Abstract
The aim of this study was to evaluate the in vitro activity of the synthetic peptide hLF1-11 against biofilm produced by clinical isolates of Candida albicans with different fluconazole susceptibility. The antibiofilm activity of the peptide hLF1-11 was assessed in terms of reduction of biofilm cellular density, metabolic activity and sessile cell viability. The extent of morphogenesis in hLF1-11 treated and untreated biofilms was also investigated microscopically. Transcription levels of genes related to cell adhesion, hyphal development and extracellular matrix production were analysed by qRT-PCR in hLF1-11 treated and untreated biofilms. Exogenous dibutyryl-cAMP (db-cAMP) was used to rescue morphogenesis in cells exposed to the peptide. The results revealed that hLF1-11 exhibited an inhibitory effect on biofilm formation by all C. albicans isolates tested in a dose-dependent manner, regardless of their fluconazole susceptibility. Visual inspection of treated or untreated biofilm cells with an inverted microscope revealed a significant reduction in hyphal formation by hLF1-11 treated cells, as early as 3 hours of incubation. Moreover, hLF1-11 showed a reduced activity on preadherent cells. hLF1-11 induced the down-regulation of biofilm and hyphal-associated genes, which were predominantly regulated via the Ras1-cAMP-Efg1 pathway. Indeed, exogenous db-cAMP restored morphogenesis in hLF1-11 treated cells. The hLF1-11 peptide significantly inhibited biofilm formation by C. albicans mainly at early stages, interfering with biofilm cellular density and metabolic activity, and affected morphogenesis through the Ras1-cAMP-Efg1 pathway. Our findings provide the first evidence that hLF1-11 could represent a potential candidate for the prevention of biofilm formation by C. albicans.
Introduction
Candida albicans is an opportunistic yeast, responsible for systemic infections in individuals with impaired immune response [1]. Nosocomial C. albicans infections are often related to the ability to produce biofilm on mucosal surfaces and implanted medical devices [2–4]. Biofilm formation is a finely regulated process, which involves multiple interconnected signalling pathways [5], leading to a structured microbial community that is attached to a surface and embedded in an exopolymeric extracellular matrix [2,6,7]. The polysaccharide matrix acts as a barrier for sessile cells, preventing the entrance of most commonly used antifungal agents, thus conferring drug resistance. Indeed, sessile C. albicans cells are up to 1000-fold more resistant to common antifungals than planktonic cells, even in the absence of specific drug-resistance genes [8,9]. Thereby, biofilm is a reservoir of viable fungal cells potentially leading to systemic infections, with a mortality rate of 40–60% [10,11].
Increasing efforts are currently underway in developing alternative strategies aimed at eradicating biofilm-related infections. Among these, antimicrobial peptides (AMPs) have been widely investigated as novel therapeutic agents [12,13].
Human lactoferrin (hLF) is an iron-binding glycoprotein (77 kDa) synthesized by mucosal gland epithelial cells and neutrophils in response to inflammatory stimuli [14]. By pepsinolysis, hLF releases lactoferricin H (residues 1 to 47) from its N-terminus, which contains two cationic domains (residues 2–5 and 28–31) [15,16]. A synthetic peptide comprising the first cationic domain of lactoferricin H, further referred to as hLF1-11, possesses a high antibacterial and antifungal activity, with a more pronounced effect in vivo [17,18]. Furthermore, hLF1-11 exerts modulatory effects on immune cells stimulating macrophages and dendritic cells, thus contributing to the clearance of infections [19,20]. In addition, previous studies have shown that hLF1-11 has no cytotoxic effects against human erythrocytes, and its administration to healthy volunteers was well tolerated [21].
Until now, the exact mechanisms of action of hLF1-11 are not fully characterised, despite mitochondria seem to be a target of hLF1-11 in C. albicans [22].
To date, no evidence is available on the role played by the hLF1-11 peptide on biofilm production by C. albicans, although immobilizing hLF1-11 onto titanium surfaces of implants reduced bacterial adhesion and biofilm formation [23].
This study was aimed at i) evaluating the potential inhibitory activity of hLF1-11 against biofilm formation of clinical isolates of C. albicans characterised by different fluconazole susceptibility, and ii) improving our understanding of the mechanism(s) underlying the hLF1-11-induced antibiofilm activity.
Materials and Methods
Strains
A panel of Candida albicans strains including ten clinical isolates collected at the U. O. Microbiologia Universitaria, Azienda Ospedaliero-Universitaria Pisana (Pisa, Italy) and the reference strain SC5314 were investigated for biofilm production. Furthermore, fluconazole susceptibility was determined by Vitek 2 system (AST-YS07 card; bioMérieux, Marcy l’Étoile, France). All strains were stored in YPD broth (Yeast Peptone Dextrose, Difco BD, Milan, Italy) supplemented with 40% (vol/vol) glycerol at -20°C and -80°C, subcultured at 37°C on YPD agar plates, and kept at 4°C until testing. After 16h incubation at 30°C, cells were washed in sodium phosphate buffer (NaPB, pH 7) and suspended at the desired concentration.
Lactoferrin peptide
The synthetic peptide corresponding to the first eleven residues of human lactoferrin (GRRRRSVQWCA; molecular mass, 1374.6 Da), further referred to as hLF1-11, was purified by Peptisyntha Inc (Torrance, CA, USA), with purity exceeding 95%. Stocks (10mM in 0.01% acetic acid; pH 3.7) were stored at -20°C.
hLF1-11-induced candidacidal activity on planktonic cells
The hLF1-11 antifungal activity was tested by the broth microdilution method according to CLSI standard M27-A3 with minor modifications [24]. Briefly, a final suspension of 1×103 cells/ml in RPMI 1640 medium (diluted 1:4 in NaPB) was adjusted to pH 7 with 0.165 3-(N-morpholino)-propanesulfonic acid (MOPS; Sigma Aldrich, St. Louis, USA) and inoculated in polystyrene, round-bottomed, 96-well microtiter plates (Falcon, Becton Dickinson BD, Milan, Italy) to reach a final volume of 100μl/well. hLF1-11 concentrations tested were 0.17-176mg/L. Following incubation at 37°C for 24h, MIC values were determined as the lowest peptide concentration inhibiting fungal growth. Two sets of independent experiments were performed, each in duplicate.
