Abstract
Objective
To determine how cognitively healthy and cognitively impaired life expectancy have changed from 2000 to 2010 among American men and women 65 years of age and over.
Methods
The prevalence of dementia, cognitive impairment without dementia (CIND), and normal cognition is determined from nationally representative data from the U.S. Health and Retirement Study (HRS). Mortality rates are from U.S. Decennial Life Table for 2000 and the U.S. annual life table for 2010. Life expectancy by cognitive status is estimated using the Sullivan method.
Results
Most of the increase in life expectancy has been concentrated in cognitively healthy years in this 10 year period. The increase in expected years cognitively intact at age 65, which exceeded that in total life expectancy, was 1.8 for men and 1.6 for women.
Conclusion
This study provides evidence suggesting that there has been a compression of cognitive morbidity.
Abbreviations: CIND, cognitive impairment without dementia; HRS, Health and Retirement Study
Keywords: Cognitive life expectancy, healthy life, healthspan, dementia, CIND
1. Introduction
In recent years a question posed by those interested in health trends has been “Are we living longer healthy lives as well as longer lives?” If we prolong life after the onset of disease or disability, life with disease and disability can be lengthened which is not really an improvement in population health. Because decreases in mortality are increasingly concentrated at older ages, and because dementia is normally a disease with onset in old age, change in mortality and change in dementia prevalence interact to affect the length of cognitively intact and cognitively impaired life expectancy. Length of life with dementia or with cognitive loss provides a good assessment of the burden of dementia and the potential value of interventions to prevent and delay cognitive loss.
Recent reports from England have shown increases in the length of cognitively healthy life expectancy at age 65 that are almost as great as the increase in life expectancy for men and greater than the increase in life expectancy for women (Jagger et al., 2015). In addition, there have been a number of reports of reductions in the prevalence of dementia both in the United States and in other countries (De Rotrou et al., 2013, Gerstorf et al., 2015, Larson et al., 2013, Langa et al., 2008, Satizabal et al., 2016, Wu et al., 2016). These lead us to expect increases in cognitively healthy life.
On the other hand, life expectancy increase in the U.S. has been relatively slow compared to that of other countries, particularly for women, which could affect relative change in the length of life with cognitive impairment (Glei, Meslé, & Vallin, 2010). An examination of life expectancy with and without impaired cognitive functioning for men and women in the U.S. in the 1990s indicated that women had longer life expectancy with cognitive impairment than men primarily because of their longer total life expectancy (Suthers, Kim, & Crimmins, 2003). The age-specific differences between men and women in the prevalence of cognitive impairment were not significant. A new look at gender differences in new cohorts with more education and using newly derived measures of dementia and cognitive impairment without dementia (CIND) is warranted.
In this analysis, we examine changes from 2000 to 2010 in the length of life with good cognition, with dementia and with cognitive impairment but without dementia (CIND). Data from the nationally representative survey of older Americans, the Health and Retirement Study (HRS), are used to estimate the prevalence of cognitive states among those 65 and older at the two dates. This is the first study to provide national estimates of life expectancy with dementia and CIND for the United States.
2. Data and methods
2.1. Data resources
Estimates of cognitive life expectancy require information on mortality and cognitive state at each date. Mortality data for this analysis are from the U.S. National Vital Statistics, the decennial life table for 2000 and the annual life table for 2010.
Data from the 2000 and 2010 Health and Retirement Study (HRS) for those 65+ were used to obtain the prevalence of cognitive states ten years apart. These data included 10,374 participants in 2000 and 9,995 in 2010 who were age 65 or older. These samples consist of both community-dwelling and nursing home residents, and both self- and proxy respondents. At each wave from 2000 to 2010, 88% or 89% of those scheduled for interview in the HRS were actually interviewed (Health and Retirement Study, 2011, for 2000 to 2008; Personal communication from HRS for 2010). Survey procedures and sample characteristics were consistent at the two dates. In 2000, 11.9% of the responses were provided by proxies; in 2010, this was true for 9.3% of responses. In 2000, 3.8% and in 2010, 4.3% of those in the samples assessed for cognition were in nursing homes. The average age of the sample at both dates was about 75 years, of whom about 58% were females.
