Abstract
DNA methylation can mimic the effects of both germline and somatic mutations for cancer predisposition genes such as BRCA1 and p16INK4a. Constitutional DNA methylation of the BRCA1 promoter has been well described and is associated with an increased risk of early-onset breast cancers that have BRCA1-mutation associated histological features. The role of methylation in the context of other breast cancer predisposition genes has been less well studied and often with conflicting or ambiguous outcomes. We examined the role of methylation in known breast cancer susceptibility genes in breast cancer predisposition and tumor development. We applied the Infinium HumanMethylation450 Beadchip (HM450K) array to blood and tumor-derived DNA from 43 women diagnosed with breast cancer before the age of 40 years and measured the methylation profiles across promoter regions of BRCA1, BRCA2, ATM, PALB2, CDH1, TP53, FANCM, CHEK2, MLH1, MSH2, MSH6 and PMS2. Prior genetic testing had demonstrated that these women did not carry a germline mutation in BRCA1, ATM, CHEK2, PALB2, TP53, BRCA2, CDH1 or FANCM. In addition to the BRCA1 promoter region, this work identified regions with variable methylation at multiple breast cancer susceptibility genes including PALB2 and MLH1. Methylation at the region of MLH1 in these breast cancers was not associated with microsatellite instability. This work informs future studies of the role of methylation in breast cancer susceptibility gene silencing.
Introduction
Aberrant methylation patterns are a well-recognized feature of tumor cells and tumorigenic pathways. This includes genomic instability induced by global hypomethylation and silencing of several tumor suppressor genes via promoter methylation, which can act in a similar manner as do germline and somatic genetic mutations. [1].
Rare germline mutations in multiple genes (BRCA1, BRCA2, ATM, PALB2, CDH1, TP53, FANCM, CHEK2, MLH1, MSH2, MSH6 and PMS2) are known to be associated with increased breast and/or ovarian cancer susceptibility and these genes are included in most commercial gene panel tests for breast and ovarian cancer susceptibility [2]. Research investigating the role of methylation as an alternate silencing mechanism for these genes has been limited.
BRCA1 promoter hypermethylation in blood and breast tumor-derived DNA has been reported in the literature. BRCA1 promoter methylation in blood-derived DNA is associated with a 3.5-fold (95% CI, 1.4–10.5) increased risk for early-onset breast cancer with histological features commonly seen in tumors arising in women with germline BRCA1 mutations [3]. The silencing of BRCA1 via promoter methylation has also been observed in breast tumors, including those arising in women with increased constitutional DNA methylation at this region [3–6]. Methylation of other breast cancer predisposition genes has been less well studied and often with conflicting outcomes.
Flanagan et al. performed methylation microarray analyses of peripheral blood DNA across genes including BRCA1, BRCA2, CHEK2, ATM, TP53, CDH1 and MLH1 and demonstrated gene body hypermethylation of ATM was associated with a 3-fold increase risk of breast cancer (P = 0.0017) [7]. Another study reported DNA methylation aberrations at an intragenic region of ATM to be associated with increased risk of breast cancer (increased risk for women in the upper quartile, OR 1.89; 95% CI 1.36–2.64; P = 2x10-4) [8].
The promoter methylation of PALB2 has been investigated in the context of breast and ovarian cancer. Potapova et al (2008) reported promoter methylation of PALB2 in approximately 8% of breast and ovarian cancers (including those with BRCA2 germline mutations), detected by methylation specific PCR and Sanger sequencing [9]. However, Mikeska et al (2013) found little evidence of PALB2 methylation in high-grade serous ovarian cancer using a methylation-sensitive high-resolution melting assay, and Poumpouridou et al (2016) were unable to detect PALB2 promoter methylation in a series of 91 breast cancers [10,11]. Studies of BRCA2 and CHEK2 have found no evidence for regulation or silencing via methylation [12,13].
The goal of this study was to examine if low levels of constitutional DNA methylation corresponding to high levels of tumor DNA methylation could be identified at breast cancer predisposition genes other than BRCA1. We included blood and tumor-derived DNA from young affected women participating in the Australian Breast Cancer Family Study (ABCFS), using the Infinium HumanMethylation450 (HM450K) beadchip assay.
Material and Methods
Study samples
Blood and corresponding tumor-derived DNA samples were prepared from 43 women diagnosed with breast cancer before the age of forty participating in a population-based case-control component of the Australian Breast Cancer Family Registry (ABCFR) [14–16]. These women had been previously screened for germline mutations in BRCA1, ATM, CHEK2, PALB2, TP53, BRCA2, CDH1, and FANCM [14,15,17–26]. A subset of women had also been assessed for methylation at the BRCA1 promoter using a site-specific MethyLight assay [3]. Written informed consent was obtained from each participant of the Australian Breast Cancer Family Study. This study was approved by the Human Research Ethics Committee of the University of Melbourne (Project 0608818) and meets the principles of the Declaration of Helsinki.
