Abstract
Purpose
MicroRNA-146a (miR-146a) has been proposed as a marker for age-associated inflammation, or “inflammaging”, acting as a negative regulator of cellular senescence and pro-inflammatory signaling pathways. However, the regulation and function of miR-146 during ocular aging remains unclear. Here we propose that miR-146 is regulated during aging of the retina and choroid, and functions in retinal pigment epithelial (RPE) cells to regulate key genes involved in inflammation and angiogenesis.
Methods
The expression of miR-146a and miR-146b was examined in the neuroretina and RPE/choroid in mice aged from 2 months to 24 months. Then, the effect of synthetic miR-146a mimetic on IL-6 and VEGF-A expression was analyzed in RPE cells treated with and without TNF-α.
Results
miR-146a and miR-146b was upregulated during aging of RPE/choroid but not neuroretina, supporting tissue-specific regulation of aging-related miRNAs in retinal tissues. Overexpression of miR-146a by miRNA mimics inhibited VEGF-A and TNF-α-induced IL-6 expression.
Conclusions
Elevation of miR-146a and miR-146b in the aging RPE/choroid but not neuroretina suggests a role for miRNAs in inflammaging in the RPE/choroid. miR-146a overexpression inhibits the expression IL-6 and VEGF-A in the RPE cells, supporting a negative feedback regulation mechanism by which inflammatory pathways may be dysregulated in RPE during aging.
Introduction
Aging represents a significant risk factor for many diseases, including cardiovascular diseases, type 2 diabetes mellitus and degenerative diseases such as age-related macular degeneration (AMD). However, the pathophysiologic basis by which age contributes to these diseases is still unclear. Recently, dysregulation of inflammatory and immune pathways have become increasingly accepted as key regulators of age-related pathophysiology. Thus, the term “inflammaging” has been used to describe a state of chronic low-grade inflammation associated with age-related diseases (1).
AMD is a degenerative disease of the central retina and the leading cause of legal blindness in adults over 55 in the United States (2). Age is one of the tops risk factors for AMD: AMD affects 14–24% of the U.S. population aged 65–74 years but 35–40% of people aged 74 years or more. AMD has both dry and wet forms (3). Wet AMD is characterized by choroidal neovascularization (CNV), a process involving abnormal growth of blood vessels from the choroid into the retina. In wet AMD, growth factors including vascular endothelial growth factor (VEGF) drive the growth of immature, leaky vessels, resulting in vision loss. By contrast, end-stage dry AMD is defined by geographic atrophy manifested by scattered or confluent areas of degeneration of retina pigment epithelial (RPE) cells. The pathophysiology of AMD is complex, with age, genetic, life style, and environmental factors each contributing to its pathogenesis. The molecular mechanism of retinal aging and its association with AMD pathogenesis remain unclear.
MicroRNAs (miRNAs or miRs) are small, non-coding RNAs that negatively regulate gene expression post-transcriptionally (4). Since their discovery in 1993, miRNAs have been shown to contribute to the pathogenesis of numerous diseases (5). The study of miRNAs in aging processes has emerged in recent years (6). One of the founding miRNAs, lin-4, has been shown to regulate lifespan in C. elegans (7). Numerous miRNAs have been shown to be significantly up- or down-regulated during aging; many of them have been identified as regulators of aging at cell, tissue or organism levels. One particular example is miR-34a, which is upregulated during aging and regulates cell senescence, life-span and aging in multiple species (8–14). The miRNA expression pattern during aging in mammals appears to be tissue-specific, which is in line with the tissue-specific aging signaling pathways (15). In the eye, age-dependent expression of miR-34a in the mouse retina and RPE cells has been observed, with steady increase from 4 months to 24 months, but a slight decrease between 24 and 32 months of age (16). Overall, the involvement of miRNAs in ocular aging is largely unknown.
