Abstract
Piceatannol, a resveratrol metabolite, is a phenolic compound found in red wine and grapes. We investigated the effect of piceatannol on renal fibrosis and histone deacetylase (HDAC) expression in a mouse model of unilateral ureteral obstruction (UUO). Fibrosis was established by UUO and piceatannol was intraperitoneally injected for 2 weeks. Piceatannol suppressed extracellular matrix (ECM) protein deposition including collagen type I and fibronectin as well as connective tissue growth factor (CTGF) and α-smooth muscle actin (α-SMA) in UUO kidneys. However, the expressions of epithelial-mesenchymal transition (EMT) marker genes, such as N-cadherin and E-cadherin, were not changed in the kidneys after UUO. Masson’s trichrome staining and fluorescence immunostaining showed that piceatannol administration attenuated collagen deposition in UUO kidneys. HDAC1, HDAC4, HDAC5, HDAC6, and HDAC10 protein expression was upregulated in UUO kidneys, whereas that of HDAC8 was downregulated. Piceatannol treatment significantly reduced HDAC4 and HDAC5 protein expression. Further, piceatannol attenuated phosphorylation of p38 mitogen-activated protein kinase (p38-MAPK) in UUO kidneys, but not that of transforming growth factor beta1-Smad2/3. These results suggest that class I HDACs and class IIa/b HDACs are involved in renal fibrosis development. Piceatannol may be a beneficial therapeutic agent for treating renal fibrosis via reduction of HDAC4 and HDAC5 protein expression or suppression of the p38-MAPK signaling pathway.
Introduction
Renal fibrosis is characterized by the accumulation of extracellular matrix (ECM) proteins, activation of myofibroblasts and fibroblasts, and tubular atrophy [1–3]. During fibrosis, interstitial fibroblast activation, pericyte differentiation, epithelial-mesenchymal transition (EMT) of tubular epithelial cells, and recruitment of fibrocytes are involved in the activation of myofibroblasts [4,5]. Unilateral ureteral obstruction (UUO) is a representative model of renal fibrosis and can be used for the evaluation of therapeutic agents for renal diseases [6,7].
Imbalance of histone deacetylase (HDAC) expression or activity is implicated in several diseases. HDACs are divided into four HDAC classes: class I HDACs (HDAC1, HDAC2, HDAC3, and HDAC8); class IIa HDACs (HDAC4, HDAC5, HDAC7, and HDAC9); class IIb HDACs (HDAC6 and HDAC10); class III (Sirt1-7); and class IV (HDAC11). HDAC inhibitors are effective in cancer, cardiac hypertrophy, and inflammation [8–10]. Furthermore, HDAC inhibitors suppress fibrosis in organs such as the heart and kidneys [11,12] as shown in vivo as well as in vitro, suggesting that HDACs may be therapeutic targets for treating fibrosis. For example, class I HDACs are activated in transforming growth factor β1- (TGF-β1) treated kidney epithelial cells [13] and are involved in the development of EMT and the ECM in fibrosis with [14] or without [15] diabetes. HDAC6, a class IIb HDAC, may be a target for hypertension-induced kidney fibrosis [16]. Class IIa HDACs (HDAC4/5/7) are related to diabetes-induced fibrosis [17].
Piceatannol, a natural polyphenolic stilbene compound, is a metabolite of resveratrol found in red wine. Piceatannol shows several biological activities, including anticancer, anti-inflammatory, anti-oxidative, anti-allergic, anti-adipogenesis, and anti-hypertrophic effects [18–25]. Piceatannol may potentially be protective against cardiovascular diseases, allergy, cancer, and inflammatory diseases. However, only few studies have shown that piceatannol plays a beneficial role in kidney diseases. For example, one study showed that piceatannol in combination with low doses of cyclosporine A prevented kidney allograft rejection [26]. More recently, another study showed a mild renoprotective effect of piceatannol in obese Zucker rats [27]. However, the effect of piceatannol on renal fibrosis and the underlying regulatory mechanism have not been fully investigated.
Resveratrol is a compound very similar to piceatannol. There are several publications related to resveratrol and renal fibrosis. For example, resveratrol inhibits renal interstitial fibrosis through a variety of mechanisms, including the regulation of AMP-activated protein kinase (AMPK)/NAPDH oxidase 4 (NOX4)/reactive oxygen species (ROS) pathway [28], downregulation of nuclear factor-κB (NF-κB) [29], or regulation of the transforming growth factor β (TGF-β) pathway [30].
Considering the protective effect of resveratrol in renal diseases, we hypothesize that piceatannol may have a beneficial effect on renal fibrosis. In this study, we investigated the effect of piceatannol on renal fibrosis in a mouse model of UUO. Furthermore, we assessed the relevance of HDAC expression and the TGF-β1-induced Smad-dependent or Smad-independent signaling pathway in the anti-fibrotic effect of piceatannol.
Materials and Methods
Animal Experiments
C57BL/6 male mice (7-week-old) weighing 20~22 g were purchased from ORIENT BIO (Gyeonggi-do, South Korea). All animal experiments were approved by the Animal Experiment Committee of the Chonnam National University Medical School (CNU IACUC-H-2015-52) and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health Publication, 8th edition, 2011). UUO was performed as follows. After anesthesia induction by using an intraperitoneal injection of ketamine (70 mg/kg) and xylazine (14 mg/kg), a midline incision was made to expose the abdominal cavity and the left ureter was ligated with 6–0 silk. The contralateral/right kidney served as a control. One day after surgery, piceatannol (50 mg/kg/day) or vehicle (1.5% DMSO in 0.9% saline/day) was intraperitoneally injected 10 times during 2 weeks.
Materials and Antibodies
Piceatannol was purchased from Future Chem (Seoul, Korea). Anti-alpha smooth muscle actin (α-SMA; 1:1000, sc-130617), anti-CTGF (1:1000, sc-14939), anti-HDAC3 (1:1000, sc-11417), anti-HDAC4 (1:1000, sc-11418), anti-HDAC5 (1:1000, sc-133225), anti-TGF-β1 (1:1000, sc-146), anti-JNK (1:1000, sc-7345), anti-ERK1 (1:1000, sc-271269), and anti-GAPDH (1:1000, sc-32233) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Primary antibodies against collagen type I (1:1000, ab34710), HDAC2 (1:1000, ab12169), HDAC8 (1:1000, ab137474), and HDAC10 (1:1000, ab53096) were purchased from Abcam (Cambridge, MA, USA). Anti-fibronectin antibody (1:1000, MA5-11981) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Anti-HDAC1 antibody (1:1000, 06–720) was purchased from Merck Millipore (Darmstadt, Germany). Anti-HDAC6 (1:1000, 7612), anti-Smad3 (1:1000, 9523), anti-Smad2 (1:1000, 3103), anti-Smad4 (1:1000, 9515), anti-p-Smad3 (1:1000, 9520), anti-p-JNK (1:1000, 9251), anti-p-p38 (1:1000, 4511), anti-p-ERK1/2 (1:1000, 4370), and anti-p38 (1:1000, 8690) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA).
Western Blot Analysis
Western blotting was performed as described previously [31]. Cell lysates were prepared with RIPA buffer (150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 50 mM Tris-HCl pH 7.5, 2 mM EDTA, 1 mM PMSF, 1 mM DTT, 1 mM Na3VO4, and 5 mM NaF) containing a protease inhibitor cocktail (Calbiochem, EMD Millipore Corp., Billerica, MA, USA). Proteins were separated using 8% SDS-PAGE and were then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were probed with the indicated antibodies and developed using Immobilon Western Detection Reagents (Millipore, Billerica, MA, USA). The Bio-ID software was used to quantify protein expression (Vilber Lourmat, Eberhardzell, Germany). All experiments were performed in triplicate.
Quantitative Real Time Polymerase Chain Reaction
Total RNA was isolated with TriZol reagent (Invitrogen Life Technologies, Waltham, MA, USA), and 1 μg of RNA was used for the reverse transcription reaction using TOPscript RT DryMIX (Enzynomics, Daejeon, South Korea). The mRNA amounts were determined using the SYBR Green PCR kit (Enzynomics, Daejeon, South Korea). The relative expression level of the indicated genes was compared to that of GAPDH by using the 2-ΔΔct method. PCR was performed using the following oligonucleotide primers: for collagen type I, sense, 5’-GAGCGGAGAGTACTGGATCG-3’, and antisense, 5’-GCTTCTTTTCCTTGGGGTTC-3’; for fibronectin, sense, 5’- GATGCACCGATTGTCAACAG-3’, and antisense, 5’-TGATCAGCATGGACCACTTC-3’; for CTGF, sense, 5’-CAAAGCAGCTGCAAATACCA-3’, and antisense, 5’- GGCCAAATGTGTCTTCCAGT-3’; for α-SMA, sense, 5’-ACTGGGACGACATGGAAAAG-3’, and antisense, 5’-AGAGGCATAGAGGGACAGCA-3’; for GAPDH, sense, 5’- GCATGGCCTTCCGTGTTCCT-3’, and antisense, 5’- CCCTGTTGCTGTAGCCGTATTCAT-3’.
Histology and Masson’s Trichrome Staining
Kidney tissues were fixed with 4% paraformaldehyde, embedded in paraffin, and cut into 3-μm-thick sections. Hematoxylin and eosin (H&E) staining was performed to assess the histological morphology. The kidney tissue section slides were incubated in Gill’s hematoxylin for 5 min, washed with tap water, incubated in 95% ethanol, and stained with eosin and phloxine for 1 min. Subsequently, the sections were dehydrated in ethanol and xylene, and were mounted with Canada balsam.
