Abstract
Oldenlandia diffusa has been empirically used as a therapeutic adjunct for the treatment of respiratory infections. To establish the basic evidence of its clinical usefulness, antimicrobial and biofilm inhibitory activities of an O. diffusa extract were examined against clinical isolates of Haemophilus influenzae, a major causative pathogen of respiratory and sensory organ infections. No significant growth inhibitory activity was observed during incubation for more than 6 h after the extract addition into a culture of H. influenzae. On the other hand, biofilm formation by H. influenzae, evaluated by a crystal violet method, was significantly and dose-dependently inhibited by the O. diffusa extract. Furthermore, the mRNA level of the biofilm-associated gene luxS of H. influenzae significantly decreased soon after the extract addition, and the suppressive effect continued for at least 2 h. At 2 h after the addition of the O. diffusa extract, the autoinducer in the culture supernatant was also significantly reduced by the O. diffusa extract in a dose-dependent manner. These results revealed that O. diffusa extract shows inhibitory activity against luxS-dependent biofilm formation but has no antimicrobial activity against planktonic cells of H. influenzae. Thus, O. diffusa extract might be useful as an adjunctive therapy for the treatment of respiratory infections caused by H. influenzae.
Introduction
In recent years, antimicrobial-resistant pathogens have become a concern for respiratory and sensory organ infections [1]. However, development of novel agents has decreased, and physicians have to deal with existing agents. Alternative complementary medicine, including traditional Chinese medicine, has attracted attention as drug therapy that does not rely on antimicrobial agents. Several traditional Chinese medicines have been empirically used for the treatment of infectious diseases, while the basic scientific evidence of their usefulness is lacking. Among traditional Chinese medicines, Oldenlandia diffusa (Odi) has been used for inflammatory and infectious diseases, such as pneumonia, appendicitis, and urinary tract infections, as an herb that clears heat and relieves toxicity [2]. Odi has been empirically administered (12–24 g/day orally) for respiratory infections, along with anti-infective medicines. Moreover, Odi has been reported to also show anticancer and immunomodulating activities [3, 4].
Haemophilus influenzae and Streptococcus pneumoniae are the major causative bacterial agents of respiratory and sensory organ infections. H. influenzae type b and S. pneumoniae infections have decreased due to routine vaccination (Hib and pneumococcal conjugate vaccines), whereas the percentage of nontypeable H. influenzae infections is on the rise [5, 6]. Among respiratory infections caused by H. influenzae, bronchitis is known to be prone to severe complications [7]. Otitis media and sinusitis, which are sensory organ infections caused by H. influenzae, can become chronic and intractable in children [7]. These intractable infections may be associated with antimicrobial resistance and biofilm formation by the bacteria [8]. Thus, inhibition of biofilm formation has been suggested to be important for preventing chronic and intractable infections. Biofilm formation by H. influenzae is known to be associated with quorum sensing (QS) via the LuxS autoinducer system and/or QS two-component control system QseBC [9, 10]. Therefore, inhibition of these systems can result in the inhibition of biofilm formation.
In this study, to establish the basic evidence of the usefulness of Odi extract (OdiE) against infections, we analyzed growth and biofilm inhibitory effects of OdiE on clinical isolates of H. influenzae.
Materials and Methods
Bacterial strains, culture conditions, and chemicals
A total of 20 H. influenzae strains were randomly selected among clinical isolates obtained at the Tokyo Medical University Hachioji Medial Center between 2011 and 2013 [11]. H. influenzae ATCC 49247 purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) was used as a reference strain. These strains were cultured at 35°C under ambient air on chocolate agar or in brain heart infusion broth supplemented with 10 μg/mL NAD and 10 μg/mL hemin (sBHI broth).
For the autoinducer (AI)-2 bioassay, we used Vibrio harveyi ATCC BAA-211 purchased from ATCC. This strain was cultured at 30°C in AI bioassay (AB) medium [10], [12].
Haku ka ja zetu sou (Iskra Industry Co., Ltd., Tokyo, Japan), which is sold as a healthy food in Japan, was used as the OdiE.
