Abstract
Background
Certain radiographic signs of a treatment response, such as cavitation, changes in density, or tumor change along a short axis, are not considered by Response Evaluation Criteria in Solid Tumors (RECIST). This study evaluates what additional prognostic information can be obtained by including these criteria in tumor assessment.
Methods
Data of 105 patients were included. Tumor cavitation was observed in 51 patients at baseline. An additional 23 patients developed tumor cavitation during treatment. A change in tumor density was the only radiographic treatment response observed in 22 patients. The only measureable treatment response in nine patients was a decrease along the short axis size of the tumor. Tumor response was assessed using various criteria.
Results
In patients with basic tumor cavitation, RECIST1.1 scores accurately predicted differences in progression‐free survival (PFS; P = 0.076) while modified (m) RECIST did not (P = 0.550). mRECIST detected a significant difference between PFS in patients with post‐therapeutic cavitation with different responses, but no significant difference using RECIST1.1 (P = 0.004 vs. P = 0.477). In patients with only tumor density changes, there was no significant difference in PFS when either RECIST1.1 or density criteria were used (P = 0.419). In patients with a change in size along the tumor's short axis, short axis criteria could predict significant difference in PFS (P = 0.004).
Conclusions
RECIST1.1 provides the best assessment of tumor response and prediction of PFS in patients with basic tumor cavitation. mRECIST provides better PFS prognostic information in patients with post‐therapeutic cavitation. Short axis criteria provides better PFS prognostic information in patients with changes in the short axis of tumor diameter. Changes in tumor density were not a useful prognostic sign.
Keywords: Cavitation, density, lung cancer, progression‐free survival, short axis
Introduction
Accurate monitoring of the changes in a tumor during treatment is crucial to predict clinical outcomes. Imaging techniques have been used to evaluate tumor response to treatment for the last 30 years. In 1981, the World Health Organization (WHO) proposed the following categories to describe tumor response: complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), and objective response (OR) if patients had either CR or PR.1 Following the introduction of these criteria, people modified the original WHO criteria to accommodate new therapies and address areas that were unclear in the original document, which led to confusion in the interpretation and comparison of clinical trial results.2 To address these problems, the European Organization for Research on Treatment of Cancer (EORTC), the National Cancer Institute (NCI), and the National Cancer Institute of Canada (NCIC) collaborated to standardize and simplify response criteria. The new criteria, known as Response Evaluation Criteria in Solid Tumors (RECIST), was published in February 2000 and later updated to version 1.1 in 2009.3, 4 While RECIST 1.1 has been widely adopted, some problems remain regarding application of the criteria to all solid tumors. For instance, when evaluating a response, much weight is placed on the changes that occur along the longest axis of target lesion, while changes in other forms are ignored. In cases where a tumor responds to treatment, reflected by changes in the short axis, density, or cavitation, RECIST 1.1 will underestimate the clinical efficacy of treatment and thus, is no longer an accurate prognostic measure. The purpose of this study is to highlight the ideal method to evaluate treatment response and predict progression‐free survival (PFS) in lung cancer patients by incorporating these changes into imaging response assessment.
Methods
Patient selection
The data of all lung cancer patients from the Cancer Hospital of Tianjin Medical University between January 2011 and December 2012, who had a target lesion of at least 1 cm, were evaluated for inclusion in the study. All patients underwent a baseline thoracic computed tomography (CT) scan within two weeks before the commencement of treatment and had the first tumor evaluation with a second CT scan within three months after receiving therapy. From this group we selected three subpopulations: patients with either a tumor cavitation present at baseline or a cavitation that appeared during treatment; patients with a ≥ 15% change in tumor density; and patients with a decrease of ≥ 30% or increase of ≥ 20% along the short axis of their tumor. Patients were excluded if they had received chemotherapy or radiation prior to enrollment or underwent surgical resection during the study period. Patient data was excluded if: it could not be retrieved from the electronic medical records query system; part of the imaging assessment was missing; or patients were lost to follow‐up.
