Abstract
Background
Epidermal growth factor receptor (EGFR) mutations occur in about 50% of Asian patients with non‐small cell lung cancer (NSCLC). Patients with advanced NSCLC and EGFR mutations derive clinical benefit from treatment with EGFR‐tyrosine kinase inhibitors (TKIs). This study assessed the efficacy and safety of adjuvant icotinib without chemotherapy in EGFR‐mutated NSCLC patients undergoing resection of stage IB–IIIA.
Methods
Our retrospective study enrolled 20 patients treated with icotinib as adjuvant therapy. Survival factors were evaluated by univariate and Cox regression analysis.
Results
The median follow‐up time was 30 months (range 24–41). At the data cut‐off, five patients (25%) had recurrence or metastasis and one patient had died of the disease. The two‐year disease‐free survival (DFS) rate was 85%. No recurrence occurred in the high‐risk stage IB subgroup during the follow‐up period. In univariate analysis, the micropapillary pattern had a statistically significant effect on DFS (P = 0.040). Multivariate logistic regression analysis showed that there was no independent predictor. Drug related adverse events (AEs) occurred in nine patients (45.0%). The most common AEs were skin‐related events and diarrhea, but were relatively mild. No grade 3 AEs or occurrences of intolerable toxicity were observed.
Conclusions
Icotinib as adjuvant therapy is effective in patients harboring EGFR mutations after complete resection, with an acceptable AE profile. Further trials with larger sample sizes might confirm the efficiency of adjuvant TKI in selected patients.
Keywords: Adjuvant therapy, epidermal growth factor receptor‐tyrosine kinase inhibitor (EGFR‐TKI), non‐small cell lung cancer (NSCLC)
Introduction
Lung cancer is the most common cancer and the leading cause of cancer death among men, while it is the second most commonly diagnosed cancer and the leading cause of cancer death among women in China.1 Surgery is the best curative treatment option for patients with non‐small‐cell lung cancer (NSCLC). Unfortunately, only one out of five patients are eligible for surgical resection, with disappointing five‐year survival rates. Cisplatin‐based chemotherapy as postoperative adjuvant treatment can improve both overall and disease‐free survival.2, 3, 4 However, patients are relatively insensitive to such treatment.
A number of randomized controlled trials have confirmed that there is a significant survival benefit associated with epidermal growth factor receptor‐tyrosine‐kinase inhibitor (EGFR‐TKI) treatment in patients with EGFR‐mutant NSCLC.5, 6, 7, 8 The progression‐free survival (PFS) rate of EGFR‐TKI treatment for EGFR‐mutant NSCLC more than doubled in comparison with patients treated with standard chemotherapy.9, 10 Nevertheless, previous studies have detected different biological characteristics between early and advanced lung cancer.11, 12 Until now, their role in early‐stage lung cancer has been far less defined.
The aim of this retrospective study was to investigate whether targeted TKI treatment could be used as effective adjuvant therapy after regular radical surgery. Icotinib is an orally administered small‐molecule reversible TKI that independently researched and developed with independent intellectual property rights in China. Icotinib is a potent and selective EGFR‐TKI and provides similar efficacy to gefitinib but with better tolerability for NSCLC patients previously treated with one or two chemotherapy agents.13, 14 We retrospectively analyzed the efficacy and safety of orally administered icotinib treatment in patients undergoing resection of stage IB (with high risk factors) to IIIA EGFR‐mutated NSCLC.