Inhibition of biofilm formation
Fungal suspensions were prepared at 2×106 cells/ml in four-fold diluted RPMI 1640 medium supplemented with glucose (2% final concentration) and buffered with MOPS. An aliquot of each yeast suspension was transferred in polystyrene, flat-bottomed, 96-well microtiter plates and incubated with different hLF1-11 concentrations (44, 88, 176mg/L) at 37°C for 24h, in a final volume of 100μl/well. The inhibitory activity of hLF1-11 on C. albicans biofilm formation was also evaluated at earlier time points, including 1.5h, 3h, 6h and, as control, 24h, using a peptide concentration of 88mg/L. A positive and negative control were included in each experiment.
After incubation, non-adhered cells were removed by washing twice with phosphate buffered saline (PBS). The antibiofilm activity of hLF1-11 was evaluated by i) spectrophotometrically measuring the biofilm cellular density; (ii) quantifying metabolic activity by XTT assay, and (iii) counting viable sessile cells (CFU/ml) after biofilm scraping from wells. Three independent experiments were performed, each in triplicate.
Biofilm cellular density
Biofilm cellular density formed in the presence or absence of the peptide was determined by measuring the optical density at 490 nm (ODλ490nm) using an automated plate reader (Model 550 Microplate Reader Bio-Rad, Milan Italy). Background optical density was subtracted from the values measured in each well.
XTT assay
XTT solution was prepared at 0.5g/L in PBS buffer and mixed with a menadione solution dissolved in acetone at a final concentration of 1μM. An aliquot of 100μl of this solution was inoculated into each well containing dry preformed biofilms and the 96-well plate was incubated in the dark at 37°C for 2h. Next, the supernatant (80μL) was transferred into a 96-well plate to measure colorimetric changes at 490 nm (Model 550 Microplate Reader).
Sessile cell viability
The recovery of sessile cells from biofilm formed on the well bottom was next evaluated. Briefly, after washings, 200μl of PBS was added to the wells and adhered cells were detached by scraping with the micropipette tip. Recovered cells were transferred to tubes containing 800μl of PBS and vortexed for 5 minutes. Suspensions were then sonicated (VWR Ultrasonic Cleanear, 230V/50-60Hz), vortexed, and serially diluted. Aliquots (200μl) of each dilution were plated on YPD agar and colony counts were performed after 24h incubation at 37°C.
hLF1-11 activity on C. albicans cell morphology
In order to assess the hLF1-11-induced effect on the architecture of biofilm formed by C. albicans, biofilms produced by four representative C. albicans strains (SC5314, CA22, CA37 and CA688) were visualised under an inverted microscope (Olympus IMT-2) at 400× magnification, following 24h exposure to the peptide.
Activity of hLF1-11 on pre-adhered C. albicans cells
C. albicans SC5314 (1×106 cells/mL) was suspended in diluted RPMI 1640 supplemented with 2% glucose and buffered with MOPS. Aliquots (100μL) of fungal suspension were inoculated into 96-well plates, and incubated for 1.5h or 6h permitting fungal adhesion. In parallel, plates were incubated for 24h to allow complete biofilm development, acting as a positive control. Following incubation, non-adhered cells were removed by washing with sterile PBS, and hLF1-11 (100μL) at various concentrations (44, 88 and 176mg/L) was added to each well. Next, plates were further incubated for 24h at 37°C for biofilm formation. Subsequently, evaluation of biofilm cellular density, metabolic activity, and cell viability was carried out as previously described. Three independent experiments were performed, each in triplicate.
Quantitative real time RT-PCR (qRT-PCR) analysis of C. albicans biofilm-related genes
qRT-PCR was used to investigate hLF1-11-induced changes in transcription level of genes related to biofilm formation. Genes involved in the Ras1-cAMP-Efg1 pathway (RAS1, EFG1, CYR1), MAP kinases pathway (HST7), Cph2-Tec1 pathway (TEC1), hyphal-specific genes (ALS3, HWP1, ECE1), and genes related to the production of extracellular matrix (GSC1, ZAP1, ADH5 and CSH1) were evaluated. C. albicans SC5314 cells (1×106 cells/mL) were grown in the absence or presence of hLF1-11 (88 mg/L) in 24-well plates for 24 h at 37°C. Next, wells were washed with PBS and adhered cells were removed from the bottom of the wells by scraping (5 wells/sample). Total RNA was extracted from sessile cells with the Nucleospin RNA (Macherey Nagel, Duren, Germany) according to manufacturer’s instructions and stored at -80°C. The quality and quantity of the extracted RNA were determined spectrophotometrically. Total RNA (1μg) was converted into cDNA with random primers in a 20μL reaction volume, using the Reverse Transcription System kit (Promega), following manufacturer’s instructions. Primer sequences used for amplification of specific genes are shown in S1 Table. qRT-PCR mixtures contained 6μL cDNA, 10μL SYBR Green Master Mix (Applied Biosystem, Life technologies, Monza, Italy), 1pMol/μL of each primer, and sterile MilliQ water to a final volume of 20μL. qRT-PCR was performed in a 96-well plates on CFX96 Touch Real-Time PCR Detection System (BioRad) (95°C for 60s, followed by 40 cycles of 95°C for 5s, 60°C for 30s). Actin (ACT1) was used as internal control. The transcription level of the selected genes was calculated using the formula of 2-ΔΔCt. Three independent experiments were performed, each in triplicate.
cAMP rescue experiments
In order to verify the role of hLF1-11 in the inhibition of Ras1-cAMP-Efg1 pathway, the effect of exogenous dibutyrylcAMP (db-cAMP; Sigma Aldrich, Milan Italy) on fungal cells treated with the peptide was evaluated. Briefly, overnight C. albicans SC5314 cells (1×106 cells/mL) were aliquoted into wells of a 24-well plate (1 mL/per well). A100 mM stock of db-cAMP solution was prepared in sterile water and stored at -20°C. Db-cAMP was added to the fungal suspension to a final concentration of 5mM, immediately following the addition of 44mg/L hLF1-11. The untreated cells with or without db-cAMP served as control. Each sample was tested in triplicate. After incubation at 37°C for 4h, cells were recovered (5,000×g for 5min) and visualised (Olympus) at a 400× magnification. The percentage of cells growing as yeast, pseudohyphae and hyphae was determined from a mean of three replicate wells.
