Abstract
Background
Polypharmacy and inappropriate medication prescriptions are associated with increased morbidity and mortality. Most interventions proposed to improve appropriate prescribing are time and resource intensive and therefore hardly applicable in daily clinical practice.
Objective
To test the efficacy of an easy-to-use checklist aimed at supporting the therapeutic reasoning of physicians in order to reduce inappropriate prescribing and polypharmacy.
Methods
We assessed the efficacy and safety of a 5-point checklist to be used by all physicians on the internal medicine wards of a Swiss hospital by comparing outcomes in 450 consecutive patients aged ≥65 years hospitalized after the introduction of the checklist, and in 450 consecutive patients ≥65 years hospitalized before the introduction of the checklist. The main measures were the proportion of patients with prescription of potentially inappropriate medications (PIMs) at discharge, according to STOPP criteria, and the number of prescribed medications at discharge, before and after the introduction of the checklist. Secondary outcomes were the prevalence of polypharmacy (≥ 5 drugs) and hyperpolypharmacy (≥ 10 drugs), and the prevalence of potentially inappropriate prescribing omissions (PPOs) according to START criteria.
Results
At admission 59% of the 900 patients were taking > 5 drugs, 13% ≥ 10 drugs, 37% had ≥ 1 PIM and 25% ≥ 1 PPO. The introduction of the checklist was associated with a significant reduction by 22% of the risk of being prescribed ≥ 1 PIM at discharge (adjusted risk ratios [RR] 0.78; 95% CI: 0.68–0.94), but not with a reduction of at least 20% of the number of drugs prescribed at discharge, nor with a reduction of the risk of PPOs at discharge.
Conclusions
The introduction of an easy-to-use 5-point checklist aimed at supporting therapeutic reasoning of physicians on internal medicine wards significantly reduced the risk of prescriptions of inappropriate medications at discharge.
Introduction
Because of the high prevalence of comorbid diseases in the higher aged, many elderly people are treated with multiple medications. The proportion of older adults exposed to polypharmacy (usually defined as concomitant prescription of ≥ 5 drugs) is rapidly increasing in the last years. This increase has several reasons, such as the rising use of cardioprotective and antidepressant medications [1] and probably also the promotion of guidelines recommending multiple drug therapy to achieve targets such as blood pressure or glycaemic control [2]. Approximately 20% to 40% of adults aged 65 and older in developed countries are prescribed ≥ 5 medications [1, 2]. Whereas polypharmacy is driven by comorbidity and may be beneficial for many patients, the number of medications used is the strongest risk factor for prescribing problems [3]. Polypharmacy results in medication nonadherence, and increases the risk of adverse drug reactions, drug-drug interactions, medication errors and of using potentially inappropriate medications (PIMs) [1]. Several studies showed that polypharmacy and inappropriate medication use are associated with adverse health outcomes, including mortality, hospitalization, falls and cognitive impairment [4–7].
Many interventions aiming at assessing and reducing the number of inappropriate medications and at optimizing appropriate prescribing in elderly people have been proposed. Appropriateness of prescribing can be assessed by explicit (criterion-based) or implicit (judgment-based) outcome measures [8]. Explicit indicators, such as lists of drugs that should be avoided in elderly people (e.g. the Beers list [9]), are usually drug-oriented or disease-oriented, do not address the burden of comorbid diseases in the individual patient and are limited due to prescribing habits across countries [8, 10]. With implicit, judgment-based approaches, clinicians use information from the patient and published work (instead e.g. of a fixed list of to-avoid-drugs) to make judgements about appropriateness. These approaches address multiple elements of medication prescribing, which are relevant for many different drugs, clinical conditions and settings [11]. They are flexible, focus on the patient, rather than on drugs or diseases, and are potentially the most sensitive, but they are time-consuming and depend on the user’s knowledge and experience [8, 11]. Deprescribing, the systematic process of identifying and tapering or discontinuing drugs in patients in which potential harms outweigh potential benefits has also been proposed as tool to reduce inappropriate polypharmacy [12]. However, most interventions described to improve appropriate prescribing in elderly people are complex, based on explicit criteria and require the presence of clinical pharmacists and/or multidisciplinary teams including for example geriatricians and other healthcare providers with specialized geriatrics training (e.g. nurses, pharmacists, psychiatrists) [8, 13]. These resources are not available in many settings. We therefore developed an intervention aimed at systematically integrating the key judgment-based elements for appropriate prescribing in the ongoing process of clinical reasoning regarding each individual patient. We used and simplified two published conceptual frameworks aiming at minimizing inappropriate medications in elderly people [14, 15] and created a checklist to be used by physicians on internal medicine wards. Finally, we assessed the efficacy and safety of this intervention and showed that the introduction of the checklist reduced the risk of prescription of inappropriate medications at discharge.
The primary aim of the study was to assess the efficacy and safety of a prescriber checklist for reducing inappropriate prescribing and polypharmacy among patients aged ≥ 65 years admitted to an internal medicine unit. Secondary aims were to assess the number of prescribed drugs, the prevalence of polypharmacy (concomitant use of ≥ 5 drugs) and hyperpolypharmacy (concomitant use of ≥ 10 drugs), to assess the prevalence of potentially inappropriate medications (PIMs) and potentially inappropriate prescribing omissions (PPOs), and to assess the prevalence of prescription and the rate of inappropriate prescription of following drugs: non-steroidal anti-inflammatory drugs (NSAID), proton pump inhibitors (PPI), systemic corticosteroids, metamizole (dipyrone) and potent opiates.
