Abstract
Purpose
Test if oxygenation kinetics correlate with the likelihood for local tumor control following fractionated radiotherapy.
Methods and Materials
We used diffuse reflectance spectroscopy to noninvasively measure tumor vascular oxygenation and total hemoglobin concentration ([THb]) associated with radiotherapy of 5 daily fractions (7.5, 9 or 13.5 Gy/day) in FaDu xenografts. Spectroscopy measurements were obtained immediately before each daily radiation fraction and during the week after radiotherapy. Oxygen saturation (SO2) and [THb] were computed using an inverse Monte Carlo model.
Results
(1) Oxygenation kinetics during and after radiotherapy, but before tumor volumes changed, were associated with local tumor control. Locally controlled tumors exhibited significantly faster increases in oxygenation after radiotherapy (days 12-15) compared with tumors that recurred locally. (2) Within the group of tumors that recurred, faster increases in oxygenation during radiotherapy (days 3-5 interval) were correlated with earlier recurrence times. An area of 0.74 under the receiver operator curve was achieved when classifying the local control tumors from all irradiated tumors using the oxygen kinetics with a logistic regression model. (3) The rate of increase in oxygenation was radiation dose dependent. Radiation doses ≤9.5 Gy/day did not initiate an increase in oxygenation whereas 13.5 Gy/day triggered significant increases in oxygenation during and after radiotherapy.
Conclusions
Additional confirmation is required in other tumor models, but these results suggest that monitoring tumor oxygenation kinetics could aid in the prediction of local tumor control after radiotherapy.
Keywords: Reoxygenation, Radiotherapy, Treatment Response, Optical spectroscopy, Hypoxia, FaDu
Introduction
Radiotherapy plays a significant role in the treatment of a wide variety of cancers (1), and particularly in the management of localized head and neck cancer (HNC) because it is a non-invasive and function-preserving modality (2). Tumor oxygenation is associated with tumor radiosensitivity, angiogenesis and metabolism (3). Hypoxic tumor cells are 3 times more resistant to radiotherapy than aerobic cells, and would dominate response if they persisted throughout a course of fractionated radiotherapy. Classic radiobiologic theory posits that once aerobic cancer cells are killed, a proportion of the remaining hypoxic cells in the tumor become reoxygenated, thereby regaining sensitivity to the next radiation fraction (4). The classic reoxygenation theory does not require a physical increase in pO2 in the tumor.
However, increases in tumor oxygenation have been reported after fractionated radiotherapy. Several studies have observed tumor increases in oxygenation, detected by microelectrode and/or immunohistochemical techniques, induced by multi-fraction radiotherapy with nude mice bearing human HNC xenografts (5-7). Hariss et al. used an oxygen sensitive probe to obtain tumor pO2 values and found that the median pO2 of irradiated tumors (10 × 4 Gy) increased with each successive radiation dose, relative to untreated controls measured at the same time (6). Maftei et al. reported decreases in hypoxic fraction assessed by pimonidazole staining, 24hr after (2 × 10Gy) irradiation in FaDu xenografts (7). Ressel et al. examined association between hypoxic fraction and treatment outcome using microelectrodes and demonstrated median pO2 values in squamous cell carcinoma xenografts increased over time after radiotherapy (5). Animals with complete tumor remission 60 days post-treatment had the lowest fraction of median pO2<10 mmHg 10 days post-treatment (5).
The invasiveness of microelectrode techniques limits measurement frequency and total number. Further, tissue damage by the implanted sensors might interfere with tumor response to radiation. In this paper, a non-invasive optical technique was used to serially measure changes in perfusion and oxygenation as assessed by total hemoglobin ([THb]) and hemoglobin saturation prior to, during and after fractionated radiotherapy in mice with FaDu xenografts. Our primary hypothesis was that oxygenation kinetics would correlate with the likelihood for local tumor control following fractionated radiotherapy. Indeed our results confirm that hypothesis. Our findings provide a strong rationale for temporal monitoring of tumor oxygenation kinetics following radiotherapy, and may identify optimal windows in which to assess the efficacy of radiotherapy, prior to discernable changes in tumor volume. To distinguish the kinetics of change in oxygenation during fractionated radiotherapy from classic reoxygenation theory, we use the term “oxygenation kinetics” throughout this manuscript.
