Abstract
Drug addiction is a chronic disorder characterized by a cycle composed of drug seeking, intoxication with drug taking and withdrawal associated with negative affect. Numerous studies have examined withdrawal/negative affect after chronic use; however, very few have examined the effect of acute administration on the negative affective state after acute drug withdrawal. One dose of amphetamine was injected into Sprague-Dawley rats. Despair behavior using the modified forced swim test (FST) and dopamine (DA) activity in the ventral tegmental area using in vivo electrophysiological recordings were studied 18, 48 and 72 h after injection of amphetamine. The effects of inactivation of the basolateral amygdala (BLA) and ketamine administration on VTA DA neuron activity and passivity in the modified FST were examined. Eighteen hours following amphetamine withdrawal, there was a substantial decrease in the number of active DA neurons, as well as an increase in time spent immobile in the modified FST, which returned to baseline after 72 h. Inactivation of the BLA after acute amphetamine prevented the decrease in DA neuron tonic activity. Injection of ketamine also prevented the decrease in DA population activity but had no effect on immobility measured in the modified FST. The data support a model in which the negative affective state following acute amphetamine withdrawal is associated with a decrease in DA neuron population activity, driven by hyperactivity of the BLA. Although ketamine reversed the hypodopaminergic state following withdrawal, the failure to reduce immobility in the modified FST indicates that different processes underlying negative emotional state may exist between depression and drug withdrawal.
Introduction
The negative affective state following the cessation of chronic drug use often drives an individual to relapse, leading to subsequent use, abuse, and addiction. Psychostimulant drugs such as amphetamine increases dopamine (DA) release from ventral tegmental area neurons, which is critical for their acute reinforcing actions (for a review, see Kuhar et al, 1991). Indeed, any states that result in reward promote DA release, which facilitates reward-driven behavior (Schultz, 1999). However, this positive emotional impact, ie, the subjective experience of pleasure, is followed by a negative affective state each time a drug is taken (Koob and Volkow, 2010). This has been described as the opponent process theory of motivation (Koob and Le Moal, 2008). The symptoms that form these negative emotional states include loss of motivation for natural reward, loss of pleasure or anhedonia, and anxiety (Koob and Le Moal, 2001; Watson et al, 1988). Persistent negative affect is also a characteristic of major depressive disorder (MDD). Here, we hypothesize that drug addiction and MDD have common neurobiological underpinnings in neural circuits regulating emotion (Conway et al, 2006; Swendsen et al, 2010).
Anhedonia has been repeatedly associated with dysfunction within the DA system (Huang and Hsiao, 2002; Wise, 1982). In animal models of depression, such as the chronic mild stress (CMS) and the learned helplessness model, we have shown previously that there is a decrease in DA activity that could be reversed by inactivation of the basolateral amygdala (BLA) (Chang and Grace, 2014) and injection of the N-methyl-D-aspartate (NMDA) antagonist and novel antidepressant ketamine (Belujon and Grace, 2014). In drug addiction, many studies have focused on the negative affective state after repeated drug administration in humans (Leventhal et al, 2008) and animal models (Der-Avakian and Markou, 2010); however, this condition appears even after a drug is taken for the first time (Koob and Le Moal, 2001). This anhedonia may drive additional drug intake that we propose is due to a decrease in DA activity. We investigated changes in VTA DA neurons after acute amphetamine administration and withdrawal. We propose that the decrease in DA activity is due to hyperactivity of the BLA, considering its role in relapse to drug-seeking behavior (Fox et al, 2008), and can be reversed by injection of ketamine.
Materials and Methods
Animals
Male Sprague-Dawley rats (300–400 g; Harlan Laboratories, Indianapolis, Indiana) were housed in pairs upon arrival on a 12-h light/dark cycle (lights on at 0700 hours) with food and water available ad libitum. All experiments were performed in accordance with the guidelines outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Drugs and Drug Infusions
Amphetamine (2 mg/kg, IP) or saline (1 ml/kg, IP) was injected 18 h (group A+18), 48 h (A+48) or 72 h (A+72) before VTA recordings or the modified forced swim test (mFST). A separate group of untreated rats was administered amphetamine immediately preceding DA neuron recordings (A+1) to determine the acute effects of amphetamine administration. In this group, amphetamine was injected immediately following anesthesia of the animal, with recordings of DA neurons performed between 1 and 3 h postinjection. Unlike the A+18 group, the amphetamine was administered to the A+1 group during anesthesia. This was necessitated given the need to perform the recordings rapidly after administration to better match the time course of the amphetamine locomotion measure. Ketamine (10 mg/kg, IP) or saline (1 ml/kg, IP) was injected immediately before VTA recordings and the mFST. For local drug infusions, tetrodotoxin (TTX, 0.25 μl, 1 μmol/l, Sigma St Louis, Missouri) or Dulbecco's phosphate buffer saline (dPBS, 0.25 μl, Sigma) was infused with a 28-gauge stainless steel cannula (PlasticsOne, Roanoke, Virginia) into the BLA (anteroposterior (AP): −3.2 mm; mediolateral (ML): +5.0 mm from bregma; dorsoventral (DV): −8.6 mm from dura) at a rate of 0.2 μl/min. The cannula was left in place for 3 min and then removed, to be followed by recordings of DA neurons.