Methods
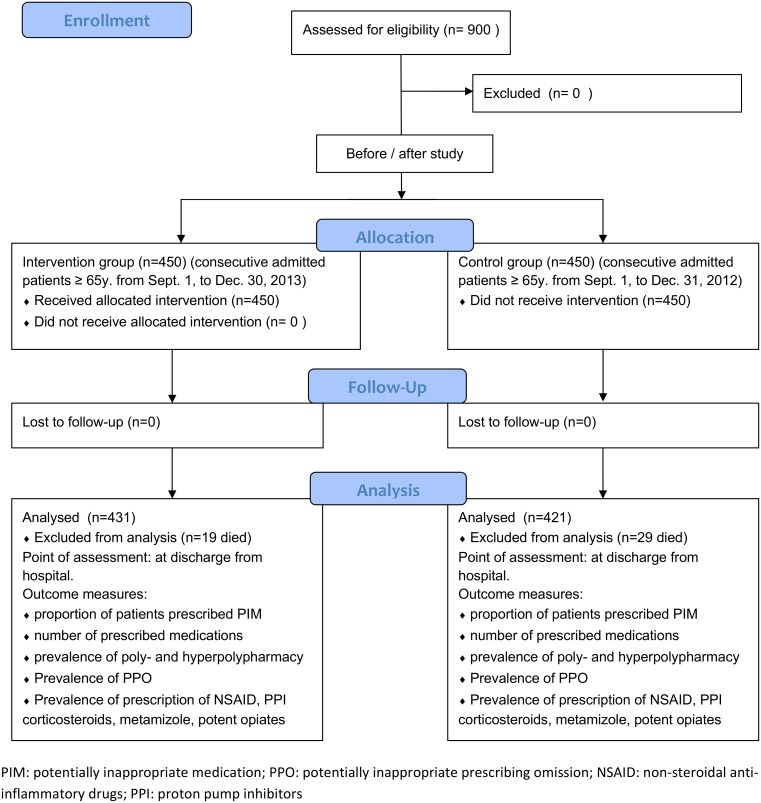
We conducted a single-center, interventional, quasi-experimental before-after study in the Division of Internal Medicine of the Kantonsspital Olten, a university-affiliated secondary-level teaching hospital with 245 beds in northwestern Switzerland. To detect an effect of 10% of the checklist (decrease in prevalence of inappropriate drug prescription and/or polypharmacy from anticipated 30% at admission to 20% at discharge) with a probability (power) of 90% at a significance level (alpha, 2-tailed) of 0.05, a sample size of 824 patients (412 in each group) is needed. Therefore, we included in the analysis the first 450 consecutive patients aged ≥ 65 years hospitalized in the Division of Internal Medicine during the period September 1st–December 30th, 2013, after the introduction of the checklist (intervention group), and compared them to the first 450 consecutive patients aged ≥ 65 years hospitalized in the same division during the same period of the previous year (September 1st–December 31th, 2012) (control group) (Fig 1). The consecutive patients were identified through the admission lists generated by the electronic hospital information system. Each patient was included only once (at the first hospitalization). Patients who died during the hospitalization were excluded from further analysis.
Fig 1. CONSORT Flow Diagram.
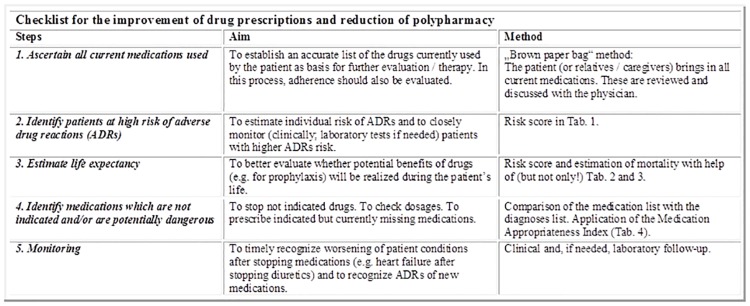
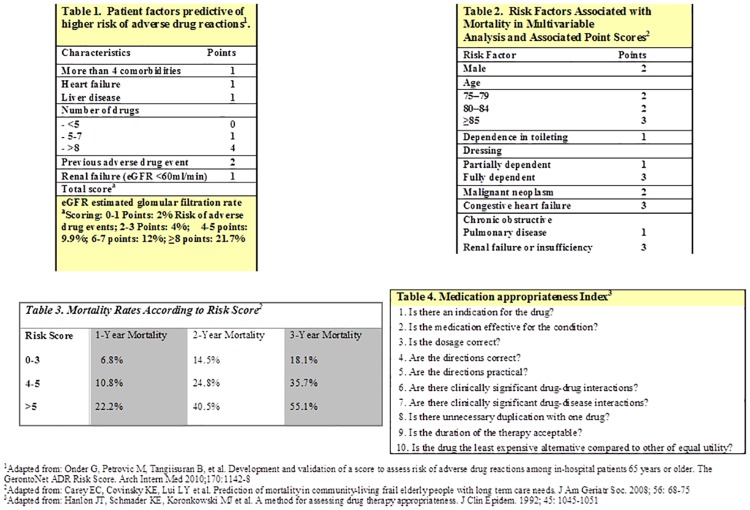
We introduced a checklist (Figs 2 and 3) aimed at supporting the therapeutic reasoning of clinicians in order to improve the quality of drug prescriptions. The checklist was based on the conceptual frameworks proposed by Scott et al. [14] and by Dovjak [15], and consisted of following 5 sequential steps: 1. ascertain all current medications used; 2. identify patients at high risk of adverse drug reactions; 3. estimate life expectancy; 4. identify medications which are not indicated and/or are potentially dangerous; 5. monitor the patient if drugs were stopped or new drugs were added. For the first 4 steps, a possible tool to be used at the discretion of the physician was proposed: for the assessment of current medications: the “brown paper bag” review [16]; to identify patients at high risk of adverse drug reactions: the gerontoNet adverse drug reactions risk score [17]; to estimate life expectancy: the prognostic index for frail elderly people described by Carey et al. [18]; and for the identification of potentially inappropriate medication: the Medication Appropriateness Index [11].
Fig 2. Checklist, page 1.
Fig 3. Checklist page 2.
The checklist was discussed and presented to all physicians of the Division of Internal Medicine during grand rounds at the beginning of the intervention period. Each physician received the checklist as a pocket-sized leaflet and was asked to systematically apply the five described steps at admission and discharge of each patient and during daily visits. The checklist was also posted on the mobile desk workstations used during ward rounds. A senior physician reiterated weekly the use of the checklist with the medical team on each ward.
