Abstract
Social isolation has been recognized as a major risk factor for morbidity and mortality in humans for more than a quarter century. The brain is the key organ of social connections and processes, however, and the same objective social relationship can be experienced as caring and protective or as exploitive and isolating. We review evidence that the perception of social isolation (i.e., loneliness) impacts brain and behavior and is a risk factor for broad-based morbidity and mortality. However, the causal role of loneliness on neural mechanisms and mortality is difficult to test conclusively in humans. Mechanistic animal studies provide a lens through which to evaluate the neurological effects of a member of a social species living chronically on the social perimeter. Experimental studies show that social isolation produces significant changes in brain structures and processes in adult social animals. These effects are not uniform across the brain or across species but instead are most evident in brain regions that reflect differences in the functional demands of solitary versus social living for a particular species. The human and animal literatures have developed independently, however, and significant gaps also exist. The current review underscores the importance of integrating human and animal research to delineate the mechanisms through which social relationships impact the brain, health, and well-being.
Keywords: social isolation, brain, animal models, loneliness, social neuroscience
Just a kind of a nightmare that your mind manufactured for you. You see we can feed the stomach with concentrates. We can supply microfilm for reading, recreation, even movies of a sort. We can pump oxygen in and waste material out, but there’s one thing we can’t simulate. That’s a very basic need. Man’s hunger for companionship. The barrier of loneliness. That’s one thing we haven’t licked yet.
— The Twilight Zone (Serling & Stevens, 1959)
For more than a quarter century, epidemiological studies have noted an association between objective measures of social isolation—typically operationalized as being unmarried, having less than monthly contact with friends and family, and/or having no participation in organizations, clubs, or religious groups—and health outcomes (e.g., House, Landis, & Umberson, 1988). The most common explanation for this association is the social control hypothesis, which posits that interactions with friends, family, and congregations incline better health behavior, which in turn decreases risks for morbidity and mortality.
Investigators in psychology and the neurosciences over the past 15 years have also addressed the association between social isolation and health. In addition to examining objective measures of isolation, as in the epidemiological literature, investigators in these areas have typically measured isolation in terms of the person’s perceptions of being socially isolated. The notion underlying this approach is that the brain is the key organ of social connections and processes (Figure 1A), and the same objective relationship (e.g., sibling, spouse) can be experienced as caring and protective or as callous and threatening (J. T. Cacioppo et al., 2000). Consistent with this notion, the number and frequency of contacts with others is not as important a predictor of feeling isolated as the quality of the social relationships (Hawkley et al., 2008; Wheeler, Reis, & Nezlek, 1983). Physical/objective social isolation can contribute to perceived social isolation/loneliness, but individuals can feel lonely in a marriage, friendship, family, or congregation. In addition to the nonsalutary nature of some relationships, the consequences of objective and perceived social isolation can differ due to individual differences in the extent to which individuals choose to form and maintain social relationships—variations that have often been analyzed in terms of broad personality traits, such as introversion (J. T. Cacioppo, Hawkley, et al., 2006). However, introversion rarely emerges as a strong risk factor for individual outcomes such as broad-based morbidity or mortality; rather, the most toxic mental and physical health effects were found to be associated with perceived isolation (i.e., loneliness; e.g., J. T. Cacioppo, Hawkley, et al., 2006; Holwerda et al., 2012; Wilson et al., 2007). Whereas introversion refers to the preference for low levels of social involvement (Eysenck, 1947), loneliness refers to the perception that one’s social relationships are inadequate in light of one’s preferences for social involvement (Weiss, 1973).
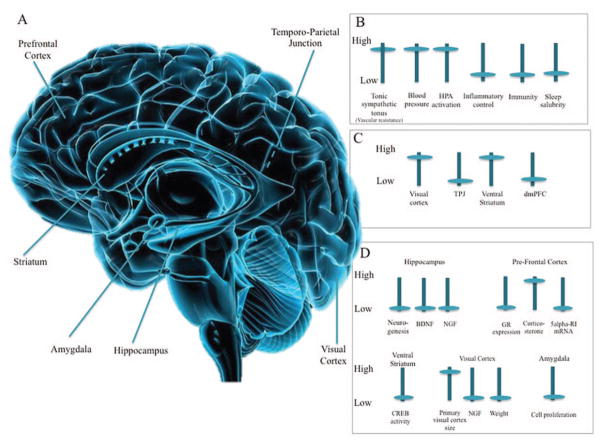
Figure 1.
Schematic representation of socially isolated brain. A: Anatomy of the isolated human brain. Sagittal view of the human brain. Main human brain areas shown to be associated with perceived social isolation (loneliness) are labeled. B: Effects of perceived social isolation on biology. Perceived social isolation (loneliness) in humans is associated with higher tonic vascular resistance, blood pressure and hypothalamic pituitary adrenocortical (HPA) activation, and lower inflammatory control, immunity, and sleep salubrity. C: Effects of perceived social isolation on human brain activation. When lonely, compared to nonlonely, individuals view unpleasant pictures of people versus objects, they show higher activation of the visual cortex and lower activation of temporo-parietal junction (TPJ). Lonely individuals also show increased activation of the ventral striatum to pleasant objects than pleasant people, while nonlonely individuals show the reverse pattern (i.e., stronger activation of the ventral striatum to pleasant people than objects). Decreased activation in the dorsomedial prefrontal cortex (dmPFC) is also observed when participants in a lonely condition view negative social scenes. D: Effects of social isolation on brain structures and mechanisms (based on animal models). Animal studies permit more invasive biological measures and manipulations. Animal studies of social isolation indicate low neurogenesis, brain-derived neurotrophic factor (BDNF), and nerve growth factor (NGF) in hippocampus; low glucocorticoid receptor (GR) expression and 5alpha RI mRNS, and high corticosterone levels in the prefrontal cortex; low cAMP response element binding protein (CREB) in the ventral striatum; large size of the primary visual cortex, and low NGF and weight of the visual cortex; and low cell proliferation in the amygdala (see Table 1 for information on these animal models). Copyright © Hank Grebe/VisualPhotos.
The causal role of loneliness on neural mechanisms and mortality is difficult to test conclusively in humans. Mechanistic animal studies are needed to evaluate the causal effects of being a member of a social species living chronically on the social perimeter, deprived of mutual assistance and companionship. There is no animal literature on perceived social isolation (loneliness) per se, but there is a large literature in which social animals are randomly assigned to either normal social living conditions or socially isolated living conditions. Our goal here is to review the literature to determine the association between loneliness, brain structures, brain processes, and mortality in humans and the experimental effects of social isolation in adult animals on brain structures and processes. The focus on the literature on adult animals is in accord with the focus in the human research on loneliness as a risk factor for morbidity and mortality in adults.
Loneliness (Perceived Social Isolation) as a Risk Factor for Morbidity and Mortality
Higher rates of morbidity and mortality in lonely than in non-lonely older adults have been reported by a number of investigators (e.g., Caspi, Harrington, Moffitt, Milne, & Poulton, 2006; Eaker, Pinsky, & Castelli, 1992; Holt-Lunstad, Smith, & Layton, 2010; Luo, Hawkley, Waite, & Cacioppo, 2012; Olsen, Olsen, Gunner-Svensson, & Waldstrom, 1991; Patterson & Veenstra, 2010; Perissinotto, Stijacic Cenzer, & Covinsky, 2012; Seeman, 2000; Thurston & Kubzansky, 2009). In 2010, a meta-analysis revealed that the odds ratio for increased mortality for loneliness is 1.45, which is approximately double the odds ratio for increased mortality for obesity and quadruple the odds ratio for air pollution (Holt-Lunstad et al., 2010). In a U.S. nationally representative sample of 2,101 adults aged 50 years and over from the 2002 to 2008 waves of the Health and Retirement Study (HRS), for instance, Luo et al. (2012) estimated the effect of loneliness at one time point on mortality over the subsequent 6 years and investigated social relationships, health behaviors, and morbidity as potential mechanisms through which loneliness affects mortality risk among older Americans. Results showed that loneliness was associated with increased mortality risk over a 6-year period and that neither health behaviors nor objective features of social relationships (e.g., marital status, proximity to friends or family) could explain the association between loneliness and mortality.
In a population-based longitudinal investigation of one especially important health behavior, physical activity, the social control hypothesis was again found to be insufficient to explain the results (Hawkley, Thisted, & Cacioppo, 2009). Specifically, declines in physical activity were predicted by loneliness, but this association was mediated by the effects of loneliness on executive functioning rather than by social control, again suggesting that neural processes may change as a function of feeling like one is on the social perimeter. This is not to mitigate the substantial effects of health behaviors on mortality, which were evident in the study by Luo et al. (2012) as well. Contrary to the social control hypothesis, however, this research indicates that the effects of loneliness on mortality are not mediated by differences in health behaviors (Luo et al., 2012; see also Seeman, 2000).
Several studies also indicate that loneliness is a risk factor for cognitive decline (Gow, Pattie, Whiteman, Whalley, & Deary, 2007) and dementia (Tilvis et al., 2004; Wilson et al., 2007). For instance, Gow et al. (2007) investigated the correlates of changes in mental ability of 488 individuals from the Lothian Birth Cohort Study who were tested at ages 11 and 79. Among the variables tested were loneliness, social support, and objective social isolation, the last measured using a social network index (e.g., presence of significant others, number of significant others). After controlling for age, IQ, gender, years of education, and social class, only loneliness was associated significantly with changes in IQ. However, Gow et al. did not address the possibility that loneliness is a consequence rather than a predictor of cognitive decline.
Two recent longitudinal studies do speak to this question. Tilvis et al. (2004) measured cognition by the Mini-Mental State Examination and the Clinical Dementia Rating at baseline and at 1-, 5-, and 10-year assessments of a population-based sample of 75- to 85-year-old individuals. Results at the 10-year follow-up assessment revealed that two biological measures and loneliness independently predicted cognitive decline. In a larger prospective study, Wilson et al. (2007) assessed 823 older adults free of dementia at enrollment. Participants completed an extensive battery of cognitive measures to assess global cognition, episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability. The lonelier the participants were, the poorer their cognitive performance within each of these domains at baseline. In addition, loneliness was associated with greater cognitive declines in every domain except working memory and episodic performance. Furthermore, 76 individuals developed dementia during the 65-month study period. Cox proportional hazards models that controlled for age, sex, and education indicated that loneliness significantly increased the risk of clinical Alzheimer’s disease, and this association was unchanged when objective social isolation and other demographic and health-related factors were included as covariates.
Contradictory results have also appeared. Shankar, Hamer, Mc-Munn, and Steptoe (2013) investigated cognitive decline/dementia in a longitudinal health survey of older adults conducted in England. A measure of loneliness and a test of short- and long-term memory and executive function were introduced in Wave 2 (N = 8,630). Four years later (Wave 4), 6,034 of these participants (69%) completed a follow-up. Data on a set of additional variables (e.g., depression, wealth as a measure of socioeconomic status, employment status, smoking, diabetes, physical activity) obtained at Wave 2 served as covariates in regression analysis to determine the association between both objective social isolation and perceived social isolation (loneliness) at Wave 2 and the performance on each of the three cognitive tasks. Results at Wave 2 indicated that higher scores for loneliness and objective isolation were associated with poorer cognitive function. After 4 years (Wave 4), the mean scores on cognition were significantly lower than at Wave 2, although mean differences were small. After adjusting for covariates, loneliness and objective isolation were associated with poor memory among those low in education, and objective isolation (but not loneliness) was associated with declines in cognitive performance.
There are several important differences between the prior studies (e.g., Gow et al., 2007; Tilvis et al., 2004; Wilson et al., 2007) and Shankar et al. (2013) that may explain this discrepancy in the finding that loneliness predicts cognitive decline in older adults. First, both experimental and longitudinal research indicates that loneliness increases depressive symptomatology (Cacioppo, Hawkley, & Thisted, 2010; J. T. Cacioppo, Hughes, Waite, Hawkley, & Thisted, 2006; Vanderweele, Hawkley, Thisted, & Cacioppo, 2011). Consistent with this research, Shankar et al. (2013) reported that loneliness (but not objective isolation) was correlated with their measure of depression. If loneliness alters cognitive functioning through its effects on depression or through a different but correlated mechanism, the inclusion of depression as a covariate in the initial analyses results in an underestimation of the true association between loneliness and cognitive decline. Second, most prior studies have investigated changes in cognition over periods of time at least twice as long as examined by Shankar et al. (2013). The extent to which the small mean changes in performance over 4 years reflect age-related cognitive declines or dementia is uncertain. Nevertheless, the Shankar et al. (2013) study raises important questions about the importance of social isolation and loneliness on cognitive function in older adults that may be addressed by animal models.
Investigations designed to identify the mechanisms underlying the association between loneliness and mortality have found that loneliness is associated not only with increased risk for age-related cognitive decline and dementia but also with increased sleep fragmentation (J. T. Cacioppo, Hawkley, Berntson, et al., 2002; Hawkley, Preacher, & Cacioppo, 2010; Jacobs, Cohen, Hammerman-Rozenberg, & Stessman, 2006; Kurina et al., 2011), increased hypothalamic pituitary adrenocortical (HPA) activity (Adam, Hawkley, Kudielka, & Cacioppo, 2006; J. T. Cacioppo et al., 2000; Doane & Adam, 2010; R. Glaser, Kiecolt-Glaser, Speicher, & Holliday, 1985; Kiecolt-Glaser et al., 1984b; Steptoe, Owen, Kunz-Ebrecht, & Brydon, 2004), altered gene expression indicative of decreased inflammatory control and increased glucocorticoid insensitivity (Cole, Hawkley, Arevalo, & Cacioppo, 2011; Cole et al., 2007), increased inflammation (Hackett, Hamer, Endrighi, Brydon, & Steptoe, 2012; Jaremka et al., 2013; Steptoe et al., 2004), elevated vascular resistance and blood pressure (J. T. Cacioppo, Hawkley, Crawford, et al., 2002; Hawkley, Burleson, Berntson, & Cacioppo, 2003; Hawkley, Masi, Berry, & Cacioppo, 2006; Hawkley, Thisted, Masi, & Cacioppo, 2010), higher rates of metabolic syndrome (Whisman, 2010), and diminished immunity (Dixon et al., 2001; K. Glaser, Evandrou, & Tomassini, 2005; Kiecolt-Glaser et al., 1984a; Pressman et al., 2005; Straits-Tröster et al., 1994; Figure 1B). Loneliness has also been associated with changes in psychological states that can contribute to morbidity and mortality, including increased depressive symptomatology (e.g., Booth, 2000; J. T. Cacioppo et al., 2010; Cacioppo, Hughes, et al. 2006b; Vanderweele et al., 2011), lower subjective well-being (e.g., Kong & You, 2013; Vanderweele, Hawkley, & Cacioppo, 2012), heightened vigilance for social threats (J. T. Cacioppo & Hawkley, 2009), and decreased executive functioning (J. T. Cacioppo et al., 2000; Hawkley et al., 2009; see also Baumeister & DeWall, 2005).
A Social Neuroscience Perspective
These investigations, along with work on the heritability of loneliness (e.g., Boomsma, Cacioppo, Muthén, Asparouhov, & Clark, 2007; Boomsma, Willemsen, Dolan, Hawkley, & Cacioppo, 2005; Distel et al., 2010; McGuire & Clifford, 2000), have led to a social neuroscience model of the association between loneliness and morbidity and mortality in humans (J. T. Cacioppo, Cacioppo, & Boomsma, 2014; J. T. Cacioppo, Hawkley, et al., 2006). Social species, by definition, create emergent organizations beyond the individual—structures ranging from dyads and families to societies. These social structures and associated behaviors evolved hand in hand with neural, hormonal, and genetic mechanisms to support them because their net effect helped these organisms survive and reproduce. Sociality carries costs (e.g., competition for food and mates; increased risk of pathogen transmission; Alexander, 1974) as well as benefits (e.g., mutual protection & assistance). The social structures and behaviors relevant to the benefits of sociality (e.g., mother–infant attachment) and those that are relevant to mitigating the costs of sociality (e.g., dominance hierarchies, signals of submission, ostracism) ultimately contribute to survival and reproduction, but they do so differently and may be instantiated in the brain in different ways.
One of the benefits of sociality is mutual protection and assistance, and being isolated or on the social perimeter can represent a dangerous circumstance. For instance, predatory fish are more likely to attack prey on the edge of a group, not because they are the slowest or weakest but because it is easier to isolate and prey upon those on the social perimeter (Ioannou, Guttal, & Couzin, 2012). According to the social neuroscience model, the brain evolved to put individuals into a short-term self-preservation mode when they find themselves without mutual protection or assistance. Among the range of neural and behavioral effects are (a) increased implicit vigilance for social threats along with increased anxiety, hostility, and social withdrawal to avoid predation; (b) increased sleep fragmentation to avoid predation during sleep; (c) elevated vascular activity, heightened HPA activity, and altered gene expression and immunity to deal with potential assaults that may arise; (d) decreased impulse control in favor of responses highest in the response hierarchy (i.e., prepotent responding) to rely on behaviors that have generally worked in the past; and (e) increased depressive symptomatology as nonverbal means of signaling the need for support and connection. Furthermore, these effects are posited to extend beyond early developmental periods, in part through mechanisms in the adult brain that permit adaptation to the functional demands of a fluid social environment. Note that these neural and behavioral responses may increase the likelihood of short-term survival, but they can carry long-term costs, especially when the experience of social isolation becomes chronic. The model, therefore, explains the effects of perceived social isolation documented in longitudinal studies of older adults through changes in brain structure and function.