DNA extraction from Guthrie card archival blood spots
Blood-derived DNA was extracted from archival dried blood spots (prepared for all participants of the ABCFR) on Whatman filter paper (GE Healthcare, United Kingdom) as previously described [27]. DNA was extracted from 28 (3.2mm diameter) punches per individual using the QIAamp 96 DNA Blood Kit (Qiagen; Hilden, Germany), as per the manufacturer’s protocol, except the DNA was eluted three times in 50μl nuclease free water to obtain a final volume of 150μl.
DNA extraction from FFPE tumor sections
A haematoxylin and eosin (H&E) stained slide, marked up by a pathologist, was used as a reference for each case. The identified tumor-enriched tissue was macrodissected from between two to ten Methyl Green stained sections per tumor using both a scalpel (Swann-Morton, Sheffield, England) and a 21-guage syringe needle (Terumo, Tokyo, Japan), as previously described [28]. DNA was extracted using the QIAamp DNA FFPE Tissue Kit as per the manufacturer’s protocol (Qiagen; Hilden, Germany), varied only by an extended tissue incubation time of 48 hours with 20μl of Proteinase K (20mg/ml) replenished at 0 and 24 hours. Extracted tumor-derived DNA was eluted twice in 15μl elution buffer to obtain a final volume of 30μl. DNA samples were stored at 4°C.
HumanMethylation450 beadchip assay
The HumanMethylation450 beadchip assay was run as previously described [27]. Briefly, the blood and tumor-derived DNA underwent sodium bisulfite modification using the EZ DNA Methylation-Gold™ Kit (Zymo Research, CA, United States), as per the manufacturer’s protocol. Bisulfite converted tumor-derived DNA was “restored” using the Infinium FFPE QC and DNA Restoration Kit as per the manufacturer’s protocol (Illumina, CA, United States). Sodium bisulfite modification was assessed using an in-house developed bisulfite-specific quantitative-PCR, as described previously [3].
The HM450K assay was performed as per the manufacturer’s protocol (Illumina, CA, United States). The extension and staining steps were performed using the TECAN automated liquid handler (Männedorf, Switzerland). The beadchips were scanned using the Illumina iScan (Illumina, CA, United States).
Data quality check and statistical analysis
Raw methylation data was imported into the R statistical environment and processed using the bioconductor package minfi 3.2 [29]. The data were filtered and normalized according to the Illumina protocol, in which the raw fluorescence data was normalized using control probes and methylation values (β-values) were calculated. Additional normalization using Subset-quantile Within Array Normalization (SWAN) was performed to adjust for technical discrepancies between Type I and Type II probes [30]. Probes with a mean detection P-value > 0.05 were excluded from further analysis. Differences of methylation between the blood and tumor-derived DNA were identified by a regression analysis using the empirical Bayes methods using limma R package. Statistical significance was estimated by FDR (false discovery rate) adjusted P-value cut-off of 0.01, calculated using moderated t-statistics. Selected breast cancer susceptibility genes were analyzed, which involved plotting the beta-methylation values at probes located at CpGs across the length of the gene. These regions were defined using UCSC Genome Browser [31].
Microsatellite instability analysis
Microsatellite Instability (MSI) was assessed for 35 pairs of matched blood and tumor-derived DNA using the Promega MSI Analysis System, Version 1.2 Kit, as per manufacturers protocol (Promega, WI, United States). Due to the highly degraded nature of the samples, 10ng of DNA was used in each reaction instead of the recommended 1-2ng, and the number of amplification cycles was increased to 30. The output data was analyzed using GeneMarker, Version 2.6.7 (SoftGenetics, PA, United States). Tumors were considered to have high levels of microsatellite instability (MSI-H) when ≥2 out of the 5 markers showed mononucleotide repeat instability, whereas tumors with <2 unstable markers were considered microsatellite stable (MSS).
Results
We assessed DNA methylation of the tumor and blood-derived samples from 43 women with early-onset breast cancer, across the genomic regions containing the breast and ovarian cancer susceptibility genes BRCA1, BRCA2, PALB2, TP53, ATM, CDH1, CHEK2, FANCM, MLH1, MSH2, MSH6, and PMS2.
