Abstract
Emerging fungal diseases are threatening ecosystems and have increased in recent decades. In corals, the prevalence and consequences of these infections have also increased in frequency and severity. Coral reefs are affected by an emerging fungal disease named aspergillosis, caused by Aspergillus sydowii. This disease and its pathogen have been reported along the Caribbean and Pacific coasts of Colombia. Despite this, an important number of coral reefs worldwide have not been investigated for the presence of this pathogen. In this work, we carried out the surveillance of the main coral reef of the Ecuadorian Pacific with a focus on the two most abundant and cosmopolitan species of this ecosystem, Leptogorgia sp. and Leptogorgia obscura. We collected 59 isolates and obtained the corresponding sequences of the Internal Transcribed Spacers (ITS) of the ribosomal DNA. These were phylogenetically analyzed using MrBayes, which indicated the presence of two isolates of the coral reef pathogen A. sydowii, as well as 16 additional species that are potentially pathogenic to corals. Although the analyzed gorgonian specimens appeared healthy, the presence of these pathogens, especially of A. sydowii, alert us to the potential risk to the health and future survival of the Pacific Ecuadorian coral ecosystem under the current scenario of increasing threats and stressors to coral reefs, such as habitat alterations by humans and global climate change.
Introduction
Coral reefs are considered one of the most biologically diverse ecosystems in the marine realm [1]. They maintain a high biomass and abundance of varied organisms [2] and provide a plethora of micro-habitats to support enormous biodiversity [3–6]. In recent decades, coral reefs have experienced increasing pressures, and are disturbed by a combination of direct human impacts, e.g., habitat fragmentation and reduction of functional diversity [7], and global climate change, e.g., increasing ocean acidification and temperature, coral bleaching, etc. [8]. These conditions make reefs more susceptible to the proliferation and development of opportunistic organisms, which take advantage of the weakened corals [9,10].
The coral disease aspergillosis has produced significant deterioration and partial and massive mortalities of coral communities in the Caribbean Sea [11–15]. The responsible pathogen is the ascomycetous fungus Aspergillus sydowii (Bainier and Sartory, 1926). The first report of this disease in gorgonians dates back to 1995 [14,15], although similar symptoms and outbreaks had been previously reported in the 1980s [16]. The ascomycete fungus A. sydowii is globally distributed and occurs in diverse environments where it survives as a soil decomposing saprotroph [17–19]. It is apparently a terrestrial fungus, but it is salt tolerant and capable of growing in the sea [20]. Moreover, A. sydowii has been reported as a food contaminant [21], and a human pathogen in immune-compromised patients [22,23]. In marine ecosystems, A. sydowii has been isolated from some gorgonian communities of the Caribbean [11,24], Colombian Pacific coasts [25], and environmental samples of the Australian coastal waters [20].
Aspergillosis causes selective mortality of large sea fans [26], and suppression of reproduction in infected individuals [27]. As a consequence, coral population levels decrease [28]. The symptoms include purpling of the tissue, galling, and lesions [11], associated with necrotic sea fan tissue [14]. Prevalence (percentage of fans infected) and disease severity (mean percentage of fan tissue affected by disease) are positively correlated with water depth, and large sea fans are more likely to be infected than small fans [15,29]. Although the origin of this disease and its epidemiology is unknown, microsatellites and phylogenetic studies reveal a pattern of global panmixia among isolates. Moreover, sea isolates are interspersed with those isolated from environmental samples [30]. Aspergillus sydowii was isolated, identified and inoculated as the causative agent of the sea fan disease (Koch’s postulates) by previous authors [11,19]. The incidence of this pathogen can be similar to other fungal species, i.e., Fusarium keratoplasticum and F. falciforme, in other animals and ecosystems [31], exacerbated by the effects of global climate change and habitat alteration by humans.
In the Ecuadorian Pacific, there are no records of A. sydowii and coral reefs appear to be healthy. Due to the current trend of expansion of fungal infections and the endangered situation of coral reefs, we performed a survey in the Machalilla gorgonian gardens, which includes the most representative gorgonian species in a hot spot of marine biodiversity in Ecuador. We investigated the presence of A. sydowii in these organisms.
Material and Methods
Sampling
Gorgonian octocoral colonies were collected by SCUBA diving from rocky bottoms located in The Frailes, Machalilla National Park (Manabí, Ecuador) (1°30'14"S 80°48'33”W). The authority who issued the permission for each location was the "Ministerio del Ambiente, Manabí (ECUADOR)" (Permit Number: N° 016 –RM–DPM–MA). Due to the absence of symptoms, we randomly selected 40 colonies from the two most abundant and cosmopolitan gorgonian species of this area (pers. obs.), Leptogorgia Milne-Edwards and Haime, 1857 [32]: Leptogorgia obscura Bielschowsky, 1929 [33], and Leptogorgia sp. (under description). The colonies were collected within a range of 10 to 15 m in depth. Samples were kept in individual sterile plastic bags and processed in the laboratory under axenic conditions.
Fungal isolation
From each colony, fragments of ca. 3 cm wide from randomly selected areas were excised using a sterile scalpel. To remove fungi not associated with the octocorals, the selected fragments were surface-sterilized with 70% ethanol for 30 s [25]. For fungal isolations, the selected fragments were transferred onto a peptone glucose agar media (PGA) [34] supplemented with penicillin (100 mg/l). In order to avoid any possible errors in the identification of coral fungi (negative control) the sea water sample was isolated. A glass-ring technique was used for isolation following the methodology described in [31]. Resulting pure cultures were maintained in PGA at 4°C. Cultures were labeled as ASP001 through ASP059 in the culture collection of the Real Jardín Botánico, Madrid, Spain (Table 1).