Measurement of growth inhibitory activity
Overnight cultures of the test strain were diluted with sBHI broth (1:100) in the presence or absence of OdiE and incubated at 35°C with shaking. The cultures were sampled at 0, 1, 2, 4, 6, 8, 12, and 24 h of incubation, and the samples were diluted with saline. The dilutions were spread on chocolate agar plates and cultured at 35°C overnight. Thereafter, the number of grown colonies was counted to calculate the number of bacterial CFU/mL in an undiluted broth culture. All experiments were performed at least three times on separate days.
Biofilm formation assay
Biofilm formation was evaluated by the crystal violet assay as reported previously [13]. Briefly, H. influenzae was cultured overnight in sBHI broth and diluted 1:100 in fresh sBHI. This suspension (100 μL) was transferred into a 96-well microtiter plate (Iwaki, Tokyo, Japan) and cultured for 24 h in the presence or absence of OdiE (2.5, 5, 10, and 20 mg/mL) at 35°C. Then, each well was washed three times with phosphate-buffered saline (PBS) to remove floating bacterial cells. The biofilms were stained for 20 min with 0.1% crystal violet and washed three times with PBS. The remaining crystal violet was dissolved with 200 μL of 95% ethanol, and the absorbance (630 nm) was measured in each well. The test was carried out using five wells per each assay and at least three times on independent occasions.
To evaluate its degradation activity on mature biofilm, OdiE was added to the biofilm formed as described above, and the plate was incubated for 3, 6, and 24 h at 35°C. The remaining biofilm was measured by staining with crystal violet as described above.
Semi-quantitative reverse transcription–PCR
To compare mRNA levels of biofilm-associated genes (luxS and qseC), we performed semi-quantitative reverse transcription (RT)–PCR. Cultures were sampled at 0, 1, 2, 4, and 6 h after the addition of OdiE (20 mg/mL). Then, total RNA was extracted from the culture using a High Pure RNA isolation kit (Roche Diagnostics, Tokyo, Japan). PCR was performed as described previously [14]. GyrB-F (GGAAAATCCTGCAGATGC), GyrB-R (AAGCAACGTACGGATGTG), luxS-F (AAAAATGAACGCACCTGCAG), luxS-R (GTACACCTAAAACATCTTGC), qseC-F (TTAAATCCGTGTAATTCCGC), and qseC-R (TGAGCGTTATTTTGTGGCAG) were used as the primers. The resulting PCR products were separated by electrophoresis, and densitometric analysis was performed using the ImageJ software (http://imagej.nih.gov/ij/). The transcriptional level of gyrB was used as an internal control.
Autoinducer bioassay
H. influenzae was cultured in 10 mL of sBHI overnight. Bacterial cells were centrifuged and resuspended in 10 mL of fresh sBHI to avoid the carryover of AI. The resultant suspension was diluted 10-fold in sBHI and cultured with shaking for 2 h after the addition of OdiE. Then, the bacterial cells were removed by centrifugation and filtration (pore size 0.45 μm).
V. harveyi ATCC BAA-211 was cultured in AB medium overnight and diluted 1:5,000 in fresh AB medium. Aliquots (100 μL) of this suspension were transferred into a black 96-well plate (STEM, Tokyo, Japan) and mixed with 10 μL of an H. influenzae supernatant diluted 1:2 with fresh BHI. The plate was incubated for 5 h at 30°C, and the bioluminescence signal was measured. V. harveyi incubated in BHI was included as a background control. All experiments were performed at least twice on separate days.
Statistical analysis
Statistical differences were assessed by Student's and Welch's t-tests using the JMP software (SAS Institute, Inc., Cary, NC, USA). P values of < 0.05 were considered statistically significant.