For Masson’s trichrome staining, after deparaffinization with xylene, the sections were treated with Bouin’s solution at 56°C for 30 min and were washed under running tap water until the sections were clear. The sections were subsequently stained with Weigert’s hematoxylin (A:B = 1:1), followed by staining with Biebrich Scarlet/Acid Fuchsin solution for 10 min and washing with distilled water. The sections were incubated with phosphotungstic acid/phosphomolybdic acid solution for 10 min and were treated with Aniline Blue solution for 15 min. They were subsequently incubated with acetic acid for 1 min and were dehydrated with ethanol and xylene. Collagen depositions, nuclei, and muscle fibers were stained blue, black, and red, respectively.
Immunofluorescence Staining
After standard histological processing procedures, immunostaining was performed using collagen type I antibody. The sections were deparaffinized in xylene and rehydrated with graded alcohol. Antigen retrieval was performed using 10 mM citrate-phosphate buffer (pH 6.0), the sections were subsequently blocked with 3% goat serum for 1 h, incubated with anti-collagen type I antibody (1:100, Abcam) at 4°C overnight, and incubated with Alexa Fluor 488 goat anti-rabbit IgG antibody (1:200, Invitrogen) for 1 h. Sudan black B solution was used to reduce autofluorescence. The sections were counterstained with DAPI and images were acquired using fluorescence microscopy (Nikon Eclipse 80i, Tokyo, Japan).
Statistics
Statistical analysis was performed using one-way ANOVA followed by the Bonferroni post-hoc test for comparative analysis between the treatment groups (GraphPad Prism, version 5.0; GraphPad Software, La Jolla, CA, USA). The data are presented as the means ± SD. A P value of <0.05 was considered statistically significant.
Results
Piceatannol reduces ECM protein and fibrosis marker expression in the UUO kidney
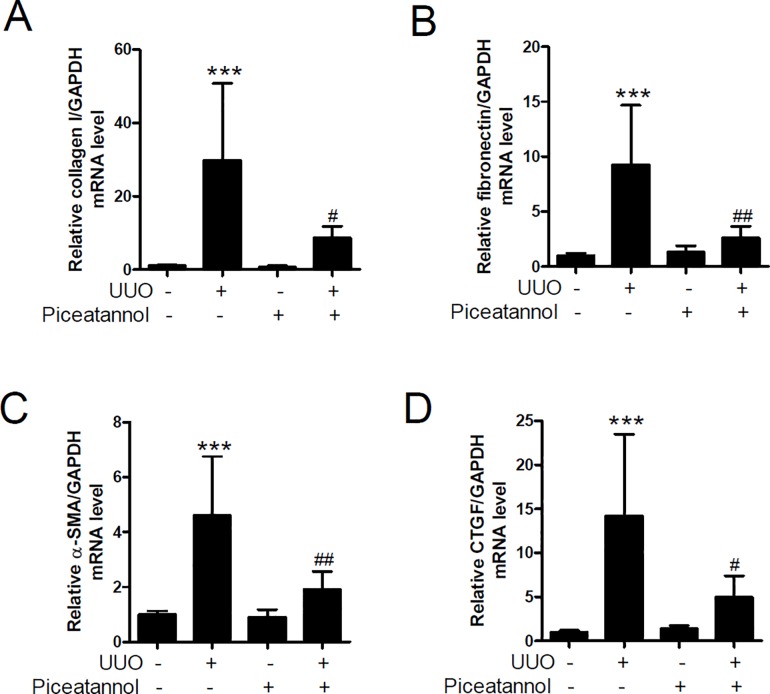
To determine whether piceatannol might have a therapeutic effect on renal fibrosis, we administrated vehicle or piceatannol (50 mg/kg/day) to UUO mice for 2 weeks. To evaluate whether piceatannol could affect the transcript levels of ECM and fibrosis markers, we performed qRT-PCR in kidney tissues. The mRNA levels of collagen type I, fibronectin, CTGF, and α-SMA were significantly enhanced in the UUO group compared with those in the control group. The increase was inhibited by piceatannol (Fig 1A–1D).
Fig 1. Piceatannol reduces the mRNA level of fibrosis-related genes in the UUO kidney.
(A-D) One day after UUO surgery, the mice received an intraperitoneal injection of vehicle or piceatannol (50 mg/kg/day) for 2 weeks. The transcription level of collagen type I (collagen type I), fibronectin, alpha smooth muscle actin (α-SMA), and connective tissue growth factor (CTGF) was determined using qRT-PCR in kidney tissues from UUO mice treated with vehicle or piceatannol. The data are expressed as the means ± SD of the mice (n = 6 per group). ***P<0.001 compared with the contralateral kidney. #P<0.05 and ##P<0.01 compared with the UUO kidney.
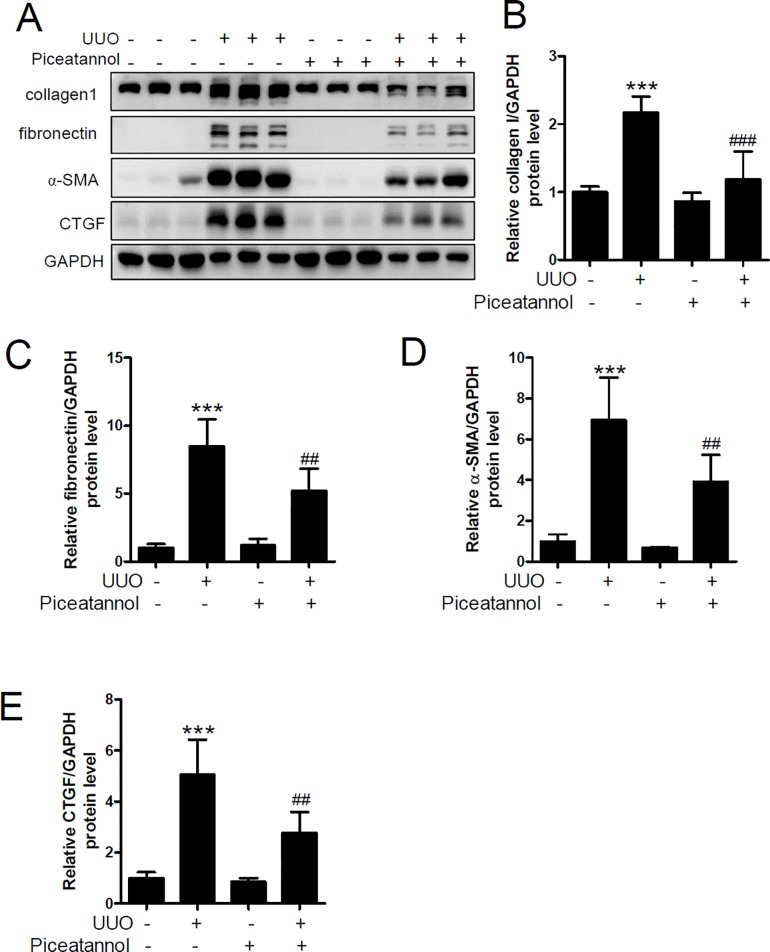
As shown in Fig 2A–2C, UUO increased the ECM proteins collagen type I and fibronectin, but the increase was significantly suppressed by piceatannol treatment. α-SMA is a myofibroblast marker involved in organ fibrosis [32]. We observed that UUO-induced α-SMA expression was significantly reduced by piceatannol administration (Fig 2A and 2D). CTGF is a matricellular protein related to tissue and wound repair and fibrotic pathology [33]. UUO-induced CTGF expression was ameliorated by treatment with piceatannol (Fig 2A and 2E). No changes in the expression of ECM proteins and fibrosis markers were observed in the control mice treated with piceatannol.
Fig 2. Piceatannol reduces extracellular matrix (ECM) protein and fibrosis marker protein expression in the UUO kidney.
One day after UUO surgery, the mice received an intraperitoneal injection of vehicle or piceatannol (50 mg/kg/day) for 2 weeks. (A) Protein lysates from kidney tissues were prepared and were analyzed using western blotting. Anti-collagen 1, fibronectin, α-SMA, and CTGF antibodies were used. GADPH was used as a loading control. Representative immunoblots. (B-E) Protein levels were quantified using densitometry. The data are expressed as the means ± SD of the mice (n = 6 per group). ***P<0.001 compared with the contralateral kidney. ##P<0.01 and ###P<0.001 compared with the UUO kidney.