Table 1. Fungal isolates from gorgonians Leptogorgia obscura and Leptogorgia sp. from the Eastern Pacific of Ecuador and the resulting molecular identification based on phylogenetic analysis.
| RJB number | Isolate number | Gorgoniidae Species | Fungus Species | GenBank Acc. Num. |
|---|---|---|---|---|
| ASP001 | GORG01 | Leptogorgia sp. | Pyrenochaetopsis leptospora | KX712403 |
| ASP002 | GORG02 | Leptogorgia sp. | Nigrospora sp. | KX712404 |
| ASP003 | GORG03 | Leptogorgia sp. | Penicillium chrysogenum | KX712405 |
| ASP004 | GORG04 | Leptogorgia sp. | Penicillium chrysogenum | KX712406 |
| ASP005 | GORG05 | Leptogorgia sp. | Penicillium chrysogenum | KX712407 |
| ASP006 | GORG07 | Leptogorgia obscura | Tritirachium sp. | KX712408 |
| ASP007 | GORG08 | Leptogorgia sp. | Penicillium chrysogenum | KX712409 |
| ASP008 | GORG09 | Leptogorgia obscura | Penicillium chrysogenum | KX712410 |
| ASP009 | GORG10 | Leptogorgia sp. | Penicillium chrysogenum | KX712411 |
| ASP010 | GORG11 | Leptogorgia obscura | Penicillium chrysogenum | KX712412 |
| ASP011 | GORG12 | Leptogorgia obscura | Fusarium longipes | KX712413 |
| ASP012 | GORG13 | Leptogorgia sp. | Penicillium chrysogenum | KX712414 |
| ASP013 | GORG16 | Leptogorgia sp. | Nigrospora sp. | KX712415 |
| ASP014 | GORG17 | Leptogorgia obscura | Lasiodiplodia pseudotheobromae | KX712416 |
| ASP015 | GORG18 | Leptogorgia obscura | Phoma sp. | KX712417 |
| ASP016 | GORG21 | Leptogorgia obscura | Penicillium chrysogenum | KX712418 |
| ASP017 | GORG22 | Leptogorgia sp. | Penicillium chrysogenum | KX712419 |
| ASP018 | GORG24 | Leptogorgia obscura | Fusarium longipes | KX712420 |
| ASP019 | GORG25 | Leptogorgia obscura | Cladosporium dominicanum | KX712421 |
| ASP020 | GORG26 | Leptogorgia obscura | Aspergillus sydowii | KX712422 |
| ASP021 | GORG27 | Leptogorgia obscura | Aspergillus sydowii | KX712423 |
| ASP022 | GORG29 | Leptogorgia obscura | Penicillium chrysogenum | KX712424 |
| ASP023 | GORG30 | Leptogorgia sp. | Penicillium chrysogenum | KX712425 |
| ASP024 | GORG31 | Leptogorgia obscura | Nigrospora sp. | KX712426 |
| ASP025 | GORG32 | Leptogorgia sp. | Aspergillus wentii | KX712427 |
| ASP026 | GORG33 | Leptogorgia sp. | Penicillium chrysogenum | KX712428 |
| ASP027 | GORG34 | Leptogorgia sp. | Penicillium chrysogenum | KX712429 |
| ASP028 | GORG35 | Leptogorgia obscura | Penicillium chrysogenum | KX712430 |
| ASP029 | GORG36 | Leptogorgia sp. | Penicillium chrysogenum | KX712431 |
| ASP030 | GORG37 | Leptogorgia sp. | Penicillium chrysogenum | KX712432 |
| ASP031 | GORG38 | Leptogorgia sp. | Cladosporium sphaerospermum | KX712433 |
| ASP032 | GORG40 | Leptogorgia obscura | Nigrospora sp. | KX712434 |
| ASP033 | GORG42 | Leptogorgia obscura | Fusarium longipes | KX712435 |
| ASP034 | GORG43 | Leptogorgia obscura | Penicillium chrysogenum | KX712436 |
| ASP035 | GORG44 | Leptogorgia obscura | Penicillium chrysogenum | KX712437 |
| ASP036 | GORG45 | Leptogorgia sp. | Penicillium chrysogenum | KX712438 |
| ASP037 | GORG46 | Leptogorgia obscura | Penicillium chrysogenum | KX712439 |
| ASP038 | GORG47 | Leptogorgia sp. | Penicillium chrysogenum | KX712440 |
| ASP039 | GORG48 | Leptogorgia obscura | Nigrospora sp. | KX712441 |
| ASP040 | GORG49 | Leptogorgia obscura | Fusarium longipes | KX712442 |
| ASP041 | GORG50 | Leptogorgia sp. | Penicillium chrysogenum | KX712443 |
| ASP042 | GORG51 | Leptogorgia sp. | Penicillium chrysogenum | KX712444 |
| ASP043 | GORG53 | Leptogorgia sp. | Aspergillus ochraceopetaliformis | KX712445 |
| ASP044 | GORG54 | Leptogorgia obscura | Nigrospora sp. | KX712446 |