Cognitive status of the 65+ population is determined through responses to a series of tests for those who are self-respondents. Responses to a set of questions to proxies and interviewer observations are the basis of ascertainment for those who are not self-respondents. Because poor cognitive functioning is one of the reasons people do not respond for themselves, it is particularly important to include these people in assessment of the national prevalence of cognitive loss. Categorizing people as having good cognition, dementia, or CIND is based on the concordance of HRS cognitive functioning scores and diagnosis of dementia and CIND in a subset of HRS respondents who had neuropsychological assessment in the Aging, Demographics, and Memory Study (ADAMS) (Crimmins et al., 2011, Langa et al., 2005). Results from a detailed neuropsychological diagnostic approach on a limited subsample of the HRS were used to develop methods for classifying people as having dementia and CIND in the larger population sample. The overall prevalence in the larger population sample is the same as would be obtained if all sample members had the neuropsychological diagnostic approach. The approach developed on this subsample of respondents for use in the larger sample was used at all the HRS waves from 2000 through 2010. In order to test the appropriateness of using the 10 year period to look at change in cognitive life expectancy, we first examine the percent with dementia and with CIND at each wave of the HRS from 2000 to 2010 to see whether the change over time looks fairly consistent.
Self-respondents’ cognitive scores can range from 0 to 27 and are based on tests of immediate recall of 10 words, delayed recall of the same 10 words, 5 trials of Serial 7s, and Backward counting (score 0–2). If a respondent does not complete all the tests, the missing measures are imputed by HRS. A detailed description of the procedures and the number of imputations over time is provided in Fisher, Hassan, Faul, Rodgers, and Weir (2015). Respondents with scores from 12 to 27 are classified as having good cognitive functioning; 0–6 is dementia; and 7–11 is CIND.
For individuals whose information is provided by proxies, the classification is based on an direct assessment of memory (0 excellent, 1 very good, 2 good, 3 fair, 4 poor); an assessment of limitations in 5 instrumental activities of daily living (IADLs) (managing money, taking medication, preparing hot meals, using phones and doing groceries) (0–5); and the interviewer assessment of difficulty completing the interview because of cognitive limitation (score 0–2 indicating none, some, prevents completion). These scores are summed and those with a score of 0–2 are classified as cognitively healthy; 3–5 as CIND; and 6–11 as having dementia. In 2000, 49.1% of those with dementia had proxy respondents; this was true for 51.6% of those with dementia in 2010. Among those who answer for themselves, most have been in the study for many waves and most of those in the 2010 sample were also in the 2000 sample. In 2000, 86% of those who answer the cognitive questions have been in the survey four or five times; in 2010, 77% have been in the survey nine or ten times.
2.2. Methods
Healthy life expectancy measures combine indicators of morbidity and mortality so that life expectancy can be divided into healthy and unhealthy expected life (Saito, Robine, & Crimmins, 2014). Here, we define healthy as life expectancy with good cognitive functioning and unhealthy as life expectancy with dementia or CIND. Cognitively healthy life expectancy reflects the average number of years at a specified age a person can expect to live with good cognitive skills given current mortality and prevalence of cognitive problems. We use the Sullivan method for computing the length of healthy and unhealthy life expectancy (Jagger et al., 2007, Saito et al., 2014).
Computation of cognitively healthy life expectancy and life expectancy with dementia or CIND is based on dividing the lifetable years lived in each age group into these three states using the prevalence of the cognitive states at each age. Years lived with dementia or CIND are summed at all ages after the specified age and divided by the number of people alive at that age to obtain life expectancy with dementia or CIND. Cognitively healthy life expectancy is determined by subtracting these two states from total life expectancy. Standard errors for the estimated values were computed using the approach provided by Jagger et al. (2007).
3. Results
3.1. Trends in prevalence of good cognitive functioning, dementia and CIND
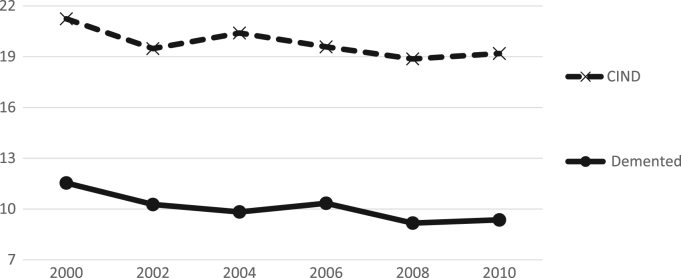
We begin with an examination of cognitive status over six points in time from 2000 to 2010 to see whether there is a somewhat consistent trend over the period before we use the endpoints in our analysis of 10 year change in cognitive life expectancy. While the trend is not linear, there does appear to be a drop over the ten years in the percent with dementia and with CIND; we believe that this provides evidence that it is worth looking further at the age-sex-specific change and combining those with changes in life expectancy (Fig. 1).
