Abstract
Background
This study was conducted to investigate the clinical significance of claudin‐1 (CLDN1) expression in patients with lung adenocarcinoma.
Methods
We examined CLDN1 protein expression by immunohistochemistry in a tissue microarray from 258 patients with lung adenocarcinoma. We investigated messenger ribonucleic acid (mRNA) expression in H358 (formerly bronchioloalveolar carcinoma) and lung adenocarcinoma cell lines (A549) by real‐time reverse transcriptase‐polymerase chain reaction.
Results
Multivariate analysis showed that prognostic factors for lung adenocarcinoma were histologic type, CLDN1, T stage and N stage. Patients with positive CLDN1 expression had a poorer prognosis than patients with negative CLDN1 expression. CLDN1 expression was correlated with Ras and epidermal growth factor receptor (EGFR) expression. Patients with positive expressions of both CLDN1 and Ras/EGFR had a poorer prognosis than patients with CLDN1 (+) Ras/EGFR(−) or CLDN1 (−) Ras/EGFR(+) and patients with negative expressions of both CLDN1 and Ras/EGFR. CLDN1 mRNA expression was lower in the H358 compared with the lung adenocarcinoma cell line (A549).
Conclusion
The combination of CLDN1 and Ras/EGFR is a valuable independent prognostic predictor for lung adenocarcinoma.
Keywords: CLDN1, EGFR, lung adenocarcinoma, Ras
Introduction
Adenocarcinoma represents the most common histological subtype of lung cancer.1 Dysfunction of the airway epithelial barrier has been reported to be involved in the development and progression of cancer, including lung cancer. The epithelial barrier is composed of two essential elements, an intact epithelial monolayer and the intercellular tight junctions that connect epithelial cells to their neighboring cells.2 Tight junctions are composed of several different components, including transmembrane, peripheral, and cytoskeletal proteins, which act in concert to control paracellular permeability. The transmembrane proteins that mediate cell‐to‐cell contacts include claudins, occludin, tricellulin, and junctional adhesion molecules. Claudins (23 kDa) have four transmembrane domains and two extracellular loops and now have a family of 27 isoforms. They are expressed differentially among various tissues, and their expression pattern impacts on epithelial barrier function. It has been reported that claudin‐1 (CLDN1), CLDN 4, CLDN 5, CLDN 14, and CLDN 18 are tightening junctional proteins with sealing function, whereas CLDN 2 and CLDN 8 are loosening junctional molecules with leaky function.3
Recent studies have suggested that CLDN1 is downregulated in lung adenocarcinoma and that low CLDN1 messenger ribonucleic acid (mRNA) expression leads to shorter overall survival (OS).4, 5 The aim of this study was to evaluate the clinical significance of CLDN1 expression in patients with lung adenocarcinoma; however, our results differed from those of other studies.4, 5
Methods
Patients and tissue samples
The study analyzed 258 paraffin‐embedded lung adenocarcinoma tissues in a tissue microarray, collected from the Tianjin Cancer Institute & Hospital, Tianjin Medical University, Tianjin, China between 2005 and 2010. Patient medical records were retrospectively reviewed to assess clinical characteristics and survival. Patients who had smoked < 100 cigarettes in their lifetime were defined as never smokers. The routine preoperative workup included pulmonary function tests, contrast chest computed tomography (CT), flexible bronchoscopy, and brain magnetic resonance imaging; 59 patients underwent positron emission tomography (PET)‐CT scans. Surgical procedures included: lobectomy or bilobectomy in 243 patients; pneumonectomy in 15; and subsequent mediastinoscopy or endobronchial ultrasound/esophageal endoscopic ultrasound‐transbronchial aspiration (EBUS/EUS‐TBNA) in 38 patients. In our hospital, if a contrast CT or PET‐CT shows no mediastinal lymph node enlargement, a cervical mediastinoscopy or EBUS/EUS‐TBNA is not routinely performed. No neoadjuvant therapy was administered to any of the 258 patients. All patients underwent systematic lymph node dissection or sampling. Patient consent and approval from the Research Ethics Committee of Tianjin Cancer Institute & Hospital of Tianjin Medical University was obtained. Histological classification was defined according to World Health Organization histologic classification. Clinicopathological variables are summarized in Table 1.