| ASP045 | GORG55 | Leptogorgia obscura | Penicillium chrysogenum | KX712447 |
| ASP046 | GORG63 | Leptogorgia sp. | Cladosporium sphaerospermum | KX712448 |
| ASP047 | GORG68 | Leptogorgia sp. | Penicillium chrysogenum | KX712449 |
| ASP048 | GORG71 | Leptogorgia obscura | Capnobotryella sp. | KX712450 |
| ASP049 | GORG76 | Leptogorgia sp. | Cladosporium sphaerospermum | KX712451 |
| ASP050 | GORG77 | Leptogorgia sp. | Cladosporium sphaerospermum | KX712452 |
| ASP051 | GORG78 | Leptogorgia sp. | Curvularia sp. | KX712453 |
| ASP052 | GORG80 | Leptogorgia sp. | Alternaria sp. | KX712454 |
| ASP053 | GORG83 | Leptogorgia obscura | Aspergillus sclerotiorum | KX712455 |
| ASP054 | GORG84 | Leptogorgia obscura | Aspergillus sclerotiorum | KX712456 |
| ASP055 | GORG85 | Leptogorgia sp. | Nigrospora sp. | KX712457 |
| ASP056 | GORG87 | Leptogorgia sp. | Cladosporium sphaerospermum | KX712458 |
| ASP057 | GORG88 | Leptogorgia sp. | Penicillium mallochii | KX712459 |
| ASP058 | GORG90 | Leptogorgia obscura | Cladosporium dominicanum | KX712460 |
| ASP059 | GORG92 | Leptogorgia sp. | Tritirachium sp. | KX712461 |
DNA extraction, PCR amplification, sequencing, and species identification
DNA was extracted from 20 mg of the fungal isolate tissues using the DNeasy extraction kit (Qiagen, Inc.) according to the manufacturer’s protocol. DNA fragments containing internal transcribed spacers ITS1 and ITS2, including 5.8S, were amplified and sequenced with primer pair ITS5/ITS4 [35]. The PCR profile was: 2.5 μl 10 x buffer, 1.4 μl 50 mM MgCl2, 1.6 μl 25 mM dNTPs, 0.5 μl of each 10 mM primer (forward and reverse), 1 μl 1 mg/ml BSA, 1 μl DNA, 0.3 μl 5 U/μl Taq polymerase, and 16.2 μl ddH2O. The PCR conditions were 1 min at 95°C, 35 cycles of 1 min at 95°C, 45 s at 58°C and 1 min at 72°C, and finally 10 min at 72°C. The amplicons were sequenced for both strands using BigDye Terminator in an ABI 3730 genetic analyzer (Applied Biosystems).
The sequences were edited and primers trimmed using the Sequencher v.4.9 program (Gene Code Corporation, Ann Arbor, MI, USA). BLAST [36] was used to compare the sequences against those existing in the National Center of Biotechnology Information (NCBI) nucleotide databases.
For species identification of the isolates, the corresponding ITS sequences were phylogenetically analyzed with a number of selected ITS sequences of reference of closely related fungal species obtained from the NCBI (see Table 2, Fig 1). To perform the phylogenetic analyses, a GTR + G + I substitution model was first obtained using the jModelTest v2.1.5 [37] program. This model was selected based on the Akaike Information Criterion (AIC). Bayesian inference and Maximum Likelihood analyses were performed using MrBayes v3.2.5 [38] and RaxML v8.0.0 [39], respectively. The Bayesian inference analysis was implemented with three runs of 20 million generations sampling one tree per 1000 replicates. For each run, eight Markov chain Monte Carlo (MCMC) simulations were conducted. These simulations were run until a critical value was reached for the topological convergence diagnostic lower than 0.005. Branch supports were evaluated by posterior probabilities after a burn-in of 25%. The Maximum Likelihood analysis was implemented with a random starting tree and clade support was assessed with 1000 bootstrap replicates. The Maximum Likelihood analysis was implemented in the graphical user interface raxmlGUI v1.5 [40]. The clade support was assessed with 1000 bootstrap replicates after selecting the best tree from 100 trees generated.
