigate the promoter activities of neuronal genes. Numerous transfection techniques have been established to deliver genes into the cells. However, efficient transfection of post-mitotic cells, including neurons, still remains a challenging task. Here, we overview the advantages and disadvantages of various techniques for the transfection of primary neurons, and provide an optimized protocol for FuGENE-6 (Promega) which allows a suitable transfection efficiency of primary neuronal cultures.
Keywords: Primary neurons, gene delivery, transfection, electroporation, nucleofection, calcium co-precipitation, lipofection, FuGENE-6
1. INTRODUCTION
Transfection is the process of introducing nucleic acids into cells. The term transfection is commonly used for non-viral gene delivery (1, 2). Transfection of animal cells typically involves opening transient pores in the cellular membrane that will allow the uptake of genetic material into the cytoplasm. Transfection may cause morphologic changes and abnormalities in target cells. The transfection of primary cortical and hippocampal neurons has proven to be a real challenge for the study of neuronal gene functions (2, 3). Over the past three decades, many transfection methods have been developed including electroporation, nucleofection, calcium phosphate co-precipitation, and lipofection.
Among these transfection methods, electroporation alters the phospholipid bilayer’s hydrophobic/hydrophilic interactions of plasma membrane by exposing cells to a voltage pulse (3–5). Applying a quick voltage shock disrupts areas of the membrane temporarily, allowing polar molecules to enter the cells through the membrane. Electroporation is generally used with freshly isolated neurons or neuronal cell lines in suspension, and requires specialized equipment which is relatively expensive. In order to avoid high cellular mortality associated with electroporation, the nucleofection technique has been developed. In conventional electroporation, the cell membrane is broken down by electric pulses and DNA from the surrounding buffer enters the cytoplasm resulting with high cell mortality. To overcome this problem, nucleofection applies cell-type-specific combinations of electric currents and solutions and thus increases the cell survival (2, 6, and 7). Although nucleofection provides high levels of transfection efficiency with low toxicity, its application is also restricted to neuronal progenitors and freshly isolated neurons in suspension.
The calcium phosphate co-precipitation method is one of the most widely used transfection methods of primary neuronal cultures (8–10). The principle of the technique relies on formation of DNA crystals with Ca2+ ions in the phosphate buffer. The crystals precipitate onto the cells during the transfection, and enter the cells by endocytosis. The technique does not require any specialized equipment. It is cost-effective and easy to optimize for the best results. Although the method is suitable to transfect neurons at all stages of differentiation, in postmitotic neurons, delivery of the DNA into the nucleus is more difficult and that leads to low transfection efficiencies (11).
Lipofection is perhaps the most popular transfection method for the gene delivery into a variety of animal cells. The technique relies on cationic lipid molecules which form unilamellar liposomes (12–15). The positively charged (cationic) lipid molecules interact with negatively charged DNA to form a DNA:lipid aggregate (12, 13). Genetic material is delivered into cells by this lipid aggregate which fuses with the negatively charged plasma membrane. In order to increase the fusion capacity, cationic lipid molecules are often combined with neutral helper lipid molecules. Many different formulations of lipofection reagents have been developed. The lipofection technique is easy to optimize and generally less toxic to the cells. Although the technique produces high transfection efficiencies in a wide range of cell types, the transfection efficiency of postmitotic neurons shows variable results based on the lipofection reagent used. In order to determine the best suitable transfection method for a particular experiment, the specific advantages and disadvantages of each technique must be considered. Each method has been proven to have their own strengths and drawbacks regarding toxicity, expression levels, cell viability, and transfection efficiency (3). In Table 1, the advantages and disadvantages of commonly used transfection techniques for primary neurons is summarized. Below we provide an optimized protocol of FuGENE-6 (Promega) which we have developed in our laboratory and yields relatively high transfection efficiencies in primary neuronal cultures.
Table 1.