EMT gene expression is not changed in the UUO kidney
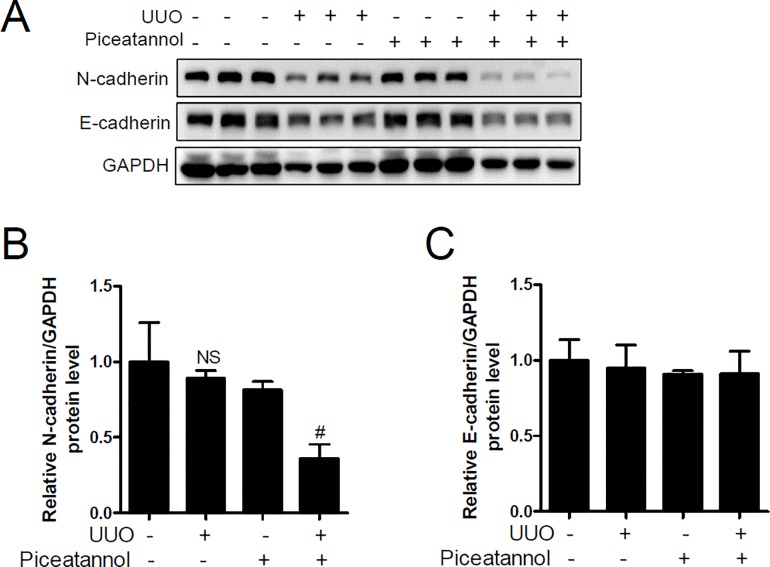
EMT is characterized by the loss of epithelial cell polarity and cell-to-cell adhesion as well as gain of mesenchymal cell migration. EMT is associated with renal fibrosis [34,35]. We performed western blot analysis to investigate whether piceatannol could affect EMT. Unexpectedly, we found that N-cadherin protein expression was not increased in UUO kidneys but that piceatannol significantly reduced N-cadherin expression (Fig 3A and 3B). In addition, we observed that E-cadherin was not downregulated in UUO kidneys (Fig 3A and 3C). Thus, these results indicated that downregulation of E-cadherin and upregulation of N-cadherin was not implicated in UUO-induced renal fibrosis.
Fig 3. Epithelial-mesenchymal transition (EMT) gene expression is not observed in the UUO kidney.
One day after UUO surgery, the mice received an intraperitoneal injection of vehicle or piceatannol (50 mg/kg/day) for 2 weeks. (A) Protein lysates from kidney tissues were prepared and were analyzed using western blotting. N-cadherin and E-cadherin antibodies were used. GADPH was used as a loading control. Representative immunoblots. (B-C) Protein levels were quantified using densitometry. The data are expressed as the means ± SD of the mice (n = 6 per group). NS indicates not significant. #P<0.05 compared with the UUO kidney.
Piceatannol does not affect UUO-induced tubular atrophy
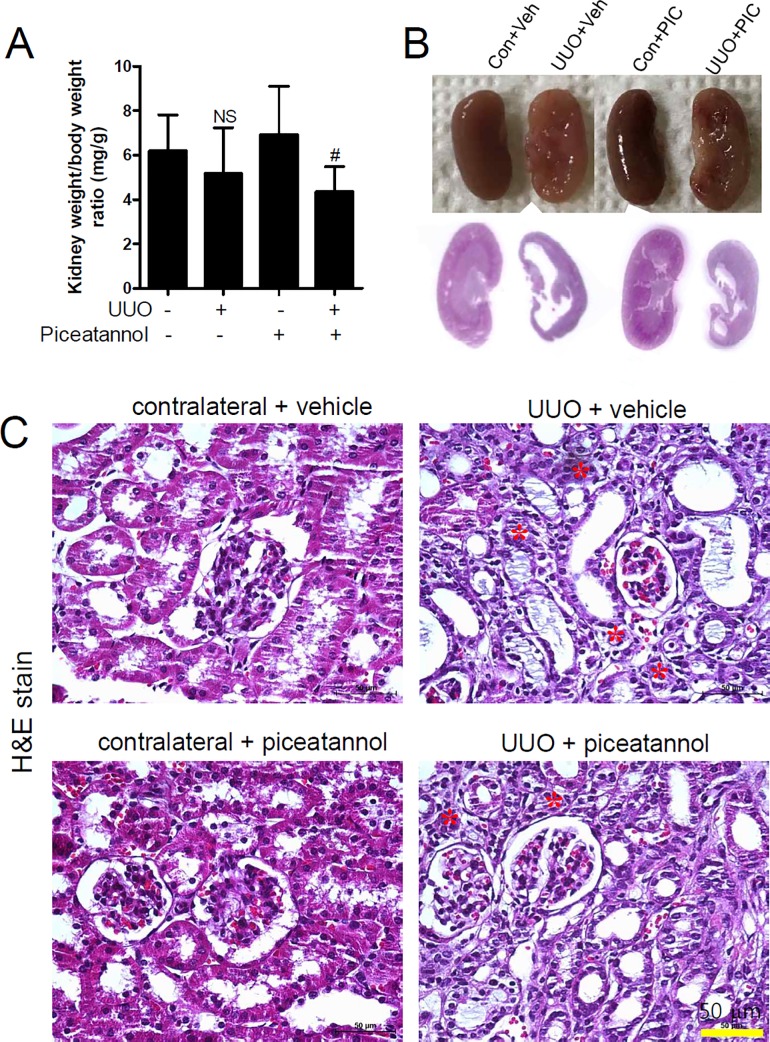
We examined the changes in kidney weight in UUO. A tendency for a decreased weight of the UUO kidney was observed compared to that of the contralateral kidney, regardless of treatment with piceatannol (Fig 4A).
Fig 4. Piceatannol does not affect UUO-induced tubular atrophy.
(A-C) One day after UUO surgery, the mice received an intraperitoneal injection of vehicle or piceatannol (50 mg/kg/day) for 2 weeks. (A) The kidney weight to body weight ratio (mg/g) is shown. (B) Representative photomicrographs of the kidneys are shown (upper panel). Left panel is organized as follows: contralateral + vehicle (Con + Veh), UUO + vehicle (UUO + Veh), contralateral + piceatannol (Con + PIC), and UUO + piceatannol (UUO + PIC). Representative images of hematoxylin & eosin (H&E) staining are shown at a lower magnification (lower panel). (C) H&E staining was performed to examine changes in the renal structure: Con + Veh, Con + PIC, UUO + Veh, and UUO + PIC. Scale bar is 50 μm. Asterisks (*, red) indicates tubular atrophy.
Severe morphological changes or a rumpled appearance were observed in the UUO kidney compared to the contralateral kidney (Fig 4B, upper panel). Histologically, the contralateral kidney had a normal cortex, outer medulla, and inner medulla structure, whereas the medulla structure of the UUO kidney was incomplete (Fig 4B, lower panel). Tubular atrophy and dilatation (Fig 4C, top right panel) were observed in the UUO kidney relative to the vehicle-treated contralateral kidney (Fig 4C, top left panel). However, these changes were not attenuated by piceatannol treatment (Fig 4C, bottom right panel).
Piceatannol ameliorates renal fibrosis in the UUO kidney
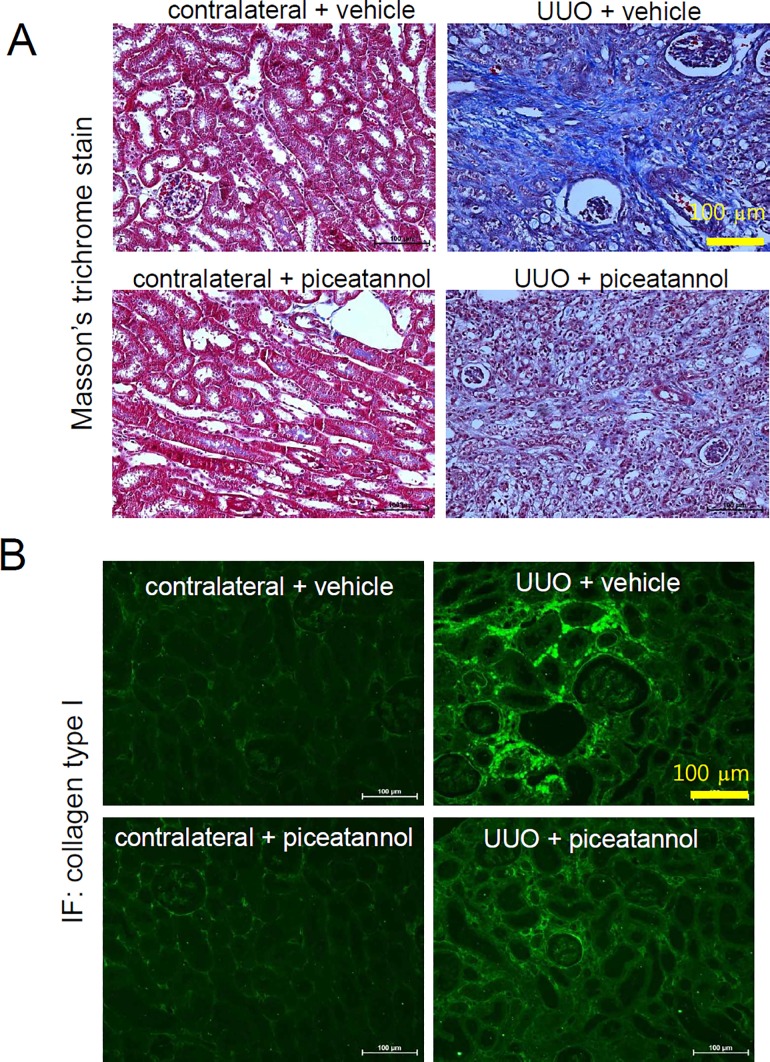
We performed Masson’s trichrome staining to further assess whether piceatannol might be a therapeutic agent for renal fibrosis. As shown in Fig 5A, deposition of interstitial collagen was not observed in the vehicle-treated contralateral kidney and piceatannol-treated contralateral kidney (left, upper panel and lower panel). Interstitial fibrosis was increased in the UUO kidney (right, upper panel), whereas it was attenuated by piceatannol treatment (right, lower panel). Immunofluorescence staining showed that collagen type I expression was increased in the peritubular and periglomerular interstitium in the UUO kidney, which was attenuated by piceatannol treatment (Fig 5B).