Methods
Patients
This study was undertaken at Xuan Wu Hospital, Capital Medical University, Beijing, China, from September 2012 to February 2014. The clinical stage of patients with lung lesions were assessed by: (i) positron emission tomography‐computed tomography (PET‐CT), or (ii) enhanced chest X‐ray CT scan, brain magnetic resonance imaging (MRI), bone scan, and abdominal ultrasound. Patients received lobectomy or bronchial wedge resection with or without lymphadenectomy, depending on whether the lung lesions were completely removed during surgery. The same surgical team performed all surgeries. Tumor specimens were collected during surgery and used for pathological diagnosis to confirm the exact pathology classification, tumor differentiation, and pathological tumor node metastasis (pTNM) stage. EGFR gene mutation was detected by real‐time quantitative polymerase chain reaction (RT‐PCR). Patients were considered eligible for inclusion in the study if they: were over 18 years of age; received surgery to completely remove the lung lesion; had histologically confirmed activating EGFR‐mutated NSCLC between stage IB (with high risk factors) and stage IIIA; had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; and adequate hematological, biochemical and organ function. Patients with high‐risk stage IB NSCLC were defined as those with poorly differentiated tumors (including lung neuroendocrine tumors, but excluding well‐differentiated neuroendocrine tumors), vascular invasion, wedge resection, tumor size >4 cm, visceral pleural involvement, or incomplete lymph node sampling. Patients were judged to have activating EGFR‐mutation positive disease if one or more of four mutations (exon 19 deletion, or 18G719X, 21L858R or 21L861Q) were detected.15 Those with a single mutation of exon 20 T790M, 20 insertions or 19D761Y were considered resistant to EGFR‐TKI and were excluded.15 Other exclusion criteria included: systemic anticancer therapy prior to and post surgery; other malignancies before or during the study; any unstable illness; and pregnancy or lactation. This study was approved by the Medical Ethics Committee of Xuan Wu Hospital, Capital Medical University and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent. All patients had received oral icotinib at a dose of 125 mg q8h/day continuously for 18 months unless either disease progression (radiographic or obvious clinical) or severe toxicity was observed, from three weeks after resection.
Assessment of the efficacy and adverse events (AEs)
Response Evaluation Criteria in Solid Tumors (RECIST) was used to evaluate the tumor responses.16 Generally, after starting with icotinib, the first two evaluations were performed every two months, and then assessed every three to four months or at overt signs of progression. Disease‐free survival (DFS) was defined as the time from surgery to the first confirmed occurrence of disease relapse or metastasis. Adverse events (AEs) were assessed according to Common Terminology Criteria for Adverse Events of the National Cancer Institute (version 4.0).17 Clinical data and outcomes were collected by searching patient medical records, consulting the doctors in charge, interviews in the clinic, and phone calls to the patients or their relatives.
Statistical analysis
SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was applied. Kaplan–Meier curves were used to describe survival data. A log‐rank test was used to determine survival differences with different baseline characteristics. The Cox proportional hazards regression model was used to identify independent factors associated with treatment outcomes. P < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 20 patients who received icotinib as adjuvant therapy were enrolled in this retrospective analysis. The median age of the population was 62 years (range 43–80). All patients were Chinese. Baseline demographics and disease characteristics are shown in Table 1. Most patients were non‐smokers and had adenocarcinoma. Among the enrolled patients, seven (35%) had high‐risk stage IB, eight (40%) had stage II, and five (25%) had stage IIIA NSCLC. Four patients had well differentiated cancer, nine moderately differentiated, two poorly differentiated, and five had unknown differentiation. Four patients had vascular invasion and five patients had micropapillary pattern (MPP) in lung adenocarcinoma (Table 2). Seventeen patients received lobectomy with lymphadenectomy, two received bronchial wedge resection with lymphadenectomy, and one patient (aged 80) received only wedge resection.
Table 1.
Patient baseline characteristics
| Characteristic | Patient n (%) |
|---|---|
| Age | |
| ≤ 60 years | 4 (20) |
| > 60 years | 16 (80) |
| Gender | |
| Male | 10 (50) |
| Female | 10 (50) |
| Histologic subtype | |
| Adenocarcinoma | 19 (95) |
| Adenosquamous carcinoma | 1 (5) |
| Smoking status | |
| Smoker | 3 (15) |
| Never smoker | 17 (85) |
| Stage | |
| IB | 7 (35) |
| IIa | 6 (30) |
| IIb | 2 (10) |
| IIIa | 5 (25) |
| EGFR status | |
| 19 exon deletion | 9 (45) |
| 21 L858R | 11(55) |
EGFR, epidermal growth factor receptor.
Table 2.
Clinical characteristics according to MPP status
| Factor | MPP | Non‐MPP |
|---|---|---|
| Gender | ||
| Male | 1 | 9 |
| Female | 4 | 6 |
| Stage | ||
| I | 2 | 5 |
| II | 2 | 6 |
| III | 1 | 4 |
| Vascular invasion | ||
| Yes | 0 | 4 |
| No | 5 | 11 |
MPP, micropapillary pattern.