Table 2. Genbank rDNA ITS reference sequences for fungal species used in phylogenetic analysis to identify fungal isolates from Ecuadorian gorgonians.
| Species | GenBank number | Isolate / strain | Type material |
|---|---|---|---|
| Penicillium chrysogenum | NR_077145 | CBS 306.48 | yes |
| Aspergillus sclerotiorum | NR_131294 | NRRL 415 | yes |
| Aspergillus ochraceopetaliformis | KF384187 | FJ120 | - |
| Aspergillus sydowii | AB267812 | CBS 593.65 | - |
| Penicillium mallochii | NR_111674 | DAOM 239917 | yes |
| Aspergillus wentii | NR_077152 | ATCC 1023 | yes |
| Lasiodiplodia pseudotheobromae | NR_111264 | CBS 116459 | yes |
| Cladosporium sphaerospermum | NR_111222 | CBS 193.54 | yes |
| Cladosporium dominicanum | NR_119603 | - | yes |
| Nigrospora oryzae | HQ607925 | ATT291 | - |
| Nigrospora sp. | KP793234 | M116 | - |
| Nigrospora sp. | JN207335 | P39E2 | - |
| Fusarium longipes | AB820724 | IFM 50036 | - |
| Tritirachium sp. | EU497949 | F13 | - |
| Alternaria sp. | KM507780 | 311a | - |
| Curvularia sp. | HE861836 | UTHSC:08–2905 | - |
| Pyrenochaetopsis leptospora | NR_119958 | - | yes |
| Phoma sp. | KF367550 | 4 BRO-2013 | - |
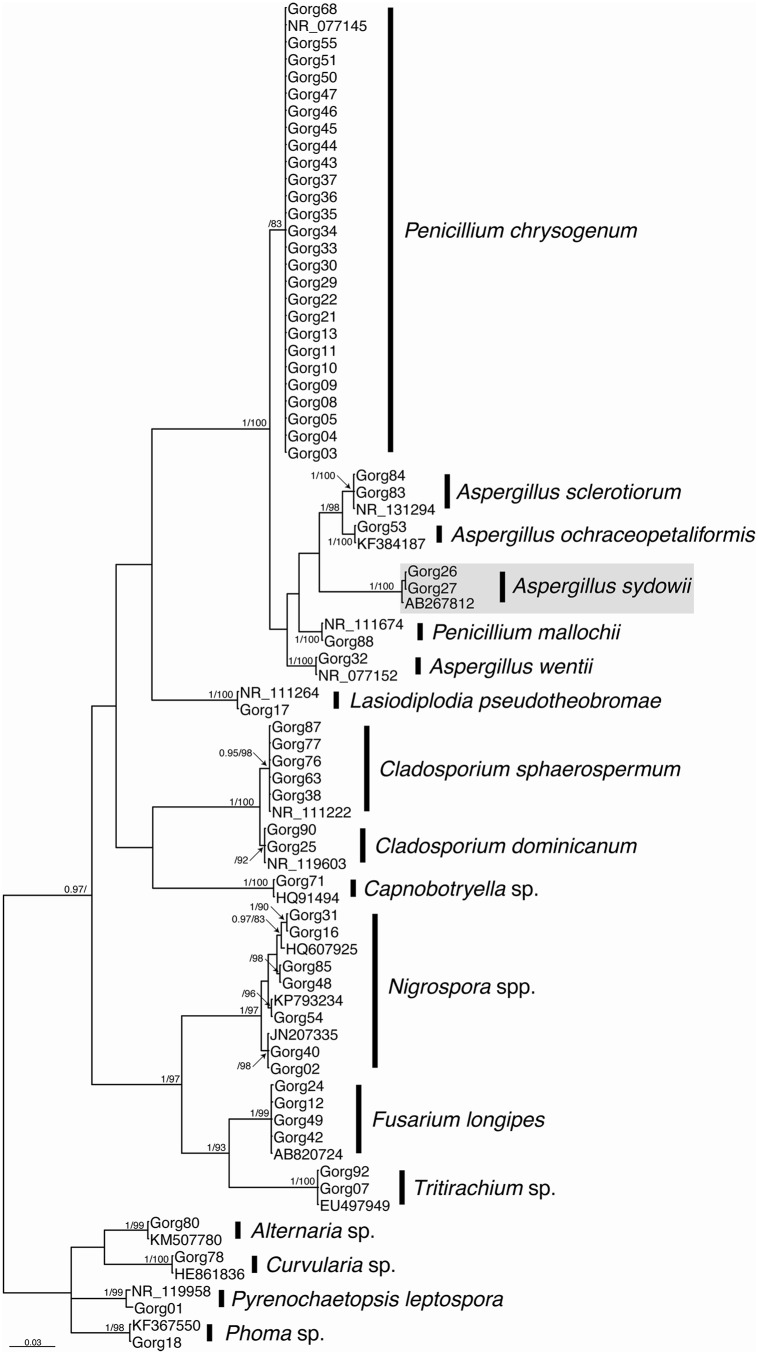
Fig 1. Bayesian out-group-rooted cladogram inferred from ITS rRNA gene sequences of fungal isolates from Leptogorgia obscura and Leptogorgia sp. from the Eastern Ecuadorian Pacific.
Numbers placed above and below the internodes are, respectively, PP and BS of the Bayesian and Maximum Likelihood analyses.
Morphological characterization
Isolates corresponding to A. sydowii according to phylogenetic analysis were morphologically characterized using scanning electron microscopy, SEM (Hitachi s3000N, Real Jardín Botánico, CSIC, Madrid, Spain). Mycelia with characteristic features were fixed in 2% glutaraldehyde for 1 h, washed in distilled sterile water, and then dehydrated for 1 h in a series of ethanol (30, 50, 70, 80, 90, 95 and 100%) solutions. The isolates dehydrated in absolute ethanol were critical-point dried and the material was sputter coated in a vacuum with an electrically conductive layer of gold to a thickness of about 80 nm. Samples were observed at a beam specimen angle of 45° with an accelerating voltage of 20kV and final aperture at 200 μm.
Results
Fungal isolation and species identification
A total of 59 fungal isolates were obtained and the phylogenetic analyses resulted in 17 phylogenetically supported clusters (Fig 1). The majority of the isolates could be assigned to a species reference sequence (from type material). These species were: Penicillium chrysogenum, P. mallochii, Aspergillus sclerotiorum, A. ochraceopetaliformis, A. sydowii, A. wentii, Lasiodiplodia pseudotheobromae, Cladosporium shpaerospermum, C. dominicanum, Fusarium longipes, and Pyrenochaetopsis leptospora. Five groups of isolates grouped with sequences of references of unknown species: Alternaria sp., Capnobotryella sp. Curvularia sp., Phoma sp., and Tritirachium sp. The sequences grouping into the cluster containing Nigrospora spp. were diverse and could not be assigned to any known ITS sequences (Table 2).
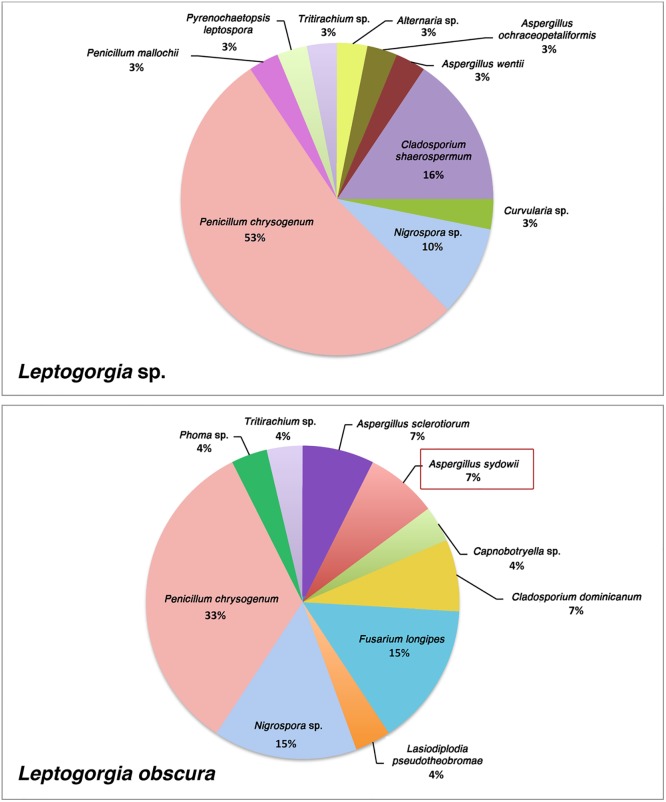
The majority of the isolates belonged to the genera Penicillium and Aspergillus (Fig 2). The most frequent species was Penicillium chrysogenum (24 out of 59), which occurred in colonies of both L. obscura and Leptogorgia sp. (Fig 2), followed by Nigorspora spp. and Penicillium granulatum (both 4 out of 59 each), to Aspergillus sydowii (2 out of 59). The species A. sydowii and Fusarium longipes were only found in L. obscura (Fig 2).