Extracellular Recordings
Recordings were performed in choral hydrate-anesthetized rats (400 mg/kg, IP, Sigma). Electrodes were lowered through nine tracks in the VTA, separated by 200 μm (AP 5.5–5.9 mm, ML 0.6–1.0 mm from bregma and DV 6.5–9.0 mm from dura). Electrophysiological characterization of DA neurons was used to ensure correct identification, based on action potential waveform, firing pattern and frequency, and location, as previously described (Belujon and Grace, 2014; Grace and Bunney, 1983; Ungless and Grace, 2012). Three parameters of activity were measured: (1) population activity, ie, the number of active DA neurons per track; (2) basal firing rate; and (3) the proportion of action potentials occurring in bursts (Grace and Bunney, 1984).
Modified Forced Swim Test
The rat FST, developed by Porsolt et al (1978) has been widely used to assess the antidepressant-like effects of the majority of antidepressant drugs (Cryan et al, 2002). Motivation is assessed by subjecting rats to an inescapable stressor and quantifying the time spent struggling (approximating escape behavior) and the time spent immobile, which has been interpreted as a sign of behavioral despair or passivity (Porsolt et al, 1978). In this test, the use of a preswim session ensures that the rat quickly adopts an immobile posture on the test day, enabling an easier observation of the tested antidepressant (for a review, see Slattery and Cryan, 2012). However, in the current study, in order to test the effect of drug use on passivity, no pretest session was performed, as described in other studies (Cryan et al, 2003). This mFST was adapted from Cryan et al (Cryan et al, 2003; Cryan and Lucki, 2000). Briefly, rats were placed individually in a plexiglas cylinder filled with water (depth: 30 cm) for 5 min and the session was videotaped. The video recordings began before the rats were placed in the cylinder and were stopped after the rats were removed from the cylinder. Rats were removed and dried off separately from their cage mate. Cage mates were reunited after both had participated in the FST. The behavior was scored and analyzed by a person blind to the treatment. Immobility behavior is described as time spent immobile with front paws not moving and the rat floating in the water. For the first group, rats were administered either saline (1 ml/kg) or amphetamine (2 mg/kg) IP and the test session occurred 18, 48 and 72 h postinjection. For the second group, rats were administered either saline (1 ml/kg, IP) or amphetamine (2 mg/kg, IP) 18 h pretesting. Thirty minutes before testing, rats were administered either saline (1 ml/kg, IP) or ketamine (10 mg/kg, IP) (Supplementary Figure S1).
Histology
Electrode placement was verified via electrophoretic ejection of Chicago Sky Blue dye (Sigma) at the recording site. Rats were euthanized with a lethal dose of chloral hydrate (additional 400 mg/kg, IP), and brains were removed. The tissue was fixed in 8% paraformaldehyde for at least 48 h and transferred to a 25% sucrose solution for cryoprotection. Once saturated, the brains were frozen and sliced coronally at 60 μm thick using a cryostat (Leica Frigocut 2800; Leica, Bannockburn, Illinois) and mounted onto gelatin–chromalum-coated slides. Tissue was stained with a combination of neutral red and cresyl violet.
Only rats with verified cannulae and electrode placements were included in the data analysis.
Analysis
For behavior, results were expressed as the mean time spent immobile over a 5-min test session (±SEM). Two-way analysis of variance (ANOVA) followed by a Dunnett's t test was performed with treatment as the between-subject factor and session as the within-subject factor.
Electrophysiological data were analyzed using a t-test or a one-way ANOVA followed by the Holm–Sidak test, with treatment as the within-subject factor. When the normality test failed, a one-way ANOVA on ranks (Kruskal–Wallis H-test) was performed. Multiple comparisons were analyzed using a two-way ANOVA followed by the Holm–Sidak test, with treatment (saline/amphetamine) as the between-subject factor and infusion or track location as the within-subject factor.
Results
Withdrawal from Acute Amphetamine Attenuates VTA DA Activity and Increases Immobility in the FST
Rats were injected with amphetamine (2 mg/kg) or saline (1 ml/kg) 18 h before VTA recordings, and saline immediately before recordings and the mFST (Figure 1). To compare the effects with the immediate effects of acute amphetamine administration, another group was injected with amphetamine immediately before VTA recordings.