Treatment and clinical follow‐up
Therapeutic protocols included platinum‐based and non‐platinum‐based chemotherapy, radiotherapy, targeted therapy, chemotherapy combined with anti‐angiogenesis therapy, chemotherapy combined with radiotherapy, and chemotherapy combined with targeted therapy and other treatments, based on National Comprehensive Cancer Network (NCCN) guidelines. Follow‐up data were obtained from the Cancer Hospital of Tianjin Medical University electronic medical records and image query system until 1 October 2014. Telephone follow‐up was conducted in some cases to verify medical records. Treatment response was evaluated by comparing the baseline CT scan to the first post‐treatment CT scan and verified with a subsequent post‐treatment CT scan one month later. PFS was defined as the number of months from the treatment start date until the date of disease progression on CT scan or death for any reason. In patients with no evidence of disease progression, the absence of disease progression was determined at the date of the last follow‐up examination.
Radiologic review
All images were obtained via 16‐multislice spiral CT scan (Siemens, Berlin, Germany). Lung cavitation was defined as the presence of an abnormal hollow space, either fluid filled or vacant within lung parenchyma.5 The circled region of interest on target lesions and tumor density were assessed by measuring Hounsfield units (HU) on the CT scan at baseline and at the time of evaluation. A full retrospective review of all radiographic imaging was performed and signs of tumor cavitation, changes in tumor density, and changes in short axis length were evaluated by three independent radiologists. Representative examples of radiographic signs are shown in Fig 1.
Figure 1.
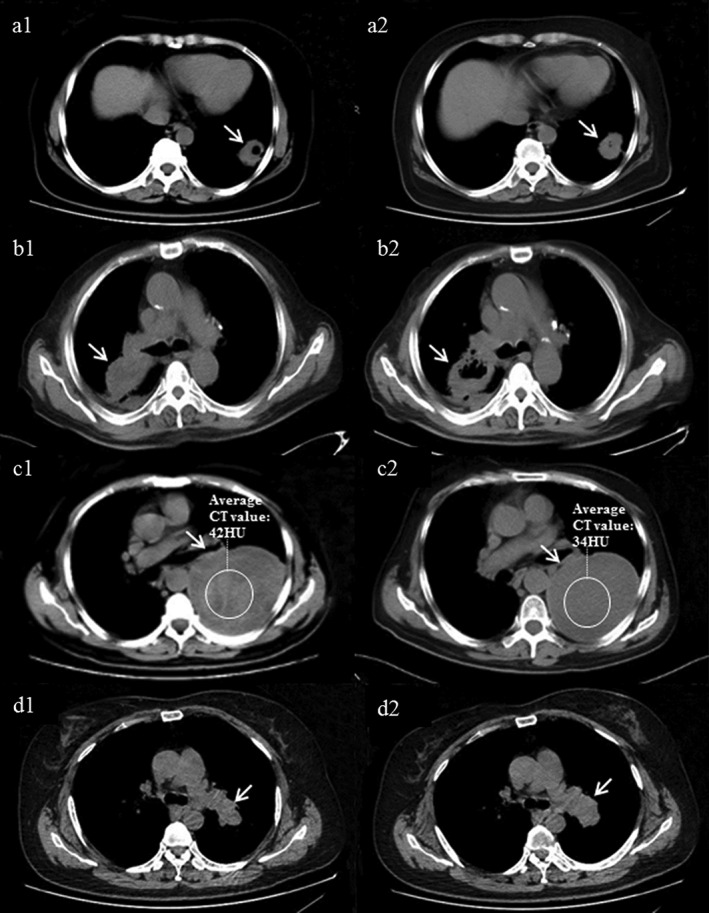
Examples of lung cancer treatment responses not quantified by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. (a) Baseline cavitation inside tumor, (b) cavitation appeared after treatment, (c) decreased tumor density after treatment without a change in tumor size, and (d) short axis of tumor decreased after treatment without change in long diameter of tumor.