Tissue specific methylation
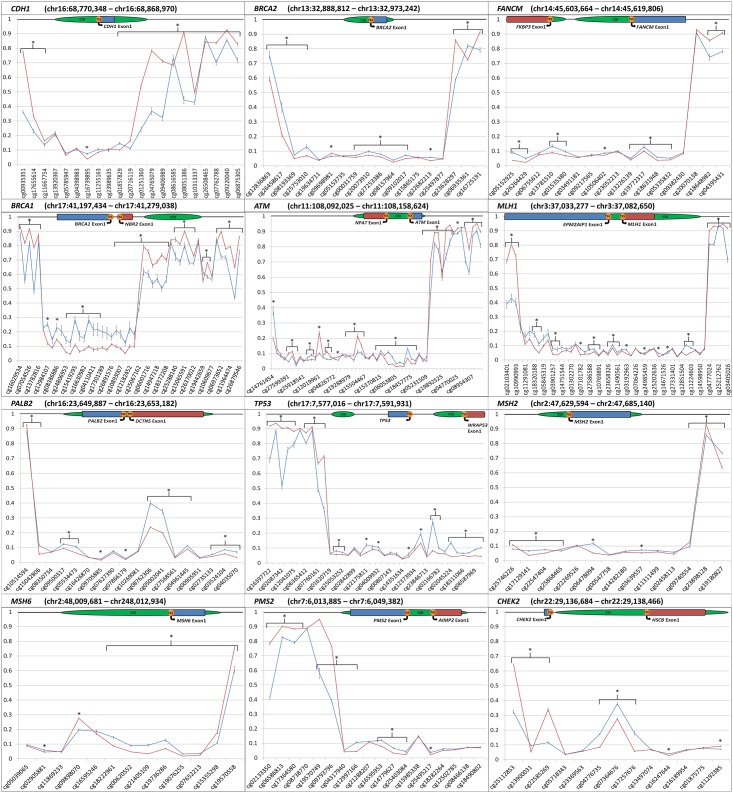
The average beta-methylation values for blood and tumor-derived DNA at the promoter regions of the selected breast cancer susceptibility genes are shown in Fig 1. Tissue specific differences in average beta-methylation levels were identified at a number of CpG probes across each of the promoter regions as indicated.
Fig 1. Comparison of average blood and tumor-derived DNA methylation across a panel of tumor suppressor genes.
Plotted β-values at CpG probes across defined genomic regions. Red line indicates average β-value for blood-derived DNA samples. Blue line indicates average β-values for tumor-derived DNA samples. * indicates significant statistical difference (p<0.01). Error bars are plotted Standard Error of Mean. Gene region schematics denote [CGI (CpG Island), TSS (Transcriptional Start Site)].
As reported previously, increased tumor-derived DNA methylation was identified across all 15 BRCA1 promoter-associated probes with an average Δβ of 11.24% (adj. P-value = 3x10-15 to 2x10-3) compared with blood-derived DNA (Fig 1) [Scott et al. manuscript under review]. Similarly, DNA methylation at 10 CpG probes across the BRCA2 promoter region was increased in the tumor-derived DNA compared with blood-derived DNA (Δβ 5.49%; adj. P-value = 8x10-9 to 1x10-5). At PALB2, the tumor-derived DNA had elevated methylation across 12 promoter associated probes (Δβ 4.67%; adj. P-value = 3x10-14 to 1x10-3) particularly at two probes located 100bp from the Transcription Start Sites (TSS), cg08762306 (Δβ 16.30%; adj. P-value = 1x10-11) and cg05002041 (Δβ 14.59%; adj. P-value = 2x10-11). Methylation in the tumor-derived DNA was increased across 13 TP53 promoter associated probes (Δβ 5.12%; adj. P-value = 5x10-21 to 1x10-3).
Increased methylation at 11 ATM promoter region probes proximal to the TSS in the tumor-derived DNA (Δβ 3.25%; adj. P-value = 1x10-20 to 9x10-3) was identified. Interestingly, this pattern of DNA methylation was reversed in nine gene body probes of ATM with an average Δβ of 12.80% (adj. P-value = 2x10-28 to 5x10-5). Overall, CpG probes encompassing CDH1 measured similar methylation levels in the two tissue types across the CpG Island (CGI) and the first exon. However, methylation was significantly increased in the blood-derived DNA both upstream and downstream of this region, with an average Δβ of 21.26% across 12 probes (adj. P-value = 9x10-33 to 5x10-4). Three consecutive CpG probes in the CHEK2 promoter region showed increased methylation (Δβ ~10.47%; adj. P-value = 2x10-18 to 5x10-13), and increases in methylation in the tumor-derived DNA were present consistently over nine FANCM promoter associated probes (Δβ 2.68%; adj. P-value = 3x10-12 to 3x10-3).
Small but significant increases (Δβ 3.49%; adj. P-value = 1x10-22 to 4x10-3) were observed over 14 probes at MLH1 (located in close relation to the CGI), and four MSH2 promoter associated probes (Δβ 3.46%; adj. P-value = 5x10-15 to 4x10-6). Tumor-derived DNA methylation at five probes across the gene body region of PMS2 was significantly decreased (Δβ 25.70%; adj. P-value = 2x10-32 to 2x10-16), and increased methylation was measured at six promoter associated probes (Δβ 3.09%; adj. P-value = 9x10-17 to 6x10-4). CpG probes at MSH6 showed consistently higher levels of methylation within the tumor-derived DNA across six probes, two of which were associated with its promoter (Δβ 4.09%; adj. P-value = 9x10-17 to 3x10-5) (Fig 1).