MiR-146 family contains miR-146a and miR-146b that have similar sequences in the mature miRNAs except for two bases toward the 3′-end. MiR-146a was the most upregulated miRNA during replicative senescence in human fibroblast cells, human umbilical vein endothelial cells (HUVEC) and human trabecular meshwork (HTM) cells (17–19). It was shown to be induced by bacterial lipopolysaccharide (LPS) and the inflammatory cytokines IL-1β and TNF-α, and functions to negatively regulate IL-6 and IL-8 expression (19–23). Moreover, miR-146a was identified as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodeling (24). Based on these studies, miR-146a has been hypothesized as a key regulator of inflammaging (25). Consistently, miR-146a was upregulated in several canine models of early-onset retinal degeneration diseases (26). Given the regulation of miR-146 by cellular senescence and its documented function in inflammation, we hypothesized that miR-146 members are regulated by aging, and function to repress inflammation in the eye. Here we report that miR-146a and miR-146b show age-dependent upregulation in the RPE/choroid but not neuroretina in mice. Furthermore, overexpression of miR-146 by miRNA mimic blocks VEGF-A expression and the induction of cytokine IL-6 by TNF-α in RPE cells. Our data implicate the miR-146 family in RPE/choroid aging, with potential implications for understanding mechanisms and therapeutic options for AMD.
Methods
Animals and tissue preparation
Animal studies were conducted in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committees at the University of Texas Southwestern Medical Center and Tulane University. C57BL/6J mice were obtained from the National Institute of Aging (NIA; Bethesda, MD). Mice were housed at 21 °C, under a 12 hr:12 hr light-dark cycle, with food and water supplied ad libitum. For retinal tissue collection, 3 male mice each from 2, 8, 12, 18 and 24 months old, were sacrificed by CO2 overdose. Immediately after euthanization, enucleated eyes were cut along the limbus to separate the ciliary body and the lens from the neuroretina and RPE/choroid tissues. The neuroretina was then separated from the RPE/choroid. The tissues were then stored in RNAlater at −20°C before RNA isolation.
ARPE-19 cell culture, miRNA mimic transfection and TNF-α treatment
Human RPE cell line (ARPE-19, CLR-2302, ATCC) was cultured in DME/F-12 medium (HyClone) supplemented with 10% FBS (HyClone) as described (27). miRNA mimetic (or mimic) transfection was performed as described in (28). Briefly 4ul of 20μM miRNA mimic or control mimic, and 4μl of Lipofectamine RNAiMAX added to 50μl optiMEM, respectively. Lipofectamine was mixed with miRNA mimic at 5 minutes later, and incubated for another 25 minutes before adding to 6-well plates for transfection. After 72 hours, cells were treated with TNF-α (10ng/ml) for 24 hours before RNA isolation. miR-146a or control mimic were synthesized from Shanghai GenePharma Co. Sequences for control mimic are: [sense] 5′-p-fUsfUfCfUfCfCGAAfCGfUGfUfCAfCsGfUsTsT-3′ and [antisense] 5′-Chol-sAsfCGfUGAfCAfCGfUfUfCGGAGAAsTsT-3′. (f: 2′-deoxy-2′-fluro nucleotides, Chol: cholesterol, p: phosphate group, s: phosphorothioate linkages). Sequences for miR-146a mimic are: [sense] 5′- p-fUsGAGAAfCfUGAAfUfUfCfCAfUGGGsfUsfU-3′ and [antisense] 5′-Chol-sfCsfCfCAfUGGAAfUfUfCAGfUfUfCfUfCAsfUsfU-3′.
RNA isolation and qRT-PCR
Total RNA was isolated from mouse tissues or cell lines using TRIzol reagent (Invitrogen) using a protocol modified from the manufacture manual. To enrich both mRNA and miRNA in the samples, 1 volume of isopropanol (instead of 0.5 volume listed in the manual) was added to the samples, and the samples were incubated at −80°C for 15 minutes before RNA precipitation. mRNA and miRNA quantitative (q) RT-PCR were performed using qScript™ cDNA Synthesis and microRNA Quantification System (Quanta Biosciences). Primers used are: IL-6: 5′-CAC ACA GAC AGC CAC TCA CC-3′ and 5′-TTT TCT GCC AGT GCC TCT TT-3′; VEGF-A: 5′-AGT GTG TGC CCA AGG A-3′ and 5′-GGT GAG GTT TGA TCC GCA TA-3′; Cyclophilin A: 5′-CCAGTGCTCAGAGCACGAAA-3′ and 5′-CCCACCGTGTTCTTCGACAT-3′; Primers for miR-146a and miR-146b were ordered from Quanta Biosciences.