Fig 2. Pie charts showing isolation frequency of different fungal isolates from each host gorgonian species, Leptogorgia obscura and Leptogorgia sp. of the Eastern Ecuadorian Pacific.
Morphological characterization
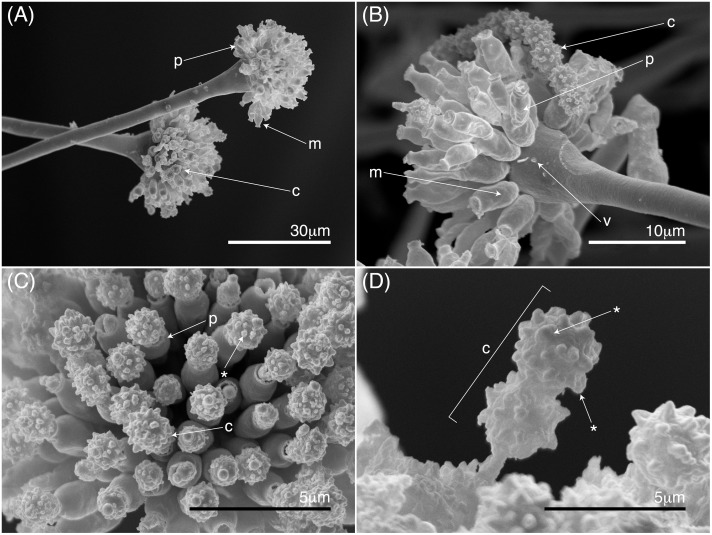
The isolates molecularly assigned to A. sydowii showed conidiophores with conidia, metulae, and phialides seen in the SEM micrographs (Fig 3). The length of the metulae ranged from 2.5 to 3.5 μm, and the breadth from 4.2 to 6.5 μm. Phialides ranged from 2.2 to 3.0 μm in length and from 3.4 to 6.1μm in breadth. The conidia were globose with a roughened or spinose ornamentation. These conidia had a diameter that ranged from 2.5 to 4.4 μm (Fig 3).
Fig 3. Scanning electron microscopy showing characteristic features of conidiophores of Aspergillus sydowii isolated from Leptogorgia obscura and Leptogorgia sp. of the Eastern Ecuadorian Pacific: (A) conidiophore structure with metule (m), phialide (p), and globose conidia (c); (B) morphological features of conidia head with a vesicle characteristic (v), metulae (m), phialide (p), and globose conidia (c); (C) phialide (p) and mature globose conidia (c) with verruculose ornamentation (*); (D) globose conidia (c) with verruculose ornamentation (*).
Discussion
The Ecuadorian Pacific coral reefs are one of the most important and unstudied marine ecosystems and biodiversity ‘hot spots’. In this study, we found that the gorgonian communities of these reefs, specifically L. obscura and Leptogorgia sp. colonies, hold a large, diverse, and mostly unknown fungal community in these hosts. Interestingly, this fungal community includes the coral pathogen A. sydowii. This constitutes the first report of this pathogen in Ecuador and the second in the Eastern Pacific. The gorgonian community appeared to be healthy and showed no symptoms of fungal disease during the sampling period. These results are similar to those of [13,41], who also found isolates of A. sydowii in healthy colonies of Gorgonia ventalina in the Caribbean. In Ecuador, this pathogen was only found in the samples of L. obscura but not in those of Leptogorgia sp. This could be due to the limited sampling and the low prevalence of this fungus in these populations (only 2 specimens out of 59). A different susceptibility of the gorgonian species could also explain this result. A previous study [14] indicated differences in the incidence of aspergillosis between two species of gorgonians, e.g., G. ventalina and G. flabellum.
Moreover, we provide new data on fungal communities of marine environments, particularly in coral reefs. Studies on fungal communities in coral reefs are scarce. In Singapore, an investigation on 10 species of gorgonian corals indicated the presence of 16 fungal genera, including Acremonium, Aspergillus, Chaetophoma, Cladosporium and Penicillium [42]; among these genera, the species Cladosporium sphaerospermum and Phoma sp. were also found in our study. In a study carried out in colonies of the Caribbean sea fan G. ventalina [43], 15 new fungal species were found, corresponding to 8 genera, including Aspergillus, Cladosporium, Gloeotinia and Penicillium. In 2008, as part of a larger sampling effort, the same authors identified 35 fungal species corresponding to 15 genera, including Aspergillus, Cladosporium, Nectria, Penicillium and Stachybotrys [13]. Among these genera, the species A. sydowii and C. sphaerospermum were also found in our study.