Treatment responses
The median follow‐up time was 30 months (range 24–41). All 20 patients recruited for the study completed the scheduled treatment and were eligible for data analysis. At the data cut‐off, five patients (25%) had recurrence or metastasis. Recurrence occurred in two patients during adjuvant treatment. Patient data is listed in Table 3. The two‐year DFS rate was 85%. One patient died of multiple organ metastases in the 25th month. The two‐year overall survival (OS) rate was 90%. One patient with mediastinal lymph node metastasis had a good response to subsequent treatment with Axitinib. We performed a subgroup analysis of DFS according to pTNM stage. No recurrence occurred in the high‐risk stage IB subgroup during the follow‐up period. The DFS rate was 62.5% in the stage II and 60% in the stage IIIA subgroups (P = 0.258). In univariate analysis, MPP had a statistically significant effect on DFS (P = 0.040; Fig 1). No significant differences in PFS were observed with respect to age (P = 0.166), smoking status (P = 0.093), stage (P = 0.258) or vascular invasion (P = 0.985). Multivariate logistic regression analysis revealed no independent predictors (Table 4). A longer follow‐up study is needed to assess the long‐term treatment responses in these 20 patients.
Table 3.
Clinical data of patients with recurrent disease
| Number | Gender | Stage | Exon | Duration of treatment | Sites of recurrence | DFS (months) |
|---|---|---|---|---|---|---|
| 1 | F | IIIa | 21 | 13 | Lymph nodes | 13 |
| 2 | M | II | 21 | 18 | Lung | 27 |
| 3 | M | IIIa | 19 | 12 | Brain, bone, and liver | 12 |
| 4 | M | II | 21 | 12 | Liver | 12 |
| 5 | M | II | 19 | 18 | Contralateral lung | 33 |
DFS, disease‐free survival; F, female; M, male.
Figure 1.
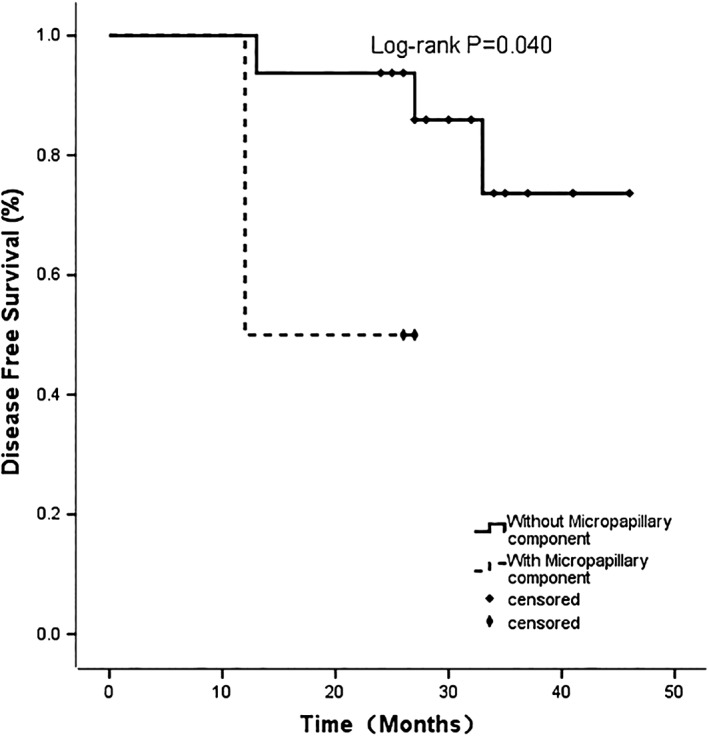
Kaplan–Meier curves for disease‐free survival by micropapillary component status.
Table 4.
Summary of multivariate analysis for disease‐free survival
| Predictor variables | Hazard ratio (95% CI) | P |
|---|---|---|
| Gender | 0.488 (0.018, 13.250) | 0.670 |
| Vascular invasion | 0.455 (0.011, 18.021) | 0.675 |
| MPP | 3.522 (0.285, 43.575 | 0.327 |
CI, confidence interval; MPP, micropapillary pattern.