Statistics
The in vitro experiment was repeated at least three times. Student’s t-tests and TWO-WAY ANOVA (followed by Tukey’s post hoc test) were used to determine statistical significance between groups. P values of less than 0.05 were considered to be statistically significant.
Results
Differential upregulation of miR-146a and miR-146b expression during Choroid/RPE aging in mice
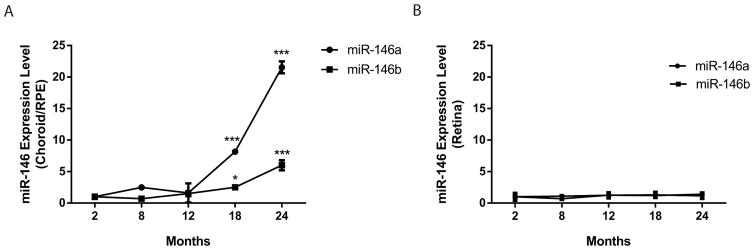
To examine the expression of miR-146a and miR-146b during retinal aging, neuroretinal tissues and choroid/RPE tissues were isolated from male mice aged at 2, 8, 12, 18 and 24 months. Quantitative (q) RT-PCR was performed, and miR-146a/b expression was normalized to U6 and compared to their expression to 2-month samples. miR-146a expression was mildly increased in 8 month choroid/RPE tissues, but significantly increased at 18 months (~8 folds) and 24 months (~22 folds) (Fig. 1A). miR-146b expression was unchanged at 8 months, but started to increase gradually from 12 to 24 months (~2 folds at 18 months and ~5 fold increase at 24 months). On the contrary, the expression of neither miR-146a nor miR-146b was significantly changed in the neuroretina in mice from 2 months to 24 months (Fig. 1B). These results suggest differential regulation of miR-146a and miR-146b expression during the aging process of neuroretina and choroid/RPE in mice.
Figure 1.
Upregulation of miR-146a and miR-146b during aging of RPE/choroid and neuroretina. Three samples were used for each age group. qRT-PCR results showed relative miR-146a and miR-146b expression in the RPE/choroid (A) and neuroretina (B). *, p<0.05 ***, p<0.001.
Inhibition of IL-6 and VEGF-A gene expression in RPE cells by miR-146a mimic
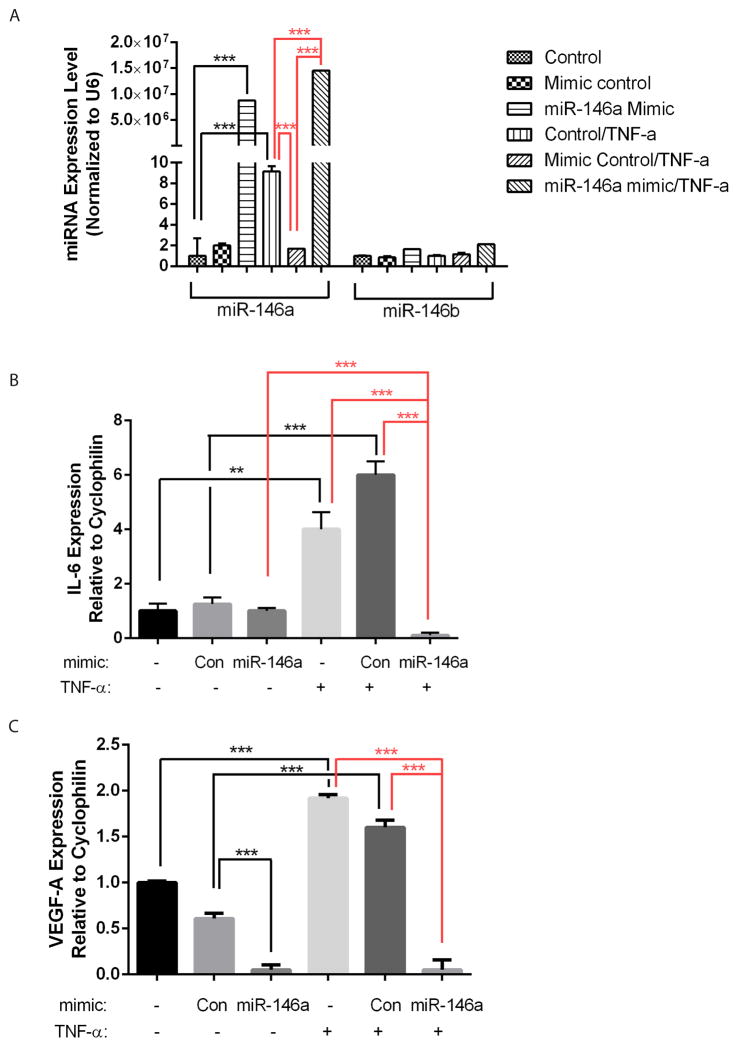
MiR-146a has been shown to be induced by inflammatory cytokines IL-1β and TNF-α, and function to target and negatively regulate IL-6 and IL-8 expression (19–22). In a recent study, miR-146a has been shown to downregulate VEGF expression in cancer cells (29). To examine the function of miR-146 in RPE cells, miR-146a or control mimic was transfected into ARPE-19 cells; cells without transfection were also used as an additional control. The cells were treated with TNF-α, and the expression of IL-6 and VEGF-A was examined by qRT-PCR, with cyclophilin A used as normalization control. As shown in Fig. 2A, compared to the controls, miR-146a mimic resulted in drastic over-expression of miR-146a but not miR-146b at either control condition or with TNF-α treatment, confirming the specificity and efficiency of miR-146a mimic. Consistent with a previous report, TNF-α preferentially induced the expression of miR-146a (~10 fold induction) but not miR-146b (23). Of note, control mimic blunted the induction of miR-146a by TNF-α, suggesting the control miRNA mimic or the transfection reagents may have some non-specific effect on miR-146a expression at the concentration used.
Figure 2.
