Abstract
Background
Video‐assisted thoracoscopic surgery (VATS) lobectomy has emerged as a safe and effective technique for treating early‐stage lung cancer. Novel three‐dimensional, high‐definition (3D HD) imaging has removed this technical obstacle and is increasingly used in laparoscopic surgery. We compared our initial experience of 3D HD VATS with standard two‐dimensional (2D) HD VATS to identify the advantages and disadvantages of 3D HD visualization in VATS.
Methods
The data of consecutive patients diagnosed with lung cancer who underwent 2D or 3D thoracoscopic lobectomy or bilobectomy at the Guangdong Lung Cancer Institute from July 2013 to October 2014 were retrospectively analyzed. Operation duration, estimated blood loss, length of postoperative stay, major complications, and mortality were recorded for each patient.
Results
In total, 359 patients were enrolled in the study. Lobectomy was performed in 339 patients and bilobectomy in 20; the 3D HD system was used for 178 of the 359 patients, and the 2D HD system for 181. Tumor size, distribution of the resected lobes, and the demographic characteristics of the patients were matched between the two groups. The mean operative time for 3D VATS was 163 minutes (range 60–330), whereas 2D VATS required 184 minutes (range 75–360; P < 0.001). The volume of blood loss was 109 and 144 mL in the 3D and 2D VATS groups, respectively (P = 0.064).
Conclusions
The new‐generation 3D HD imaging system is feasible and safe for thoracic lobectomy. The 3D system required a shorter operative duration.
Keywords: Lobectomy, three‐dimensional, video‐assisted thoracic surgery
Introduction
Video‐assisted thoracoscopic surgery (VATS) lobectomy has emerged as a safe and effective technique for treating early‐stage lung cancer.1, 2 However, conventional VATS is a complex procedure limited by the technical challenges associated with two‐dimensional (2D) monitors and cameras and, thus, cannot provide stereoscopic information, which is considered to be important for precise manual task execution.3 Improvements in video imaging have led to the development of three‐dimensional (3D) visualization systems. However, early prototypes entailed several technical flaws, and 3D imaging, therefore, came to be regarded as a technique that compromises image quality. Previous studies comparing 2D and 3D visualization have reported contradictory results.4, 5, 6 With advances in 3D technology, the latest 3D high‐definition (HD) imaging systems now provide significantly improved picture quality and resolution, similar to those of 2D displays. Several studies have shown the advantages of 3D HD over conventional 2D HD in terms of improving surgeon performance in standardized basic skills tasks.7, 8, 9 More recent studies have suggested a possible advantage of these new‐generation 3D HD systems during laparoscopy.10, 11, 12
As an emerging imaging system and technique, 3D thoracoscopic surgery has also been of interest to thoracic surgeons in clinical practice. However, few studies have assessed the feasibility, safety, and surgical outcomes of this new technique. The aim of this study was to compare the results of thoracoscopic lobectomy using 2D HD and 3D HD visualization systems.
Methods
Patient selection
We performed a retrospective analysis of patient medical records and operative data of consecutive patients with lung cancer who underwent thoracoscopic lobectomy or bilobectomy from July 2013 to October 2014 at the Guangdong Lung Cancer Institute. We excluded patients with large (> 6 cm) tumors and those who had undergone pneumonectomy and sleeve lobotomy. Operation duration, estimated blood loss, length of postoperative stay, all major complications, and mortality were recorded for each patient. Informed consent was obtained from all patients.
Surgical procedures
Four surgeons with varying levels of experience in our department contributed cases to this study and had equal opportunity to use the 3D system. All procedures were performed using the Karl Storz 30° 3D TIPCAM System or the Karl Storz 30° 2D HD System (Karl Storz, GmbH & Co. KG, Tuttlingen, Germany). No selection criteria were employed for use of the 3D platform; either the 2D or 3D system was used for all procedures, depending on availability, during simultaneous operations.