Fig. 1.
Percent with dementia and CIND among those 65+ HRS: 2000–2010.
When we examine the change over ten years by gender, we find a significant increase in the prevalence of good cognitive functioning among both men (4.45 percentage points) and women (3.41 percentage points) in the 65+ population (Table 1); there was also a decrease of 2.60 percentage points in dementia prevalence among men and 1.99 percentage points among women. In addition, there was a decrease in the prevalence of CIND among both men and women, but it is not statistically significant.
Table 1.
Prevalence (%) with good cognition, dementia and CIND 2000 and 2010.
| Males |
| With good cognition | CIND | Dementia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2010 | Change 2010–2000 | 2000 | 2010 | Change 2010–2000 | 2000 | 2010 | Change 2010–2000 | ||
| Total | 67.28 (65.91–68.65) | 71.73 (70.43–73.03) | 4.45* | 22.39 (21.18–23.61) | 20.55 (19.39–21.72) | −2.84 | 10.32 (9.44–11.21) | 7.72 (6.94–8.49) | −2.60* | |
| 65–69 | 80.39 (78.34–82.43) | 83.55 (81.25–85.85) | 3.16 | 13.72 (11.95–15.50) | 13.49 (11.37–15.61) | −0.23 | 5.89 (4.68–7.10) | 2.96 (1.91–4.03) | −2.93* | |
| 70–74 | 70.24 (67.56–72.92) | 78.51 76.33–80.68) | 8.27* | 23.13 (20.66–25.61) | 16.63 (14.66–18.61) | −6.50* | 6.63 (5.17–8.09) | 4.86 (3.72–6.00) | −1.77 | |
| 75–79 | 64.62 (61.56–67.68) | 67.90 (65.03–70.78) | 3.28 | 24.73 (21.97–27.49) | 22.76 (20.18–25.34) | −1.97 | 10.65 (8.68–12.62) | 9.34 (7.55–11.13) | −1.31 | |
| 80–84 | 53.94 (49.84–58.05) | 60.13 (56.39–63.87) | 6.19 | 29.95 (26.18–33.72) | 28.70 (25.25–32.16) | −1.25 | 16.11 (13.08–19.14) | 11.16 (8.76–13.57) | −4.95 | |
| 85+ | 38.13 (33.54–42.73) | 40.73 (36.61–44.84) | 2.60 | 33.64 (29.18–38.11) | 37.42 (33.37–41.48) | 3.78 | 28.22 (23.97–32.48) | 21.85 (18.39–25.32) | −6.37 | |
| Females | ||||||||||
|
With good cognition |
CIND |
Dementia |
||||||||
| 2000 | 2010 | Change 2000–2010 | 2000 | 2010 | Change 2000–2010 | 2000 | 2010 | Change 2000–2010 | ||
| Total | 66.82 (65.64–67.99) | 70.23 (69.11–71.36) | 3.41* | 20.39 (19.39–21.39) | 18.97 (18.00–19.93) | −1.42 | 12.79 (11.96–13.63) | 10.80 (10.03–11.56) | −1.99* | |
| 65–69 | 85.32 (83.67–86.98) | 85.64 (83.81–87.47) | 0.32 | 11.42 (9.93–12.91) | 11.54 (9.87–13.21) | 0.12 | 3.26 (2.42–4.09) | 2.63 (1.76–3.51) | −0.63 | |
| 70–74 | 74.39 (72.03–76.75) | 80.31 (78.42–82.19) | 5.92* | 18.98 (16.86–21.10) | 15.12 (13.42–16.82) | −3.86* | 6.63 (5.29–7.98) | 4.57 (3.59–5.56) | −2.06 | |
| 75–79 | 67.59 (64.98–70.19) | 72.66 (70.25–75.07) | 5.07* | 21.03 (18.77–23.30) | 19.31 (17.18–21.45) | −1.72 | 11.38 (9.62–13.15) | 8.03 (6.56–9.50) | −3.35* | |
| 80–84 | 55.66 (52.52–58.81) | 62.59 (59.34–65.83) | 6.93* | 25.58 (22.81–28.34) | 22.37 (19.58–25.17) | −3.21 | 18.76 (16.29–21.24) | 15.04 (12.64–17.44) | −3.72 | |
| 85+ | 29.88 (26.89–32.87) | 35.72 (32.78–38.66) | 5.84 | 32.99 (29.91–36.06) | 32.87 (29.98–35.75) | −0.12 | 37.13 (33.98–40.29) | 31.42 (28.57–34.27) | −5.71 | |
All age groups within the 65+ group of both men and women experienced an increase in the prevalence of good cognition. Among women, the increase was significant in all but the youngest (65–69) and the oldest (85+); among men, the increase was only significant for the 70–74. While there was a consistent decrease in dementia across age-sex groups, it was only significant for the youngest men and women 75–79.