Expression of miR-146a, miR-146b, IL-6 and VEGF-A in ARPE-19 cells after miRNA mimic transfection and TNF-α treatment. qRT-PCR results showed miR-146a and -b expression (A), IL-6 expression (B) and VEGF-A expression (C) in ARPE-19 cells with/without miR-146a mimic transfection and TNF-α treatment. **, p<0.01; ***, p<0.001.
After setting up the system, the expression of IL-6 and VEGF-A was examined by qRT-PCR. Without TNF-α treatment, neither control mimic nor miR-146a mimic significantly affected IL-6 expression compared to the non-transfection control (Fig. 2B). However, TNF-α induced IL-6 expression completely blunted upon miR-146a mimic transfection.
When VEGF-A expression was analyzed, control mimics repressed VEGF-A expression to ~60% compared to the non-transfection control at the basal condition, again suggesting non-specific effects. Meanwhile, miR-146a mimic reduced expression of VEGF-A expression to less than 11% compared to the both controls. In addition, TNF-α also significantly increased the expression of VEGF-A expression, and this upregulation was reversed by miR-146a mimic transfection.
Taken together, these data demonstrate that miR-146a is induced by the inflammatory cytokine TNF-α, and miR-146a mimic represses the expression of inflammatory mediators IL-6 and VEGF-A in ARPE-19 cells.
Discussion
We provide evidence that the expression of miR-146a and miR-146b shows age-dependent regulation in the RPE/choroid but not the neuroretina in mice. Moreover, overexpression of miR-146a by miRNA mimic inhibits TNF-α-induced IL-6 expression and the expression of VEGF-A. Together, these data support a hypothesis for tissue-specific inflammaging mechanisms in RPE/choroid. In addition, our finding that miR-146a inhibits VEGF-A and TNF-α-induced IL-6 expression may have implications in understanding the mechanism and therapeutics of AMD.
miR-146a as marker for RPE/choroid aging and inflammaging
How miRNAs are involved in ocular aging remains unclear. miR-34a upregulation in the blood and the brain have been identified as a senescence marker for brain tissue in mice (30). In the neuroretina and the RPE/choroid, miR-34a expression was recently shown to peak at 24 months of age in mice, but decreased gradually from 24–32 months of age, suggesting regulation of miRNAs during aging (16). Since miR-34a expression is not strictly age-dependent during neuroretina or RPE/choroid aging, we set to search for additional miRNAs involved in retinal aging. We found that miR-146a and miR-146b expression level increases steadily from 2 months to 48 months in the mouse RPE/choroid. MiR-146a has been shown to be highly up-regulated in senescent human fibroblast cells, HUVEC and HTM cells (17–19), and circulating miR-146a has been observed in aged individuals and in patients age-related diseases (17). Collectively, our work is consistent with prior studies, implicating miR-146 as a candidate marker for RPE/choroid aging.