Tumor response assessment according to various criteria
Response Evaluation Criteria in Solid Tumors 1.1 were used to evaluate the treatment response in all patients and compared with the modified criteria in each patient subgroup.4 Patients with cavitation at either baseline or during treatment were evaluated using modified (m)RECIST.6 mRECIST measurements were identical to those of RECIST 1.1 except that tumor volume was assessed by subtracting tumor cavitation. The longest diameter of cavitation was subtracted from the longest diameter of the whole lesion in each plane so that only solid components of the tumor were measured (Fig 2). In patients with only a change in density observed on imaging, density criteria were applied to evaluate treatment efficacy. In density criteria, PR is defined as a greater than 15% decrease in tumor density, PD by a greater than 15% increase, and SD as density changes less than 15% in either direction.7 In patients with only a change in the diameter of the short axis of the tumor observed on imaging, response was evaluated using short axis criteria, measuring the single short axis of the tumor compared with RECIST 1.1 criteria.8 All other aspects of tumor response were assessed using regular RECIST 1.1 criteria.
Figure 2.
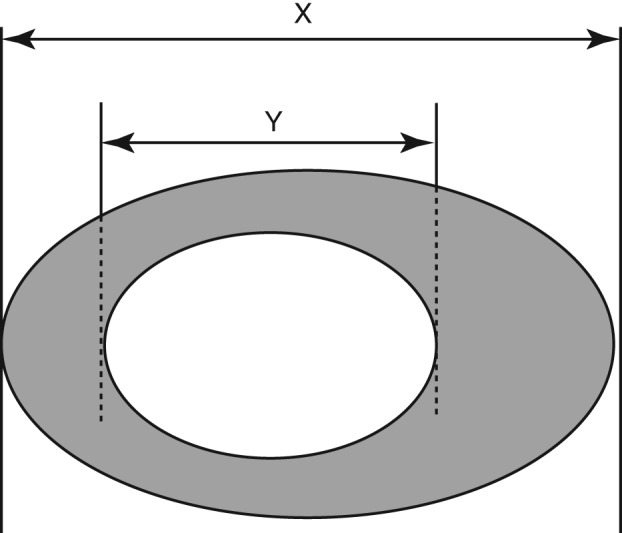
Diagram depicting target lesion measured by Response Evaluation Criteria in Solid Tumors (RECIST) and modified (m)RECIST. X, the longest diameter of total lesion. Y, the longest diameter of cavitation in tumor. RECIST measurement: only X was calculated; mRECIST measurement: the value X‐Y was calculated.
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). A log rank test was used to compare the PFS in patients with different responses from treatment. A P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
The data of 105 patients were identified based on inclusion criteria. Tumor cavitation was observed in 51 patients at baseline. An additional 23 patients developed tumor cavitation during treatment; 16 (69.6%) were treated with more than two cycles of platinum‐based chemotherapy. A change in tumor density was the only radiographic treatment response observed in 22 patients. The only measureable treatment response in nine patients was a decrease along the short axis of the tumor. There were no statistically significant differences in patient demographics, stage, or treatment between these subgroups (Table 1).
Table 1.
Baseline characteristics and treatment for 105 patients with special image signs
| Baseline characteristics and treatment | Baseline cavitation | Post‐therapeutic cavitation | Change in density only | Change in short axis only | |
|---|---|---|---|---|---|
| Number of patients | 51 | 23 | 22 | 9 | |
| Age (years) | Median | 66 | 59 | 62.5 | 61 |
| Range | 41–84 | 43–87 | 49–78 | 52–76 | |
| Gender | Male | 37 (72.5%) | 21 (91.3%) | 10 (45.5%) | 2 (22.2%) |
| Female | 14 (27.5%) | 2 (8.7%) | 12 (54.5%) | 7 (77.8%) | |
| Histology | Squamous‐cell carcinoma | 33 (64.7%) | 13 (56.5%) | 8 (36.4%) | 3 (33.3%) |
| Adenocarcinoma | 13 (25.5%) | 6 (26.1%) | 12 (54.5%) | 4 (44.4%) | |
| Small cell | 3 (5.9%) | 4 (17.4%) | 2 (9.1%) | 2 (22.2%) | |
| Adenosquamous carcinoma | 2 (3.9%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Stage | I‐IIIA | 8 (15.7%) | 2 (8.7%) | 3 (13.6%) | 3 (33.3%) |
| IIIB | 2 (3.9%) | 4 (17.4%) | 1 (4.5%) | 0 (0%) | |