Table 1.
Five‐year OS and DFS of patients with lung adenocarcinoma
| Variables | n | 5‐year OS (%) | P | 5‐year DFS (%) | P |
|---|---|---|---|---|---|
| Pathology | |||||
| AIS | 25 | 65.2 | 0.020 | 65.2 | 0.023 |
| MIA + LPA | 123 | 45.0 | 61.3 | ||
| ADE | 110 | 36.7 | 33.8 | ||
| Ras | |||||
| − | 106 | 54.9 | 0.000 | 54.8 | 0.000 |
| + | 152 | 34.9 | 31.3 | ||
| Claudin‐1 | |||||
| − | 106 | 54.8 | 0.001 | 53.4 | 0.001 |
| + | 152 | 34.6 | 32.5 | ||
| EGFR | |||||
| − | 149 | 46.2 | 0.199 | 45.5 | 0.156 |
| + | 109 | 38.4 | 34.1 | ||
| Gender | |||||
| Male | 123 | 37.4 | 0.074 | 36.1 | 0.075 |
| Female | 135 | 48.6 | 46.1 | ||
| Age | |||||
| ≤ 60 | 142 | 43.0 | 0.941 | 42.0 | 0.920 |
| > 60 | 116 | 43.0 | 40.5 | ||
| Smoking | |||||
| No | 138 | 47.6 | 0.036 | 45.6 | 0.039 |
| Yes | 120 | 38.0 | 36.8 | ||
| T stage | 0.000 | ||||
| T1 | 111 | 60.9 | 0.000 | 56.9 | |
| T2 | 88 | 39.4 | 39.8 | ||
| T3, 4 | 59 | 16.8 | 16.0 | ||
| N stage | 0.000 | ||||
| N0 | 140 | 58.1 | 0.000 | 55.7 | |
| N1 | 31 | 37.1 | 34.8 | ||
| N2 | 87 | 21.0 | 20.8 | ||
| Adjuvant chemotherapy | |||||
| No | 98 | 52.6 | 0.062 | 51.7 | 0.013 |
| Yes | 160 | 39.0 | 35.0 | ||
| Laterality | |||||
| Left | 111 | 41.3 | 0.889 | 40.4 | 0.957 |
| Right | 147 | 44.8 | 41.9 | ||
| Location | |||||
| Peripheral | 219 | 44.5 | 0.587 | 42.3 | 0.427 |
| Central | 39 | 36.0 | 35.0 | ||
| Operation | |||||
| Lobectomy | 243 | 44.9 | 0.019 | 42.7 | 0.016 |
| Pneumonectomy | 15 | 17.8 | 20.0 | ||
ADE, invasive adenocarcinoma not including LPA; AIS, adenocarcinoma in situ; DFS, disease‐free survival; LPA, lepidic predominant adenocarcinoma; MIA, minimally invasive adenocarcinoma; OS, overall survival.
Lung cancer cell lines were either originally purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA; H358), or obtained from Dr. Zhenyi Ma (Tianjin Medical University, Tianjin, China; A549).
