Abstract
Purpose of review
To review the prevalence, causes and functional significance of vitamin B12 deficiency in vulnerable subpopulations including older adults and the developing embryo.
Recent findings
It is becoming increasingly recognized that the susceptibility to vitamin B12 deficiency may change throughout the life cycle, with the developing embryo and older adults exhibiting elevated risk. Recent data implicate low vitamin B12 status as a risk factor for birth defects resulting from improper neural tube development. The potential for vitamin supplementation and/or food fortification to ameliorate the risk of deficiency in these subpopulations is discussed.
Summary
The prevalence and impact of vitamin B12 deficiency varies throughout the life cycle, with older adults and potentially the developing embryo having the greatest risk and susceptibility. Additional research is needed to develop effective public health interventions that address the unique causes of this nutritional deficiency, which differ among at-risk subpopulations.
Keywords: anemia, birth defects, cobalamin, older adults, vitamin B12
Introduction
The importance of vitamin B12 nutritional status throughout the life cycle is increasingly recognized especially in two vulnerable populations, older adults and pregnant women. The clinical manifestations of severe and persistent vitamin B12 deficiency on reversible hematological changes and irreversible loss of neurological function in older adults have been recognized for decades. More recently it has been discovered that low vitamin B12 status is more prevalent than previously thought [1,2,3•]. The effect of low vitamin B12 status, which results in altered cellular metabolism, on age-related disease and functional decline including cognition, cardiovascular disease and bone health, is an active area of investigation [2,4]. In the early life cycle, the developing embryo may be particularly susceptible to vitamin B12 deficiency, and there is new emerging evidence that vitamin B12 status is involved in the etiology of neural tube defects, which are common birth defects resulting from failure in neural tube closure during very early human development [5••]. The importance of vitamin B12 nutrition in human physiology and health is reviewed in light of current considerations to initiate public health interventions to prevent vitamin B12-associated pathologies in vulnerable subpopulations[6•].
Vitamin B12
Vitamin B12 is a member of the water-soluble B-vitamin family and therefore is an essential nutrient that must be acquired from the diet [7]. Vitamin B12 belongs to a class of naturally occurring colbalt-containing compounds known as cobalamins, which contain a planar corrin ring that binds a single colbalt atom. Colbalt is the functional part of vitamin B12, which serves as an enzyme cofactor for two vitamin B12-dependent, enzyme-catalyzed reactions in mammals. During cellular metabolism, the colbalt atom reacts with chemical substrates, which occupy the β-axial position of the corrin ring. The various forms of vitamin B12 are named by the occupancy of the β-axial ligand, and include methylcobalamin, deoxyadenosylcobalamin, hydroxocobalamin, aquocobalamin and cyanocobalamin. Methylcobalamin and deoxyadenosylcobalamin are the two biologically functional cobalamin forms that participate in human metabolism. Cyanocobalamin is a synthetic and stable form of vitamin B12 and the form most commonly found in vitamin B12 nutritional supplements and fortified food. It is converted to biologically active forms of the vitamin once imported into cells; methylcobalamin is also present in some vitamin supplements.
Physiological function of vitamin B12
Vitamin B12 affects many cellular processes, but its deficiency has the greatest impact on the generation of new blood cells and neurological function. At the cellular level, vitamin B12 is a required cofactor for only two metabolic enzymes, methionine synthase and L-methylmalonyl-coenzyme A mutase, and these are the only two known functions for this vitamin in human physiology. Methionine synthetase generates methylcobalamin, and the methyl group is then used to convert the amino acid homocysteine to the amino acid methionine. Methionine synthase also requires the B-vitamin folate in the form of 5-methyltetrahydrofolate, to generate methylcobalamin. Methionine synthase serves two important functions. First, it prevents homocysteine from accumulating systemically in tissues and serum, which is a risk factor for vascular disease, stroke and certain cancers [8]. Second, it generates the required amino acid methionine, which is essential for protein synthesis and through its conversion to S-adenosylmethionine, is involved in numerous cellular methylation reactions, which are required for the synthesis of many biological molecules including phospholipids and neurotransmitters, and plays important roles in the regulation of gene expression and protein function. Impairment in methionine synthase activity, which occurs during vitamin B12 deficiency, results in elevated plasma homocysteine [9], impaired cellular methylation, and indirectly inhibits DNA synthesis by ‘trapping’ cellular folate and making it unavailable for the synthesis of deoxyribonucleotides, resulting in hematologic abnormalities [10•]. The second enzyme that requires vitamin B12, L-methyl-malonylcoenzyme A mutase, utilizes deoxyadenosylcobalamin to convert L-methylmalonyl-coenzyme A to succinyl-coenzyme A, a reaction involved in the metabolism of branch chain amino acids and odd chain fatty acids. Loss of L-methylmalonyl-coenzyme A mutase activity, as can occur during vitamin B12 deficiency, induces methylmalonicacidemia, which is characterized by blood elevated levels of methylmalonic acid in serum.