| IV | 41 (80.4%) | 17 (73.9%) | 18 (81.8%) | 6 (66.7%) | |
| Treatment | Platinum‐based chemotherapy | 28 (54.9%) | 16 (69.6%) | 17 (77.3%) | 4 (44.4%) |
| Non‐platinum based chemotherapy | 7 (13.7%) | 1 (4.3%) | 0 (0%) | 0 (0%) | |
| Radiotherapy | 5 (9.8%) | 2 (8.7%) | 2 (9.1%) | 3 (33.3%) | |
| Targeted therapy | 0 (0%) | 0 (0%) | 1 (4.5%) | 0 (0%) | |
| Chemotherapy combined with anti‐angiogenesis therapy | 2 (3.9%) | 1 (4.3%) | 1 (4.5%) | 0 (0%) | |
| Chemotherapy combined with radiotherapy | 2 (3.9%) | 1 (4.3%) | 0 (0%) | 0 (0%) | |
| Chemotherapy combined with targeted therapy | 2 (3.9%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Other treatments | 5 (9.8%) | 2 (8.7%) | 1 (4.5%) | 2 (22.2%) | |
| Period of first evaluation (months) | Median | 1.5 | 1.5 | C1.3 | 1.7 |
| PFS (months) | Median | 7.3 | 5.1 | 8.5 | 10.3 |
| 95% CI | 5.6–9.0 | 1.2–9.0 | 6.5–10.5 | 4.9–15.7 | |
CI, confidence interval; PFS, progression‐free survival.
Response evaluation of patients with cavitation at baseline
We compared patient data using classic RECIST 1.1 versus mRECIST to evaluate treatment effects in tumors with cavitation at baseline. In this subset, OR was 78.4% using RECIST 1.1 and 60.8% by mRECIST. The PFS of patients with different response grades was compared between RECIST 1.1 and mRECIST (Table 2). RECIST 1.1 (P = 0.076) more accurately predicted PFS than mRECIST (P = 0.550), although these findings were not statistically significant (Fig 3a). There was a significant difference in PFS of patients whose response was categorized as SD compared with PD by RECIST 1.1 (P = 0.012), with longer PFS in patients with SD than those with PD. There was also a significant difference between the PFS of patients with PR + SD responses versus those with PD (P = 0.032; Fig 3b), and a marginally significant difference in the PFS of patients with SD versus those with PR + PD (P = 0.053; Fig 3c), with SD patients experiencing longer PFS. There were no differences in PFS between patients with PR and SD or PR and PD. In contrast, when tumor response was evaluated using mRECIST criteria, we did not observe any significant differences in PFS between patients with PD versus SD.
Table 2.
Best response rates using RECIST 1.1 and mRECIST criteria of assessment in patients with baseline and post‐therapeutic cavitation
| Response | RECIST 1.1 | mRECIST | ||||
|---|---|---|---|---|---|---|
| No. of patients (%) | Median PFS (months) | P | No. of patients (%) | Median PFS (months) | P | |
| Patients with baseline cavitation | 0.076† | 0.550† | ||||
| PR | 8 (15.7) | 4.5 | 0.409‡ | 14 (27.5) | 6.4 | 0.258‡ |
| SD | 32 (62.7) | 8.2 | 0.574§ | 17 (33.3) | 8.2 | 0.913§ |
| PD | 11 (21.6) | 4.3 | 0.012¶ | 20 (39.2) | 5.0 | 0.407¶ |
| PR + SD | 40 (78.4) | 7.9 | 0.657†† | 31 (60.8) | 8.0 | 0.549†† |
| SD + PD | 43 (84.3) | 7.3 | 0.032‡‡ | 37 (72.5) | 6.5 | 0.575‡‡ |
| PR + PD | 19 (37.3) | 4.3 | 0.053§§ | 34 (66.7) | 6.2 | 0.276§§ |
| Patients with post‐therapeutic cavitation | 0.477† | 0.004† | ||||
| PR | 2 (8.7) | 4.9 | 0.200‡ | 15 (65.2) | 8.0 | 0.566‡ |
| SD | 12 (52.2) | 6.9 | 0.557§ | 2 (8.7) | 12.9 | 0.003§ |
| PD | 9 (39.1) | 3.6 | 0.507¶ | 6 (26.1) | 3.5 | 0.034¶ |
| PR + SD | 14 (60.9) | 6.6 | 0.298†† | 17 (73.9) | 8.7 | 0.184†† |
| SD + PD | 21 (91.3) | 4.8 | 0.972‡‡ | 8 (34.8) | 3.6 | 0.001‡‡ |
| PR + PD | 11 (47.8) | 3.6 | 0.369§§ | 21 (91.3) | 4.7 | 0.373§§ |
PR versus SD versus PD.