Two corollaries to this reasoning are noteworthy here. First, the brain is posited to be the central organ for forming, monitoring, maintaining, repairing, and replacing salutary connections with others. This is true for humans, and it should be true for other species for whom sociality has been a central feature of life for millions of years. The removal of mutual protection and assistance (e.g., social isolation), therefore, should affect brain structures and/or functions and produce biological and behavioral effects in nonhuman animals, perhaps especially those closest in terms of phylogeny. Second, human research on loneliness has emphasized the importance of attributional reasoning, with loneliness resulting from the discrepancy between the interpersonal interactions that are desired and those that are achieved (Peplau, Russell, & Heim, 1979). If there are deep evolutionary roots tilting the human brain and biology toward short-term self-preservation when a person feels socially isolated, then at least part of what is triggered when individuals feel socially isolated should be nonconscious. For instance, feeling socially isolated increases the explicit desire to connect with others, but it also appears to produce an implicit hypervigilance for social threats (cf. J. T. Cacioppo et al., 2014)—perhaps an adaptation of the predator evasion and aggressiveness documented previously in socially isolated rodents (Hofer, 2009; Kaushal, Nair, Gozal, & Ramesh, 2012). This priming for social threats, in turn, can lead to attentional, confirmatory, and memory biases that lead an individual to think and act toward others in a more negative fashion, which in turn can increase negative interactions with others (e.g., Duck, Pond, & Leatham, 1994; Rotenberg, 1994; Rotenberg, Gruman, & Ariganello, 2002), fuel feelings of social isolation (J. T. Cacioppo et al., 2014; Lau & Gruen, 1992; Rotenberg & Kmill, 1992), and spread across a social network (J. T. Cacioppo, Fowler, & Christakis, 2009)—all while leaving the lonely individual feeling as if he or she had little or no responsibility for the hostile interactions with others.
Evidence from behavioral and functional magnetic resonance imaging (fMRI) studies also supports the notion that loneliness increases attention to negative social stimuli (e.g., social threats) and to self-preservation. Using a modified emotional Stroop task, lonely participants, relative to nonlonely participants, showed greater Stroop interference specifically for negative social words relative to negative nonsocial words (Shintel, Cacioppo, & Nusbaum, 2006). Stroop interference is used to gauge the implicit processing of stimuli, so these results suggest that loneliness is associated with a heightened accessibility of negative social information. Consistent with this interpretation, Yamada and Decety (2009) investigated the effects of subliminal priming on the detection of painful facial expressions. Using measures of sensitivity (discriminability) and bias from signal detection theory, Yamada and Decety found that although the pain was more easily detected in dislikable than likable faces overall, lonely individuals were more sensitive to the presence of pain in dislikable faces than were nonlonely individuals.
In an fMRI study, lonely and nonlonely participants were exposed to pleasant or unpleasant social or nonsocial images. Activation of the visual cortex to the presentation of unpleasant social, in contrast to nonsocial, pictures was directly related to the loneliness of the participant, indicative of greater visual attention to the negative social stimuli (J. T. Cacioppo, Norris, Decety, Monteleone, & Nusbaum, 2009). Despite the greater attention given to negative social stimuli, lonely, in contrast to nonlonely, individuals may be more likely to focus on their own short-term self-preservation in negative social circumstances. Consistent with this notion, activation in the temporo-parietal junction (TPJ)—a region that has been found previously to be activated in theory of mind tasks and in tasks in which individuals take the perspective of another—was inversely related to the loneliness of the participant when they viewed unpleasant pictures of people versus objects (Figure 1C).
Recent research suggests that loneliness is related to appetitive social information processing, as well. The ventral striatum, a key component of the mesolimbic dopamine system, is rich in dopaminergic neurons and is critical in reward processing and learning (Delgado, Miller, Inati, & Phelps, 2005; O’Doherty, 2004). The ventral striatum is activated by primary rewards such as stimulant drugs (Leyton, 2007), abstinence-induced cravings for primary rewards (Wang et al., 2007), and secondary rewards such as money (Seymour, Daw, Dayan, Singer, & Dolan, 2007). Evidence that social reward also activates the ventral striatum has begun to accumulate in studies of sexual desire (S. Cacioppo, Bianchi-Demicheli, Frum, Pfaus, & Lewis, 2012), social cooperation (Rilling et al., 2002), social comparison (Fliessbach et al., 2007), and punitive altruism (De Quervain et al., 2004). J. T. Cacioppo, Norris, et al. (2009) investigated how an individual’s loneliness was related to the differential activation of the ventral striatum to pleasant social versus matched nonsocial images. Lonely individuals showed stronger activation of the ventral striatum to pleasant pictures of objects than to equally pleasant pictures of people, whereas nonlonely individuals showed stronger activation of the ventral striatum when exposed to pleasant pictures of people than of objects (Figure 1C).
The neural correlates of social rejection have also been investigated. Eisenberger, Liberman, and Williams (2003) published the first neuroimaging study of social rejection in a sample of 13 participants, showing that rejection led to increased activity in the dorsal anterior cingulate cortex (dACC), insula, and the right ventral prefrontal cortex (vPFC) regions. These results were interpreted as evidence that social rejection operates on the pain matrix to produce social pain (Eisenberger et al., 2003; Eisenberger, 2012). Contrarian views have been espoused, however (e.g., Somerville, Heatherton, & Kelley, 2006). The early studies of social rejection were characterized by small sample sizes, which can lead to unreliable effects (Button et al., 2013). A recent meta-analysis based on a statistical multilevel kernel density analysis (MKDA) of Cyberball neuroimaging studies with 244 participants failed to support the claim that social rejection operates on the same pain matrix as nociceptive stimuli (S. Cacioppo et al., 2013). The MKDA of the neuroimaging studies was repeated for studies in which participants relived a romantic rejection to test whether the pain matrix was activated if the rejection were more meaningful. Results again failed to support the notion that rejection activates the same neural matrix identified in studies of physical pain. Although more research is needed to clarify this literature, the region of the anterior cingulate that was reliably activated in these studies is in line with Somerville et al.’s (2006) results and suggestion that social rejection operates on attentional mechanisms.
Using fMRI, Powers, Wagner, Norris, and Heatherton (2013) investigated the neural effects of social exclusion on participants’ response to positive or negative social scenes. Following a personality survey and feedback while in the scanner that was putatively based on their answers, participants were randomly assigned to receive one of two conditions prior to the experimental session. Half of the participants were told their future lives would be isolated and lonely (social exclusion), whereas the other half were told that their lives would be filled with long-lasting, stable relationships (social inclusion). All participants also received Barnum statements (personality feedback typically believed by the average person) to increase the credibility of the experimental manipulation. Participants were then scanned while viewing pictorial stimuli that varied in valence and sociality. Results indicated that a region of the dorsomedial prefrontal cortex (dmPFC) previously shown to be involved in mentalizing (i.e., thinking about the mental states of other individuals) was less active in participants in the social exclusion condition than in participants in the social inclusion condition when viewing negative social scenes (Figure 1C). Moreover, the dmPFC activity in participants in the social exclusion condition was least active in response to negative social scenes, intermediate in response to neutral social scenes, and most active in response to positive social scenes—as would be expected if manipulation of social exclusion promoted a short-term self-preservation mode.
One study to date has examined the association between loneliness and brain size. In a study of 108 healthy adults, Kanai, Bahrami, Duchaine, et al. (2012) reported that loneliness was correlated negatively with gray matter density in the left posterior superior temporal sulcus (pSTS), an area involved in biological motion and social perception. Kanai, Bahrami, Roylance, and Rees (2012) had previously demonstrated that the smaller the size of a participant’s online social network, the smaller the pSTS, middle temporal gyrus, and entorhinal cortex—brain regions involved in social perception and associative memory. Kanai, Bahrami, Duchaine, et al. (2012) examined whether the association between loneliness and pSTS size could be explained by social network size (an index of objective social isolation), empathy, or anxiety. Results showed that factoring out these variables did not change the correlation between loneliness and pSTS size. Moreover, consistent with the notion that loneliness is related to differences in social perception rather than social contact, Kanai, Bahrami, Duchaine, et al. (2012) found that loneliness and pSTS size were related to poorer performance on gaze perception, and gaze perception performance mediated the association between loneliness and pSTS.
The premise underlying the research in humans on loneliness and health outcomes is that the brain is the key organ of social connections and processes. Although the evidence from the human literature is suggestive, mechanistic animal studies may provide useful information on the effects of a member of a social species being deprived of mutual assistance and companionship on brain structures and processes. We therefore turn next to a review of experimental investigations in adult social animals on the effects of isolation on brain structures and processes.
It should be noted that the human and animal literatures on social isolation developed independently, with an emphasis in the human literature on the potential role of social relationships/isolation on social cognition, morbidity and mortality (e.g., J. T. Cacioppo & Patrick, 2008), and an emphasis in the animal literature on the effects of environmental enrichment/isolation on brain plasticity and learning (e.g., Markham & Greenough, 2004; Rosenzweig, Bennett, Hebert, & Morimoto, 1978) or social isolation as a model of behavioral disorders (e.g., depression, anxiety, schizophrenia, aggressive behavior; Nin, Martinez, Pibiri, Nelson, & Pinna, 2011; Valzelli, 1973; Wallace et al., 2009). Both human and animal research suggests that loneliness (perceived isolation) reflects the discrepancy between the preferred and actual social conditions rather than objective social isolation (see J. T. Cacioppo et al., in press; Capitanio, Hawkley, Cole, & Cacioppo, in press). Our goal here is not to provide a definitive answer to the question of how loneliness affects morbidity and mortality in humans but to determine whether the animal literature on social isolation may have something to contribute to the answer. Animal models enable the experimental manipulation of the social environment (e.g., separation from conspecifics, a preferred partner, or a nonpreferred partner) and biological targets (e.g., genes, neurochemistry, neural regions) in a more controlled, standardized fashion for longer periods of duration than possible in human studies; animal studies also permit the collection of more invasive measures of brain structures and processes. Animal models on the effects of social loss on depression, anxiety, and aggression may be especially relevant in light of the human evidence that (a) social loss leads to loneliness to the extent that the loss of the relationship was not a preferred outcome (Perlman & Peplau, 1981); and (b) loneliness leads to increased depressive symptomatology, anxiety, and hostility (cf. J. T. Cacioppo et al., in press). We hope the current review contributes to interest in and the development of animal models for investigating the separable effects of objective and perceived social isolation on brain structures and processes and the role of perceived isolation (loneliness) on morbidity and mortality.
Social Isolation: Animal Models and Paradigms
Nonhuman primates are often considered to be the closest match to humans in terms of genetic, behavioral, biological, and social similarity, but animal models of the neurological effects of social isolation fall along the full spectrum of the phylogenetic tree. In the invertebrate literature on social isolation, much of the research has focused on two species: the fruit fly, Drosophila melanogaster, and the desert locust, Schistocerca gregaria. The fruit fly has been an attractive model due to its short lifespan and the simplicity of its neuroarchitecture. The desert locust, in contrast, has been an attractive model because of its natural ability to change from a solitary state to a gregarious state (Breuer, Hoste, & De Loof, 2003; Burrows, Rogers, & Ott, 2011; Ott & Rogers, 2010; Ott et al., 2012; Rahman et al., 2003; Rogers et al., 2004) and the problems (e.g., famine) that swarming desert locusts can cause when in the gregarious state.
Among the adult vertebrate models of social isolation are voles (Fowler, Liu, Ouimet, & Wang, 2002; Grippo, Cushing, & Carter 2007; Grippo, Gerena, & Huang, 2007; Lieberwirth, Liu, Jia, & Wang, 2012; Pournajafi-Nazarloo & Partoo, 2011), gerbils (Ågren & Meyerson, 1978; Pickles, Hagan, Jones, & Hendrie, 2012), canaries (Lehongre, Aubin, & Del Negro, 2009; Terleph, Lu, & Vicario, 2008), zebra finches (Banerjee & Adkins-Regan, 2011; Barnea, Mishal, & Nottebohm, 2006; Lipkind, Nottebohm, Rado, & Barnea, 2002; Terleph et al., 2008), and nonhuman primates (e.g., Coelho, Carey, & Shade, 1991; Eaton, Kelley, Axthelm, Iliff-Sizemore, & Shiigi, 1994; Gilbert & Baker, 2011; Gust, Gordon, Brodie, & McGuire 1994; Gust, Gordon, & Hambright, 1993; Li et al., 2013; Niehoff, Bergmann, & Weinbauer, 2010; Sapolsky, Alberts, & Altmann, 1997; Shively, Clarkson, & Kaplan, 1989; Shively et al., 2005; A. S. Smith, Birnie, & French, 2011; T. E. Smith & French, 1997; Suomi, Eisele, Grady, & Harlow, 1975), but the most common animal models of social isolation are the ubiquitous laboratory rats (e.g., Altman & Das, 1964; Barrientos et al., 2003; Bennett, Diamond, Krech, & Rosenzweig, 1964; Bennett, Rosenzweig, & Diamond, 1969; Bhide & Bedi, 1984; Bjørnebekk, Mathé, Gruber, & Brené, 2007; Diamond, Ingham, Johnson, Bennett, & Rosenzweig, 1976; Diamond, Johnson, Ingham, Rosenzweig, & Bennett, 1975; Diamond, Krech, & Rosenzweig, 1964; Diamond et al., 1966; Diamond, Lindner & Raymond, 1967; Diamond, Rosenzweig, Bennett, Lindner, & Lyon, 1972; Djordjevic et al., 2010; Ferland & Schrader, 2011; Garrido et al., 2013; A. K. Mohammed, Winblad, Ebendal, & Lärkfors, 1990; Pham et al., 1999; Scaccianoce et al., 2006; Stranahan, Khalil, & Gould, 2006; Van Gool, Pronker, Mirmiran, & Uylings, 1987) and mice (e.g., Berry et al., 2012; Modigh, 1973; Valzelli, 1973). Research on the neurological effects of social isolation in these animal models has relied on experimental manipulations, controlling for other aspects of the environment (e.g., amount of space available, complexity of the environment, thermoregulation).1 Tables 1–3 and Figure 1D provide a representative summary of the effects of social isolation on brain structures and processes in various adult animal models.
Table 1.
Effects of Social Isolation on Brain Structures and Processes in Adult Social Invertebrates
| Studya | Speciesb | Age at testingc,d | Social isolation duration |
Sample size (by gender)e
|
Primary dependent variable |
Primary effect of social isolation |
|
|---|---|---|---|---|---|---|---|
| Social isolation group | Comparison group | ||||||
| Desert locusts | |||||||
| Ott & Rogers, 2010 | Schistocerca gregaria Forskål | Adult (after 5th & final moult) | 3rd-gen. isolated desert locusts | 9 male | Long-term gregarious males (n = 9 | Brain proportions |
|
| Rogers et al., 2004 | Schistocerca gregaria Forskål | Adult (5–10 days after final moult) | 2nd-gen. isolated desert locusts | 9 (split approximately evenly between sexes) | Long-term gregarious subjects (n = 9), split approximately evenly between sexes | 13 different neurotransmitters and/or neuromodulators in the central nervous system |
|
|
| |||||||
| Drosophila melanogaster | |||||||
| Heisenberg et al., 1995 | WT Berlin | Adult | 19 days | Female | Group of females housed in a socially enrichedf flight cage (n = 200) | Size of mushroom bodies of the calyx, optic lobe without lamina, central brain without calyces and optic lobes, the central complex without protocerebral bridge |
|
| Technau, 1984 | WT Kapelle | Adult | 21 days | 22 female | Group of WT Kapelle housed in a socially enriched environmentf (control group; n = 17 male, 20 female) + a visually deprived group (n = 14 female) + an olfactory deprived group (n = 9 male, 10 female)f | Number of mushroom body fibers |
|
|
| |||||||
| Honeybees | |||||||
| Maleszka et al., 2009 | Apis mellifera ligustica | 27, 57, and 93 days oldg | 8 days | 10 bees (workers) per group (with the exception of 27 day hive bees for which 7 were measured) | Group of honeybees (workers) caged in a social environment (control group; n = 50) + group of honeybees with dead bees (n = 50 live bees and 5 freeze-dried dead bees) + a pair composed of an isolated bee with a single dead bee | Mushroom body volume in the lip, collar, and basal ring regions of the calyx, peduncles, and lobes, but excluding the Kenyon cell body volumes located within the cup of each calyx |
|
Note. WT = wild type; gen. = generation; GABA = γ–aminobutyric acid.
Authors are listed alphabetically.
Specified to level of detail in the original paper.
All animals fall in the adult range for their species. Age is provided when available.
