Abstract
Social isolation has been recognized as a major risk factor for morbidity and mortality in humans for more than a quarter of a century. Although the focus of research has been on objective social roles and health behavior, the brain is the key organ for forming, monitoring, maintaining, repairing, and replacing salutary connections with others. Accordingly, population-based longitudinal research indicates that perceived social isolation (loneliness) is a risk factor for morbidity and mortality independent of objective social isolation and health behavior. Human and animal investigations of neuroendocrine stress mechanisms that may be involved suggest that (a) chronic social isolation increases the activation of the hypothalamic pituitary adrenocortical axis, and (b) these effects are more dependent on the disruption of a social bond between a significant pair than objective isolation per se. The relational factors and neuroendocrine, neurobiological, and genetic mechanisms that may contribute to the association between perceived isolation and mortality are reviewed.
Keywords: social endocrinology, social neuroscience, social genomics, social isolation, loneliness, animal models
INTRODUCTION
Chronic social isolation has long been recognized as a risk factor for broad-based morbidity and mortality. The early evidence for this association came from epidemiological studies, where social isolation has typically been defined in terms of objective features of the social environment such as the absence of a spouse, having less than monthly contact with friends and family, and/or having no participation in organizations, clubs, or religious groups (e.g., House et al. 1988). At that time, health behaviors were already known to have a strong impact on morbidity and mortality, and the primary explanation for the association between isolation and mortality—the social control hypothesis—emphasized the impact of friends and family on a person’s health behaviors. Specifically, the hypothesis posits that internalized obligations to, and the overt influence of, network members (e.g., spouses, family members, friends) encourage individuals to exhibit good health behaviors such as adequate sleep, diet, exercise, and compliance with medical regimens, and discourage individuals from health-damaging behaviors such as smoking, excessive eating, drug abuse, and excessive alcohol consumption (House 2001, Umberson 1987). In sum, the social control hypothesis places the focus on the social control of a person’s health behaviors.
SOCIAL ISOLATION: A SOCIAL NEUROSCIENCE PERSPECTIVE
A contrasting perspective that places social endocrinology front and center begins with the proposition that the brain is the key organ for forming, monitoring, maintaining, repairing, and replacing salutary connections with others as well as regulating physiological processes relevant to morbidity and mortality (Cacioppo & Berntson 1992). The human brain does not simply respond to stimuli (including people) in an invariant fashion, but rather it categorizes, abstracts, interprets, and evaluates incoming stimuli in light of current states and goals as well as prior knowledge and predispositions.
The demographic and environmental factors associated negatively with perceived social isolation [or what Weiss (1973) termed loneliness] include marriage, having offspring, higher levels of education, and larger number of siblings (Distel et al. 2010), whereas those factors related positively to loneliness include male gender, physical health symptoms, chronic work or social stress, small social network, and lack of a spousal confidant (e.g., Hawkley et al. 2008).1 However, the same objective social relationship (e.g., spouse) can be perceived as caring and protective or as exploitive and isolating based on a host of factors including an individual’s prior experiences, current attributions, and overall preference for social contact. Moreover, people may find themselves with others who heighten their sense of threat and isolation (e.g., an untrustworthy sibling or an arch enemy), or they may choose to be alone at times while still feeling connected to others (e.g., a new mother taking a break from caregiving). Accordingly, the association between indices of perceived and objective social isolation is mediated by the perceived quality of social relationships, and perceived social isolation (i.e., loneliness) has been found to predict increased morbidity and mortality (e.g., Caspi et al. 2006, Holt-Lunstad et al. 2010, Patterson & Veenstra 2010, Penninx et al. 1997, Seeman 2000) even after adjusting for objective social isolation and health behaviors (Luo et al. 2012, Luo & Waite 2014; see also Hawkley et al. 2009).
Why is the perception of social isolation important to consider? Sociality has costs (e.g., competition for food and mates, exploitation, increased risk of pathogen transmission) as well as benefits (e.g., mutual protection and assistance, transmission of foraging skills). The social structures and behaviors relevant to mitigating the costs of sociality (e.g., dominance hierarchies, signals of submission, ostracism, punitive altruism) and those relevant to garnering the benefits of sociality (e.g., mother-infant attachment, cheating) ultimately contribute to survival and reproduction, but they do so differently and appear to be instantiated differently in the brain. Human and animal research on the effects of social isolation on the brain suggests the involvement of multiple, functionally distinct brain mechanisms including neural mechanisms involved in social threat surveillance and aversion (e.g., amygdala, anterior insula, anterior cingulate), social reward (e.g., ventral striatum), and attention to one’s self-preservation in a social context (e.g., orbitofrontal cortex, medial pre-frontal cortex, superior temporal sulcus, temporal parietal junction) (Bickart et al. 2012; Cacioppo et al. 2009, 2012, 2013; Eisenberger & Cole 2012; Klumpp et al. 2012).
In many contexts across human history, a chief threat to a person’s reproductive success and survival has come from other humans. The perception of isolation from others—of being on the social perimeter—is not only unhappy but also signals danger across phylogeny. Fish have evolved to swim to the middle of the group when predators approach (Ioannou et al. 2012), mice housed in social isolation rather than in pairs show sleep disruptions and reduced slow wave sleep (Kaushal et al. 2012), and prairie voles when isolated from their partner and subsequently placed in an open field show less exploratory behavior and more predator evasion (Grippo et al. 2014). These behaviors reflect an increased emphasis on self-preservation when on the social perimeter, an emphasis that increases the likelihood of survival. For instance, fish on the edge of a school are more likely to be attacked by predatory fish, not because they are the slowest or weakest, but because it is easier to isolate and prey upon those on the social perimeter (Ioannou et al. 2012).
These behavioral results suggest a more general principle, specifically, that perceived social isolation activates neural, neuroendocrine, and behavioral responses that promote short-term self-preservation. Among the range of neural and behavioral effects of perceived isolation documented in human adults are an increased implicit vigilance for social threats along with increased anxiety, hostility, and social withdrawal; increased sleep fragmentation and daytime fatigue; increased vascular resistance and altered gene expression and immunity; decreased impulse control in favor of responses highest in the response hierarchy (i.e., prepotent responding); increased negativity and depressive symptomatology; and increased age-related cognitive decline and risk of dementia (cf. Cacioppo & Hawkley 2009).
Indeed, growing evidence indicates that loneliness increases attention to negative social stimuli (e.g., social threats, rejection, exclusion). For instance, lonely compared to nonlonely individuals worry more about being evaluated negatively and feel more threatened in social situations (even when they are not more likely to be rejected; Jones et al. 1981), and these differences are found when loneliness is measured across individuals or is manipulated experimentally (Cacioppo et al. 2006). The effects of loneliness on attention to potential social threats appear to be largely implicit. In a modified emotional Stroop task, lonely participants relative to nonlonely participants show greater Stroop interference for negative social compared to negative nonsocial words (see review by Cacioppo & Hawkley 2009). Stroop interference is used to gauge the implicit processing of stimuli, so these results suggest that loneliness is associated with a heightened accessibility of negative social information. Consistent with this reasoning, Yamada & Decety (2009) investigated the effects of subliminal priming on the detection of painful facial expressions and found that lonely individuals are more sensitive to the presence of pain in dislikable faces than are nonlonely individuals.
Functional magnetic resonance imaging research also indicates that loneliness is associated with greater activation of the visual cortex in response to negative social images in contrast to negative nonsocial images (Cacioppo et al. 2009), and eye tracking research similarly shows that individuals high in loneliness are more likely to first fixate on and to spend a greater proportion of their initial viewing time looking at socially threatening stimuli in a social scene, whereas individuals low in loneliness are more likely to first fixate on and spend a greater proportion of their initial viewing time looking at positive stimuli in a social scene (Bangee et al. 2014). Further evidence for the effect of perceived isolation on nonconscious processes in humans comes from cross-sectional and longitudinal research showing that loneliness predicts more fragmented sleep (Cacioppo et al. 2002a, Kurina et al. 2011). Finally, whether measured in a hospital laboratory (Cacioppo et al. 2002b) or over the course of a normal day using ambulatory procedures (Hawkley et al. 2003), loneliness is associated with elevated tonic vascular resistance—a marker of threat surveillance (Mendes et al. 2002).
These changes observed in human and animal studies support short-term self-preservation by preparing the individual to detect and defend against any potential assault as well as to identify and solicit any socially mediated resources (e.g., food, shelter, reproductive opportunities) that may become available. These effects extend beyond early developmental periods, in part through mechanisms in the adult brain that permit adaptation to the functional demands of a fluid social environment. Although the function of these physiological and behavioral adjustments may be to increase the likelihood of short-term survival, they carry long-term costs, especially when the perception of social isolation becomes chronic.
To the extent that the brain is the central organ for evaluating interpersonal relationships, the neuroendocrine system becomes an important system through which perceived social isolation may operate, at least in part, to affect morbidity and mortality. We begin with a brief description of the two major neuroendocrine axes that respond to stressors—the sympathetic adrenomedullary (SAM) axis and the hypothalamic-pituitary-adrenocortical (HPA) axis, and we examine the regulation of these axes by prefrontal and limbic regions of the central nervous system. We then summarize the human literature on the association between the perception of loneliness and neuroendocrine activity, emphasizing where possible the research designed to investigate the putative causal role of perceived isolation on neuroendocrine regulation.
Although the evidence from the human literature is suggestive, mechanistic animal studies in which adult animals are experimentally assigned to normal or socially isolated housing conditions are important for evaluating the causal effects of an individual being deprived of mutual assistance and companionship on neuroendocrine activity. We therefore also review representative animal investigations on the effects of isolation on neuroendocrine responses and briefly discuss recent literature on the impact of direct sympathetic innervation of lymphoid tissue (i.e., tissue responsible for the production of lymphocytes and antibodies). We focus on experimental studies involving adult mammals because we seek to determine the possible role of the HPA and SAM axes in the association between perceived isolation and mortality in adults. We conclude with discussions of inconsistencies in the extant literature as well as the neurobiological mechanisms that may have been conserved across phylogeny to produce the sympathetic and neuroendocrine effects of perceived social isolation. Although also pertinent, a review of the oxytocinergic system and relevant animal and human literature is beyond the scope of this article. However, interested readers may wish to consult recent reviews of oxytocin and its effects on social endocrinology and behavior (e.g., Heinrichs et al. 2009, Insel 2010, Love 2014, Olff et al. 2013, Ross & Young 2009, Taylor 2006).