Immunohistochemical staining and assessment
Paraffin sections (4 μm) from samples were deparaffinized in 100% xylene and rehydrated in descending ethanol dilutions according to standard protocols. Heat‐induced antigen retrieval was performed in ethylene‐diamine‐tetraacetic acid buffer (pH 9.0) for three minutes at 100°C. Endogenous peroxidase activity and non‐specific antigens were blocked with peroxidase blocking reagent containing 3% hydrogen peroxide and serum, followed by incubation with each antibody: rabbit anti‐CLDN1 (Abcam, Cambridge, UK) at a dilution of 1:200; mouse anti‐epidermal growth factor receptor (EGFR; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:100; and rabbit anti‐Ras (Bioss, Beijing, China) at a dilution of 1:100, overnight at 4°C. After washing, the sections were incubated with biotin‐labeled secondary antibody for 40 minutes at 37°C and were subsequently incubated with streptavidin‐conjugated horseradish peroxidase. The peroxidase reaction was developed using 3,3‐diaminobenzidine chromogen solution in 3,3'‐diaminobenzidine‐tetrahydrochloride (DAB) buffer substrate (ChemMate EnVision Detection Kit, Dako, Carpintaria, CA, USA). Sections were visualized with DAB counterstained with hematoxylin, mounted in neutral gum, and analyzed using a bright field microscope.6
Two independent investigators performed the analysis of immunohistochemical staining. Extensiveness and intensity of staining in tumor cells were assessed for each sample using a semiquantitative scale. The extent of tumor staining was scored as follows: 0 (< 5% immunoreactive), 1+ (< 33% immunoreactive), 2+ (33–66% immunoreactive), and 3+ (> 66% immunoreactive). The staining intensity was graded using the following scale: 0 (negative), 1+ (weak), 2+ (moderate), and 3+ (strong). For each tumor, a combined score was calculated by multiplying the scores for extensiveness and intensity, using the following scale: 0+ = score 0, 1+ = 1–3, 2+ = 4–6, 3 + = 7–9. Scores 0+ and 1+ were defined as negative, while scores 2+ and 3+ were considered positive expression.
Quantitative real‐time reverse transcription‐polymerase chain reaction
Quantitative real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) was used to test the levels of CLDN1 expression in lung cancer cells. Real‐time RT‐PCR was performed on CLDN1 and the control gene, glyceraldehyde 3‐phosphate dehydrogenase (GAPDH), with the use of specific TaqMan probes and primer sets. Gene expression was quantified in relation to GAPDH expression using sequence detector software with the relative quantification method (Applied Biosystems, Foster City, CA, USA). Briefly, RNA (2 μg/reaction) was used to generate cDNA and then the appropriate individual pairs of oligonucleotides (40 pmol/reaction) for the test genes were used to amplify DNA. Semiquantitative PCR was performed using 100 μL reaction volumes and taking 33 μL aliquots at 25, 30, and 35 cycles. GAPDH mRNA expression was determined for RNA samples to control for variations in RNA quantity.7
Statistical analysis
The χ2 test was used to evaluate differences between the groups. Survival curves were obtained by the Kaplan–Meier method. Survival values were compared using the log‐rank test. The Cox proportional hazards ratio model was used to investigate the simultaneous effect of multiple predictors on survival. All statistical tests were two‐sided, and a P value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient survival
Claudin‐1 and EGFR showed membrane expression (Fig 1a,b, respectively), while Ras was expressed in the cytoplasm (Fig 1c). Univariate analysis revealed that pathologic subtype, CLDN1 expression , Ras, smoking, T stage, and lymph node involvement were prognostic factors (P = 0.020, P = 0.001, P = 0.000, P = 0.036, P = 0.000, P = 0.000 for 5‐year OS; P = 0.023, P = 0.001/ P = 0.000, P = 0.039, P = 0.000, P = 0.000 for 5‐year disease‐free survival [DFS], respectively; Fig 2). Patients with positive expressions of CLDN1/Ras had significantly poorer survival than those with negative expression (Table 1). Table 2 summarizes the multivariate analysis of the prognostic value of the prognostic factors, which were determined by univariate analysis (P < 0.05) of OS in the 258 patients. In this study, a significant OS value was observed for pathologic subtype (P = 0.026), CLDN1 expression (P = 0.008), T stage (P = 0.014), and lymph node involvement (P = 0.003).
Figure 1.
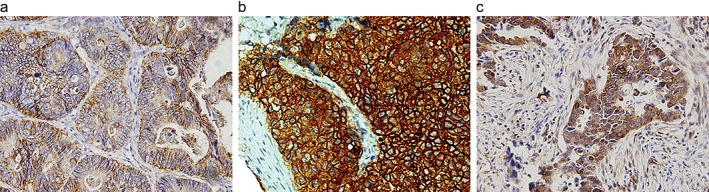
Typical photomicrographs show Claudin‐1 (CLDN1), epidermal growth factor receptor (EGFR), and Ras immunohistochemistry expression in tissue microarray. Diaminobenzidine solution is the stain used in all images. (a) CLDN‐1+ (original magnification × 100); (b) EGFR+ (original magnification × 100); (c) Ras+ (original magnification × 100).