The primary outcomes of the study were the proportion of patients prescribed PIMs at discharge, according to STOPP criteria [19], and the total number of prescribed medications at discharge, before and after the introduction of the checklist. Secondary outcomes (evaluated at discharge), were the prevalence of polypharmacy (concomitant use of ≥ 5 drugs) and hyperpolypharmacy (concomitant use of ≥ 10 drugs), the prevalence of PPOs (according to START criteria [19]), the prevalence of prescription and the rate of inappropriate prescription of following drugs: non-steroidal anti-inflammatory drugs (NSAID); proton pump inhibitors (PPI); systemic corticosteroids; metamizole (Novalgin™); potent opiates. In addition we assessed the in-hospital mortality rate and the all-cause re-hospitalization rate at 30 days after discharge.
Basic demographic data and information on diagnoses, medications, duration of hospitalization, admission to the intensive care unit, re-hospitalization within 30 days after discharge, and in-hospital death were collected from the electronic patient records of the hospital. The medication of each patient at admission and discharge was reviewed independently by two of the investigators, who assessed the medication appropriateness by chart review according to STOPP and START criteria [19]. Discrepancies were resolved by discussion. Data were recorded on a standardized case report form and anonymized before statistical analysis. The group assignment of each patient (intervention vs. control) was not reported on the case report form. However, it was not possible to blind the two investigators assessing medication appropriateness, because charts used for chart review contained hospitalization dates, dates of laboratory examinations etc.
Basic demographic characteristics, co-morbidities, clinical and laboratory parameters as well as the type and number of drugs prescribed at admission and discharge were compared according to the intervention using the chi-squared test or Fisher’s exact test for categorical variables as appropriate, and the non-parametric Wilcoxon-Mann-Whitney U test for continuous variables. As the outcome event was common (> 10%) we estimated relative risks and risk ratios (RR) instead of odds ratios (OR), since there might be an overestimation of the effect of the intervention when using OR [20–23]. Because of the failed convergence by the log-binomial logistic method in building multivariate models, we used Poisson regression models with a robust error variance [20] to estimate the effect of the checklist on prescription of at least 20% less drugs at discharge compared to admission, as well as risk factors of prescribing at least one inappropriate drug or missing to prescribe at least one appropriate drug at discharge. Risk factors of being re-hospitalized after discharge were also assessed using Poisson regression models with a robust error variance. Bivariable Poisson regression analysis was used to preselect independent variables when the Wald statistic was p<0.05. If the Phi correlation coefficient between two variables was ≥0.8, the variable with the lowest Wald statistic was excluded from further analysis. Thereafter, we used a backward stepwise multivariable Poisson regression analysis on the selected variables to form the prediction model (entry criteria = p<0.05; removal criteria = p≥0.10). We retained those variables that are known to be associated with the higher probability of drug prescription in the literature (older age, male sex, comorbidities, i.e. Charlton comorbidity index). Likelihood ratio tests were used to measure goodness of the fit of the regression models. Results are presented as crude and adjusted risk ratios (RR) after adjusting for potential confounders as indicated. Finally, we checked the models for any interactions. Data were analyzed using an intention-to-intervention approach, where all subjects in the intervention group were compared regardless of whether checklist has been used by the physician in charge. All analyses were performed using STATA software version 13 for Windows (Stata Corp, College Station, Texas, USA).
The local research ethics committee (Kantonale Ethikkommission Aargau / Solothurn; N. 2013/039) approved the study and accepted the protocol as a quality improvement project aimed at improving the application of recognized standards of care, waiving the requirement to obtain informed consent.
Trial Registration: ClinicalTrials.gov NCT02712268.
Results
General characteristics of the study population before and after the intervention are shown in Table 1. Overall, in this 900 patients with a median age between 76 (intervention group) and 79 years (control group) 59% of patients were taking > 5 drugs and 13% ≥ 10 drugs at admission. At discharge, after excluding 48 patients who died during hospitalization, the percentages were 72% (patients taking > 5 drugs) and 21% (patients taking ≥ 10 drugs), respectively. About 37% of the 900 patients had ≥ 1 potentially inappropriate medication (PIM) and 25% ≥ 1 missing potentially appropriate medication (PPO) at admission. At discharge, of 852 patients who did not die during the hospitalization, 266 (31%) still had ≥ 1 PIM and 160 (19%) ≥ 1 PPO (Table 1). Overall, at admission 8% of patients had inappropriate prescription of NSAID and 11% inappropriate prescription of PPI (Table 1).
Table 1. General characteristics and outcome measures of the 450 patients hospitalized before (“without intervention”) and the 450 patients hospitalized after (“with intervention”) the introduction of the checklist.