Methylation in tumor-derived DNA samples
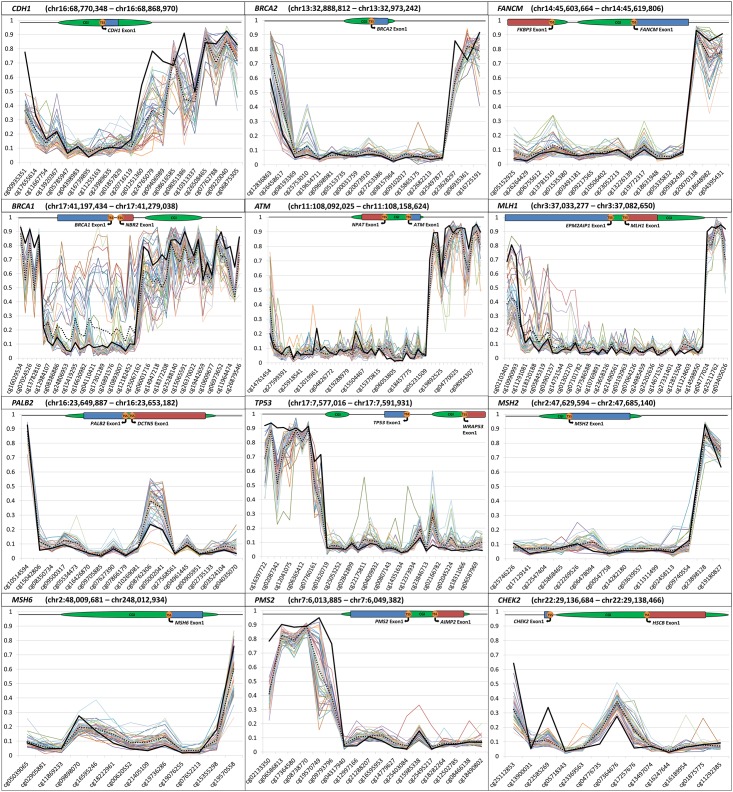
We assessed the individual methylation pattern of each tumor-derived DNA sample across the genomic regions of interest (Fig 2).
Fig 2. Sample tumor DNA methylation across a panel of tumor suppressor genes.
Plotted β-values at CpG probes across defined genomic regions. Each colored line indicates β-values for an individual tumor-derived DNA sample. Solid black line indicates average β-values for blood-derived DNA. Dotted black line indicates average β-values for tumor-derived DNA. Gene region schematics denote [CGI (CpG Island), TSS (Transcriptional Start Site)].
As previously reported, 11 of the 43 samples had notably higher levels of methylation across 18 probes within the BRCA1 promoter region. These 11 samples showed large increases in average methylation levels across the 18 probes when compared with the remaining 32 tumor-derived DNA samples (Δβ 25.03%), and a further increase when compared with the blood-derived DNA samples (Δβ 33.66%) (Scott et al. manuscript under review).
Extending the analysis to the other gene promoter regions of interest found that some tumor samples had distinct methylation profiles. For the TP53 promoter region, one tumor showed notable increases in methylation across 13 probes when compared with the other tumor-derived DNAs (Δβ 14.65%). Two probes (cg22175811 and cg25896754), had a large average Δβ of 42.22%. In the PMS2 promoter region, one sample had a large increase in methylation across 5 promoter-associated probes when compared with the remaining tumor-derived (Δβ ~14.66%) and blood-derived DNA samples (Δβ 15.44%). Both tumor samples with outlying methylation patterns at TP53 and PMS2 were infiltrating ductal carcinomas (grade III). Thirty seven of the 43 tumor samples (86%) were infiltrating ductal tumors.
For PALB2, 40 out of 43 most tumor-derived DNA samples had a higher level of methylation at two promoter probes (cg08762306 and cg05002041) when compared with the average blood-derived DNA methylation. However, one infiltrating ductal carcinoma had a large decrease in methylation compared with the other tumor (Δβ 37.88%) and blood-derived (Δβ 21.78%) DNA samples. Another high grade infiltrating ductal tumor-derived DNA sample had a consistent increase in methylation at FANCM across 12 consecutive probes when compared with the remaining 42 tumor samples (Δβ 8.99%). Two probes located in adjacent CGIs within the FANCM promoter region had a large degree of variation in tumor-derived DNA methylation. Methylation levels ranged from 34.12% to 5.09% at the probe upstream to the TSS (cg13781510), and 29.97% to 6.31% downstream to the TSS (cg19772317).
For CDH1, there was a large variation in methylation between the individual tumor-derived DNA samples, with levels ranging from 4.50% to 67.60% across three probes. BRCA2 contained one promoter associated probe which also had large variation between the tumor-derived DNA samples, ranging from 4.49% to 41.88%.
For the MSH6 promoter region, one tumor-derived DNA sample showed a consistently higher methylation level across 11 probes, located within a CGI and its north shore, when compared to the remaining 42 (Δβ ~8.37%). This sample also had increased methylation across the BRCA1-promoter region, and corresponded to an atypical medullary tumor. Interestingly, this sample also had a consistent increase in methylation across a number of other genes including PALB2, TP53, CDH1, CHEK2, MSH2 and FANCM.
Two tumor-derived DNA samples were highly methylated at MLH1 across 14 probes located proximal to a CGI nearby the promoter region. Methylation was greatly increased when compared to the other 41 tumor-derived DNA samples (average Δβ ~31.63%), in addition to the averaged blood-derived DNA values (Δβ ~35.46%). One sample was in infiltrating ductal tumor (grade II), whereas the other was an atypical medullary cancer (grade III) (Fig 2).