All animals were reared in normal social/crowded conditions except solitarious desert locusts, which were reared in isolation for two or three generations.
Sample size/gender as specified in the original paper.
See original paper for further details.
Worker bees reach adulthood at about 21 days of age (range 18–22 days; Wheeler & Robinson).
Table 3.
Effects of Social Isolation on Brain Structures and Processes in Adult Social Rodents
| Studya | Speciesb | Age at testingc | Social isolation duration | Sample size (per gender)d
|
Primary dependent variable | Primary effect of social isolation | |
|---|---|---|---|---|---|---|---|
| Social isolation group | Comparison group | ||||||
| Mice | |||||||
| Agís-Balboa et al., 2007 | Swiss-Webster | Adulte | 4 weeks | Male | Group housed male mice | Expression of 5α-reductase Type I (5α-RI) and 3α-hydroxysteroid dehydrogenase (3α-HSD) mRNA in different brain regions |
|
| Berry et al., 2012 | C57BL/6J strain | Adult | 21 days | 12 male | Group housed male mice (n = 16) + group housed male mice submitted to stressf (n = 16) | Ratio of adrenal weight to body weight; brain-derived neurotrophic factor (BDNF) levels measured in the hypothalamus, hippocampus, frontal cortex, striatum, & midbrain on 8 subjects per group randomly chosen from pool of subjects |
|
| Liu et al., 2012 | C57Bl/6J strain | Adult | 2–8 weeks | 6 male, 9 female | Group housed male mice (n = 5) & female mice (n = 12) | Myelination changes |
|
| Pinna et al., 2003 | Swiss-Webster | Adult | 1 day–8 weeks | Male & female | Group housed mice (n = 5–6) | 5α-reductase Type I mRNA |
|
| Rats | |||||||
| Barrientos et al., 2003 | Sprague-Dawley | Adultg | 6 hr | 2–6 male | 2 male rats housed together with 2 other rats (Experiment 1) | BDNF mRNA expression |
|
| Barrot et al., 2005 | Sprague-Dawley | 7–8 weeks of age | 10–14 weeks | 6 male | 7 male rats housed together (2 per cage) | cAMP response element (CRE) binding protein (CREB) activity |
|
| Bhide & Bedi, 1984 | Lister hooded | 85 days of age | 30 days | 12 rats | 12 rats group housed in a large metal cage with 6–10 toysf | Cortical depth, nuclear diameter, and numerical density of neurons and glial cells; perikaryal size and volume; forebrain weight and size |
|
| Bjørnebekk et al., 2007 | Flinders sensitive line & Sprague-Dawley | 29–30 weeks of age | 7 weeks | 8 Flinders sensitive line females and 8 Sprague-Dawley females | 8 Flinders sensitive line females group housed together (4 per cage) 8 Sprague-Dawley females group housed together (4 per cage) |
Survival of proliferating cells in the dentate gyrus of the hippocampus, as measured with BrdU labeled cells, brain-derived neurotrophic factor (BDNF), neuropeptide Y (NPY), and serotonin 5HT2A receptor mRNA |
|
| Diamond et al., 1972 | S1 strain | 60 and 105 days of age | 30–80 days | 25 60-day-old male and 18 105-day-old male | A group of 25 60-day-old male rats and 18 105-day-old male rats group housed together (10–12 rats per cage) | Cortical depth in frontal, somesthetic, and occipital cortex |
|
| Diamond et al., 1975 | S1 strain | 60 and 105 days of age | 30–80 days | 19 60-day-old male and 18 105-day-old male | 19 60-day-old male rats and 18 105-day-old male rats group housed together (10–12 rats per cage for each age range) with access to toysf | Cortical neuronal nuclei and perikarya dimensions |
|
| Diamond et al., 1976 | Rats | 60 and 105 days of age | 1, 4, or 30 days for the 60-day-old rats; 80 days for the 105-day-old rats | A total of 266 rats | 60- and 105-day-old rats (total n = 266f) group housed together per age range | Cortical depth in motor, somesthetic, and occipital cortex |
|
| Djordjevic et al., 2010 | Wistar | 3 months of age | 21 days | Male | A group of unstressed animals (control group) + a group of rats exposed to an acute stress (i.e., 30-min immobilization period) + a group exposed to a combined stress (i.e., a 21-day social isolation period followed by a 30-min immobilization period) In each comparison group, rats were housed 4 per cage |
Glucocorticoid receptor (GR) and nuclear factor kappa B (NFkB) protein and mRNA expression, and DNA defragmentation in the prefrontal cortex (PFC) |
|
| Garrido et al., 2013 | Wistar | 3 months of age | 12 weeks | Male | A group of male rats housed together (10–12 animals per cage) with 2 running wheels, tunnels, and different objectsf | Corticosterone, dopamine, and acetylcholine responses to acute restraint stress in the PFC of the awake rat and the mRNA levels of GRs in the PFC |
|
| A. K. Mohammed et al., 1990 | Sprague-Dawley | 52 days of age | 30 days | 12 male (6 of which were submitted to 1 day of automatic activity cages + 3 days in the Morris water maze after social isolation) | 12 male group housed together (6 per cage) + 12 other male group housed together (3 per cage). Half of the animals of each group were submitted to 1 day of automatic activity cages + 3 days in the Morris water maze after social isolation | Nerve growth factor (NGF) content in the cortex, hippocampus, and septum |
|
| Moser et al., 1997 | Rats | Adultg | 14–18 days | 4–5 male | 10–11 male rats spatially trained together in a complex environment 4 hr per day and then housed in pairs + 6–7 nontrained pair housed male rats | Spine density on a sample of oblique secondary branches of apical dendrites of CA1 pyramidal cells of the hippocampus; distribution of new spines on basal dendrites |
|
| Nilsson et al., 1999 | Sprague-Dawley | 9 weeks of age | 4 weeks | 10 female | 12 female group housed (6 per cage) with toysf | Proliferating cells in the dentate gyrus of the hippocampus, as measured with BrdU labeled cells |
|
| Pham et al., 1999 | Sprague-Dawley | 2 months of ageg | 1 year | 15 male | 16 male rats housed together (8 per cage) with toysf | Nerve growth factor (NGF) levels in hippocampus, visual cortex, entorhinal cortex, and hypothalamus |
|
| Rosenzweig et al., 1968 | S3 strain (Experiments I–IV); S1 strain (Experiment V) | 60 days of age | 55 days (Experiment I) 56 days (Experiment II) 54 days (Experiment III) 31 days (Experiment IV) 30 days (Experiment V) |
10 male (Experiments I & III) 11 male (Experiment II & IV) |
10–11 male rats group housed together for 24 hr with toys (24 hr enriched condition) + 10–11 male rats group housed together for 4.5 hr with toys + 10–11 male rats group housed together for 2.5 hr with toysf (Experiments I–II) A group of male rats (n = 10) housed together with toysf for 2 hr, while the other 22 hr of the day were spent in 3-per-cage group housing (Experiment III) A group of male rats (n = 11) housed together (5–6 per cage) for 2 hr after receiving saline injection, with access to toys for 90 min, while the other 22 hr of the day were spent in social isolation (Experiments IV and V) |
Brain weight and brain chemistry of acetylcholinesterase (AChE) and cholinesterase (ChE) |
|
| Scaccianoce et al., 2006 | Sprague-Dawley | 2 months of age | 8 weeks | 8 male | 8 pair housed male rats | Brain-derived neurotrophic factor (BDNF) expression in the hippocampus, striatum, and prefrontal cortex |
|
| Spritzer et al., 2011 | Sprague-Dawley | 55 days old | 34 days | 8 male after a sham surgery; 8 male rats after castration (Experiment 1); 8 castrated male receiving daily testosterone injection; 8 castrated male receiving daily sesame oil injections | 8 male rats pair housed after a sham surgery; 8 male rats pair housed after castration (Experiment 1); 8 castrated male pair housed with daily testosterone injection; 8 castrated male pair housed with daily sesame oil injection (Experiment 2) | Number of BrdU labeled cells in the granule cell layer and subgranular zone of the dentate gyrus; volume of brain regions; serum testosterone levels | Experiment 1
|
| Stranahan et al., 2006 | Sprague-Dawley | Adult | 12 days | 6 male | Group housed male rats (3 per cage)f with or without running activity | Number of BrdU labeled cells in the dentate gyrus |
|
| Wallace et al., 2009 | Sprague-Dawley | 7–8 weeks | 10–12 weeks | Male | Pair housed | cAMP response element (CRE) binding protein (CREB) activity in the shell of the nucleus accumbens (NAcSh) |
|
| Westenbroek et al., 2004 | Wistar | 3 weeks | 3 months of age | 10 male and 10 female | 14 male and 14 female were housed in unisex groups of four rats | Survival of proliferating cells in the dentate gyrus of the hippocampus |
|
| Voles | |||||||
| Fowler et al. 2002 | Prairie voles (Microtus ochrogaster) | 85–135 days of ageh | 2 days or 3 weeks | 7 female for the 2-day social isolation condition; 7 female for the 3-week social isolation condition (Experiment 1); 5 female for the 2-day social isolation condition (Experiment 2) | Housed with an unfamiliar male (n = 7 for the 2-day condition; n = 6 for the 3-week condition, Experiment 1; n = 6, Experiment 2; male exposure groups); housed with an unfamiliar female (n = 6 for the 2-day condition; n = 8 for the 3-week condition, Experiment 1; n = 6, Experiment 2; female exposure groups) | Number of proliferating BrdU-labeled cells (Experiment 1) and apoptosis rate and DNA fragmentation (Experiment 2) in the subventricular zone and dentate gyrus of the hippocampus |
|
| Grippo, Cushing, & Carter, 2007 | Prairie voles (Microtus ochrogaster) | 60–120 days of age | 60 days | 10 female | 10 pair housed female | Numbers of corticotropin-releasing factor-and oxytocin-immunoreactive cells in the paraventricular nucleus of the hypothalamus, and circulating levels of hormones and peptide in response to an acute social stressor (resident–intruder test). |
|
| Grippo Gerena, & Huang, 2007 | Prairie voles (Microtus ochrogaster) | 60–90 days of age | 4 weeks | 8 female and 8 male | 8 female and 8 male pair housed with a same-sex sibling | Basal central (in hypothalamic paraventricular nucleus, PVN), and plasma circulating hormones and peptides; c-Fos expression in PVN |
|
| Lieberwirth et al., 2012 | Prairie voles (Microtus ochrogaster) | 90–120 days of age | 14 days | 8–9 female | 8–9 female pair housed with a same-sex vole | Cell proliferation and survival, neuronal differentiation, and cell death in subregions of the amygdala and hippocampus |
|
| Pournajafi-Nazarloo & Partoo, 2011 | Prairie voles (Microtus ochrogaster) | 2 months of age | 1 hr total (single social isolation) or 1 hr every day for 4 weeks (repeated social isolation) or 4 continuous weeks (chronic social isolation) | 8 per group | Animals were subjected to single, repeated, or chronic social isolation | Corticotropin-releasing hormone (CRH) receptor (CRH-R1 mRNA) and Type 2 CRH receptor (CRH-R2 mRNA) expression in the hypothalamus, hippocampus, and pituitary gland |
|
Authors are listed alphabetically.
Specified to level of detail in the original paper.
All animals fall in the adult range for their species and were reared in normal social group conditions. Age is provided when available.
Sample size/gender as specified in the original paper.
Mice reach adulthood at about 2 months of age (National Institutes of Health, 2013).
See original paper for further details.
Rats reach adulthood at about 6 weeks of age (Sengupta, 2013).
Voles reach adulthood at about 45–50 days of age (Mateo et al., 1994).
Effects of Social Isolation on Brain Volume
Sociality and social complexity have been posited to be a driving force for the evolution of primate brain size (Dunbar, 1998, 2003; Dunbar & Shultz, 2007; Lihoreau, Latty, & Chittka, 2012; Semendeferi et al., 2011). Dating back to the 19th century, scientists including Charles Darwin (1874) and Ramón y Cajal (1895) speculated that brain size was associated with the social environment in which a species resided (see A. H. Mohammed et al., 2002, for review). The social brain hypothesis posits that the complexity of the social environment across species is related to brain size and connectivity—that is, to the information processing capacity of the brain. Investigations of the social brain hypothesis in primates have produced provocative and generally supportive results (Dunbar, 1998, 2003; Dunbar & Shultz, 2007), though the evidence is less compelling in Hymenoptera (Farris & Schulmeister, 2011) and Carnivora (Finarelli & Flynn, 2009). The more pertinent question here, however, is the extent to which social isolation has effects within species on adult brain structures and processes.
Because the brain is energetically expensive, it has been posited that specific brain regions should enlarge only when needed to meet functional demands (Niven & Laughlin 2008). If social isolation follows the rule of “use it or lose it,” regional neuroanatomical adjustments should occur contingent on the demands of social versus isolated living conditions. Consistent with this reasoning, experimental studies of social isolation or solitary states on brain size indicate that the effects are not uniform across the brain but instead are most evident in brain regions that reflect differences in the functional demands of solitary versus social living for that particular species. The desert locust transforms from a solitary to a social state, with the solitary state producing an approximately 30% reduction in locust brain size (which comprises a midbrain flanked by paired optic lobes; Burrows et al., 2011; Ott & Rogers, 2010). Despite having a smaller brain overall, the solitary locust has disproportionally large primary visual and olfactory neuropils, putatively due to the increased individual predation risk and the need for the solitary locust to detect visual stimuli at a greater distance (Burrows et al., 2011). By contrast, the gregarious locust has a larger midbrain to optic lobe ratio, and within both the visual and olfactory systems, higher multimodal integration centers are disproportionately larger than the primary sensory neuropils (Burrows et al., 2011; Ott & Rogers, 2010). The central complex, an important multimodal sensory and sensorimotor integration center, is also considerably larger in gregarious locusts. Together, these results suggest the modulation of the size of specific brain regions based on environmental demands (Burrows et al., 2011).
Similar reductions in regional brain size in socially isolated animals have been found in other insects, including Drosophila melanogaster (Technau, 1984) and several species of honeybees (e.g., Maleszka, Barron, Helliwell, & Maleszka, 2009; A. R. Smith, Seid, Jiménez, & Wcislo, 2010; Withers, Day, Talbot, Dobson, & Wallace, 2008; Withers, Fahrbach, & Robinson, 1993; cf. Lihoreau et al., 2012). For instance, the mushroom bodies (MBs) in Drosophila melanogaster are involved in olfactory learning, multisensory integration, and memory (cf. Heisenberg, 1998, for review). Technau (1984) showed that socially isolated adult female wild-type Kapelle Drosophila melanogaster have fewer MB fibers than do members of a control group (see Table 1). Heisenberg, Heusipp, and Wanke (1995) then repeated Technau’s (1984) experiment and showed that the calyces of socially isolated females are 21% smaller than the calyces of Drosophila melanogaster housed in a socially enriched flight cage. These differences were also accompanied by volume changes in the lamina, medulla, lobula, and the central brain (Table 1). However, in another experiment, Heisenberg et al. failed to replicate any brain size differences between isolated and social Drosophila melanogaster (see Heisenberg et al., 1995, for details). The authors attributed these inconsistencies to the fact that brain size is influenced by multiple factors. In another series of experiments in a more controlled environment (e.g., controlled atmosphere, filtered air, constant humidity, normal light:dark cycle), Heisenberg et al. (1995) showed that: (a) the adult brain of the Drosophila melanogaster can change in volume as a function of living conditions (including social isolation); (b) these effects are not uniform across the entire brain; and (c) these changes could not be explained by olfactory inputs alone.
Like Drosophila melanogaster, when insects such as bees or ants switch from performing tasks within a confined social environment to foraging in a more complex social environment, they are exposed to additional sensory stimuli and navigation requirements. This increase in social and sensory demands is associated with an increase in the volume of the MBs in ants (Gronenberg, Heeren, & Hölldobler, 1996; Kühn-Bühlmann & Wehner, 2006), sweat bees (Megalopta genalis; A. R. Smith et al., 2010), and worker honeybees (e.g., Withers et al., 1993, 2008). For instance, when adult worker honeybees change from working in the hive to working outside the hive, this change is associated with a significant growth of their MBs (Ismail, Robinson, & Fahrbach, 2006; Withers et al., 1993). Conversely, social isolation of adult honeybees produces a decline in MBs volume (Maleszka et al., 2009; see Table 1). Together, these studies of arthropods suggest that the behavioral demands of group living and social foraging influence the phenotypic expression of regional brain size, and in turn that social isolation changes the volume of brain regions involved in these social behaviors. More generally, these studies suggest that socially related neural plasticity follows the heuristic that the size and complexity of specific brain regions vary in adulthood with the functional and behavioral demands placed on them by the environment (Lihoreau et al., 2012; Niven & Laughlin, 2008; O’Donnell, Clifford, & Molina, 2011; Withers et al., 1993). The study of invertebrates may be a valuable model for abstracting principles involved in the effects of perceived social isolation on the brain.