Treatment‐related side effects
Drug related AEs occurred in nine of the 20 patients (45%; Table 5). The most common AEs were skin‐related events and diarrhea. The incidence of acne‐like rash and diarrhea were 30% and 20%, respectively. Other common AEs included dry skin, oral ulcer, nausea, fatigue, and elevated alanine transaminase/aspartate transaminase. However, these side effects were relatively mild, assessed mainly as grade 1, with a very small number receiving a grade of 2; while no grade 3 side effects or occurrences of intolerable toxicity were observed. No possible drug‐related interstitial lung disease or drug related death was noted and no patient required a dose reduction because of AEs.
Table 5.
Adverse events related to treatment
| Adverse events | Grade 1 | Grade 2 |
|---|---|---|
| Acne‐like rash | 4 (20%) | 2 (10%) |
| Dry skin | 2 (10%) | 1 (5%) |
| Oral ulcer | 1 (5%) | 0 |
| Diarrhea | 4 (20%) | 0 |
| Elevated ALT/AST | 0 | 2 (10%) |
| Nausea | 2 (10%) | 0 |
ALT, alanine transaminase; AST, aspartate transaminase.
Discussion
Successful adjuvant targeting therapies have been reported in other cancer fields, such as imatinib for the treatment of gastrointestinal stromal tumors and trastuzumab for breast cancer. Some Asian studies have shown that EGFR mutation frequency (about 50%) in early stage NSCLC was similar to that in advanced lung cancer patients.18, 19, 20 Therefore, EGFR‐TKI adjuvant therapy is expected to clear away residual tumor cells.
Investigation of adjuvant EGFR‐TKI for resected NSCLC began with a large randomized trial, designed to assess EGFR‐TKIs in early stage NSCLC.21 No significant differences in OS or DFS were detected in either of the groups (gefitinib and placebo groups, respectively); however the EGFR subgroup reported poor results. The possible explanations for such poor results include: (i) the median treatment duration was shorter than usual and did not allow sufficient time for the TKIs to demonstrate their efficacy; and (ii) a low EGFR mutation rate was observed in this study (15/503, 3%) most likely because there was no confirmation of EGFR status in the study population. 22 However, there is also a hypothesis that there might be a lower EGFR mutation rate in early stages of NSCLC and that, in general, early and advanced NSCLC might have different biologic characteristics. Overall, the appropriate selection of patients with EGFR mutation is necessary to avoid misleading conclusions.
Two retrospective analyses of patients with completely resected NSCLC stages I–III with EGFR mutation demonstrated that patients who were treated with an EGFR‐TKI presented improved two‐year DFS rates (P = 0.06).23, 24 There was no statistical difference in OS; the two‐year OS was ≥90% in both the EGFR‐TKI and chemotherapy groups. These results lead to conjecture that EGFR mutant NSCLC patients may benefit after R0 surgery, thus, planned prospective analysis must be conducted in this population in order to determine treatment benefit. Our results were consistent with the results of these studies, regarding two‐year DFS (89% vs. 85%) and two‐year OS rates (96% vs. 90%). Recently, a group of authors from Peking University Cancer Hospital and Institute published their results from a retrospective study, which included 257 patients with completely resected adenocarcinoma stages I–IIIA.18 Among them, 138 patients had EGFR mutations; 31 patients received adjuvant TKI, while 27 received exclusive TKI therapy with a median treatment duration of 18 months. The EGFR‐TKIs included gefitinib, erlotinib, and icotinib. Patients with EGFR‐positive mutations who received adjuvant TKIs achieved longer DFS (P = 0.033). However, adjuvant TKI therapy did not have an impact on OS between the groups (P = 0.258), although patients who received TKIs had better three‐year OS (92.5% vs. 81%).