MSI analysis
As MLH1 promoter methylation has been shown to be associated with microsatellite instability (MSI), we tested the tumor derived DNA for evidence of these replication errors. MSI was examined for 35/43 (81%) paired blood and tumor-derived DNA samples, including those with increased MLH1 and MSH6 methylation, using five microsatellite markers: NR-21, NR-24, BAT-25, BAT-26, and MONO-27. No evidence of MSI was observed in any of the 35 samples tested (data not shown).
Discussion
In this study, we assessed DNA methylation profiles of both blood and tumor-derived DNA from 43 early-onset breast cancer cases at known breast cancer predisposition genes.
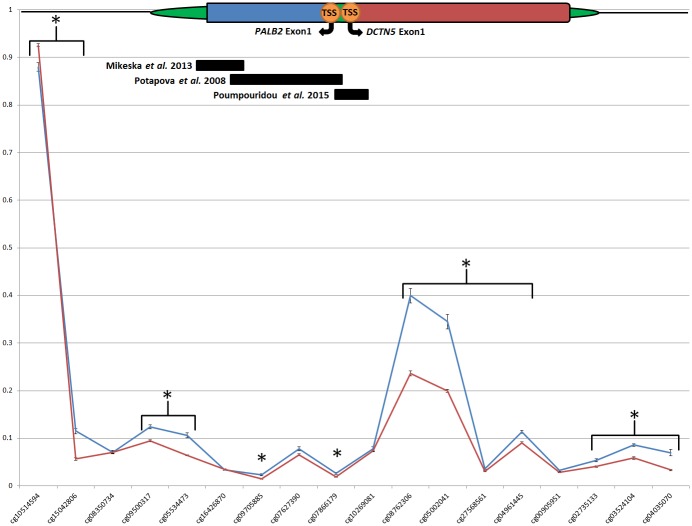
We found variable methylation in tumor-derived DNA at two PALB2 promoter-associated CpG probes (cg08762306 and cg05002041). Three previous studies have investigated PALB2 promoter methylation and each has targeted a different site within the CGI that encompasses the promoter region. Potapova et al (2008) found that 10/130 (~8%) sporadic breast and ovarian tumors presented with hypermethylation within the core promoter region of exon1 near the TSS [9]. Mikeska et al (2013) examined high-grade serous ovarian cancers and found none to be hypermethylated [10]. Poumpouridou et al (2016) analyzed 91 sporadic fresh-frozen breast tissues and also found none to be methylated and almost all (95.6%) to express PALB2 [11]. The region this study found to be methylated was not targeted by any of the above studies (Fig 3). This new data should be used to inform the design of targeted methylation assessment of the PALB2 promoter region in the future.
Fig 3. DNA methylation at the PALB2 promoter region.
Plotted β-values for each sample at each CpG probe across PALB2 (chr16:23,649,887 –chr16:23,653,182). Red line indicates average β -value for blood-derived DNA samples. Blue line indicates average β-value for tumor-derived DNA samples. * indicates significant statistical difference (p<0.01). Solid black bars indicate regions previously screened for methylation. Gene region schematic denotes [CGI (CpG Island), TSS (Transcriptional Start Site)].
Studies of methylation and TP53 in breast cancer are currently limited, although hypermethylation has been reported in epithelial ovarian and cervical cancers [32,33]. These two studies examined a region of TP53 that is encompassed by six HM450K CpG probes between chr17:7,590,728 and chr17:7,591,011. We observed no aberrant methylation in the tumor-derived DNA from this study at this region, however, marginal increased methylation was observed in the CpG probes 939bp downstream of this previously studied region (Fig 1). Increased methylation may be disrupting the normal mechanism of gene transcription, potentially contributing to tumorigenesis in these early-onset breast cancers. These CpGs may also vary in levels of methylation between different tumor tissue types.