The phenotypic changes in brain morphology in response to the social environment are not restricted to invertebrates. Social isolation-dependent brain plasticity has also been observed in the adult avian brain (e.g., zebra finches, Taeniopygia guttata), for instance, where socially isolated songbirds, compared to communally housed songbirds, have fewer new neurons in brain areas involved in vocal communication (e.g., the neostriatum caudale (NC; Lipkind et al., 2002). Barnea et al. (2006) replicated this finding for the NC and also found isolated, compared to communally housed, songbirds had fewer neurons in the hippocampal complex, an area involved in memory and spatial information processing (see Table 2).
Table 2.
Effects of Social Isolation on Brain Structures and Processes in Adult Social Birds
| Studya | Speciesb | Age at testingc | Social isolation duration | Sample size (per gender)d
|
Primary dependent variable | Primary effect of social isolation | |
|---|---|---|---|---|---|---|---|
| Social isolation group | Comparison group | ||||||
| Barnea et al., 2006 | Zebra finches | 4–5 months of agee | 40, 60, or 150 days | 5–7 male birds for each time period (40 days: n = 5; 60 days: n = 6; 150 days: n = 5) | 18 experimental zebra finches housed for 40 (n = 5), 60 (n = 6), or 150 days (n = 7) with a group (n = 40–45) of adult male and female zebra finches | Volume and number of total neurons per mm3 in nidopallium caudale (NC) and hippocampal complex (HC) Number, volume, and diameter of [3H]-thymidine labeled neurons in NC and HC |
|
| Lipkind et al., 2002 | Zebra finches (Taeniopygia guttata) | 4–5 months of agee | 40 days | 5 male, 5 female | Male–female pairs (n = 5 pairs, 1 male & 1 female) + a group of 10 experimental birds (5 male, 5 female) housed with a group (n = 40–45) of adult male and female zebra finches | Number and diameter of [3H]-thymidine labeled neurons in neostriatum caudale |
|
Authors are listed alphabetically.
Specified to level of detail in the original paper.
All animals fall in the adult range for their species. Age is provided when available.
Sample size/gender as specified in the original paper.
Zebra finches reach adulthood at about 100 days of age (Day et al., 2009).
Similarly, experimental studies in rodents suggest that rodents that are housed alone, in contrast to those housed in groups, develop a smaller cerebral cortex, smaller cell bodies, shorter synapses, and fewer glial cells in specific regions of the brain known to be important for sensorimotor integration and social behaviors (e.g., Bhide & Bedi, 1984; Bjørnebekk et al. 2007; Garrido et al., 2013; Rosenzweig, Love, & Bennett, 1968; for reviews, see Van Praag, Kempermann, & Gage, 2000; Rosenzweig & Bennett, 1996). Bhide and Bedi (1984), for instance, replicated work showing that the forebrain of rats that underwent social isolation between 85 and 115 days of age was significantly lighter and shorter in length than controls. However, contrary to other prior studies (e.g., Diamond et al., 1972, 1975, 1976; for review, see Diamond, 2001), no significant differences in cortical depth were observed between the isolated and group housed animals, except in one brain section of the left occipital cortex (area 17) where the superior colliculus was clearly defined and the hippocampus was continuous as it extended ventrally. Among the different variables that might explain these discrepancies are age and duration of social isolation (see Diamond, 2001, for review), as a short period of social isolation may have different effects on a young adult rat compared to an older adult rat (e.g., Diamond, 2001; Diamond et al., 1972; see Table 3).
Despite the discrepancies that exist in this literature, several robust effects of social isolation have emerged—effects that have been found in Rodentia from the family Muridae, which includes rats and mice (for reviews, cf. A. H. Mohammed et al., 2002; Praag et al., 2000; Rosenzweig & Bennett, 1996) and in the extended Rodentia family (i.e., the Cricetidae family). For instance, socially isolated rodents, relative to controls, show regional brain changes in several areas that are involved in the processing of social information, memory, sensorimotor integration and spatial information processing, such as the prefrontal cortex (Djordjevic, Adzic, Djordjevic, & Radojcic, 2010), the occipital cortex (e.g., Diamond et al., 1975, 1976), and the hippocampus (Moser, Trommald, Egeland, & Andersen, 1997).
Neurogenesis
The observed effects of social isolation on the morphology of the adult brain has led to the hypothesis that social isolation also affects the rate and fate of new cell proliferation in the adult brain (Praag et al., 2000; see also Gheusi, Ortega-Perez, Murray, & Lledo, 2009). Consistent with this hypothesis, studies in fish, birds, and mammals indicate that enriched social environments and complex social interactions (such as those observed during the breeding season) enhance cell proliferation and neurogenesis in the brain, notably in the regions critical for social interaction, memory, and communication (cf. Dunlap & Chung, 2013; Dunlap, Chung, & Castellano, 2013; Dunlap, Silva, & Chung, 2011; Goldman & Nottebohm, 1983; Lieberwirth & Wang, 2012; Zupanc, 2008; Zupanc & Sirbulescu, 2011). Conversely, social isolation reduces brain cell proliferation in various taxa, such as birds (e.g., Barnea et al., 2006; Lipkind et al., 2002) and prairie voles, Microtus ochrogaster (Fowler et al., 2002; Lieberwirth et al., 2012). Importantly, inconsistencies also exist in this literature. In a study of female Sprague Dawley and Flinders Sensitive Line (FSL) adult rats, Bjørnebekk et al. (2007) found that 7 weeks of individual housing, relative to group housing, led to increased (rather than decreased) cell proliferation in the hippocampus in FSL rats but had no effect on cell proliferation in Sprague Dawley rats. Westenbroek, Den Boer, Veenhuis, and Ter Horst (2004) investigated the effects of social isolation and chronic foot shock stress on hippocampal neurogenesis in male and female Wistar rats and found that social isolation, especially when combined with chronic stress, decreased cell proliferation in males but had the opposite effect in females, suggesting that neurosteroids may play a role.
More generally, the effects of social isolation on adult neurogenesis may interact with a variety of factors for reasons that are not yet fully understood. For instance, Stranahan et al. (2006) found that exercise reduced hippocampal neurogenesis in socially isolated male rats, whereas exercise increased neurogenesis in group housed rats. Subsequent research extended this result to female rats (Leasure & Decker, 2009) but not to mice (Kannangara, Webber, Gil-Mophapel, & Christie, 2009). Spritzer, Ibler, Inglis, and Curtis (2011) investigated the potential interactive effects of sex steroids on neurogenesis in adult male Sprague Dawley rats. The animals were either socially isolated or pair housed for 34 days; testosterone was manipulated by bilateral castration or sham castration; and neurogenesis was subsequently measured in one of the primary areas of adult neurogenesis, the dentate gyrus. Castration decreased new cell proliferation, an effect that was evident primarily in the animals that were socially isolated. In a second experiment, all rats were castrated and either socially isolated or pair housed. In addition, the animals received daily injections of either testosterone propionate or vehicle. Contrary to the results of their first study, Spritzer et al. (2011) found that social isolation and testosterone each decreased neurogenesis in the dentate gyrus. The testosterone injections in Spritzer et al.’s second experiment produced supraphysiological surges of testosterone, which may explain why their results for adult neurogenesis were contrary to what has been observed previously for normal circulating levels of testosterone.
Research has also been done on the effects of isolation on myelination in the prefrontal cortex (PFC) of mice (Liu et al., 2012; Makinodan, Rosen, Ito, & Corfas, 2012). Contrary to PFC myelination in early life, Liu et al. (2012) determined that the ongoing myelination that occurs in the adult PFC represents a form of myelin plasticity to adapt brain structures and functions to environmental demands. Adult mice were either singly housed or housed in groups of five mice per cage for 2–8 weeks (Liu et al., 2012). Results showed that after 8 weeks, there were no differences in locomotion between the socially isolated and group housed mice, but the isolated, relative to group housed, mice spent less time interacting with a conspecific mouse (a sign of social withdrawal), had thinner myelin sheaths in PFC, and showed decreased myelin gene transcripts and proteins in PFC. To determine whether the isolation-induced hypomyelination was due to delayed myelin formation, Liu et al. examined nuclear chromatin condensation. Results showed that the presence of axons with thinner myelin in the isolated mice was associated with oligodendrocytes with immature nuclear chromatin and with a lower proportion of heterochromatin. These results were interpreted in terms of a model of myelin plasticity wherein prolonged social isolation, via neuronally derived signals (neuregulins or unknown factors), produces changes in the nuclear heterochromatin (which plays a role in the expression of genes) of oligodendrocytes in PFC, which slow myelin formation.
Liu et al. (2012) repeated their study using a shorter period of social isolation (15 days rather than 8 weeks) and found slightly decreased myelin thickness in the PFC as well as features of oligodendrocytes that are characteristic of immature myelin (e.g., decreased percentage of heterochromatin). No differences were observed in social withdrawal, however, possibly indicating that the changes in heterochromatin in PFC precede and may play a role in the changes in social behavior. To test reversibility (i.e., plasticity), mice previously isolated for 8 weeks were group housed for 4 weeks. As predicted by the adult myelin plasticity model, in which myelination plasticity serves to adapt brain function to environmental demands, the myelin transcripts in PFC and social behavior returned to control levels in the social reintegration group.
Research on neurosteroids, transcription factors, and growth factors has begun to identify additional molecular mechanisms through which social isolation may impact neurogenesis in parts of the adult brain. Social isolation has been shown to reduce levels of brain-derived neurotropic factor (BDNF)2 in rats (e.g., Nilsson, Perfilieva, Johansson, Orwar, & Eriksson, 1999; Scaccianoce et al., 2006) and mice (Berry et al., 2012). In rats, Scaccianoce et al. (2006) showed that the significant reduction in BDNF protein concentrations was observed in the hippocampus but not in the striatum and prefrontal cortex.
Allopregnanolone is a GABAA receptor active neurosteroid that facilitates the inhibitory actions of GABA and up-regulates BDNF in the adult mouse (Nin et al., 2011; Pinna, 2010). Male adult mice socially isolated for at least 4 weeks, compared to group housed male mice, show reductions in levels of allopregnanolone in hippocampal CA3 pyramidal neurons and glutamatergic granular cells of the dentate gyrus, cortical pyramidal neurons (layers V–VI), and neurons in the basolateral amygdala—reductions that are attributable to the effects of social isolation on a specific enzyme involved in the biosynthesis of allopregnanolone, 5α-reductase (Agís-Balboa, Pinna, Kadriu, Costa, & Guidotti, 2007; Nin et al., 2011).
The levels of allopregnanolone were also found to correlate with the heightened levels of aggressive behavior found in the socially isolated, compared to group housed, mice, and the infusion of allopregnanolone into the basolateral amygdala mitigated these behavioral effects (Nelson & Pinna, 2011). Reductions in levels of allopregnanolone by social isolation, and corresponding reductions in its facilitative effects on GABA, may also play a role in the decreased susceptibility to barbiturates and other γ-aminobutyric acid (GABA) mimetic drugs found in socially isolated male mice (Guidotti et al., 2001). Work by Pinna and colleagues (e.g., Pinna, Done, Matsumoto, Costa, & Guidotti, 2003; cf. Nin et al., 2011) further indicates that fluoxetine administration reduces the differences in corticolimbic levels of allopregnanolone and the mRNA expression of BDNF observed between socially isolated and group housed mice. Although 5-HT1A receptors may be involved, the involvement of other 5-HT receptor subtypes needs clarification (e.g., Sánchez, Arnt, Hyttel, & Moltzen, 1993). One hypothesis is that fluoxetine ameliorates the behavioral effects of social isolation through its effects on allopregnanolone levels rather than by inhibiting serotonin reuptake—that is, SSRIs may be effective through their actions as selective brain steroidogenic stimulants (Nin et al., 2011).
Early growth response transcription factor genes (Egr-1 to Egr-4) have been implicated in the regulation of synaptic plasticity and long-term memory formation in rodents (e.g., Lee, Everitt, & Thomas, 2004). Egr-1, for instance, is thought to trigger experience-dependent modifications in synapses, and its expression is down-regulated in the visual cortex by dark adaptation (Mataga, Fujishima, Condie, & Hensch, 2001). Matsumoto, Ono, Ouchi, Tsushima, and Murakami (2012) investigated the effects of social isolation on Egr-1 gene expression in male mice. Animals were sacrificed 3, 7, or 56 days after social isolation (or group housing), and whole brain tissues were extracted and analyzed. Results showed that the expression of Egr-1 is down-regulated by social isolation as early as 7 days after isolation, whereas Egr-2 to Egr-4 protein levels were not affected. Moreover, the effect of social isolation on Egr-1 protein levels was not uniform across the brain but rather was limited to the cerebral cortex and particularly to the frontal cortex.
cAMP response element-binding protein (CREB), a cellular transcription factor that is involved in neuroplasticity and long-term memory formation, has also been found to be affected by long-term social isolation in adult rodents. Building on prior work showing that the activity of the transcription factor CREB in the shell of the nucleus accumbens (NAcSh) is a key regulator of responses to emotional stimuli, Barrot et al. (2005) tested and found that socially isolating adult rats for 10–12 weeks induced anxiety and decreased CREB activity in the NAcSh and that this local reduction in CREB activity mediated the isolation-induced increases in anxious behavior. Wallace et al. (2009) also compared adult rats that were socially isolated or pair-housed for 10–12 weeks and found increased anxiety and depressive behavior as well as reduced CREB activity in the NAcSh in the socially isolated, relative to pair-housed, animals. The isolation-induced differences in CREB activity were related to the anxiety-like behaviors but not to the depressive behaviors. Chronic administration of imipramine, a tricyclic antidepressant, normalized CREB activity in the NAcSh and reversed the anxiety-like and depressive-like behaviors in the socially isolated rats. The analysis of DNA expression arrays suggested that social isolation reduced CREB activity in the NAcSh, which served to up-regulate several K+ channels and depress the excitability of NAcSh neurons, leading to anxiety-like behaviors. The extent to which CREB activity in other brain regions, such as the hippocampus, might be related to the depressive-like effects of social isolation is yet to be determined.
Studies in which nonhuman primates are socially isolated are less common and have generally focused on the effects of early life social isolation.3 The few studies of social isolation that have been performed in adult nonhuman primates have focused on physiological measures rather than neural measures. Socially isolated, compared to normally housed, nonhuman primates were characterized by (a) elevated hypothalamic pituitary adrenocortical activity, as indexed by basal hypercortisolism (Sapolsky et al., 1997), a rise of salivary cortisol levels (Cross, Pines, & Rogers, 2004), and urinary cortisol excretion (A. S. Smith et al., 2011; T. E. Smith & French, 1997); (b) greater depressive-like behavior (Li et al., 2013; Shively et al., 2005; Suomi et al., 1975); and (c) lower heart rates and higher blood pressure. In an interesting variation on this study design, Coelho et al. (1991) measured cardiovascular activity in adult male baboons when they were housed (a) individually (Weeks 1 and 4); (b) with two socially familiar companions (i.e., the experimental animal had at least 4 years of previously established and nonhostile history with the companion; Weeks 2 and 5); and (c) with two socially unfamiliar animals (Weeks 3 and 6). The last two conditions, therefore, controlled for the presence of conspecifics but varied the salubrity of the relationship between the experimental animal and the companion animals. Results showed that blood pressure was higher and heart rate was lower when the experimental animals were individually housed than housed with familiar companions. The presence of conspecifics was not sufficient, however; instead the nature of the relationship between the experimental and companion animals mattered: Blood pressure was higher and heart rate was higher when the animals were housed with unfamiliar than with familiar companions (Coelho et al., 1991). The neuroendocrine and pressor effects observed in the nonhuman primate studies are reminiscent of the longitudinal findings putatively attributable to perceived social isolation in the human literature, but additional research is needed, especially to determine the neural mechanisms underlying these autonomic and neuroendocrine effects.
Neurological studies on primates are also needed to test the notion that adult neurogenesis occurs in response to the functional demands of solitary versus social living for each particular species. The understanding of the dynamic interplay between social isolation and adult neurogenesis within and between species would contribute to our understanding of how the social brain matrix has evolved along the phylogenetic tree—that is, from olfactory brain regions and optical lobes in insects to higher order brain areas of social cognition in humans. Furthermore, given the influence of social isolation on neurotropins documented in studies of rodents and the critical role of neurotrophic factors in neuroprotection, expanding this research to nonhuman primates may contribute to our understanding of the influence of the social environment on the aging brain and on cell death following brain trauma.