Materials and Methods
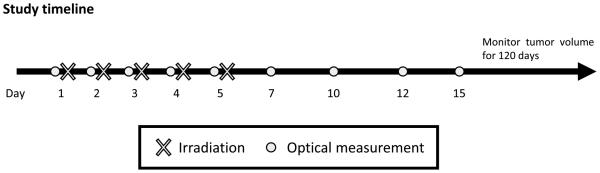
Mouse protocols were approved by the Institutional Animal Care and Use Committee. Fig. 1 shows the study time line for these studies.
Figure 1. Study time line the experiment.
Optical measurements were obtained during and after radiotherapy. Tumor volumes were monitored for 120 days to confirm treatment outcomes.
Fractionated radiotherapy of FaDu HNSCCs xenografts
Approximately 1×106 FaDu cells were injected s.c. into the right flank of nude mouse (nu/nu) to initiate tumor growth. Radiotherapy commenced when tumor volumes reached 100-400mm3. Thereafter, tumor volume was measured 2-3 times a week for the first two weeks after the start of radiotherapy, and then 1-2 times a week for up to 120 days after the first day of radiotherapy, or until the tumor volumes reached 5 times the volume measured on the first day of radiotherapy.
Mice were irradiated with five daily fractions of radiation from 7.5-13.5 Gy per fraction, using a commercial X-RAD320 irradiator (Precision X-Ray, Bradford, CT). The unit produced a collimated X-ray beam (with mean energy of 110 kV) at a dose rate of 0.64 Gy/min. Mice were anesthetized via isoflurane during irradiation and only the tumor area was irradiated. In each experiment, mice were randomly assigned 3:1 to irradiated and non-irradiated control groups.
Optical Measurement Schedule
Vascular oxygenation (SO2%) and [THb] were computed from tissue diffuse reflectance spectra (DRS) collected on a portable optical instrument (8) (9). The sensing depth of the probe was determined to be 1.2 mm with a Monte Carlo (MC) simulation (10). Tissue DRS were obtained from all mice before each radiation fraction, during radiotherapy and after radiotherapy, on days 7, 10, 12 and 15. (Fig. 1). Immediately prior to the measurements, DRS were obtained at five random sites on the tumor of each mouse. The mean DRS was analyzed using an inverse MC model to compute SO2% and [THb]. Follow-up values of SO2% and [THb] were divided by their baseline to obtain baseline-corrected values. Change in the baseline-corrected SO2% (f-SO2) across an interval of time from t1 to t2 was defined as [f-SO2(t2) minus f-SO2(t1)], where t1 and t2 are two selected time points.
All mice that underwent radiotherapy were assessed for local tumor recurrence up to 120 days post treatment. Treated mice with no visible tumor for at least 50 days were classified as local control (LC); treated mice that showed local recurrence within the 50 day period were classified as local failure (LF). For LF mice, time-to-failure was defined as the earliest time at which the recurrent lesion had increasing volume for two consecutive observations. Non-irradiated tumor bearing mice formed the control group (CTL).
Statistical analysis
A repeated measures model was used to test for a difference among CTL, LC and LF groups on the quadratic trajectory of SO2% across time. The Wilcoxon rank-sum test was used to test for group differences on the rate of change across various intervals of time (for example, day 3 to 5, day 7 to 10, and day 12 to 15). The Spearman correlation coefficient was used to assess the correlation between the rate of change in optical endpoints over various time intervals and time to failure mice only in the LF group. To investigate the dose dependency of oxygenation kinetics, mice receiving 7.5 and 9.5 Gy were combined (low radiation dose group). SO2% of the low radiation dose group and the high radiation dose group (13.5 Gy) were compared to CTL using Wilcoxon rank-sum test. All tests were two-tailed with alpha of 0.05. Logistic regression models were built to predict LF within all treated mice. The models were built based on rate of changes in f-SO2 across various time intervals. A leave-one-out cross validation technique was used to generate receiver operator curves (ROCs) from which the area under the curve (AUC) was computed. Data analysis was conducted using MATLAB (Mathworks Inc., Natick, MA). Logistic regression models were computed with the SAS software (SAS Institute Inc., Cary, NC, USA).
Results
Oxygenation kinetics are associated with local control rate after radiotherapy
Table 1 summarizes the number of mice in LC (n=10), LF (n=31), and control groups (n=17) according to dose of radiation received. Within the LF group, the longest lesion free time was 35 days. Thus, we defined LC as any animal that remained disease free at 50 days after treatment.