Statistical analysis
Data were expressed as means±standard error of the mean (S.E.M.). Results were evaluated by one-way ANOVA test, followed by the Tukey-Kramer post-hoc test, using GraphPad Instat software (version 6.05 for Windows, La Jolla, CA USA). The level of significance was set at a P value of < 0.05.
Results
hLF1-11-induced candidacidal activity on planktonic cells
The hLF1-11-induced candidacidal activity on planktonic cells of ten clinical isolates and the reference strain SC5314 was evaluated (Table 1). All strains exhibited similar susceptibility to hLF1-11 with MIC values ranging from 22 to 44 mg/L (Table 1). No difference in hLF1-11 MIC values was found between fluconazole-resistant (CA688), and -susceptible clinical isolates (Table 1).
Table 1. MICs of fluconazole and hLF1-11 against planktonic cells of C. albicans clinical isolates and the reference strain SC5314.
| Strain | Source | Fluconazole MIC (mg/L)a | hLF1-11 MIC (mg/L) |
|---|---|---|---|
| SC5314 | Reference strain | ≤1 (S) | 22 |
| CA28 | Blood culture | ≤1 (S) | 22 |
| CA688 | Bronchoalveolar lavage | 64 (R) | 22 |
| CA22 | Oral swab | ≤1 (S) | 22 |
| CA5 | Oral swab | ≤1 (S) | 44 |
| CA31 | Oral swab | ≤1 (S) | 44 |
| CA37 | Vaginal swab | ≤1 (S) | 44 |
| CA3 | Vaginal swab | ≤1 (S) | 22 |
| CA17 | Vaginal swab | ≤1 (S) | 22 |
| CA18 | Vaginal swab | ≤1 (S) | 44 |
| CA35 | Vaginal swab | ≤1 (S) | 22 |
aInterpretation of fluconazole susceptibility was performed according to the EUCAST (2015) clinical interpretive breakpoints: ≤2mg/L Susceptible (S), >4mg/L Resistant (R)
hLF1-11 antibiofilm activity
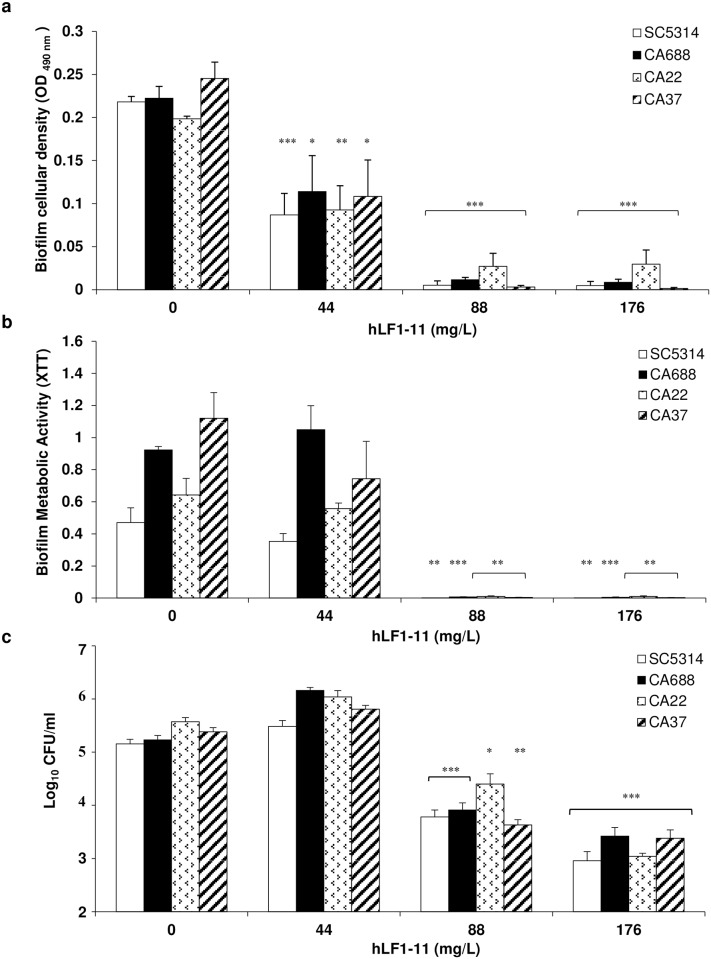
The antibiofilm activity of hLF1-11 was evaluated against C. albicans reference strain SC5314 and on the clinical isolates (CA688, CA37, CA22) previously selected based on different RAPD profiles (data not shown), propensity to produce biofilm (data not shown), and fluconazole susceptibility (Table 1). Fungal cells were incubated in the absence and presence of different peptide concentrations (44, 88 and 176mg/L). hLF1-11 induced a significant reduction in biofilm cellular density, metabolic activity and number of viable cells, (P<0.05), in all the tested strains (Fig 1). The antibiofilm activity was shown to occur in a dose-dependent manner. The results revealed a 50% reduction in cellular density values after incubation with 44 mg/L hLF1-11, compared to the untreated biofilm and a 100% reduction with doses higher than 88mg/L (Fig 1a). Furthermore, a significant reduction in metabolic activity was observed in the presence of the peptide at 88 and 176mg/L (100%; P <0.05), compared to the untreated control (Fig 1b), while no statistically significant reduction was observed at the lowest concentration (44mg/L). The hLF1-11 peptide also induced 1- and 2-log reduction in cell viability (CFU/mL) at 88 and 176mg/L, respectively (Fig 1c). No decrease in cell viability was observed at the lowest dose of hLF1-11 tested.