| Characteristic | Without intervention n = 450 | With intervention n = 450 | p-valuea | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Age (median, IQR) | 79 | 73–84 | 76 | 71–83 | 0.004 | |
| Males | 198 | 44.0 | 213 | 47.3 | 0.31 | |
| Living at home | 408 | 90.7 | 415 | 92.2 | 0.40 | |
| Number of diagnoses (median, IQR) | 8 | 6–11 | 7 | 5–10 | 0.001 | |
| Charlson Comorbidity Index | 0 | 86 | 19.1 | 94 | 20.9 | 0.43 |
| 1–2 | 197 | 43.8 | 195 | 43.3 | ||
| 3–4 | 115 | 25.6 | 98 | 21.8 | ||
| 5 | 52 | 11.5 | 63 | 14.0 | ||
| Number of drugs at admission | <5 | 172 | 38.2 | 196 | 43.6 | 0.07 |
| 5–9 | 208 | 46.2 | 205 | 45.6 | ||
| ≥10 | 70 | 15.6 | 49 | 10.9 | ||
| Type of drugs at admission | NSAID | 59 | 13.1 | 35 | 7.8 | 0.008 |
| PPI | 149 | 33.1 | 130 | 28.9 | 0.17 | |
| Corticosteroids | 34 | 7.6 | 37 | 8.2 | 0.72 | |
| Metamizole | 13 | 2.9 | 9 | 2.0 | 0.38 | |
| Opiate | 32 | 7.1 | 27 | 6.0 | 0.50 | |
| ≥1 potential inappropriate drugs at admission | 196 | 43.7 | 138 | 30.6 | <0.001 | |
| ≥1 missing appropriate drug at admission | 118 | 26.3 | 104 | 23.1 | 0.26 | |
| Inappropriate drugs at admission | NSAID | 46 | 10.2 | 27 | 6.0 | 0.02 |
| PPI | 57 | 12.7 | 45 | 9.9 | 0.20 | |
| Corticosteroids | 11 | 2.5 | 8 | 1.8 | 0.48 | |
| Metamizole | 11 | 2.5 | 5 | 1.1 | 0.10 | |
| Opiate | 9 | 2.0 | 4 | 0.9 | 0.13 | |
| Intensive care unit stay | 61 | 13.6 | 43 | 9.5 | 0.06 | |
| Hospitalization, days (median, IQR) | 7 | 4–10 | 6 | 3–9 | 0.02 | |
| Number of drugs at dischargeb | <5 | 109 | 25.9 | 125 | 29.0 | 0.58 |
| 5–9 | 222 | 52.7 | 215 | 49.9 | ||
| ≥10 | 90 | 21.4 | 91 | 21.1 | ||
| Type of drugs at dischargeb | NSAID | 10 | 2.4 | 18 | 4.2 | 0.14 |
| PPI | 173 | 41.1 | 160 | 37.1 | 0.24 | |
| Corticosteroids | 57 | 13.5 | 49 | 11.4 | 0.34 | |
| Metamizole | 0 | - | 1 | 0.2 | - | |
| Opiate | 42 | 10.0 | 52 | 12.1 | 0.33 | |
| ≥1 potential inappropriate drugs at dischargeb | 164 | 39.0 | 102 | 23.7 | <0.001 | |
| ≥1 missing appropriate drug at dischargeb | 88 | 20.9 | 72 | 16.7 | 0.12 | |
| Inappropriate drug at dischargeb | NSAID | 4 | 1.0 | 9 | 2.1 | 0.14 |
| PPI | 67 | 15.9 | 41 | 9.5 | 0.005 | |
| Corticosteroids | 10 | 2.4 | 3 | 0.7 | 0.04 | |
| Metamizole | 0 | - | 1 | 0.2 | - | |
| Opiate | 3 | 0.7 | 2 | 0.5 | 0.49 | |
| Rehospitalization within 30 daysb | 54 | 12.8 | 43 | 10.0 | 0.19 | |
| In-hospital death | 29 | 6.4 | 19 | 4.2 | 0.14 | |
IQR: interquartile range. NSAID: non-steroidal anti-inflammatory drugs. PPI: proton pump inhibitors.
achi-squared test or Fisher’s exact test for categorical variables; Wilcoxon-Mann-Whitney U test for continuos variables.
bTotal number of patients at discharge = 852 (group without intervention: 421; group with intervention: 431). Total in-hospital death: 48.
Patients in the after-intervention period were younger (median age 76 years vs. 79), had less diagnoses (median number of diagnoses: 7 vs. 8) and a shorter length of stay in the hospital (6 vs. 7 days) than patients in the before-intervention period (Table 1).
The intervention with the checklist was associated with a significant reduction by 22% of the risk of being prescribed ≥ 1 PIM at discharge (adjusted RR 0.78; 95% CI: 0.68–0.94 in the multivariate analysis) (Table 2), but not with a reduction of at least 20% of the number of drugs prescribed at discharge (Table 3), nor with a reduction of the risk of missing potentially appropriate drug prescriptions at discharge (Table 4). A higher risk for prescription of PIM was more likely in patients with PIM at admission, and with an increasing number of diagnoses (test for trend, p<0.001) and medications (test for trend, p<0.001). The analysis of inappropriate prescriptions of NSAID, PPI, corticosteroids, metamizole and opiates at discharge (Table 1) indicated that the intervention with the checklist significantly reduced the risk of inappropriate prescription of PPI at discharge (adjusted RR 0.72, 95% CI: 0.44–0.88, p = 0.010, in the multivariate analysis; data not shown).
Table 2. Risk factors for prescription of at least 1 inappropriate drug at discharge.
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-valuea | Adj. RR | 95% CI | p-valuea | ||
| Age (years) | <70 | 1 | - | - | 1 | - | - |
| 70–80 | 1.12 | 0.84–1.49 | 0.439 | 1.05 | 0.84–1.31 | 0.584 | |
| >80 | 1.27 | 0.95–1.67 | 0.106 | 0.88 | 0.70–1.10 | 0.551 | |
| Sex | Male | 1 | - | - | 1 | - | - |
| Female | 1.21 | 0.98–1.48 | 0.067 | 1.09 | 0.93–1.28 | 0.300 | |
| Living condition | At home | 1 | - | - | 1 | - | - |
| Institutionalized | 1.80 | 1.41–2.31 | <0.001 | 1.21 | 0.99–1.49 | 0.068 | |
| Charlson Comorbidity Index ≥1 | 1.08 | 0.88–1.32 | 0.480 | 0.87 | 0.73–1.03 | 0.103 | |
| Number of diagnoses at admission | |||||||
| ≤5 | 1 | - | - | 1 | - | - | |
| 6–8 | 1.71 | 1.20–2.43 | 0.003 | 0.92 | 0.68–1.23 | 0.584 | |
| ≥9 | 2.35 | 1.69–3.27 | <0.001 | 1.09 | 0.81–1.48 | 0.551 | |
| Number of drugs at admission | |||||||
| ≤2 | 1 | - | - | 1 | - | - | |
| 3–5 | 2.05 | 1.24–3.39 | 0.005 | 1.24 | 0.84–1.93 | 0.334 | |
| 6–7 | 3.91 | 2.41–6.36 | <0.001 | 1.67 | 1.09–2.56 | 0.018 | |
| ≥8 | 4.81 | 3.02–7.68 | <0.001 | 1.68 | 1.09–2.55 | 0.016 | |
| Inappropriate medication at admission | 9.86 | 7.22–13.47 | <0.001 | 8.22 | 5.96–11.37 | <0.001 | |
| Hospital stay (days), for each additional week | 1.13 | 1.02–1.24 | 0.018 | 1.06 | 0.96–1.15 | 0.237 | |
| Intervention (checklist) | 0.61 | 0.49–0.75 | <0.001 | 0.78 | 0.68–0.94 | 0.005 | |
RR: risk ratios. CI: confidence interval. Adj. RR: adjusted risk ratios for all variables listed.
aPoisson regression models with a robust error variance, Likelihood Ratio Test.