In addition, there are a series of randomized controlled prospective trials on EGFR mutated patients who received adjuvant chemotherapy with or without EGFR‐TKI consolidation therapy. Most results indicated that adjuvant chemotherapy plus EGFR‐TKI had better DFS compared with chemotherapy alone.25, 26, 27
A series of studies have demonstrated that MPP is an unfavorable prognostic factor in early stage adenocarcinoma.28, 29, 30, 31 However, other survival analyses have indicated that patients with MPP harboring EGFR mutations were reported to have better survival when they received TKI treatment compared with those who received either no treatment or conventional platinum‐based chemotherapy.32 , 33 In other words, EGFR‐mutated patients with MPP may benefit from the application of EGFR‐TKIs, which subsequently control the disease. In our study, five patients had MPP and none had any other poor prognostic factors, such as vascular invasion or poor differentiation. However, according to our univariate analysis, these patients with MPP had significantly shorter DFS than patients without MPP. Therefore, it is important to focus on EGFR‐mutated patients with MPP at the early stage of lung cancer, as they are more likely to experience recurrence, even after receiving EGFR‐TKI treatment.
In our study, the patients who received oral icotinib tolerated the drug well and no dose reductions or interruptions were necessary. The incidence of icotinib‐related AEs was 45% and no incidence of grade 3 or 4 AEs was observed. The rate of AEs in our study was notably better than the rate reported in the ICOGEN study, where the incidence of drug‐related AEs in icotinib‐treated patients was 61%.13 However, the ICOGEN study included more advanced stage patients and the residual tumor cells in our cohort of resected NSCLC patients are obviously smaller than that in advanced patients. Because our patients had fewer residual tumor cells, the EGFR‐TKIs targeting cells with EGFR mutations had a less significant AEs.
The role of EGFR‐TKIs in early stage NSCLC patients with EGFR activating mutations has not yet been established or validated. Although our study was limited by the short follow‐up period and small number of patients, our results regarding efficacy and AEs were promising, and further prospective studies with larger patient populations recruited from multiple centers might establish the clinical efficiency of adjuvant TKI in selected patients. We look forward to the results of two ongoing randomized controlled trials (Chinese CTONG1104 and Japanese WJOG6410L) focused on adjuvant TKI monotherapy compared with adjuvant chemotherapy in sensitive EGFR‐mutated stage II–III NSCLC patients.
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank all of the participating patients in this study.
References
- 1. Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 2. Pignon JP, Tribodet H, Scagliotti GV et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008; 26: 3552–9. [DOI] [PubMed] [Google Scholar]
- 3. Butts CA, Ding K, Seymour L et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non‐small‐cell lung cancer: Updated survival analysis of JBR‐10. J Clin Oncol 2010; 28: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arriagada R, Dunant A, Pignon JP et al. Long‐term results of the international adjuvant lung cancer trial evaluating adjuvant cisplatin‐based chemotherapy in resected lung cancer. J Clin Oncol 2010; 28: 35–42. [DOI] [PubMed] [Google Scholar]
- 5. Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–22. [DOI] [PubMed] [Google Scholar]
- 6. Han JY, Park K, Kim SW et al. First‐SIGNAL: First‐line single‐agent iressa versus gemcitabine and cisplatin trial in never‐smokers with adenocarcinoma of the lung. J Clin Oncol 2012; 30: 1122–8. [DOI] [PubMed] [Google Scholar]
- 7. Mok TS, Wu YL, Thongprasert S et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 8. Maemondo M, Inoue A, Kobayashi K et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 9. Zhou C, Wu YL, Chen G et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–42. [DOI] [PubMed] [Google Scholar]
- 10. Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 11. Yu ZH, Lin SH, Balter P, Zhang L, Dong L. A comparison of tumor motion characteristics between early stage and locally advanced stage lung cancers. Radiother Oncol 2012; 104: 33–8. [DOI] [PubMed] [Google Scholar]
- 12. Cedres S, Nuñez I, Longo M et al. Serum tumor markers CEA, CYFRA21‐1, and CA‐125 are associated with worse prognosis in advanced non‐small‐cell lung cancer (NSCLC). Clin Lung Cancer 2011; 12: 172–9. [DOI] [PubMed] [Google Scholar]