Vitamin B12 absorption
Vitamin B12 absorption is a highly complex process that often becomes less efficient with age, and involves the stomach, pancreas and small intestine. In healthy adults, about half of the ingested vitamin B12 present in food is absorbed into the body [9], but loss of function in any of these organs impairs vitamin B12 absorption potentially leading to vitamin B12 deficiency. In the stomach, secreted gastric acid and pepsin are essential to liberate vitamin B12 from the proteins in food that bind it tightly. Once liberated from food, vitamin B12 is bound in the stomach by R-proteins (otherwise known as haptocorrins), which carry the vitamin to the intestine. The R-proteins are degraded in the small intestine by proteases secreted from the pancreas, thereby liberating the vitamin B12. The vitamin B12 does not remain free in the intestine, but rather binds to another carrier protein called intrinsic factor, which is secreted by the parietal cells in the stomach. Intrinsic factor cannot bind vitamin B12 in the acidic environment of the stomach, but tightly binds the vitamin in the alkaline pH of the intestine. The vitamin B12-intrinsic factor complex is transported into the enterocyte by specific receptors. From the enterocyte, it enters circulation where it is bound by a serum protein termed transcobalamin II (TCII) and enters cells via a TCII receptor.
Dietary sources of vitamin B12 and causes and consequences of deficiency
Bacteria are the only organisms capable of vitamin B12 biosynthesis and are ultimately responsible for the presence of vitamin B12 in animal source foods including fish, meat, eggs and dairy products. In healthy individuals, nutritional vitamin B12 deficiency is uncommon, in part because total body stores in adults can exceed 2500 μg and daily turnover is slow [2]. Therefore, adults have several years’ worth of vitamin B12 stores. The vitamin B12 RDA for adults is 2.4 μg/day [11]. Mild vitamin B12 deficiency in otherwise healthy individuals can result from dietary patterns that result in insufficient dietary intake of animal source foods including strict vegetarian and vegan diets, or can be caused by changes in stomach function resulting from aging and/or pharmaceutical use [12]. Mild vitamin B12 deficiency does not elicit clinical symptoms but can be diagnosed by measurement of plasma and serum levels of vitamin B12, methylmalonic acid, total homocysteine and/or holoTCII, although methylmalonic acid is the best diagnostic test for vitamin B12 deficiency [9]. Up to 38% of older adults may exhibit mild vitamin B12 deficiency and depleted vitamin B12 stores [9].
The capacity to absorb vitamin B12 from a food-based diet decreases in older adults and over time can result in the food-cobalamin malabsorption syndrome, characterized by mild vitamin B12 deficiency, decreased whole body stores and metabolic disturbances [2,13]. This syndrome can potentially place the individual at greater risk for severe vitamin B12 deficiencies. The primary cause of malabsorption in older adults is diminished acid secretions in the stomach leading to decreased capacity to extract vitamin B12 bound to food proteins. The use of histamine H2-receptor antagonist, pharmaceuticals that decrease gastric acid secretion parietal cells, including cimetidine (Tagamet), famotidine (Pepcid), nizatidine (Axid) and ranitidine (Zantac) may exacerbate impairments in vitamin B12 absorption from food in older adults, but rarely induce deficiency alone. Proton pump inhibitors, which include omeprazole (Prilosec, Losec), lansoprazole (Prevacid), rabeprazole (Aciphex), pantoprazole (Protonix, Pantoloc), and esomeprazole (Nexium) block the secretion of gastric acid and pepsin and therefore may be more likely to impair vitamin B12 absorption because they can induce the complete absence of gastric acid secretion. These medicinal agents do not block the absorption of vitamin B12 from supplements because supplemental vitamin B12 is not usually protein bound. Because up to 30% of adults over 51 years of age have atrophic gastritis with low stomach acid excretion, it is recommended that they meet the RDA for vitamin B12 with supplements and/or fortified foods. Short-term consumption of diets lacking vitamin B12 and/or use of medications is usually insufficient to trigger severe cobalamin deficiency.