THE NEUROENDOCRINE STRESS AXES
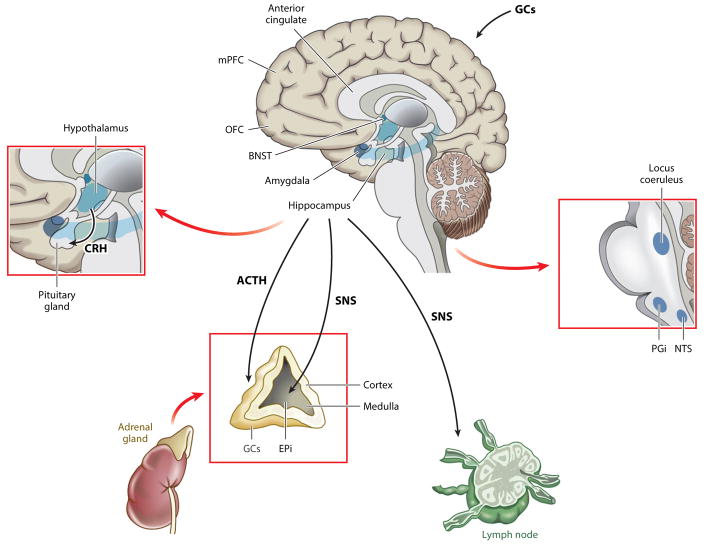
Schematics of the SAM and HPA axes are depicted in Figure 1. A cascade of signals travels from the prefrontal cortex and limbic regions (e.g., amygdala, bed nucleus of the stria terminalis) to the brain stem (e.g., locus coeruleus) and to the paraventricular nucleus of the hypothalamus. The sympathetic nervous system (SNS) includes (a) sympathetic nerve fibers that directly innervate most major organ systems and locally release the catecholamine neurotransmitter norepinephrine, and (b) an adrenal-medullary (SAM) component mediated by splanchnic nerve innervation of the chromaffin cells of the adrenal medulla, which releases catecholamines into the bloodstream. The direct innervation of the adrenal medulla by the SNS permits rapid neuroendocrine responses to acute stressors, and most of the circulating epinephrine (but only a small percentage of circulating norepinephrine) comes from the adrenal medulla (see Figure 1).
Figure 1.
Schematics of the hypothalamic-pituitary-adrenocortical (HPA) axis, the sympathetic adrenomedullary (SAM) axis, and the innervation of the lymph node tissue by the sympathetic nervous system (SNS). The HPA axis controls circulating glucocorticoid (GC) levels through a cascade that starts with signals from the prefrontal cortex [e.g., medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC)] and limbic regions [e.g., amygdala, bed nucleus stria terminalis (BNST)] to the paraventricular nucleus of the hypothalamus, which secretes corticotropin-releasing hormone (CRH) into the hypophyseal portal circulatory system. This activity stimulates the anterior pituitary to release adrenocorticotropic hormone (ACTH). ACTH travels through the blood to the adrenal cortex, where it acts on melanocortin type 2 receptors to stimulate the secretion of GC hormones (cortisol in humans and most mammals; corticosterone in rodents) into circulation. GC regulation is accomplished systemically via a negative feedback loop involving higher structures of the HPA axis (notably the hippocampus), whereby increases in circulating cortisol concentrations inhibit CRH secretion from the hypothalamus and diminish the production of ACTH in the pituitary gland by binding to glucocorticoid and mineralocorticoid receptors (GR and MR, respectively); both processes lead to a decrease in cortisol secretion from the adrenal gland. The SAM axis controls circulating epinephrine (EPi) levels. The SNS, through preganglionic neurons (the splanchnic nerve), projects from the central nervous system directly to cells in the adrenal medulla, which secretes primarily EPi (in addition to smaller amounts of norepinephrine and dopamine) into the circulatory system, where it serves to heighten metabolism and increase available energy. In addition, there is direct SNS nerve fiber delivery of norepinephrine into immune system organs such as the lymph nodes, spleen, and thymus; immune cells coordinate responses to tissue injury and infection. Artwork courtesy of Tianyi Li, adapted for publication by Annual Reviews.
The HPA axis is sensitive to the interpretation by the brain of threats and stressors, and it influences a wide range of physiological, behavioral, and health outcomes (e.g., Charmandari et al. 2005, Hostinar et al. 2014, McEwen & Gianaros 2011, Sapolsky et al. 2000). Unlike the adrenal medulla of the SAM axis, the adrenal cortex of the HPA axis is necessary for survival, and the HPA axis includes a negative feedback mechanism to limit its circulating hormonal outputs. The cascade of signals from prefrontal cortex and limbic regions to the paraventricular nucleus of the hypothalamus triggers the secretion of corticotropin-releasing hormone (CRH) into the hypophyseal portal circulatory system. CRH has hypothalamic and extrahypothalamic actions, including the promotion of the release of adrenocorticotropic hormone (ACTH) by the anterior pituitary gland into circulation (see Figure 1).
ACTH travels through the blood to the adrenal cortex, where it stimulates the secretion of glucocorticoid hormones (cortisol in humans and most mammals, corticosterone in rodents) into circulation. The vast majority of circulating cortisol is bound to large proteins (e.g., cortisol binding globulin, albumin), and only a small fraction of unbound cortisol is thought to be biologically active—that is, to be free to bind to glucocorticoid receptors. This is important because the proportion of the glucocorticoids that is biologically active differs across tissues (e.g., salivary, blood, serum, urine), which means that assays from these tissues can reflect different aspects of HPA functioning. Assays of salivary cortisol have become popular in human behavioral and biomedical research because cortisol levels measured in saliva are correlated with unbound cortisol levels in serum or plasma.
Glucocorticoids are small, lipophilic molecules that cross the blood-brain barrier, where they are involved in a number of processes including neuronal cell birth, differentiation, apoptosis, dendritic arborization, and synaptic function (McEwen & Gianaros 2011, Riedemann et al. 2010). Circulating glucocorticoids that pass through the blood-brain barrier also regulate HPA activation by acting on glucocorticoid receptors in the hippocampus (McEwen & Gianaros 2011). Specifically, the hippocampus, through inhibitory projections to the paraventricular nucleus in the hypothalamus, contributes to the maintenance of cortisol concentrations within bounds by inhibiting the secretion of CRH from the hypothalamus as well as the production of ACTH in the pituitary gland (Chrousos 2009, Hawkley et al. 2012, Hostinar et al. 2014).
Glucocorticoids are released in a pulsatile fashion across the day to regulate numerous physiological processes including energy mobilization, inflammation, reproduction, and immune functioning. The release of these glucocorticoids has a circadian rhythm, with levels highest in the morning and lowest in the evening. Significant stressors can also alter HPA activity, for instance by increasing the frequency or magnitude of the pulsatile release either transiently or chronically (thereby producing transient or chronic changes in circulating cortisol levels), altering the maximal cortisol concentrations observed approximately 30 to 45 minutes after awakening (termed the cortisol awakening response), or flattening the circadian rhythm.
A major focus in recent years has been on the environmental factors early in life that have lasting effects on HPA functioning and stress reactivity (e.g., Hostinar et al. 2014, Meaney & Szyf 2005). However, the HPA axis in adults remains responsive to metabolic needs, physiological inputs, and psychogenic stressors including social-evaluative threats (Dickerson & Kemeny 2004), and alterations of the activity of the adult HPA axis are associated with numerous deleterious psychological and physical health outcomes (Chrousos 2009, Fries et al. 2009, Gunnar & Vazquez 2001) (for an overview of gene regulation by the HPA axis in adults, see sidebar Gene Regulation by the HPA Axis).
GENE REGULATION BY THE HPA AXIS.
Glucocorticoids regulate a diverse array of physiologic processes by simultaneously altering the transcription of hundreds of genes. Following HPA axis activation, glucocorticoids circulate through the bloodstream to reach virtually every cell type in the body. Glucocorticoid molecules are small and easily diffuse across cell membranes and into the cytoplasm, where they can bind to intracellular glucocorticoid receptors (GRs). Glucocorticoid binding prompts GRs to dissociate from their resting antagonist molecules and traffic into the nucleus of the cell, where they can bind to genes that contain specific DNA sequences called glucocorticoid response elements (GREs; a typical GRE is G.ACA…TGT.C, where “…” can be any nucleotide). In many cases, GR binding to a GRE serves to flag a gene for transcription into RNA and translation into a protein that can alter cellular function. Many metabolic effects of glucocorticoids are mediated by such transcriptional induction of genes involved in glucose production. Some anti-inflammatory effects of glucocorticoids are mediated by transcriptional induction of molecules that inhibit immune responses. GR molecules can also inhibit the transcription of specific genes either by binding to their DNA sequences in locations that block access by other stimulatory molecules or by binding to stimulatory molecules in the cytoplasm and blocking their translocation to the nucleus. For example, many anti-inflammatory effects of glucocorticoids are mediated by GR antagonism of the proinflammatory transcription factors NF-κB and AP-1. GR transcriptional repression also mediates the negative feedback loop in the hypothalamus that prevents accumulation of excessive glucocorticoid levels. The combination of strong transcriptional activation of some gene sets and transcriptional repression of other gene sets allows one specific hormonal signal to influence a diverse array of biological processes in a wide range of different cell types. GR signaling is itself subject to inhibition by other cellular signaling pathways via phosphorylation of GR proteins in the cytoplasm and by transcriptional downregulation of the NR3C1gene that encodes the GR protein. These dynamics can result in a state of glucocorticoid resistance in which normal or high levels of HPA activity have little or no effect on cellular function because the GR fails to translate the hormonal stimulus into a gene transcriptional response. Several studies now suggest that social threat in general, and loneliness in particular, is associated with glucocorticoid resistance and a complementary increase in proinflammatory gene expression that may contribute to some of the adverse health outcomes associated with perceived social isolation.
Neuroendocrine outputs are regulated by brain circuits, which translate perceptual and evaluative processes into specific patterns of hormonal release. The prefrontal cortex modulates attention, working memory, conflicting inputs, and emotion regulation as well as integrates information from plans (e.g., goals) and prior knowledge, information from peripheral afferents, and information from the environment—including the social environment—to coordinate neural, hormonal, and behavioral responses (Hostinar et al. 2014, McEwen & Gianaros 2011). The prefrontal cortex also plays a role in orchestrating anticipatory neural, hormonal, and behavioral responses to minimize threats and perturbations. Environmental challenges and stressors can also increase the release of dopamine and acetylcholine in the prefrontal cortex; dopamine and acetylcholine then play a role in modulating anxiety (Berntson et al. 2003), attention, and working memory (e.g., Sarter & Bruno 1997).2
Importantly, the prefrontal cortex has extensive neuroanatomical and functional connectivity with the limbic system, which in turn permits the modulation of HPA activity by the resulting environmental appraisals, including appraisals of the quality of companionship and mutual assistance available in the social environment—a strong determinant of perceived social isolation (Hawkley et al. 2008). Within the limbic system, the central and medial nuclei of the amygdala and the bed nucleus of the stria terminalis (BNST) are connected by cells throughout the stria terminalis, and both the amygdala and the BNST project to hypothalamic and brain stem areas that mediate autonomic, neuroendocrine, and behavioral responses to aversive or threatening stimuli (Walker & Davis 2008). The BNST, like the amygdala, is composed of multiple distinct subnuclei, which differentially regulate HPA activation (Choi et al. 2007, Ulrich-Lai & Herman 2009). Connections also exist between the hippocampus and BNST; the hippocampus modulates the actions of the BNST through glutamate, whereas the amygdala acts on the BNST through CRH and gamma-aminobutyric acid (Riedemann et al. 2010).