Fig 1. Effect of hLF1-11 on biofilm formation by four representative C. albicans strains.
C. albicans cells (1x106 cells/ml) were co-incubated with various concentrations of hLF1-11 for 24h at 37°C. After incubation, the antibiofilm activity of the peptide was assessed in terms of (a) biofilm cellular density reduction, (b) metabolic activity by the XTT assay, and c reduction of sessile cell viability. Data are expressed as the mean of three independent experiments ± SEM. SC5314 open bars, CA688 closed bars, CA22 dotted bars, CA37 diagonally hatched bars * P≤0.05, ** P<0.01, *** P<0.001, as compared to the untreated control biofilms.
Kinetics of hLF1-11 antibiofilm activity
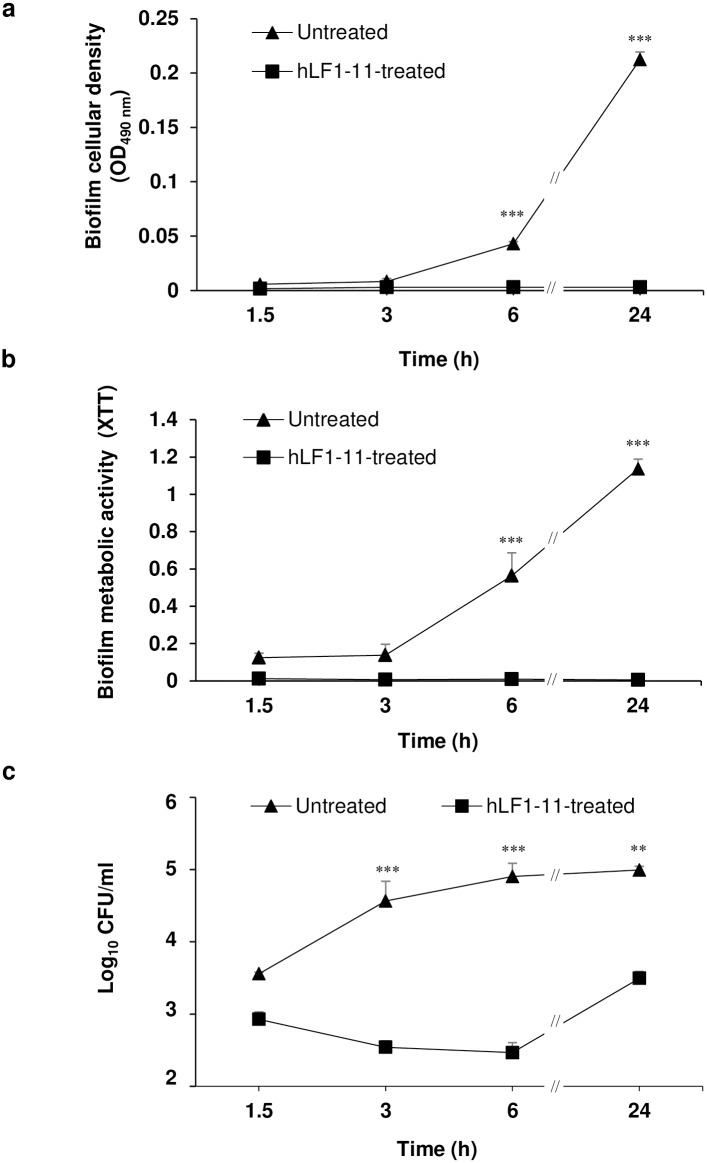
In order to assess whether the hLF1-11 antibiofilm activity after a 24-hour incubation could also be observed at earlier stages, the peptide (88mg/L) and fungal cells were incubated for 1.5h, 3h, 6h and, as a control, 24h. Evaluation of early antibiofilm activity was performed considering the same parameters as previously described. Data obtained from kinetic experiments demonstrated that hLF1-11 induced a reduction in biofilm formation after a 3h incubation (Fig 2a and 2b). Interestingly, a complete abolishment of biofilm metabolic activity was observed as early as 1.5h, which persisted up to 24h. In addition, hLF1-11 caused a 2-log reduction in sessile cell viability after a 3h incubation period, compared to untreated biofilms. This reduction decreased to 1-log following a 24h incubation period.
Fig 2. Kinetics of hLF1-11 activity on biofilm formation by four representative C. albicans strains.
C. albicans SC5314 cells (1x106 cells/ml) were co-incubated with hLF1-11 (88mg/L) for different time periods (1.5, 3, 6 and 24h) at 37°C. Following incubation, the antibiofilm activity of the peptide was assessed in terms of (a) biofilm cellular density reduction, (b) metabolic activity by the XTT assay, and (c) reduction of sessile cell viability. Data are expressed as the mean of three independent experiments ± SEM. hLF1-11-treated sample (square symbol), untreated sample (triangle symbol) ** P<0.01, *** P<0.001, as compared to the untreated control biofilms.