Mechanistically, MiR-146a is induced by the Toll-like receptor (TLR) ligand LPS and the inflammatory cytokines IL-1β and TNF-α, and functions to negatively regulate IL-6 and IL-8 expression (19–23). Two key adapter molecules downstream of TLRs, TRAF6 and IRAK1, have been confirmed as direct targets of miR-146a (31). These data suggest a negative regulatory loop where miR-146a gene is upregulated by NF-κB and downregulates IRAK1 and TRAF6 to reduce NF-κB activity. Based on these studies, miR-146a was proposed as to be one of the key miRNAs involved in inflammaging (32). Differentially increased expression of inflammatory genes and proteins has been observed in models of neuronretina/RPE/choroid aging (33, 34). The normal aging process of the neuroretina is known to be less obvious than that of the choroid based on the fact that fewer age-related disease is associated with the retina, which correlates with the upregulation of miR-146a in the choroid/RPE but not neuroretina. Our findings that miR-146a is upregulated by inflammatory cytokine TNF-α and by choroid/RPE aging suggest a heightened inflammation in the RPE/choroid during aging, further supporting the inflammaging theory in the RPE/choroid. Future studies should address the regulation and function of miR-146 in AMD patients and in animal models of ocular disease.
Taken together, our data support the hypothesis that miR-146a/b is a marker for both aging and inflammaging in the RPE/choroid. Limitations of the study include the small sample size, limited time points and the lack of in vivo miR-146a functional studies. Future studies are needed to dissect the regulation of miR-146a/b in choroid and RPE separately, and rigorously test the inflammaging theory in RPE/Choroid.
Function and implications of miR-146a in RPE cells
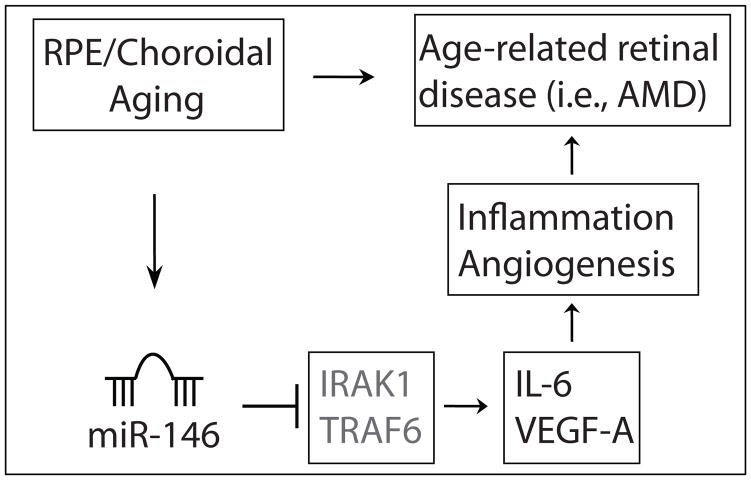
miR-146a has been shown to repress multiple genes associated with inflammation, including IRAK1, TRAF6, IL-6 and IL-8 (19–23). It has been recently shown to inhibit cancer metastasis by downregulating VEGF expression in cancer cells (29). Our results that miR-146a mimic represses VEGF-A expression and TNF-α induced IL-6 expression in ARPE-19 cells is consistent with the previous results using other cell types. Upregulation of miR-146a/b in senescent fibroblasts and HTM cells has been shown to downregulate the expression of multiple genes involved in the inflammatory response. Our data suggest that miR-146a serves as a negative feedback mechanism to prevent excessive inflammatory gene expression in the RPE cells (Fig. 3). Future study is required to determine whether miR-146a can regulate inflammatory pathways in aging RPE cells in vivo.