3.2. Life expectancy change
There was modest improvement in life expectancy over the 10 years. At age 65, life expectancy for men increased by 1.6 years and for women by 1.2 years (Table 2). At age 85, life expectancy for both men and women increased by 0.3 years.
Table 2.
Life Expectancy 2000 and 2010: total, with good cognition, with CIND, with dementia: Health and Retirement Study.
| Expectation of life in years |
Males |
Females |
||||
|---|---|---|---|---|---|---|
| 2000 | 2010 | Change 2010–2000 | 2000 | 2010 | Change 2010–2000 | |
| At age 65 | ||||||
| Total | 16.1 | 17.7 | 1.6 | 19.1 | 20.3 | 1.2 |
| With good cognition | 10.7 (10.4–10.9) | 12.5 (12.3–12.7) | 1.8* | 12.5 (12.3–12.7) | 14.1 (13.9–14.4) | 1.6* |
| CIND | 3.7 (3.5–3.9) | 3.7 (3.5–4.0) | 0.0 | 4.0 (3.8–4.2) | 3.9 (3.7–4.1) | −0.1 |
| Dementia | 1.8 (1.6–1.9) | 1.4 (1.3–1.6) | −0.4 | 2.6 (2.5–2.8) | 2.3 (2.1–2.4) | −0.3* |
| At age 85 | ||||||
| Total | 5.5 | 5.8 | 0.3 | 6.6 | 6.9 | 0.3 |
| With good cognition | 2.1 (1.8–2.3) | 2.4 (2.2–2.6) | 0.3 | 2.0 (1.8–2.2) | 2.5 (2.3–2.7) | 0.5* |
| CIND | 1.8 (1.6–2.1) | 2.2 (1.9–2.4) | 0.4 | 2.2 (2.0–2.4) | 2.3 (2.1–2.5) | 0.1 |
| Dementia | 1.5 (1.3–1.8) | 1.2 (1.0–1.5) | −0.3 | 2.5 (2.2–2.7) | 2.2 (2.0–2.4) | −0.3 |
Note: Sum of life expectancy in states sometimes does not add to total expectation of life because of rounding.
3.3. Cognitively healthy life expectancy change
There was a significant increase in the length of cognitively healthy life expectancy at age 65 for both men and women. The increase in expected years with good cognition was 1.8 years for men and 1.6 years for women; the increase in cognitively healthy life expectancy exceeds that in total life expectancy. At age 85, both men and women also had increased cognitively intact life expectancy of 0.3 years for men and 0.5 years for women. The change is significant for women but for men there is some overlap in the estimated confidence intervals at the two dates. For women at age 85, the increase in life expectancy with good cognition was greater than the increase in total life expectancy, and for men at age 85 these increases were equal.
3.4. Life expectancy with dementia and CIND change
Expected years of life expectancy with dementia at age 65 were reduced by 0.3 for women and 0.4 for men, only the decrease among females is significant. This is the same reduction as at age 85 indicating that the reduction was really concentrated at the oldest ages. There was no significant change in the estimated life expectancy with CIND between 2000 and 2010 for either men or women.
3.5. Change in proportion of life with and without cognitive problems
More of both men and women's expected life (4 percentage points more) after age 65 is cognitively healthy; and less is spent with dementia (a decrease of 3.3 percentage points for men and 2.3 percentage points for women) (Table 3). There is also an increase in the proportion of life with good cognitive functioning after age 85 (3.2 percentage points for men and 5.9 percentage points for women). The decline in the proportion of life with dementia after age 85 is quite marked: 6.6 percentage points for men and 6.0 percentage points for women.
Table 3.