Most suspicious N2 metastases revealed in positron emission tomography‐computed tomography (PET/CT) or CT scan were routinely examined by endobronchial ultrasound (EBUS), while mediastinoscopy was usually performed in cases in which EBUS could not procure sufficient tissue. All patients underwent general anesthesia using a double‐lumen endotracheal tube for lung isolation. VATS lobectomy was performed with the patient in the lateral decubitus position, usually using a double port, non‐rib‐spreading technique. A 3–5 cm access incision was placed at the fourth or fifth intercostal space at the anterior axillary line. A 1–2 cm camera port was placed at the seventh or eighth intercostal space at the anterior axillary line; this port was also used for stapler insertion if necessary (Fig 1). Individual vascular and bronchial structures were divided using an endoscopic stapling device, and standard thoracoscopic instruments were used. Lymphadenectomy was performed immediately after extracting the lobe in a bag from the chest cavity if a diagnosis of malignancy had been made or suspicion of malignancy was present. All patients underwent systematic removal of lymph nodes from at least three stations of N2 nodes, according to the International Association for the Study of Lung Cancer classification. The ethics committee and the institutional review board of the Guangdong Lung Cancer Institute approved the study.
Figure 1.
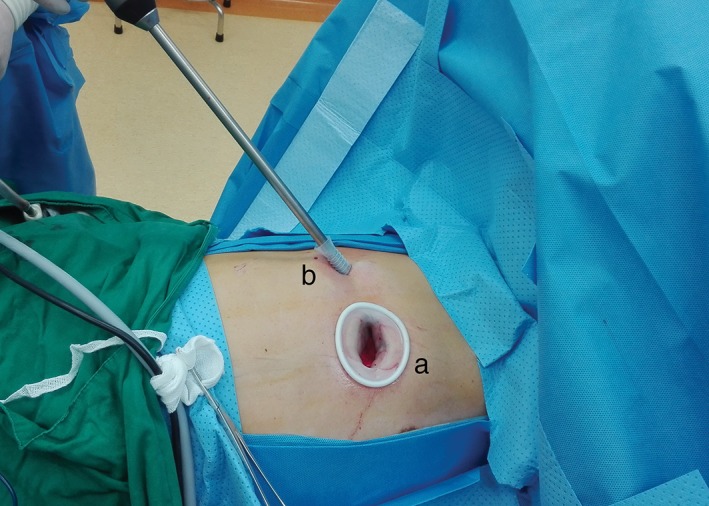
Incisions for right upper thoracoscopic lobectomy. (a) procedure incision (with a lap‐protector) in the fourth intercostal space in the anterior axillary line. (b) A 1 cm trocar for the endoscopic instrument in the fifth intercostal space in the posterior‐axillary line.
Statistical analysis
Statistical analysis was performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). Wilcoxon and Mann–Whitney tests were used to analyze continuous variables, such as operation duration, length of hospital stay, and tube duration. Differences in the distributions of gender, tumor location, histology, tumor stage, and surgeons in the 2D and 3D groups were evaluated using the chi‐squared test. We performed an independent sample t‐test to compare age, blood loss, number of lymph nodes, and tumor diameter between the groups. A P value of < 0.05 was considered to indicate statistical significance.
Results
Video‐assisted thoracoscopic surgery lobectomy or bilobectomy was attempted in 359 patients for non‐small‐cell lung cancer (NSCLC) from July 2013 to October 2014. Surgery was converted to open thoracotomy in 11 patients. The median chest tube duration for the entire group was 3.6 days (range 1–43), and the median length of stay was 7.1 days (range 2–56). In total, 178 patients underwent 3D VATS and 181 underwent standard 2D VATS. The two groups were matched for age, tumor size, and tumor location (Table 1).
Table 1.