Transfection methods commanly used to transfect primary neurons: advantages and disadvantages.
| Advantages | Disadvantages | |
|---|---|---|
|
| ||
| Electroporation | Relatively high transfection efficiencies; | Only used for fresly isolated neurons in suspension; |
| less optimization required; | expensive material; | |
| simple and quick transfection; | relatively higher toxicity; | |
| expression of the genes starts within hours. | requires specialized equipment. | |
|
| ||
| Nucleofection | Delivers genes directly into the nucleus; | Only used for fresly isolated neurons in suspension; |
| very high transfection efficiencies; | requires expensive material and equipment; | |
| higher expression levels; | requires optimization of the material; | |
| requires specialized equipment; | ||
| relatively low toxicity. | ||
|
| ||
| Ca2+ phosphate co-precipitation | Can be used for cultured primary neurons; | Relatively low transfection efficiencies. |
| no pecialized equipment is required; | ||
| little optimization for different plasmids; | ||
| low toxicity when optimized; | ||
| cost-effective. | ||
|
| ||
| Lipofection | Can be used for cultured primary neurons | Needs optimization for higher transfection efficiencies |
| Simple and quick transfection procedure; | depending on reagent used, some toxicity is observed. | |
| provides reproducible transfection efficiencies; | ||
| high transfection efficiency depending on reagent; | ||
| cost-effective. | ||
2. MATERIALS
-
2.1
Freshly isolated or post-mitotic neurons.
-
2.2
6-well cell culture-grade plastic dishes.
-
2.3
Neuronal Growth Medium (NGM): Prepare Neuro-Basal Medium supplemented with B27 (2%), L-Glutamine (250 μM), Penicillin/Streptomycin (50 μg/ml), and amphotericin b (2.5 μg/ml).
-
2.4
Opti-MEM serum free media (Life Technologies).
-
2.5
FuGENE-6 transfection reagent (Promega).
-
2.6
Plastic pipets.
-
2.7
pLEGFP-C1 plasmid.
-
2.8
37°C, 5% CO2 humidified tissue culture incubator.
-
2.9
Sterile tissue culture hood.
-
2.10
Sterile 1.5 ml microcentrifuge tubes.
3. METHODS
-
3.1
Plate and maintain neurons in 6-well tissue culture dishes at a density of 60–80% confluency.
-
3.2
Place Opti-MEM serum free media at 37°C for at least 1 hour prior transfection. Opti-MEM is provided in 500 ml bottles. To maintain the quality and freshness of the media, aliquot cold Opti-MEM in 15 or 50 ml sterile tubes based on amount to be needed for the transfection.
-
3.3
FuGENE6 is stored at 4C°. Bring FuGENE6 at room temperature before usage.
-
3.4
Transfection mixture should be prepared in tissue culture hood where contamination risk is minimized. Prepare and label sterile 1.5 ml microcentrifuge tubes based on transfection conditions.
-
3.5
Place 100 μl of Opti-MEM media into the 1.5 ml microcentrifuge tubes, add 18 μl FuGENE6 to form transfection mixture A (TM-A). Add the transfection reagent directly to the optimum. Put the tip directly into the Opti-MEM do not allow the reagent to touch any other plastic parts of the tube. FuGENE 6 may bind to the plastic and limit the effectiveness of the transfection.
-
3.6
Mix the TM-A by gentle pipetting. Do not vortex or centrifuge the TM-A mixture.
-
3.7
In a separate tube, prepare transfection mixture B (TM-B). Dilute DNA construct (pLEGFP-C1 plasmid) in 100 μl of opti-MEM media as 60ng/μl (total DNA amount for each well is 6 μg).
-
3.8
Incubate TM-A and TM-B in the cell culture hood for 5 minutes, and carefully combine TM-B with TM-A in the TM-A tube to obtain primary transfection mixture (PTM). Mix the PTM by gentle pipetting. Do not vortex or centrifuge the PTM mixture.
-
3.9
Incubate PTM at room temperature for 30 minutes. This will allow proper time for the formation of DNA:liposome complexes.
-
3.10
Remove media from primary neuronal cultures, and rinse the cells with 2ml warm Opti-MEM.