Figure 2.
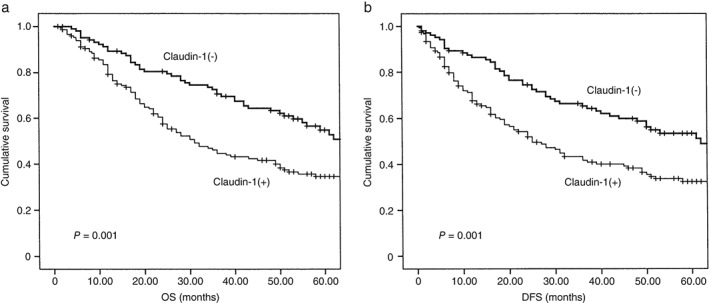
Relationship between Claudin‐1 (CLDN1) expression and survival in patients with lung adenocarcinoma. Patients with negative CLDN1 expression had better (a) overall survival (OS) and (b) and disease‐free survival (DFS) than those with positive expression (P = 0.001).
Table 2.
Cox proportional hazard survival analyses for patients with lung adenocarcinoma
| Variables | Hazard ratio (95% CI) | P |
|---|---|---|
| Pathology | ||
| AIS vs. MIA + LPA vs. ADE | 0.729 (0.552–0.962) | 0.026 |
| Ras | ||
| − vs. + | 0.735 (0.509–1.062) | 0.100 |
| CLDN1 | ||
| − vs. + | 0.613 (0.428–0.879) | 0.008 |
| Smoking | ||
| − vs. + | 0.963 (0.895–1.762) | 0.909 |
| T stage | ||
| T1 vs. T2 vs. T3, 4 | 0.736 (0.575–0.942) | 0.014 |
| N stage | ||
| N0 vs. N1 vs. N2 | 0.729 (0.583–0.897) | 0.003 |
| Operation | ||
| Lobectomy vs. pneumonectomy | 0.963 (0.510–1.822) | 0.909 |
ADE, invasive adenocarcinoma not including LPA; AIS, adenocarcinoma in situ; CI, confidence interval; CLDN1, claudin‐1; LPA, lepidic predominant adenocarcinoma; MIA, minimally invasive adenocarcinoma.
Correlation between claudin‐1 (CLDN1) and clinical‐pathological characteristics
Because our results indicated that CLDN1 was a poor prognostic factor, we investigated the relationship between CLDN1 and other factors that may affect prognostic outcome. Our results demonstrated that CLDN1 expression was correlated with Ras (P = 0.000) and EGFR expression (P = 0.000). There was no association between CLDN1 and other clinical prognostic factors, such as T or N stage (Table 3).
Table 3.
Correlation between Claudin‐1 and clinical‐pathologic characteristics
| Variables | CLDN1 | P | |
|---|---|---|---|
| − | + | ||
| Pathology | |||
| AIS | 8 | 17 | 0.326 |
| MIA + LPA | 56 | 67 | |
| ADE | 42 | 68 | |
| Ras | |||
| − | 59 | 47 | 0.000 |
| + | 47 | 105 | |
| EGFR | |||
| − | 81 | 68 | 0.000 |
| + | 25 | 84 | |
| Gender | |||
| Male | 43 | 80 | 0.056 |
| Female | 63 | 72 | |
| Age | |||
| ≤ 60 | 64 | 78 | 0.150 |
| > 60 | 42 | 74 | |
| Smoker | |||
| No | 60 | 78 | 0.402 |
| Yes | 46 | 74 | |
| T stage | |||
| T1 | 49 | 62 | 0.423 |
| T2 | 37 | 51 | |
| T3, 4 | 20 | 39 | |
| N stage | |||
| N0 | 60 | 80 | 0.092 |
| N1 | 17 | 14 | |
| N2 | 29 | 58 | |
| Adjuvant chemotherapy | |||
| No | 39 | 59 | 0.742 |
| Yes | 67 | 93 | |
| Laterality | |||
| Left | 48 | 63 | 0.540 |
| Right | 58 | 89 | |
| Location | |||
| Peripheral | 88 | 131 | 0.485 |
| Central | 18 | 21 | |
| Operation | |||
| Lobectomy | 8 | 7 | 0.320 |
| Pneumonectomy | 98 | 145 | |
ADE, invasive adenocarcinoma not including LPA; AIS, adenocarcinoma in situ; LDN1, claudin‐1; EGFR, epidermal growth factor receptor; LPA, lepidic predominant adenocarcinoma; MIA, minimally invasive adenocarcinoma.