PR versus SD.
PR versus PD.
SD versus PD.
PR versus (SD + PD).
(PR + SD) versus PD.
SD versus (PR + PD).
mRECIST, modified Response Evaluation Criteria in Solid Tumors; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Figure 3.
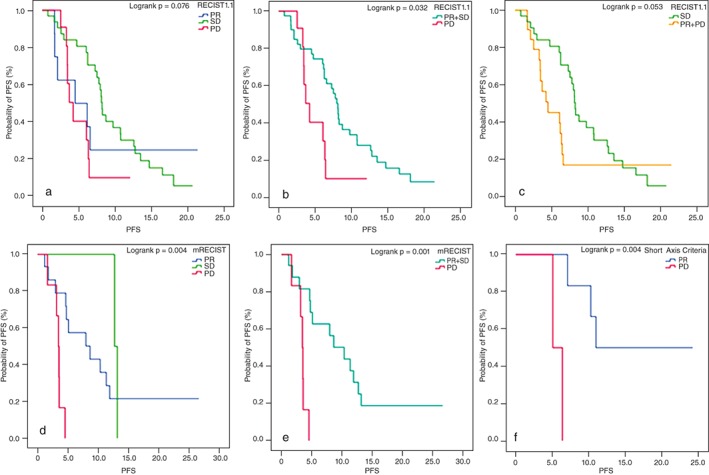
Progression‐free survival (PFS) in different efficacies in using different criteria to evaluate response in patients with special image signs. (a–c) PFS of patients with different responses in using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 in cases with baseline cavitation. (a) Partial response (PR) versus stable disease (SD) versus progressive disease (PD) as 4.5 versus 8.2 versus 4.3 months, P = 0.076; SD versus PD as 8.2 versus 4.3 months, P = 0.012; (b) (PR + SD) versus PD as 7.9 versus 4.3 months, P = 0.032; (c) SD versus (PR + PD) as 8.2 versus 4.3 months, P = 0.053; (d, e) PFS of patients with different responses in using mRECIST in cases with post‐therapeutic cavitation. (d) PR versus SD versus PD as 8.0 versus 12.9 versus 3.5 months, P = 0.004; PR versus PD as 8.0 versus 3.5 months, P = 0.003; SD versus PD as 12.9 versus 3.5 months, P = 0.034. (e) (PR + SD) versus PD as 8.7 versus 3.5 months, P = 0.001; (f) PFS of patients with different responses in using short axis criteria in cases with single change in the short axis of tumor; PR vs. PD as 11.0 versus 5.8 months, P = 0.004.
Response evaluation of patients with post‐therapeutic cavitation
Among patients with post‐therapeutic cavitation, mRECIST predicted differences in PFS between the response groups (P = 0.004; Fig 3d). Specifically, there were significant differences in the PFS of patients with PR versus PD (P = 0.003) and SD versus PD (P = 0.034), with longer PFS in patients with PR or SD than those with PD. There was also a significant difference in the PFS of patients with PR + SD versus PD (P = 0.001; Fig 3e). There was no significant difference in PFS between patients with PR and SD. RECIST 1.1 tumor response evaluations, in comparison, did not yield any significant difference in PFS between the groups.