Social Stimuli as Therapeutic Following Neural Insult
The role of social isolation is evident in animal models of brain injury. For example among hypertensive rodents, social isolation in the pre- and perioperative period before induction of stroke by middle cerebral artery occlusion (MCAO; Craft et al., 2005; Karelina, Norman, Zhang, & DeVries, 2009; Karelina, Norman, Zhang, Morris, et al., 2009; Karelina et al., 2011) impairs infarct size and functional recovery (Craft et al., 2005; Venna et al., 2012) compared to animals housed in an enriched social environment. Similar effects are also reported for poststroke social isolation (Dahlqvist et al., 2003; Johansson & Ohlsson, 1996; McKenzie, Diamond, Greer, Woo, & Telles, 1990; Ohlsson & Johansson, 1995; Risedal et al., 2002). Both social isolation (e.g., Sapolsky et al., 1997) and cerebral ischemia activate the HPA axis (DeVries, Joh, et al., 2001) and inflammatory mechanisms (An et al., 2013), which in turn can impact infarct size and functional recovery (Craft et al., 2005; DeVries, Nelson, et al., 2001; Sugo et al., 2002). Social isolation before or after stroke could possibly act on stroke outcome through mechanisms that involve dendritic structures in the contralateral hemisphere (Johansson & Belichenko, 2002), altered gene expression (Karelina, Norman, Zhang, et al., 2009), cell death in specific brain areas (Farrell, Evans, & Corbett, 2001), and/or altered HPA activity (Craft et al., 2005). For instance, Craft et al. (2005) showed that mice that were socially isolated for 2 weeks before and throughout a 7-day reperfusion period after a transient focal cerebral ischemia by MCAO had larger infarcts and greater functional deficits (as measured by contralateral paws) than did pair-housed mice. In Craft et al.’s study, social isolation did not have an effect on intra-ischemic or post-ischemic corticosterone concentration, which suggests that corticosteroids are not sufficient to explain the effects of social isolation on ischemic outcome. However, intra-ischemic C-reactive protein (CRP) levels—an index of inflammation (Szalai, Nataf, Hu, & Barnum, 2002) and a potential risk-factor for stroke (Chaudhuri et al., 2013; Lindsberg & Grau, 2003; VanGilder et al., 2014) and increased cerebral infarct size (Gill, Kemp, Sabin, & Pepys, 2004)—were higher in socially isolated male mice relative to pair-housed male mice (Craft et al., 2005).
Karelina, Norman, Zhang, et al. (2009) established a causative role for neuroinflammation as a mediator of the effects of social isolation on stroke severity. As in Craft et al. (2005), adult male mice were either socially isolated or pair-housed for 2 weeks prior to the MCAO-induced stroke and throughout a reperfusion period. Karelina, Norman, Zhang, et al. (2009) replicated Craft et al.’s finding of a larger infarct size in isolated animals compared to pair-housed controls. The increase in ischemic damage in socially isolated mice was accompanied by a decrease in poststroke survival rate (40% for socially isolated mice compared to 100% in socially housed mice) and an altered neuroinflammatory response, as measured by IL-6. Specifically, peripheral IL-6 was higher in socially isolated than pair housed mice, whereas central IL-6 was down-regulated in isolated relative to pair-housed mice. Conflicting data exist on the functional role of cytokines in cell death, but elevations in peripheral IL-6 levels are generally regarded as proinflammatory. Importantly, central administration of IL-6 neutralizing antibody increased central IL-6 levels in the pair-housed mice, and this was shown to eliminate the differences in serum (peripheral) IL-6 and in infarct size between socially isolated and pair-housed mice (Karelina, Norman, Zhang, et al., 2009). Relatedly, Venna et al. (2012) replicated the increased serum IL-6 levels in isolated, relative to pair-housed, mice. Moreover, they found that social isolation per se did not affect nuclear factor-kappaB (NF-κB) transcriptional activity but that stroke increased NF-κB signaling, with this increase being greater in the socially isolated than pair-housed mice. The enhanced NF-κB activity was also associated with the effects of social isolation on stroke outcome, and inhibition of the NF-κB signaling difference using a pharmacological inhibitor (PDTC) eliminated the differences in stroke outcome between the socially isolated and pair-housed mice (Venna et al., 2012). Together, these results indicate that NF-κB signaling mediates the detrimental effects of social isolation on cell death following stroke.
NF-κB is an upstream regulator of several signaling pathways including IL-6 and oxytocin. Oxytocin (OT) is a nonapeptide produced in the paraventricular and supraoptic nuclei of the hypothalamus and released during positive social interactions. Karelina et al. (2011) investigated the OT mRNA levels in mice that had been socially isolated or pair-housed for 1 week. Results confirmed that OT mRNA levels were lower in the socially isolated than pair-housed mice. To investigate the possibility that the effects of social isolation on NF-κB signaling operated at least in part through OT, Karelina et al. (2011) compared the effects of MCAO on socially isolated versus pair-housed mice treated with exogenous OT, an oxytocin receptor antagonist (OTA), or vehicle beginning 1 week prior to the ischemic event. Replicating their prior results, the socially isolated mice given the vehicle showed larger infarct sizes (greater cell death) 72 hr after MCAO than pair-housed mice given the vehicle. Moreover, OT administration eliminated the effects of social isolation on infarct size by decreasing the infarct size observed in socially isolated mice, whereas OTA administration eliminated the effects of social isolation on infarct size by increasing the infarct size observed in pair-housed mice. These results indicate that OT serves a neuroprotective function following cerebral ischemia. Karelina et al. (2011) suggested that this neuroprotective effect was through a suppressive action of OT on microglia, a key instigator of the development of neuroinflammation following cerebral ischemia. Further studies are needed to better understand the interaction between microglia, neuroprotection, oxytocin, IL-6, CRP and social isolation in animals as well as in humans.
Sleep
Most animal research concerns the effects of social isolation on brain structures or processes during waking states. However, male adult mice who were housed individually for 4–8 weeks, compared to group housed mice, showed shorter pentobarbital-induced sleeping time (Baumel, DeFero, & Lal, 1970; Watanabe, Ohdo, Ishikawa, & Ogawa, 1992), indicating a decrease in the hypnotic activity of barbiturates. Several mechanisms have been implicated in this effect. For instance, Matsumoto, Ojima, and Watanabe (1996) demonstrated that neurosteroids that modulate GABA are involved; Dong, Matsumoto, Tohda, and Watanabe (1999) demonstrated that endogenous ligands for benzodiazepine receptors (e.g., diazepam binding inhibitor, octadecaneuropeptide) may be involved; and Ojima, Matsumoto, Tohda, and Watanabe (1995) demonstrated that the noradrenergic modulation of the central corticotropin releasing factor (CRF) system may be involved. A pharmacological inter-action between CRF and GABAA-benzodiazepine systems has been found so these studies suggest that changes in the level of proteins with the ability to modulate GABAA receptor function underlie the social isolation-induced differences in hypnotic activity of pentobarbital in mice.
Importantly, in light of human research showing an association between loneliness and sleep efficiency (e.g., J. T. Cacioppo, Hawkley, Berntson, et al., 2002; Kurina et al., 2011), Kaushal et al. (2012) reported that adult mice who were socially isolated for 5 weeks, compared to pair-housed, showed a marked reduction in EEG delta power in NREM sleep during baseline conditions. The socially isolated mice also showed a blunted homeostatic sleep response to acute sleep deprivation. Both isolated and pair-housed mice showed increases in EEG delta power in NREM sleep following sleep deprivation, but this increase in EEG delta power did not persist throughout the dark period in socially isolated mice, indicating less deep sleep and poorer sleep quality compared to matched pair-housed mice. This difference was still evident 18 hr after deprivation, when recordings were terminated.
Conclusion
Perhaps one of the more remarkable observations in this review is that the emphasis on the brain as the key organ for understanding the association between social relationships and health marks a departure from the status quo. Over a quarter century ago, House et al. (1988) published a landmark review of prospective epidemiological studies of social isolation in humans. They reported that social isolation was a significant risk factor for broad-based morbidity and mortality—a finding that subsequent research has confirmed (Holt-Lunstad et al., 2010). What was especially surprising was that social isolation was as strong a risk factor for morbidity and mortality as smoking, obesity, sedentary lifestyle, and high blood pressure (House et al., 1988). The social control hypothesis was posited to explain the effect of isolation. Social control theory holds that internalized obligations to, and the overt influence of, network members tend to discourage poor health behaviors and encourage good health behaviors (e.g., Umberson, 1987). For instance, among women, direct social control (i.e., “How often does anyone tell or remind you to do anything to protect your health?”) predicted increased physical activity 3 years later (Umberson, 1992). Being married is associated with an increased likelihood of engaging in health-promoting behaviors such as exercise (Pettee et al., 2006; Satariano, Haight, & Tager, 2002; Schmitz, French, & Jeffery, 1997), presumably because marital partners exert some influence over these behaviors (Umberson, 1987, 1992).
The focus on the objective nature of social relationships (e.g., marriage, contact with friends or family), the social control hypothesis, and parsimony together led to the impression that neuroscientific and animal studies were irrelevant to understanding the causal role of social relationships on morbidity and mortality. However, the brain is the central organ for forming, monitoring, maintaining, repairing, and replacing salutary connections with others; this is true for humans and is likely to be true for other species for whom sociality has been a central feature of life for millions of years. The human brain does not simply respond to stimuli in an invariant fashion but rather categorizes, abstracts, and evaluates incoming stimuli in light of current states and goals as well as prior experiences and predispositions. For instance, the same objective social relationship (e.g., a marriage) can be experienced as caring and protective or as exploitive and isolating based on a host of factors including the person’s prior experiences, current attributions, and overall preference for social contact. The current review shows a sizeable literature has emerged over the past 15 years showing that (a) the perception of social isolation—or what Weiss (1973) termed loneliness—is a major risk factor for morbidity and mortality above and beyond objective social isolation; and (b) the social control hypothesis is not sufficient to explain the association between loneliness and mortality (e.g., Luo et al., 2012; Seeman, 2000).
It is difficult in human studies alone to delineate fully the causal role of perceived social isolation on morbidity and mortality or the mechanisms through which perceived isolation has such effects. Our goal here is not so much to provide a definitive answer to the question of how (perceived) social isolation affects morbidity and mortality in humans but to determine whether the animal literature may have something to contribute to the answer. As noted in this review, the human and animal literatures on social isolation developed independently to address somewhat different questions. The human research has tended to focus on social isolation (objective and perceived) to investigate how the social environment affects social perception, morbidity, and mortality, whereas the animal literature has tended to focus on social isolation to investigate brain plasticity/learning or various behavioral disorders (e.g., depression, anxiety, schizophrenia, aggression). As a result, the extant animal literature does not provide a final answer to the question of causation or underlying mechanisms in humans.
There are also important inconsistencies in the animal literature regarding precisely what are the effects of social isolation on the brain. The incredible complexity of social life within and across species and gender, the plethora of brain mechanisms needed to make sense of and respond to an ever-changing social world, and the still nascent level of understanding of the social brain may explain in part these apparent inconsistencies. The typically small sample sizes and underpowered studies, coupled with an emphasis on null hypothesis testing, may also be contributing to some of the inconsistencies in the animal literature.4 Nevertheless, the present review indicates that (a) social isolation (or a solitary state) has significant effects on brain structure and processes in adult social animals; (b) these effects are not uniform across the brain or across species but instead tend to be most evident in brain regions that permit adaptation to differences in the functional demands of solitary versus social living for a particular species; and (c) the effects are not simply weakened recapitulations of what is observed as a result of isolation during development.
The review underscores the potential value of animal studies of social isolation for investigating the mechanisms through which social relationships impact the brain, health, and well-being. For instance, experimental studies designed to examine the short-term effects of loneliness suggest that it increases depressive symptomatology, anxiety, aggressiveness, and impulsive responding (e.g., Baumeister & DeWall, 2005; J. T. Cacioppo, et al., 2006), and longitudinal studies suggest that loneliness increases depressive symptomatology (e.g., J. T. Cacioppo et al., 2010; Vanderweele et al., 2011), sleep fragmentation (Hawkley, Preacher, & Cacioppo, 2010), blood pressure (Hawkley, Thisted, et al., 2010), hypothalamic pituitary adrenocortical activity (Adam et al., 2006; Hawkley, Cole, Capitanio, Norman, & Cacioppo, 2012), cognitive decline and dementia (e.g., Wilson et al., 2007), and mortality (e.g., Luo et al., 2012). Experimental studies in nonhuman animals indicate that social isolation, compared to pair or group housing, increases depressive, anxiety, and aggressive behaviors (e.g., Nin et al., 2011; Norman et al., 2010); predator evasion (e.g., Hofer, 2009; Kaushal et al., 2012); sleep impairments (Kaushal et al., 2012); and neuroinflammation and cell death following experimental cerebral ischemia (Karelina, Norman, Zhang, & DeVries, 2009; Karelina, Norman, Zhang, Morris, et al., 2009; Karelina et al., 2011; Venna et al., 2012); glucocorticoid levels (e.g., Cross et al., 2004; Sapolsky et al., 1997; A. S. Smith et al., 2011); and blood pressure (Coelho et al., 1991). What is needed are more explicitly integrated animal and human studies to determine whether these similarities are superficial or extend to underlying mechanisms.
As but one example, the large prospective study by Wilson et al. (2007) showed that loneliness at baseline predicted greater cognitive declines in multiple cognitive domains and increased risk for dementia even after controlling for objective social isolation, education, gender, age, and other health-related factors. Brain autopsies were available for 67% of the participants who died during the study. Of these, 30% had a clinical diagnosis of Alzheimer’s disease. Loneliness and the neuropathological measures derived from the brain autopsy were each inversely related to global cognition at the last assessment prior to death, but loneliness was unrelated to the neuropathological measures. Although the mechanism underlying the association between loneliness and cognitive decline has not yet been identified, there are two lines of evidence that point to neuroinflammation as possibly playing a role. As reviewed above, studies in mice have found that social isolation decreases central anti-inflammatory responses and survival rate and increase the infarct size and edema development, following the induction of stroke in mice (Karelina, Norman, Zhang, & DeVries, 2009; Karelina, Norman, Zhang, et al., 2009; Venna et al., 2012). Moreover, research in humans has shown loneliness to be related to increased gene expression of proinflammatory NF-κB transcripts (Cole et al., 2007, 2011) and inflammation (e.g., Jaremka et al., 2013).
If the brain is such a key organ for social connections and processes, does the perception that one is socially isolated impact brain structures and processes? The extant research reveals significant associations between loneliness and regional gray matter density (Kanai, Bahrami, Duchaine, et al., 2012), regional brain activation to social in contrast to nonsocial stimuli (J. T. Cacioppo, Norris, et al., 2009), age-related cognitive decline (Gow et al., 2007), and the onset of dementia in an elderly sample (Wilson et al., 2007), but this literature is still very limited. The extant experimental research in adult rodents showing, for instance, isolation-induced differences in myelination in the prefrontal cortex (Liu et al., 2012) and concentrations of neurosteroids (e.g., Nin et al., 2011) and growth factors (BDNF, CREB, Egr-1; e.g., Matsumoto et al., 2012; Wallace et al., 2009), as well as evidence that these differences are related to behaviors (e.g., social withdrawal, aggressive behavior, anxiety & depressive behavior) that also characterize lonely individuals, provide fertile ground on which to build collaborative human investigations.
In sum, in ontogeny and across phylogeny, members of many species need the aid and companionship of others to survive and prosper. Animal studies of social isolation are an important complement to human studies because randomization and experimental manipulations of isolation in humans are limited in intensity and duration by the possible damaging effects, and the manipulations and measurements of brain structures and function that are appropriate for human studies limits the mechanisms that can be investigated. A closer integration of human and animal research would help overcome these limitations. The integration of human and animal research also holds promise for the development of biomarkers and more effective interventions for loneliness to improve well-being and to mitigate the deleterious behavioral, neurological and health effects. The first episode of The Twilight Zone, entitled “Where is Everybody?” aired 55 years ago and ended by noting that loneliness is a problem that has yet to be solved. Thirty-six years ago, the U.S. President’s Commission on Mental Health (1978) reported that “Until recently, the role of affiliative and supportive structures to help ease the pain of depression, loneliness, and helplessness has been minimal. . . . The need for integration of health and social services is nowhere so clear or so urgent as in the care of the elderly” (p. 8). The development of effective interventions for loneliness is still needed (see review by Masi, Chen, Hawkley, & Cacioppo, 2011) and is a timely goal given increasing prevalence of social isolation in industrialized countries. According to the U.S. Census Bureau, about 31 million Americans were living alone in 2010. By 2050, the U.S. Census Bureau (2009) projects this number to range between 43.2 and 57 million Americans. Perceived social isolation has also increased, with a recent report indicating that loneliness now affects over 40% of older adults in the United States (Perissinotto et al., 2012). Given an aging population in industrialized nations and the rise in the prevalence of perceived isolation, the integration of animal and human studies of isolation to determine underlying mechanisms and treatments is needed now more than ever before.
Acknowledgments
This research was supported by National Institute on Aging Grant R01-AG033590. We are grateful to Richard Suzman for his feedback and support.
Footnotes
Because individual housing is known to be deleterious in these non-human social species, current guidelines recommend that social isolation should be kept to a minimum.
Neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), play a major role in brain plasticity, neuroprotection, and brain functions and are good candidates for transducing the effects of adverse social events, such as social isolation, into changes in brain function. Aberrations in its expressions have been implicated in brain disorders (Cirulli et al., 2009).