Patient characteristics and clinical outcomes
| Variable | 3D | 2D | P |
|---|---|---|---|
| Gender (male/female) | 109/69 | 106/76 | 0.563 |
| Age (mean, years) | 61.1 | 60.6 | 0.687 |
| Lobes | |||
| Right upper | 58 | 60 | — |
| Right middle | 9 | 10 | — |
| Right lower | 29 | 21 | — |
| Right upper and middle | 3 | 3 | — |
| Right middle and lower | 5 | 9 | — |
| Left upper | 42 | 51 | — |
| Left lower | 32 | 27 | 0.717 |
| Stage of NSCLC | |||
| I | 99 | 106 | — |
| II | 40 | 34 | — |
| IIIA | 31 | 33 | — |
| IIIB | 1 | 1 | — |
| IV | 7 | 7 | 0.943 |
| Tumor size (cm) | 2.82 | 2.78 | 0.951 |
| Operation time (minutes) | 163 | 184 | < 0.001 |
| Lymph node number | 21.3 | 19.5 | 0.064 |
| Estimated blood loss (mL) | 109 | 144 | 0.064 |
| Tube duration (days) | 3.6 | 3.5 | 0.618 |
| Length of stay (days) | 6.9 | 7.1 | 0.399 |
2D, two dimensional; 3D, three‐dimensional; NSCLC, non‐small cell lung cancer.
The mean age of the patients in the 3D group was 61.1 years (range 29–83), compared with 60.6 years (range 35–81) in the 2D group. The mean operative time using the 3D system was 163 minutes (range 60–330gtfr), while that of the 2D system was 184 minutes (range 75–360; P < 0.001). The mean number of harvested pulmonary and mediastinal lymph nodes in the 3D group was 21.3 compared with 19.5 in the 2D (P = 0.064). N2 lymph node metastasis occurred in 31 cases in the 2D group and 33 cases in the 3D. There were seven cases of stage IV; five cases had unexpected pleural metastasis detected during surgery, while two were N0 patients with solitary brain metastases. The estimated volume of blood loss was 109 mL in the 3D group and 144 mL in the 2D (P = 0.064). No differences were found in tube duration or length of stay between the groups (Table 1). Five emergent conversions to thoracotomy as a result of intraoperative bleeding occurred in the 3D group (three for pulmonary arterial bleeding, two for venous bleeding), and six occurred in the 2D group (four for pulmonary arterial bleeding, two for venous bleeding); however, no significant difference existed between the groups (P = 0.781). Four patients in the 3D group and eight in the 2D required an intraoperative or postoperative blood transfusion.
About 16% of patients (29/178) in the 3D group had major complications that required treatment, versus 15% (24/181) in the 2D group. Thirteen of these patients had more than one complication (Table 2). The most common complication was prolonged tube duration (air leakage or serous drainage) in 8.9% of patients in the 3D group and in 9.9% of patients in the 2D group. Two in‐hospital deaths occurred in the 2D group: one as a result of acute respiratory distress syndrome of unknown cause and the other because of respiratory failure; the latter patient had marginal pulmonary function, was sent to the intensive care unit after surgery for low oxygen saturation, and died of respiratory failure a week later. A patient in the 3D group died of pneumonia two weeks after discharge.
Table 2.
Major complications after anatomic resection by video‐assisted thoracic surgery
| Complication | 3D | 2D | P |
|---|---|---|---|
| None | 149/178 | 157/181 | — |
| Complication | — | ||
| Air leak (≥ 6 days) | 8 | 8 | — |
| Serous drainage (≥ 6 days) | 8 | 10 | — |
| Pneumonia | 4 | 5 | — |
| Atrial fibrillation | 7 | 4 | — |
| Chylothorax | 2 | 2 | — |
| Pulmonary embolism | 0 | 1 | — |
| Postoperative massive bleeding | 5 | 3 | — |
| Heart failure | 1 | 0 | — |
| Stroke | 1 | 1 | 0.459 |
2D, two dimensional; 3D, three‐dimensional.
Discussion
As an emerging imaging system and technique, 3D thoracoscopic surgery is of interest to thoracic surgeons in clinical practice. However, only limited data exist on clinical experience with the 3D imaging system in video‐assisted lobectomy. A recent study of 18 patients undergoing 3D thoracoscopic surgery reported that the 3D HD system significantly reduces surgical time from 176 to 145 minutes; however, the tube duration, number of lymph node stations, and upstaging were similar between the groups.13 We observed similar results in the present case series; lobectomies using the 3D system required less time than those using the 2D imaging system (163 vs. 184 minutes, respectively; P < 0.001). The volume of blood loss was also smaller in the 3D group, but there was no statistically significant difference (109 vs. 144 mL, respectively; P = 0.064). Although the blood loss volume was not significantly different between the two groups, we believe that this difference could become statistically significant with a larger population size.