Results and Discussion
Oldenlandia diffusa extract does not inhibit growth of Haemophilus influenzae
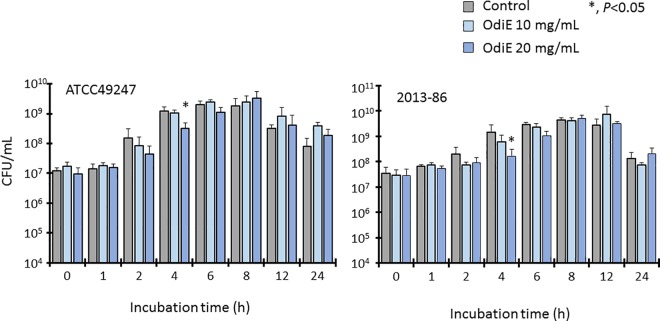
To determine whether OdiE could inhibit the growth of H. influenzae, H. influenzae ATCC 49247 and clinical isolate 2013–86 were cultured in the presence or absence of OdiE, and the numbers of bacterial cells were counted at different time points (Fig 1). In the presence of 20 mg/mL of OdiE, the number of bacterial cells decreased at 4 h of incubation for both strains, but there were no significant differences with the control after 6 h. Moreover, the numbers of bacterial cells did not decrease in the presence of 10 mg/mL of OdiE. These data suggested that OdiE had a weak antibacterial activity, which was not sufficient to inhibit the growth of H. influenzae.
Fig 1. Growth of H. influenzae with or without Oldenlandia diffusa extract.
Each experiment was performed three times on separate occasions, and the data are shown as the mean ± standard deviation (SD). The P value was calculated by the Welch's t-test. OdiE, Oldenlandia diffusa extract
Oldenlandia diffusa extract inhibits biofilm formation by Haemophilus influenzae
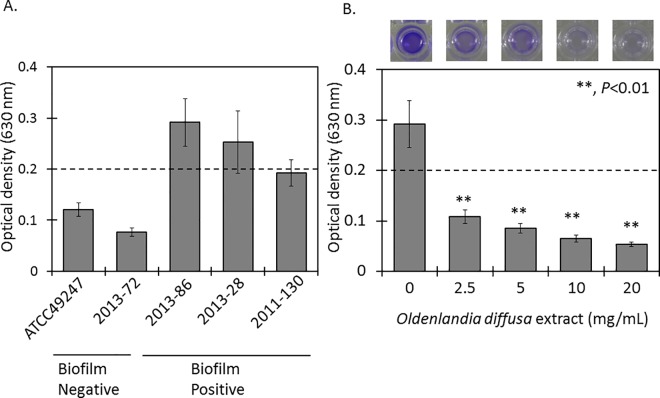
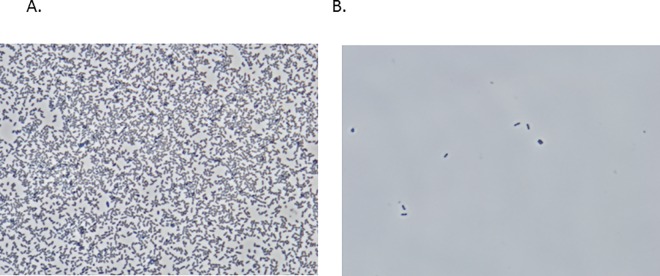
To examine the effect of OdiE on biofilm formation, we first screened the 20 clinical isolates selected for their biofilm formation ability by the crystal violet assay (Data not shown). Based on this screening, two isolates (2013–86 and 2013–28) with a biofilm formation ability and one isolate (2011–130) with a weak biofilm formation ability were selected (Fig 2). All isolates were typed as nontypeable. Two isolates showed susceptibility to β-lactams, macrolides, and levofloxacin, and one isolate showed resistance to β-lactams (Table 1). To test whether OdiE could inhibit biofilm formation, the biofilm amount was measured in the presence or absence of OdiE. Biofilm formation by H. influenzae 2013–86 was significantly reduced in the presence of OdiE in a concentration-dependent manner (Fig 2, P < 0.01). Furthermore, we observed H. influenzae 2013–86 under a phase-contrast microscope (×1,000) to examine the presence of bacterial cells after washing the wells with PBS. In the presence of 20 mg/mL of OdiE, the number of bacterial cells was clearly smaller than that in the control (Fig 3), indicating that the formation of biofilm was suppressed by the addition of OdiE. Similar results were obtained for the other biofilm-forming H. influenzae clinical isolates (Table 1).
Fig 2. Biofilm formation assay.