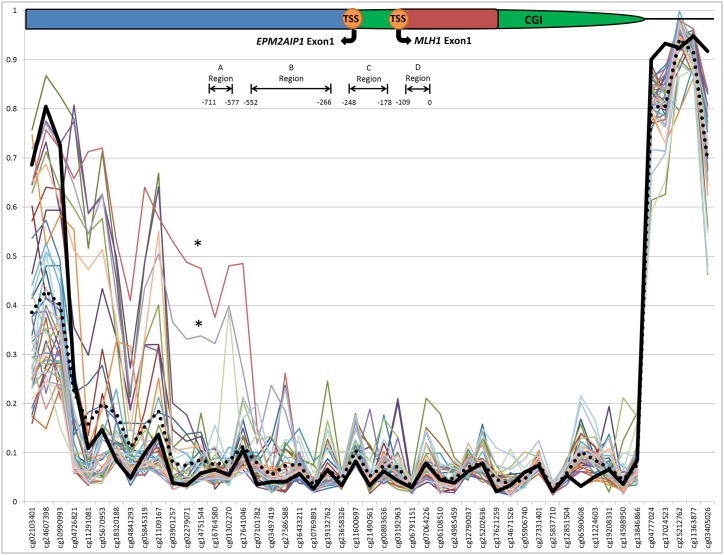
Disruption to the normal function of the DNA mismatch repair (MMR) machinery causes MSI in ~15% of colorectal cancer cases, and has also been reported in sporadic breast tumors [34,35]. Loss of MMR function can result from either carrying a germline mutation in one of the MMR genes (Lynch syndrome), or by hypermethylation of the promoter region of MLH1 in the tumor [36,37]. Two breast tumor-derived DNA samples from this study were highly methylated at MLH1 across 14 probes when compared with the remaining 41 tumor-derived DNA samples (average Δβ ~31.63%). These 14 highly methylated probes were located across previously defined regions ‘A’ (-711 to -577) and ‘B’ (-552 to -266) upstream to the start codon of MLH1 (chr3:37,033,779–37,034,673), although not within the ‘C’ region (-248 to -178) (Fig 4) [38]. Previous studies have found that methylation of the ‘C’ region of the promoter as opposed to the ‘A’ region is more critical for the transcriptional silencing of MLH1, and is associated with MSI-H status and loss of MLH1 protein expression in colorectal cancer [39]. Methylation of region ‘B’ may be important in silencing MLH1 expression, however further research is required to define the function of this region. In this study, increases in methylation were found within the ‘A’ and ‘B’ regions and therefore was consistent with our finding of absence of MSI-H in these two breast tumors.
Fig 4. Sample tumor DNA methylation at the MLH1 promoter region.
Plotted β-values for each sample at each CpG probe across MLH1 (chr3:37,033,277 –chr3:37,082,650). Solid black line indicates average β-values for blood-derived DNA. Dotted black line indicates average β-values for tumor-derived DNA. Schematic of four MLH1 promoter regions previously defined by Deng et al, base pair number is in relation to the start codon (ATG) Regions. * highlights tumor-derived DNA samples showing increased MLH1 methylation. Gene region schematic denotes [CGI (CpG Island), TSS (Transcriptional Start Site)]
Methylation at the BRCA1 promoter in blood derived DNA is associated with risk of breast cancer with distinct histological features [3]. We tested for associations between methylation marks in the additional genes and histological features: mitotic index, nuclear grade, tubule formation, a trabecular growth pattern (primary or secondary), a syncytial growth pattern, pushing margins (>50%), circumscribed, necrosis, moderate or intense lymphocytic infiltrate but no clear associations were identified.
This study has limitations. First, the study is small and the molecular events being investigated could be very rare in breast cancer predisposition and tumorigenesis. Larger studies are needed to further test for i) methylation as a silencing mechanism involved with these genes and breast cancer predisposition, ii) methylation as a silencing mechanism involved with these genes and breast cancer progression and iii) for associations between methylation marks and histological features.
Second, HM450K array data derived from adjacent non-tumour ductal epithelium (preferably matched to the women whose tumors were included in this study), would have been an informative reference. Unfortunately, accessing normal breast material is a significant challenge, especially when using archival FFPE tumor material [40] and the necessary resources were not available for this study. However, as demonstrated by the BRCA1 example (see Fig 2; BRCA1) much information is gained by tumor/tumor and tumor/blood comparisons and it is highly unlikely that a methylation event at one of these breast cancer predisposition genes would be involved in all tumor and blood derived DNA samples included in this study. Indeed, aberrant methylation at BRCA1 is evident in only eleven tumor DNA samples (and the corresponding blood derived DNA samples) despite the highly selected nature of the samples (early onset breast cancer).
Third, although we have indirectly tested the relevance of the observed MLH1 methylation (by testing for microsatellite instability), we do not have any direct evidence that the methylation patterns described for these breast cancer predisposition genes correspond to a change in gene or protein expression (due to a lack of suitable material from which to collect this data).
Application of the HM450K beadchip assay has enabled a finer description of DNA methylation at genomic regions of interest to breast cancer susceptibility and tumor progression. This work informs future studies investigating the role of methylation in breast cancer susceptibility gene silencing.
Supporting Information
(CSV)
Acknowledgments
We extend our thanks to the many women and their families who generously participated in the Australian Breast Cancer Family Study and consented to allow us access to their pathology material.
Data Availability
All relevant data are within the paper and the supporting information files.
Funding Statement
The Australia site of Breast Cancer Family Registry was supported by the National Health and Medical Research Council of Australia, the New South Wales Cancer Council, the Victorian Health Promotion Foundation (Australia) and grant UM1 CA164920 from the USA National Cancer Institute. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products, or organizations imply endorsement by the USA Government or the BCFR. JLH is a Senior Principle Research Fellow and MCS is a Senior Research Fellow of the NHMRC. CMS is a recipient of a Genetic Epidemiology Laboratory Honors Scholarship, Department of Pathology, The University of Melbourne.