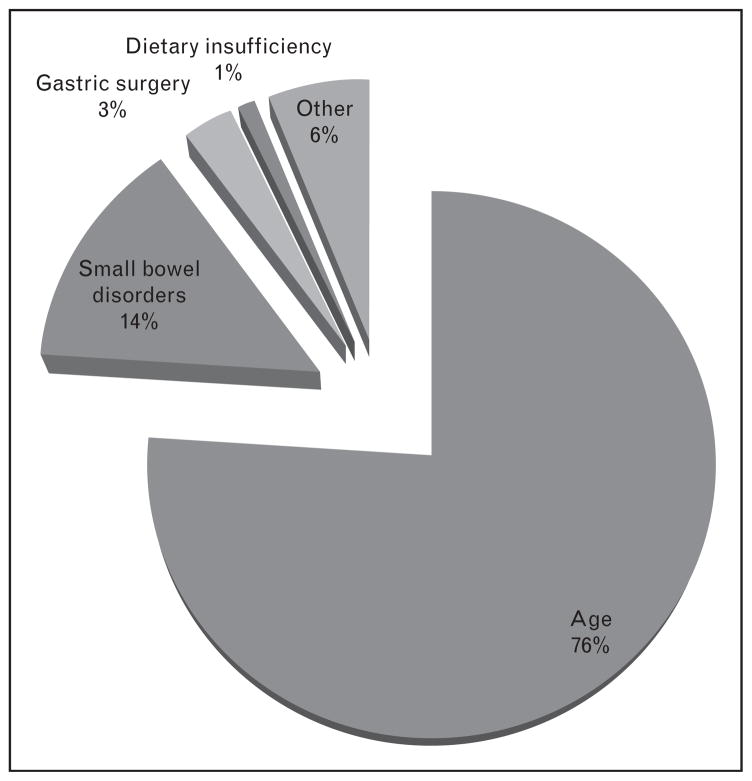
Severe vitamin B12 deficiency often manifests as colalamin-deficiency anemia, neuropathy and myelopathy. It is observed almost exclusively in older adults. Colalamin-deficiency anemia, which has been observed in nearly 2% of older adults [14], is otherwise known as pernicious anemia [2] and is caused by either a loss of parietal cells, small intestine disorders, genetic mutations [15], and/or gastric surgery, all of which can independently disrupt intrinsic factor-mediated vitamin B12 absorption [2] (Fig. 1). Up to 90% of vitamin B12-deficient patients exhibit neurological complications that are not always fully reversed following vitamin B12 administration [2,11]. Sometimes, neurological symptoms are the only clinical manifestation of vitamin B12 deficiency. Neurological complications gradually progress with the nutritional deficiency, and include peripheral neuropathies (tingling, numbness in limbs), motor disturbances, visual disturbances and cognitive impairments including memory loss, disorientation and frank dementia. Successful treatment and management requires lifelong oral supplemental vitamin B12 administration (1.0 mg/day) or periodic intramuscular injections. Such high doses of supplemental vitamin B12 are usually required because loss of intrinsic factor mediated absorption requires absorption by passive diffusion from the gut [7]. Severe vitamin B12 deficiency can be induced by prolonged exposure to nitrous oxide anesthesia, which oxidizes and thereby inactivates vitamin B12 [16].
Figure 1.