Table 1.
Outcome in each radiation dose group
| Number of mice per dose level |
||||
| Group | 7.5 (37.5) Gy |
9.5 (47.5) Gy |
13.5 (67.5) Gy |
Total |
|
| ||||
| CTL | 4 | 6 | 7 | 17 |
|
| ||||
| LF | 6 | 2 | 2 | 10 |
|
| ||||
| LC | 4 | 13 | 14 | 31 |
|
| ||||
| Total | 14 | 21 | 23 | 58 |
Seventeen mice were in the control group. A total of 10 and 31 mice achieved LC and LF, respectively. Radiation was administered in 5 daily fractions at the doses indicated. The dose per fraction and total dose, in parentheses, are shown.
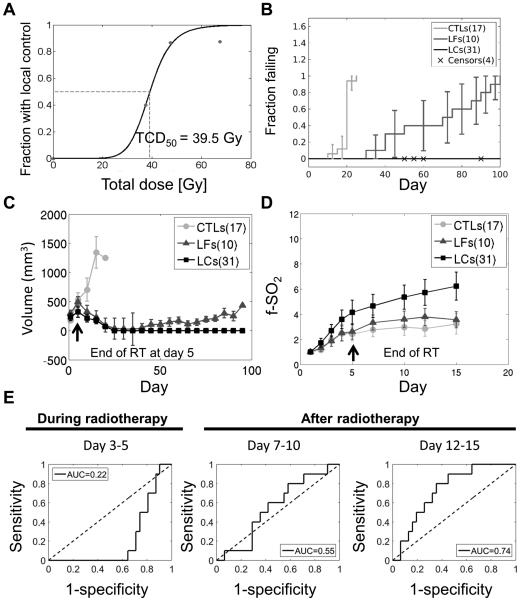
Fig. 2 demonstrates that oxygenation kinetics are associated with therapy outcomes. A radiation dose-effect curve for local tumor control was generated with data fitted to a Hill equation (Fig. 2A). The dose required to achieve local control in 50% of the animals (TCD50) was 38.5 Gy. Fig. 2B and Fig 2C show the time to local failure and tumor volumes for each group respectively. Irradiation resulted in an overall increase in SO2% for the LC and LF groups relative to the control group (Fig. 2D). Within irradiated mice, tumors in the LC group achieved higher SO2% compared to the LF group, particularly after completion of the radiotherapy course. The 2-degree of freedom test showed a significant difference (p<0.001) among groups in f-SO2 trajectory across time. The LC group showed a moderate increase and then a decrease in [THb] across the two-week period. Overall, an early increase in the mean f-[THb] for the LC and LF groups suggests an increase in the overall blood volume or perfusion as a result of radiotherapy (supplementary Fig. 1). A similar, latent increase of the mean f-[THb] in the control group may be related to tumor-directed angiogenesis. The two-degree test for a difference among groups in trajectory across time had a p-value of 0.02.
Figure 2. Optical measurements of change in oxygenation kinetics prior to measureable volume changes differentiates tumors that achieve local control from those that fail.
A. Radiation dose-effect curve for local tumor control. The solid line in Fig. 2A was constructed by fitting the data to the Hill equation.
B. Time to local failure for local failure (LF), local control (LC) and control groups (CTL). Error bars show the 95% confidence intervals.
C. Differences in tumor volumes between local control and local failure groups are visible 40 days after radiotherapy
D. Optical measurements of tumor oxygenation kinetics during radiotherapy and after completion of radiotherapy. Tumor oxygenation kinetics were higher in the local control group than in the local failure group starting 5 days after radiotherapy. At this time point, there is no significant change in tumor volume. Error bars represent standard error of the mean.
E. Area under the receiver operating curve (AUC) computed from the logistic regression analysis for classifying the LF mice. The regression model was built on the change of baseline-corrected vascular oxygenation (f-SO2) obtained from different time intervals. A leave-one-out cross validation technique was used.