Percent of life expectancy in cognitive states.
|
Males |
Females |
|||||
|---|---|---|---|---|---|---|
| 2000 | 2010 | Change 2010–2000 | 2000 | 2010 | Change 2010–2000 | |
| At age 65 | ||||||
| With good cognition | 66.5 | 70.6 | 3.9 | 65.4 | 69.5 | 4.1 |
| CIND | 23.0 | 20.9 | −2.1 | 20.9 | 19.2 | −1.7 |
| Dementia | 11.2 | 7.9 | −3.3 | 13.6 | 11.3 | −2.3 |
| At age 85 | ||||||
| With good cognition | 38.2 | 41.4 | 3.2 | 30.3 | 36.2 | 5.9 |
| CIND | 32.7 | 37.9 | 5.2 | 33.3 | 33.3 | 0.0 |
| Dementia | 27.3 | 20.7 | −6.6 | 37.9 | 31.9 | −6.0 |
3.6. Differences between men and women
While our interest in this paper is primarily change over time, our estimates of dementia and CIND for men and women provide national estimates for the population of the United States on sex differences in both the prevalence and life expectancy with dementia, CIND, and good cognitive functioning. In 2010, across all ages, men and women 65 and over have the same proportion with good cognition (71.73% for men and 70.23% for women); the percentage with dementia is higher for women (10.80% versus 7.72%). At each age group less than 85, the prevalence of dementia is similar for men and women; only above age 85 do women have higher levels of dementia. This higher prevalence of dementia and longer life expectancy above age 85 lead to an extra year of expected life with dementia for women (2.2 versus 1.2 for men).
4. Discussion
We report an increase in cognitively healthy life expectancy and a decrease in life expectancy with dementia. If we define the compression of morbidity as an increase in the proportion of life with good cognition, there is a suggestion of a compression of cognitive morbidity at the older ages in the United States from 2000 to 2010. This is an important indicator of healthspan improvement. The increase in cognitively healthy life expectancy and the reduction in dementia may reflect influences from earlier life as well as current health conditions. Early life is thought to have a particularly strong influence on cognitive functioning (Katsnelson, 2015); and improvements in health, nutrition and education of cohorts in early life may contribute to the reduction of dementia and better cognitive functioning in later life (Crimmins and Saito, 2001, Matthews et al., 2009). However, recent research from Europe has implicated cognitive activity in midlife, as evidenced in complex occupations, and late life social engagement as additional protectors of cognitive functioning (Marioni et al., 2012, Marioni et al., 2014). In addition, recent changes in hypertension and cholesterol control in the older population may play a role in reducing dementia.
The data from this study provide the first national estimates of life expectancy with dementia because the study was based on data from a large national sample of self-respondents tested for cognitive ability, reports from proxies for those who could not be tested, and a smaller subsample with detailed neuropsychological testing for developing diagnostic criteria. While the sample has many strengths we should mention one limitation. In a longitudinal cohort study where people are interviewed every two years, respondents have been more exposed to the cognitive questions in later years. It is possible that with more practice, they become better at answering the questions.
Changes in healthy life expectancy provide a good summary of how the average expected life cycle might change. Our data show a reduction in the time spent with dementia that should result in a reduction in the number of years when care and support is needed for an average individual. However, the projected future increase in the national burden of dementia is related to increases in the number of people in the older age groups, which will only partially be offset by the improvement of the average period spent with dementia.
Acknowledgements
Support for the HRS was primarily provided by the National Institute on Aging of the National Institutes of Health (U01 AG009740). Analysis was partially supported by a grant from the US National Institute on Aging (P30 AG17265) and a grant from the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research 25293121). Authors are responsible for the content of the article.
Contributor Information
Eileen M. Crimmins, Email: crimmin@usc.edu.
Yasuhiko Saito, Email: saito.yasuhilo@nihon-u.ac.jp.
Jung Ki Kim, Email: jungk@usc.edu.