Table 3. Predictors for prescription of at least 20% less drugs at discharge compared to admission.
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-valuea | Adj. RR | 95% CI | p-valuea | ||
| Age (years) | <70 | 1 | - | - | 1 | - | - |
| 70–80 | 1.02 | 0.61–1.72 | 0.930 | 0.99 | 0.59–1.68 | 0.991 | |
| >80 | 1.00 | 0.59–1.71 | 0.985 | 0.83 | 0.47–1.48 | 0.537 | |
| Sex | Male | 1 | - | - | 1 | - | - |
| Female | 1.07 | 0.72–1.58 | 0.731 | 1.50 | 0.82–2.72 | 0.182 | |
| Living condition | At home | 1 | - | - | 1 | - | - |
| Institutionalized | 1.70 | 0.97–2.95 | 0.062 | 1.36 | 1.07–1.74 | 0.013 | |
| Charlson Comorbidity Index ≥1 | 0.89 | 0.59–1.35 | 0.593 | 0.85 | 0.47–1.31 | 0.470 | |
| Number of diagnoses at admission | |||||||
| ≤5 | 1 | - | - | 1 | - | - | |
| 6–8 | 1.09 | 0.65–1.85 | 0.726 | 0.91 | 0.52–1.58 | 0.729 | |
| ≥9 | 1.04 | 0.62–1.74 | 0.878 | 0.85 | 0.42–1.33 | 0.333 | |
| Number of drugs at admission | |||||||
| ≤2 | 1 | - | - | 1 | - | - | |
| 3–5 | 3.31 | 1.52–7.23 | 0.003 | 3.54 | 1.62–7.75 | 0.002 | |
| 6–7 | 1.24 | 0.47–3.25 | 0.662 | 1.39 | 0.52–3.71 | 0.504 | |
| ≥8 | 3.41 | 1.55–7.49 | 0.002 | 3.94 | 1.75–8.87 | 0.001 | |
| Hospital stay (days), for each additional week | 0.99 | 0.78–1.24 | 0.924 | 0.98 | 0.77–1.25 | 0.904 | |
| Intervention (checklist) | 0.80 | 0.54–1.18 | 0.266 | 0.79 | 0.53–1.16 | 0.233 | |
RR: risk ratios. CI: confidence interval. Adj. RR: adjusted risk ratios for all variables listed.
aPoisson regression models with a robust error variance, Likelihood Ratio Test.
Table 4. Risk factors for missing prescription of at least 1 appropriate drug at discharge.
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-valuea | Adj. RR | 95% CI | p-valuea | ||
| Age (years) | <70 | 1 | - | - | 1 | - | - |
| 70–80 | 1.42 | 0.93–2.16 | 0.101 | 1.32 | 0.87–1.99 | 0.187 | |
| >80 | 1.48 | 0.97–2.27 | 0.069 | 1.23 | 0.80–1.89 | 0.351 | |
| Sex | Male | 1 | - | - | 1 | - | - |
| Female | 1.11 | 0.84–1.47 | 0.467 | 1.08 | 0.81–1.45 | 0.585 | |
| Living condition | At home | 1 | - | - | 1 | - | - |
| Institutionalized | 0.99 | 0.59–1.64 | 0.959 | 0.75 | 0.44–1.26 | 0.272 | |
| Charlson Comorbidity Index ≥1 | 1.11 | 0.83–1.48 | 0.469 | 0.91 | 0.67–1.23 | 0.554 | |
| Number of diagnoses at admission | |||||||
| ≤5 | 1 | - | - | 1 | - | - | |
| 6–8 | 1.81 | 1.10–2.96 | 0.019 | 1.53 | 0.90–2.58 | 0.113 | |
| ≥9 | 2.61 | 1.64–4.15 | <0.001 | 1.99 | 1.19–3.32 | 0.008 | |
| Number of drugs at admission | |||||||
| ≤2 | 1 | - | - | 1 | - | - | |
| 3–5 | 1.10 | 0.65–1.86 | 0.713 | 0.94 | 0.55–1.61 | 0.821 | |
| 6–7 | 2.13 | 1.30–3.51 | 0.003 | 1.71 | 1.01–2.89 | 0.044 | |
| ≥8 | 2.26 | 1.41–3.63 | 0.001 | 1.74 | 1.04–2.91 | 0.036 | |
| Hospital stay (days), for each additional week | 1.06 | 0.90–1.23 | 0.452 | 0.99 | 0.83–1.18 | 0.925 | |
| Intervention (checklist) | 0.80 | 0.60–1.06 | 0.122 | 0.85 | 0.65–1.13 | 0.266 | |
RR: risk ratios. CI: confidence interval. Adj. RR: adjusted risk ratios for all variables listed.
aPoisson regression models with a robust error variance, Likelihood Ratio Test.
A higher number of prescribed drugs at admission and not living at home were independently associated with a reduction ≥ 20% of prescribed drugs at discharge (Table 3). A higher number of prescribed drugs and in particular inappropriate medication at admission were associated with a higher risk of prescription of ≥ 1 PIM at discharge (Table 2). A higher number of diagnoses and of prescribed drugs at admission were significantly associated with a higher risk of missing prescription of potentially appropriate medications at discharge (Table 4). The intervention was neither associated with an increased risk of re-hospitalization at 30 days after discharge (Table 5) nor with an increased risk of in-hospital death (Table 6).
Table 5. Risk factors for rehospitalization within 30 days after discharge.