Response evaluation in patients with a change in tumor density as the only radiographic response
Both RECIST 1.1 and density criteria were used to evaluate the response in 22 patients in which the only radiographic response was a change in tumor density (Table 3). When efficacy was evaluated using RECIST 1.1, all patients had SD, except one with PD who developed distant metastasis. There were no significant differences in PFS (P = 0.235). Evaluating treatment response based on density criteria identified 15 patients with PR and seven patients with PD; however no significant difference in PFS was found (P = 0.419).
Table 3.
Best response rates using RECIST 1.1, density criteria, and short axis criteria of assessment in patients with single changes in density and short axis in tumor
| Response | RECIST1.1 | Density Criteria | Short Axis Criteria | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients (%) | Median PFS (months) | P | No. of patients (%) | Median PFS (months) | P | No. of Patients (%) | Median PFS (months) | P | |
| Patients with single change in density | 0.235† | 0.419‡ | |||||||
| PR | 0 (0) | – | 15 (68.2) | 9.4 | |||||
| SD | 21 (95.5) | 8.5 | 0 (0) | – | |||||
| PD | 1 (4.5) | 15.0 | 7 (31.8) | 7.9 | |||||
| Patients with single change in short axis | 0.004‡ | ||||||||
| PR | 0 (0) | – | 7 (77.8) | 11.0 | |||||
| SD | 9 (100) | 10.3 | 0 (0) | – | |||||
| PD | 0 (0) | – | 2 (22.2) | 5.8 | |||||
SD versus PD.
PR versus PD.
RECIST, Response Evaluation Criteria in Solid Tumors; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease
Response evaluation in patients with a change in the diameter of the short axis of the tumor as the only radiographic response
Patients whose only measureable tumor response was a change in the diameter of the short axis of their tumor were evaluated with RECIST 1.1 and short axis criteria (Table 3). In these patients, RECIST 1.1 detected no differences and determined that all patients had SD. However, using short axis criteria, seven patients were considered to have a PR and two patients were identified with PD. There was significant difference in the PFS of these nine patients with PR and PD (P = 0.004; Fig 3f).
Discussion
Response Evaluation Criteria in Solid Tumors 1.1 is the internationally recognized method of evaluating tumor response in clinical trials, as it predicts survival outcomes in patients with solid tumors; however, its accuracy is limited in certain circumstances.3, 4 Measuring tumor diameter in one dimension is inappropriate in some solid neoplasms, such as peripherally growing tumors like malignant pleural mesothelioma.9, 10 Changes in tumor size do not reflect changes in tumor biology and anatomic changes will occur more slowly than functional changes within a tumor, for example, in gastrointestinal stromal tumors treated with imatinib.11 Furthermore, tumor cavitation and changes in density are signs of a response to treatment. RECIST 1.1 may misjudge efficacy and prognosis. Following treatment, a variety of tumor responses may be visible on imaging; therefore, the currently recognized radiologic scoring system needs to be optimized to most accurately predict outcome and survival when changes other than to the longest axis of the target lesion are observed.
In a study of 53 non‐small cell lung cancer patients treated with an angiogenesis inhibitor, Crabb et al. proposed that the assessment of tumor response might be improved by incorporating tumor cavitation into the calculation of tumor volume. This proposed change in tumor volume assessment has the potential to alter the outcomes of key efficacy parameters in clinical trials.6 Lee et al. suggested measuring only the solid components of a tumor as a modified criterion. Using this modified criterion, the authors demonstrated statistically significant correlations between response rates and prognosis.12 Evidence that mRECIST assessment can be superior to RECIST in evaluating the efficacy of anti‐angiogenesis therapy in patients with tumor cavitation has been confirmed in other studies.13
A change in tumor density after treatment was demonstrated in a study of a gastrointestinal stromal tumor treated with imatinib. Choi et al. proposed using a combination of tumor size and density (HU) to produce modified criteria.7 This criterion was promoted as an early response predictor in patients with metastatic malignant melanoma treated with vemurafenib and to predict long‐term prognosis in patients with advanced hepatocellular carcinoma treated with sorafenib.14, 15
In 1984, Glazer first proposed that lymph nodes could be assessed by measuring the shortest dimension. He argued that the actual spatial location of lymph nodes may interfere with correct measurement of the long and short axis on CT because the longest diameter of lymph nodes can be measured accurately only if it lies parallel to the CT image plane. In surgical pathology specimens, in most cases, cross sectional CT imaging does not align perfectly with the long axis of lymph nodes; therefore, it is difficult to accurately measure the longest diameter.16 As such, the short axis is a more reliable parameter to determine the size of lymph nodes.8 As precise and repeatable measurement of lymph nodes is vital to evaluate treatment response, RECIST 1.1 criteria adopted the short axis measurement for lymph nodes, although it is accepted that long axis measurement is more meaningful in judging tumor response.4 It remains unknown if we can evaluate efficacy by measuring the short axis when target lesions only change in size along the short axis.