Combination of CLDN1 and Ras/epidermal growth factor receptor
Because CLDN1 is associated with poor prognosis and has an association with Ras/EGFR, we hypothesized that Ras and EGFR signal transduction pathways may regulate the role of CLDN1 in terms of prognosis. We conducted survival analysis with a combination of these factors. Our results indicated that both CLDN1(+) and Ras(+) prognoses were worse than CLDN1(+) Ras(−), CLDN1(−) Ras(+), and CLDN1(−) Ras (−); and both CLDN1(+) and EGFR(+) prognoses were worse than CLDN1(+) EGFR(−), CLDN1(−) EGFR(+), and CLDN1(−) EGFR(−) (Tables 4, 5, Figs 3, 4).
Table 4.
Survival analysis of the combination of Claudin‐1 and Ras
| Variables | n | 5‐year OS (%) | P | 5‐year DFS (%) | P |
|---|---|---|---|---|---|
| Claudin1−/Ras− | 59 | 57.6 | 0.000 | 58.0 | 0.000 |
| Claudin1+/Ras − and Claudin1−/Ras + | 94 | 51.4 | — | 49.3 | — |
| Claudin1+/Ras + | 105 | 27.2 | — | 24.1 | — |
DFS, disease‐free survival; OS, overall survival.
Table 5.
Survival analysis of the combination of Claudin‐1 and EGFR
| Variables | n | 5‐year OS (%) | P | 5‐year DFS (%) | P |
|---|---|---|---|---|---|
| Claudin1−/EGFR− | 59 | 57.6 | 0.000 | 58.0 | 0.000 |
| Claudin1+/EGFR− and Claudin1−/EGFR+ | 94 | 51.4 | — | 49.3 | — |
| Claudin1+/EGFR+ | 105 | 27.2 | — | 24.1 | — |
DFS, disease‐free survival; EGFR, epidermal growth factor receptor; OS, overall survival.
Figure 3.
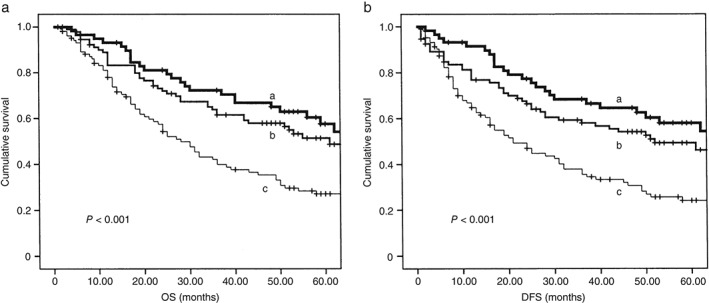
Prognosis of patients with both Claudin‐1 (CLDN1)(+) and Ras(+) were worse than those with CLDN1(+) Ras(−), CLDN1 (−) Ras (+), and CLDN1 (−) Ras(−) (a) Overall survival (OS), (b) disease‐free survival (DFS). a: CLDN1 −/Ras−, b: CLDN1 +/Ras− and CLDN1 −/Ras+, and c: CLDN1 +/Ras+.
Figure 4.

Prognosis of patients with both Claudin‐1 (CLDN1)(+) and epidermal growth factor receptor (EGFR)(+) were worse than those with CLDN1 (+) EGFR(−),CLDN1 (−) EGFR(+) and CLDN1 (−) EGFR(−). (a) Overall survival, (b) disease‐free survival (DFS). a: CLDN1−/EGFR‐\r\n, b: CLDN1+/EGFR− and CLDN1−/EGFR+\r\n and c: CLDN1+/EGFR.