References
- Crimmins E.M., Kim J.K., Langa K.M., Weir D.R. Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66(Suppl 1):i162–i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E.M., Saito Y. Trends in healthy life expectancy in the United States, 1970–1990: Gender, racial, and educational differences. Social Science Medicine. 2001;52(11):1629–1641. doi: 10.1016/s0277-9536(00)00273-2. [DOI] [PubMed] [Google Scholar]
- De Rotrou J., Wu Y.H., Mabire J.B., Moulin F., de Jong L.W., Rigaud A.S., Hanon O., Vidal J.S. Does cognitive function increase over time in the healthy elderly? PloS One. 2013;8(11):1–8. doi: 10.1371/journal.pone.0078646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, G.G., Hassan, H., Faul, J.D., Rodgers, W.L., Weir, D.R. (2015). Health and Retirement study imputation of cognitive functioning measures: 1992–2012 (Final Release Version) Data Description Prepared by Survey Research Center University of Michigan Ann Arbor, MI, April 20, 2015, Retrieved from 〈http://hrsonline.isr.umich.edu/modules/meta/xyear/cogimp/desc/ COGIMPdd.pdf〉
- Gerstorf D., Hülür G., Drewelies J., Eibich P., Duezel S., Demuth I., Paolo G., Steinhagen-Thiessen E., Wagner G.G., Lindenberger U. Secular changes in late-life cognition and well-being: Towards a long bright future with a short brisk ending? Psychology and Aging. 2015;30(2):301. doi: 10.1037/pag0000016. [DOI] [PubMed] [Google Scholar]
- Glei D.A., Meslé F., Vallin J. Diverging trends in life expectancy at age 50: A look at causes of death. In: Crimmins E.M., Preston S.H., Cohen B., editors. International differences in mortality at older ages: Dimensions and sources (pp. 17–67). National Research Council, Committee on Population, Division of Behavioral and Social Sciences and Education. The National Academies Press; Washington, DC: 2010. [PubMed] [Google Scholar]
- Health and Retirement Study (2011). Sample sizes and response rates. 〈http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf/〉 Accessed 16.08.31
- Jagger C., Matthews F.E., Wohland P., Fouweather T., Stephan B.C., Robinson L., Arther An, Brayne C., Medical Research Council Cognitive Function and Ageing Collaboration A comparison of health expectancies over two decades in England: Results of the Cognitive Function and Ageing Study I and II. The Lancet. 2015:1–8. doi: 10.1016/S0140-6736(15)00947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger C., Matthews R., Matthews F., Robinson T., Robine J.M., Brayne C. The burden of diseases on disability-free life expectancy in later life. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(4):408–414. doi: 10.1093/gerona/62.4.408. [DOI] [PubMed] [Google Scholar]
- Katsnelson A. News feature: The neuroscience of poverty. Proceedings of the National Academy of Sciences. 2015;112(51):15530–15532. doi: 10.1073/pnas.1522683112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa K.M., Plassman B.L., Wallace R.B., Herzog A.R., Heeringa S.G., Ofstedal M.B., Burke J.R., Fisher G.G., Fultz N.H., Hurd M.D., Potter G.G., Rodgers W.L., Steffens D.C., Weir D.R., Willis R.J. The aging, demographics, and memory study: Study design and methods. Neuroepidemiology. 2005;25(4):181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- Langa K.M., Larson E.B., Karlawish J.H., Cutler D.M., Kabeto M.U., Kim S.Y., Rosen A.B. Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers & Dementia. 2008;4(2):134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E.B., Yaffe K., Langa K.M. New insights into the dementia epidemic. New England Journal of Medicine. 2013;369(24):2275–2277. doi: 10.1056/NEJMp1311405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni R.E., Proust-Lima C., Amieva H., Brayne C., Matthews F.E., Dartigues J.F., Jacqmin-Gadda H. Cognitive lifestyle jointly predicts longitudinal cognitive decline and mortality risk. European Journal of Epidemiology. 2014;29:211–219. doi: 10.1007/s10654-014-9881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni R.E., Valenzuela M.J., Van den Hout A., Brayne C., Matthews F.E. Active cognitive lifestyle is associated with positive cognitive health transitions and compression of morbidity from age sixty-five. PLoS ONE. 2012;7(12):e50940. doi: 10.1371/journal.pone.0050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews F.E., Jagger C., Miller L.L., Brayne C., CFAS M. Education differences in life expectancy with cognitive impairment. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64(1):125–131. doi: 10.1093/gerona/gln003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Robine J.M., Crimmins E.M. The methods and materials of health expectancy. Statistical Journal of the IAOS. 2014;30(3):209–223. doi: 10.3233/SJI-140840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal C., Beiser A., Chouraki V., Chene G., Dufouil C., Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. New England Journal of Medicine. 2016;374(6):523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthers K., Kim J.K., Crimmins E. Life expectancy with cognitive impairment in the older population of the United States. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2003;58(3):S179–S186. doi: 10.1093/geronb/58.3.s179. [DOI] [PubMed] [Google Scholar]
- Wu Y.T., Fratiglioni L., Matthews F.E., Lobo A., Breteler M.M., Skoog I., Brayne C. Dementia in Western Europe: Epidemiological evidence and implications for policy making. The Lancet Neurology. 2016;15(1):116–124. doi: 10.1016/S1474-4422(15)00092-7. [DOI] [PubMed] [Google Scholar]