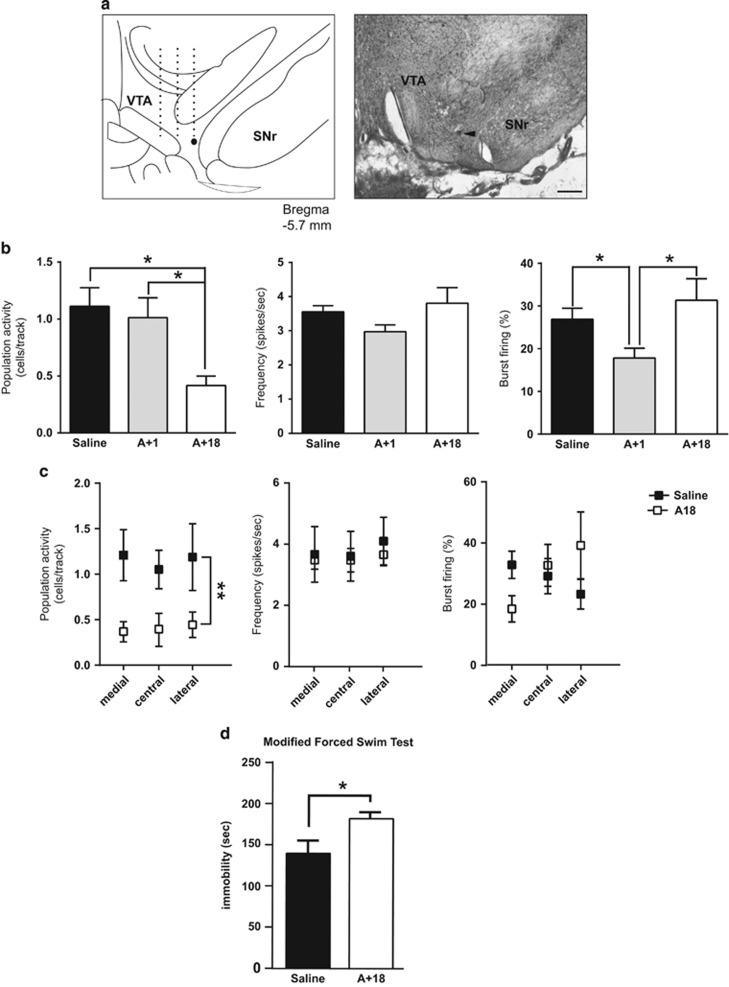
Figure 1.
Withdrawal from amphetamine injection decreased VTA DA neuron population activity and increased immobility in the modified forced swim test. (a) Example illustrating the locations of the last three of nine electrode tracks in the VTA (left) and representative Chicago Sky Blue dye deposited at the last recording site. Arrowhead indicates the dye deposit (right). Scale bar: 500 μm. Coronal sections images from Paxinos and Watson (Berridge et al, 2009). (b) Number of spontaneously active DA neurons per electrode track (left), firing rate (middle), and burst firing (right). There is a significant decrease in the number of spontaneously firing DA neurons 18 h after amphetamine injection but not at 1 h postinjection. In contrast, 1 h postinjection there is a significant decrease in burst firing. (c) The decreased number of spontaneously active DA neurons was significant across the medial–lateral extent of the VTA (medial, central and lateral) VTA (amphetamine=white squares) compared with saline rats (black squares). The firing rate and bursting activity were not different across the medial–lateral extent of the VTA (middle and right panel, respectively). (d) The amphetamine rats exhibited more time spent immobile compared with saline rats in the modified forced swim test. Error bars are±SEM. *p<0.05, **p<0.01. Abbreviations: SNr: subtanstia nigra pars reticulate; VTA, ventral tegmental area.
Acute amphetamine without withdrawal (A+1, n=7 rats, 57 neurons) had no effect on DA population activity in comparison to control rats (population activity: control, 1.11±0.16 cells/track; A+1: 1.01±0.17; 5% confidence interval (CI): 0.19–2.04), whereas the amphetamine withdrawal group (A+18, n=6 rats, 19 neurons) exhibited 60% fewer DA neurons firing compared with the control group (saline, n=8 rats, 79 neurons) (F2,18=5.611, p<0.05). No differences in average firing rate among the three groups were observed (F2,152=2.937, p=0.06); post hoc analysis revealed a significant decrease in the percentage of spikes in bursts after acute amphetamine (A+1) in comparison to control and A+18 rats (F2,152=3.743, p=0.445, p<0.05) (Figure 1b). The decrease in the A+18 amphetamine-withdrawn rats was significant across all tracks (F1,35=11.98, p<0.01, Figure 1c), with no difference in the number of active DA neurons/track across the medial–lateral extent of the VTA (F2,35<1). There was no difference in the percentage of spikes in bursts or firing frequency (F2,79=1.996, p=0.1426 and F2,79<1, respectively) when examined with respect to the medial–lateral location with the VTA between the A+18 and control rats (Figure 1c). In a separate group of rats, the A+18 group (n=10) exhibited increased immobility in the mFST, showing significantly more time immobile over the 5-min test period in comparison to saline-injected rats (n=10) (t18=0.0218, p<0.05, Figure 1d).