Messenger ribonucleic acid expression was lower in the H358 cell line compared with A549
The International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society sponsored a new international multidisciplinary classification, in which non‐mucinous bronchioloalveolar carcinoma (BAC, ≤ 3 cm) is classified as adenocarcinoma in situ (AIS, formerly pure BAC).1 Some authors have suggested that atypical adenomatous hyperplasia (AAH), AIS, minimally invasive adenocarcinoma (MIA, formerly BAC with focal invasion), and lepidic predominant adenocarcinoma (LPA, formerly adenocarcinoma with BAC features) represent the developmental sequence of bronchioloalveolar stem cells, rather than a classification system.8, 9
We hypothesized that CLDN1 expression was higher in adenocarcinoma than in AIS and MIA. mRNA expression in CLDN1 was lower in the H358 cell line compared with the lung adenocarcinoma cell line, A549 (Fig 5, Table 6).
Figure 5.
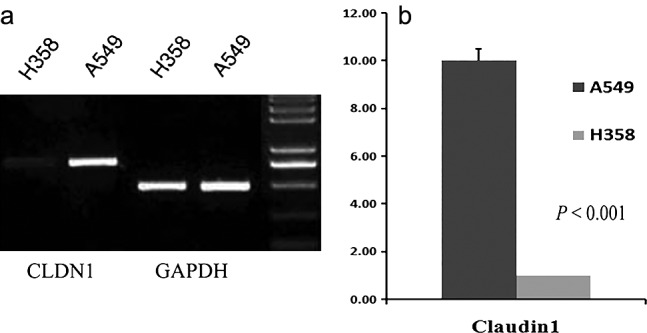
(a) The messenger ribonucleic acid (mRNA) expression of Claudin‐1 (CLDN1) in H358 and A549 cell lines. (b) The mRNA expression of CLDN1 was lower in the H358 cell line compared with the lung adenocarcinoma cell line (P < 0.001). GAPDH, glyceraldehyde 3‐phosphate dehydrogenase.
Table 6.
Claudin‐1 primers for RT‐PCR
| Gene | Primer (5′–3′) | Genbank | Product size (bp) | |
|---|---|---|---|---|
| CLDN1 | Forward | GATAGCAATCTTTGTGGCCACCGT | NC_000003.12 | 205 |
| Reverse | TTCGTACCTGGCATTGACTGGG | — | — | |
CLDN1, claudin‐1; RT‐PCR, reverse transcriptase‐polymerase chain reaction.
Discussion
Disruption of the cell‐cell junction and detachment of tumor cells from the primary site is the first step of cancer cell invasion and metastasis. Therefore, CLDN1, a key component of tight junctions, which is abnormally regulated in lung adenocarcinoma, plays an important role in cancer progression and prognosis in lung adenocarcinoma.
Studies have suggested that the role of CLDN1 in the behavior of cancer cells differs in different types of cancers, and even in the same cancer. Several studies have reported a robust increase in CLDN1 expression in colon cancer.10, 11, 12 The causal association of high CLDN1 expression with cancer progression, invasion, and metastasis has been demonstrated in colon and colorectal cancer lesions.12, 13 Other studies have reported that low CLDN1 expression is associated with lymphatic involvement, histological differentiation, the extent of the poorly differentiated component, and reduced DFS and OS in cancers.14, 15, 16
Our study demonstrated different results than those found in previous studies; however, the reason for this is unclear.4, 5 Warrier et al. reported that CLDN1 was expressed both in the cytoplasm and at the plasma membrane in breast carcinoma cells.17 Cytoplasmic predominant expression of CLDN1 is associated with a favorable prognosis. In contrast, membranous predominant expression is associated with larger tumors, histological grade 3, and a poor outcome; however, we did not find a correlation between this kind of expression location and clinical outcome. We hypothesized that other molecular pathways associated with lung cancer development may affect the role of CLDN1. We examined Ras and EGFR protein expression by immunohistochemistry in the tissue microarray of 258 patients. We found that CLDN1 expression was correlated with Ras and EGFR, and the combination of CLDN1 and Ras/EGFR had more powerful clinical significance. Therefore, we suggest that Ras and EGFR signal pathways may regulate the role of CLDN1 in lung adenocarcinoma. If CLDN1 does not activate Ras and EGFR signal pathways, it may not negatively affect the clinical outcome in lung adenocarcinoma.