hLF1-11 effect on C. albicans cell morphology
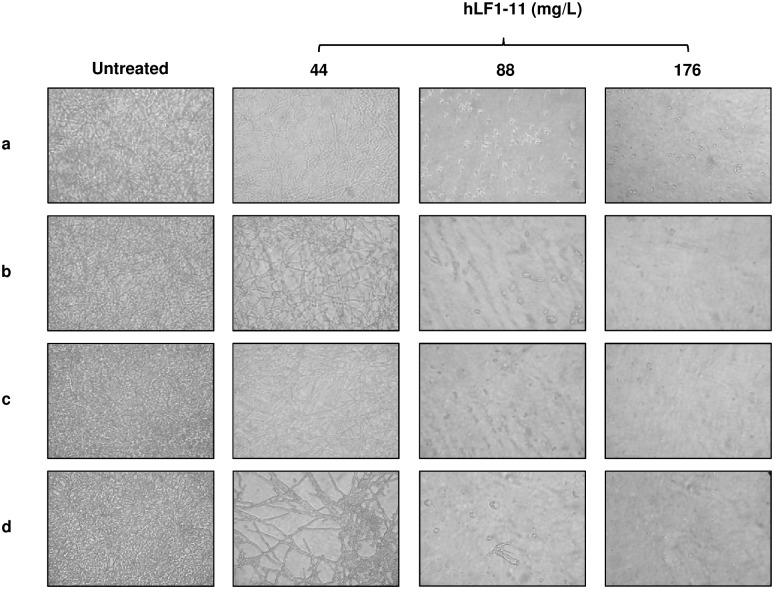
The hLF1-11 effect on biofilm architecture of the 4 C. albicans strains was evaluated using an inverted microscope, following a 24h incubation in the presence/absence of the peptide. The peptide induced a dose-dependent reduction in the number of hyphae compared to untreated cells for all the strains tested (Fig 3a–3d). Indeed, microscopic observation in the absence of the peptide revealed a very thick layer of mature biofilm, consisting of hyphae and matrix exopolysaccharide. In the presence of hLF1-11 at 44mg/L the biofilm layer appeared less compact and homogeneous, with isolated hyphae. At higher concentrations (88 and 176mg/L), the peptide completely inhibited biofilm formation, with fungal cells predominantly growing as yeast.
Fig 3. Effect of hLF1-11 on biofilm architecture of C. albicans strains.
Inverted microscope images (400× magnification) of (a) C. albicans SC5314 and three clinical isolates, (b) CA688, (c) CA22, and (d) CA37, untreated and treated with 44mg/L, 88mg/L and 176mg/L hLF1-11. C. albicans strains were co-incubated with the different hLF1-11 concentrations at 37°C for 24h.
hLF1-11 effect on pre-adhered C. albicans cells
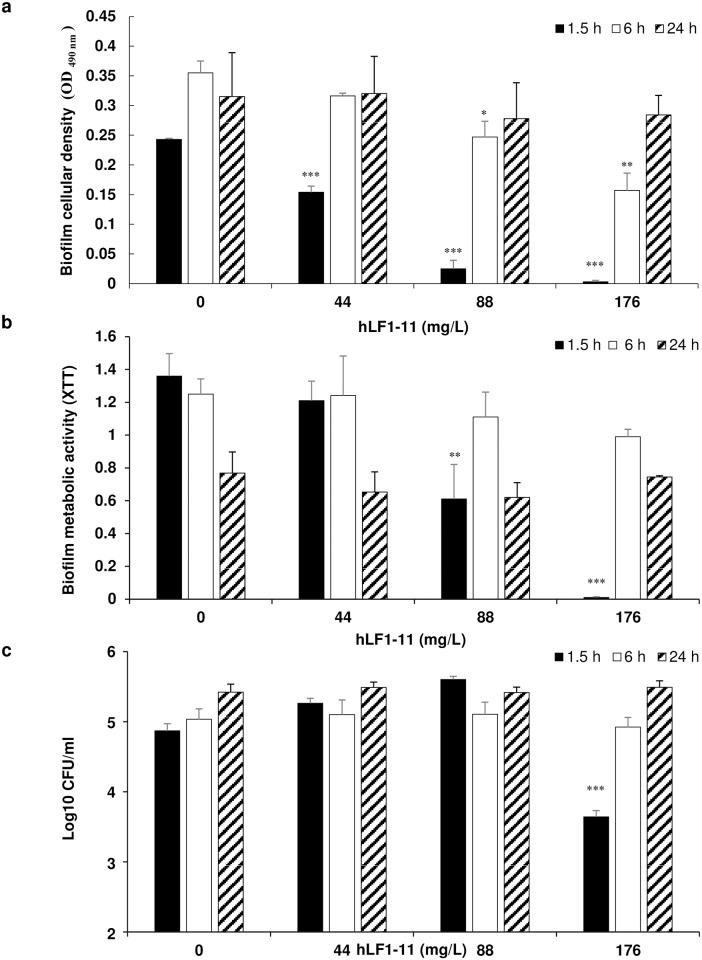
In order to evaluate whether the peptide could act on pre-adhered cells, cells were incubated for 1.5 or 6h to allow adhesion to the 96-well plate or, as a control, 24h, prior to addition of different hLF1-11 concentrations (44, 88, and 176mg/L) and incubated for further 24h at 37°C. The results obtained indicate that the addition of hLF1-11 to C. albicans cells after 1.5h adhesion induced a significant dose-dependent decrease in cellular density and metabolic activity of the biofilm formed and about 1.5 log reduction (P<0.001) in colony count compared to the control with the highest peptide concentration tested (176mg/L). Addition of hLF1-11 to cells after a 6h adhesion phase revealed a significant decrease in biofilm cellular density at the concentrations of 88 and 176mg/L (Fig 4a), but no decrease in biofilm metabolic activity or in the number of sessile viable cells at all the concentrations tested (Fig 4b and 4c). As expected, no change was observed on preformed biofilm (Fig 4a–4c).
Fig 4. Effect of hLF1-11 on pre-adhered C. albicans cells.
C. albicans SC5314 cells were allowed to adhere for different times (1.5h, 6h and 24h) and then various concentrations of hLF1-11 were added and incubated for 24h at 37°C. Following incubation, the antibiofilm activity of the peptide was assessed in terms of (a) biofilm cellular density reduction, (b) metabolic activity by the XTT assay, and (c) reduction of sessile cell viability. Data are expressed as the mean of three independent experiments ± SEM. 1.5h closed bars, 6h open bars, 24h diagonally hatched bars * P≤0.05, ** P<0.01, *** P<0.001, as compared to the untreated control biofilms.