The amygdala and the BNST are involved in fear and anxiety conditioning, respectively (Davis 1998)—two acquired behaviors that permit anticipatory responses to a potentially threatening situation. The amygdala appears to be especially important for rapid-onset, short-duration behaviors that occur in response to specific threats, whereas the BNST appears to mediate slower-onset, longer-lasting responses that frequently accompany sustained threats (or the surveillance for threats) and that may persist even after threat termination (Walker et al. 2003). Outputs from the basolateral amygdala activate medial portions of the central amygdala to rapidly elicit phasic fear responses via projections to the hypothalamus and brain stem. The basolateral amygdala also projects to the lateral portion of the BNST, which contributes to a slower-developing, more sustained response (Walker & Davis 2008). We return to this distinction of the temporal effects of the amygdala and BNST on HPA activity in the Concluding Remarks section.
NEUROENDOCRINE ACTIVITY AND PERCEIVED SOCIAL ISOLATION (LONELINESS)
The extant human research suggests that perceived social isolation (loneliness) and social threats are associated most consistently with activity of the HPA axis (cf. Dickerson et al. 2011, Hawkley et al. 2012). Some data also suggest an association between perceived social isolation and increased circulating levels of catecholamines, although the SAM findings are less numerous and consistent (e.g., Edwards et al. 2010, Hawkley et al. 2006) and may be attributable at least in part to differences in perceived stress rather than perceived isolation per se (Hawkley et al. 2006).
In an early set of studies of medical students, loneliness was found to be associated with poorer cellular immune competence, as indexed by significantly higher Epstein-Barr virus antibody titers (Glaser et al. 1985) and natural killer cell activity (Kiecolt-Glaser et al. 1984a). To investigate whether the HPA axis might be involved, Kiecolt-Glaser et al. (1984b) investigated the association between loneliness and urinary cortisol levels in newly admitted nonpsychotic psychiatric inpatients. Loneliness and stressful life events were measured by self-report, and a median split was performed on each self-report measure to divide participants into high or low groups on loneliness and high and low groups on recent stressful life events. Analyses indicated that inpatients in the high lonely group had significantly higher levels of urinary cortisol than inpatients in the low lonely group, whereas the inpatients grouped in terms of high or low levels of recent stressful life events did not differ in urinary cortisol levels. Assays of natural killer cell activity and blastogenesis (cell proliferation to the mitogen, phytohemagglutinin) were lower in the lonely than nonlonely groups, and loneliness was found to be the best predictor of these immune measures, although the correlations were low.
Subsequent investigations suggest that loneliness is typically associated with higher levels of HPA activation, although the strength of the association may vary depending on the chronicity of loneliness, the specific tissue assayed, the parameter used to gauge HPA activity, the time of day of the measurements, and the reliability (e.g., number) of the measurements. Using an experience sampling methodology, Cacioppo et al. (2000) measured salivary cortisol levels in undergraduate students at nine random points during a normal day. Results indicated that loneliness was positively correlated with salivary cortisol levels, but this association reached statistical significance only for chronic loneliness. Interestingly, the percent of time spent alone was not associated with salivary cortisol levels. Using a similar methodology at four points in time across the day, Pressman et al. (2005) similarly found loneliness to be related to salivary cortisol levels, although this association reached statistical significance only for salivary cortisol levels measured an hour after awakening and at night. Subsequent work has confirmed that the association between loneliness and overall salivary cortisol levels is generally positive but small (Edwards et al. 2010, Hawkley et al. 2006, Steptoe et al. 2004).
As mentioned above, cortisol levels are characterized by a strong basal diurnal rhythm, with levels high in the morning and typically increasing 50% to 60% in the first 30 to 45 minutes after awakening (i.e., the cortisol awakening response), dropping rapidly over the first few hours after waking, and then declining more slowly across the rest of the day until finally reaching a low point around midnight (e.g., Adam 2006). The variations in HPA activity across the day are often much larger than those found between groups or in response to quotidian stressors, making the time and conditions of measurement important considerations. Steptoe et al. (2004) reported that differences in loneliness across respondents, controlling for waking salivary cortisol value, gender, socioeconomic status, smoking, time of waking, and body mass, were associated with the cortisol awakening response, with higher levels of loneliness associated with larger cortisol increases.
Associations identified in cross-sectional studies do not address the causal role of perceived social isolation. To address this limitation, Adam and colleagues (2006) measured salivary cortisol at waking, 30 minutes after waking (the cortisol awakening response), and at bedtime, and loneliness was measured using an end-of-day diary each day for three days in a longitudinal, population-based study of older adults. Multilevel growth-curve modeling was used to estimate three HPA indices for each person: waking cortisol levels, slope from waking to bedtime, and size of the cortisol awakening response. Results averaged across the three days replicated those of Steptoe et al. (2004), showing that loneliness was related to larger cortisol awakening responses. When across-day (i.e., longitudinal) analyses were performed, loneliness predicted the size of the cortisol awakening response the following day independent of other variables such as demographic factors, nervousness, or perceived stress, whereas the cortisol awakening response did not predict the subsequent levels of loneliness. These longitudinal results were replicated in a study of high school students (Doane & Adam 2010); in addition, Doane & Adam (2010) found that momentary and daily assessments of loneliness were associated with momentary salivary cortisol levels, and trait loneliness was associated with a flattening of the diurnal cortisol rhythm.
Glucocorticoids (e.g., cortisol) influence a wide range of physiological functions that include glucose regulation, metabolism, inflammatory control, cardiovascular activity (e.g., endothelial function, atherosclerosis), cellular and humoral immunity, reproductive processes, and neurodegeneration and apoptosis. Among these effects (e.g., carbohydrate metabolism) are relatively quick-acting nongenomic effects (Borski 2000), but most are mediated by slower-acting genomic effects, where up to 20% of the expressed genome in a tissue is susceptible to the direct and indirect influences of glucocorticoids, estrogens, and androgens (Chrousos 2009, Hawkley et al. 2012). For instance, cortisol acts on the glucocorticoid receptors in leukocytes, leading to a suppression of proinflammatory gene networks [e.g., blocking of nuclear factor (NF)-κB-mediated transcription of proinflammatory cytokine genes such as IL1B, IL6, IL8, and TNF ]. Although negative feedback mechanisms in the brain operate to constrain cortisol concentrations, animal models of social disruption suggest that social factors can lead to glucocorticoid resistance in which the glucocorticoid receptor becomes less efficient in transducing endogenous glucocorticoid signals (e.g., Cole et al. 2009, Hanke et al. 2012, Pace et al. 2007, Powell et al. 2013), thereby increasing an inflammatory biology that can contribute to the development of diseases ranging from type II diabetes and atherosclerosis to neurodegeneration and tumor metastasis. Mechanistic studies have shown that the effects of social threat on glucocorticoid resistance are mediated in part by sympathetically induced alterations in immune cell production (hematopoiesis) (Hanke et al. 2012, Powell et al. 2013).
Given the association between loneliness and HPA activity, Cole (2008) investigated the extent to which loneliness was associated with glucocorticoid resistance using data from a nationally representative sample of adults ages 54 and older from Taiwan. Cortisol, through its effects on the glucocorticoid receptors in leukocytes, normally stimulates an increase in the concentrations of neutrophils and a decrease in the concentrations of lymphocytes and monocytes in circulating blood. Cole (2008) used the strength of the glucocorticoid regulation of the circulating neutrophil:lymphocyte ratio and of the circulating neutrophil:monocyte ratio as a marker for receptor functional activity in leukocytes. The rationale is that the extent to which the glucocorticoid receptors become insensitive (resistant) to glucocorticoid signals should be reflected in an attenuation of the established positive correlation between cortisol levels and the circulating neutrophil:lymphocyte and neutrophil:monocyte ratios. Cole (2008) found that loneliness was associated with smaller neutrophil:lymphocyte and neutrophil:monocyte ratios, consistent with leukocyte glucocorticoid resistance.
Research has also linked loneliness to a proinflammatory gene expression profile (see sidebar Gene Regulation by the HPA Axis). Genome-wide microarray analyses revealed a reduction in the expression of genes bearing glucocorticoid receptor response elements, an upregulation of proinflammatory gene transcripts (e.g., mRNAs encoding proinflammatory cytokines and other inflammatory mediators, and bioinformatic indications of activated NF-κB transcription factor), and a downregulation of anti-inflammatory markers (e.g., bioinformatic indications of reduced transcriptional activity of the glucocorticoid receptor) in middle- and older-age adults who are high in loneliness compared with those low in loneliness (Cole et al. 2007, 2011). A reduction in glucocorticoid receptor signaling has a permissive effect on NF-κB activation (Almawi & Melemedjian 2002), so the impaired transcription of glucocorticoid receptor–regulated genes may also indicate an upstream activation of proinflammatory transcription factors that could contribute to the increased risk of inflammatory disease in chronically lonely individuals.3
Although a significant body of human research, including longitudinal studies, suggests that perceived social isolation affects the HPA axis, inflammation, and immunity, the causal role of social isolation is difficult to test conclusively in humans. The idea that the brain is the key organ of social connections and processes should be true for other species for which sociality has been a central feature of life for millions of years. Mechanistic animal studies therefore may provide a more direct test of the causal effects of a member of a social species being deprived of companionship and mutual assistance. There is not an animal literature on loneliness per se, but there is a large literature in which social animals are randomly assigned either to normal social living conditions or to socially isolated living conditions. We turn next to this literature, specifically experimental studies of the effects of social isolation on HPA and SAM activity in adult animals. As the review shows—and paralleling the research on perceived isolation in humans—the nature of the relationship that is disrupted by isolating an animal and the duration of isolation are important influences on the neuroendocrine response to social isolation.
ANIMAL STUDIES OF NEUROENDOCRINE ACTIVITY AS A FUNCTION OF SOCIAL ISOLATION
Correlational research in adult baboons indicates that relative social isolation (i.e., negative deviations from median values on a composite measure of social connectedness) is associated with elevated levels of basal cortisol (Sapolsky et al. 1997) (see Table 1). A major advantage of using animal models is the ability to experimentally manipulate social isolation from conspecifics, controlling for other aspects of the environment (e.g., amount of space available, complexity of the environment, thermoregulation), to investigate its effects on the SAM and HPA axes. Experimental studies in animals have manipulated social isolation acutely (e.g., social isolation for one hour, sometimes repeated daily) and chronically (e.g., social isolation for days or weeks).
Table 1.