Other studies have also suggested that CLDN1 plays a role in cancer via different signal transduction pathways. Warrier et al. suggested that CLDN1 is a direct target of Hedgehog pathway activation in breast cancer.17 The transcription factor, RUNX3, is a gastric tumor suppressor; CLDN1 has gastric tumor suppressive activity and is a direct transcriptional target of RUNX3. CLDN1 is downregulated during the epithelial‐mesenchymal transition (EMT) and has a causal role in the EMT in human liver cells.18 c‐Abl‐protein kinase Cδ (PKCδ) and matrix metalloproteinase‐2 expression are involved in the acquisition of invasive capacity associated with CLDN1 in human liver and non‐invasive human hepatocellular carcinoma cells.19, 20
In lung cancer cells, CLDN1 small interfering RNA significantly reduced tumor necrosis factor‐enhanced cell migration and fibroblast‐like morphology. Furthermore, CLDN1 overexpression enhanced cell migration in human lung cancer cells.21 AAH, AIS, MIA, and LPA might represent the developmental sequence of bronchioloalveolar stem cells, rather than a classification system. During the malignant transformation of normal colonic epithelium to colon adenocarcinoma, CLDN1 levels increased.22 We hypothesized that CLDN1 expression was higher in lung adenocarcinoma and LPA; however, our immunohistochemistry results showed no difference in protein expression. mRNA expression was lower in the H358 compared with the lung adenocarcinoma cell line (A549), which may be attributed to the relatively low number of BAC cases (25) compared with the high number of lung adenocarcinoma cases (233) in this study. No subgroup analysis of BAC, such as pathologic subtype (mucinous or non‐mucinous) or tumor size was conducted, because of the small number of cases.
Our results indicate that CLDN1 is associated with poor prognosis and has an association with Ras/EGFR. The combination of CLDN1 and Ras/EGFR had more powerful clinical significance. CLDN1 may be involved in the progression of lung adenocarcinoma and Ras and EGFR signal pathways may regulate its role.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We thank Dr. Zhenyi Ma for his contribution of the lung adenocarcinoma cell line (A549). This research was supported by the National Natural Science Foundation of China (NO. 81201649, NO. 81470137) and the Key Program for Anti‐cancer Research of Tianjin Municipal Science and Technology Commission (12ZCDZSY15400).
References
- 1. Travis WD, Brambilla E, Noguchi M et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ogasawara N, Kojima T, Go M et al. PPAR gamma agonists upregulate the barrier function of tight junctions via a PKC pathway in human nasal epithelial cells. Pharmacol Res 2010; 61: 489–98. [DOI] [PubMed] [Google Scholar]
- 3. Schulzke JD, Günzel D, John LJ, Fromm M. Perspectives on tight junction research. Ann N Y Acad Sci 2012; 1257: 1–19. [DOI] [PubMed] [Google Scholar]
- 4. Paschoud S, Bongiovanni M, Pache JC, Citi S. Claudin‐1 and claudin‐5 expression patterns differentiate lung squamous cell carcinomas from adenocarcinomas. Mod Pathol 2007; 20: 947–54. [DOI] [PubMed] [Google Scholar]
- 5. Chao YC, Pan SH, Yang SC et al. Claudin‐1 is a metastasis suppressor and correlates with clinical outcome in lung adenocarcinoma. Am J Respir Crit Care Med 2009; 179: 123–33. [DOI] [PubMed] [Google Scholar]
- 6. Jiang R, Jin Z, Liu Z, Sun L, Wang L, Li K. Correlation of activated STAT3 expression with clinicopathologic features in lung adenocarcinoma and squamous cell carcinoma. Mol Diagn Ther 2011; 15: 347–52. [DOI] [PubMed] [Google Scholar]