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-valuea | Adj. RR | 95% CI | p-valuea | ||
| Age (years) | <70 | 1 | - | - | 1 | - | - |
| 70–80 | 1.02 | 0.63–1.67 | 0.934 | 0.98 | 0.60–1.61 | 0.956 | |
| >80 | 0.91 | 0.54–1.52 | 0.718 | 0.86 | 0.50–1.47 | 0.573 | |
| Sex | Male | 1 | - | - | 1 | - | - |
| Female | 1.06 | 0.72–1.53 | 0.781 | 1.17 | 0.80–1.74 | 0.416 | |
| Living condition | At home | 1 | - | - | 1 | - | - |
| Institutionalized | 0.61 | 0.26–1.44 | 0.259 | 0.61 | 0.25–1.45 | 0.264 | |
| Charlson Comorbidity Index ≥1 | 1.58 | 1.09–2.30 | 0.016 | 1.51 | 0.99–2.30 | 0.051 | |
| Number of diagnoses at admission | |||||||
| ≤5 | 1 | - | - | 1 | - | - | |
| 6–8 | 1.63 | 0.92–2.89 | 0.091 | 1.83 | 0.99–3.36 | 0.050 | |
| ≥9 | 1.65 | 0.94–2.89 | 0.079 | 1.68 | 0.90–3.14 | 0.101 | |
| Number of drugs at admission at admission | |||||||
| ≤2 | 1 | - | - | 1 | - | - | |
| 3–5 | 0.66 | 0.39–1.11 | 0.117 | 0.55 | 0.32–0.95 | 0.031 | |
| 6–7 | 0.71 | 0.40–1.28 | 0.260 | 0.56 | 0.31–1.02 | 0.059 | |
| ≥8 | 0.92 | 0.56–1.52 | 0.742 | 0.68 | 0.38–1.22 | 0.202 | |
| Hospital stay (days), for each additional week | 1.23 | 1.05–1.44 | 0.009 | 1.15 | 0.97–1.37 | 0.103 | |
| Intervention (checklist) | 0.78 | 0.53–1.13 | 0.192 | 0.80 | 0.55–1.16 | 0.233 | |
RR: risk ratios. CI: confidence interval. Adj. RR: adjusted risk ratios for all variables listed.
aPoisson regression models with a robust error variance, Likelihood Ratio Test.
Table 6. Risk factors for in-hospital death.
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-valuea | Adj. RR | 95% CI | p-valuea | ||
| Age (years) | <70 | 1 | - | - | 1 | - | - |
| 70–80 | 2.12 | 0.611–7.35 | 0.236 | 1.99 | 0.57–6.96 | 0.281 | |
| >80 | 5.69 | 1.77–18.3 | 0.004 | 5.08 | 1.56–16.5 | 0.007 | |
| Sex | Male | 1 | - | - | 1 | - | - |
| Female | 0.99 | 0.57–1.73 | 0.981 | 1.17 | 0.67–2.04 | 0.587 | |
| Living condition | At home | 1 | - | - | 1 | - | - |
| Institutionalized | 1.82 | 0.85–3.93 | 0.124 | 0.61 | 0.25–1.45 | 0.264 | |
| Charlson Comorbidity Index ≥1 | 4.69 | 2.52–8.75 | <0.001 | 4.94 | 2.48–9.84 | <0.001 | |
| Number of diagnoses at admission | |||||||
| ≤5 | 1 | - | - | 1 | - | - | |
| 6–8 | 1.71 | 0.73–3.99 | 0.216 | 0.95 | 0.41–2.35 | 0.924 | |
| ≥9 | 1.72 | 0.74–3.97 | 0.201 | 0.91 | 0.37–2.21 | 0.839 | |
| Number of drugs at admission | |||||||
| ≤2 | 1 | - | - | 1 | - | - | |
| 3–5 | 1.60 | 0.64–3.99 | 0.308 | 1.12 | 0.46–2.68 | 0.807 | |
| 6–7 | 1.57 | 0.58–4.23 | 0.370 | 0.97 | 0.37–2.55 | 0.959 | |
| ≥8 | 1.66 | 0.66–4.20 | 0.282 | 0.73 | 0.27–1.93 | 0.530 | |
| Hospital stay (days), for each additional week | 1.04 | 0.76–1.42 | 0.791 | 0.85 | 0.57–1.27 | 0.432 | |
| Intervention (checklist) | 0.66 | 0.37–1.15 | 0.142 | 0.73 | 0.43–1.26 | 0.269 | |
RR: risk ratios. CI: confidence interval. Adj. RR: adjusted risk ratios for all variables listed.
aPoisson regression models with a robust error variance, Likelihood Ratio Test.
Discussion
In our study involving 900 hospitalized patients aged 65 years and older with a high prevalence of polypharmacy, we were able to show that a simple intervention such as the introduction of a 5-steps checklist aimed at supporting the therapeutic reasoning of the treating physician significantly reduced by 22% the risk for the patient of being prescribed ≥ 1 PIM at discharge. Many interventions to improve appropriate prescribing in elderly people have been described in the literature and have been recently reviewed [8, 13, 24]. The effect of our intervention is comparable to the effect of other more complex and time-consuming interventions [8, 13, 24]. Dalleur et al. [25] reported for example that specific STOPP recommendations provided to hospital physicians doubled the reduction of PIMs at discharge in frail inpatients ≥ 75 years old. Recommendations were provided to the ward physician by a geriatrician of an inpatient geriatric consultation team who systematically screened the list of medications on admission for PIMs using STOPP criteria [19]. However, the proportion of patients having ≥ 1 PIM at discharge did not differ between intervention and control group (23% vs. 16%; OR 1.5, 95% CI 0.49–4.89) [25]. In their review Patterson et al. [13] conclude that interventions to improve appropriate polypharmacy, such as pharmaceutical care, appear beneficial in terms of reducing inappropriate prescribing. Eleven of 12 studies included in this review analyzed complex, multi-faceted interventions involving pharmacists and/or specialized physicians (e.g. geriatricians). One intervention consisted of computerized decision support.
Our intervention was not associated with a significant (>20%) reduction of the number of prescribed drugs at discharge (compared to admission) (Adj. RR 0.79, 95% CI 0.53–1.16) (Table 3). This is not surprising, since hospitalized patients are usually treated with additional drugs for the acute problem leading to admission. This result is also in line with former studies showing even an increase in the number of drugs between admission and discharge [26] and points out that polypharmacy is driven by polymorbidity. A higher number of prescribed drugs at admission and not living at home (but in an institution such as e.g. a nursing home) were significantly associated with a reduction ≥ 20% of prescribed drugs at discharge (Table 3). In patients with many medications, the pressure to reduce the number of prescribed drugs may be higher (e.g. because of adverse events, interactions or adherence problems). Moreover, we showed that a higher number of prescribed drugs at admission was significantly associated with a higher risk of prescription of ≥ 1 PIM (Table 2), that should be stopped.