References
- 1.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 2003;349:2042–54. 10.1056/NEJMra023075 [DOI] [PubMed] [Google Scholar]
- 2.Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 2015;372:2243–57. 10.1056/NEJMsr1501341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong EM, Southey MC, Fox SB, Brown MA, Dowty JG, Jenkins MA, et al. Constitutional methylation of the BRCA1 promoter is specifically associated with BRCA1 mutation-associated pathology in early-onset breast cancer. Cancer Prev Res (Phila) 2011;4:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, Lerma E, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst 2000;92:564–9. [DOI] [PubMed] [Google Scholar]
- 5.Esteller M, Fraga MF, Guo M, Garcia-Foncillas J, Hedenfalk I, Godwin AK, et al. DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum Mol Genet 2001;10:3001–7. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe Y, Maeda I, Oikawa R, Wu W, Tsuchiya K, Miyoshi Y, et al. Aberrant DNA methylation status of DNA repair genes in breast cancer treated with neoadjuvant chemotherapy. Genes Cells 2013;18:1120–30. 10.1111/gtc.12100 [DOI] [PubMed] [Google Scholar]
- 7.Flanagan JM, Munoz-Alegre M, Henderson S, Tang T, Sun P, Johnson N, et al. Gene-body hypermethylation of ATM in peripheral blood DNA of bilateral breast cancer patients. Hum Mol Genet 2009;18:1332–42. 10.1093/hmg/ddp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan K, Garcia-Closas M, Orr N, Fletcher O, Jones M, Ashworth A, et al. Intragenic ATM Methylation in Peripheral Blood DNA as a Biomarker of Breast Cancer Risk. Cancer Research 2012;72:2304–13. 10.1158/0008-5472.CAN-11-3157 [DOI] [PubMed] [Google Scholar]
- 9.Potapova A, Hoffman AM, Godwin AK, Al-Saleem T, Cairns P. Promoter hypermethylation of the PALB2 susceptibility gene in inherited and sporadic breast and ovarian cancer. Cancer Res 2008;68:998–1002. 10.1158/0008-5472.CAN-07-2418 [DOI] [PubMed] [Google Scholar]
- 10.Mikeska T, Alsop K, Group AOCS, Mitchell G, Bowtell DDL, Dobrovic A. No evidence for PALB2 methylation in high-grade serous ovarian cancer. Journal of Ovarian Research 2013;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poumpouridou N, Acha-Sagredo A, Goutas N, Vlachodimitropoulos D, Chatziioannidou I, Lianidou E, et al. Development and validation of molecular methodologies to assess PALB2 expression in sporadic breast cancer. Clin Biochem 2016;49:253–9. 10.1016/j.clinbiochem.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 12.Ramalho EA, Silva-Filho JL, Cartaxo MF, Cavalcanti CB, Rego MJ, Oliveira MB, et al. Assessment of changes in the BRCA2 and P53 genes in breast invasive ductal carcinoma in northeast Brazil. Biol Res 2014;47:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams LH, Choong D, Johnson SA, Campbell IG. Genetic and epigenetic analysis of CHEK2 in sporadic breast, colon, and ovarian cancers. Clin Cancer Res 2006;12:6967–72. 10.1158/1078-0432.CCR-06-1770 [DOI] [PubMed] [Google Scholar]
- 14.Hopper JL, Southey MC, Dite GS, Jolley DJ, Giles GG, McCredie MR, et al. Population-based estimate of the average age-specific cumulative risk of breast cancer for a defined set of protein-truncating mutations in BRCA1 and BRCA2. Australian Breast Cancer Family Study. Cancer Epidemiol Biomarkers Prev 1999;8:741–7. [PubMed] [Google Scholar]
- 15.Dite GS, Jenkins MA, Southey MC, Hocking JS, Giles GG, McCredie MR, et al. Familial risks, early-onset breast cancer, and BRCA1 and BRCA2 germline mutations. J Natl Cancer Inst 2003;95:448–57. [DOI] [PubMed] [Google Scholar]
- 16.John EM, Hopper JL, Beck JC, Knight JA, Neuhausen SL, Senie RT, et al. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res 2004;6:R375–89. 10.1186/bcr801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apicella C, Dowty JG, Dite GS, Jenkins MA, Senie RT, Daly MB, et al. Validation study of the LAMBDA model for predicting the BRCA1 or BRCA2 mutation carrier status of North American Ashkenazi Jewish women. Clin Genet 2007;72:87–97. 10.1111/j.1399-0004.2007.00841.x [DOI] [PubMed] [Google Scholar]
- 18.Leong T, Whitty J, Keilar M, Mifsud S, Ramsay J, Birrell G, et al. Mutation analysis of BRCA1 and BRCA2 cancer predisposition genes in radiation hypersensitive cancer patients. Int J Radiat Oncol Biol Phys 2000;48:959–65. [DOI] [PubMed] [Google Scholar]
- 19.Andrulis IL, Anton-Culver H, Beck J, Bove B, Boyd J, Buys S, et al. Comparison of DNA- and RNA-based methods for detection of truncating BRCA1 mutations. Hum Mutat 2002;20:65–73. 10.1002/humu.10097 [DOI] [PubMed] [Google Scholar]