- 7. Zhang ZF, Pei BX, Wang AL et al. Expressions of CLDN1 and insulin‐like growth factor 2 are associated with poor prognosis in stage N2 non‐small cell lung cancer. Chin Med J (Engl) 2013; 126 (19): 3668–74. [PubMed] [Google Scholar]
- 8. Soh J, Toyooka S, Ichihara S et al. Sequential molecular changes during multistage pathogenesis of small peripheral adenocarcinomas of the lung. J Thorac Oncol 2008; 3: 340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raz DJ, He B, Rosell R, Jablons DM. Bronchioloalveolar carcinoma: A review. Clin Lung Cancer 2006; 7: 313–22. [DOI] [PubMed] [Google Scholar]
- 10. Dhawan P, Singh AB, Deane NG et al. Claudin‐1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest 2005; 115 (7): 1765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ouban A, Hamdan H, Hakam A, Ahmed AA. Claudin‐1 expression in squamous cell carcinomas of different organs: Comparative study of cancerous tissues and normal controls. Int J Surg Pathol 2012; 20 (2): 132–8. [DOI] [PubMed] [Google Scholar]
- 12. Kinugasa T, Akagi Y, Ochi T et al. Increased claudin‐1 protein expression in hepatic metastatic lesions of colorectal cancer. Anticancer Res 2012; 32 (6): 2309–14. [PubMed] [Google Scholar]
- 13. Krishnan M, Singh AB, Smith JJ et al. HDAC inhibitors regulate claudin‐1 expression in colon cancer cells through modulation of mRNA stability. Oncogene 2010; 29 (2): 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stache C, Hölsken A, Fahlbusch R et al. Tight junction protein claudin‐1 is differentially expressed in craniopharyngioma subtypes and indicates invasive tumor growth. Neuro Oncol 2014; 16: 256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shibutani M, Noda E, Maeda K, Nagahara H, Ohtani H, Hirakawa K. Low expression of claudin‐1 and presence of poorly‐differentiated tumor clusters correlate with poor prognosis in colorectal cancer. Anticancer Res 2013; 33: 3301–6. [PubMed] [Google Scholar]
- 16. Abdelzaher E, Rizk AM, Bessa SS, Omer KM. Predictive value of immunohistochemical expression of claudin‐1 in colonic carcinoma. J Egypt Natl Canc Inst 2011; 23 (4): 123–31. [DOI] [PubMed] [Google Scholar]
- 17. Warrier S, Sellinger C, Beith JM et al. Claudin‐1 as a novel transcriptional target of hedgehog signaling and a predictor for outcome in breast cancer. 2013 ASCO Annual Meeting ProceedingsJ Clin Oncol 2013; 31 (Suppl.): Abstract 1053. [Google Scholar]
- 18. Chang TL, Ito K, Ko TK et al. Claudin‐1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells. Gastroenterology 2010; 138 (1): 255–65. [DOI] [PubMed] [Google Scholar]
- 19. Suh Y, Yoon CH, Kim RK et al. Claudin‐1 induces epithelial‐mesenchymal transition through activation of the c‐Abl‐ERK signaling pathway in human liver cells. Oncogene 2013; 32 (41): 4873–82. [DOI] [PubMed] [Google Scholar]
- 20. Yoon CH, Kim MJ, Park MJ et al. Claudin‐1 acts through c‐Abl‐Protein Kinase Cδ (PKCδ) signaling and has a causal role in the acquisition of invasive capacity in human liver cells. J Biol Chem 2010; 285: 226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shiozaki A, Bai XH, Shen‐Tu G et al. Claudin 1 mediates TNF alpha‐induced gene expression and cell migration in human lung carcinoma cells. PLoS One 2012; 7 (5): e38049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh AB, Sharma A, Smith JJ et al. Claudin‐1 up‐regulates the repressor ZEB‐1 to inhibit E‐cadherin expression in colon cancer cells. Gastroenterology 2011; 141 (6): 2140–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