Outcomes using the 3D HD system in other laparoscopic surgeries have been reported. Several laboratory studies compared 3D with 2D laparoscopic visualization with contradictory results. Cicione et al. reported that new‐generation 3D imaging facilitated the surgical performance of urologic surgeons without a laparoscopic background; however, those with an established laparoscopic background did not recognize an advantage in using the 3D system.8 Storz et al. showed that 3D imaging allows the surgeon to work faster with greater accuracy, and similar results were reported in a student group.7 Lusch et al. evaluated students, residents, and expert surgeons and found that the 3D laparoscopic camera equipment resulted in a significant improvement in depth perception, spatial location, and precision of surgical performance; the authors found that even expert laparoscopic surgeons may benefit from 3D imaging.14 Wilhelm et al. reported that for surgeons with varying experience levels in laparoscopy, novel 3D displays improve laparoscopic interventions as a result of faster performance and higher precision.3
In recent years, several studies have compared new‐generation 3D HD with 2D HD visualization in laparoscopic surgery. One study reported that 3D visualization reduced operation duration from 284 minutes using the 2D HD system to 225 minutes in laparoscopic liver resection, with no significant difference in blood loss.12 Aykan et al. reported a shorter operating duration and reduced blood loss in the 3D HD group in a cohort of patients who underwent laparoscopic radical prostatectomy.11 Bagan et al. compared 3D HD thoracoscopic surgery with 2D HD lobectomy and reported that the 3D HD system significantly reduced surgical duration.13 Our results show that the 3D system has the advantage of a shorter operating duration for thoracic surgery, similar to that of 3D imaging for laparoscopy and thoracoscopy. Based on our own and the experiences of other authors, we conclude that the better visualization of the 3D system reaps benefits, such as better depth perception that cannot be achieved with traditional 2D systems without an increase in visual strain. This benefit could reduce the incidence of intraoperative complications and facilitate the identification of small vessels.12
In our cohort, the incidence of major complications and emergent conversion rates were similar between the 3D and 2D VATS groups. About 16% of patients (29/178) in the 3D group had major complications that required treatment versus 15% (24/181) in the 2D. Emergent conversion rates were 2.8% in the 3D group and 3.3% in the 2D. In a large series performed by experienced thoracoscopic surgeons, emergent conversion rates were as low as 1.6–2.5.15, 16
Systematic lymph node dissection is an essential part of lung resection for lung cancer because inadequate lymph node dissection results in inappropriate staging. The mean number of harvested pulmonary and mediastinal lymph nodes were 21.3 in the 3D group and 19.5 in the 2D (P = 0.064), similar to previous reports.17, 18 Mediastinal staging is important for determining the treatment method for NSCLC patients. Sayar et al. reported that extended cervical mediastinoscopy has an adequate negative predictive value and accuracy in determining metastasis to the aortopulmonary window lymph node.19 In our study, eight cases were treated with VATS lobectomy followed by mediastinoscopy, five cases in the 3D group and three in the 2D. We consider 3D thoracoscopic surgery to be feasible and safe for thoracic lobectomy of lung tumors, but no data are yet available on the long‐term survival of these patients.
Three‐dimensional technology is one of the major benefits of robotically assisted surgery. The da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) is a telerobotic system with a true 3D endoscope that provides a high‐resolution binocular view of the surgical field.20 The da Vinci Surgical System has been applied to an increasing number of thoracoscopic lobectomies and has been considered feasible and safe; however, no published data comparing robot thoracoscopic lobectomy with 3D‐assisted lobectomy exists.21, 22, 23, 24 The da Vinci Surgical System allows for the improved dexterity and filtration of surgeon tremors, with the ergonomic advantage of sitting comfortably at a console, allowing the surgeon to perform for a longer period of time with less fatigue.25 However, the drawbacks of the da Vinci system are the high cost of implementation and maintenance, lack of tactile feedback to the surgeon, the bulk of the robotic arms, and the presence of long, thick cords.25, 26 The use of robotic technology is currently far from universal. The 3D HD system may be an effective intermediate step between the standard 2D system and robot‐assisted surgery, combining the low cost of the 2D imaging system with the 3D technology of the da Vinci Surgical System, especially for centers in developing countries.