DA Activity and Immobility in the FST Returned to Baseline 72 h following Amphetamine Withdrawal
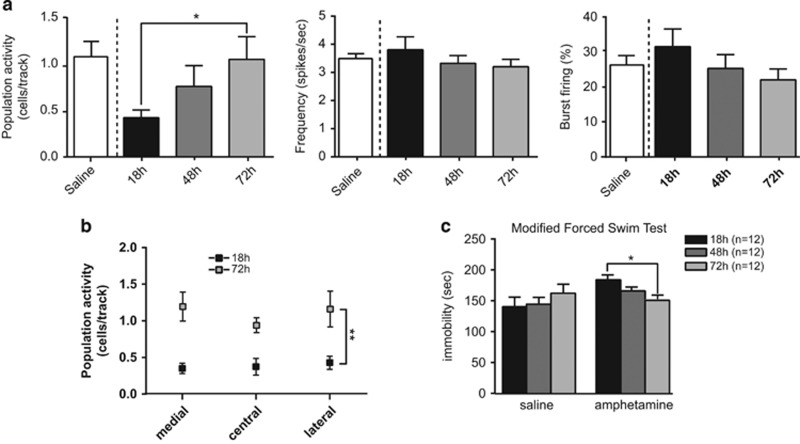
The duration of the attenuation in DA neuron activity was evaluated at 48 h (n=5 rats, 29 neurons) and 72 h (n=6 rats, 54 neurons) after amphetamine injection (Figure 2). A return to the baseline was observed 72 h after amphetamine injection but not at 48 h (one-way ANOVA on ranks, H=6.068, p<0.05). No differences in average firing rate (F2,99<1) or percentage of spikes in bursts (F2,99=1.109, p=0.3339) were observed (Figure 2a). The decrease was significant across all tracks (F1,28=13.18, p<0.01, Figure 2b), and no difference was observed across the medial–lateral extent of the VTA (F2,28<1) when comparing the 18 and 72 h time points. Similarly, in the mFST, after 72 h there was a significant decrease in immobility in comparison to 18 h (F2,18=10.17, p<0.01), the duration of immobility returning to levels comparable to saline animals (saline 72 h: 162.6±13.88 s spent immobile; A+72: 151.0±6.4 s; 5% CI=76.6–248.6). Post hoc analysis revealed no significant difference in the saline group between 18, 48 and 72 h.
Figure 2.
The amphetamine withdrawal-induced decrease in DA neuron population activity and immobility time in the modified FST recovers to baseline after 72 h. (a) The number of spontaneously active DA neurons per electrode track (left), firing rate (middle), and burst firing (right). The DA population activity is restored 72 h after amphetamine injection but not at 48 h. (b) The number of active DA neurons is restored to baseline across the medial–lateral extent of the VTA (medial, central, lateral) to levels comparable to saline animals when tested 72 h after amphetamine injection. (c) The immobility time in the modified forced swim test is restored to levels comparable to saline rats 72 h after amphetamine injection. Error bars are±SEM. *p<0.05, **p<0.01.
The Amphetamine-Induced Decrease in DA Neuron Activity is Reversed by Inactivation of the BLA
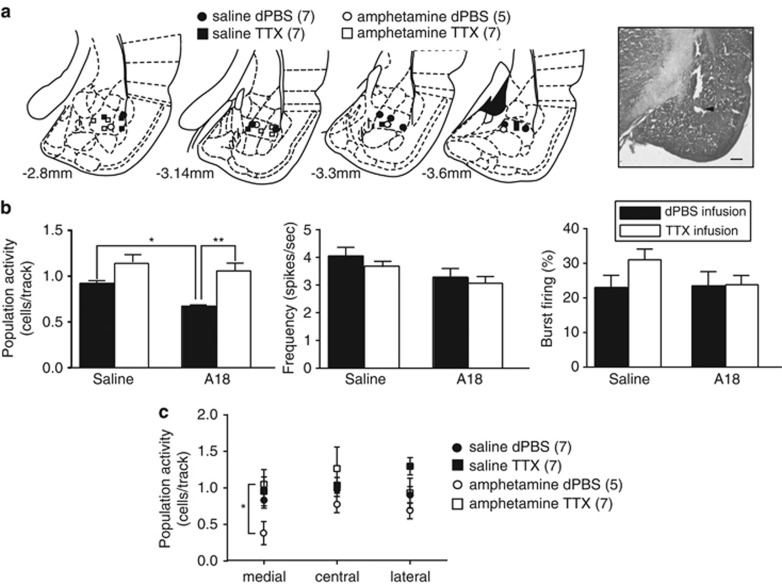
We have shown previously in the CMS animal model of depression that the decreased in DA activity in the VTA was due to hyperactivity of the BLA (Chang and Grace, 2014). As BLA neurons are also activated during drug self-administration and by stimuli previously paired with intravenous delivery of the psychostimulant cocaine (Carelli et al, 2003), we tested whether the decrease in DA neuron activity after withdrawal from acute amphetamine was also dependent on the BLA (Figure 3). Rats received injection of saline or amphetamine 18 h before VTA recordings and were given local BLA infusion of dPBS (saline: n=7 rats, 37 neurons; amphetamine: n=5 rats, 28 neurons) or TTX (saline: n=7 rats, 70 neurons; amphetamine: n=7 rats, 56 neurons) (Figure 3a) immediately before recording VTA DA neuron activity. Inactivation of the BLA reversed the decrease in DA activity in amphetamine-treated rats to levels that were comparable to saline animals (significant main effect of ‘treatment' (F1,22=6.375) and significant main effect of ‘infusion' (F1,22=20.91), Figure 3b). There was no significant interaction between ‘treatment' and ‘infusion' (F1,22=2.066). Post hoc analysis revealed that DA neuron population activity was significantly lower in the amphetamine–dPBS group compared with control–dPBS and amphetamine–TTX (p<0.05 and p<0.01, respectively), with no significant difference between saline–dPBS and saline–TTX. Inactivation of the BLA had no effect on the firing rate (F1,187=1.233, p=0.2682) and percentage of spikes in burst (F1,187=1.199, p=0.2750) in comparison to dPBS infusion in both groups.