Biofilm formation was evaluated by the crystal violet assay. (A), Screening for biofilm formation. H. influenzae ATCC 49247 and 2013–72 represented non-biofilm-forming bacteria. (B), Biofilm formation with or without OdiE. The upper panel shows a photograph of each well after adding 95% ethanol. Each experiment was performed three times on separate occasions, and the data are shown as the mean ± SD. P values were calculated by the Student's t-test. Dotted line shows the cutoff value for biofilm formation in this study.
Table 1. Antimicrobial susceptibility and biofilm formation ability of Haemophilus influenzae clinical isolates.
| Strain | Isolation site | Serotypea | Minimum inhibitory concentration (μg/mL)b | Biofilm formation (OD630) c | P valued | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | Ampicillin sulbactam | Cefotaxime | Clarithromycin | Azithromycin | Levofloxacin | Without OdiE | With OdiE (20 mg/mL) | ||||
| 2013–28 | Nasal cavity | Nontypeable | 0.125 | 0.125 | ≤0.063 | 2 | 0.25 | ≤0.063 | 0.253 ± 0.061 | 0.096± 0.032 | <0.001 |
| 2013–86 | Nasal cavity | Nontypeable | 0.25 | 0.25 | ≤0.063 | 4 | 0.5 | ≤0.063 | 0.292 ± 0.047 | 0.098 ± 0.032 | <0.001 |
| 2011–130 | Nasal cavity | Nontypeable | 8 | 4 | 1 | 2 | 0.125 | ≤0.063 | 0.193 ± 0.025 | 0.104 ± 0.039 | <0.001 |
aSerotype was determined by PCR
bminimum inhibitory concentration was measured by the broth dilution method according to the Clinical and Laboratory Standards Institute guidelines
cmean ± SD
dP values were calculated by the Student's t-test.
Fig 3. Phase-contrast light microscopic images of H. influenzae 2013–86 (magnification ×1,000).
H. influenzae 2013–86 was cultured in a 24-well plate, and each well was washed with PBS three times to remove planktonic bacteria. (A) without OdiE (B) with 20 mg/mL of OdiE.
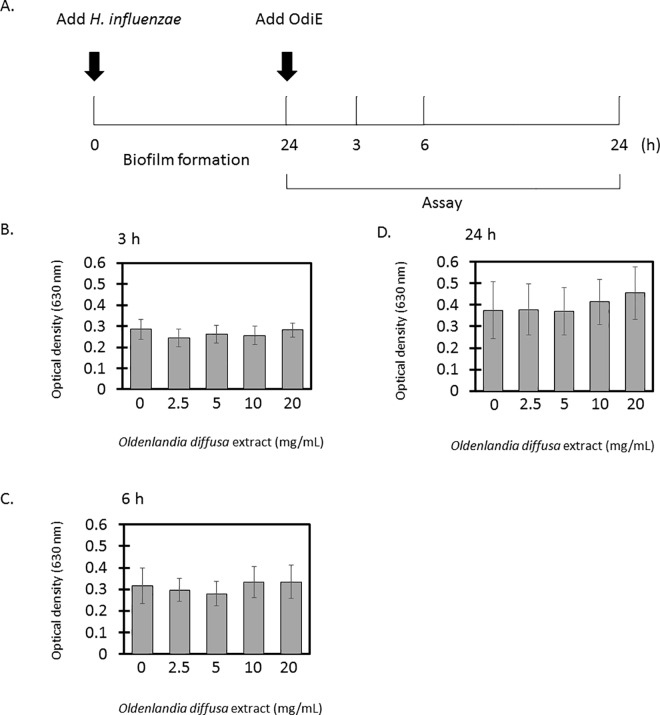
To test whether OdiE could exfoliate the formed biofilm, OdiE was added to the formed biofilm, and the amount of biofilm was quantified. The amount of biofilm did not decrease at any concentration of OdiE (Fig 4). These data showed that OdiE could inhibit the formation of biofilm by H. influenzae but could not exfoliate the formed biofilm.
Fig 4. Exfoliative activity of OdiE on biofilm of H. influenzae 2013–86.
(A) analysis scheme (B), 3 h after adding OdiE (C) 6 h after adding OdiE (D) 24 h after adding OdiE. Each experiment was performed three times on separate occasions, and the data are shown as the mean ± SD. The P value was calculated by the Student's t-test.