Effect of social isolation across phylogeny
| References (alphabetical order) |
Species | Age at testing |
Social isolation duration |
Sample size (per gender) of the social isolation group* |
Socially isolated from |
Sample size (per gender) of the comparison group(s) |
Primary dependent variable(s) |
Primary effect(s) of social isolation |
|---|---|---|---|---|---|---|---|---|
| Voles | ||||||||
| Bosch et al. (2009) | Prairie voles (Microtus ochrogaster) | 70–100 days | 5 days | Males | Female partner or male sibling | Paired with either unfamiliar female or male siblings for a total of 10 days | Plasma adrenocorticotropic hormone (ACTH) and corticosterone levels + corticotropin-releasing hormone (CRH) mRNA in the medial bed nucleus of the stria terminalis (mBNST) |
Corticosterone levels Social isolation from a female partner increases basal corticosterone levels
Pairing with a female increases CRH mRNA
|
| Grippo et al. (2007b) | Prairie voles (Microtus ochrogaster) | 60–90 days | 4 weeks | Experiment 1: 8 females and 8 males | Same-sex sibling | Experiment 1: 8 females and 8 males pair housed with a same-sex sibling | Experiment 1: plasma levels of oxytocin, ACTH, and CRH and corticosterone levels + c-Fos expression in hypothalamic paraventricular nucleus (PVN) |
Corticosterone levels
|
| Klein et al. (1997) | Meadow voles (Microtus pennsylvanicus) and prairie voles (Microtus ochrogaster) | 90–120 days | 28 days | Experiment 2: prairie voles: 6 males, 6 females; meadow voles: 6 males, 6 females | Same-sex littermates | Experiment 2: pair housed with same-sex conspecific (6–10 per group), or pair housed with opposite-sex conspecific (6–7 per group), or group housed with four per cage with same-sex conspecific (12 per group) or opposite-sex conspecifics (2 males, 2 females per cage; 6–8 per group) | Experiment 2: corticosterone levels |
Corticosterone levels
|
| McNeal et al. (2014) | Prairie voles (Microtus ochrogaster) | 60–90 days | 5 days | Experiment 1: 9 males Experiment 2: 10 males, 10 females |
Experiment 1: female partner Experiment 2: partner |
Experiment 1: pair-housed controls (8 males, 8 females) Experiment 2: pair-housed controls (10 males, 10 females) |
Experiment 1: resting cardiac parameters + autonomic nervous system Experiment 2: plasma ACTH and corticosterone levels |
Resting cardiac parameters (experiment 1)
|
| Pournajafi-Nazarloo & Partoo (2011) | Prairie voles (Microtus ochrogaster) | 2 months | 1 hour total (single social isolation), or 1 hour every day for 4 weeks (repeated social isolation), or 4 continuous weeks (chronic social isolation) | Males and females; 8 per group | Same-sex sibling | Handling without isolation (HAN group) or pair housed with a same-sex sibling partner; 8 per group | Plasma levels of corticosterone levels + CRH, type 1 CRH receptor (CRH-R1 mRNA) + type 2 CRH receptor (CRH-R2 mRNA) expression in the hypothalamus, hippocampus, and pituitary gland |
Corticosterone levels
|
| Stowe et al. (2005) | Experiment 1: prairie voles (Microtus ochrogaster) and meadow voles | 3–4 months | 24 hours or 2 weeks | Experiment 1: males (9 prairie, 6 meadow for the 24-hour group; 9 prairie, 6 meadow for the 2-week group) | Same-sex sibling | Experiment 1: a no-social-isolation group (no-isolation group; 9 prairie, 8 meadow) in which subjects were housed with a same-sex sibling and transferred to the testing room for 24 hours prior to being tested + a group of animals serving as controls for handling (control group; 8 prairie voles, 6 meadow voles) | Levels of plasma corticosterone + c-Fos-labeled cells were examined in brain areas involved in anxiety and social behaviors, i.e., the medial (MeA), anterior cortical (ACo), and central (CeA) subnuclei of the amygdala (AMYG); BNST (including the anterior dorsal and anterior ventral parts); lateral septum (LS) (intermediate); paraventricular nucleus (PVN); ventromedial hypothalamus (VMH); medial preoptic area (MPOA); anterior hypothalamus (aHYP); and prefrontal cortex (PFC) |
Corticosterone levels
|
| Experiment 3: prairie voles (Microtus ochrogaster) | 3–4 months | 24 hours or 2 weeks | Experiment 3: males (8 for the 24-hour group, 8 for the 2-week group) | Same-sex sibling | Experiment 3: control animals were housed with a male cage mate (n = 8) |
Levels of Fos-immunoreactivity (Fos-ir) expression
|
||
| Hamsters | ||||||||
| Castro & Matt (1997) | Siberian dwarf hamster (Phodopus sungorus) | 3 months | 4 weeks | 15 males | Female partner after 3 weeks of pair bonding | 13 pairs of mate-housed animals | Plasma cortisol and catecholamine levels |
Cortisol levels
|
| Rats | ||||||||
| Djordjevic et al. (2010) | Wistar rats | 3 months | 21 days | Males | 3 other same-sex rats | A group of unstressed animals (control group) + a group of rats exposed to an acute stress (i.e., 30-minute immobilization period) + a group exposed to a combined stress (i.e., a 21-day social isolation period followed by a 30-minute immobilization period). In each comparison group, rats were housed 4 per cage | Corticosterone and catecholamine levels + glucocorticoid receptor (GR) and nuclear factor kappa B (NF-κB) protein and mRNA expression, neural cell adhesion molecule (NCAM) mRNA, polysialylated (PSA)-NCAM protein, protein expression of Bax and Bcl-2, and DNA defragmentation in the PFC |
Corticosterone levels
|
| Dronjak & Gavrilovic (2006) | Wistar rats | 3 months | 21 days | Males | 3 other same-sex rats | A control group of 4 animals in a cage | Plasma catecholamine levels |
Catecholamine levels
|
| Dronjak et al. (2004) | Wistar rats | Adult | 21 days | 6 males | 5 other rats | A control group of 6 animals in a cage + a group of 12 animals housed per cage (crowding group) | Changes in plasma levels of ACTH, CORT, and catecholamine as well as in cytosol GR and heat shock protein 70 (Hsp70) in hippocampus |
Corticosterone levels
|
| Ferland & Schrader (2011) | Wistar rats | 56 days | Overnight social isolation for 14 days | Males | Cage mate | One group of paired rats assigned to overnight social crowding (social crowding group: 6 rats per cage) and one group of paired rats assigned to a nonstressed (control group) condition | CORT levels at 0-, 5-, 15-, 30-, or 90-minute intervals after separation from cage mate |
Corticosterone levels
|
| Garrido et al. (2012) | Wistar rats | 3 months | 12 weeks | Males | 9–11 other male rats | A group of male rats housed together (10–12 animals per cage) with two running wheels, tunnels, and different objects | Corticosterone and the mRNA levels of GRs in the PFC |
Corticosterone levels
|
| Gavrilovic et al. (2010) | Wistar rats | 11 weeks | 12 weeks | Males | 3 other male rats | Group-housed rats | Plasma epinephrine and norepinephrine |
Catecholamine levels
|
| Zlatković & Filipović (2012) | Wistar rats | 2–3 months | 21 days | Males | 3 other male rats | A group of unstressed rats + a group of rats submitted to an acute stressor (2 hours of immobilization or cold) + a group subjected to a combined stressor (social isolation followed by acute stressor) | Serum corticosterone levels; ratio of proapoptotic to antiapoptotic proteins (e.g., Bax protein/Bcl-2) and cytosolic/ mitochondrial levels in relation to cytosolic (NO) metabolites (nitrates and nitrites) and p53 protein redistribution between cytosolic and mitochondrial compartments in the PFC and hippocampus (HIPP) |
Corticosterone levels
|
| Zlatković & Filipović (2013) | Wistar rats | 2–3 months | 21 days | 6 males | 3 other male rats | A group of unstressed rats (n = 6) + a group of rats submitted to an acute stressor (2 hours of immobilization or cold, n = 6 per stressor) + a group subjected to a combined stressor (social isolation followed by acute stressor, n = 6 per stressor) | Serum corticosterone levels; Hsp70 concentrations; cytosolic and neuronal distributions of NF-κB as a transcriptional factor for inducible nitric oxide synthase (iNOS) and neuronal nitric oxide synthase (nNOS) synthesis in PFC; protein expression of cytosolic Hsp70i as a suppressor of NF-κB activation |
Corticosterone levels
|
| Nonhuman primates | ||||||||
| Cross et al. (2004) | Marmosets (Callithrix jacchus) | 5–9 years | 15 minutes | 4 males and 4 females | A group of 20 other marmosets | Relative to their preisolation cortisol levels | Salivary cortisol levels |
Cortisol levels
|
| Mendoza & Mason (1986a) | Squirrel monkeys (Saimiri) and titi monkeys (Callicebus) | Adult | 1 hour in home cage | 10 heterosexual pairs of Saimiri, seven Callicebus (5 males and 2 females), and 17 Saimiri (9 males and 8 females) | Mate for at least 39.3 months for Callicebus (range: 2 to 113) and 41.6 months for Saimiri (range: 3 to 110) | Relative to basal levels | Plasma cortisol levels |
Cortisol levels
|
| Sapolsky et al. (1997) | Yellow baboons (Papio cynocephalus) | Adult | 2 months of observation | 12 males | Relative to median values of the group | Socially connected animals | Cortisol concentrations from blood samples |
Cortisol levels
|
| Smith et al. (2011) | Geoffroy’s tufted-ear marmosets (Callithrix geoffroyi) | 3.1 years on average (SE = 0.3) | 6 to 20 weeks | 4 male and 4 female marmosets that were removed from their natal group and paired with a novel, opposite-sex conspecific after a period of social isolation (ISO-P) | Natal group (4 males and 4 females) | Natal group (4 males and 4 females) + marmosets that were removed from their natal group and immediately paired with a novel, opposite-sex conspecific (Natal-P: 2 males, 3 females) | Urinary cortisol levels |
Cortisol levels
|
| Smith & French (1997) | Wied’s black- tufted-ear marmosets (Callithrix kuhlii) | 1.2–10.2 years | 11 hours | 9 females and 7 males | Marmosets housed in family groups, or breeding pairs, or trios that had been established for at least 6 months | A group of animals (4 males and 4 females) housed in their home cage with their normal social or family group (control group) + a group of animals that were held in gloved hands for 5 minutes (handling stressor) and then socially isolated for 11 hours (H + SI group) | Urinary cortisol levels |
Cortisol levels
|
| Dogs | ||||||||
| Tuber et al. (1996) | Mongrel dogs (Canis familiaris) | 7–9 years | 4 hours of walk alone in a familiar environment (alone home condition) or in a novel environment (alone novel condition) | 3 males and 5 females | From a familiar dog they’ve known for 7–9 years (either with a same-sex peer or an opposite-sex peer) | Control condition (kennel mates were walked together in a familiar environment) + in-person novel condition (dog alone placed in a novel environment with a familiar human caretaker) | Plasma glucocorticoid levels |
Glucocorticoid levels
|
| Cows | ||||||||
| Higashiyama et al. (2009) | Shorthorn cows | 2–12 years | 3 days | 6 cows | 13 other cows | Compared to their basal-level preisolation | Urinary cortisol and catecholamine levels |
Cortisol levels
|
| Munksgaard & Simonsen (1996) | Friesian cows | Adult | 4 and 8 weeks | 6 cows | 29 other cows | A control group kept in stalls; a group deprived of lying down from 900 to 1600 and 2200 to 0500 | Plasma cortisol and ACTH levels |
Cortisol levels
|
| Rushen et al. (1999) | Holstein cows | Adult | 15 minutes of social isolation; injection with saline | 12 cows | 11 other cows | Each cow was subjected to different treatments (with a 2-day to 4-day interval) in a balanced order following a Latin square design. Control treatment condition: cow was injected with saline, then walked to the door of the room containing the isolation chamber at t = 0 minutes, but then returned to its stall for 15 minutes, after which it was again walked to the door of the room containing the isolation chamber and then returned |
Plasma cortisol levels |
Cortisol levels
|
| Sheep | ||||||||
| Parrot et al. (1988) | Wethers of the Clun Forest breed | Adult | 105 minutes. Each sheep was tested with or without mirror panel | 6 males | Group of sheep | Relative to baseline levels | Plasma cortisol levels |
Cortisol levels
|
| Goats | ||||||||
| Carbonaro et al. (1992) | Nubian and Alpine dairy goats | Adult | 30 minutes | 4 Nubian and 4 Alpine female goats | 3 other same-sex peers and then a peer with which the experimental animal was paired for 8 days prior to isolation | 4 Nubian and 4 Alpine female goats that were paired with an experimental animal | Plasma concentrations of cortisol, thyroxine (T4), triiodothyronine (T3), norepinephrine (NOR), and epinephrine (EPI) at 0 minutes (prior to isolation); 10, 20, and 30 minutes (during isolation); and 40, 50, and 60 minutes (after return to their group) |
Cortisol levels
|
Unless specified, the authors did not report the number of animals tested in their methods sections.