The risk of missing prescription of an appropriate medication at discharge was not reduced by our intervention (Adj. RR 0.85, 95% CI 0.65–1.13) (Table 4). This might be explained by the fact that our intervention primarily focused on reducing inappropriate polypharmacy and the number of prescribed drugs. The introduction of the checklist appeared to be safe, at least in the short term: it was neither associated with an increased risk of in-hospital death nor with an increased risk of re-hospitalization at 30 days after discharge.
We also analyzed the prescription of a few drugs which may be problematic particularly in elderly patients (NSAIDs, systemic corticosteroids, potent opiates), are frequently prescribed often with unclear indication (PPI), or are even considered by several experts to be contraindicated at all, such as metamizole, a controversial NSAID marketed since 1922, that is popular and increasingly used in many countries (including Switzerland), but has been banned in several others (e.g. USA, UK, Canada) because of its association with potentially life threatening agranulocytosis. The introduction of the checklist significantly reduced the risk of inappropriate prescription of PPI at discharge by 28% (adjusted RR 0.72, 95% CI: 0.44–0.88).
We found a high prevalence of polypharmacy (59%) and hyperpolypharmacy (13%), as well as a high rate of potentially inappropriate drug prescriptions (PIM: 37%) and missing potentially appropriate drug prescriptions (PPO: 25%) in 900 patients ≥ 65 years admitted to a division of internal medicine in Switzerland. These results confirm the urgent need of strategies to improve adequacy and safety of drug prescription in elderly patients and are comparable to findings of other studies in other countries. In Europe and Australia the prevalence of polypharmacy and hyperpolypharmacy in elderly hospitalized patients varied in several studies between 52% and 76% for polypharmacy, and between 11% and 24% for hyperpolypharmacy. Between 35% and 77% of patients in these studies were prescribed PIM and the rate of PPO was between 51% and 63% [7, 27–30]. In the USA a cross-sectional study performed in 2007 and including more than 460,000 veterans age 65 and older found that 26% were taking ≥ 1 PIM [3], and among more than 13,000 adults aged ≥ 65 years participating in the National Health & Nutrition Examination Survey the proportion of participants taking ≥ 5 medications tripled from 13% to 39% between 1988 and 2010, while use of PIM decreased from 28% to 15% [1]. Both these US studies included only persons not residing in an inpatient facility and defined PIM according to Beers’ criteria. Only little information was previously available on the situation in Switzerland: among 150 patients aged ≥ 65 years who were admitted to the acute geriatric medicine unit at the Geneva University Hospital 67% were prescribed ≥ 6 and 21% > 10 medications. The PIM prevalence rate was 77% and the PPO rate 65% [27]. A previous study included 800 elderly patients (≥ 65 years) admitted to a general medical or geriatric ward at the University Hospital Basel. The PIM rate according to Beers criteria was overall 18% [31]. Finally, a recent study based on claims data from the largest health insurance in Switzerland and including community-dwelling adults reported for persons older than 65 years a polypharmacy rate (≥ 5 medications) of 41% and a PIM rate of 21% according to 2003 Beers criteria or the PRISCUS list [32]. The lower rates of PIM and polypharmacy reported in the two US studies and in the last two Swiss studies mentioned above in comparison to our results and the cited European and Australian studies may be explained by differences in the examined populations (patients admitted to the hospital versus community-dwelling adults) and by the use of different PIM definitions, since STOPP criteria have a higher sensitivity than e.g. Beers criteria [28, 29].
Our study has several limitations. A randomization was not possible because of the contamination effect, since all physicians of the Division of Internal Medicine rotate on all wards of the division every 1–2 months. In order to compensate for the inherent limitations of a before-after study design we analyzed the most relevant variables influencing drug prescribing, such as indicators of severity of illness and polymorbidity, and accounted for seasonal variations by comparing the patients in the intervention group with patients admitted in the same months of the previous year. Still, the patients in the control group had more diagnoses (8 vs. 7), had a slightly longer hospitalization (median 7 vs. 6 days) and were older (median age 79 vs. 76 years) than in the intervention group. However, we believe that these differences do not reflect relevant differences in the two collectives (e.g. regarding the disease burden), since other more significant characteristics, such as the Charlson Comorbidity Index and the number of drugs at admission were similar. The differences between the two groups might be explained by organizational changes in the Swiss health care system. In 2012 the reimbursement system for the hospitals was switched from a system based on daily fees to the new national Swiss diagnoses related groups (DRG) system, which is a flat rate system providing a fixed remuneration per patient based on diagnoses, procedures, and additional factors (such as age and comorbidities). This system encourages hospitals to shorten length of stay and first analyses suggest that the introduction of the new DRG-system led in Switzerland to a reduction of the duration of hospitalization particularly for elderly patients [33]. In addition, with the new DRG-system also the reimbursement for acute geriatric care and geriatric rehabilitation was changed, leading to the creation in several hospitals (including the Kantonsspital Olten) of new acute geriatric units. It is possible, that during the second part of our study, particularly very old patients have more frequently been admitted to the new acute geriatric unit, than to the division of internal medicine, explaining the difference in age between the study group with and without intervention.
The present study has also several strengths such as the size of the collective studied and the use of STOPP criteria, which require thorough chart-review but appear to be more sensitive and clinically relevant than for example Beers criteria [28, 29].
In conclusion, the introduction of an easy-to-use 5-point checklist aimed at supporting therapeutic reasoning of physicians on internal medicine wards significantly reduced the risk of prescriptions of inappropriate medications at discharge.