Figure 3.
The amphetamine withdrawal-induced decrease in VTA DA neuron population activity is reversed by inactivation of the BLA. (a) Cannulae placements for all rats included in this analysis (left) and representative example of cannula track (right). Scale bar: 500 μm. Coronal section images from Paxinos and Watson (Berridge et al, 2009). (b) The number of spontaneously active DA neurons per electrode track (left), firing rate (middle), and burst firing (right). DA neuron population activity is restored to baseline levels following inactivation of the BLA. (c) BLA inactivation restored DA neuron population activity across the medial–lateral extent of the VTA. Error bars are±SEM. *p<0.05, **p<0.01. Abbreviations: BLA, basolateral amygdala; dPBS, Dulbecco's phosphate buffer saline; TTX, tetrodotoxin.
Administration of the NMDA Antagonist Ketamine Reverses the Amphetamine-Induced Decrease in DA Neuron Activity but not the Increased Immobility in the FST
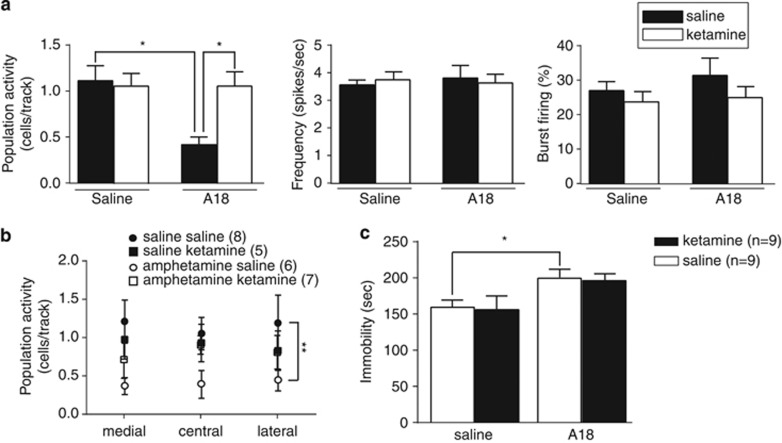
We have shown previously in the learned helplessness model of depression that escape behavior and DA activity were restored to baseline by injection of the novel antidepressant drug ketamine. In a separate set of experiments, we examined whether the noncompetitive NMDA antagonist ketamine (10 mg/kg) impacted DA neuron activity and immobility in the FST 18 h after amphetamine injection (Figure 4). We observed a significant interaction between ‘treatment' and ‘injection' (F1,22=5.814, p<0.05) and a significant main effect of ‘treatment' (F1,22=5.887, p<0.05). Post hoc analysis revealed that DA neuron population activity was significantly lower in the amphetamine–saline group (n=6 rats, 19 neurons) compared with saline–saline group (n=8 rats, 79 neurons) and the amphetamine–ketamine group (n=6 rats, 54 neurons). There was no difference between the saline–ketamine and saline–saline groups (n=6 rats, 40 neurons). No differences in average firing rate (all F1,188<0.1) and the percentage of spikes in bursts (‘treatment': F1,188<0.1; ‘injection': F1,188<1; ‘interaction': F1,188=1.679, p=0.1966) were observed. In contrast, in the mFST, ketamine did not affect immobility in either the saline or the amphetamine groups (‘injection': F1,32<1, ‘interaction': F1,32<1). There was a significant main effect of ‘treatment' (F1,32=10.17, p<0.01) in which amphetamine rats show an increased time of immobility during the 5-min test session.
Figure 4.
Ketamine reverses the amphetamine-induced decrease in DA neuron population activity to levels comparable to saline rats without affecting the increased immobility in the FST. (a) The number of spontaneously active DA neurons per electrode track (left), firing rate (middle), and burst firing (right). The amphetamine-induced decrease in DA neuron population activity is reversed to levels comparable to saline rats by injection of ketamine. (b) This restoration of activity occurred across the medial–lateral extent of the VTA. (c) In contrast, ketamine did not alter the amphetamine withdrawal-induced increase in the immobility time in the modified forced swim test and did not affect immobility in saline rats. Error bars are±SEM. *p<0.05.