Figure 3.
Working model. In this model, RPE/choroid aging induces the expression of miR-146a, which in turn represses the IL-6 and VEGF-A expression in RPE cells. Therefore miR-146a functions to alleviate the inflammatory and angiogenic response in the aging RPE/choroid. Regulation of IL-6 and VEGF-A is likely mediated by its target genes IRAK1, TRAF6, which function in the NFκB pathway.
VEGF plays an important role in the pathological angiogenesis and vascular permeability in wet AMD, and anti-VEGF agents are the current mainstay in treating wet AMD (35–37). Therefore, our findings that miR-146a is upregulated during RPE/choroid aging in vivo and miR-146a mimic negatively regulates the expression of inflammatory and angiogenic genes may have implication in the therapeutics for AMD. Our published data and others have shown important function of miRNAs in the vascular system (28, 38–43). It would be thus interesting to test the function of miR-146a in both inflammation and angiogenesis in models of ocular and vascular disease.
Acknowledgments
S.W. was supported by a startup fund from Tulane University, NIH Grant EY021862, a career development award from the Research to Prevent Blindness foundation, and a Bright Focus Foundation Award in Age-related Macular Degeneration. We would also like to thank Kyle M. Koster for technical and editorial assistance.
References
- 1.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflammaging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J Eye Diseases Prevalence Research G. Prevalence of age-related macular degeneration in the United States. Archives of ophthalmology. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 3.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 6.Smith-Vikos T, Slack FJ. MicroRNAs and their roles in aging. J Cell Sci. 2012;125:7–17. doi: 10.1242/jcs.099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehm M, Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005;310:1954–1957. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Chen W, Miao R, Zhou Y, Wang Z, Zhang L, Wan Y, Dong Y, Qu K, Liu C. miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget. 2015;6:3988–4004. doi: 10.18632/oncotarget.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci U S A. 2007;104:15472–15477. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab. 2010;299:E110–116. doi: 10.1152/ajpendo.00192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Chen D, He Y, Melendez A, Feng Z, Hong Q, Bai X, Li Q, Cai G, Wang J, Chen X. MiR-34 modulates Caenorhabditis elegans lifespan via repressing the autophagy gene atg9. Age. 2013;35:11–22. doi: 10.1007/s11357-011-9324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu N, Landreh M, Cao K, Abe M, Hendriks GJ, Kennerdell JR, Zhu Y, Wang LS, Bonini NM. The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature. 2012;482:519–523. doi: 10.1038/nature10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Liu H, McGee J, Walsh EJ, Soukup GA, He DZ. Identifying microRNAs involved in degeneration of the organ of corti during age-related hearing loss. PLoS One. 2013;8:e62786. doi: 10.1371/journal.pone.0062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Treguer K, Carmona G, Bonauer A, Horrevoets AJ, Didier N, Girmatsion Z, Biliczki P, Ehrlich JR, Katus HA, Muller OJ, Potente M, Zeiher AM, Hermeking H, Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 15.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 16.Smit-McBride Z, Forward KI, Nguyen AT, Bordbari MH, Oltjen SL, Hjelmeland LM. Age-dependent increase in miRNA-34a expression in the posterior pole of the mouse eye. Mol Vis. 2014;20:1569–1578. [PMC free article] [PubMed] [Google Scholar]
- 17.Olivieri F, Rippo MR, Monsurro V, Salvioli S, Capri M, Procopio AD, Franceschi C. MicroRNAs linking inflammaging, cellular senescence and cancer. Ageing research reviews. 2013;12:1056–1068. doi: 10.1016/j.arr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Modulation of inflammatory markers by miR-146a during replicative senescence in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2010;51:2976–2985. doi: 10.1167/iovs.09-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, Lithgow GJ, Campisi J. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany NY) 2009;1:402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moschos SA, Williams AE, Perry MM, Birrell MA, Belvisi MG, Lindsay MA. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genomics. 