In the Pacific, the only sampled coral reef area studied for fungal community composition was at Chocó, Colombia [25]. This area is located near the coral reef investigated in our study. They found 59 fungal species in the sea fan Pacifigorgia spp. corresponding to 13 fungal genera (e.g., Aspergillus, Penicillium). In our work, we only found A. sydowii and A. sclerotiorum, two species already reported in the eastern Pacific [25]. Thus, all other fungal species identified in our study represent the first report of this species in the Pacific gorgonians. We also found species that had been previously reported in gorgonians. These included Penicillium chrysogenum [13,25,44] and Cladosporium sphaerospermum [43]. However, the role of pathogens of these species remains unknown.
We found some fungal species that had never been described in gorgonians, including Aspergillus fumigatus (previously described in soil, air, water, food, plants and organic matter), Capnobotryella sp. (previously described in lichens and pumpkins), Fusarium longipes (previously described in soil of tropical regions), Lasiodiplodia theobromae (previously described causing damage in vascular plants), Phoma sp. (described from soil, as saprophytes on various plants, and as pathogens in plants and humans) and Pyrenochaetopsis leptospora (soil borne and mainly associated with gramineous plants). The pathogenicity of these fungal species to gorgonians is unknown. However, whether these species are also characteristic of marine environments or originate from land as agricultural runoff and move to marine environments via discharges from rivers is unknown.
Other species found in this work have been previously found in marine environments but not in gorgonians or coral reefs. For example, Aspergillus ochraceopetaliformis and Alternaria sp. were found in deep-sea environments [45,46], Nigrospora sp. in sea anemones [47], or saline environments in general, such as Penicillium mallochii was before isolated from the guts of tropical leaf-eating caterpillars in Costa Rica [48].
In spite of the presence of known pathogens, such as A. sydowii, and a wide diversity of fungal pathogens in the Ecuadorian coral reefs, we did not observe any damage or mortality. The factors that lead to aspergillosis or the development of other fungal pathogens on coral reefs are unknown. In phylogenetically related fungal pathogens, some environmental stressors influence the development of disease [31,49]. It was suggested that coral aspergillosis could be enhanced by abiotic factors [50,51] and speculated that this disease could be the result of specific combinations of environmental factors (e.g., humidity, UV, temperature, aerosol concentrations) or of large-scale climate patterns (oceanic currents) [50]. Specifically, the microclimatic parameter of temperature has been shown to be involved in the gorgonia-A. sydowii interaction by promoting the growth and activity of the pathogen and reducing the efficacy of host defenses [24,25].
Thus far, studies on coral diseases, such as Aspergillosis, are limited by the lack of isolates from marine sources prior to epidemics [30]. The detection of these pathogens in healthy Ecuadorian gorgonian gardens is, therefore, of key importance since it indicates the presence of A. sydowii and other potential coral pathogens in asymptomatic colonies. This can help in further investigations aiming to decipher the factors leading to gorgonian disease and an eventual deterioration of this ecosystem. Alert models on diverse environmental stressors and the monitoring of the health of coral reefs are crucial for a better understanding of the development of aspergillosis disease. Future studies on this pathogen and the factors triggering disease require the comparison of healthy coral reefs with the presence of A. sydowii to those affected by this pathogen.
Understanding where and to what extent potentially harmful organisms exist is of crucial importance to the design of adequate conservation plans. Fungal diseases currently represent one of the main threats to biodiversity worldwide [52] and corals are no exception. The results presented here contribute to a better understanding of the biodiversity of fungal communities in coral reefs and the construction of data-baselines for the presence and incidence of these fungal pathogens in natural systems and in particular coral reefs.
Acknowledgments
We thank the Museo Ecuatoriano de Ciencias Naturales, Machalilla National Park and Ministerio del Ambiente of Ecuador (Manabí) for participation and collection permits (N° 016 –RM–DPM–MA). Thanks to Michel Guerrero and his team (Exploramar Diving) for his special interest from the early stages of our research. Special thanks to Micaela Peña for unconditional help and support. This research was partially supported by a grant from the Spanish Ministry of Economy and Competitiveness (CTM2014-57949-R).
Data Availability
Cultures were labeled as ASP001 through ASP059 in the culture collection of the Real Jardín Botánico, Madrid, Spain. The molecular data are published in GenBank (GenBank number are included in the manuscript).
Funding Statement
This research was only partially supported by a grant from the Spanish Ministry of Economy and Competitiveness (CTM2014-57949-R). The authors have not had any additional funding and coauthors have supported the sampling and sequencing from other projects. Both author and coauthors have currently not funding to pay publication fees.