The rate of f-SO2 change was evaluated over three specific time-intervals relative to the onset of radiotherapy (day 3-5, day 7-10 and day 12-15). These intervals were chosen to represent time frames during radiotherapy, shortly after radiotherapy was completed and an interval after radiotherapy, but before any discernable change in tumor volume, respectively. Table 2 summarizes the rate of f-SO2 changes over the three time intervals for LC, LF and CTL groups. A negative rate of f-SO2 change indicates a decrease in SO2% during the time interval. The LC group showed a positive rate of change in f-SO2 in all three time-intervals. The rate of the f-SO2 change in LC group was significantly higher than in the CTL group in the day 7-10 interval (p=0.01) and was significantly higher than the LF group in the day 12-15 interval (p<0.01). In addition, rate of the f-SO2 change in the control group was significantly higher than the LF group in the day 12-15 interval (p=0.05). The cross-validated AUC computed from logistic regression models built from rate of the f-SO2 changes in day 3-5, 7-10 and 12-15 intervals were 0.22, 0.55 and 0.74 respectively. Fig. 2 E shows the corresponding ROC computed from each interval.
Table 2.
The oxygenation kinetics after radiotherapy is associated with tumor recurrence.
| Group | Rate of f-SO2 change | ||
|---|---|---|---|
| Days 3-5 | Days 7-10 | Days 12-15 | |
| CTL (17) | 0.238 (0.039) | 0.039 (0.017) | −0.047 ¶ (0.022) |
| LF (10) | 0.377 (0.048) | 0.087(0.019) | −0.076 (0.017) |
| LC (31) | 0.727 (0.047) | 0.23 † (0.01) | 0.169§ (0.01) |
The mean (standard error) of the rate of change in baseline-corrected vascular oxygenation (f-SO2) across various time intervals, per group are shown. The p-values were computed from Wilcoxon rank-sum test for comparing the rate of change in f- SO2. Spearman’s correlations (r) between the rate of f-SO2 change and the tumor recurrence times are also shown.
Rate of f-SO2 change in LC is significantly higher than in the control (CTL) group in the interval from Day7-10 (p = 0.01).
Rate of the f-SO2 change in CTL is significantly higher than LF in the interval from Day 12-15 (p = 0.05).
Rate of the f-SO2 change for LC is significantly higher than for LF in the interval from Day 12-15 (p = 0.01).
There is a strong association between rate of change of SO2% and time to tumor recurrence
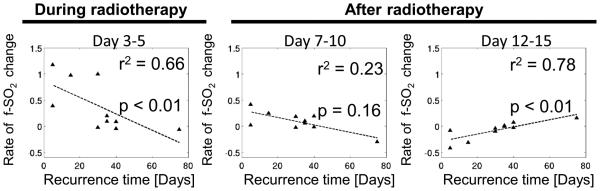
The association between rates of change of f- SO2 and tumor recurrence time was evaluated within the LF group. Of the 10 LFs, 6 were from the 7.5 Gy group, 2 from the 9.5 Gy group and 2 from the 13.5 group. Rate of change in f-SO2 during radiotherapy from day 3 to day 5 was negatively correlated with time to recurrence while the rate of change in f-SO2 after radiotherapy from days 12 to day 15 was positively correlated with time to recurrence (Fig. 3). In other words, within the LF group, tumors with shorter recurrence times exhibited a faster increase in oxygenation during radiotherapy and a slower increase in oxygenation after radiotherapy. No significant correlation was found between rate of change of f- SO2 across days 7 to 10 and recurrence time. In the control group, there were no significant correlations between changes in f-SO2 and the rate of tumor growth across any time intervals (data not shown).
Figure 3. Oxygenation rate is significantly correlated with recurrence time within local failure group.
The rate of change f-SO2 after radiotherapy from day 12 to day 15 was positively correlated with tumor recurrence time. The rate of change in f-SO2 from day 3 to day 5 during radiotherapy was negatively correlated with time until tumor recurrence. Within the local failure mice, tumors that showed higher oxygenation profiles during radiotherapy tended to recur faster.
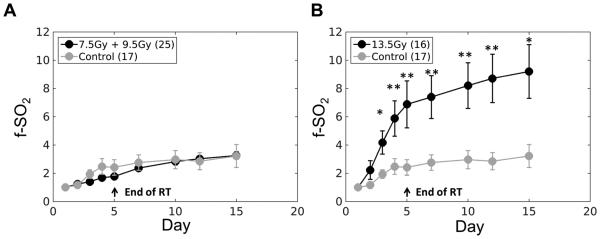
Low dose radiation does not initiate an increase in oxygenation
Fig. 4 shows oxygenation kinetics for mice irradiated with the lowest two radiation doses (7.5 Gy and 9.5 Gy combined) and highest radiation dose (13.5 Gy). The f-SO2 of mice receiving high dose radiation was significantly higher than control mice after day 2 (p<0.05 for day 3 and 15, p<0.01 for day 3, 4, 5, 7, 10 and 12). Statistical significance was not observed when comparing oxygenation kinetics of tumors receiving 7.5 and 9 Gy to control tumors. The oxygenation kinetics are shown in supplementary Fig. 2.