Transcriptional profiles of biofilm associated genes
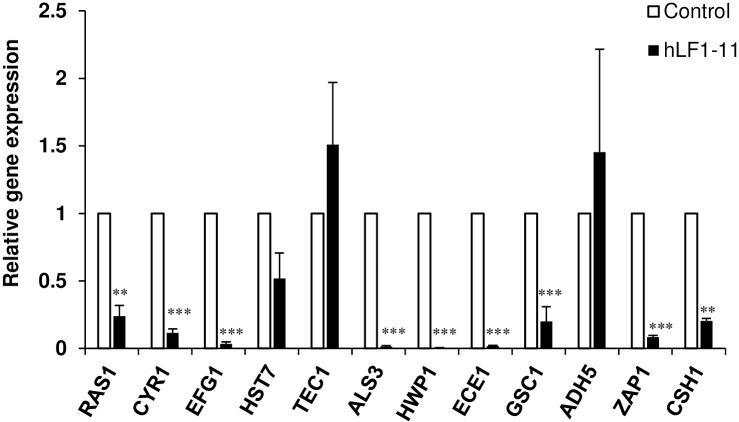
The hLF1-11-induced changes at the transcriptional level of twelve genes related to biofilm formation was assessed by qRT-PCR. The expression level of each gene was analysed in cells exposed to the peptide (88mg/L) for 24h and compared to untreated cells (Fig 5). Analysis of the actin-normalised data showed that the peptide induced a significant decrease in expression level of the following genes: RAS1, CYR1, EFG1, ALS3, HWP1, ECE1, GSC1, ZAP1, and CSH1, while hLF1-11 did not influence the expression of HST7, TEC1, and ADH5 genes. Furthermore, the latter showed a variable level of expression compared to the untreated control, in all experimental replicates.
Fig 5. Quantitative real time RT-PCR analysis of C. albicans biofilm-related genes.
C. albicans SC5314 biofilms were treated with 88mg/L of hLF1-11 in 24-well plates for 24h at 37°C and the expression of the target genes was determined qRT-PCR. Level of gene expression (closed column) is presented as fold change relative to the control group (untreated biofilms, open column). ACT1 expression was used for normalization. Assays were performed in triplicate and bars represent means ± S.E.M from three independent experiments. ** P<0.01 *** P<0.001.
Rescue of morphogenesis by exogenous cAMP
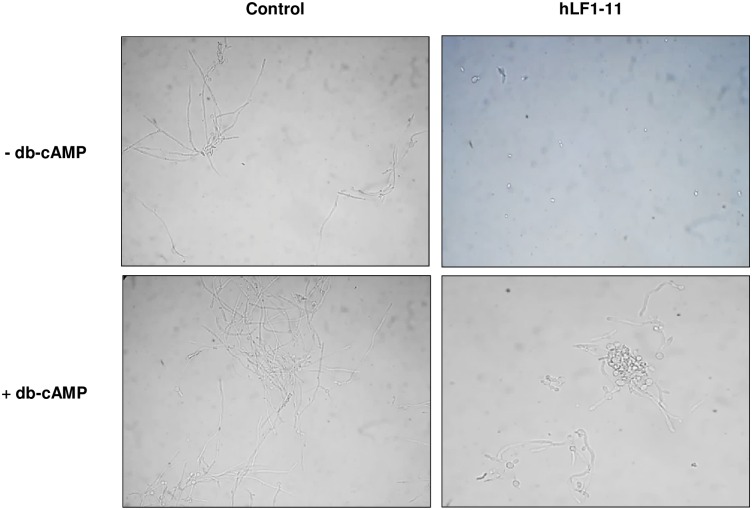
To verify the role of hLF1-11 in Ras1-cAMP-Efg1 pathway inhibition, exogenous db-cAMP was administered to fungal cells immediately after treatment with the hLF1-11 peptide (44mg/L) and microscopically evaluated after a 4h incubation. hLF1-11-treated cells used as a control were mainly in the yeast form (Fig 6). db-cAMP addition to hLF1-11-treated cells resulted in the recovery of filamentous growth. Fungal cell counts revealed a predominance of cells in the yeast form (80%), with a few pseudohyphae (20%) and no hyphae in the hLF1-11-treated sample, without exogenous db-cAMP. Notably, incubation with db-cAMP partially restored morphogenesis, with a percentage of filamentous forms (pseudohyphae and hyphae) rising to 60% (data not shown).
Fig 6. Exogenous db-cAMP restored the hLF1-11-inhibited hyphal formation.
A cell suspension of C. albicans SC5314 (1x106 cells/ml) was incubated at 37°C with db-cAMP (5mM) immediately following the addition of 44mg/L hLF1-11. Peptide free cells with or without db-cAMP served as control. After 4h, cells were recovered from the wells by centrifugation and visually inspected by microscopy (400 × magnification).
Discussion
The main finding from the present study demonstrates that hLF1-11 can significantly inhibit C. albicans biofilm formation. This conclusion is based on the following findings. First, a reduction of total cellular density, metabolic activity and cell viability was observed in a dose-dependent manner after a 24-hour incubation. Second, kinetics experiments indicated that hLF1-11 inhibits early stages of biofilm formation in C. albicans, starting from 3h, where an approximate 2-log reduction in sessile cell viability was observed, although a significant reduction of all biofilm parameters was shown after 6h.
However, the hLF1-11 inhibitory effect was only observed in biofilm cellular density in the presence of pre-adhered cells (1.5-6h). Moreover, C. albicans mature biofilm was unaffected following a 24h incubation with the peptide. Accordingly, other antimicrobial peptides, such as β-peptides and LL37, exerted a direct antibiofilm activity on the first stages of C. albicans biofilm formation [25–27].
Third, hLF1-11 inhibited C. albicans morphogenesis in a dose-dependent manner, in agreement with previous results in vitro and in vivo [18].