References
- 1.Enochs I, Hockensmith G. Effects of coral mortality on the community composition of cryptic metazoans associated with Pocillopora damicornis. Proc 11th Int Coral Reef Symp. 2008;26: 1368–1372. Available: http://www.nova.edu/ncri/11icrs/proceedings/files/m26-08.pdf [Google Scholar]
- 2.Sanchez J, Dueñas LF. Diversidad y evolución de octocorales. Hipótesis, apuntes científicos uniandinos. 2012;12: 42–46. [Google Scholar]
- 3.Buhl M, Mortensen P. Symbiosis in Deep-Water Corals. Symbiosis. 2004;37: 33–61. [Google Scholar]
- 4.Thrush SF, Dayton PK. Disturbance to marine benthic habitats by trawling and dredging: implications for marine biodiversity. Annu Rev Ecol Syst. 2002;33: 449–473. 10.1146/annurev.ecolsys.33.010802.150515 [DOI] [Google Scholar]
- 5.Tsounis G, Rossi S, Gili JM, Arntz W. Population structure of an exploited benthic cnidarian: the case study of red coral (Corallium rubrum L.). Mar Biol. 2006;149: 1059–1070. 10.1007/s00227-006-0302-8 [DOI] [Google Scholar]
- 6.Gates RD, Ainsworth TD. The nature and taxonomic composition of coral symbiomes as drivers of performance limits in scleractinian corals. J Exp Mar Bio Ecol. Elsevier B.V.; 2011;408: 94–101. 10.1016/j.jembe.2011.07.029 [DOI] [Google Scholar]
- 7.Nyström M, Folke C, Moberg F. Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol Evol. 2000;15: 413–417. 10.1016/S0169-5347(00)01948-0 [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson C. Status of coral reefs of the World: 2008. Status Coral Reefs World 2008. 2008; 5–19. [Google Scholar]
- 9.Szmant AM. Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline? Estuaries. Coastal and Estuarine Research Federation; 2002;25: 743–766. Available: http://www.jstor.org/stable/1353030 [Google Scholar]
- 10.Contreras AC, Ortegón-Aznar I, Mota AT, Suárez-Salazar J. Cambio de fase coral-algas en el arrecife de coral de Mahahual, en el Caribe Mexicano 64th Gulf Caribb Fish Inst. 2011; 28–31. Available: http://nsgl.gso.uri.edu/flsgp/flsgpw11001/papers/010.pdf [Google Scholar]
- 11.Smith GW, Ives LD, Nagelkerken IA, Ritchle KB. Caribbean sea-fan mortalities. Nature. 1996. p. 487 10.1038/383487a08606764 [DOI] [Google Scholar]
- 12.Sánchez JA, Gómez CE, Escobar D, Dueñas LF. Diversidad, abundancia y amenazas de los octocorales de la Isla Malpelo, Pacífico Oriental Tropical, Colombia. Bol Invest Mar Cost. 2011;40: 139–154. [Google Scholar]
- 13.Toledo-Hernández C, Zuluaga-Montero A, Bones-González A, Rodríguez JA, Sabat AM, Bayman P. Fungi in healthy and diseased sea fans (Gorgonia ventalina): is Aspergillus sydowii always the pathogen? Coral Reefs. 2008;27: 707–714. 10.1007/s00338-008-0387-2 [DOI] [Google Scholar]
- 14.Nagelkerken I, Buchan K, Smith GW, Bonair K, Bush P, Garzón-Farreira J, et al. Widespread disease in caribbean sea fans I. Spreading and general characteristics. In: Lessios HA, Macintyre IG, editors. Proceedings of the 8th International Coral Reef Symposium Vol 1. Smithsonia. Panama; 1997. pp. 679–682.
- 15.Nagelkerken I, Buchan K, Smith GW, Bonair K, Bush P, Garzón-Ferreira J, et al. Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. Mar Ecol Prog Ser. 1997;160: 255–263. 10.3354/meps160255 [DOI] [Google Scholar]
- 16.Garzón-Ferreira J, Zea S. A mass mortality of Gorgonia ventalina (Cnidaria: Gorgonidae) in the Santa Marta area, Caribbean coast of Colombia. Bull Mar Sci. 1992;50: 522–526. [Google Scholar]
- 17.Klinch MA. Identification of common Aspergillus species Centraalbureau voor Schimmelcultures. Utrecht, Netherlands; 2002. [Google Scholar]
- 18.Raper KB, Fenell DI. The genus Aspergillus. In: Williams, Wilkins, editors. Baltimore, USA; 1965. [Google Scholar]
- 19.Geiser DM, Taylor JW, Ritchie KB, Smith GW. Cause of sea fan death in the West Indies. Nature. 1998;394: 137–138. 10.1038/280799671296 [DOI] [Google Scholar]
- 20.Hallegraeff G, Coman F, Davies C, Hayashi A, McLeod D, Slotwinski A, et al. Australian dust storm associated with extensive Aspergillus sydowii fungal “Bloom” in coastal waters. Appl Environ Microbiol. 2014;80: 3315–3320. 10.1128/AEM.04118-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang XY, Zhang Y, Xu XY, Qi SH. Diverse deep-sea fungi from the south china sea and their antimicrobial activity Curr Microbiol. 2013;67: 525–530. 10.1007/s00284-013-0394-6 [DOI] [PubMed] [Google Scholar]
- 22.de Hoog GS, Guarro J, Gené J, Figueras MJ. Atlas of clinical fungi. 2nd ed Utrecht/Reus: Centraalbureau voor Schimmelcultures/Universitat Rovira i Virgili; 2000. [Google Scholar]
- 23.Nagarajan C, Thayanidhi P, Kindo AJ, Ramaraj V, Mohanty S, Arunachalam R. Fungal Rhinosinusitis: Report of uncommon Aspergillus species as etiological agents. Int J Case Reports Images. 2014;5: 13 10.5348/ijcri-2014-01-430-CS-3 [DOI] [Google Scholar]
- 24.Alker AP, Smith GW, Kim K. Characterization of Aspergillus sydowii (Thom et Church), a fungal pathogen of Caribbean sea fan corals. Hydrobiologia. 2001;460: 105–111. 10.1023/A:1013145524136 [DOI] [Google Scholar]
- 25.Barrero-Canosa J, Dueñas LF, Sánchez JA. Isolation of potential fungal pathogens in gorgonian corals at the Tropical Eastern Pacific. Coral Reefs. 2013;32: 35–41. 10.1007/s00338-012-0972-2 [DOI] [Google Scholar]
- 26.Kim K, Harvell CD. The rise and fall of six a six-year coral-fungal epizootic. Am Nat. 2004;164: S52–S63. 10.1086/424609 [DOI] [PubMed] [Google Scholar]
- 27.Petes LE, Harvell CD, Peters EC, Webb MAH, Mullen KM. Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Mar Ecol Prog Ser. 2003;264: 167–171. 10.3354/meps264167 [DOI] [Google Scholar]