Discussion
In this study, we found that withdrawal from acute amphetamine induces a decrease in DA population activity in the VTA, as well as an increased immobility time in the mFST, which are restored 72 h later. We also found that inactivation of the BLA prevented the decrease in DA neuron tonic firing, and ketamine reversed the decrease in DA population activity but not the time spent in immobility.
When administered acutely, all drugs of abuse, such as cocaine or amphetamine, have reinforcing properties that is reflected by a decrease in the reward threshold (Kornetsky and Esposito, 1979). The acute rewarding properties of psychostimulant drugs have long been known to depend on activation of the mesolimbic DA system (Koob, 1992; Nestler, 2005). Indeed, it has been shown previously that lesions of the mesolimbic DA system block the reinforcing effects of cocaine and amphetamine (McGregor and Roberts, 1993). Moreover, microdialysis studies have shown an increase in extracellular DA levels in the nucleus accumbens after acute administration of psychostimulants (Bradberry and Roth, 1989; Carboni et al, 1989). Because of activation of DA autoreceptors and long-loop feedback pathways from forebrain structures, these drugs also inhibit DA cell firing (Einhorn et al, 1988; Lodge and Grace, 2005) via activation of D1 and D2 receptors (Shi et al, 2000). In the present study, we show a decrease in the percentage of spikes in bursts of DA neurons after acute amphetamine (A+1), consistent with previous work (Lodge and Grace, 2005).
After chronic administration and during withdrawal, psychostimulants increase reward threshold (for a review, see Koob and Volkow, 2010). Indeed, imaging studies have reported a decrease in DA D2 receptor expression, as well as a decrease in DA release, consistent with DA hypofunction (Martinez et al, 2004, 2005). It is likely that this DA hypofunction contributes to anhedonia; ie, a decreased sensitivity to rewarding events (for a review, see Der-Avakian and Markou, 2012) and therefore an inability to experience pleasure, described in addicted individuals after long-term withdrawal (Leventhal et al, 2008; Volkow et al, 2007), as well as in animal models (Der-Avakian and Markou, 2010). Therefore, following chronic use, the positive affect following drug administration is followed by the opposite process to maintain homeostasis in brain systems: a negative affective state which has been advanced in the opponent process theory of motivation (for a review, see Koob and Le Moal, 2008). It has been hypothesized that this effect appears after the positive hedonic response following even initial acute drug administration (Bardo et al, 1999; Koob and Le Moal, 2008). As the majority of studies have investigated homeostatic changes following chronic drug use, in the present study we examined changes in VTA DA neuron activity in the acute phase after drug administration, which is proposed to predispose an individual to seeking and taking of drugs in the earliest phases of its use. We found that 18 h following a single injection of amphetamine, there is a significant decrease in DA neuron population activity, as well as an increase in immobility time, in the mFST. Although the FST is not an animal model of depression per se (Nestler and Hyman, 2010), it is is a validated model to assess behavioral despair and passivity test, and is useful when an extended training period is not feasible as is the case for sucrose preference. Indeed, when rats are exposed to water, after initial escape behavior by active swimming and climbing, they show an immobile passive behavior (Slattery and Cryan, 2012).
In animal models of MDDs, in which anhedonia is a core symptom, a decrease in DA neuron activity in the VTA has been described by our group (Belujon and Grace, 2014; Chang and Grace, 2014) and others (Tye et al, 2013). Indeed, we have shown previously in the CMS model (Chang and Grace, 2014) a decrease in DA activity in the VTA, as well as an increase in immobility time, in the FST. Moreover, in the learned helplessness model, a decrease in DA activity, as well as failure to escape, has been described (Belujon and Grace, 2014). Using optogenetic techniques, Tye et al (2013) have observed in CMS rats a robust increase in kick frequency in the FST following activation of DA neurons in the VTA, which was not time locked to the light pulses, suggesting a role for dopaminergic tone, rather than bursting activity, in this behavior. This is consistent with the decrease in the population activity of DA VTA neurons associated with an increase in immobility time in the FST 18 h following acute injection of amphetamine in the current study.