Research on acute social isolation shows it typically produces an acute neuroendocrine response. Studies in monogamous prairie voles, for instance, show that a single acute (e.g., one hour) or repeated acute (e.g., one hour per day for four weeks) social isolation from a group or from a same-sex sibling increases corticosterone levels (e.g., Pournajafi-Nazarloo & Partoo 2011). This finding is in line with a large body of studies describing the separation of an animal from conspecifics as a stressor (Garrido et al. 2012; Zlatković & Filipović 2012, 2013). Studies in Wistar rats provide information about the temporal dynamics of the effect of repeated acute social isolation on levels of corticosterone: Levels peak at the 5- and 15-minute intervals, then plateau through the 30-minute interval, and finally return to baseline after 90 minutes of social isolation (Ferland & Schrader 2011). Similar temporal dynamics in the effects of acute social isolation on cortisol also have been found in cows (Rushen et al. 1999) and sheep (Parrot et al. 1988) (see Table 1).
In the marmoset, acute and chronic isolation have been shown to increase levels of basal cortisol. Adult marmosets exposed to a brief 15-minute period of social isolation (Cross et al. 2004) and to 11 hours of social isolation (Smith & French 1997), relative to normally housed animals, exhibited increased cortisol levels. Prolonged social isolation (6–20 weeks) in adult Geoffroy marmosets prior to cohabitation with an opposite-sex partner, compared to the animals that had remained with their natal group prior to cohabitation, exhibited higher cortisol levels that remained elevated over the course of the 90-day cohabitation period (Smith et al. 2011).
Studies in rats similarly suggest that chronic social isolation increases corticosterone levels when experimental animals are socially isolated from a group of same-sex rats (Djordjevic et al. 2010; Dronjak et al. 2004; Garrido et al. 2012; Zlatković & Filipović 2012, 2013), but inconsistencies have also been observed (cf. Pournajafi-Nazarloo & Partoo 2011). There are two important factors to consider in this literature, however. First, most investigations use small sample sizes due to concerns about cost and animal welfare. There is a growing appreciation for an unintended consequence of small sample sizes, however. As Button et al. (2013) detail, a small sample size reduces the likelihood of detecting a true effect (due to low statistical power), increases the likelihood that the effect size of a true effect is overestimated (due to the use of p < 0.05 to identify when an effect has been “detected” and the larger sampling error associated with smaller sample sizes), and increases the likelihood that a statistically significant effect is not truly different from zero (due to differences in the base rates for tests of true and untrue effects). The predictable outcome is a literature with somewhat inconsistent results. Despite this inconsistency in statistical significance, meta-analyses of an unbiased literature nevertheless can produce a cumulative science because true causal effects should produce a more consistent pattern of findings (i.e., effect sizes) across studies than effects attributable simply to sampling error.
Second, and in line with human research indicating that the meaning of the presence or absence of a conspecific is an important determinant of the resulting HPA response, the effect of social isolation on the HPA axis in animals may not be a general effect but may depend on the social structure and dynamics of the species—that is, the brain’s interpretation of the social environment. For instance, studies in monogamous prairie voles show that animals that are chronically isolated from their pair-bonded partner show increased corticosterone levels (e.g., Bosch et al. 2009, McNeal et al. 2014) and higher corticosterone levels after a resident-intruder test (Grippo et al. 2007a), whereas prairie voles that are chronically isolated from a conspecific for whom partner preference is low (e.g., same-sex sibling) show no such increase in corticosterone levels (Bosch et al. 2009, Grippo et al. 2007b, Klein et al. 1997, Pournajafi-Nazarloo & Partoo 2011, Stowe et al. 2005). Similar effects have been found in other monogamous species, such as Siberian dwarf hamsters (Castro & Matt 1997) and nonhuman primates (Mendoza & Mason 1986a,b; Smith & French 1997).
The importance of conspecific preference is nicely illustrated in research by Mendoza & Mason (1986a,b), who tested the strength and quality of the relationship (with different measures such as social distance between cage mates and proximity within arm’s reach) among members of two species: the monogamous titi monkeys, which are known to form strong mutual pair bonds, and the polygynous squirrel monkey. Members of both species had been housed in heterosexual pairs for several months but were found to respond differently to social isolation. Following one hour of social isolation from their pair mates, the normally monogamous titi monkeys (for whom partner preference is high) showed a significant increase in plasma cortisol, whereas the normally polygynous squirrel monkeys (for whom partner preference is relatively low) did not (Mendoza & Mason 1986a).
The titi monkey and the squirrel monkey do not differ simply in terms of their HPA reactivity. The titi monkeys show elevated HPA activity when isolated from their monogamous partner, but they do not show HPA activation when separated from their infant (Mendoza & Mason 1986b). In contrast, the HPA axis in the squirrel monkeys is unresponsive to isolation from polygamous partners or adult peers (Hennessy 1986, Mendoza et al. 1992), but the separation of squirrel monkey mothers from their infant produces significant increases in plasma cortisol levels in both the mother and the infant (Coe et al. 1978, Mendoza et al. 1978, Vogt & Levine 1980).
These results are consistent with the notion that it is not the objective presence of or absence of a conspecific that determines HPA activation but rather the brain’s interpretation of the presence or absence of the conspecific. Paralleling this specific pair-bond effect, adult domesticated dogs (Canis familiaris), who show “vocalization and destructiveness immediately after their owner’s departure, intense greeting on reunion, and a persistent shadowing to maintain proximity to the owner during other times” (Tuber et al. 1996, p. 103), have reduced glucocorticoid levels in the presence of their human caretaker, even when placed in a novel environment, whereas the presence of a long-term familiar (either a same-sex or an opposite-sex) kennel mate does not reduce their stress in a novel environment (Tuber et al. 1996).
A few studies have investigated the effects of social isolation on glucocorticoid receptors. For instance, chronic social isolation from same-sex peers in rats elevates nuclear glucocorticoid protein in prefrontal cortex (Djordjevic et al. 2010), downregulates glucocorticoid receptor expression in the prefrontal cortex (Djordjevic et al. 2010), and decreases cytosolic glucocorticoid receptors in the hippocampus (Dronjak et al. 2004). Although only suggestive, these results are consistent with the hypothesis that chronic social isolation contributes to glucocorticoid resistance and a corresponding reduction in the negative feedback that constrains HPA activation.
Although most of the published research on chronic social isolation and stress hormones in adult animals has focused on the HPA axis, several studies have measured SAM activity. As in the human literature, the effects of chronic social isolation on SAM activity and plasma catecholamine levels are less consistent across studies than are the effects of chronic isolation on HPA (see Table 1). Castro & Matt (1997), for instance, studied male Siberian dwarf hamsters to investigate the effects of four weeks of social isolation from a female partner versus pair housing with the female partner on plasma cortisol, catecholamine, and testosterone levels. The isolated males showed elevated plasma cortisol levels but similar levels of epinephrine and testosterone (and lower levels of norepinephrine) compared to pair-housed males. In a study of Wistar rats, Dronjak et al. (2004) measured HPA and SAM activity to investigate the effects of three housing conditions: one animal per cage (social isolation), 6 animals per cage (normal housing), and 12 animals per cage (social crowding). Chronic social isolation increased basal levels of ACTH and corticosterone, whereas no effect of social isolation (or social crowding) was found for basal catecholamine levels. Gavrilovic and colleagues (2010), in contrast, reported increased plasma levels of epinephrine and norepinephrine in adult male Wistar rats following 12 weeks of social isolation. A study of neuroendocrine responses to acute isolation in adult female dairy goats also documented increased norepinephrine levels but no change in epinephrine or cortisol levels (Carbonaro et al. 1992). Experimentally imposed social isolation thus can have different effects in various animal models; this may be due to species- and sex-related differences in the natural social conditions of the animal populations studied and resulting differences in the contrast condition created by experimental social isolation (which can sometimes result in reduced physical activity and conspecific aggression, particularly in males) and small sample sizes.
Finally, there is evidence in the animal literature that the chronic social isolation of an adult animal from preferred partners enhances neuroendocrine responsiveness to acute stressors. Although contrary evidence exists (cf. Djordjevic et al. 2010), chronic social isolation in rodents relative to control animals has been shown to increase catecholamine (Dronjak et al. 2004; cf. Dronjak & Gavrilovic 2006) and corticosterone responses to acute stressors (Dronjak et al. 2004, Ferland & Schrader 2011, Grippo et al. 2007b).