Fig 5. Piceatannol decreases renal fibrosis in the UUO kidney.
(A-B) One day after UUO surgery, mice were received an intraperitoneal injection of vehicle or piceatannol (50 mg/kg/day) for 2 weeks. (A) Masson’s trichrome staining was performed to examine interstitial fibrosis: contralateral + vehicle, contralateral + piceatannol, UUO + vehicle, and UUO + piceatannol. Scale bar is 100 μm. (B) Immunofluorescent staining using anti-collagen type I antibody. A representative photomicrograph is shown. Scale bar is 100 μm.
HDAC1 protein expression is upregulated in the UUO kidney
Dysregulation of HDACs is associated with several diseases [36]. We performed western blot analysis to investigate whether HDAC expression was changed in the UUO kidney.
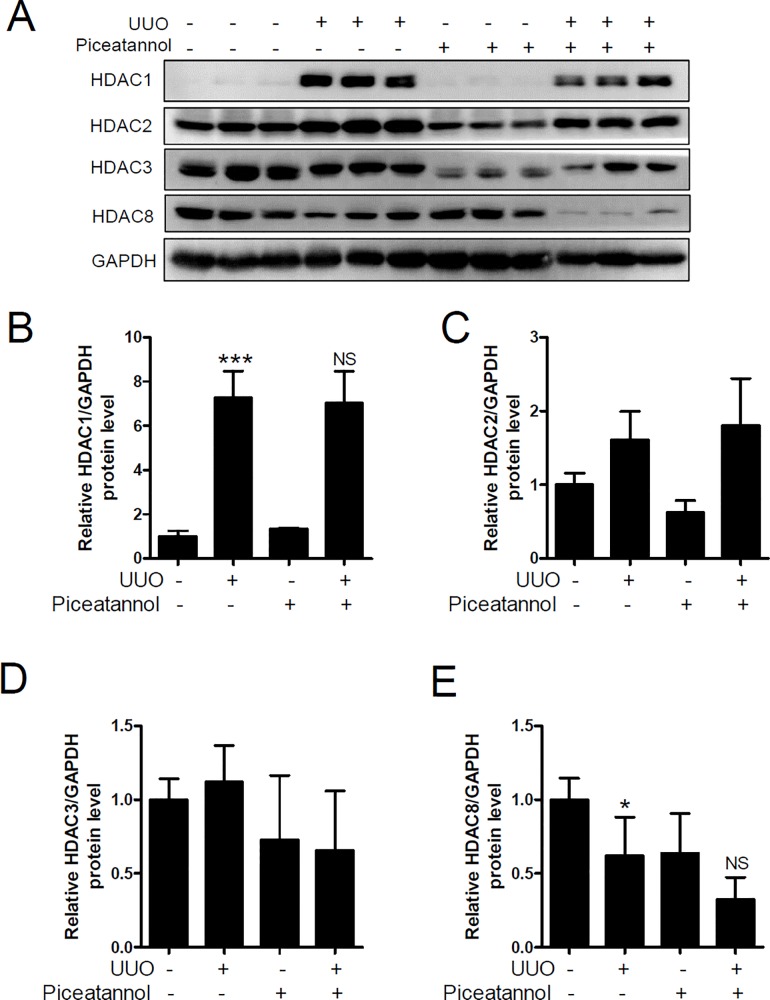
First, we examined the protein expression of class I HDACs including HDAC1, HDAC2, HDAC3, and HDAC8. As shown in Fig 6A, HDAC1 protein levels were significantly increased in the UUO kidney than that in the contralateral kidney. However, piceatannol treatment did not reduce the level of HDAC1 protein (Fig 6B). HDAC2 and HDAC3 protein expression was unchanged in the UUO kidney (Fig 6C and 6D), whereas that of HDAC8 was decreased in the UUO kidney (Fig 6E). These results indicate that class I HDACs may not play a major role in UUO-induced renal fibrosis.
Fig 6. HDAC1 protein expression is upregulated in the UUO kidney.
(A) Kidney protein lysates were analyzed using western blotting. Antibodies against HDAC1, HDAC2, HDAC3, and HDAC8 were used. GAPDH was used as a loading control. (B-E) Quantification analysis was performed using densitometry. The data are expressed as the means ± SD of the mice (n = 6 per group). *P<0.05 and ***P<0.001 compared with the contralateral kidney. NS indicates not significant.
Piceatannol attenuates UUO-induced HDAC4 and HDAC5 protein expression
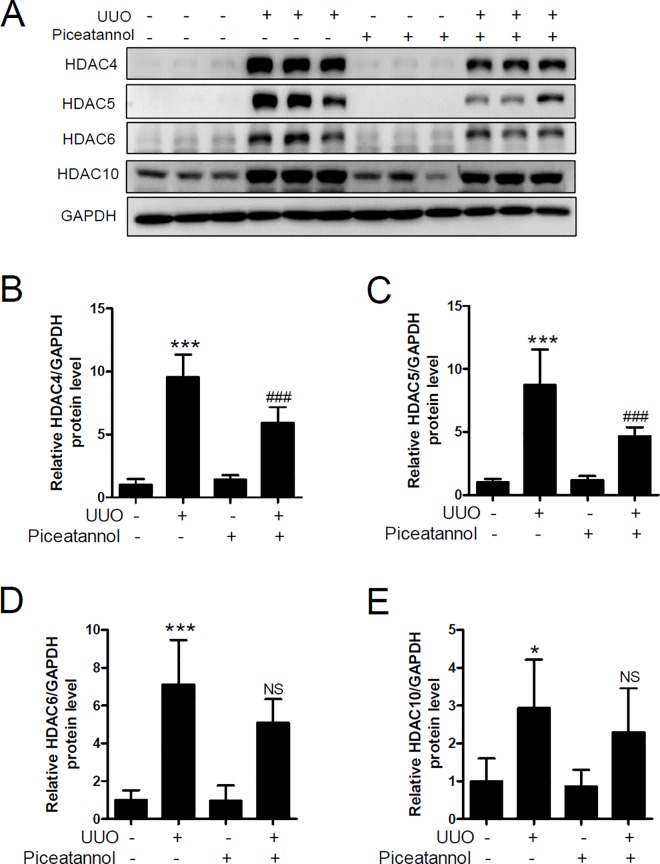
Class II HDACs are divided into two subgroups. Class IIa includes HDAC4, HDAC5, HDAC7, and HDAC9; class IIb includes HDAC6 and HDAC10 [37]. We next examined expression of HDAC4, HDAC5, HDAC6, and HDAC10 in the UUO kidney. As shown in Fig 7A, the protein levels of HDAC4, HDAC5, HDAC6, and HDAC10 were significantly increased in the UUO kidney compared to those in the contralateral kidney. Treatment with piceatannol reduced the UUO-induced protein expression of HDAC4 and HDAC5 (Fig 7B and 7C) but not that of HDAC6 and HDAC10 (Fig 7D and 7E).
Fig 7. Piceatannol attenuates the expression of UUO-induced renal HDAC5/HDAC6 protein.
(A) Kidney lysates were used for western blot analysis. Antibodies against HDAC4, HDAC5, HDAC6, and HDAC10 were used. GAPDH was used as a loading control. (B-E) Quantification analysis was performed using densitometry. The data are expressed as the means ± SD of the mice (n = 6 per group). *P<0.05 and ***P<0.001 compared with the contralateral kidney. ###P <0.001 compared with the UUO kidney. NS indicates not significant compared with the UUO kidney.