- 20.Southey MC, Tesoriero AA, Andersen CR, Jennings KM, Brown SM, Dite GS, et al. BRCA1 mutations and other sequence variants in a population-based sample of Australian women with breast cancer. Br J Cancer 1999;79:34–9. 10.1038/sj.bjc.6690008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith LD, Tesoriero AA, Ramus SJ, Dite G, Royce SG, Giles GG, et al. BRCA1 promoter deletions in young women with breast cancer and a strong family history: a population-based study. Eur J Cancer 2007;43:823–7. 10.1016/j.ejca.2007.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuhausen SL, Ozcelik H, Southey MC, John EM, Godwin AK, Chung W, et al. BRCA1 and BRCA2 mutation carriers in the Breast Cancer Family Registry: an open resource for collaborative research. Breast Cancer Res Treat 2009;116:379–86. 10.1007/s10549-008-0153-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Southey MC, Teo ZL, Dowty JG, Odefrey FA, Park DJ, Tischkowitz M, et al. A PALB2 mutation associated with high risk of breast cancer. Breast Cancer Res 2010;12:R109 10.1186/bcr2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldgar DE, Healey S, Dowty JG, Da Silva L, Chen X, Spurdle AB, et al. Rare variants in the ATM gene and risk of breast cancer. Breast Cancer Res 2011;13:R73 10.1186/bcr2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Calvez-Kelm F, Lesueur F, Damiola F, Vallee M, Voegele C, Babikyan D, et al. Rare, evolutionarily unlikely missense substitutions in CHEK2 contribute to breast cancer susceptibility: results from a breast cancer family registry case-control mutation-screening study. Breast Cancer Res 2011;13:R6 10.1186/bcr2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mouchawar J, Korch C, Byers T, Pitts TM, Li E, McCredie MR, et al. Population-based estimate of the contribution of TP53 mutations to subgroups of early-onset breast cancer: Australian Breast Cancer Family Study. Cancer Res 2010;70:4795–800. 10.1158/0008-5472.CAN-09-0851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joo JE, Wong EM, Baglietto L, Jung CH, Tsimiklis H, Park DJ, et al. The use of DNA from archival dried blood spots with the Infinium HumanMethylation450 array. Bmc Biotechnology 2013;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong EM, Joo JE, McLean CA, Baglietto L, English DR, Severi G, et al. Tools for translational epigenetic studies involving formalin-fixed paraffin-embedded human tissue: applying the Infinium HumanMethyation450 Beadchip assay to large population-based studies. BMC Res Notes 2015;8:543 10.1186/s13104-015-1487-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014;30:1363–9. 10.1093/bioinformatics/btu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maksimovic J, Gagnon-Bartsch JA, Speed TP, Oshlack A. Removing unwanted variation in a differential methylation analysis of Illumina HumanMethylation450 array data. Nucleic Acids Res 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Research 2002;12:996–1006. 10.1101/gr.229102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chmelarova M, Krepinska E, Spacek J, Laco J, Beranek M, Palicka V. Methylation in the p53 promoter in epithelial ovarian cancer. Clin Transl Oncol 2013;15:160–3. 10.1007/s12094-012-0894-z [DOI] [PubMed] [Google Scholar]
- 33.Jha AK, Nikbakht M, Jain V, Sehgal A, Capalash N, Kaur J. Promoter hypermethylation of p73 and p53 genes in cervical cancer patients among north Indian population. Molecular Biology Reports 2012;39:9145–57. 10.1007/s11033-012-1787-5 [DOI] [PubMed] [Google Scholar]
- 34.Murata H, Khattar NH, Kang Y, Gu L, Li GM. Genetic and epigenetic modification of mismatch repair genes hMSH2 and hMLH1 in sporadic breast cancer with microsatellite instability. Oncogene 2002;21:5696–703. 10.1038/sj.onc.1205683 [DOI] [PubMed] [Google Scholar]
- 35.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–7. 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ten Broeke SW, Brohet RM, Tops CM, van der Klift HM, Velthuizen ME, Bernstein I, et al. Lynch syndrome caused by germline PMS2 mutations: delineating the cancer risk. J Clin Oncol 2015;33:319–25. 10.1200/JCO.2014.57.8088 [DOI] [PubMed] [Google Scholar]
- 37.Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011;305:2304–10. 10.1001/jama.2011.743 [DOI] [PubMed] [Google Scholar]
- 38.Deng G, Chen A, Hong J, Chae HS, Kim YS. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res 1999;59:2029–33. [PubMed] [Google Scholar]
- 39.Parsons MT, Buchanan DD, Thompson B, Young JP, Spurdle AB. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification. J Med Genet 2012;49:151–7. 10.1136/jmedgenet-2011-100714 [DOI] [PubMed] [Google Scholar]
- 40.Wong EM, Joo JE, McLean CA, Baglietto L, English DR, Severi G, et al. Analysis of the breast cancer methylome using formalin-fixed parafin-embedded tumour. Breast Cancer Res Treat. 2016. September 7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
Data Availability Statement
All relevant data are within the paper and the supporting information files.