Previous studies on the prognostic value of tumor cavitation have mostly focused on cavitation following treatment with angiogenesis inhibitors, rather than cavitation at baseline. A superior radiographic method to evaluate tumors with elements of cavitation prior to treatment has not yet been determined. It is also unknown if isolated changes in tumor density or tumor regression along the short axis of the tumor indicate a meaningful treatment response.
In this study, the RECIST 1.1 response in patients with cavitation at baseline predicted significant differences in PFS. Patients with SD had longer PFS compared with patients with PR, while patients with PD had the shortest PFS. However, there were no significant differences in PFS between patients in different mRECIST efficacy groups. In patients with post‐therapeutic cavitation, PFS was not significantly different between RECIST 1.1 response groups, but there was a significant difference in the PFS of patients with different responses when the evaluation was made by mRECIST criteria. This suggests that RECIST 1.1 is best for evaluating patients with baseline cavitation, while mRECIST may be a more accurate method to predict PFS in patients who develop post‐therapeutic cavitation. Cavitation at baseline most often represents a rapidly expanding tumor that outgrows its blood supply and develops central necrosis, but does not represent tumor response to treatment compared with post‐therapeutic cavitation, which signifies a treatment response.17 Recent studies have generally recommended that mRECIST should be used in lung cancer with cavitation, but only applies to cases that have developed cavitation after treatment with anti‐angiogenic agents. However, our study found that the mRECIST also has significance in predicting PFS in lung cancer patients who develop cavitation after chemotherapy or radiation. Thus, we suggest that more research should be conducted to further test this theory. In our study, most patients who developed cavitation after treatment received strong platinum‐based doublet chemotherapy, from which we would expect a strong treatment response. Therefore, the timing of central necrosis is an important factor in determining if RECIST can be used to predict PFS.
In patients whose only response to treatment was a change in tumor density, there was no significant difference in PFS between evaluations made by RECIST 1.1 or density criteria. In the authors’ opinion, changes in tumor density fall along the same spectrum as the process of tumor cavitation, but demonstrate a less significant change and are not a strong enough indicator of tumor response to affect PFS. RECIST 1.1 detected SD in all patients whose 8tumors only changed in size along the short axis. However, within this same group of patients, we identified those with PR and PD using short axis criteria. There was a significant difference in the PFS of patients with PR and PD, with longer PFS seen in patients with PR. In these patients, using short axis criteria to predict PFS is more accurate than RECIST 1.1. James et al. studied the relationship between a tumor's greatest diameter, the product of dual diameter measurements, and the actual number of tumor cells and discovered that the relationship between greatest diameter and the number of tumor cells was the strongest.18 Therefore, if both long and short diameters change after treatment, the long diameter would be the best measure of tumor response; however, in situations where only the short axis changes, the short axis alone may more accurately reflect tumor response and PFS.
In conclusion, RECIST 1.1 is the best predictor of PFS in patients with baseline cavitation, mRECIST should be applied to account for tumor volume in patients with post‐therapeutic cavitation, and short axis criteria is the best predictor of PFS when the only response to treatment is a change in the short axis of the tumor. Isolated changes in tumor density did not effectively predict PFS. Given the results of this study, using only RECIST 1.1 to evaluate treatment response may underestimate treatment response. More research is needed to confirm these findings.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was supported by the Tianjin Science and Technology Committee (12ZCDSY15600), the National Science and Technology Major Project (2013ZX), the National Science and Technology Project (81372517), and the CSCO Project (Y‐s2014‐001).