Piceatannol attenuates UUO-induced p38-MAPK activation but not TGF-β1-Smad2/3 pathway activation
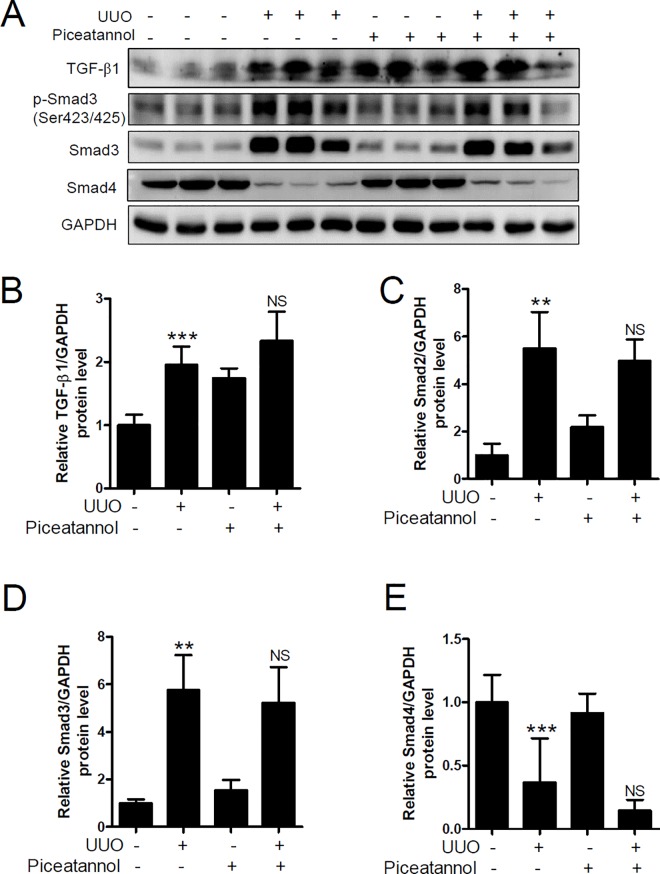
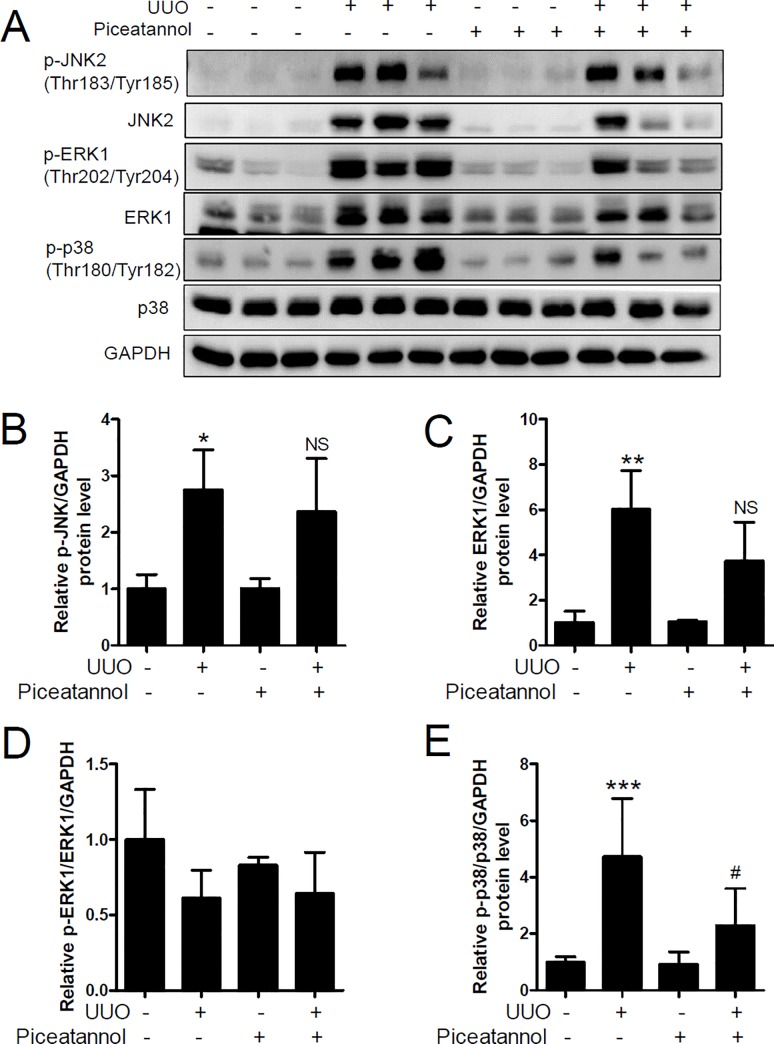
TGF-β/Smad signaling is a critical mediator of renal fibrosis [38]. We performed western blot analysis to determine whether piceatannol affected TGF-β/Smad signaling. Protein expression of TGF-β1, Smad2, and Smad3 was significantly increased in the UUO kidney (Fig 8A–8D), and was not reduced by piceatannol treatment. Similarly, UUO-induced phosphorylation of Smad3 (Ser423/425) was not suppressed by piceatannol treatment. In contrast, Smad4 protein expression significantly reduced in the UUO kidney (Fig 8A and 8E). TGF-β/Smad signaling interacts with MAPK signaling in renal fibrosis [39–42]. To assess whether piceatannol affected TGF-β1-induced MAPK signaling, we examined the protein expression of JNK2, ERK1, and p38 in the UUO kidney. As shown in Fig 9A–9E, the phosphorylated state of JNK2 (Thr183/Tyr185), ERK1 (Thr202/Tyr204), and p38 (Thr180/Tyr182) was increased in the UUO kidney compared to that in the contralateral kidney. Expression of phosphorylated JNK2 and ERK1 proteins was not reduced by piceatannol treatment. Unexpectedly, we observed that the expression of non-phosphorylated JNK2 and ERK1, except p38-MAPK, also increased in the UUO kidney compared to that in the control kidney (Fig 9A). Of note, the ratio of phosphorylated p38 to total p38 significantly reduced in the piceatannol-treated UUO kidney compared to that in the UUO kidney (Fig 9A and 9F).
Fig 8. Piceatannol does not suppress TGF-β1-Smad signaling in the UUO kidney.
(A) Kidney lysates were used for western blot analysis. Antibodies against TGF-β1, p-Smad3 (Ser423/425), Smad3, Smad2, and Smad4 were used. GAPDH was used as a loading control. (B-E) Quantification analysis was performed using densitometry. The data are expressed as the means ± SD of the mice (n = 6 per group). **P<0.01 and ***P<0.001 compared with the contralateral kidney. NS indicates not significant compared with the UUO kidney.
Fig 9. Piceatannol attenuates phosphorylated p38 MAPK expression in the UUO kidney.
(A) Kidney lysates were used for western blot analysis. Antibodies against p-JNK2 (Thr183/Tyr185), JNK2, p-ERK1 (Thr202/Tyr204), ERK1, p-p38 (Thr180/Tyr182), and p38 were used. GAPDH was used as a loading control. (B-E) Quantification analysis was performed using densitometry. The data are expressed as the means ± SD of the mice (n = 6 per group). *P<0.05, **P<0.01, and ***P<0.001 compared with the contralateral kidney. #P <0.05 compared with the UUO kidney. NS indicates not significant compared with the UUO kidney.
Discussion
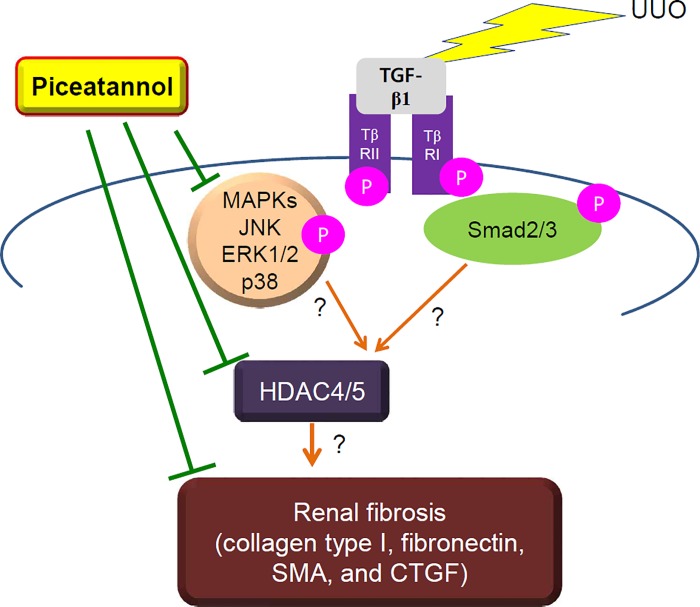
The present study demonstrates that piceatannol attenuates renal fibrosis in a mouse model of UUO. As explained in Fig 10, we suggest that the anti-fibrotic effect of piceatannol is related to the inhibition of the TGF-β1/Smad-independent pathway but not to that of the TGF-β1/Smad-dependent pathway in the UUO-induced fibrosis model. Unexpectedly, piceatannol treatment did not reduce the upregulation of TGF-β1, Smad2, and Smad3 as well as that of phosphorylated Smad3 expression in the UUO kidney. Of note, piceatannol attenuated phosphorylated p38-MAPK in the UUO kidney. Our findings indicate that the anti-fibrotic effect of piceatannol may be associated with downregulation of class IIb HDAC protein expression (HDAC4/5). However, we could not demonstrate a link between HDAC4/5 and MAPK signaling or TGF-β1/Smad signaling, nor a direct association between HDAC4/5 and renal fibrosis.
Fig 10. Piceatannol suppresses renal fibrosis by downregulation of the p38-MAPK/HDAC4/5 pathway.
Fibrotic stress such as UUO can induce TGF-β1 expression, which causes phosphorylation and upregulation of Smad2/3 or MAPK (JNK2, ERK1/2, or p38) protein expression. It also increases the expression of class II HDACs (HDAC4/5). Piceatannol attenuates the activation of p38-MAPK signaling and the increased HDAC4/5 expression, but not the activation of the TGF-β1/Smad3 signaling pathway. However, whether a direct association between HDAC4/5 and MAPK signaling or between HDAC4/5 and renal fibrosis exists remains unknown. TβRII and TβRI indicate TGFβ receptor II and TGFβ receptor I, respectively.
UUO kidney tissues exhibit interstitial fibrosis and severe morphological changes. Masson’s trichrome staining showed that the increased accumulation of collagen in the UUO kidney was ameliorated by piceatannol treatment. Piceatannol reduced the collagen type I mRNA and protein level in the obstructed kidney 14 days after UUO surgery. Immunofluorescence staining demonstrated that renal collagen type I expression was decreased by piceatannol treatment. Fibronectin is another ECM glycoprotein involved in cell adhesion, wound healing, and fibrosis [43,44]. In our study, the contralateral kidney showed a low fibronectin expression, whereas the expression was greatly induced in the UUO kidney. Piceatannol treatment significantly suppressed fibronectin mRNA and protein expression in the UUO kidney. Fibroblast-to-myofibroblast transition is implicated in renal fibrosis [45]. α-SMA is a representative marker of myofibroblast activation [46]. We found increased α-SMA mRNA and protein expression in the UUO kidney, and this was reduced by piceatannol treatment. CTGF showed results similar to those observed for α-SMA. The UUO-induced increase in fibrosis-related gene expression including collagen type I, fibronectin, α-SMA, and CTGF was consistent with that found in previous studies [47–49]. The upregulation of fibrosis-related genes is related to changes in renal structure in the UUO kidney. Tubular atrophy and dilatation was observed in the UUO kidney as determined using H&E staining. These morphological changes were consistent with those shown in previous studies [50–52]. N-cadherin is a marker of mesenchymal changes in fibrosis, whereas E-cadherin is a hallmark of epithelial features [35]. In the present study, we did not observe a change in EMT marker gene expression in the UUO kidney.