Nonhuman primate research has primarily focused on maternal separation (i.e., partial social isolation from mother but not necessarily from peers) as a model of social deprivation (Harlow & Suomi, 1971; Harlow & Zimmermann, 1959; Suomi, 1997; Suomi & Harlow, 1972) rather than total social isolation per se (animals reared in total social isolation i.e., with no parents and no peers; Griffin & Harlow, 1966; Harlow, Dodsworth, & Harlow, 1965; Mason & Sponholz, 1963) and has been almost exclusively concerned with the early experience of these conditions. In line with studies on social isolation in adults of other species, however, results on maternal separation indicate a specific altered activity of subcortical regions, including a decreased phosphorylation of neurofilament protein in the dentate gyrus of the hippocampal formation (Siegel et al., 1993), changes in the chemoarchitecture of striatal structures such as the caudate and putamen (Martin, Spicer, Lewis, Gluck, & Cork, 1991), and abnormalities in the amygdala and bed nucleus stria terminalis (Gudsnuk & Champagne, 2011; Martin et al., 1991).
Button et al. (2013) provided a lucid discussion of this issue in the realm of neuroimaging, but the statistical issues, observations, and recommendations are applicable to the experimental neurobiological literature as well.
Contributor Information
Stephanie Cacioppo, University of Chicago.
John P. Capitanio, University of California, Davis
John T. Cacioppo, University of Chicago
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences, USA. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proceedings of the National Academy of Sciences, USA. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ågren G, Meyerson BJ. Long-term effects of social deprivation during early adulthood in the Mongolian gerbil (Meriones unguiculatus) Zeitschrift für Tierpsychologie. 1978;47:422–431. [PubMed] [Google Scholar]
- Alexander RD. The evolution of social behavior. Annual Review of Ecology, Evolution, and Systematics. 1974;5:352–383. doi: 10.1146/annurev.es.05.110174.001545. [DOI] [Google Scholar]
- Altman J, Das GD. Autoradiographic examination of the effects of enriched environment on the rate of glial multiplication in the adult rat brain. Nature. 1964;204:1161–1163. doi: 10.1038/2041161a0. [DOI] [PubMed] [Google Scholar]
- An C, Shi Y, Li P, Hu X, Gan Y, Stetler RA, … Chen J. Molecular dialogs between the ischemic brain and the peripheral immune system: Dualistic roles in injury and repair. Progress in Neurobiology. 2013;115:6–24. doi: 10.1016/j.pneurobio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SB, Adkins-Regan E. Effect of isolation and conspecific presence in a novel environment on corticosterone concentrations in a social avian species, the zebra finch (Taeniopygia guttata) Hormones and Behavior. 2011;60:233–238. doi: 10.1016/j.yhbeh.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Barnea A, Mishal A, Nottebohm F. Social and spatial changes induce multiple survival regimes for new neurons in two regions of the adult brain: An anatomical representation of time? Behavioural Brain Research. 2006;167:63–74. doi: 10.1016/j.bbr.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/S0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- Barrot M, Wallace DL, Bolaños CA, Graham DL, Perrotti LI, Neve RL, … Nestler EJ. Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proceedings of the National Academy of Sciences, USA. 2005;102:8357–8362. doi: 10.1073/pnas.0500587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister R, DeWall N. The inner dimension of social exclusion: Intelligent thought and self-regulation among rejected persons. In: Williams KD, Forgas JP, von Hippel W, editors. The social outcast: Ostracism, social exclusion, rejection, and bullying. New York, NY: Psychology Press; 2005. pp. 53–76. [Google Scholar]
- Baumel I, DeFero JJ, Lal H. Alterations in brain sensitivity and barbiturate metabolism unrelated to aggression in socially deprived mice. Psychopharmacologia. 1970;18:320–324. doi: 10.1007/BF00412678. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity of brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Rosenzweig MR, Diamond MC. Rat brain: Effects of environmental enrichment on wet and dry weights. Science. 1969;163:825–826. doi: 10.1126/science.163.3869.825. [DOI] [PubMed] [Google Scholar]
- Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, Cirulli F. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. 2012;37:762–772. doi: 10.1016/j.psyneuen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Bhide PG, Bedi KS. The effects of a lengthy period of environmental diversity on well-fed and previously undernourished rats. II. Synapse-to-neuron ratios. Journal of Comparative Neurology. 1984;227:305–310. doi: 10.1002/cne.902270213. [DOI] [PubMed] [Google Scholar]
- Bjørnebekk A, Mathé AA, Gruber SHM, Brené S. Social isolation increases number of newly proliferated cells in hippocampus in female Flinders Sensitive Line rats. Hippocampus. 2007;17:1193–1200. doi: 10.1002/hipo.20352. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Cacioppo JT, Muthén B, Asparouhov T, Clark S. Longitudinal genetic analysis for loneliness in Dutch twins. Twin Research and Human Genetics. 2007;10:267–273. doi: 10.1375/twin.10.2.267. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Willemsen G, Dolan CV, Hawkley LC, Cacioppo JT. Genetic and environmental contributions to loneliness in adults: The Netherlands Twin Register Study. Behavior Genetics. 2005;35:745–752. doi: 10.1007/s10519-005-6040-8. [DOI] [PubMed] [Google Scholar]
- Booth R. Loneliness as a component of psychiatric disorders. Medscape General Medicine. 2000;2:1–7. Retrieved from http://www.medscape.com/viewarticle/430545. [Google Scholar]
- Breuer M, Hoste B, De Loof A. The endocrine control of phase transition: Some new aspects. Physiological Entomology. 2003;28:3–10. doi: 10.1046/j.1365-3032.2003.00313.x. [DOI] [Google Scholar]
- Burrows M, Rogers SM, Ott SR. Epigenetic remodelling of brain, body and behaviour during phase change in locusts. Neural Systems & Circuits. 2011;1(11) doi: 10.1186/2042-1001-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Boomsma DI. Evolutionary mechanisms for loneliness. Cognition & Emotion. 2014;28:3–21. doi: 10.1080/02699931.2013.837379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Cole SW, Capitanio JP, Goossens L, Boomsma DI. Loneliness across phylogeny and a call for comparative studies and animal models. Perspectives on Psychological Science. doi: 10.1177/1745691614564876. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Ernst JM, Burleson MH, McClintock MK, Malarkey WB, Hawkley LC, … Berntson GG. Lonely traits and concomitant physiological processes: The MacArthur social neuroscience studies. International Journal of Psychophysiology. 2000;35:143–154. doi: 10.1016/S0167-8760(99)00049-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Fowler JH, Christakis NA. Alone in the crowd: The structure and spread of loneliness in a large social network. Journal of Personality and Social Psychology. 2009;97:977–991. doi: 10.1037/a0016076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends in Cognitive Sciences. 2009;13:447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG, Ernst JM, Gibbs AC, Stickgold R, Hobson JA. Do lonely days invade the nights? Potential social modulation of sleep efficiency. Psychological Science. 2002;13:384–387. doi: 10.1111/j.0956-7976.2002.00469.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, … Berntson GG. Loneliness and health: Potential mechanisms. Psychosomatic Medicine. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Ernst JM, Burleson M, Berntson GG, Nouriani B, Spiegel D. Loneliness within a nomological net: An evolutionary perspective. Journal of Research in Personality. 2006;40:1054–1085. doi: 10.1016/j.jrp.2005.11.007. [DOI] [Google Scholar]
- Cacioppo JT, Hawkley LC, Thisted RA. Perceived social isolation makes me sad: Five-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychology and Aging. 2010;25:453–463. doi: 10.1037/a0017216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: Cross sectional and longitudinal analyses. Psychology and Aging. 2006;21:140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Norris CJ, Decety J, Monteleone G, Nusbaum H. In the eye of the beholder: Individual differences in perceived social isolation predict regional brain activation to social stimuli. Journal of Cognitive Neuroscience. 2009;21:83–92. doi: 10.1162/jocn.2009.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Patrick B. Loneliness: Human nature and the need for social connection. New York, NY: Norton; 2008. [Google Scholar]
- Cacioppo S, Bianchi-Demicheli F, Frum C, Pfaus JG, Lewis JW. The common neural bases between sexual desire and love: A multilevel kernel density fMRI analysis. Journal of Sexual Medicine. 2012;9:1048–1054. doi: 10.1111/j.1743-6109.2012.02651.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, Cacioppo JT. A quantitative meta-analysis of functional imaging studies of social rejection. Scientific Reports. 2013;3:2027. doi: 10.1038/srep02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Hawkley LC, Cole SW, Cacioppo JT. A behavioral taxonomy of loneliness in monkeys and rhesus monkeys. PLoS ONE. doi: 10.1371/journal.pone.0110307. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Moffitt TE, Milne BJ, Poulton R. Socially isolated children 20 years later: Risk of cardiovascular disease. Archives of Pediatrics & Adolescent Medicine. 2006;160:805–811. doi: 10.1001/archpedi.160.8.805. [DOI] [PubMed] [Google Scholar]
- Chaudhuri JR, Mridula KR, Umamahesh M, Swathi A, Balaraju B, Bandaru VC. High sensitivity C-reactive protein levels in acute ischemic stroke and subtypes: A study from a tertiary care center. Iran Journal of Neurology. 2013;12:92–97. [PMC free article] [PubMed] [Google Scholar]
- Cirulli F, Francia N, Berry A, Aloe L, Alleva E, Suomi SJ. Early life stress as a risk factor for mental health: Role of neurotrophins from rodents to non-human primates. Neuroscience and Biobehavioral Reviews. 2009;33:573–585. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho AM, Carey KD, Shade RE. Assessing the effects of social environment on blood pressure and heart rates of baboons. American Journal of Primatology. 1991;23:257–267. doi: 10.1002/ajp.1350230406. [DOI] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JMG, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proceedings of the National Academy of Sciences, USA. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biology. 2007;8(9) doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft TKS, Glasper ER, McCullough L, Zhang N, Sugo N, Otsuka T, … DeVries AC. Social interaction improves experimental stroke outcome. Stroke: A Journal of Cerebral Circulation. 2005;36:2006–2011. doi: 10.1161/01.STR.0000177538.17687.54. [DOI] [PubMed] [Google Scholar]
- Cross N, Pines MK, Rogers LJ. Saliva sampling to assess cortisol levels in unrestrained common marmosets and the effect of behavioral stress. American Journal of Primatology. 2004;62:107–114. doi: 10.1002/ajp.20005. [DOI] [PubMed] [Google Scholar]
- Dahlqvist P, Ronnback A, Risedal A, Nergardh R, Johansson IM, Seckl JR, … Olsson T. Effects of postischemic environment on transcription factor and serotonin receptor expression after permanent focal cortical ischemia in rats. Neuroscience. 2003;119:643–652. doi: 10.1016/S0306-4522(03)00195-7. [DOI] [PubMed] [Google Scholar]
- Darwin C. The descent of man and selection in relation to sex. 2. New York, NY: Merrill & Baker; 1874. [DOI] [Google Scholar]
- Day NF, Kinnischtzke AK, Adam M, Nick TA. Daily and developmental modulation of “premotor” activity in the birdsong system. Developmental Neurobiology. 2009;69:796–810. doi: 10.1002/dneu.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. NeuroImage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- de Quervain DJF, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, Fehr E. The neural basis of altruistic punishment. Science. 2004;305:1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Joh HD, Bernard O, Hattori K, Hurn PD, Traystman RJ, Alkayed NJ. Social stress exacerbates stroke outcome by suppressing Bcl-2 expression. Proceedings of the National Academy of Sciences, USA. 2001;98:11824–11828. doi: 10.1073/pnas.201215298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Nelson RJ, Traystman RJ, Hurn PD. Cognitive and behavioral assessment in experimental stroke research: Will it prove useful? Neuroscience and Biobehavioral Reviews. 2001;25:325–342. doi: 10.1016/S0149-7634(01)00017-3. [DOI] [PubMed] [Google Scholar]
- Diamond MC. Response of the brain to enrichment. Anais da Academia Brasileira de Ciências. 2001;73:211–220. doi: 10.1590/S0001-37652001000200006. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Ingham CA, Johnson RE, Bennett EL, Rosenzweig MR. Effects of environment on morphology of rat cerebral cortex and hippocampus. Journal of Neurobiology. 1976;7:75–85. doi: 10.1002/neu.480070108. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Johnson R, Ingham C, Rosenzweig MR, Bennett EL. Effects of differential experience on neuronal nuclear and perikarya dimensions in the rat cerebral cortex. Behavioral Biology. 1975;15:107–111. doi: 10.1016/S0091-6773(75)92144-6. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Krech D, Rosenzweig MR. The effects of an enriched environment on the histology of the rat cerebral cortex. Journal of Comparative Neurology. 1964;123:111–120. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Law F, Rhodes H, Lindner B, Rosenzweig MR, Krech D, Bennett EL. Increases in cortical depth and glia numbers in rats subjected to enriched environment. Journal of Comparative Neurology. 1966;128:117–126. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Lindner B, Raymond A. Extensive cortical depth measurements and neuron size increases in the cortex of environmentally enriched rats. Journal of Comparative Neurology. 1967;131:357–364. doi: 10.1002/cne.901310305. [DOI] [Google Scholar]
- Diamond MC, Rosenzweig MR, Bennett EL, Lindner B, Lyon L. Effects of environmental enrichment and impoverishment on rat cerebral cortex. Journal of Neurobiology. 1972;3:47–64. doi: 10.1002/neu.480030105. [DOI] [PubMed] [Google Scholar]
- Distel MA, Rebollo-Mesa I, Abdellaoui A, Derom CA, Willemsen G, Cacioppo JT, Boomsma DI. Familial resemblance for loneliness. Behavior Genetics. 2010;40:480–494. doi: 10.1007/s10519-010-9341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D, Cruess S, Kilbourn K, Klimas N, Fletcher MA, Ironson G, … Antoni MH. Social support mediates loneliness and human herpesvirus Type 6 (HHV-6) antibody titers. Journal of Applied Social Psychology. 2001;31:1111–1132. doi: 10.1111/j.1559-1816.2001.tb02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic A, Adzic M, Djordjevic J, Radojcic MB. Chronic social isolation suppresses proplastic response and promotes proapoptotic signalling in prefrontal cortex of Wistar rats. Journal of Neuroscience Research. 2010;88:2524–2533. doi: 10.1002/jnr.22403. [DOI] [PubMed] [Google Scholar]
- Doane LD, Adam EK. Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendocrinology. 2010;35:430–441. doi: 10.1016/j.psyneuen.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Tohda M, Watanabe H. Involvement of diazepam binding inhibitor and its fragment octadecaneuropeptide in social isolation stress-induced decrease in pentobarbital sleep in mice. Life Sciences. 1999;64:1779–1784. doi: 10.1016/S0024-3205(99)00116-2. [DOI] [PubMed] [Google Scholar]
- Duck S, Pond K, Leatham G. Loneliness and the evaluation of relational events. Journal of Social and Personal Relationships. 1994;11:253–276. doi: 10.1177/0265407594112006. [DOI] [Google Scholar]
- Dunbar RIM. The social brain hypothesis. Evolutionary Anthropology. 1998;6:178–190. doi:10.1002/(SICI)1520-6505(1998)6: 5%3C178::AID-EVAN5%3E3.3.CO;2-P. [Google Scholar]
- Dunbar RIM. Evolution of the social brain. Science. 2003;302:1160–1161. doi: 10.1126/science.1092116. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, Chung M. Social novelty enhances brain cell proliferation, cell survival, and chirp production in an electric fish, Apteronotus leptorhynchus. Developmental Neurobiology. 2013;73:324–332. doi: 10.1002/dneu.22063. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, Chung M, Castellano JF. Influence of long-term social interaction on chirping behavior, steroid levels and neurogenesis in weakly electric fish. The Journal of Experimental Biology. 2013;216:2434–2441. doi: 10.1242/jeb.082875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap KD, Silva AC, Chung M. Environmental complexity, seasonality and brain cell proliferation in a weakly electric fish, Brachyhypopomus gauderio. The Journal of Experimental Biology. 2011;214:794–805. doi: 10.1242/jeb.051037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaker ED, Pinsky J, Castelli WP. Myocardial infarction and coronary death among women: Psychosocial predictors from a 20-year follow-up of women in the Framingham Study. American Journal of Epidemiology. 1992;135:854–864. doi: 10.1093/oxfordjournals.aje.a116381. [DOI] [PubMed] [Google Scholar]
- Eaton GG, Kelley ST, Axthelm MK, Iliff-Sizemore SA, Shiigi SM. Psychological well-being in paired adult female rhesus (Macaca mulatta) American Journal of Primatology. 1994;33:89–99. doi: 10.1002/ajp.1350330204. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Liberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. Dimensions of personality. London, England: Paul, Trench, Trubner; 1947. [Google Scholar]
- Farrell R, Evans S, Corbett D. Environmental enrichment enhances recovery of function but exacerbates ischemic cell death. Neuroscience. 2001;107:585–592. doi: 10.1016/S0306-4522(01)00386-4. [DOI] [PubMed] [Google Scholar]