Figure 4. 7.5 Gy and 9.5 Gy fractions cannot initiate an increase in oxygenation.
A. Baseline-corrected vascular oxygenation (f-SO2) for tumors irradiated with 7.5 Gy and 9.5 Gy was not significantly different from the control tumors during and after radiotherapy B. f-SO2 of tumors irradiated with 13.5 Gy was significantly higher than control tumors after day 2 (p<0.05 for day 3 and 15, p<0.01 for day 3, 4, 5, 7, 10 and 12). P values were computed with Wilcoxon rank-sum test. Error bars show standard error.
Discussion
Tumor hypoxia is considered a major factor in predicting radiotherapy treatment outcome since hypoxic tumor cells are 3-fold more resistant to irradiation than aerobic cells (11). However, it is not clear whether kinetic changes in tumor oxygenation during or after radiotherapy are related to the probability of achieving local tumor control. Clinical studies using polarographic microelectrodes to measure tumor hypoxia show the efficacy of radiotherapy is negatively influenced by the extent of pre-treatment tumor hypoxia (12-16). Nevertheless, it has been difficult to evaluate the kinetics of oxygenation because other methods to measure tumor oxygenation kinetics are invasive (microelectrodes) or quite expensive (PET). Reoxygenation during chemoradiotherapy (CRT) was associated with treatment outcome in one study of HNC patients (17). Brizel et al. used microelectrodes to show that reoxygenation early in the course of thermoradiotherapy of soft tissue sarcomas was associated with a favorable response to treatment (18). In another study, no evidence for reoxygenation after the first 10-15Gy was seen in patients with HNC treated with fractionated CRT (16). Optical spectroscopy is relatively inexpensive and completely non-invasive. Using optical spectroscopy, this study, for the first time, reports daily serial tumor vascular oxygenation measurements in mice, during and after fractionated radiotherapy.
LC and LF mice exhibited significantly different trajectories in oxygenation kinetics– LCs demonstrated significantly improved oxygenation compared to LFs, 12-15 days after the first day of a 5 day fractionated course of radiation therapy. These changes occurred at a time when tumor volumes between the LC and LF mice were not significantly different. The classification performance is better when the model was built on the parameters computed from the day 12-15 than from day 3-5 and day 7-10 intervals. LCs showed improved oxygenation and blood perfusion whereas LF demonstrated a decrease or no improvement in oxygenation and blood perfusion in the day 12-15 interval. The results are consistent with the concept that the improvement in oxygenation observed after radiotherapy may be the result of a decreased oxygen consumption in the tumor due to tumor cell death. However, these effects occurred prior to measureable reduction in tumor volume. Secomb et al. previously demonstrated that relatively minor changes in oxygen consumption rate(10-30% reduction), as could occur with a cell loss of equal magnitude can dramatically reduce tumor hypoxia. Cell loss of this magnitude would not likely be detectable on a tumor volume measurement (19). There was no consistent relationship between [THb] and change in SO2 . These results suggest that perfusion change is not directly responsible for the changes in SO2 . This is further evidence that changes in oxygen consumption rate are likely influencing the oxygenation kinetics. Secomb et al. have shown previously that oxygen consumption rate has a more dynamic effect on oxygen transport than changes in perfusion (20).
The relationship between the observed oxygenation rates and time-to-failure from day 3 to day 5 during radiotherapy is strikingly different from day 12 to day 15 after radiotherapy for the mice in the LF group (Fig. 3). The negative relationship between the changes in tumor oxygenation during radiotherapy and time of tumor recurrence (days 3-5) might be explained by the upregulation of HIF-1(21). It was previously reported that a HIF-1 target gene, VEGF, is upregulated 24-48 hours after radiotherapy. The upregulation of VEGF protects endothelial cells from death (21,22). The upregulation of HIF-1 may have mediated switch from aerobic to anaerobic metabolism, which could have further protected tumor cells from death (23). Zhong et al. previously reported that upregulation of HIF-1 after radiotherapy protects tumor microvessels and promotes a switch to anaerobic metabolism (24).