Studies of the effects of piceatannol on renal fibrosis are limited to obese rat models, while the effects of resveratrol have been studied in several animal models, including models of renal ischemia-reperfusion injury, UUO, diabetes (db/db mice), hypertension (spontaneously hypertensive rats, SHR), renovascular hypertension, and obesity (obese Zucker rats) (Table 1). As shown in Table 1, resveratrol has a protective effect against renal fibrosis and renal injury. Therefore, we expect that piceatannol may have similar renal protective effects as those observed for resveratrol.
Table 1. Pharmacological effects of piceatannol and resveratrol on in vivo models of renal diseases.
| Compound | Effects | Target organ | Animal models | References |
|---|---|---|---|---|
| Piceatannol | Mild renoprotective effect | Kidney | Obese Zucker rats | Llarena et al., 2016 [27] |
| Piceatannol | Preventive graft rejection | Kidney | ACI-to-Lewis rats | Fernandez et al., 2002 [26] |
| Resveratrol | • RenoprotectionReduction of renal interstitial fibrosis • Inhibition of EMT and renal fibrosis |
Kidney | UUO rats | • Yang et al., 2016 [29] • Zhang et al., 2016 [67] • Bai et al., 2014 [68] |
| Resveratrol | Attenuation of renal interstitial fibrosis | Kidney | db/db mice | • He et al., 2016 [28] • Yan et al., 2016 [69] |
| Resveratrol | Amelioration of renal injury and tubulointerstitial fibrosis | Kidney | SHR rats | Xue et al., 2016 [70] |
| Resveratrol captopril | Improvement of aortic remodeling and fibrosis | Aorta | 2K1C Goldblatt rats | Natalin et al., 2016 [71] |
| Resveratrol | • Attenuation of renal injury and fibrosis • Protective renal fibrosis |
Kidney | • UUO mice or I/R injury mice • UUO mice • UUO mice |
• Xiao et al., 2016 [30] • Liang et al., 2014 [72] • Li et al., 2010 [48] |
| Resveratrol | Protective renal fibrosis | Kidney | 5/6th nephrectomized rats | Huang et al., 2014 [73] |
| Resveratrol | • Attenuation of diabetic nephropathy • Reduction of renal fibrosis |
Kidney | Streptozotocin-treated rats | • Wen et al., 2013 [74] • Chen et al., 2011 [75] |
| Resveratrol | Renoprotection | Kidney | Unilateral nephrectomized rats | Sener at al., 2006 [76] |
UUO: unilateral ureteral obstruction
EMT: epithelial-mesenchymal transition
I/R injury: ischemia-reperfusion injury
db/db: spontaneous type 2 diabetic animal model
SHR: spontaneously hypertensive rats
2K1C: two-kidney, one-clip model
HDACs are enzymes that remove the acetyl group at the N-terminal region of histone and non-histone proteins [53]. Dysfunction of HDACs is involved in a variety of diseases [53]. For example, overexpression of HDAC1, HDAC2, HDAC4, HDAC6, and HDAC7 has been observed in various cancers. To the best of our knowledge, the present study is the first to show that HDAC1 and most members of class IIa/b HDACs are associated with renal fibrosis. Among the class I HDACs, HDAC1 expression increased in the UUO kidney, which was not reduced by piceatannol treatment. The observed UUO-induced HDAC1 expression was in accord with the finding of a previous report [15]. Further, Tian et al. reported that HDAC1 mRNA and protein expression increased in aristolochic acid I-induced renal fibrosis [54]. Of the class II HDACs assessed, the protein expression of HDAC4, HDAC5, HDAC6, and HDAC10 highly increased in the UUO kidney. Interestingly, HDAC4 and HDAC5 protein expression was downregulated by piceatannol treatment. Considering the findings that the anti-fibrotic effect of piceatannol was related to the reduced expression of HDAC4 and HDAC5, future studies are needed to determine the effect of class IIa-selective HDAC inhibitors (for example LMK235, a selective HDAC4 and HDAC5 inhibitor) in the UUO model. In addition, it is important to investigate whether the anti-fibrotic effect of piceatannol is related to inhibition of HDAC activity. Pharmacological HDAC inhibitors such as trichostatin A or MS-275 were protective against renal fibrosis in a rodent model of UUO [55–57], indicative of an anti-fibrotic effect of HDAC inhibitors via inhibition of HDAC enzyme activity.
Natural products (piceatannol and resveratrol) are known activators of SIRT1, a class III HDAC. Both these polyphenol compounds increased SIRT1 mRNA and protein expression in the THP-1 monocytic cell line [58]. Piceatannol is also known as a dietary HDAC activator, and binds to a conserved N-terminal domain in SIRT1 [59]. In the present study, we did not examine SIRT1 expression in the UUO kidney. However, a recent study showed that renal fibrosis was inhibited by treatment of UUO mice with the SIRT1/2 inhibitor sirtinol or SIRT1 inhibitor EX527 [60]. This result raises doubts about the protective role of SIRT1 in renal fibrosis.
However, our results showed that administration of piceatannol reduced the UUO-induced class II HDACs (HDAC4 and 5) protein levels. This finding indicates that piceatannol may be considered to be acting a dietary HDAC inhibitor rather than a HDAC activator in this model. As mentioned above, our findings are supported by several studies showing that renal fibrosis was attenuated by the pan-HDAC inhibitor TSA [57], class I HDAC inhibitor MS-275 [56], and HDAC6-selective inhibitor Tubastatin A [16].
Piceatannol is a metabolite of resveratrol and is present in red wine, grapes, berries, and several plants. Sim fruit (Rhodomyrtus tomentosa) has a higher piceatannol content than that of blueberries and red grapes [61]. Piceatannol inhibits cancer, inflammation, cardiac hypertrophy, and adipogenesis [24,62,63]. Furthermore, a recent study showed a renoprotective effect of piceatannol by attenuating early-stage nephropathy associated with obesity [64], suggesting the potential efficacy of piceatannol in preventing renal fibrosis.
The area under the plasma concentration curve (AUC) represents the bioavailability of a drug as determined using the plasma drug concentrations. The AUC for piceatannol (8.6 μmol/h/L) was higher than that for resveratrol (4.1 μmol/h/L), suggesting that piceatannol has a higher metabolic stability [65]. A recent report showed enhanced absorption of piceatannol in rats when complexed with α-cyclodextrin [66]. Therefore, we hypothesize that piceatannol can be a useful phytochemical compound to treat or prevent renal fibrosis without causing side-effects.
In summary, our data show that piceatannol can suppress renal fibrosis as well as the expression of fibrosis-related genes in the UUO kidney. The anti-fibrotic effect of piceatannol may be associated with the downregulation of HDAC4 and HDAC5 in kidney fibrosis. Piceatannol inhibits the p38-MAPK signaling pathway, but not the TGF-β/Smad-dependent pathway. Taken together, we suggest that piceatannol can be a potential therapeutic agent in the treatment of renal fibrosis development.
Abbreviations
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- ERK1/2
extracellular signal-regulated kinase
- HDAC
histone deacetylase
- H&E
hematoxylin and eosin
- JNK
c-Jun terminal kinase
- MAPK
mitogen-activated protein kinase
- α-SMA
α-smooth muscle actin
- TGF-β1
transforming growth factor beta1
- TβRI
TGFβ receptor I
- TβRII
TGFβ receptor II
- UUO
unilateral ureteral obstruction
- qRT-PCR
quantitative real time polymerase chain reaction
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1527). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A01056798). The funders had no role in study design, data collection and analysis, decision to publish, or preparataion of the manuscript.