Oldenlandia diffusa extract inhibits biofilm formation by suppressing LuxS
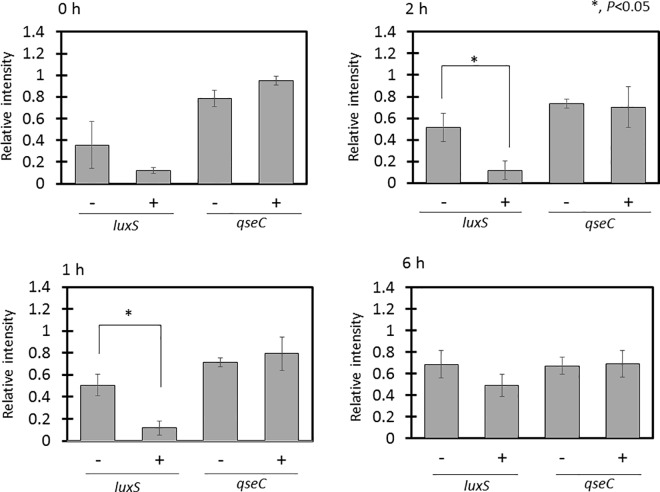
LuxS and the two-component control system QseBC are known to be involved in biofilm formation by H. influenzae [15]. Therefore, we investigated whether OdiE affects the mRNA levels of these two genes (luxS and qseC) using RT–PCR. The mRNA level of luxS was significantly reduced soon after the OdiE addition, and the suppressive effect continued for at least 2 h, whereas the mRNA level of qseC did not change (Fig 5). These results suggested that OdiE inhibits biofilm formation by suppressing its early stage via suppression of luxS expression.
Fig 5. Transcript levels of H. influenzae 2013–86 biofilm-related genes.
Transcript levels of luxS and qseC were evaluated by semi-quantitative RT–PCR. Relative expression was calculated in comparison with the transcription level of gyrB. Each experiment was performed three times on separate occasions, and the data are shown as the mean ± SD. The P value was calculated by the Student's t-test.
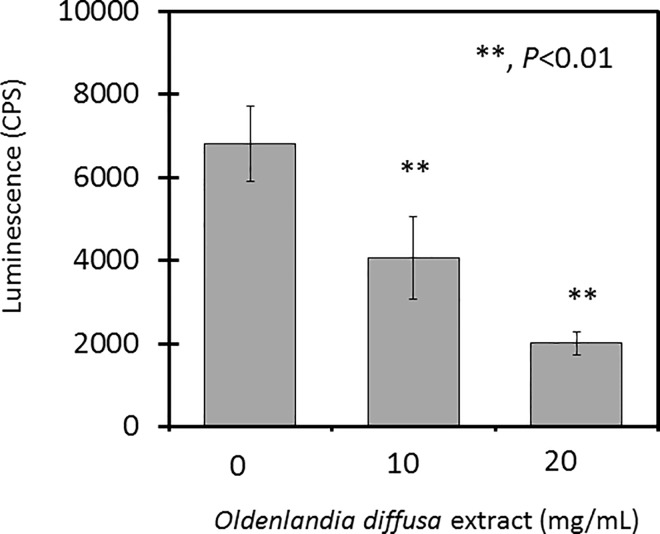
The luxS gene is known to be essential for the generation of AI, which is the signal molecule of QS [16]. Since the amount of luxS mRNA was reduced by the OdiE addition, we investigated whether the amount of AI was changed using the AI assay with V. harveyi ATCC BAA-211. By the addition of OdiE, the luminescence was significantly reduced in a dose-dependent manner (Fig 6), indicating that the amount of AI was reduced. Therefore, these data showed that OdiE reduced the secretion of AI by inhibiting the transcription of luxS in H. influenzae, which resulted in a biofilm suppression effect.
Fig 6. Oldenlandia diffusa extract inhibits autoinducer production.
The autoinducer in the medium was measured by a bioassay using Vibrio harveyi ATCC BAA-211. Each experiment was performed twice on separate occasions, and the data are shown as the mean ± SD. The P value was calculated by the Student's t-test.