When mice were stratified by radiation dose, mice that received the highest dose of radiation had significantly different tumor oxygenation kinetics than mice that received the lower doses of radiation (Fig. 4). These results suggest that a faster increase in oxygenation can be triggered by higher radiation doses. The difference in oxygenation kinetics may be related to sensitivity of endothelial cells to radiation (25). Garcia-Barros et al. showed that tumors in endothelial cell apoptosis-resistant mice were relatively radioresistant because their endothelial cells do not undergo apoptosis via activation of the acid sphingomyelinase pathway (25). Moreover, doses of radiotherapy <10 Gy did not induce endothelial cell apoptosis in wild type mice. (25-27). Below 10Gy, a decrease in endothelial cell kill combined with the increase in HIF-1 expression, discussed above, may offer radioprotection to tumor cells. If tumor cells are radioprotected, cell mass would remain relatively large and oxygen consumption rates would be maintained (28).
DRS provides label-free, non-invasive, simple and cost-effective means to quantitatively and non-invasively measure and quantify tissue hypoxia in vivo (29-32). It is ideal for serial assessments of tumor hypoxia/perfusion prior to, during and after the radiotherapy. Early prediction of treatment failure could lead to clinical decisions about more aggressive treatments, thereby improving likelihood for a favorable treatment outcome.
Supplementary Material
Summary.
This work examines the kinetics of tumor oxygenation during and after fractionated radiotherapy. These oxygenation kinetics show differential associations with local tumor control outcome. Such relationships have not been described previously.
Acknowledgment
The views expressed in this article are those of WTL, and JKS. They do not necessarily represent the views of the Department of Veterans Affairs or the United States government.
Grant support
The work was funded by NIH grants CA40355-26-28, 5K99CA140783-02 and from generous support of the Alexander and Margaret Stewart Trust, Duke Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflicts of interest.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians. 2014;64(4):252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Seiwert TY, Salama JK, Vokes EE. The chemoradiation paradigm in head and neck cancer. Nat Clin Pract Oncol. 2007;4(3):156–71. doi: 10.1038/ncponc0750. [DOI] [PubMed] [Google Scholar]
- 3.Hockel M, Vaupel P. Biological consequences of tumor hypoxia. Semin Oncol. 2001;28(2):36–41. [PubMed] [Google Scholar]
- 4.Kallman RF. Phenomenon of Reoxygenation and Its Implications for Fractionated Radiotherapy. Radiology. 1972;105(1):135. doi: 10.1148/105.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Ressel A, Weiss C, Feyerabend T. Tumor oxygenation after radiotherapy, chemotherapy, and/or hyperthermia predicts tumor free survival. Int J Radiat Oncol Biol Phys. 2001;49(4):1119–25. doi: 10.1016/s0360-3016(00)01523-6. [DOI] [PubMed] [Google Scholar]
- 6.Harriss W, Bezak E, Yeoh E, Hermans M. Measurement of reoxygenation during fractionated radiotherapy in head and neck squamous cell carcinoma xenografts. Australasian Physical & Engineering Science in Medicine. 2010;33(3):251–63. doi: 10.1007/s13246-010-0032-6. [DOI] [PubMed] [Google Scholar]
- 7.Maftei CA, Bayer C, Shi K, Astner ST, Vaupel P. Changes in the fraction of total hypoxia and hypoxia subtypes in human squamous cell carcinomas upon fractionated irradiation: evaluation using pattern recognition in microcirculatory supply units. Radiother Oncol. 2011;101(1):209–16. doi: 10.1016/j.radonc.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Vishwanath K, Chang K, Klein D, Deng Y, Chang VC, Ramanujam N. Portable, Fiber-Based Diffuse Reflection Spectroscopy (DRS) Systems for Estimating Tissue Optical Properties. Applied Spectroscopy. 2011;65(2):206–15. doi: 10.1366/10-06052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer GM, Ramanujam N. Monte Carlo-based inverse model for calculating tissue optical properties. Part I: Theory and validation on synthetic phantoms. Applied optics. 2006;45(5):1062–71. doi: 10.1364/ao.45.001062. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Ramanujam N. Scaling method for fast Monte Carlo simulation of diffuse reflectance spectra from multilayered turbid media. Journal of the Optical Society of America A, Optics, image science, and vision. 2007;24(4):1011–25. doi: 10.1364/josaa.24.001011. [DOI] [PubMed] [Google Scholar]