Supporting Information
(DOC)
(PDF)
Acknowledgments
The results of this study were presented in part at the Annual Congress of the Swiss Society of General Internal Medicine (section of the 10 best posters), Basel, Switzerland, May 25–27, 2016.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Charlesworth CJ, Smit E, Lee DS, Alramadhan F, Odden MC. Polypharmacy Among Adults Aged 65 Years and Older in the United States: 1988–2010. J Gerontol A Biol Sci Med Sci. 2015;70(8):989–95. 10.1093/gerona/glv013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995–2010. BMC Med. 2015;13:74 10.1186/s12916-015-0322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman MA, Miao Y, Boscardin WJ, Komaiko KD, Schwartz JB. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29(10):1379–86. 10.1007/s11606-014-2924-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried TR, O'Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–72. 10.1111/jgs.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau DT, Kasper JD, Potter DE, Lyles A, Bennett RG. Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch Intern Med. 2005;165(1):68–74. 10.1001/archinte.165.1.68 [DOI] [PubMed] [Google Scholar]
- 6.Price SD, Holman CD, Sanfilippo FM, Emery JD. Association between potentially inappropriate medications from the Beers criteria and the risk of unplanned hospitalization in elderly patients. Ann Pharmacother. 2014;48(1):6–16. 10.1177/1060028013504904 [DOI] [PubMed] [Google Scholar]
- 7.Dalleur O, Spinewine A, Henrard S, Losseau C, Speybroeck N, Boland B. Inappropriate prescribing and related hospital admissions in frail older persons according to the STOPP and START criteria. Drugs Aging. 2012;29(10):829–37. 10.1007/s40266-012-0016-1 [DOI] [PubMed] [Google Scholar]
- 8.Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173–84. 10.1016/S0140-6736(07)61091-5 [DOI] [PubMed] [Google Scholar]
- 9.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–24. 10.1001/archinte.163.22.2716 [DOI] [PubMed] [Google Scholar]
- 10.Fialova D, Topinkova E, Gambassi G, Finne-Soveri H, Jonsson PV, Carpenter I, et al. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA. 2005;293(11):1348–58. 10.1001/jama.293.11.1348 [DOI] [PubMed] [Google Scholar]
- 11.Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45(10):1045–51. [DOI] [PubMed] [Google Scholar]
- 12.Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–34. 10.1001/jamainternmed.2015.0324 [DOI] [PubMed] [Google Scholar]
- 13.Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014;10:CD008165. [DOI] [PubMed] [Google Scholar]
- 14.Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med. 2012;125(6):529–37.e4. 10.1016/j.amjmed.2011.09.021 [DOI] [PubMed] [Google Scholar]
- 15.Dovjak P. Tools in polypharmacy. Current evidence from observational and controlled studies. Z Gerontol Geriatr. 2012;45(6):468–72. 10.1007/s00391-012-0362-y [DOI] [PubMed] [Google Scholar]
- 16.Bayoumi I, Howard M, Holbrook AM, Schabort I. Interventions to improve medication reconciliation in primary care. Ann Pharmacother. 2009;43(10):1667–75. 10.1345/aph.1M059 [DOI] [PubMed] [Google Scholar]
- 17.Onder G, Petrovic M, Tangiisuran B, Meinardi MC, Markito-Notenboom WP, Somers A, et al. Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: the GerontoNet ADR risk score. Arch Intern Med. 2010;170(13):1142–8. 10.1001/archinternmed.2010.153 [DOI] [PubMed] [Google Scholar]
- 18.Carey EC, Covinsky KE, Lui LY, Eng C, Sands LP, Walter LC. Prediction of mortality in community-living frail elderly people with long-term care needs. J Am Geriatr Soc. 2008;56(1):68–75. 10.1111/j.1532-5415.2007.01496.x [DOI] [PubMed] [Google Scholar]
- 19.Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83. [DOI] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 21.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160(4):301–5. 10.1093/aje/kwh221 [DOI] [PubMed] [Google Scholar]
- 22.Cook TD. Advanced statistics: up with odds ratios! A case for odds ratios when outcomes are common. Acad Emerg Med. 2002;9(12):1430–4. [DOI] [PubMed] [Google Scholar]
- 23.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–3. [DOI] [PubMed] [Google Scholar]
- 24.Topinkova E, Baeyens JP, Michel JP, Lang PO. Evidence-based strategies for the optimization of pharmacotherapy in older people. Drugs Aging. 2012;29(6):477–94. 10.2165/11632400-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 25.Dalleur O, Boland B, Losseau C, Henrard S, Wouters D, Speybroeck N, et al. Reduction of potentially inappropriate medications using the STOPP criteria in frail older inpatients: a randomised controlled study. Drugs Aging. 2014;31(4):291–8. 10.1007/s40266-014-0157-5 [DOI] [PubMed] [Google Scholar]
- 26.Sganga F, Landi F, Vetrano DL, Corsonello A, Lattanzio F, Bernabei R, et al. Impact of hospitalization on modification of drug regimens: Results of the Criteria to Assess Appropriate Medication Use Among Elderly Complex Patients study. Geriatr Gerontol Int. 2015. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher P, Lang PO, Cherubini A, Topinkova E, Cruz-Jentoft A, Montero Errasquin B, et al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol. 2011;67(11):1175–88. 10.1007/s00228-011-1061-0 [DOI] [PubMed] [Google Scholar]
- 28.Gallagher P, O'Mahony D. STOPP (Screening Tool of Older Persons' potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers' criteria. Age Ageing. 2008;37(6):673–9. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton H, Gallagher P, Ryan C, Byrne S, O'Mahony D. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171(11):1013–9. 10.1001/archinternmed.2011.215 [DOI] [PubMed] [Google Scholar]
- 30.Hubbard RE, Peel NM, Scott IA, Martin JH, Smith A, Pillans PI, et al. Polypharmacy among inpatients aged 70 years or older in Australia. Med J Aust. 2015;202(7):373–7. [DOI] [PubMed] [Google Scholar]
- 31.Egger SS, Bachmann A, Hubmann N, Schlienger RG, Krahenbuhl S. Prevalence of potentially inappropriate medication use in elderly patients: comparison between general medical and geriatric wards. Drugs Aging. 2006;23(10):823–37. [DOI] [PubMed] [Google Scholar]
- 32.Blozik E, Rapold R, von Overbeck J, Reich O. Polypharmacy and potentially inappropriate medication in the adult, community-dwelling population in Switzerland. Drugs Aging. 2013;30(7):561–8. 10.1007/s40266-013-0073-0 [DOI] [PubMed] [Google Scholar]
- 33.Health FOoP. Evaluation der KVG-Revision im Bereich der Spitalfinanzierung. Zwischenresultate. Bericht des BAG an den Bundesrat. Bern, 13. Mai 2015. 2015 [cited 2015 November 23, 2015]. http://www.bag.admin.ch/evaluation/01759/07350/12642/index.html?lang=de.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
Data Availability Statement
All relevant data are within the paper.