ANIMAL AND HUMAN STUDIES IN RETROSPECT
The cumulative human and animal research suggests that perceived social isolation—that is, chronic isolation from a meaningful (e.g., pair-bonded) conspecific rather than isolation per se—is associated with increased HPA activity. Moreover, longitudinal studies in humans and experimental studies in animals indicate that perceived isolation has a causal effect on the HPA axis. Important differences are also apparent. The animal research, for instance, suggests that chronic social isolation between meaningful pairs not only elevates basal levels of glucocorticoids (see Table 1) but also tends to enhance the neuroendocrine response to an acute stressor (i.e., stress reactivity)—an effect not typically observed in the human literature. Most quotidian stressors in industrialized societies are neither extreme nor life threatening. As Sapolsky (2001) noted, people in contemporary societies are not getting their ulcers from being chased by saber-toothed tigers, they are inventing social stressors. Accordingly, the acute stressors used commonly in human studies are relatively mild (e.g., public speaking, serial subtraction) models of the stressors encountered in modern societies. In contrast, the acute stressors used in animal studies are relatively severe (e.g., two hours of immobilization simulating the collapse of a burrow, two hours in a 4°C chamber). The difference in the effects of chronic social isolation on stress reactivity in the human and animal literatures, therefore, may be attributable to the use of relatively mild acute stressors in human studies. This raises two testable hypotheses: (a) that chronic social isolation from a meaningful social partner enhances stress reactivity in an animal model for intense but not for mild acute laboratory stressors, and (b) given that exposure to extreme acute stressors in modern societies is rare for most individuals, the effects of perceived social isolation on basal HPA functioning may be more deleterious for human health and longevity than are its effects on HPA and SAM reactivity to acute stressors.
The most appropriate animal model for investigating the mechanisms underlying perceived isolation and mortality may depend not only on the nature of the relationship between conspecifics but also on the specific mechanism under scrutiny. For example, social isolation of male adult rodents is generally associated with a substantial reduction in physical activity (and attending decreases in activity-related SNS activity) and a notable decrease in fighting and other overtly aggressive behavior. Once reintroduced into social settings, isolated male rodents often display a greater propensity for dominant/aggressive behavior (Blanchard et al. 2001), which has parallels in the increased negativity/hostility profile observed in lonely individuals but possibly less so in the socially withdrawn/anxious/depressed profile observed in lonely humans (Cacioppo et al. 2006). In small rodent models, repeated social threat from an aggressive conspecific may also model important aspects of the chronic sense of social threat and hostility seen in lonely humans. The animal model for repeated social threat activates neuroendocrine responses in both the HPA and SAM axes, and it also induces proinflammatory/glucocorticoid-resistant immune dynamics (Hanke et al. 2012, Powell et al. 2013) analogous to those observed in lonely humans (Cole 2008; Cole et al. 2007, 2011). Experimental molecular studies show that the proinflammatory gene-regulation dynamics observed in mouse paradigms involving repeated social threat derive in part from catecholamine-mediated alterations in immune cell development within the bone marrow, which generates a population of glucocorticoid-resistant monocytes that are primed for hyperinflammatory responses as they subsequently circulate throughout the body (Hanke et al. 2012, Powell et al. 2013). This pattern is similar to the immunologic effects observed in lonely humans (Cole et al. 2007, 2011), but it is not observed in rodents subject to objective social isolation.
To the extent that human loneliness stems from a chronic sense of social threat and a diminished reward from social interactions (Cacioppo & Patrick 2008, Cacioppo et al. 2014), nonhuman primate models of repeated low-grade social threat may also help illuminate the neural and biological consequences of experienced isolation in humans. Several studies in rhesus macaques have shown that unstable social conditions (experimentally preventing the development of a stable social hierarchy) confer risk for greater mortality due to viral infection (Capitanio et al. 1998, Capitanio & Lerche 1998) and induce both socially anxious behavior and immunoregulatory alterations that resemble those observed in lonely humans (Sloan et al. 2007). Experimentally imposed social instability also induces SNS innervation of the lymph node tissues in which immune cells coordinate responses to tissue injury and infection (Sloan et al. 2007, 2008) even though social instability does not appear to alter circulating SAM catecholamine levels. Such observations suggest that nonhuman primate models may provide an ethologically valid context for analyzing the effects of perceived social isolation and may play an important role in identifying the most appropriate small rodent models for mechanistic investigations.
PUTATIVE UNDERLYING NEUROBIOLOGICAL MECHANISMS
The distinction between the effects of the amygdala versus the BNST on HPA activity may also be relevant to understanding how social isolation affects neuroendocrine activity and mortality in contemporary society. There is now a sizable literature in humans and animals for social buffering, including an attenuation of the sympathetic and HPA response to a stressor (Cacioppo et al. 1998, Hostinar et al. 2014). As noted above, however, social buffering has not been a particularly robust finding in human studies of the effects of perceived social isolation on autonomic and neuroendocrine activity in adults. Instead, perceived social isolation has typically been associated with changes in tonic functioning such as basal differences in sympathetic vascular tonus (as gauged by vascular resistance), cortisol awakening responses, elevated evening cortisol levels, circulating glucocorticoid levels, and decreased glucocorticoid receptor sensitivity (e.g., Cacioppo et al. 2003, Hawkley et al. 2012).
In an early test of the buffering hypothesis, cardiovascular activity was measured in healthy young adults who were high or low in loneliness prior to and during a series of laboratory stressors. Analyses revealed two main effects—higher vascular resistance in lonely than nonlonely participants and higher vascular resistance during the stressors than during the baseline—whereas the interaction did not approach significance (Cacioppo et al. 2002b). That is, there was no difference between these groups in stress reactivity. The basal differences in vascular resistance between lonely and nonlonely participants were also apparent when participants performed postural adjustments (sitting, standing; Cacioppo et al. 2002b) and during rest whether in the laboratory or during the course of a normal day (Hawkley et al. 2003). In a similar study, Steptoe et al. (2004) reported the interaction to be significant, but it held only for women and only for diastolic blood pressure, not systolic blood pressure or heart rate.
Rather than the social buffering of stressors, several studies suggest that perceived social isolation may diminish the generally salubrious effects of interacting with others. In an experience sampling study, undergraduate students were just as likely to interact with other people whether or not they felt socially isolated. For those who felt isolated, the interactions were rated as being of poorer quality and as providing less support and comfort (Hawkley et al. 2003). Importantly, the presence of others did not differentially affect the ratings of the severity of stressors for individuals who did and did not feel socially isolated; instead, social interactions, which themselves are a potential uplift and a source of pleasure for most individuals, were experienced less positively by individuals who felt socially isolated. These behavioral findings suggest that perceived social isolation may both increase surveillance for social threats and decrease the rewards that one derives from interpersonal relationships. Consistent with this idea, a functional magnetic resonance imaging study found that perceived isolation was associated with (a) stronger activity in the visual cortex in response to unpleasant social relative to unpleasant nonsocial visual stimuli and (b) weaker activity in the ventral striatal area in response to pleasant social compared to pleasant nonsocial visual stimuli (Cacioppo et al. 2009).
Both the amygdala and the BNST are involved in HPA adjustments in conditions that permit anticipatory or preparatory responses to a potentially threatening situation. The amygdala is especially important for rapid-onset, short-duration behaviors that occur in response to specific threats, whereas the BNST appears to mediate slower-onset, longer-lasting responses that frequently accompany sustained threats and that may persist even after threat termination (Walker & Davis 2008). These differences raise the possibility that the BNST plays a key role in the effects of perceived social isolation from a significant conspecific on basal HPA functioning. CRH is produced not only by neurons in the medial parvocellular region of the paraventricular nucleus of the hypothalamus but also by cells in the lateral central amygdala that release CRH into the lateral BNST (Walker & Davis 2008). The BNST, through projections to the brain stem and paraventricular nucleus of the hypothalamus, produces neuroendocrine and autonomic responses that appear as changes in relatively tonic activity.
The receptors for CRH, namely CRHR1 and CRHR2, are differentially distributed in the brain (the former are widely distributed, whereas the latter are found in only a few nuclei including the central amygdala and BNST). The anxiogenic effects of CRH are mediated by CRHR1, whereas anxiogenic and anxiolytic effects are mediated by CRHR2. The HPA axis is also under the influence of oxytocin and vasopressin, and these hormones exert opposite effects on the HPA axis, with oxytocin decreasing and vasopressin increasing HPA axis activity (De Boer et al. 2012). Given the prevalence of oxytocin receptors in the BNST, central amygdala, and paraventricular nucleus of the hypothalamus, Dabrowska et al. (2011) investigated the distribution of CRHR2 in the BNST, paraventricular nucleus, and supraoptic nucleus of the hypothalamus in relation to oxytocin, oxytocin receptors, CRH, and arginine-vasopressin. Their results indicated a reciprocal neuroanatomical relationship between CRH-containing neurons in the BNST and oxytocin-containing neurons in the hypothalamus. Moreover, the colocalization of CRHR2 and oxytocin in hypothalamic neurons and in axon terminals throughout the BNST suggests that the BNST is involved in a potential feedback loop between the hypothalamic oxytocin system and the forebrain CRH system (Dabrowska et al. 2011). How precisely this feedback loop operates is not fully known, but given the role of oxytocin in pair bonding and in suppressing HPA activity, one might posit that the presence of companionship and mutual assistance lowers HPA activation in part through its effects on the BNST and the hypothalamic oxytocin system or, conversely, that the removal from or absence of companionship and mutual assistance raises HPA activation in part through its effects on the BNST and the hypothalamic oxytocin system.
Other mechanisms, such as the development of glucocorticoid resistance, also warrant further empirical investigation. In these studies, it will be important to distinguish between the SAM neuroendocrine component of sympathetic activation (which does not seem to be consistently associated with loneliness or glucocorticoid resistance and other proinflammatory dynamics) and the effects of direct SNS nerve fiber delivery of norepinephrine into immune system organs such as spleen, lymph nodes, and thymus, and into diseased tissues such as tumors (Lutgendorf et al. 2009, 2011; Sloan et al. 2007, 2008). Studies examining systemic SAM catecholamine levels in parallel with localized SNS-derived catecholamines have found a surprising degree of discontinuity between the two (Lutgendorf et al. 2009, 2011), and social processes appear to be much more strongly related to the latter (as are immunobiological alterations in animal models; Sloan et al. 2007).
CONCLUDING REMARKS
Social isolation has been recognized as a major risk factor for morbidity and mortality in humans for more than a quarter of a century. The brain is the key organ of social connections and processes, however, and the same objective social relationship can be experienced as caring and protective or as exploitive and isolating. The extant evidence indicates that the perception of social isolation (i.e., loneliness) is also a risk factor for broad-based morbidity (both physical and psychological) and mortality. However, the causal role of loneliness on neural and neuroendocrine mechanisms is difficult to test conclusively in humans. Mechanistic animal studies provide a means to evaluate the effects of social isolation on the HPA axis, autonomic functioning, and SAM axis. Adult animal studies of the effects of social isolation on HPA and SAM activity are reminiscent of two findings in the human literature: (a) chronic social isolation is associated with relatively consistent increases in HPA axis activity but little alteration in SAM catecholamine activity, and (b) the effects of chronic social isolation appear to be more dependent on the disruption of a social bond between a significant social pair (e.g., as indexed by behavioral measures of partner preference in animals or rated quality of relationships in humans) than isolation from others per se. The experimental research in adult animals further demonstrates that social isolation can have a causal effect on neuroendocrine functioning.