- 13. Shi Y, Zhang L, Liu X et al. Icotinib versus gefitinib in previously treated advanced non‐small‐cell lung cancer (ICOGEN): A randomised, double‐blind phase 3 non‐inferiority trial. Lancet Oncol 2013; 14: 953–61. [DOI] [PubMed] [Google Scholar]
- 14. Tan F, Shen X, Wang D et al. Icotinib (BPI‐2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. Lung Cancer 2012; 76: 177–82. [DOI] [PubMed] [Google Scholar]
- 15. Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non‐small cell lung cancer. Clin Cancer Res 2006; 12: 7232–41. [DOI] [PubMed] [Google Scholar]
- 16. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 17. Trotti A, Colevas AD, Setser A et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13: 176–81. [DOI] [PubMed] [Google Scholar]
- 18. Lv C, An C, Feng Q et al. A retrospective study of stage I to IIIa lung adenocarcinoma after resection: What is the optimal adjuvant modality for patients with an EGFR mutation? Clin Lung Cancer 2015; 16: e173–81. [DOI] [PubMed] [Google Scholar]
- 19. Shi Y, Au JS, Thongprasert S et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014; 9: 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Isaka T, Yokose T, Ito H et al. Correlations between the EGFR mutation status and clinicopathological features of clinical stage I lung adenocarcinoma. Medicine (Baltimore) 2015; 94: e1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goss GD, O'Callaghan C, Lorimer I et al. Gefitinib versus placebo in completely resected non‐small‐cell lung cancer: Results of the NCIC CTG BR19 study. J Clin Oncol 2013; 31: 3320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhai H, Zhong W, Yang X, Wu YL. Neoadjuvant and adjuvant epidermal growth factor receptor tyrosine kinase inhibitor (EGFR‐TKI) therapy for lung cancer. Transl Lung Cancer Res 2015; 4: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janjigian YY, Park BJ, Zakowski MF et al. Impact on disease‐free survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor EGFR mutations. J Thorac Oncol 2011; 6: 569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D'Angelo SP, Janjigian YY, Ahye N et al. Distinct clinical course of EGFR‐mutant resected lung cancers: Results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol 2012; 7: 1815–22. [DOI] [PubMed] [Google Scholar]
- 25. Pennell NA, JW N, Chaft JE. SELECT: A multicenter phase II trial of adjuvant erlotinib in resected early‐stage EGFR mutation‐positive NSCLC. . 2014 ASCO Annual Meeting ProceedingsJ Clin Oncol 2014; 32 (Suppl. 5): Abstr 7514. [Google Scholar]
- 26. Feng S, Wang Y, Cai K et al. Randomized adjuvant chemotherapy of EGFR‐mutated non‐small cell lung cancer patients with or without icotinib consolidation therapy. PLoS One 2015; 10 (10): e0140794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li N, Ou W, Ye X et al. Pemetrexed‐carboplatin adjuvant chemotherapy with or without gefitinib in resected stage IIIA‐N2 non‐small cell lung cancer harbouring EGFR mutations: A randomized, phase II study. Ann Surg Oncol 2014; 21: 2091–6. [DOI] [PubMed] [Google Scholar]
- 28. Xu CH, Wang W, Wei Y et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification in stage IB lung adenocarcinoma. Eur J Surg Oncol 2015; 41: 1430–6. [DOI] [PubMed] [Google Scholar]
- 29. Yang F, Chen K, Liao Y et al. Risk factors of recurrence for resected T1aN0M0 invasive lung adenocarcinoma: A clinicopathologic study of 177 patients. World J Surg Oncol 2014; 12: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun Y, Yu X, Shi X, Hong W, Zhao J, Shi L. Correlation of survival and EGFR mutation with predominant histologic subtype according to the new lung adenocarcinoma classification in stage IB patients. World J Surg Oncol 2014; 12: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, Sun Y, Xiang J, Zhang Y, Hu H, Chen H. A clinicopathologic prediction model for postoperative recurrence in stage Ia non‐small cell lung cancer. J Thorac Cardiovasc Surg 2014; 148: 1193–9. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y, Wang R, Cai D et al. A comprehensive investigation of molecular features and prognosis of lung adenocarcinoma with micropapillary component. J Thorac Oncol 2014; 9: 1772–8. [DOI] [PubMed] [Google Scholar]
- 33. Sumiyoshi S, Yoshizawa A, Sonobe M et al. Pulmonary adenocarcinomas with micropapillary component significantly correlate with recurrence, but can be well controlled with EGFR tyrosine kinase inhibitors in the early stages. Lung Cancer 2013; 81: 53–9. [DOI] [PubMed] [Google Scholar]