In the CMS model, we have shown that the decrease in DA population activity was prevented by inactivation of the BLA, suggesting that hyperactivation of this structure may be responsible for hypodopaminergia in depression. In the current study, inactivation of the BLA also prevented the decrease in VTA activity following acute withdrawal. Amphetamine administration alone does not induce changes in synaptic strength in the BLA (Rademacher et al, 2010), although changes in neuronal activity have been described (Wang and Rebec, 1996). This suggests that the drug alone does not induce changes in synaptic plasticity in the BLA. However, increased numbers of synapses have been described in the mPFC after psychostimulant exposure (Morshedi et al, 2009). Psychostimulant withdrawal has also been shown to alter the activity state in the PFC and nucleus accumbens (Onn and Grace, 2000). In particular, an increased bursting activity. as well as increased firing rate, has been described in PFC neurons after amphetamine withdrawal (Onn and Grace, 2000). mPFC projections to the BLA are excitatory (Smith et al, 2000), and the main target of mPFC neurons are projection neurons in the BLA (Smith et al, 2000). Therefore, changes in PFC function after psychostimulant administration is likely to have an influence on information processing in downstream structures such as the BLA, as previously described in animal models of depression (Chang and Grace, 2014; Moreines et al, 2014). It should be noted that the BLA sends very few direct projections to DA neurons in the VTA, suggesting intermediate structure, such as the ventral pallidum (VP) (Chang and Grace, 2014), in mediating these effects. Indeed, the BLA sends direct excitatory projections to the VP (Maslowski-Cobuzzi and Napier, 1994), a key structure in providing an inhibitory GABAergic influence on the VTA (Wu et al, 1996) and specifically on the population activity, with no effect on firing rate or bursting activity (Floresco et al, 2003), as described in the current study. Moreover, we have previously shown that a BLA-induced inhibition of VTA DA population activity can be reversed by blocking glutamate input into the VP (Chang and Grace, 2014). Although this does not rule out a third intervening glutamatergic area between the BLA and the VP, these data strongly support a BLA–VP system as a strong candidate in mediating the inhibitory influence of the BLA on the VTA.
A recent advance in the study of depression shows that one low dose of ketamine, a functional noncompetitive NMDA antagonist, relieves symptoms in treatment-resistant depressed patients within hours, which can last for up to 10 days (Zarate et al, 2006). As acute amphetamine withdrawal and depression seem to involve a common neural substrate, we tested the effect of ketamine on DA VTA activity and the FST after acute amphetamine administration. Consistent with the learned helplessness model of depression (Belujon and Grace, 2014), ketamine prevented the hypodopaminergia 18 h after amphetamine administration in the current study. However, there was no effect of ketamine on the increased immobility time. In animal models of depression, ketamine has been shown to reverse despair behavior and anhedonia, supposedly via an effect on the hippocampus. Indeed, ketamine rapidly increases mTOR-dependent synaptogenesis in the hippocampus (Garcia et al, 2008) and increases hippocampal brain-derived neurotrophic factor (BDNF) and mammalian target of rapamycin levels during the FST in rats (Tizabi et al, 2012).
In the current study, although ketamine reversed hypodopaminergia during withdrawal, the failure to reduce immmobility in the FST indicates that different processes may be present between depression-like behaviors and drug withdrawal. Synaptic alterations in DA VTA neurons have been described 24 h after a single injection of cocaine (Ungless et al, 2001); however, it remains unknown whether VTA synapses are altered in depression models. Moreover, an increased tyrosine kinase B (TrkB)-like immunoreactivity in the hippocampus has been observed in amphetamine-induced conditioned place preference but not in the delayed-paired amphetamine condition (Shen et al, 2006), suggesting that the environmental context in which the drug has been paired has a crucial role in these synaptic alterations. TrkB is activated by BDNF and can induce spine formation and dendritic growth (McAllister et al, 1999), which increases synaptic strength, and is proposed to be the mechanism by which ketamine exerts its antidepressant action in the hippocampus. In the current study, amphetamine injection was not paired to a specific context, suggesting different changes in synaptic properties in structures such as the hippocampus that do not involve BDNF, which may limit the effect of ketamine on passivity in the FST in the present study. Although the present study highlighted the key role of the DA system after acute amphetamine withdrawal, other systems, such as the serotoninergic (Horner et al, 2009) and norepinephrine (Koob, 2009) systems, could also be involved in the negative state during withdrawal. Indeed, serotonin systems, particularly those involving serotonin 5-HT1B receptor activation in the nucleus accumbens, also have been implicated in the acute reinforcing effects of psychostimulant drugs.
Taken together, the present data suggest that either ketamine administration or BLA inactivation circumvents the decrease in DA neuron tonic firing that we propose underlies the negative withdrawal state in addiction. Therefore, the negative state, because of a DA deficit, could be reversed by ketamine administration, which would circumvent the seeking and taking of more drugs after a single use of amphetamine. An important extension would be to examine the time course of the deficit state following different periods of chronic amphetamine administration and withdrawal; indeed, the model by Koob (Koob and Le Moal, 2008) would suggest that the longer periods of administration would be associated with protracted periods of anhedonia.
Funding and Disclosure
Dr Grace received funds from Johnson and Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, and Asubio. The other authors declare no conflict of interest.
Acknowledgments
We thank Niki MacMurdo for technical assistance. This work was funded by the National Institute on Drug Abuse CEBRA R21 Award (DA036328) (to AAG).