The incredible complexity of social life within and across species, the plethora of brain mechanisms needed to make sense of and respond to an ever-changing social world, and the still nascent level of understanding of the social brain underscore the importance of integrating human and animal research to determine which specific animals and paradigms are best for modeling a specific process or mechanism and delineating the pathways through which social relationships, or their absence, impact health and longevity. Experimental animal models of repeated social threat (but not chronic social isolation) have been found to generate immunobiological dynamics that resemble those observed in lonely human beings and thus may provide an experimental framework in which to analyze the increased risk of inflammation-related diseases observed in the human social epidemiology of loneliness. In these studies, functional alterations in the HPA axis (glucocorticoid resistance) and the SNS (innervation of immune system organs regulating leukocyte development) interact to promote a proinflammatory “defensive regime” in gene expression that ultimately increases the risk of chronic illnesses such as cardiovascular, neurodegenerative, and neoplastic diseases while simultaneously undermining resistance to viral infections. The correspondence of the behavioral, neurobiological, and genomic effects of repeated social threat in animals and those of human loneliness suggests that it may be important for future studies to define more precisely the specific brain dynamics and the specific cognitive processes that are most engaged by perceived social isolation. To date it is clear that a full understanding of the core psychological and biological features of human loneliness requires a consideration of the brain’s interpretation of the social environment.
Acknowledgments
Preparation of this article was supported by the National Institute on Aging Grant No. R37-AG033590 and by Department of the Army Award #W81XWH-11-2-0114. We wish to express our gratitude to Richard Suzman for his feedback and support.
Footnotes
Ethnic differences in loneliness tend to be attributable primarily to differences in socioeconomic status, and the (inverse) association between income and loneliness is explicable in terms of marital status, with loneliness lower and family income higher in married than unmarried individuals (cf. Hawkley et al. 2008).
Vagal afferents convey visceral information to the nucleus tractus solitarus, the major visceral relay nucleus of the brain stem (cf. Berntson et al. 2003). The nucleus tractus solitarus issues a direct noradrenergic projection to forebrain areas such as the amygdala, and via an excitatory input to the paragigantocellularis can also activate the ascending noradrenergic system arising in the locus coeruleus (Figure 1). The locus coeruleus, in turn, projects to the basal forebrain cholinergic system as well as to the amygdala and cortex. Thus, there are noradrenergic and cholinergic projections through which afferent information can impact appraisals of environmental circumstances, stimuli, and events (Berntson et al. 2003). Norepinephrine is principally synthesized in the brain in the locus coeruleus and—in addition to serotonin released from the raphe nuclei and dopamine from the ventral tegmental area, nucleus accumbens, striatum, and substantia nigra—has modulatory effects on the cortical and limbic regions involved in the control of the HPA axis (Riedemann et al. 2010).
Although not the only factor in the activation of NF-κB, glucocorticoids do play a key role. NF-κB is normally sequestered in the cytoplasm by inhibitory protein IκB. Glucocorticoids induce the activation of IκB. NF-κB can also be activated by cytokines [e.g., tumor necrosis factor (TNF)-alpha and interleukin (IL)-1] and microbial and viral infections. These immune challenges activate IκB kinases, which in turn phosphorylate IκB. Phosphorylation of IκB releases a nuclear localization signal on NF-κB, and once NF-κB is in the nucleus, it actively stimulates the transcription of proinflammatory genes encoding cytokines, cell adhesion molecules, antimicrobial molecules, and cell death mediators.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Stephanie Cacioppo, Email: Cacioppo@uchicago.edu.
John P. Capitanio, Email: jpcapitanio@ucdavis.edu.
Steven W. Cole, Email: Coles@ucla.edu.
LITERATURE CITED
- Adam EK. Transactions among trait and state emotion and adolescent diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–79. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci USA. 2006;103:17058–63. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almawi WY, Melemedjian OK. Negative regulation of nuclear factor-κB activation and function by glucocorticoids. J Mol Endocrinol. 2002;28:69–78. doi: 10.1677/jme.0.0280069. [DOI] [PubMed] [Google Scholar]
- Bangee M, Harris RA, Bridges N, Rotenberg KJ, Qualter P. Loneliness and attention to social threat in young adults: findings from an eye tracker study. Personal Individ Differ. 2014;63:16–23. [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. Eur J Neurosci. 2003;18:2103–9. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Feldman Barrett L, Dickerson BC. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J Neurosci. 2012;32:14729–41. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–71. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Borski RJ. Nongenomic membrane actions of glucocorticoids in vertebrates. Trends Endocrinol Metab. 2000;11:427–36. doi: 10.1016/s1043-2760(00)00325-8. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–15. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–76. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Social psychological contributions to the decade of the brain: doctrine of multilevel analysis. Am Psychol. 1992;47:1019–28. doi: 10.1037//0003-066x.47.8.1019. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, et al. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Ann N Y Acad Sci. 1998;840:664–73. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Norris CJ, Gollan JK. The evaluative space model. In: Van Lange P, Kruglanski A, Higgins ET, editors. Handbook of Theories of Social Psychology. Vol. 1. Thousand Oaks, CA: Sage; 2012. pp. 50–72. [Google Scholar]
- Cacioppo JT, Cacioppo S, Boomsma D. Evolutionary mechanisms for loneliness. Cogn Emot. 2014;28:3–21. doi: 10.1080/02699931.2013.837379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Ernst JM, Burleson MH, McClintock MK, Malarkey WB, et al. Lonely traits and concomitant physiological processes: the MacArthur social neuroscience studies. Int J Psychophysiol. 2000;35:143–54. doi: 10.1016/s0167-8760(99)00049-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn Sci. 2009;13:447–54. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG. The anatomy of loneliness. Curr Dir Psychol Sci. 2003;12:71–74. [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG, Ernst JM, Gibbs AC, et al. Lonely days invade the nights: social modulation of sleep efficiency. Psychol Sci. 2002a;13:384–87. doi: 10.1111/1467-9280.00469. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, et al. Loneliness and health: potential mechanisms. Psychosom Med. 2002b;64:407–17. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Ernst JM, Burleson M, Berntson GG, et al. Loneliness within a nomological net: an evolutionary perspective. J Res Personal. 2006;40:1054–85. [Google Scholar]
- Cacioppo JT, Norris CJ, Decety J, Monteleone G, Nusbaum H. In the eye of the beholder: Individual differences in perceived social isolation predict regional brain activation to social stimuli. J Cogn Neurosci. 2009;21:83–92. doi: 10.1162/jocn.2009.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Patrick B. Loneliness: Human Nature and the Need for Social Connection. New York: Norton; 2008. [Google Scholar]
- Cacioppo S, Frum C, Asp E, Weiss R, Lewis JW, Cacioppo JT. A quantitative meta-analysis of functional imaging studies of social rejection. Sci Rep. 2013;3:2027. doi: 10.1038/srep02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Lerche NW. Social separation, housing relocation, and survival in simian AIDS: a retrospective analysis. Psychosom Med. 1998;60:235–44. doi: 10.1097/00006842-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW, Mason WA. Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proc Natl Acad Sci. 1998;95:4714–19. doi: 10.1073/pnas.95.8.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro DA, Friend TH, Dellmeier GR, Nuti LC. Behavioral and physiological responses of dairy goats to isolation. Physiol Behav. 1992;51:297–301. doi: 10.1016/0031-9384(92)90144-q. [DOI] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Moffitt TE, Milne BJ, Poulton R. Socially isolated children 20 years later: risk of cardiovascular disease. Arch Pediatr Adolesc Med. 2006;160(8):805–11. doi: 10.1001/archpedi.160.8.805. [DOI] [PubMed] [Google Scholar]
- Castro WL, Matt KS. Neuroendocrine correlates of separation stress in the Siberian dwarf hamster (Phodopus sungorus) Physiol Behav. 1997;61:477–84. doi: 10.1016/s0031-9384(96)00456-8. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–84. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–34. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–81. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Coe CL, Mendoza SP, Smotherman WP, Levine S. Mother-infant attachment in the squirrel monkey: adrenal response to separation. Behav Biol. 1978;22:256–63. doi: 10.1016/s0091-6773(78)92305-2. [DOI] [PubMed] [Google Scholar]
- Cole SW. Social regulation of leukocyte homeostasis: the role of glucocorticoid sensitivity. Brain Behav Immun. 2008;22:1049–65. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JMG, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci USA. 2011;108:3080–85. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Mendoza SP, Capitanio JP. Social stress desensitizes lymphocytes to regulation by endogenous glucocorticoids: insights from in vivo cell trafficking dynamics in rhesus macaques. Psychosom Med. 2009;71:591–97. doi: 10.1097/PSY.0b013e3181aa95a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross N, Pines MK, Rogers LJ. Saliva sampling to assess cortisol levels in unrestrained common marmosets and the effect of behavioral stress. Am J Primatol. 2004;62:107–14. doi: 10.1002/ajp.20005. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo J, McDonald AJ, et al. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and bed nucleus of the stria terminalis of the rat: implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–26. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–47. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- De Boer A, Van Buel EM, Ter Horst GJ. Love is more than just a kiss: a neurobiological perspective on love and affection. Neuroscience. 2012;201:114–24. doi: 10.1016/j.neuroscience.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. Physiological effects of social threat: implications for health. In: Decety J, Cacioppo JT, editors. Handbook of Social Neuroscience. New York: Oxford Univ. Press; 2011. pp. 787–803. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Distel MA, Rebollo-Mesa I, Abdellaoui A, Derom CA, Willemsen G, et al. Familial resemblance for loneliness. Behav Genet. 2010;40:480–94. doi: 10.1007/s10519-010-9341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic A, Adzic M, Djordjevic J, Radojcic M. Chronic social isolation suppresses proplastic response and promotes proapoptotic signaling in prefrontal cortex of Wistar rats. J Neurosci Res. 2010;88:2524–33. doi: 10.1002/jnr.22403. [DOI] [PubMed] [Google Scholar]
- Doane LD, Adam EK. Loneliness and cortisol: momentary, day-to-day, and trait associations. Psychoneuroendocrinology. 2010;35:430–41. doi: 10.1016/j.psyneuen.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronjak S, Gavrilović L. Effects of stress on catecholamine stores in central and peripheral tissues of long-term socially isolated rats. Braz J Med Biol Res. 2006;39:785–90. doi: 10.1590/s0100-879x2006000600011. [DOI] [PubMed] [Google Scholar]