-
3.11
The volume of PTM is about ~ 220 μl (this will change slightly based on DNA amount). Add 280μl more Opti-MEM media to bring the volume to the total of 500 μl (final PTM volume).
-
3.12
Add the PTM mixture directly (slowly and drop-wise) onto the cells while swirling media in cells to mix.
-
3.13
Place cells back into the tissue culture incubator and incubate for 6 hours. It is important to swirl the transfection media on cells every hour during the transfection process. That will help to yield higher transfection efficiencies.
-
3.14
After 6 hours of incubation, remove the transfection mixture, and add 2ml fresh growth media onto cells.
-
3.15
Green fluorescein protein (GFP) expression will typically start around 6h post-transfection, and will reach to a pick at 48–72h.
4 NOTES
-
4.1
Isolation and preparation of neuronal cultures for the transfection is a crucial step (use appropriate protocols for the isolation of cortical and hippocampal neurons). Neurons should be isolated and plated in sterile tissue culture plates at a density of 50–80% and maintained in a humidified tissue culture incubator. Using too low a cell density may cause cells to grow poorly. If the cultures are too dense cells, this may result in contact inhibition which negatively affects the uptake of DNA. Cultures should be free of contamination, and grown in appropriate medium with all the necessary growth factors.
-
4.2
Use high-quality of DNA which is free of RNA, proteins, and endotoxins. The DNA should be of transfection-quality. This can be achieved by using a high quality plasmid preparation kit such as PureYield Plasmid Maxiprep Systems. The A260/A280 ratio of the DNA should be 1.7–1.9.
-
4.3
The optimal amount of DNA is crucially important. Determining the right amount of the plasmid DNA for transfection is important to obtain high transfection efficiencies. The amount of DNA will vary widely depending upon the transfection reagent, DNA type, number of cells, and the surface area of the tissue culture dishes. Table 2 summarizes the optimized composition of PTM used to transfect primary neurons via FuGENE-6 in different size of tissue culture plates. [TABLE 2]
-
4.4
Attaining an optimal ratio of FuGENE-6 to the DNA is also very important. Ratios of 3:1 and 2:1 work well for primary neurons, but ratios outside this range may be optimal for a particular experiment or application.
-
4.5
Toxicity is an important consideration. FuGENE-6 transfection reagent is one of the less toxic methods of DNA transfection into cells. In the event of cell death, optimize conditions as follows. Lower the amount of input DNA and FuGENE-6 while holding the DNA:FuGENE-6 ratio constant. Increasing the cell density on the plates may also eliminate the associated toxicity. In the case of excessive cell death, possible toxicity of the gene products should also be considered. That can be addressed by using a control plasmid for transfections performed in parallel.
Table 2.
Reference table for PTM composition for different size of tissue culture plates.
| Growth area | Total DNA amount | PT-A volume | PT-B volume | FuGENE-6 volume | Final PTM volume | |
|---|---|---|---|---|---|---|
| 96-well plate | 0.32 cm2 | 0.375 μg | 6 μl | 6 μl | 1.2 μl | 24 μl |
| 24-well plate | 2.0 cm2 | 1.5 μg | 25 μl | 25 μl | 4.5 μl | 125 μl |
| 12-well plate | 3.8 cm2 | 3.0 μg | 50 μl | 50 μl | 19 μl | 250 μl |
| 6-well plate | 9.6 cm2 | 6.0 μg | 100 μl | 100 μl | 18 μl | 500 μl |
| 60 mm dishes | 21.3 cm2 | 12 μg | 200 μl | 200 μl | 36 μl | 1000 μl |
| 100 mm dishes | 58.1 cm2 | 20 μg | 400 μl | 400 μl | 60 μl | 2000 μl |
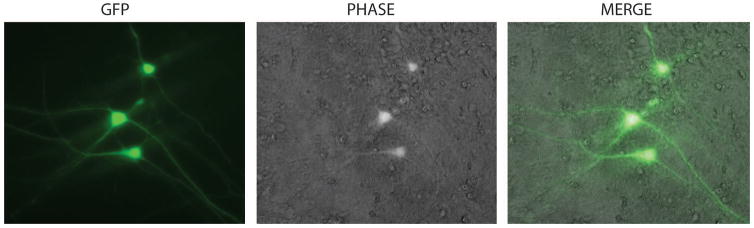
Figure 1.