- 11.Begg AC. Predicting recurrence after radiotherapy in head and neck cancer. Seminars in radiation oncology. 2012;22(2):108–18. doi: 10.1016/j.semradonc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77(1):18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 13.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38(2):285–9. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 14.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Research. 1989;49:6449–65. [PubMed] [Google Scholar]
- 15.Moeller BJ, Richardson RA, Dewhirst MW. Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007;26(2):241–8. doi: 10.1007/s10555-007-9056-0. [DOI] [PubMed] [Google Scholar]
- 16.Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53(2):113–7. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 17.Dietz A, Vanselow B, Rudat V, Conradt C, Weidauer H, Kallinowski F, et al. Prognostic impact of reoxygenation in advanced cancer of the head and neck during the initial course of chemoradiation or radiotherapy alone. Head Neck-J Sci Spec. 2003;25(1):50–58. doi: 10.1002/hed.10177. [DOI] [PubMed] [Google Scholar]
- 18.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Dodge RK, Charles HC, et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Research. 1996;56(23):5347–50. [PubMed] [Google Scholar]
- 19.Secomb TW, Hsu R, Ong ET, Gross JF, Dewhirst MW. Analysis of the Effects of Oxygen-Supply and Demand on Hypoxic Fraction in Tumors. Acta Oncol. 1995;34(3):313–16. doi: 10.3109/02841869509093981. [DOI] [PubMed] [Google Scholar]
- 20.Secomb TW, Hsu R, Ong ET, Gross JF, Dewhirst MW. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta Oncol. 1995;34(3):313–6. doi: 10.3109/02841869509093981. [DOI] [PubMed] [Google Scholar]
- 21.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5(5):429–41. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 22.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59(14):3374–8. [PubMed] [Google Scholar]
- 23.Meijer TWH, Kaanders JHAM, Span PN, Bussink J. Targeting Hypoxia, HIF-1, and Tumor Glucose Metabolism to Improve Radiotherapy Efficacy. Clinical Cancer Research. 2012;18(20):5585–94. doi: 10.1158/1078-0432.CCR-12-0858. [DOI] [PubMed] [Google Scholar]
- 24.Zhong J, Rajaram N, Brizel DM, Frees AE, Ramanujam N, Haberle I, et al. Radiation induces aerobic glycolysis through reactive oxygen species. Radiotherapy and Oncology. 2013;106(3):390–96. doi: 10.1016/j.radonc.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–59. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 26.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22(37):5897–906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Barros M, Thin TH, Maj J, Cordon-Cardo C, Haimovitz-Friedman A, Fuks Z, et al. Impact of stromal sensitivity on radiation response of tumors implanted in SCID hosts revisited. Cancer Res. 2010;70(20):8179–86. doi: 10.1158/0008-5472.CAN-10-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norbury CJ, Zhivotovsky B. DNA damage-induced apoptosis. Oncogene. 2004;23(16):2797–808. doi: 10.1038/sj.onc.1207532. [DOI] [PubMed] [Google Scholar]
- 29.Chitneni SK, Palmer GM, Zalutsky MR, Dewhirst MW. Molecular imaging of hypoxia. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52(2):165–8. doi: 10.2967/jnumed.110.075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manzoor AA, Yuan H, Palmer GM, Viglianti BL, Dewhirst MW. Weissleder R, Ross BD, Rehemtulla A, Gambhir SS, editors. Imaging Hypoxia. Molecular Imaging: Principles and Practice. 2010:756–80. [Google Scholar]
- 31.Hu FY, Vishwanath K, Beumer HW, Puscas L, Afshari HR, Esclamado RM, et al. Assessment of the sensitivity and specificity of tissue-specific-based and anatomical-based optical biomarkers for rapid detection of human head and neck squamous cell carcinoma. Oral Oncol. 2014;50(9):848–56. doi: 10.1016/j.oraloncology.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu FY, Vishwanath K, Lo J, Erkanli A, Mulvey C, Lee WT, et al. Rapid Determination of Oxygen Saturation and Vascularity for Cancer Detection. Plos One. 2013;8(12) doi: 10.1371/journal.pone.0082977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.