2007;8:240. doi: 10.1186/1471-2164-8-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry MM, Moschos SA, Williams AE, Shepherd NJ, Larner-Svensson HM, Lindsay MA. Rapid changes in microRNA-146a expression negatively regulate the IL-1beta-induced inflammatory response in human lung alveolar epithelial cells. J Immunol. 2008;180:5689–5698. doi: 10.4049/jimmunol.180.8.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutty RK, Nagineni CN, Samuel W, Vijayasarathy C, Jaworski C, Duncan T, Cameron JE, Flemington EK, Hooks JJ, Redmond TM. Differential regulation of microRNA-146a and microRNA-146b-5p in human retinal pigment epithelial cells by interleukin-1beta, tumor necrosis factor-alpha, and interferon-gamma. Mol Vis. 2013;19:737–750. [PMC free article] [PubMed] [Google Scholar]
- 24.Olivieri F, Lazzarini R, Recchioni R, Marcheselli F, Rippo MR, Di Nuzzo S, Albertini MC, Graciotti L, Babini L, Mariotti S, Spada G, Abbatecola AM, Antonicelli R, Franceschi C, Procopio AD. MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age. 2013;35:1157–1172. doi: 10.1007/s11357-012-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivieri F, Rippo MR, Prattichizzo F, Babini L, Graciotti L, Recchioni R, Procopio AD. Toll like receptor signaling in “inflammaging”: microRNA as new players. Immunity & ageing : I & A. 2013;10:11. doi: 10.1186/1742-4933-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genini S, Guziewicz KE, Beltran WA, Aguirre GD. Altered miRNA expression in canine retinas during normal development and in models of retinal degeneration. BMC Genomics. 2014;15:172. doi: 10.1186/1471-2164-15-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanus J, Zhang H, Wang Z, Liu Q, Zhou Q, Wang S. Induction of necrotic cell death by oxidative stress in retinal pigment epithelial cells. Cell Death Dis. 2013;4:e965. doi: 10.1038/cddis.2013.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Developmental cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Zhang Y, Sun XX, Ma X, Chen ZN. microRNA-146a inhibits cancer metastasis by downregulating VEGF through dual pathways in hepatocellular carcinoma. Molecular Cancer. 2015:14. doi: 10.1186/1476-4598-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Khanna A, Li N, Wang E. Circulatory miR34a as an RNAbased, noninvasive biomarker for brain aging. Aging (Albany NY) 2011;3:985–1002. doi: 10.18632/aging.100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou J, Wang P, Lin L, Liu X, Ma F, An H, Wang Z, Cao X. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 32.Rippo MR, Olivieri F, Monsurro V, Prattichizzo F, Albertini MC, Procopio AD. MitomiRs in human inflammaging: A hypothesis involving miR-181a, miR-34a and miR-146a. Experimental gerontology. 2014;56:154–163. doi: 10.1016/j.exger.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Chen M, Muckersie E, Forrester JV, Xu HP. Immune Activation in Retinal Aging: A Gene Expression Study. Invest Ophth Vis Sci. 2010;51:5888–5896. doi: 10.1167/iovs.09-5103. [DOI] [PubMed] [Google Scholar]
- 34.Steinle JJ, Sharma S, Smith CP, McFayden-Ketchum LS. Normal Aging Involves Modulation of Specific Inflammatory Markers in the Rat Retina and Choroid. J Gerontol a-Biol. 2009;64:325–331. doi: 10.1093/gerona/gln052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, Group AS. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 37.Zampros I, Praidou A, Brazitikos P, Ekonomidis P, Androudi S. Antivascular endothelial growth factor agents for neovascular age-related macular degeneration. Journal of ophthalmology. 2012;2012:319728. doi: 10.1155/2012/319728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol (Oxf) 2015;213:60–83. doi: 10.1111/apha.12416. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Q, Gallagher R, Ufret-Vincenty R, Li X, Olson EN, Wang S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Q, Anderson C, Hanus J, Zhao F, Ma J, Yoshimura A, Wang S. Strand and cell-type specific function of microRNA-126 in angiogenesis. Mol Ther. 2016 doi: 10.1038/mt.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, Weber C. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Q, Anderson C, Zhang H, Li X, Inglis F, Jayagopal A, Wang S. Repression of choroidal neovascularization through actin cytoskeleton pathways by microRNA-24. Mol Ther. 2014;22:378–389. doi: 10.1038/mt.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Olson EN. AngiomiRs--key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]