Causes of severe vitamin B12 deficiency
The developing embryo may also be at risk for vitamin B12 deficiency. There is increasing evidence from cross-sectional studies that some neural tube defect affected pregnancies that are not prevented by maternal folic acid supplementation and/or wheat flour folic acid fortification may result from vitamin B12 deficiency, although no randomized control trails have been conducted [5••]. Serum vitamin B12 levels decrease significantly in the first trimester of pregnancy and to a greater degree than can be accounted for by hemodilution. Only circulating, newly absorbed maternal vitamin B12 is available to the developing embryo and fetus, and therefore the developing embryo may be sensitive to variations in maternal dietary vitamin B12 intake independent of maternal vitamin B12 stores [11]. The RDA for pregnant women is 2.6 μg/day [11].
Vitamin B12 deficiency and neurological disease
Vitamin B12 deficiency and/or age-related impairments in its function are increasing recognized as contributing to age-related cognitive decline, both subtle deficits and frank dementia. Neurological function declines with age; incidence of dementia increases with age, with a prevalence of 25% in adults 85–89 years of age [17]. Numerous prospective and cross-sectional studies have shown that elevations in total plasma homocysteine, vitamin B12 deficiency, as well as deficiencies in other B-vitamins that interact with vitamin B12 in metabolizing homocysteine, are risk factors for cognitive decline and more severe dementia, although not all studies have shown positive associations between blood status indicators of vitamin B12 and dementia [18]. Although mechanisms have yet to be established, vascular disease, ischemia, and cerebral cortex atrophy as indicated from CT scans, have been implicated as contributing factors. Other etiologies have been proposed from animal studies. Mice fed B-vitamin deficient diets exhibit elevated plasma homocysteine and impaired performance on the Morris Water Maze without observable neurodegeneration [19]. Rather, changes were seen in the cellular methylation potential of the cells indicating a metabolic rather than a structural etiology. It has also been suggested that elevated levels of homocysteine itself may be neurotoxic [17]. More studies are needed to determine the benefits of vitamin B12 and other B-vitamin supplementation on cerebrovascular disease, dementia and more sublte cognitive decline [20].
Conclusion
The high prevalence of vitamin B12 deficiencies in the elderly and increasing evidence that that low maternal vitamin B12 status in pregnant women may be a risk factor in birth defects has stimulated interest in considering the potential benefits of mandatory fortification of enriched flour with vitamin B12 [6•] No adverse effects are known to be associated with excess vitamin B12 intake from food or supplements in healthy individuals, except for individuals at risk for hereditary Leber’s optic atrophy disease who should not be exposed to cyanocobalamin because of a reduced capacity to detoxify the cyanide that is liberated in the cell [11]. However, there are a number of considerations that will need to be addressed before universal fortification of the food supply is advanced, including the potential for public health benefit [5••,6•]. The prevention of developmental defects by vitamin B12, in combination with folic acid, must be validated through a multicenter randomized, controlled trail [5••]. Furthermore, a better understanding of the adverse health consequences resulting from sustained mild, subclinical vitamin B12 deficiency in older adults is needed, as well as the responsiveness of food-bound cobalamin malabsorption to small oral vitamin B12 consumed with a meal [10•].
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 108).