The limitations of our study include its retrospective design, which made it difficult to control for selection bias during treatment allocation between the 3D and 2D systems. The present study provides a realistic perspective of daily clinical practice. Whether the extension of 3D visualization to surgeons without a thoracoscopic background will shorten the learning curve of VATS remains to be determined.
In conclusion, our results show benefits of using 3D HD visualization over the 2D system. The 3D system improved surgical precision and was tolerated well by the surgeons without tool change overtime and the need for additional training. The evolution of VATS lobectomy is partly driven by technological advancement and the refinement of surgical instruments. In view of the growing popularity of robot‐assisted VATS, evaluations of 3D HD with robotic systems are needed.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81302033), the Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (Grant No. 2012A061400006), the Special Fund for Research in the Public Interest from National Health and Family Planning Commission of PRC (Grant No. 201402031), and the Research Fund from Guangzhou Science and Technology Bureau (Grant No. 2011Y2‐00014).
References
- 1. Dziedzic D, Orlowski T. The role of VATS in lung cancer surgery: Current status and prospects for development. Minim Invasive Surg 2015; 2015: 938430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee PC, Nasar A, Port JL et al. Long‐term survival after lobectomy for non‐small cell lung cancer by video‐assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013; 96: 951–60. [DOI] [PubMed] [Google Scholar]
- 3. Wilhelm D, Reiser S, Kohn N et al. Comparative evaluation of HD 2D/3D laparoscopic monitors and benchmarking to a theoretically ideal 3D pseudodisplay: Even well‐experienced laparoscopists perform better with 3D. Surg Endosc 2014; 28: 2387–97. [DOI] [PubMed] [Google Scholar]
- 4. Chan AC, Chung SC, Yim AP, Lau JY, Ng EK, Li AK. Comparison of two‐dimensional vs three‐dimensional camera systems in laparoscopic surgery. Surg Endosc 1997; 11: 438–40. [DOI] [PubMed] [Google Scholar]
- 5. McDougall EM, Soble JJ, Wolf JS Jr, Nakada SY, Elashry OM, Clayman RV. Comparison of three‐dimensional and two‐dimensional laparoscopic video systems. J Endourol 1996; 10: 371–4. [DOI] [PubMed] [Google Scholar]
- 6. Dion YM, Gaillard F. Visual integration of data and basic motor skills under laparoscopy. Influence of 2‐D and 3‐D video‐camera systems. Surg Endosc 1997; 11: 995–1000. [DOI] [PubMed] [Google Scholar]
- 7. Storz P, Buess GF, Kunert W, Kirschniak A. 3D HD versus 2D HD: Surgical task efficiency in standardised phantom tasks. Surg Endosc 2012; 26: 1454–60. [DOI] [PubMed] [Google Scholar]
- 8. Cicione A, Autorino R, Breda A et al. Three‐dimensional vs standard laparoscopy: Comparative assessment using a validated program for laparoscopic urologic skills. Urology 2013; 82: 1444–50. [DOI] [PubMed] [Google Scholar]
- 9. Wagner OJ, Hagen M, Kurmann A, Horgan S, Candinas D, Vorburger SA. Three‐dimensional vision enhances task performance independently of the surgical method. Surg Endosc 2012; 26: 2961–8. [DOI] [PubMed] [Google Scholar]
- 10. Honeck P, Wendt‐Nordahl G, Rassweiler J, Knoll T. Three‐dimensional laparoscopic imaging improves surgical performance on standardized ex‐vivo laparoscopic tasks. J Endourol 2012; 26: 1085–8. [DOI] [PubMed] [Google Scholar]