- 28.Rypien K. The origins and spread of Aspergillus sydowii, an opportunistic pathogen of caribbean gorgonian corals. Faculty of the Graduate School of Cornell University; 2008. [Google Scholar]
- 29.Kim K, Harvell CD. Aspergillosis of sea fan corals: disease dynamics in the Florida Keys In: Porter JW, Porter K, editors. The Everglades, Florida Bay, and coral reefs of the Florida Keys: an Ecosystem Sourcebook. CRC Press; New York; 2002. pp. 813–824. [Google Scholar]
- 30.Rypien KL, Andras JP, Harvell CD. Globally panmictic population structure in the opportunistic fungal pathogen Aspergillus sydowii. Mol Ecol. 2008;17: 4068–4078. 10.1111/j.1365-294X.2008.03894.x [DOI] [PubMed] [Google Scholar]
- 31.Sarmiento-Ramirez JM, Abella-Perez E, Phillott AD, Sim J, Van West P, Martin MP, et al. Global distribution of two fungal pathogens threatening endangered sea turtles. PLoS One. 2014;9 10.1371/journal.pone.0085853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milne-Edwards H, Haime J. Histoire naturelle des coralliaires ou, Polypes proprement dits. Paris: à la Libraire Encyclopédique de Roret; 1857. 10.5962/bhl.title.11574 [DOI]
- 33.Bielschowsky E. Die Gorgonarien Westindien. 6. Die Familie Gorgoniidae, zugleich eine Revision. Zool Jahrbücher, Suppl 1929;16: 63–234. [Google Scholar]
- 34.Söderhäll K, Svensson E, Unestam T. Chitinase and protease activities in germinating zoospore cysts of a parasitic fungus, Aphanomyces astaci, Oomycetes. Mycopathologia. 1978;64: 9–11. 10.1007/BF00443081 [DOI] [Google Scholar]
- 35.White TJ, Bruns S, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics PCR Protocols: A guide to methods and applications. 1990. pp. 315–322. citeulike-article-id:671166 [Google Scholar]
- 36.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215: 403–10. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 37.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and high-performance computing. Nat Methods. 2012;9: 772 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17: 754–755. 10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- 39.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. Oxford University Press; 2014;30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silvestro D, Michalak I. RaxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 2012;12: 335–337. 10.1007/s13127-011-0056-0 [DOI] [Google Scholar]
- 41.Zuluaga-Montero A, Toledo-Hernández C, Rodríguez JA, Sabat AM, Bayman P. Spatial variation in fungal communities isolated from healthy and diseased sea fans Gorgonia ventalina and seawater. Aquat Biol. 2009;8: 151–160. 10.3354/ab00218 [DOI] [Google Scholar]
- 42.Koh LL, Tan TK, Chou LM, Goh NKC. Fungi associated with gorgonians in Singapore. In: Moosa MK, Soemodihardjo S, Soegiarto A, Romimohtarto K, Nontji A, Suharsono S, editors. Proceedings of the Ninth International Coral Reef Symposium. 2000. pp. 521–526.
- 43.Toledo-Hernández C, Bones-González A, Ortiz-Vázquez OE, Sabat AM, Bayman P. Fungi in the sea fan Gorgonia ventalina: diversity and sampling strategies. Coral Reefs. 2007;26: 725–730. 10.1007/s00338-007-0252-8 [DOI] [Google Scholar]
- 44.Moree WJ, McConnell OJ, Nguyen DD, Sanchez LM, Yang Y-L, Zhao X, et al. Microbiota of healthy corals are active against fungi in a light-dependent manner. ACS Chem Biol. American Chemical Society; 2014;9: 2300–2308. 10.1021/cb500432j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Tang G, Xu X, Nong X, Qi S-H. Insights into deep-sea sediment fungal communities from the east indian ocean using targeted environmental sequencing combined with traditional cultivation. Chaturvedi V, editor. PLoS One. 2014;9: e109118 10.1371/journal.pone.0109118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landy ET, Jones GM. What is the fungal diversity of marine ecosystems in Europe? Mycologist. 2006;20: 15–21. 10.1016/j.mycol.2005.11.010 [DOI] [Google Scholar]
- 47.Yang K-L, Wei M-Y, Shao C-L, Fu X-M, Guo Z-Y, Xu R-F, et al. Antibacterial anthraquinone derivatives from a sea anemone-derived fungus Nigrospora sp. J Nat Prod. American Chemical Society; 2012;75: 935–941. 10.1021/np300103w [DOI] [PubMed] [Google Scholar]
- 48.Rivera KG, Díaz J, Chavarría-Díaz F, Garcia M, Urb M, Thorn RG, et al. Penicillium mallochii and P. guanacastense, two new species isolated from Costa Rican caterpillars. Mycotaxon. 2012;119: 315–328. 10.5248/119.315 [DOI] [Google Scholar]
- 49.Diéguez-Uribeondo J, Förster H, Adaskaveg JE. Effect of wetness duration and temperature on the development of anthracnose on selected almond tissues and comparison of cultivar susceptibility. Phytopathology. 2011;101: 1013–20. 10.1094/PHYTO-07-10-0193 [DOI] [PubMed] [Google Scholar]
- 50.Green EP, Bruckner AW. The significance of coral disease epizootiology for coral reef conservation. Biol Conserv. 2000;96: 347–361. 10.1016/S0006-3207(00)00073-2 [DOI] [Google Scholar]
- 51.Rypien KL. African dust is an unlikely source of Aspergillus sydowii, the causative agent of sea fan disease. Mar Ecol Prog Ser. 2008;367: 125–131. 10.3354/meps07600 [DOI] [Google Scholar]
- 52.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484: 186–194. 10.1038/nature10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Cultures were labeled as ASP001 through ASP059 in the culture collection of the Real Jardín Botánico, Madrid, Spain. The molecular data are published in GenBank (GenBank number are included in the manuscript).