- Dronjak S, Gavrilović L, Filipović D, Radojčić MB. Immobilization and cold stress affect sympatho-adrenomedullary system and pituitary-adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol Behav. 2004;81:409–15. doi: 10.1016/j.physbeh.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Edwards KM, Bosch JA, Engeland CG, Cacioppo JT, Marucha PT. Elevated macrophage migration inhibitory factor (MIF) is associated with depressive symptoms, blunted cortisol reactivity to acute stress, and lowered morning cortisol. Brain Behav Immun. 2010;24:1202–8. doi: 10.1016/j.bbi.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–74. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Ferland CL, Schrader LA. Cage mate separation in pair-housed male rats evokes an acute stress corticosterone response. Neurosci Lett. 2011;489:154–58. doi: 10.1016/j.neulet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Garrido P, De Blas M, Ronzoni G, Cordero I, Antón M, et al. Differential effects of environmental enrichment and isolation housing on the hormonal and neurochemical responses to stress in the prefrontal cortex of the adult rat: relationship to working and emotional memories. J Neural Transm. 2012;120:829–43. doi: 10.1007/s00702-012-0935-3. [DOI] [PubMed] [Google Scholar]
- Gavrilovic L, Spasojevic N, Dronjak S. Chronic individual housing-induced stress decreased expression of catecholamine biosynthetic enzyme genes and proteins in spleen of adult rats. Neuroimmunomodulation. 2010;17:265–69. doi: 10.1159/000290042. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. J Behav Med. 1985;8:249–60. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007a;69:149–57. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, et al. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007b;32:966–80. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Ihm E, Wardwell J, McNeal N, Scotti ML, et al. The effects of environmental enrichment on depressive and anxiety-relevant behaviors in socially isolated prairie voles. Psychosom Med. 2014;76:277–84. doi: 10.1097/PSY.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Low cortisol and a flattening of expected daytime rhythm: potential índices of risk in human development. Dev Psychopathol. 2001;13:515–38. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. 2012;26:1150–59. doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Burleson MH, Berntson GG, Cacioppo JT. Loneliness in everyday life: cardiovascular activity, psychosocial context, and health behaviors. J Personal Soc Psychol. 2003;85:105–20. doi: 10.1037/0022-3514.85.1.105. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cole SW, Capitanio JP, Norman GJ, Cacioppo JT. Effects of social isolation on glucocorticoid regulation in social mammals. Horm Behav. 2012;62:314–23. doi: 10.1016/j.yhbeh.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Hughes ME, Waite LJ, Masi CM, Thisted RA, Cacioppo JT. From social structure factors to perceptions of relationship quality and loneliness: the Chicago Health, Aging, and Social Relations Study. J Gerontol Soc Sci. 2008;63B:S375–84. doi: 10.1093/geronb/63.6.s375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol Aging. 2006;21:152–64. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Thisted RA, Cacioppo JT. Loneliness predicts reduced physical activity: cross-sectional and longitudinal analyses. Health Psychol. 2009;28:354–63. doi: 10.1037/a0014400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–57. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Effects of social partners on pituitary-adrenal activity during novelty exposure in adult female squirrel monkeys. Physiol Behav. 1986;38:803–7. doi: 10.1016/0031-9384(86)90046-6. [DOI] [PubMed] [Google Scholar]
- Higashiyama Y, Nashiki M, Narita H. Urinary catecholamine and cortisol responses of Japanese Shorthorn cows to social isolation. Asian-Aust J Anim Sci. 2009;22:1437–40. [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLOS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140:256–82. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS. Social isolation kills, but how and why? Psychosom Med. 2001;63:273–74. doi: 10.1097/00006842-200103000-00011. [DOI] [PubMed] [Google Scholar]
- House JS, Landis K, Umberson D. Social relationships and health. Science. 1988;241:540–45. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Insel T. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–79. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou CC, Guttal V, Couzin ID. Predatory fish select for coordinated collective motion in virtual prey. Science. 2012;337(6099):1212–15. doi: 10.1126/science.1218919. [DOI] [PubMed] [Google Scholar]
- Jones WH, Freemon JE, Goswick RA. The persistence of loneliness: self and other determinants. J Personal. 1981;49:27–48. [Google Scholar]
- Kaushal N, Nair D, Gozal D, Ramesh V. Socially isolated mice exhibit a blunted homeostatic sleep response to acute sleep deprivation compared to socially paired mice. Brain Res. 2012;1454:65–79. doi: 10.1016/j.brainres.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Garner W, Speicher CE, Penn GM, Holliday JE, Glaser R. Psychosocial modifiers of immunocompetence in medical students. Psychosom Med. 1984a;46:7–14. doi: 10.1097/00006842-198401000-00003. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Ricker D, George J, Messick G, Speicher CE, et al. Urinary cortisol levels, cellular immunocompetency, and loneliness in psychiatric inpatients. Psychosom Med. 1984b;46:15–23. doi: 10.1097/00006842-198401000-00004. [DOI] [PubMed] [Google Scholar]
- Klein SL, Hairston JE, DeVries AC, Nelson RJ. Social environment and steroid hormones affect species and sex differences in immune function among voles. Horm Behav. 1997;32:30–39. doi: 10.1006/hbeh.1997.1402. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol. 2012;89:273–76. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurina LM, Knutson KL, Hawkley LC, Cacioppo JT, Lauderdale DS, Ober C. Loneliness is associated with sleep fragmentation in a communal society. Sleep. 2011;34:1519–26. doi: 10.5665/sleep.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love TM. Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav. 2014;119:49–60. doi: 10.1016/j.pbb.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Hawkley LC, Waite LJ, Cacioppo JT. Loneliness, health, and mortality in old age: a national longitudinal study. Soc Sci Med. 2012;74:907–14. doi: 10.1016/j.socscimed.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Waite LJ. Loneliness and mortality among older adults in China. J Gerontol B Psychol Sci Soc Sci. 2014;69:633–45. doi: 10.1093/geronb/gbu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun. 2011;25:250–55. doi: 10.1016/j.bbi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, DeGeest K, Sung CY, Arevalo JM, Penedo F, et al. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun. 2009;23:176–83. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–23. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–45. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Scotti ML, Wardwell J, Chandler DL, Bates SL, et al. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton Neurosci. 2014;180:9–16. doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB, Blascovich J, Lickel B, Hunter S. Challenge and threat during social interactions with white and black men. Personal Soc Psychol Bull. 2002;28:939–52. [Google Scholar]
- Mendoza SP, Hennessy MB, Lyons DM. Distinct immediate and prolonged effects of separation on plasma cortisol levels in adult female squirrel monkeys. Psychobiology. 1992;20:300–6. [Google Scholar]
- Mendoza SP, Mason WA. Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiol Behav. 1986a;38:795–801. doi: 10.1016/0031-9384(86)90045-4. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Parental division of labour and differentiation of attachments in a monogamous primate (Callicebus moloch) Anim Behav. 1986b;34:1336–47. [Google Scholar]
- Mendoza SP, Smotherman WP, Miner MT, Kaplan J, Levine S. Pituitary-adrenal response to separation in mother and infant squirrel monkeys. Dev Psychobiol. 1978;11:169–75. doi: 10.1002/dev.420110209. [DOI] [PubMed] [Google Scholar]
- Munksgaard L, Simonsen HB. Behavioral and pituitary adrenal-axis responses of dairy cows to social isolation and deprivation of lying down. J Anim Sci. 1996;74:769–78. doi: 10.2527/1996.744769x. [DOI] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, et al. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–94. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Pace TW, Hu F, Miller A. Cytokine-effects on glucocorticoid receptor function: relevance of glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrot RF, Houpt KA, Mission BH. Modification of the responses of sheep to isolation stress by the use of mirror panels. Appl Anim Behav Sci. 1988;19:331–38. [Google Scholar]
- Patterson AC, Veenstra G. Loneliness and risk of mortality: a longitudinal investigation in Alameda County, California. Soc Sci Med. 2010;71:181–86. doi: 10.1016/j.socscimed.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Penninx BWJH, van Tiburg T, Kriegsman DMW, Deeg DJH, Boeke AJP, van Eijk JTM. Effects of social support and personal coping resources on mortality in older age: the Longitudinal Aging Study Amsterdam. Am J Epidemiol. 1997;146:510–19. doi: 10.1093/oxfordjournals.aje.a009305. [DOI] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Partoo L. Effects of social isolation on mRNA expression for corticotrophin-releasing hormone receptors in prairie voles. Psychoneuroendocrinology. 2011;36:780–89. doi: 10.1016/j.psyneuen.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110:16574–79. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychol. 2005;24:297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- Riedemann T, Patchev AV, Cho K, Almeida O. Corticosteroids: way upstream. Mol Brain. 2010;3:2. doi: 10.1186/1756-6606-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–47. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushen J, Boissy A, Terlouw EM, de Passillé AM. Opioid peptides and behavioral and physiological responses of dairy cows to social isolation in unfamiliar surroundings. J Anim Sci. 1999;77:2918–24. doi: 10.2527/1999.77112918x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, neurodegeneration and individual differences. Lecture at Washington State Univ; 2001. Oct 10, http://en.wikiquote.org/wiki/Robert_Sapolsky. [Google Scholar]
- Sapolsky RM, Alberts SC, Altmann J. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch Gen Psychiatry. 1997;54:1137–43. doi: 10.1001/archpsyc.1997.01830240097014. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. Am J Health Promot. 2000;14:362–70. doi: 10.4278/0890-1171-14.6.362. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Capitanio JP, Cole SW. Stress-induced remodeling of lymphoid innervation. Brain Behav Immun. 2008;22:15–21. doi: 10.1016/j.bbi.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J Neurosci. 2007;27:8857–65. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Birnie AK, French JA. Social isolation affects partner-directed social behavior and cortisol during pair formation in marmosets, Callithrix geoffroyi. Physiol Behav. 2011;104:955–61. doi: 10.1016/j.physbeh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys (Callithrix kuhli) Physiol Behav. 1997;62:225–32. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Stowe J, Liu Y, Curtis JT, Freeman ME, Wang Z. Species differences in anxiety-related responses in male prairie and meadow voles: the effects of social isolation. Physiol Behav. 2005;86:369–78. doi: 10.1016/j.physbeh.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Tend and befriend: biobehavioral bases of affiliation under stress. Curr Dir Psychol Sci. 2006;15:273–77. [Google Scholar]
- Tuber DS, Sanders S, Hennessy MB, Miller JA. Behavioral and glucocorticoid responses of adult domestic dogs (Canis familiaris) to companionship and social separation. J Comp Psychol. 1996;110:103–8. doi: 10.1037/0735-7036.110.1.103. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D. Family status and health behaviors: social control as a dimension of social integration. J Health Soc Behav. 1987;28:306–19. [PubMed] [Google Scholar]
- Vogt JL, Levine S. Response of mother and infant squirrel monkeys to separation and disturbance. Physiol Behav. 1980;24:829–32. doi: 10.1016/0031-9384(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Weiss RS. Loneliness: The Experience of Emotional and Social Isolation. Cambridge, MA: MIT Press; 1973. [Google Scholar]
- Yamada M, Decety J. Unconscious affective processing and empathy: an investigation of subliminal priming on the detection of painful facial expressions. Pain. 2009;143:71–75. doi: 10.1016/j.pain.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Zlatković J, Filipović D. Bax and B-cell-lymphoma 2 mediate proaptotic signaling following chronic isolation stress in rat brain. Neuroscience. 2012;223:238–45. doi: 10.1016/j.neuroscience.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Zlatković J, Filipović D. Chronic social isolation induces NF-κB activation and upregulation of iNOS protein expression in rat prefrontal cortex. Neurochem Int. 2013;63:172–79. doi: 10.1016/j.neuint.2013.06.002. [DOI] [PubMed] [Google Scholar]