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Bardo MT, Valone JM, Bevins RA (1999). Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology (Berl) 143: 39–46. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA (2014). Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76: 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW (2009). Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol 9: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Roth RH (1989). Cocaine increases extracellular dopamine in rat nucleus accumbens and ventral tegmental area as shown by in vivo microdialysis. Neurosci Lett 103: 97–102. [DOI] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G (1989). Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience 28: 653–661. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Williams JG, Hollander JA (2003). Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. J Neurosci 23: 8204–8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Grace AA (2014). Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry 76: 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF (2006). Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 67: 247–257. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A (2003). Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry 54: 49–58. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Lucki I (2000). 5-HT4 receptors do not mediate the antidepressant-like behavioral effects of fluoxetine in a modified forced swim test. Eur J Pharmacol 409: 295–299. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I (2002). Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23: 238–245. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A (2010). Withdrawal from chronic exposure to amphetamine, but not nicotine, leads to an immediate and enduring deficit in motivated behavior without affecting social interaction in rats. Behav Pharmacol 21: 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A (2012). The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 35: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ (1988). Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci 8: 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA (2003). Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6: 968–973. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R (2008). Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology 33: 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC et al (2008). Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 32: 140–144. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS (1983). Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neuroscience 10: 301–315. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS (1984). The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner KA, Noble ES, Lauterbach EC (2009). Differential regulation of prodynophin, c-fos, and serotonin transporter mRNA following withdrawal from a chronic, escalating dose regimen of D-amphetamine. Synapse 63: 257–268. [DOI] [PubMed] [Google Scholar]
- Huang AC, Hsiao S (2002). Haloperidol attenuates rewarding and aversively conditioned suppression of saccharin solution intake: reevaluation of the anhedonia hypothesis of dopamine blocking. Behav Neurosci 116: 646–650. [DOI] [PubMed] [Google Scholar]
- Koob GF (1992). Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 13: 177–184. [DOI] [PubMed] [Google Scholar]
- Koob GF (2009). Brain stress systems in the amygdala and addiction. Brain Res 1293: 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24: 97–129. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2008). Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci 363: 3113–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU (1979). Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc 38: 2473–2476. [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW (1991). The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14: 299–302. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I et al (2008). Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. Am J Addict 17: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA (2005). Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol Exp Ther 314: 201–206. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y et al (2004). Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology 29: 1190–1202. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A et al (2005). Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 58: 779–786. [DOI] [PubMed] [Google Scholar]
- Maslowski-Cobuzzi RJ, Napier TC (1994). Activation of dopaminergic neurons modulates ventral pallidal responses evoked by amygdala stimulation. Neuroscience 62: 1103–1119. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC (1999). Neurotrophins and synaptic plasticity. Annu Rev Neurosci 22: 295–318. [DOI] [PubMed] [Google Scholar]
- McGregor A, Roberts DC (1993). Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res 624: 245–252. [DOI] [PubMed] [Google Scholar]
- Moreines JL, Owrutsky WL, Grace AA (2014) Infralimbic Prefrontal Cortex Modulation of Dopaminergic System Function in Chronic Mild Stress Model of Depression Program No 71113 Neuroscience Meeting Planner Society for Neuroscience: Washington, DC, USA, 2014. [Google Scholar]
- Morshedi MM, Rademacher DJ, Meredith GE (2009). Increased synapses in the medial prefrontal cortex are associated with repeated amphetamine administration. Synapse 63: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2005). Is there a common molecular pathway for addiction? Nat Neurosci 8: 1445–1449. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE (2010). Animal models of neuropsychiatric disorders. Nat Neurosci 13: 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn SP, Grace AA (2000). Amphetamine withdrawal alters bistable states and cellular coupling in rat prefrontal cortex and nucleus accumbens neurons recorded in vivo. J Neurosci 20: 2332–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M (1978). Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47: 379–391. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Rosenkranz JA, Morshedi MM, Sullivan EM, Meredith GE (2010). Amphetamine-associated contextual learning is accompanied by structural and functional plasticity in the basolateral amygdala. J Neurosci 30: 4676–4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (1999). The reward signal of midbrain dopamine neurons. News Physiol Sci 14: 249–255. [DOI] [PubMed] [Google Scholar]
- Shen F, Meredith GE, Napier TC (2006). Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus. J Neurosci 26: 11041–11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Smith PL, Bunney BS (2000). Endogenous DA-mediated feedback inhibition of DA neurons: involvement of both D(1)- and D(2)-like receptors. Synapse 35: 111–119. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF (2012). Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc 7: 1009–1014. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D (2000). Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol 416: 496–508. [PubMed] [Google Scholar]
- Swendsen J, Conway KP, Degenhardt L, Glantz M, Jin R, Merikangas KR et al (2010). Mental disorders as risk factors for substance use, abuse and dependence: results from the 10-year follow-up of the National Comorbidity Survey. Addiction 105: 1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L (2012). Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience 213: 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J et al (2013). Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493: 537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA (2012). Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35: 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411: 583–587. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M et al (2007). Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci 27: 12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Rebec GV (1996). Amygdaloid neurons respond to clozapine rather than haloperidol in behaving rats pretreated with intra-amygdaloid amphetamine. Brain Res 711: 64–72. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wise RA (1982). Neuroleptics and operant behavior: the anhedonia hypothesis 39–87.
- Wu M, Hrycyshyn AW, Brudzynski SM (1996). Subpallidal outputs to the nucleus accumbens and the ventral tegmental area: anatomical and electrophysiological studies. Brain Res 740: 151–161. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.