Odi has been empirically used as an adjunctive agent for treatments of respiratory infections, such as common cold, for a long time. Interestingly, the unique features revealed in this study, i.e., the OdiE ability to inhibit the biofilm formation but not the growth of H. influenzae, are consistent with the empirical usage of OdiE. Therefore, our study confirmed the empirical role of OdiE through basic research.
In the bacteriological aspect, agents exhibiting such properties have been rare. In general, a drug that shows biofilm inhibitory effects also shows antimicrobial activity [17], [18]. For example, macrolides are known as antimicrobial agents that also show a biofilm inhibitory activity. In fact, macrolides have been used to treat chronic sinusitis for a long time [19]. The use of antimicrobial agents has been known to lead to an increase of resistant strains by preventing growth of susceptible strains [20]. Actually, macrolide resistance has been increasing not only among H. influenzae strains but also among S. pneumoniae and Mycoplasma pneumoniae [1]. Since OdiE does not affect bacterial growth, the risk of the emergence of resistant bacteria may be very low.
For an adjunctive therapy agent, its toxicity to humans is a very important factor. A previous study has indicated that OdiE showed toxic effects on cancer cell lines but not on normal cell lines [21], and the IC50 values ranged from 7 to 25 mg/mL [21]. Therefore, this strongly suggested that OdiE did not show toxicity for normal cells.
Thus, our data suggested that Odi might have preventive effects on chronic and intractable infections caused by H. influenzae via prevention of biofilm formation. In the future, it is necessary to analyze the action of Odi against other respiratory pathogenic bacteria, such as S. pneumoniae and Moraxella catarrhalis.
Our study indicated that O. diffusa could inhibit the biofilm formation by H. influenzae via suppression of luxS transcription, which supports the usefulness of the herb as an adjunctive therapy against respiratory infections.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Morozumi M, Chiba N, Okada T, Sakata H, Matsubara K, Iwata S, et al. Antibiotic susceptibility in relation to genotype of Streptococcus pneumoniae, Haemophilus influenzae, and Mycoplasma pneumoniae responsible for community-acquired pneumonia in children. J Infect Chemother. 2013;19(3):432–40. 10.1007/s10156-012-0500-x [DOI] [PubMed] [Google Scholar]
- 2.Chinese Materia Medica. Teng J, editor. China: People's Medical Publishing House; 2007.
- 3.Song YH, Jeong SJ, Kwon HY, Kim B, Kim SH, Yoo DY. Ursolic acid from Oldenlandia diffusa induces apoptosis via activation of caspases and phosphorylation of glycogen synthase kinase 3 beta in SK-OV-3 ovarian cancer cells. Biol Pharm Bull. 2012;35(7):1022–8. [DOI] [PubMed] [Google Scholar]
- 4.Chung HS, Jeong HJ, Hong SH, Kim MS, Kim SJ, Song BK, et al. Induction of nitric oxide synthase by Oldenlandia diffusa in mouse peritoneal macrophages. Biol Pharm Bull. 2002;25(9):1142–6. [DOI] [PubMed] [Google Scholar]
- 5.Wiertsema SP, Kirkham LA, Corscadden KJ, Mowe EN, Bowman JM, Jacoby P, et al. Predominance of nontypeable Haemophilus influenzae in children with otitis media following introduction of a 3+0 pneumococcal conjugate vaccine schedule. Vaccine. 2011;29(32):5163–70. 10.1016/j.vaccine.2011.05.035 [DOI] [PubMed] [Google Scholar]
- 6.Benninger MS. Acute bacterial rhinosinusitis and otitis media: changes in pathogenicity following widespread use of pneumococcal conjugate vaccine. Otolaryngo Head Neck Surg. 2008;138(3):274–8. [DOI] [PubMed] [Google Scholar]
- 7.Murphy TF, Apicella MA. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis. 1987;9(1):1–15. [DOI] [PubMed] [Google Scholar]