In order to better understand the mechanisms underlying the observed antibiofilm effect of hLF1-11 on C. albicans, we also investigated the transcriptional profiles of genes associated to cell adhesion, hyphal growth and extracellular matrix production, under biofilm-inducing conditions. The main signaling pathways involved in the induction of hyphae and biofilm formation are the Ras1-cAMP-Efg1 and MAP kinase [5,28,29]. Interestingly, hLF1-11 significantly decreased expression levels of RAS1, CYR1, EFG1, which are members of the Ras1-cAMP-Efg1 pathway. Ras1 GTPase stimulates Cyr1 adenylate cyclase enzyme to synthesize cAMP, which promotes activation of the Efg1 transcription factor. The latter plays an important role in the regulation of some hypha-specific genes, such as ALS3, HWP1 and ECE1, which are essential factors of C. albicans adhesion [30] as well as biofilm formation [29,31–33]. As expected, transcription of ALS3, HWP1 and ECE1 was also repressed. To confirm these results, exogenous cAMP was added in order to evaluate whether hyphal growth could be restored in hLF1-11-treated C. albicans cells. The results showed that db-cAMP could partially restore C. albicans morphogenesis. This finding is in agreement with previous studies showing an antibiofilm activity effect exerted via the inhibition of the Ras1-cAMP-Efg1 pathway [34–36].
In contrast, the hLF1-11 peptide did not affect transcriptional levels of HST7 and TEC1 genes. HST7 codifies a protein kinase belonging to the MAP kinase pathway, whereas TEC1 encodes a transcription factor that positively regulates the expression of hypha-specific genes, independently from the two pathways described above [37]. On the other hand, C. albicans morphogenesis is regulated by complex networks responding to a wide variety of extracellular stimuli [38].
Although the composition of the extracellular matrix of C. albicans biofilm is still not completely characterized, the presence of carbohydrates, proteins and nucleic acids has already been described [39]. The main extracellular carbohydrate identified is β-1,3-glucan, synthesized by the β-1,3-glucan synthase (Gsc1). For this reason, GSC1 transcript was included as an index of the matrix production [40]. As expected, the hLF1-11 peptide down regulated GSC1 gene. Yet, little is known about the regulation of biofilm matrix production, which relies upon complex regulatory mechanisms involving several transcription factors. C. albicans Zap1 (zinc-responsive activator protein) negatively regulated matrix production to promote cellular dispersion. Zap1 directly activates the expression of CSH1 and IFD6, both of which inhibit matrix accumulation, and indirectly represses the expression of other alcohol dehydrogenase genes, such as ADH5, which promotes matrix production [41] We found that hLF1-11 reduced transcript levels of ZAP1 and CSH1, whereas it did not influence the expression of ADH5. It is worth noting that Zap1 is also required for efficient C. albicans morphogenesis, in agreement with the finding that C. albicans ZAP1 mutant mainly grows as yeast in biofilm (S1 Fig) [41,42]. Consistently, microscopic examination revealed that hLF1-11-treated C. albicans cells predominantly grew as yeast after a 24h incubation.
Overall, these results indicate that hLF1-11 inhibits early stages of biofilm formation in C. albicans, by affecting yeast-hypha transition. In this regard, hLF1-11 seems to be a promising candidate for a potential use as coating agent of prosthetic medical devices, as described for other antimicrobial peptides [43,44].
Supporting Information
(PDF)
(PDF)
Acknowledgments
We would like to thank Dr Colin Gerard Egan for revising the manuscript for English language.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Italian Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR), research grant number 2012WJSX8K_005.
References
- 1.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. United States; 2007;20: 133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. United States; 2001;183: 5385–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. Characterization of mucosal Candida albicans biofilms. PLoS One. 2009;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasan F, Xess I, Wang X, Jain N, Fries BC. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. France; 2009;11: 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas S, Van Dijck P, Datta A. Environmental Sensing and Signal Transduction Pathways Regulating Morphopathogenic Determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71: 348–376. 10.1128/MMBR.00009-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. United States; 2002;15: 167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. England; 2003;11: 30–36. [DOI] [PubMed] [Google Scholar]
- 8.Ramage G, Saville SP, Thomas DP, López-Ribot JL. Candida biofilms: an update. Eukaryot Cell. 2005;4: 633–8. 10.1128/EC.4.4.633-638.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonhomme J, d’Enfert C. Candida albicans biofilms: building a heterogeneous, drug-tolerant environment. Curr Opin Microbiol. England; 2013;16: 398–403. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel RP, Gennings C. Bloodstream infections due to Candida species in the intensive care unit: identifying especially high-risk patients to determine prevention strategies. Clin Infect Dis. United States; 2005;41 Suppl 6: S389–93. [DOI] [PubMed] [Google Scholar]
- 11.Viudes A, Peman J, Canton E, Ubeda P, Lopez-Ribot JL, Gobernado M. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur J Clin Microbiol Infect Dis. Germany; 2002;21: 767–774. [DOI] [PubMed] [Google Scholar]
- 12.Hancock REW, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. United States; 2006;24: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 13.Fox JL. Antimicrobial peptides stage a comeback. Nat Biotechnol. 2013;31: 379–382. 10.1038/nbt.2572 [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Limmon G V, Imani F, Teng C. Induction of lactoferrin gene expression by innate immune stimuli in mouse mammary epithelial HC-11 cells. Biochimie. France; 2009;91: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuijens JH, van Berkel PH, Schanbacher FL. Structure and biological actions of lactoferrin. J Mammary Gland Biol Neoplasia. UNITED STATES; 1996;1: 285–295. [DOI] [PubMed] [Google Scholar]