References
- 1.Qi W., Chen X., Poronnik P., Pollock C. A. The renal cortical fibroblast in renal tubulointerstitial fibrosis. Int J Biochem Cell Biol 2006, 38,1–5. 10.1016/j.biocel.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Nakatsuji S., Yamate J., Sakuma S. Macrophages, myofibroblasts, and extracellular matrix accumulation in interstitial fibrosis of chronic progressive nephropathy in aged rats. Vet Pathol 1998, 35,352–360. [DOI] [PubMed] [Google Scholar]
- 3.Moon J. A., Kim H. T., Cho I. S., Sheen Y. Y., Kim D. K. IN-1130, a novel transforming growth factor-beta type I receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in obstructive nephropathy. Kidney Int 2006, 70,1234–1243. 10.1038/sj.ki.5001775 [DOI] [PubMed] [Google Scholar]
- 4.Kanasaki K., Taduri G., Koya D. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol (Lausanne) 2013, 4,7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisberg M., Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol 2013, 304,C216–225. 10.1152/ajpcell.00328.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takenaka M., Machida N., Ida N., Satoh N., Kurumatani H., Yamane Y. Effect of beraprost sodium (BPS) in a new rat partial unilateral ureteral obstruction model. Prostaglandins Leukot Essent Fatty Acids 2009, 80,263–267. 10.1016/j.plefa.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 7.Chevalier R. L., Forbes M. S., Thornhill B. A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 2009, 75,1145–1152. 10.1038/ki.2009.86 [DOI] [PubMed] [Google Scholar]
- 8.Ma N., Luo Y., Wang Y., Liao C., Ye W. C., Jiang S. Selective Histone Deacetylase Inhibitors with Anticancer Activity. Curr Top Med Chem 2016, 16,415–426. [DOI] [PubMed] [Google Scholar]
- 9.Kee H. J., Sohn I. S., Nam K. I., Park J. E., Qian Y. R., Yin Z. et al. , Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation 2006, 113,51–59. 10.1161/CIRCULATIONAHA.105.559724 [DOI] [PubMed] [Google Scholar]
- 10.Leoni F., Fossati G., Lewis E. C., Lee J. K., Porro G., Pagani P. et al. , The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol Med 2005, 11,1–15. 10.2119/2006-00005.Dinarello [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kee H. J., Bae E. H., Park S., Lee K. E., Suh S. H., Kim S. W. et al. , HDAC inhibition suppresses cardiac hypertrophy and fibrosis in DOCA-salt hypertensive rats via regulation of HDAC6/HDAC8 enzyme activity. Kidney Blood Press Res 2013, 37,229–239. 10.1159/000350148 [DOI] [PubMed] [Google Scholar]
- 12.Liu N., Zhuang S. Treatment of chronic kidney diseases with histone deacetylase inhibitors. Front Physiol 2015, 6,121 10.3389/fphys.2015.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi S. Y., Kee H. J., Kurz T., Hansen F. K., Ryu Y., Kim G. R. et al. , Class I HDACs specifically regulate E-cadherin expression in human renal epithelial cells. J Cell Mol Med 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noh H., Oh E. Y., Seo J. Y., Yu M. R., Kim Y. O., Ha H. et al. , Histone deacetylase-2 is a key regulator of diabetes- and transforming growth factor-beta1-induced renal injury. Am J Physiol Renal Physiol 2009, 297,F729–739. 10.1152/ajprenal.00086.2009 [DOI] [PubMed] [Google Scholar]
- 15.Marumo T., Hishikawa K., Yoshikawa M., Hirahashi J., Kawachi S., Fujita T. Histone deacetylase modulates the proinflammatory and -fibrotic changes in tubulointerstitial injury. Am J Physiol Renal Physiol 2010, 298,F133–141. 10.1152/ajprenal.00400.2009 [DOI] [PubMed] [Google Scholar]
- 16.Choi S. Y., Ryu Y., Kee H. J., Cho S. N., Kim G. R., Cho J. Y. et al. , Tubastatin A suppresses renal fibrosis via regulation of epigenetic histone modification and Smad3-dependent fibrotic genes. Vascul Pharmacol 2015, 72,130–140. 10.1016/j.vph.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 17.Khan S., Jena G., Tikoo K. Sodium valproate ameliorates diabetes-induced fibrosis and renal damage by the inhibition of histone deacetylases in diabetic rat. Exp Mol Pathol 2015, 98,230–239. 10.1016/j.yexmp.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 18.Seyed M. A., Jantan I., Bukhari S. N., Vijayaraghavan K. A Comprehensive Review on the Chemotherapeutic Potential of Piceatannol for Cancer Treatment, with Mechanistic Insights. J Agric Food Chem 2016, 64,725–737. 10.1021/acs.jafc.5b05993 [DOI] [PubMed] [Google Scholar]
- 19.Ko Y. J., Kim H. H., Kim E. J., Katakura Y., Lee W. S., Kim G. S. et al. , Piceatannol inhibits mast cell-mediated allergic inflammation. Int J Mol Med 2013, 31,951–958. 10.3892/ijmm.2013.1283 [DOI] [PubMed] [Google Scholar]
- 20.Kwon J. Y., Seo S. G., Heo Y. S., Yue S., Cheng J. X., Lee K. W. et al. , Piceatannol, natural polyphenolic stilbene, inhibits adipogenesis via modulation of mitotic clonal expansion and insulin receptor-dependent insulin signaling in early phase of differentiation. J Biol Chem 2012, 287,11566–11578. 10.1074/jbc.M111.259721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kee H. J., Park S., Kang W., Lim K. S., Kim J. H., Ahn Y. et al. , Piceatannol attenuates cardiac hypertrophy in an animal model through regulation of the expression and binding of the transcription factor GATA binding factor 6. FEBS Lett 2014, 588,1529–1536. 10.1016/j.febslet.2014.03.027 [DOI] [PubMed] [Google Scholar]
- 22.Son Y., Chung H. T., Pae H. O. Differential effects of resveratrol and its natural analogs, piceatannol and 3,5,4'-trans-trimethoxystilbene, on anti-inflammatory heme oxigenase-1 expression in RAW264.7 macrophages. Biofactors 2014, 40,138–145. 10.1002/biof.1108 [DOI] [PubMed] [Google Scholar]
- 23.Jeong S. O., Son Y., Lee J. H., Cheong Y. K., Park S. H., Chung H. T. et al. , Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol Med Rep 2015, 12,937–944. 10.3892/mmr.2015.3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko H. S., Lee H. J., Kim S. H., Lee E. O. Piceatannol suppresses breast cancer cell invasion through the inhibition of MMP-9: involvement of PI3K/AKT and NF-kappaB pathways. J Agric Food Chem 2012, 60,4083–4089. 10.1021/jf205171g [DOI] [PubMed] [Google Scholar]
- 25.Piotrowska H., Kucinska M., Murias M. Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat Res 2012, 750,60–82. 10.1016/j.mrrev.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 26.Fernandez L. A., Torrealba J., Yagci G., Ishido N., Tsuchida M., Tae Kim H. et al. , Piceatannol in combination with low doses of cyclosporine A prolongs kidney allograft survival in a stringent rat transplantation model. Transplantation 2002, 74,1609–1617. 10.1097/01.TP.0000041447.91134.24 [DOI] [PubMed] [Google Scholar]
- 27.Llarena M., Andrade F., Hasnaoui M., Portillo M. P., Perez-Matute P., Arbones-Mainar J. M. et al. , Potential renoprotective effects of piceatannol in ameliorating the early-stage nephropathy associated with obesity in obese Zucker rats. J Physiol Biochem 2016, 72,555–566. 10.1007/s13105-015-0457-1 [DOI] [PubMed] [Google Scholar]
- 28.He T., Xiong J., Nie L., Yu Y., Guan X., Xu X. et al. , Resveratrol inhibits renal interstitial fibrosis in diabetic nephropathy by regulating AMPK/NOX4/ROS pathway. J Mol Med (Berl) 2016. [DOI] [PubMed] [Google Scholar]
- 29.Yang S. Y., Lin S. L., Chen Y. M., Wu V. C., Yang W. S., Wu K. D. Downregulation of angiotensin type 1 receptor and nuclear factor-kappaB by sirtuin 1 contributes to renoprotection in unilateral ureteral obstruction. Sci Rep 2016, 6,33705 10.1038/srep33705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Z., Chen C., Meng T., Zhang W., Zhou Q. Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-beta pathway on matrix metalloproteinase 7. Exp Biol Med (Maywood) 2016, 241,140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kee H. J., Kim J. R., Nam K. I., Park H. Y., Shin S., Kim J. C. et al. , Enhancer of polycomb1, a novel homeodomain only protein-binding partner, induces skeletal muscle differentiation. J Biol Chem 2007, 282,7700–7709. 10.1074/jbc.M611198200 [DOI] [PubMed] [Google Scholar]
- 32.Desai V. D., Hsia H. C., Schwarzbauer J. E. Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS One 2014, 9,e86865 10.1371/journal.pone.0086865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brigstock D. R. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal 2010, 4,1–4. 10.1007/s12079-009-0071-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalluri R., Neilson E. G. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003, 112,1776–1784. 10.1172/JCI20530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriz W., Kaissling B., Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest 2011, 121,468–474. 10.1172/JCI44595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takagi D., Nakamaru Y., Suzuki M., Fukuda S. Dysregulation of histone deacetylase and histone acetyltransferase in development of Wegener's granulomatosis. Ann Otol Rhinol Laryngol 2012, 121,816–820. [DOI] [PubMed] [Google Scholar]
- 37.Yang X. J., Gregoire S. Class II histone deacetylases: from sequence to function, regulation, and clinical implication. Mol Cell Biol 2005, 25,2873–2884. 10.1128/MCB.25.8.2873-2884.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng X. M., Tang P. M., Li J., Lan H. Y. TGF-beta/Smad signaling in renal fibrosis. Front Physiol 2015, 6,82 10.3389/fphys.2015.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma F. Y., Tesch G. H., Nikolic-Paterson D. J. ASK1/p38 signaling in renal tubular epithelial cells promotes renal fibrosis in the mouse obstructed kidney. Am J Physiol Renal Physiol 2014, 307,F1263–1273. 10.1152/ajprenal.00211.2014 [DOI] [PubMed] [Google Scholar]