- Farris SM, Schulmeister S. Parasitoidism, not sociality, is associated with the evolution of elaborate mushroom bodies in the brains of hymenopteran insects. Proceedings of the Royal Society: Biological Sciences. 2011;278:940–951. doi: 10.1098/rspb.2010.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland CL, Schrader LA. Cage mate separation in pair-housed male rats evokes an acute stress corticosterone response. Neuroscience Letters. 2011;489:154–158. doi: 10.1016/j.neulet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finarelli JA, Flynn JJ. Brain-size evolution and sociality in Carnivora. Proceedings of the National Academy of Sciences, USA. 2009;106:9345–9349. doi: 10.1073/pnas.0901780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliessbach K, Weber B, Trautner P, Dohmen T, Sunde U, Elger CE, Falk A. Social comparison affects reward-related brain activity in the human ventral striatum. Science. 2007;318:1305–1308. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. Journal of Neurobiology. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Garrido P, De Blas M, Ronzoni G, Cordero I, Anton M, Gine E, … Mora F. Differential effects of environmental enrichment and isolation housing on the hormonal and neurochemical responses to stress in the prefrontal cortex of the adult rat: Relationship to working and emotional memories. Journal of Neural Transmission. 2013;120:829–843. doi: 10.1007/s00702-012-0935-3. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Ortega-Perez I, Murray K, Lledo P. A niche for adult neurogenesis in social behavior. Behavioural Brain Research. 2009;200:315–322. doi: 10.1016/j.bbr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Gilbert MH, Baker KC. Social buffering in adult male rhesus macaques (Macaca mulatta): Effects of stressful events in single vs. pair housing. Journal of Medical Primatology. 2011;40:71–78. doi: 10.1111/j.1600-0684.2010.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R, Kemp JA, Sabin C, Pepys MB. Human C-reactive protein increases cerebral infarct size after middle cerebral artery occlusion in adult rats. Journal of Cerebral Blood Flow & Metabolism. 2004;24:1214–1218. doi: 10.1097/01.WCB.0000136517.61642.99. [DOI] [PubMed] [Google Scholar]
- Glaser K, Evandrou M, Tomassini C. The health consequences of multiple roles at older ages in the UK. Health and Social Care in the Community. 2005;13:470–477. doi: 10.1111/j.1365-2524.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. Journal of Behavioral Medicine. 1985;8:249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proceedings of the National Academy of Sciences, USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow AJ, Pattie A, Whiteman MC, Whalley LJ, Deary IJ. Social support and successful ageing: Investigating the relationships with lifetime cognitive change and life satisfaction. Journal of Individual Differences. 2007;28:103–115. doi: 10.1027/1614-0001.28.3.103. [DOI] [Google Scholar]
- Griffin GA, Harlow HF. Effects of 3 months of total social deprivation on social adjustment and learning in the rhesus monkey. Child Development. 1966;37:533–547. doi: 10.2307/1126677. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic Medicine. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenberg W, Heeren S, Hölldobler B. Age-dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. Journal of Experimental Biology. 1996;199:2011–2019. doi: 10.2307/2937655. [DOI] [PubMed] [Google Scholar]
- Gudsnuk KM, Champagne FA. Epigenetic effects of early developmental experiences. Clinics in Perinatology. 2011;38:703–717. doi: 10.1016/j.clp.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially isolated mouse: A model to study the putative role of allopregnanolone and 5a-dihydroprogesterone in psychiatric disorders. Brain Research Reviews. 2001;37:110–115. doi: 10.1016/S0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Brodie AR, McGuire HM. Effect of a preferred companion in modulating stress in adult female rhesus monkeys. Physiology & Behavior. 1994;55:681–684. doi: 10.1016/0031-9384(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Hambright MK. Response to removal from and return to a social group in adult male rhesus monkeys. Physiology & Behavior. 1993;53:599–602. doi: 10.1016/0031-9384(93)90159-D. [DOI] [PubMed] [Google Scholar]
- Hackett RA, Hamer M, Endrighi R, Brydon L, Steptoe A. Loneliness and stress-related inflammatory and neuroendocrine responses in older men and women. Psychoneuroendocrinology. 2012;37:1801–1809. doi: 10.1016/j.psyneuen.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Dodsworth RO, Harlow MK. Total social isolation in monkeys. Proceedings of the National Academy of Sciences, USA. 1965;54:90–9697. doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Suomi SJ. Social recovery by isolation-reared monkeys. Proceedings of the National Academy of Sciences, USA. 1971;68:1534–1538. doi: 10.1073/pnas.68.7.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Zimmermann RR. Affectional responses in the infant monkey. Science. 1959;130:421–432. doi: 10.1126/science.130.3373.421. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Burleson MH, Berntson GG, Cacioppo JT. Loneliness in everyday life: Cardiovascular activity, psychosocial context, and health behaviors. Journal of Personality and Social Psychology. 2003;85:105–120. doi: 10.1037/0022-3514.85.1.105. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cole SW, Capitanio JP, Norman GJ, Cacioppo JT. Effects of social isolation on glucocorticoid regulation in social mammals. Hormones and Behavior. 2012;62:314–323. doi: 10.1016/j.yhbeh.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Hughes ME, Waite LJ, Masi CM, Thisted RA, Cacioppo JT. From social structure factors to perceptions of relationship quality and loneliness: The Chicago Health, Aging, and Social Relations Study. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2008;63:S375–S384. doi: 10.1093/geronb/63.6.S375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychology and Aging. 2006;21:152–164. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Preacher KJ, Cacioppo JT. Loneliness impairs daytime functioning but not sleep duration. Health Psychology. 2010;29:124–129. doi: 10.1037/a0018646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Thisted RA, Cacioppo JT. Loneliness predicts reduced physical activity: Cross-sectional & longitudinal analyses. Health Psychology. 2009;28:354–363. doi: 10.1037/a0014400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Thisted RA, Masi CM, Cacioppo JT. Loneliness predicts increased blood pressure: 5-year cross-lagged analyses in middle-aged and older adults. Psychology and Aging. 2010;25:132–141. doi: 10.1037/a0017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. What do the mushroom bodies do for the insect brain? An introduction. Learning & Memory. 1998;5:1–10. [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M, Heusipp M, Wanke C. Structural plasticity in the Drosophila brain. Journal of Neuroscience. 1995;15:1951–1960. doi: 10.1523/JNEUROSCI.15-03-01951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA. Developmental neuroscience. In: Berntson GG, Cacioppo JT, editors. Handbook of neuroscience for the behavioral sciences. Hoboken, NJ: Wiley; 2009. pp. 12–31. [DOI] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda TJ, Beekman ATF, Deeg DJH, Stek ML, Van Tilburg TG, Visser PJ, … Schoevers RA. Increased risk of mortality associated with social isolation in older men: Only when feeling lonely? Results from the Amsterdam Study of the Elderly (AM-STEL) Psychological Medicine. 2012;42:843–853. doi: 10.1017/S00.33291711001772. [DOI] [PubMed] [Google Scholar]
- House JS, Landis K, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Ioannou CC, Guttal V, Couzin ID. Predatory fish select for coordinated collective motion in virtual prey. Science. 2012;337:1212–1215. doi: 10.1126/science.1218919. [DOI] [PubMed] [Google Scholar]
- Ismail N, Robinson GE, Fahrbach SE. Stimulation of muscarinic receptors mimics experience-dependent plasticity in the honeybee brain. Proceedings of the National Academy of Sciences, USA. 2006;103:207–211. doi: 10.1073/pnas.0508318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JM, Cohen A, Hammerman-Rozenberg R, Stessman J. Global sleep satisfaction of older people: The Jerusalem Cohort Study. Journal of the American Geriatrics Society. 2006;54:325–329. doi: 10.1111/j.1532-5415.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- Jaremka LM, Fagundes CP, Peng J, Bennett JM, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Loneliness promotes inflammation during acute stress. Psychological Science. 2013;24:1089–1097. doi: 10.1177/0956797612464059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: Effect of environmental enrichment on intact and postischemic rat brain. Journal of Cerebral Blood Flow and Metabolism. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Johansson BB, Ohlsson AL. Environment, social interaction, and physical activity as determinants of functional outcome after cerebral infarction in the rat. Experimental Neurology. 1996;139:322–327. doi: 10.1006/exnr.1996.0106. [DOI] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Duchaine B, Janik A, Banissy MJ, Rees G. Brain structure links loneliness to social perception. Current Biology. 2012;22:1975–1979. doi: 10.1016/j.cub.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proceedings of the Royal Society: Biological Sciences. 2012;279:1327–1334. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara TS, Webber A, Gil-Mophapel J, Christie BR. Stress differentially regulates the effects of voluntary exercise on cell proliferation in the dentate gyrus of mice. Hippocampus. 2009;19:889–897. doi: 10.1002/hipo.20514. [DOI] [PubMed] [Google Scholar]
- Karelina K, Norman GJ, Zhang N, DeVries AC. Social contact influences histological and behavioral outcomes following cerebral ischemia. Experimental Neurology. 2009;220:276–282. doi: 10.1016/j.expneurol.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proceedings of the National Academy of Sciences, USA. 2009;106:5895–5900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, Stuller KA, Jarrett B, Zhang N, Wells J, Norman GJ, DeVries AC. Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke: A Journal of Cerebral Circulation. 2011;42:3606–3611. doi: 10.1161/STROKEAHA.111.628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Nair D, Gozal D, Ramesh V. Socially isolated mice exhibit a blunted homeostatic sleep response to acute sleep deprivation compared to socially paired mice. Brain Research. 2012;1454:65–79. doi: 10.1016/j.brainres.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Garner W, Speicher CE, Penn GM, Holliday JE, Glaser R. Psychosocial modifiers of immunocompetence in medical students. Psychosomatic Medicine. 1984a;46:7–14. doi: 10.1097/00006842-198401000-00003. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Ricker D, George J, Messick G, Speicher CE, Garner W, Glaser R. Urinary cortisol levels, cellular immunocompetency, and loneliness in psychiatric inpatients. Psychosomatic Medicine. 1984b;46:15–23. doi: 10.1097/00006842-198401000-00004. [DOI] [PubMed] [Google Scholar]
- Kong F, You X. Loneliness and self-esteem as mediators between social support and life satisfaction in late adolescence. Social Indicators Research. 2013;110:271–279. doi: 10.1007/s11205-011-9930-6. [DOI] [Google Scholar]
- Kühn-Bühlmann S, Wehner R. Age-dependent and task-related volume changes in the mushroom bodies of visually guided desert ants, Cataglyphis bicolor. Journal of Neurobiology. 2006;66:511–521. doi: 10.1002/neu.20235. [DOI] [PubMed] [Google Scholar]
- Kurina LM, Knutson KL, Hawkley LC, Cacioppo JT, Lauderdale DS, Ober C. Loneliness is associated with sleep fragmentation in a communal society. Sleep: Journal of Sleep and Sleep Disorders Research. 2011;34:1519–1526. doi: 10.5665/sleep.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S, Gruen GE. The social stigma of loneliness: Effect of target person’s and perceiver’s sex. Personality and Social Psychology Bulletin. 1992;18:182–189. doi: 10.1177/0146167292182009. [DOI] [Google Scholar]
- Leasure JL, Decker L. Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus. 2009;19:907–912. doi: 10.1002/hipo.20563. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Lehongre K, Aubin T, Del Negro C. Influence of social conditions in song sharing in the adult canary. Animal Cognition. 2009;12:823–832. doi: 10.1007/s10071-009-0241-0. [DOI] [PubMed] [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31:1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Li X, Xu F, Xie L, Ji Y, Cheng K, Zhou Q, … Xie P. Depression-like behavioral phenotypes by social and social plus visual isolation in the adult female Macaca fascicularis. PLoS One. 2013;8:e73293. doi: 10.1371/journal.pone.0073293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Hormones and Behavior. 2012;62:357–366. doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z. The social environment and neurogenesis in the adult mammalian brain. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihoreau M, Latty T, Chittka L. An exploration of the social brain hypothesis in insects. Frontiers in Physiology. 2012;3 doi: 10.3389/fphys.2012.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34:2518–2532. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- Lipkind D, Nottebohm F, Rado R, Barnea A. Social change affects the survival of new neurons in the forebrain of adult songbirds. Behavioural Brain Research. 2002;133:31–43. doi: 10.1016/S0166-4328(01)00416-8. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, … Casaccia P. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature Neuroscience. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Hawkley LC, Waite LJ, Cacioppo JT. Loneliness, health, and mortality in old age: A national longitudinal study. Social Science & Medicine. 2012;74:907–914. doi: 10.1016/j.socscimed.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleszka J, Barron AB, Helliwell PG, Maleszka R. Effect of age, behaviour and social environment on honey bee brain plasticity. Journal of Comparative Physiology A. 2009;195:733–740. doi: 10.1007/s00359-009-0449-0. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biology. 2004;1:351–363. doi: 10.1017/S1740925X05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Spicer DM, Lewis MH, Gluck JP, Cork LC. Social deprivation of infant rhesus monkeys alters the chemo-architecture of the brain: I. Subcortical regions. Journal of Neuroscience. 1991;11:3344–3358. doi: 10.1017/S1740925X05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi CM, Chen HY, Hawkley LC, Cacioppo JT. A meta-analysis of interventions to reduce loneliness. Personality and Social Psychology Review. 2011;15:219–266. doi: 10.1177/1088868310377394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Sponholz RR. Behavior of rhesus monkeys raised in isolation. Journal of Psychiatric Research. 1963;1:299–306. doi: 10.1016/0022-3956(63)90005-0. [DOI] [PubMed] [Google Scholar]
- Mataga N, Fujishima S, Condie BG, Hensch TK. Experience-dependent plasticity of mouse visual cortex in the absence of the neuronal activity-dependent marker egr1/zif268. The Journal of Neuroscience. 2001;21:9724–9732. doi: 10.1523/JNEUROSCI.21-24-09724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo JM, Holmes WG, Bell AM, Turner M. Sexual maturation in male prairie voles: Effects of the social environment. Physiology & Behavior. 1994;56:299–304. doi: 10.1016/0031-9384(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Ojima K, Watanabe H. Neurosteroidal modulation of social isolation-induced decrease in pentobarbital sleep in mice. Brain Research. 1996;708:1–6. doi: 10.1016/0006-8993(95)01277-X. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Ono K, Ouchi H, Tsushima R, Murakami Y. Social isolation stress down-regulates cortical early growth response 1 (Egr-1) expression in mice. Neuroscience Research. 2012;73:257–262. doi: 10.1016/j.neures.2012.04.004. [DOI] [PubMed] [Google Scholar]
- McGuire S, Clifford J. Genetic and environmental contributions to loneliness in children. Psychological Science. 2000;11:487–491. doi: 10.1111/1467-9280.00293. [DOI] [PubMed] [Google Scholar]
- McKenzie A, Diamond MC, Greer ER, Woo L, Telles T. The effects of enriched environment on neural recovery following lesioning of the forelimb area of rat cortex. Paper presented at the annual meeting of the American Physical Therapy Association Annual Conference; Anaheim, CA. 1990. Jun, [Google Scholar]
- Modigh K. Effects of isolation and fighting in mice on the rate of synthesis of noradrenaline, dopamine and 5-hydroxytryptamine in the brain. Psychopharmacologia. 1973;33:1–17. doi: 10.1007/BF00428790. [DOI] [PubMed] [Google Scholar]
- Mohammed AH, Zhu S, Darmophil S, Hjerling-Leffler J, Ernfors P, Winblad B, … Bogdanovic N. Environmental enrichment and the brain. Progress in Brain Research. 2002;138:109–133. doi: 10.1016/S0079-6123(02)38074-9. [DOI] [PubMed] [Google Scholar]
- Mohammed AK, Winblad B, Ebendal T, Lärkfors L. Environmental influence on behaviour and nerve growth factor in the brain. Brain Research. 1990;528:62–72. doi: 10.1016/0006-8993(90)90195-H. [DOI] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Egeland T, Andersen P. Spatial training in a complex environment and isolation alter the spine distribution differently in rat CA1 pyramidal cells. Journal of Comparative Neurology. 1997;380:373–381. doi: 10.1002/(sici)1096-9861(19970414)380:3<373::aid-cne6>3.0.co;2-#. doi:10.1002/(SICI)1096-9861 (19970414)380:3<373::AID-CNE6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Munching mice: Reference manual. 2013 Retrieved from http://science.education.nih.gov/supplements/nih4/energy/activities/508/mice-ref/mice_ref.htm.