- 11. Aykan S, Singhal P, Nguyen DP et al. Perioperative, pathologic, and early continence outcomes comparing three‐dimensional and two‐dimensional display systems for laparoscopic radical prostatectomy–a retrospective, single‐surgeon study. J Endourol 2014; 28: 539–43. [DOI] [PubMed] [Google Scholar]
- 12. Velayutham V, Fuks D, Nomi T, Kawaguchi Y, Gayet B. 3D visualization reduces operating time when compared to high‐definition 2D in laparoscopic liver resection: A case‐matched study. Surg Endosc 2016; 30: 147–53. [DOI] [PubMed] [Google Scholar]
- 13. Bagan P, De Dominicis F, Hernigou J et al. Complete thoracoscopic lobectomy for cancer: Comparative study of three‐dimensional high‐definition with two‐dimensional high‐definition video systems. Interact Cardiovasc Thorac Surg 2015; 20: 820–3. [DOI] [PubMed] [Google Scholar]
- 14. Lusch A, Bucur PL, Menhadji AD et al. Evaluation of the impact of three‐dimensional vision on laparoscopic performance. J Endourol 2014; 28: 261–6. [DOI] [PubMed] [Google Scholar]
- 15. McKenna RJ Jr, Houck W, Fuller CB. Video‐assisted thoracic surgery lobectomy: Experience with 1,100 cases. Ann Thorac Surg 2006; 81: 421–5. [DOI] [PubMed] [Google Scholar]
- 16. Onaitis MW, Petersen RP, Balderson SS et al. Thoracoscopic lobectomy is a safe and versatile procedure: Experience with 500 consecutive patients. Ann Surg 2006; 244: 420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riquet M, Legras A, Mordant P et al. Number of mediastinal lymph nodes in non‐small cell lung cancer: A Gaussian curve, not a prognostic factor. Ann Thorac Surg 2014; 98: 224–31. [DOI] [PubMed] [Google Scholar]
- 18. Scott WJ, Allen MS, Darling G et al. Video‐assisted thoracic surgery versus open lobectomy for lung cancer: A secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010; 139: 976–81. [DOI] [PubMed] [Google Scholar]
- 19. Sayar A, Çitak N, Büyükkale S et al. Impact of extended cervical mediastinoscopy in staging of left lung carcinoma. Thorac Cancer 2013; 4: 361–8. [DOI] [PubMed] [Google Scholar]
- 20. Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Review of early clinical results. Surg Endosc 2002; 16: 1389–402. [DOI] [PubMed] [Google Scholar]
- 21. Louie BE, Farivar AS, Aye RW, Vallières E. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video‐assisted thoracoscopic surgery cases. Ann Thorac Surg 2012; 93: 1598–604. [DOI] [PubMed] [Google Scholar]
- 22. Kent M, Wang T, Whyte R, Curran T, Flores R, Gangadharan S. Open, video‐assisted thoracic surgery, and robotic lobectomy: Review of a national database. Ann Thorac Surg 2014; 97: 236–42. [DOI] [PubMed] [Google Scholar]
- 23. Gharagozloo F, Margolis M, Tempesta B, Strother E, Najam F. Robot‐assisted lobectomy for early‐stage lung cancer: Report of 100 consecutive cases. Ann Thorac Surg 2009; 88: 380–4. [DOI] [PubMed] [Google Scholar]
- 24. Park BJ, Flores RM, Rusch VW. Robotic assistance for video‐assisted thoracic surgical lobectomy: Technique and initial results. J Thorac Cardiovasc Surg 2006; 131: 54–9. [DOI] [PubMed] [Google Scholar]
- 25. Nezhat C, Lewis M, Kotikela S et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril 2010; 94: 2758–60. [DOI] [PubMed] [Google Scholar]
- 26. Bodner JC, Zitt M, Ott H et al. Robotic‐assisted thoracoscopic surgery (RATS) for benign and malignant esophageal tumors. Ann Thorac Surg 2005; 80: 1202–6. [DOI] [PubMed] [Google Scholar]