- 40.Li Z., Liu X., Wang B., Nie Y., Wen J., Wang Q. et al. , Pirfenidone suppresses MAPK signaling pathway to reverse epithelial-mesenchymal transition and renal fibrosis. Nephrology (Carlton) 2016. [DOI] [PubMed] [Google Scholar]
- 41.Xu W., Shao X., Tian L., Gu L., Zhang M., Wang Q. et al. , Astragaloside IV ameliorates renal fibrosis via the inhibition of mitogen-activated protein kinases and antiapoptosis in vivo and in vitro. J Pharmacol Exp Ther 2014, 350,552–562. 10.1124/jpet.114.214205 [DOI] [PubMed] [Google Scholar]
- 42.Meng X. M., Nikolic-Paterson D. J., Lan H. Y. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 2016, 12,325–338. 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- 43.Altrock E., Sens C., Wuerfel C., Vasel M., Kawelke N., Dooley S. et al. , Inhibition of fibronectin deposition improves experimental liver fibrosis. J Hepatol 2015, 62,625–633. 10.1016/j.jhep.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 44.Lenselink E. A. Role of fibronectin in normal wound healing. Int Wound J 2015, 12,313–316. 10.1111/iwj.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strutz F., Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol 2006, 17,2992–2998. 10.1681/ASN.2006050420 [DOI] [PubMed] [Google Scholar]
- 46.Rao K. B., Malathi N., Narashiman S., Rajan S. T. Evaluation of myofibroblasts by expression of alpha smooth muscle actin: a marker in fibrosis, dysplasia and carcinoma. J Clin Diagn Res 2014, 8,ZC14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J., Hwang I., Lee J. H., Lee H. W., Jeong L. S., Ha H. The selective A3AR antagonist LJ-1888 ameliorates UUO-induced tubulointerstitial fibrosis. Am J Pathol 2013, 183,1488–1497. 10.1016/j.ajpath.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 48.Li J., Qu X., Ricardo S. D., Bertram J. F., Nikolic-Paterson D. J. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol 2010, 177,1065–1071. 10.2353/ajpath.2010.090923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pradere J. P., Klein J., Gres S., Guigne C., Neau E., Valet P. et al. , LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol 2007, 18,3110–3118. 10.1681/ASN.2007020196 [DOI] [PubMed] [Google Scholar]
- 50.Jang H. S., Padanilam B. J. Simultaneous deletion of Bax and Bak is required to prevent apoptosis and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 2015, 309,F540–550. 10.1152/ajprenal.00170.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tasanarong A., Kongkham S., Thitiarchakul S., Eiam-Ong S. Vitamin E ameliorates renal fibrosis in ureteral obstruction: role of maintaining BMP-7 during epithelial-to-mesenchymal transition. J Med Assoc Thai 2011, 94 Suppl 7,S10–18. [PubMed] [Google Scholar]
- 52.Pulskens W. P., Rampanelli E., Teske G. J., Butter L. M., Claessen N., Luirink I. K. et al. , TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J Am Soc Nephrol 2010, 21,1299–1308. 10.1681/ASN.2009070722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bassett S. A., Barnett M. P. The role of dietary histone deacetylases (HDACs) inhibitors in health and disease. Nutrients 2014, 6,4273–4301. 10.3390/nu6104273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian Y., Yang Y., Gao L., Zhao H., Peng X., Zhang Z. et al. , Expression of histone deacetylase-1 and p300 in aristolochic acid nephropathy models. Toxicol Mech Methods 2014, 24,377–384. 10.3109/15376516.2014.920448 [DOI] [PubMed] [Google Scholar]
- 55.Manson S. R., Song J. B., Hruska K. A., Austin P. F. HDAC dependent transcriptional repression of Bmp-7 potentiates TGF-beta mediated renal fibrosis in obstructive uropathy. J Urol 2014, 191,242–252. 10.1016/j.juro.2013.06.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu N., He S., Ma L., Ponnusamy M., Tang J., Tolbert E. et al. , Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling. PLoS One 2013, 8,e54001 10.1371/journal.pone.0054001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pang M., Kothapally J., Mao H., Tolbert E., Ponnusamy M., Chin Y. E. et al. , Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 2009, 297,F996–F1005. 10.1152/ajprenal.00282.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawakami S., Kinoshita Y., Maruki-Uchida H., Yanae K., Sai M., Ito T. Piceatannol and its metabolite, isorhapontigenin, induce SIRT1 expression in THP-1 human monocytic cell line. Nutrients 2014, 6,4794–4804. 10.3390/nu6114794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinclair D. A., Guarente L. Small-molecule allosteric activators of sirtuins. Annu Rev Pharmacol Toxicol 2014, 54,363–380. 10.1146/annurev-pharmtox-010611-134657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ponnusamy M., Zhou X., Yan Y., Tang J., Tolbert E., Zhao T. C. et al. , Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. J Pharmacol Exp Ther 2014, 350,243–256. 10.1124/jpet.113.212076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lai T. N., Herent M. F., Quetin-Leclercq J., Nguyen T. B., Rogez H., Larondelle Y. et al. , Piceatannol, a potent bioactive stilbene, as major phenolic component in Rhodomyrtus tomentosa. Food Chem 2013, 138,1421–1430. 10.1016/j.foodchem.2012.10.125 [DOI] [PubMed] [Google Scholar]
- 62.Song H., Jung J. I., Cho H. J., Her S., Kwon S. H., Yu R. et al. , Inhibition of tumor progression by oral piceatannol in mouse 4T1 mammary cancer is associated with decreased angiogenesis and macrophage infiltration. J Nutr Biochem 2015, 26,1368–1378. 10.1016/j.jnutbio.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 63.Kita Y., Miura Y., Yagasaki K. Antiproliferative and anti-invasive effect of piceatannol, a polyphenol present in grapes and wine, against hepatoma AH109A cells. J Biomed Biotechnol 2012, 2012,672416 10.1155/2012/672416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Llarena M., Andrade F., Hasnaoui M., Portillo M. P., Perez-Matute P., Arbones-Mainar J. M. et al. , Potential renoprotective effects of piceatannol in ameliorating the early-stage nephropathy associated with obesity in obese Zucker rats. J Physiol Biochem 2015. [DOI] [PubMed] [Google Scholar]
- 65.Setoguchi Y., Oritani Y., Ito R., Inagaki H., Maruki-Uchida H., Ichiyanagi T. et al. , Absorption and metabolism of piceatannol in rats. J Agric Food Chem 2014, 62,2541–2548. 10.1021/jf404694y [DOI] [PubMed] [Google Scholar]
- 66.Inagaki H., Ito R., Setoguchi Y., Oritani Y., Ito T. Administration of Piceatannol Complexed with alpha-Cyclodextrin Improves Its Absorption in Rats. J Agric Food Chem 2016, 64,3557–3563. 10.1021/acs.jafc.6b00398 [DOI] [PubMed] [Google Scholar]
- 67.Zhang C., Zhou Y., Lu Y., Wang D. Regulation of eIF2alpha expression and renal interstitial fibrosis by resveratrol in rat renal tissue after unilateral ureteral obstruction. Ren Fail 2016, 38,622–628. 10.3109/0886022X.2016.1149774 [DOI] [PubMed] [Google Scholar]
- 68.Bai Y., Lu H., Wu C., Liang Y., Wang S., Lin C. et al. , Resveratrol inhibits epithelial-mesenchymal transition and renal fibrosis by antagonizing the hedgehog signaling pathway. Biochem Pharmacol 2014, 92,484–493. 10.1016/j.bcp.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 69.Yan C., Xu W., Huang Y., Li M., Shen Y., You H. et al. , HRD1-Mediated IGF-1R Ubiquitination Contributes to Renal Protection of Resveratrol in db/db Mice. Mol Endocrinol 2016, 30,600–613. 10.1210/me.2015-1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xue H. Y., Yuan L., Cao Y. J., Fan Y. P., Chen X. L., Huang X. Z. Resveratrol ameliorates renal injury in spontaneously hypertensive rats by inhibiting renal micro-inflammation. Biosci Rep 2016, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Natalin H. M., Garcia A. F., Ramalho L. N., Restini C. B. Resveratrol improves vasoprotective effects of captopril on aortic remodeling and fibrosis triggered by renovascular hypertension. Cardiovasc Pathol 2016, 25,116–119. 10.1016/j.carpath.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 72.Liang J., Tian S., Han J., Xiong P. Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. Ren Fail 2014, 36,285–291. 10.3109/0886022X.2013.844644 [DOI] [PubMed] [Google Scholar]
- 73.Huang X. Z., Wen D., Zhang M., Xie Q., Ma L., Guan Y. et al. , Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-beta/Smad3 pathway. J Cell Biochem 2014, 115,996–1005. 10.1002/jcb.24748 [DOI] [PubMed] [Google Scholar]
- 74.Wen D., Huang X., Zhang M., Zhang L., Chen J., Gu Y. et al. , Resveratrol attenuates diabetic nephropathy via modulating angiogenesis. PLoS One 2013, 8,e82336 10.1371/journal.pone.0082336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen K. H., Hung C. C., Hsu H. H., Jing Y. H., Yang C. W., Chen J. K. Resveratrol ameliorates early diabetic nephropathy associated with suppression of augmented TGF-beta/smad and ERK1/2 signaling in streptozotocin-induced diabetic rats. Chem Biol Interact 2011, 190,45–53. 10.1016/j.cbi.2011.01.033 [DOI] [PubMed] [Google Scholar]
- 76.Sener G., Tugtepe H., Yuksel M., Cetinel S., Gedik N., Yegen B. C. Resveratrol improves ischemia/reperfusion-induced oxidative renal injury in rats. Arch Med Res 2006, 37,822–829. 10.1016/j.arcmed.2006.04.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.