- Nelson M, Pinna G. S-norfluoxetine infused into the basolateral amygdala increases allopregnanolone levels and reduces aggression in socially isolated mice. Neuropharmacology. 2011;60:1154–1159. doi: 10.1016/j.neuropharm.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehoff MO, Bergmann M, Weinbauer GF. Effects of social housing of sexually mature male cynomolgus monkeys during general and reproductive toxicity evaluation. Reproductive Toxicology. 2010;29:57–67. doi: 10.1016/j.reprotox.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. Journal of Neurobiology. 1999;39:569–578. doi: 10.1002/(SICI)1097-4695(19990615)39:4<569::AID-NEU10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Nin MS, Martinez LA, Pibiri F, Nelson M, Pinna G. Neurosteroids reduce social isolation-induced behavior deficits: A proposed link with neurosteroid-mediated upregulation of BDNF expression. Frontiers in Endocrinology. 2011;2:1–12. doi: 10.3389/fendo.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. Journal of Experimental Biology. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Zhang N, Morris JS, Karelina K, Berntson GG, DeVries AC. Social interaction modulates autonomic, inflammatory, and depressive-like responses to cardiac arrest and cardiopulmonary resuscitation. Proceedings of the National Academy of Sciences, USA. 2010;107:16342–16347. doi: 10.1073/pnas.1007583107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O’Donnell S, Clifford M, Molina Y. Comparative analysis of constraints and caste differences in brain investment among social paper wasps. Proceedings of the National Academy of Sciences, USA. 2011;108:7107–7112. doi: 10.1073/pnas.1017566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson AL, Johansson BB. Environment influences functional outcome of cerebral infarction in rats. Stroke. 1995;26:644–649. doi: 10.1161/01.STR.26.4.644. [DOI] [PubMed] [Google Scholar]
- Ojima K, Matsumoto K, Tohda M, Watanabe H. Hyperactivity of central noradrenergic and CRF systems is involved in social isolation-induced decrease in pentobarbital sleep. Brain Research. 1995;684:87–94. doi: 10.1016/0006-8993(95)00388-7. [DOI] [PubMed] [Google Scholar]
- Olsen RB, Olsen J, Gunner-Svensson F, Waldstrom B. Social networks and longevity: A 14-year follow-up study among elderly in Denmark. Social Science & Medicine. 1991;33:1189–1195. doi: 10.1016/0277-9536(91)90235-5. [DOI] [PubMed] [Google Scholar]
- Ott SR, Rogers SM. Gregarious desert locusts have substantially larger brains with altered proportions compared with the solitarious phase. Proceedings of the Royal Society B: Biological Sciences. 2010;277:3087–3096. doi: 10.1098/rspb.2010.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott SR, Verlinden H, Rogers SM, Brighton CH, Quah PS, Vleugels RK, … Vanden Broeck J. Critical role for protein kinase A in the acquisition of gregarious behavior in desert locust. Proceedings of the National Academy of Sciences, USA. 2012;109:E381–E387. doi: 10.1073/pnas.1114990109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AC, Veenstra G. Loneliness and risk of mortality: A longitudinal investigation in Alameda County, California. Social Science & Medicine. 2010;71:181–186. doi: 10.1016/j.socscimed.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Peplau LA, Russell D, Heim M. The experience of loneliness. In: Frieze IH, Bar-Tal D, Carroll JS, editors. New approaches to social problems: Applications of attribution theory. San Francisco, CA: Jossey-Bass; 1979. pp. 53–78. [Google Scholar]
- Perissinotto CM, Stijacic Cenzer I, Covinsky KE. Loneliness in older persons: A predictor of functional decline and death. Archives of Internal Medicine. 2012;172:1078–1083. doi: 10.1001/archinternmed.2012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D, Peplau LA. Toward a social psychology of loneliness. In: Duck S, Gilmour R, editors. Personal relationships in disorder. London, England: Academic Press; 1981. pp. 31–56. [Google Scholar]
- Pettee KK, Brach JS, Kriska AM, Boudreau R, Richardson CR, Colbert LH, … Newman AB. Influence of marital status on physical activity levels among older adults. Medicine and Science in Sports and Exercise. 2006;38:541–546. doi: 10.1249/01.mss.0000191346.95244.f7. [DOI] [PubMed] [Google Scholar]
- Pham TM, Ickes B, Albeck D, Söderström S, Granholm AC, Mohammed AH. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94:279–286. doi: 10.1016/S0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- Pickles AR, Hagan JJ, Jones DNC, Hendrie CA. Short-term individual housing induced social deficits in female Mongolian gerbils: Attenuation by chronic but not acute imipramine. Neuropharmacology. 2012;62:1993–1998. doi: 10.1016/j.neuropharm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Pinna G. In a mouse model relevant for post-traumatic stress disorder, selective brain steroidogenic stimulants (SBSS) improve behavioral deficits by normalizing allopregnanolone biosynthesis. Behavioural Pharmacology. 2010;21:438–450. doi: 10.1097/FBP.0b013e32833d8ba0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Done E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proceedings of the National Academy of Sciences, USA. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Partoo L. Effects of social isolation on mRNA expression for corticotrophin-releasing hormone receptors in prairie voles. Psychoneuroendocrinology. 2011;36:780–789. doi: 10.1016/j.psyneuen.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KE, Wagner DD, Norris CJ, Heatherton TF. Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Social, Cognitive, and Affective Neuroscience. 2013;8:151–157. doi: 10.1093/scan/nsr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychology. 2005;24:297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Vandingenen A, Begum M, Breuer M, De Loof A, Huybrechts R. Search for phase specific genes in the brain of desert locust, Schistocerca gregaria (Orthoptera: Acrididae) by differential display polymerase chain reaction. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2003;135:221–228. doi: 10.1016/S1095-6433(03)00050-3. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. In: Les nouvelles idées sur la structure du système nerveux chez l’homme et chez les vertébrés [New ideas about the structure of the nervous system in humans and in vertebrates] Azoulay L, translator. Paris, France: Reinwald; 1895. [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/S0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Risedal A, Mattsson B, Dahlqvist P, Nordborg C, Olsson T, Johansson BB. Environmental influences on functional outcome after a cortical infarct in the rat. Brain Research Bulletin. 2002;58:315–321. doi: 10.1016/S0361-9230(02)00796-7. [DOI] [PubMed] [Google Scholar]
- Rogers SM, Matheson T, Sasaki K, Kendrick K, Simpson SJ, Burrows M. Substantial changes in central nervous system neurotransmitters and neuromodulators accompany phase change in the locust. Journal of Experimental Biology. 2004;207:3603–3617. doi: 10.1242/jeb.01183. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: Effects of training and experience on brain and behavior. Behavioural Brain Research. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Research. 1978;153:563–576. doi: 10.1016/0006-8993(78)90340-2. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Love W, Bennett EL. Effects of a few hours a day of enriched experience on brain chemistry and brain weights. Physiology & Behavior. 1968;3:819–825. doi: 10.1016/0031-9384(68)90161-3. [DOI] [Google Scholar]
- Rotenberg K. Loneliness and interpersonal trust. Journal of Social and Clinical Psychology. 1994;13:152–173. doi: 10.1521/jscp.1994.13.2.152. [DOI] [Google Scholar]
- Rotenberg KJ, Gruman JA, Ariganello M. Behavioral confirmation of the loneliness stereotype. Basic and Applied Social Psychology. 2002;24:81–89. doi: 10.1207/S15324834BASP2402_1. [DOI] [Google Scholar]
- Rotenberg KJ, Kmill J. Perception of lonely and non-lonely persons as a function of individual differences in loneliness. Journal of Social and Personal Relationships. 1992;9:325–330. doi: 10.1177/0265407592092009. [DOI] [Google Scholar]
- Sánchez C, Arnt J, Hyttel J, Moltzen EK. The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology. 1993;110:53–59. doi: 10.1007/BF02246950. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Alberts SC, Altmann J. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Archives of General Psychiatry. 1997;54:1137–1143. doi: 10.1001/archpsyc.1997.01830240097014. [DOI] [PubMed] [Google Scholar]
- Satariano WA, Haight TJ, Tager IB. Living arrangements and participation in leisure-time physical activities in an older population. Journal of Aging and Health. 2002;14:427–451. doi: 10.1177/089826402237177. [DOI] [PubMed] [Google Scholar]
- Scaccianoce S, Del Bianco P, Paolone G, Caprioli D, Modafferi AME, Nencini P, Badiani A. Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behavioural Brain Research. 2006;168:323–325. doi: 10.1016/j.bbr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Schmitz K, French SA, Jeffery RW. Correlates of changes in leisure time physical activity over 2 years: The Healthy Worker Project. Preventive Medicine. 1997;26:570–579. doi: 10.1006/pmed.1997.0178. [DOI] [PubMed] [Google Scholar]
- Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. American Journal of Health Promotion. 2000;14:362–370. doi: 10.4278/0890-1171-14.6.362. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Teffer K, Buxhoeveden DP, Park MS, Bludau S, Amunts K, … Buckwalter J. Spatial organization of neurons in the frontal pole sets humans apart from great apes. Cerebral Cortex. 2011;21:1485–1497. doi: 10.1093/cercor/bhq191. [DOI] [PubMed] [Google Scholar]
- Sengupta P. The laboratory rat: Relating its age with human’s. International Journal of Preventive Medicine. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- Serling R, Writer, Stevens R., Director . Where is everybody? [Television series episode] In: Serling R Executive producer, editor. The twilight zone. New York, NY: Columbia Broadcasting System; 1959. [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. The Journal of Neuroscience. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Hamer M, McMunn A, Steptoe A. Social isolation and loneliness: Relationships with cognitive function during 4 years of follow-up in the English Longitudinal Study of Ageing. Psychosomatic Medicine. 2013;75:161–170. doi: 10.1097/PSY.0b013e31827f09cd. [DOI] [PubMed] [Google Scholar]
- Shintel H, Cacioppo JT, Nusbaum H. Accentuate the negative, eliminate the positive? Individual differences in attentional bias to positive and negative information. Paper presented at the 47th Annual Meeting of the Psychonomic Society; Houston, TX. 2006. Nov, [Google Scholar]
- Shively CA, Clarkson TB, Kaplan JR. Social deprivation and coronary artery atherosclerosis in female cynomologous monkeys. Atherosclerosis. 1989;77:69–76. doi: 10.1016/0021-9150(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biological Psychology. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Ginsberg SD, Hof PR, Foote SL, Young WG, Kraemer GW, … Morrison JH. Effects of social deprivation in prepubescent rhesus monkeys: Immunohistochemical analysis of the neurofilament protein triplet in the hippocampal formation. Brain Research. 1993;619:299–305. doi: 10.1016/0006-8993(93)91624-2. [DOI] [PubMed] [Google Scholar]
- Smith AS, Birnie AK, French JA. Social isolation affects partner-directed social behavior and cortisol during pair formation in marmosets, Callithrix geoffroyi. Physiology & Behavior. 2011;104:955–961. doi: 10.1016/j.physbeh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Seid MA, Jiménez LC, Wcislo WT. Socially induced development in a facultatively eusocial sweat bee Megalopta genalis (Halictidae) Proceedings of the Royal Society B: Biological Sciences. 2010;277:2157–2163. doi: 10.1098/rspb.2010.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys (Callithrix kuhli) Physiology & Behavior. 1997;62:225–232. doi: 10.1016/S0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Ibler E, Inglis W, Curtis MG. Testosterone and social isolation influence adult neurogenesis in the dentate gyrus of male rats. Neuroscience. 2011;195:180–190. doi: 10.1016/j.neuroscience.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Straits-Tröster KA, Patterson TL, Semple SJ, Temoshok L, Roth PG, McCutchan JA, … Grant I. The relationship between loneliness, interpersonal competence, and immunologic status in HIV-infected men. Psychology & Health. 1994;9:205–219. doi: 10.1080/08870449408407481. [DOI] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature Neuroscience. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugo N, Hurn PD, Morahan MB, Hattori K, Traystman RJ, DeVries AC. Social stress exacerbates focal cerebral ischemia in mice. Stroke. 2002;33:1660–1664. doi: 10.1161/01.STR.0000016967.76805.BF. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: Evidence from primate studies. British Medical Bulletin. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Eisele CD, Grady SA, Harlow HF. Depressive behavior in adult monkeys following separation from family environment. Journal of Abnormal Psychology. 1975;84:576–578. doi: 10.1037/h0077066. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Harlow HF. Social rehabilitation of isolate-reared monkeys. Developmental Psychology. 1972;6:487–496. doi: 10.1037/h0032545. [DOI] [Google Scholar]
- Szalai AJ, Nataf S, Hu XZ, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. Journal of Immunology. 2002;168:5792–5797. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- Technau GM. Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex and experience. Journal of Neurogenetics. 1984;1:113–126. doi: 10.3109/01677068409107077. [DOI] [PubMed] [Google Scholar]
- Terleph TA, Lu K, Vicario DS. Response properties of the auditory telencephalon in songbirds change with recent experience and season. PLoS One. 2008;3:e2854. doi: 10.1371/journal.pone.0002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston RC, Kubzansky LD. Women, loneliness, and incident coronary heart disease. Psychosomatic Medicine. 2009;71:836–842. doi: 10.1097/PSY.0b013e3181b40efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilvis RS, Kähönen-Väre MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. The Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2004;59:M268–M274. doi: 10.1093/gerona/59.3.M268. [DOI] [PubMed] [Google Scholar]
- Umberson D. Family status and health behaviors: Social control as a dimension of social integration. Journal of Health and Social Behavior. 1987;28:306–319. doi: 10.2307/2136848. [DOI] [PubMed] [Google Scholar]
- Umberson D. Gender, marital status and the social control of health behavior. Social Science & Medicine. 1992;34:907–917. doi: 10.1016/0277-9536(92)90259-S. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. American Community Survey, 2009. 2009 Retrieved from http://www.census.gov/acs/www/
- U.S. President’s Commission on Mental Health. Report to the President from the President’s Commission on Mental Health. 1–4. Washington, DC: U. S. Government Printing Office; 1978. [Google Scholar]
- Valzelli L. The “isolation syndrome” in mice. Psychopharmacologia. 1973;31:305–320. doi: 10.1007/BF00421275. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ, Hawkley LC, Cacioppo JT. On the reciprocal relationship between loneliness and subjective well-being. American Journal of Epidemiology. 2012;176:777–784. doi: 10.1093/aje/kws173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, Hawkley LC, Thisted RA, Cacioppo JT. A marginal structural model analysis for loneliness: Implications for intervention trials and clinical practice. Journal of Clinical and Consulting and Clinical Psychology. 2011;79:225–235. doi: 10.1037/a0022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGilder RL, Davidov DM, Stinehart KR, Huber JD, Turner RC, Wilson KS, … Barr TL. C-reactive protein and long-term ischemic stroke prognosis. Journal of Clinical Neuroscience. 2014;21:547–553. doi: 10.1016/j.jocn.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gool WA, Pronker HF, Mirmiran M, Uylings HB. Effect of housing in an enriched environment on the size of the cerebral cortex in young and old rats. Experimental Neurology. 1987;96:225–232. doi: 10.1016/0014-4886(87)90185-3. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature Reviews Neuroscience. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Venna VR, Weston G, Benashski SE, Tarabishy S, Liu F, Li J, … McCullough LD. NF-κB contributes to the detrimental effects of social isolation after experimental stroke. Acta Neuropathologica. 2012;124:425–438. doi: 10.1007/s00401-012-0990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iñiguez SD, … Nestler EJ. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nature Neuroscience. 2009;12:200–209. doi: 10.1038/nn2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, … Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. Journal of Neuroscience. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Ohdo S, Ishikawa M, Ogawa N. Effects of social isolation on pentobarbital activity in mice: Relationship to racemate levels and enantiomer levels in brain. Journal of Pharmacology and Experimental Therapeutics. 1992;263:1036–1045. [PubMed] [Google Scholar]
- Weiss RS. Loneliness: The experience of emotional and social isolation. Cambridge, MA: MIT Press; 1973. [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Research Bulletin. 2004;64:303–308. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wheeler L, Reis H, Nezlek J. Loneliness, social interaction, and sex roles. Journal of Personality and Social Psychology. 1983;45:943–953. doi: 10.1037/0022-3514.45.4.943. [DOI] [PubMed] [Google Scholar]
- Wheeler MM, Robinson GE. Diet-dependent gene expression in honey bees: Honey vs. sucrose or high fructose corn syrup. Scientific Reports. 2014;4:5726. doi: 10.1038/srep05726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA. Loneliness and the metabolic syndrome in a population-based sample of middle-aged and older adults. Health Psychology. 2010;29:550–554. doi: 10.1037/a0020760. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, … Bennett DA. Loneliness and risk of Alzheimer disease. Archives of General Psychiatry. 2007;64:234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- Withers GS, Day NF, Talbot EF, Dobson HEM, Wallace CS. Experience-dependent plasticity in the mushroom bodies of the solitary bee Osmia lignaria (Megachilidae) Developmental Neurobiology. 2008;68:73–82. doi: 10.1002/dneu.20574. [DOI] [PubMed] [Google Scholar]
- Withers GS, Fahrbach S, Robinson G. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature. 1993;364:238–240. doi: 10.1038/364238a0. [DOI] [PubMed] [Google Scholar]
- Yamada M, Decety J. Unconscious affective processing and empathy: An investigation of subliminal priming on the detection of painful facial expressions. Pain. 2009;143:71–75. doi: 10.1016/j.pain.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Zupanc GKH. Adult neurogenesis in teleost fish. In: Gage FH, Kempermann G, Song H, editors. Adult neurogenesis. New York, NY: Cold Spring Harbor Laboratory Press; 2008. pp. 571–592. [Google Scholar]
- Zupanc GKH, Sirbulescu RF. Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. European Journal of Neuroscience. 2011;34:917–929. doi: 10.1111/j.1460-9568.2011.07854.x. [DOI] [PubMed] [Google Scholar]