GFP expression in post-mitotic neurons 72 hours after transfection with FuGENE-6. Primary neurons were plated in 6-well tissue culture plates at a density of 60% confluency and transfected with pLEGFP-C1 plasmid as described in methods section.
References
- 1.http://www.promega.com/resources/product-guides-and-selectors/protocols-and-applications-guide/transfection/.
- 2.Zeitelhofer M, John P, Vessey JP, Thomas S, Kiebler M, Dahm R. Transfection of Cultured Primary Neurons via Nucleofection. Current Protocols in Neuroscience. 2009:4.32.1–4.32.21. doi: 10.1002/0471142301.ns0432s47. [DOI] [PubMed] [Google Scholar]
- 3.Karra D, Dahm R. Transfection Techniques for Neuronal Cells. J Neurosci. 2010;30(18):6171–7. doi: 10.1523/JNEUROSCI.0183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Washbourne P, McAllister AK. Techniques for gene transfer into neurons. Curr Opin Neurobiol. 2002;12:566–573. doi: 10.1016/s0959-4388(02)00365-3. [DOI] [PubMed] [Google Scholar]
- 5.Dib-Hajj SD, Choi JS, Macala LJ, Tyrrell L, Black JA, Cummins TR, Waxman SG. Transfection of rat or mouse neurons by biolistics or electroporation. Nat Protoc. 2009;4:1118–1126. doi: 10.1038/nprot.2009.90. [DOI] [PubMed] [Google Scholar]
- 6.Gresch O, Engel FB, Nesic D, Tran TT, England HM, Hickman ES, Korner I, Gan L, Chen S, Castro-Obregon S, Hammermann R, Wolf J, Müller-Hartmann H, Nix M, Siebenkotten G, Kraus G, Lun K. New non-viral method for gene transfer into primary cells. Methods. 2004;33:151–163. doi: 10.1016/j.ymeth.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Gartner A, Collin L, Lalli G. Nucleofection of Primary Neurons. Methods in Enzymology. 2006;406:374–388. doi: 10.1016/S0076-6879(06)06027-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu LY, Arumäe U. Survival assay of transiently transfected dopaminergic neurons. J Neurosci Methods. 2008;169(1):8–15. doi: 10.1016/j.jneumeth.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Gabellini N, Minozzi MC, Leon A, Dal Toso R. Nerve growth factor transcriptional control of c-fos promoter transfected in cultured spinal sensory neurons. J Cell Biol. 1992;118(1):131–8. doi: 10.1083/jcb.118.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetze B, Grunewald B, Baldassa S, Kiebler M. Chemically controlled formation of a DNA/calcium phosphate coprecipitate: applicationfor transfection of mature hippocampal neurons. J Neurobiol. 2004;60:517–525. doi: 10.1002/neu.20073. [DOI] [PubMed] [Google Scholar]
- 12.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, Ramsey P, Martin M, Felgner PL. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994;269(4):2550–61. [PubMed] [Google Scholar]
- 14.Wiesenhofer B, Humpel C. Lipid-Mediated Gene Transfer into Primary Neurons Using FuGene: Comparison to C6 Glioma Cells and Primary GliaExperimental. Neurology. 2000;164:38–44. doi: 10.1006/exnr.2000.7414. [DOI] [PubMed] [Google Scholar]
- 15.Ohki EC, Tilkins ML, Ciccarone VC, Price PJ. Improving the transfection efficiency of post-mitotic neurons. Journal of Neuroscience Methods. 2001;112:95–99. doi: 10.1016/s0165-0270(01)00441-1. [DOI] [PubMed] [Google Scholar]