- 16.Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. NETHERLANDS; 1992;1121: 130–136. [DOI] [PubMed] [Google Scholar]
- 17.Nibbering PH, Ravensbergen E, Welling MM, van Berkel LA, van Berkel PH, Pauwels EK, et al. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect Immun. United States; 2001;69: 1469–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupetti A, Brouwer CPJM, Bogaards SJP, Welling MM, de Heer E, Campa M, et al. Human lactoferrin-derived peptide’s antifungal activities against disseminated Candida albicans infection. J Infect Dis. United States; 2007;196: 1416–1424. [DOI] [PubMed] [Google Scholar]
- 19.van der Does AM, Joosten SA, Vroomans E, Bogaards SJP, van Meijgaarden KE, Ottenhoff THM, et al. The antimicrobial peptide hLF1-11 drives monocyte-dendritic cell differentiation toward dendritic cells that promote antifungal responses and enhance Th17 polarization. J Innate Immun. Switzerland; 2012;4: 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Does AM, Bogaards SJP, Jonk L, Wulferink M, Velders MP, Nibbering PH. The human lactoferrin-derived peptide hLF1-11 primes monocytes for an enhanced TLR-mediated immune response. Biometals. Netherlands; 2010;23: 493–505. [DOI] [PubMed] [Google Scholar]
- 21.Velden WJ, Van Der FM, van Iersel TMP, Blijlevens NM a, Donnelly JP. Safety and tolerability of the antimicrobial peptide human lactoferrin 1–11 (hLF1-11). BMC Med. 2009;7: 44 10.1186/1741-7015-7-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupetti A, Paulusma-Annema A, Senesi S, Campa M, Van Dissel JT, Nibbering PH. Internal thiols and reactive oxygen species in candidacidal activity exerted by an N-terminal peptide of human lactoferrin. Antimicrob Agents Chemother. United States; 2002;46: 1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godoy-Gallardo M, Mas-Moruno C, Fernandez-Calderon MC, Perez-Giraldo C, Manero JM, Albericio F, et al. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. England; 2014;10: 3522–3534. [DOI] [PubMed] [Google Scholar]
- 24.Clinical LSI. Reference Method for Broth Dilution antifungal susceptibility testing of yeasts, 3rd edition, Approved Standard M27-A3. Clinical Laboratory Standards Institute, Wayne, PA: 2008. [Google Scholar]
- 25.Raman N, Lee MR, Lynn DM, Palecek SP. Antifungal activity of 14-helical?? -peptides against planktonic cells and biofilms of Candida species. Pharmaceuticals. 2015;8: 483–503. 10.3390/ph8030483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai PW, Yang CY, Chang HT, Lan CY. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS One. 2011;6: e17755 10.1371/journal.pone.0017755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vriens K, Cools TL, Harvey PJ, Craik DJ, Spincemaille P, Cassiman D, et al. Synergistic Activity of the Plant Defensin HsAFP1 and Caspofungin against Candida albicans Biofilms and Planktonic Cultures. PLoS One. United States; 2015;10: e0132701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramage G, VandeWalle K, Lopez-Ribot JL, Wickes BL. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol Lett. Netherlands; 2002;214: 95–100. [DOI] [PubMed] [Google Scholar]
- 29.Leberer E, Harcus D, Dignard D, Johnson L, Ushinsky S, Thomas DY, et al. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol Microbiol. 2001;42: 673–687. [DOI] [PubMed] [Google Scholar]
- 30.Tronchin G, Pihet M, Lopes-Bezerra LM, Bouchara J-P. Adherence mechanisms in human pathogenic fungi. Med Mycol. 2008;46: 749–72. 10.1080/13693780802206435 [DOI] [PubMed] [Google Scholar]
- 31.Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283: 1535–8. [DOI] [PubMed] [Google Scholar]
- 32.Nobile CJ, Nett JE, Andes DR, Mitchell AP. Function of Candida albicans adhesin hwp1 in biofilm formation. Eukaryot Cell. 2006;5: 1604–1610. 10.1128/EC.00194-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan DA, Sundstrom P. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol. England; 2009;4: 1263–1270. [DOI] [PubMed] [Google Scholar]
- 34.Sun L, Liao K, Wang D. Effects of magnolol and honokiol on adhesion, yeast-hyphal transition, and formation of biofilm by Candida albicans. PLoS One. United States; 2015;10: e0117695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theberge S, Semlali A, Alamri A, Leung KP, Rouabhia M. C. albicans growth, transition, biofilm formation, and gene expression modulation by antimicrobial decapeptide KSL-W. BMC Microbiol. BMC Microbiology; 2013;13: 246 10.1186/1471-2180-13-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao L-X, Li D-D, Hu D-D, Hu G-H, Yan L, Wang Y, et al. Effect of tetrandrine against Candida albicans biofilms. PLoS One. United States; 2013;8: e79671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane S, Zhou S, Pan T, Dai Q, Liu H. The basic helix-loop-helix transcription factor Cph2 regulates hyphal development in Candida albicans partly via TEC1. Mol Cell Biol. 2001;21: 6418–28. 10.1128/MCB.21.19.6418-6428.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. England; 2011;9: 737–748. [DOI] [PubMed] [Google Scholar]
- 39.Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: Chemical composition and role in drug resistance. J Med Microbiol. 2006;55: 999–1008. 10.1099/jmm.0.46569-0 [DOI] [PubMed] [Google Scholar]
- 40.Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, et al. A Candida Biofilm-Induced Pathway for Matrix Glucan Delivery: Implications for Drug Resistance. PLoS Pathog. 2012;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nobile CJ, Nett JE, Hernday AD, Homann OR, Deneault JS, Nantel A, et al. Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol. 2009;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim MJ, Kil M, Jung JH, Kim J. Roles of zinc-responsive transcription factor Csr1 in filamentous growth of the pathogenic yeast Candida albicans. J Microbiol Biotechnol. 2008;18: 242–247. [PubMed] [Google Scholar]
- 43.Alves D, Olívia Pereira M. Mini-review: Antimicrobial peptides and enzymes as promising candidates to functionalize biomaterial surfaces. Biofouling. 2014;30: 483–499. 10.1080/08927014.2014.889120 [DOI] [PubMed] [Google Scholar]
- 44.Tati S, Li R, Puri S, Kumar R, Davidow P, Edgerton M. Histatin 5-spermidine conjugates have enhanced fungicidal activity and efficacy as a topical therapeutic for oral candidiasis. Antimicrob Agents Chemother. 2014;58: 756–766. 10.1128/AAC.01851-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.