- 1.McLean E, de Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull. 2008;29(2 Suppl):S38–S51. doi: 10.1177/15648265080292S107. [DOI] [PubMed] [Google Scholar]
- 2.Carmel R. Nutritional anemias and the elderly. Semin Hematol. 2008;45:225–234. doi: 10.1053/j.seminhematol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 3•.Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr. 2009;89:693S–696S. doi: 10.3945/ajcn.2008.26947A. Illustrates the magnitude of vitamin B12 deficiency. [DOI] [PubMed] [Google Scholar]
- 4.Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev. 2008;66:250–255. doi: 10.1111/j.1753-4887.2008.00031.x. [DOI] [PubMed] [Google Scholar]
- 5••.Thompson MD, Cole DE, Ray JG. Vitamin B-12 and neural tube defects: the Canadian experience. Am J Clin Nutr. 2009;89:697S–701S. doi: 10.3945/ajcn.2008.26947B. Insights into the potential need for vitamin B12 food fortification to prevent birth defects. [DOI] [PubMed] [Google Scholar]
- 6•.Green R. Is it time for vitamin B-12 fortification? What are the questions? Am J Clin Nutr. 2009;89:712S–716S. doi: 10.3945/ajcn.2008.26947E. Highlights approaches needed to address the need for vitamin B12 fortification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baik HW, Russell RM. Vitamin B12 deficiency in the elderly. Annu Rev Nutr. 1999;19:357–377. doi: 10.1146/annurev.nutr.19.1.357. [DOI] [PubMed] [Google Scholar]
- 8.Kerr MA, Livingstone B, Bates CJ, Bradbury I, et al. Folate, related B vitamins, and homocysteine in childhood and adolescence: potential implications for disease risk in later life. Pediatrics. 2009;123:627–635. doi: 10.1542/peds.2008-1049. [DOI] [PubMed] [Google Scholar]
- 9.Hoey L, Strain JJ, McNulty H. Studies of biomarker responses to intervention with vitamin B-12: a systematic review of randomized controlled trials. Am J Clin Nutr. 2009;89:1981S–1996S. doi: 10.3945/ajcn.2009.27230C. [DOI] [PubMed] [Google Scholar]
- 10•.Carmel R. Efficacy and safety of fortification and supplementation with vitamin B12: biochemical and physiological effects. Food Nutr Bull. 2008;29(2 Suppl):S177–S187. doi: 10.1177/15648265080292S121. This is a clinical perspective on the approaches to treat and prevent vitamin B12 deficiency. [DOI] [PubMed] [Google Scholar]
- 11.Food and Nutrition Board IoM. Dietary reference intakes for thiamin, roboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin and choline. National Academy Press; Washington, D.C: 1998. pp. 306–348. [PubMed] [Google Scholar]
- 12.Andres E, Federici L, Serraj K, Kaltenbach G. Update of nutrient-deficiency anemia in elderly patients. Eur J Intern Med. 2008;19:488–493. doi: 10.1016/j.ejim.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Dali-Youcef N, Andres E. An update on cobalamin deficiency in adults. QJM. 2009;102:17–28. doi: 10.1093/qjmed/hcn138. [DOI] [PubMed] [Google Scholar]
- 14.Carmel R. Megaloblastic anemias. Curr Opin Hematol. 1994;1:107–112. [PubMed] [Google Scholar]
- 15.Remacha AF, Del Rio E, Sarda MP, Canals C, et al. Role of (Glu –> Arg, Q5R) mutation of the intrinsic factor in pernicious anemia and other causes of low vitamin B12. Ann Hematol. 2008;87:599–600. doi: 10.1007/s00277-008-0465-0. [DOI] [PubMed] [Google Scholar]
- 16.Singer MA, Lazaridis C, Nations SP, Wolfe GI. Reversible nitrous oxide-induced myeloneuropathy with pernicious anemia: case report and literature review. Muscle Nerve. 2008;37:125–129. doi: 10.1002/mus.20840. [DOI] [PubMed] [Google Scholar]
- 17.Smith AD. The worldwide challenge of the dementias: a role for B vitamins and homocysteine? Food Nutr Bull. 2008;29(2 Suppl):S143–S172. doi: 10.1177/15648265080292S119. [DOI] [PubMed] [Google Scholar]
- 18.Kivipelto M, Annerbo S, Hultdin J, et al. Homocysteine and holo-transcobalamin and the risk of dementia and Alzheimers disease: a prospective study. Eur J Neurol. 2009;16:808–813. doi: 10.1111/j.1468-1331.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 19.Troen AM, Shukitt-Hale B, Chao WH, et al. The cognitive impact of nutritional homocysteinemia in apolipoprotein-E deficient mice. J Alzheimers Dis. 2006;9:381–392. doi: 10.3233/jad-2006-9403. [DOI] [PubMed] [Google Scholar]
- 20.Stanger O, Fowler B, Piertzik K, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. 2009;9:1393–1412. doi: 10.1586/ern.09.75. [DOI] [PubMed] [Google Scholar]