References
- 1. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14. [DOI] [PubMed] [Google Scholar]
- 2. Tonkin K, Tritchler D, Tannock I. Criteria of tumor response used in clinical trials of chemotherapy. J Clin Oncol 1985; 3: 870–5. [DOI] [PubMed] [Google Scholar]
- 3. Therasse P, Arbuck SG, Eisenhauer EA et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 4. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 5. Pentheroudakis G, Kostadima L, Fountzilas G et al. Cavitating squamous cell lung carcinoma‐distinct entity or not? Analysis of radiologic, histologic, and clinical features. Lung Cancer 2004; 45: 349–55. [DOI] [PubMed] [Google Scholar]
- 6. Crabb SJ, Patsios D, Sauerbrei E et al. Tumor cavitation: Impact on objective response evaluation in trials of angiogenesis inhibitors in non‐small‐cell lung cancer. J Clin Oncol 2009; 27: 404–10. [DOI] [PubMed] [Google Scholar]
- 7. Choi H. Critical issues in response evaluation on computed tomography: Lessons from the gastrointestinal stromal tumor model. Curr Oncol Rep 2005; 7: 307–11. [DOI] [PubMed] [Google Scholar]
- 8. Schwartz LH, Bogaerts J, Ford R et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer 2009; 45: 261–7. [DOI] [PubMed] [Google Scholar]
- 9. Tropine A, Dellani PD, Glaser M et al. Differentiation of fibroblastic meningiomas from other benign subtypes using diffusion tensor imaging. J Magn Reson Imaging 2007; 25: 703–8. [DOI] [PubMed] [Google Scholar]
- 10. van Klaveren RJ, Aerts JG, de Bruin H, Giaccone G, Manegold C, van Meerbeeck JP. Inadequacy of the RECIST criteria for response evaluation in patients with malignant pleural mesothelioma. Lung Cancer 2004; 43: 63–9. [DOI] [PubMed] [Google Scholar]
- 11. Choi H, Charnsangavej C, de Castro FS et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: A quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 2004; 183: 1619–28. [DOI] [PubMed] [Google Scholar]
- 12. Lee HY, Lee KS, Ahn MJ et al. New CT response criteria in non‐small cell lung cancer: Proposal and application in EGFR tyrosine kinase inhibitor therapy. Lung Cancer 2011; 73: 63–9. [DOI] [PubMed] [Google Scholar]
- 13. Huang C, Wang X, Wang J et al. Incidence and clinical implication of tumor cavitation in patients with advanced non‐small cell lung cancer induced by Endostar, an angiogenesis inhibitor. Thorac Cancer 2014; 5: 438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uhrig M, Hassel JC, Schlemmer HP, Ganten MK. Therapy response assessment in metastatic melanoma patients treated with a BRAF inhibitor: Adapted Choi criteria can reflect early therapy response better than does RECIST. Acad Radiol 2013; 20: 423–9. [DOI] [PubMed] [Google Scholar]
- 15. Ronot M, Bouattour M, Wassermann J et al. Alternative response criteria (Choi, European Association for the Study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist 2014; 19: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glazer GM, Orringer MB, Gross BH, Quint LE. The mediastinum in non‐small cell lung cancer: CT‐surgical correlation. AJR Am J Roentgenol 1984; 142: 1101–5. [DOI] [PubMed] [Google Scholar]
- 17. Gasinska A, Kolodziejski L, Niemiec J, Dyczek S. Clinical significance of biological differences between cavitated and solid form of squamous cell lung cancer. Lung Cancer 2005; 49: 171–9. [DOI] [PubMed] [Google Scholar]
- 18. James K, Eisenhauer E, Christian M et al. Measuring response in solid tumors: Unidimensional versus bidimensional measurement. J Natl Cancer Inst 1999; 91: 523–8. [DOI] [PubMed] [Google Scholar]