- 8.Erwin AL, Smith AL. Nontypeable Haemophilus influenzae: understanding virulence and commensal behavior. Trends Microbiol. 2007;15(8):355–62. 10.1016/j.tim.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 9.Novotny LA, Jurcisek JA, Ward MO Jr., Jordan ZB, Goodman SD, Bakaletz LO. Antibodies against the majority subunit of type IV Pili disperse nontypeable Haemophilus influenzae biofilms in a LuxS-dependent manner and confer therapeutic resolution of experimental otitis media. Mol Microbiol. 2015;96(2):276–92. PubMed Central PMCID: PMC4423401. 10.1111/mmi.12934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unal CM, Singh B, Fleury C, Singh K, Chavez de Paz L, Svensater G, et al. QseC controls biofilm formation of non-typeable Haemophilus influenzae in addition to an AI-2-dependent mechanism. Int J Med Microbiol. 2012;302(6):261–9. 10.1016/j.ijmm.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 11.Wajima T, Seyama S, Nakamura Y, Kashima C, Nakaminami H, Ushio M, et al. Prevalence of macrolide-non-susceptible isolates among beta-lactamase-negative ampicillin-resistant Haemophilus influenzae in a tertiary care hospital in Japan. J Glob Antimicrob Resist. 2016;6:22–6. 10.1016/j.jgar.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 12.Vilchez R, Lemme A, Thiel V, Schulz S, Sztajer H, Wagner-Dobler I. Analysing traces of autoinducer-2 requires standardization of the Vibrio harveyi bioassay. Anal Bioanal Chem. 2007;387(2):489–96. 10.1007/s00216-006-0824-4 [DOI] [PubMed] [Google Scholar]
- 13.Puig C, Marti S, Hermans PW, de Jonge MI, Ardanuy C, Linares J, et al. Incorporation of phosphorylcholine into the lipooligosaccharide of nontypeable Haemophilus influenzae does not correlate with the level of biofilm formation in vitro. Infect Immun. 2014;82(4):1591–9. PubMed Central PMCID: PMC3993405. 10.1128/IAI.01445-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seyama S, Wajima T, Nakaminami H, Noguchi N. Clarithromycin Resistance Mechanisms of Epidemic beta-Lactamase-Nonproducing Ampicillin-Resistant Haemophilus influenzae Strains in Japan. Antimicrob Agents Chemother. 2016;60(5):3207–10. PubMed Central PMCID: PMC4862528. 10.1128/AAC.00163-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armbruster CE, Hong W, Pang B, Dew KE, Juneau RA, Byrd MS, et al. LuxS promotes biofilm maturation and persistence of nontypeable Haemophilus influenzae in vivo via modulation of lipooligosaccharides on the bacterial surface. Infect Immun. 2009;77(9):4081–91. PubMed Central PMCID: PMC2738029. 10.1128/IAI.00320-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardie KR, Heurlier K. Establishing bacterial communities by 'word of mouth': LuxS and autoinducer 2 in biofilm development. Nature Rev Microbiol. 2008;6(8):635–43. [DOI] [PubMed] [Google Scholar]
- 17.Kalia M, Yadav VK, Singh PK, Sharma D, Pandey H, Narvi SS, et al. Effect of Cinnamon Oil on Quorum Sensing-Controlled Virulence Factors and Biofilm Formation in Pseudomonas aeruginosa. PLoS one. 2015;10(8):e0135495 PubMed Central PMCID: PMC4532483. 10.1371/journal.pone.0135495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaue Y, Domon H, Oda M, Takenaka S, Kubo M, Fukuyama Y, et al. Anti-biofilm and bactericidal effects of magnolia bark-derived magnolol and honokiol on Streptococcus mutans. Microbiol Immunol. 2016;60(1):10–6. 10.1111/1348-0421.12343 [DOI] [PubMed] [Google Scholar]
- 19.Iino Y, Yoshida N, Kato T, Kakizaki K, Miyazawa T, Kakuta H. Clinical effects of clarithromycin on persistent inflammation following Haemophilus influenzae-positive acute otitis media. Acta Otolaryngol. 2015;135(3):217–25. 10.3109/00016489.2014.975893 [DOI] [PubMed] [Google Scholar]
- 20.Goossens H. Antibiotic consumption and link to resistance. Clin Microbiol Infect. 2009;15 Suppl 3:12–5. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Zhang D, Yi J, Shao J. Anticancer activities of Oldenlandia diffusa. J Herb Pharmacother. 2004;4(1):21–33. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.