Abstract
Although evidence exists that changes in sensorimotor function occur with aging, changes in the bilateral coordination of the upper extremities is less understood. Here, we review the behavioral and neural evidence of declines in bilateral coordination as well as the implications these deficits have on function and physical rehabilitation. We begin with an introduction to the two major forms of bilateral coordination, symmetric and non-symmetric and their sub-groupings. After discussing the motor performance changes with age in symmetric tasks, we address age-related changes in motor lateralization that may affect the bilateral coordination of non-symmetric coordination. This is followed by a discussion of the contributions of cognitive, sensory, and cortical changes with age that influence and underlie bilateral motor performance. Finally, age-related changes in motor learning of bilateral movements are also considered. In general, most age related changes are found in complex symmetric movements but, surprisingly, there is a dearth of information about changes in the more challenging and ubiquitous non-symmetric bilateral movements. Future investigations should focus on broadening the understanding of age-related changes in complex, functionally relevant bilateral movements, such that the real-world implications of these changes may be derived.
Keywords: upper extremity, bilateral coordination, aging
Introduction
As we age, it is easy to see the physical effects on our ability to walk, run, and our effort to reduce the possibility of falling; but it is more difficult to notice the changes in upper extremity function and the impact on everyday life. Upper extremity motor function has been identified as an important predictor of both disability and mortality [1], [2]. Since everyday activities rely primarily on bimanual movements in both young and older adults [3], [4], an understanding of the age-related impairments in bilateral arm coordination leading to functional limitations in the activities of daily living [5]–[7] is essential. Age-related deficits in bilateral coordination not only represent a significant functional problem for older individuals [4], but also correlate with dementia and early-stage Alzheimer’s disease [8]–[10]. Specifically, declines in bilateral hand motor function were found to be indicative of cognitive impairments and therefore assessment of bilateral hand function may contribute to the differentiation and early diagnosis of dementia subtypes [9]. For this review, we focus on age-related changes in the control of bilateral task performance. We begin with a description of the different forms of bilateral coordination, followed by a review of age-related changes within each form. Next, we discuss changes in key contributors to bilateral control, including cognition, sensation, and cortical structure and function. Finally, we will consider the age-related changes in motor learning of bilateral tasks and the implications these changes have for rehabilitation. We acknowledge that declines in motor performance with age result from the degeneration of multiple interacting central and peripheral systems [11]–[13]. However, peripheral changes, such as loss of muscle strength, are not directly covered in this review since loss of muscle mass in both arms does not necessarily affect bilateral coordination.
Forms of Bilateral Coordination
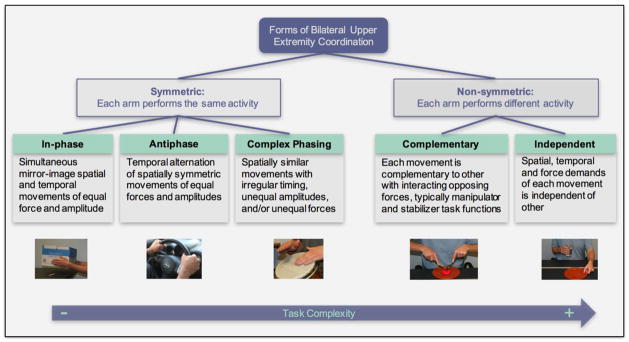
There are different ways of categorizing bilateral tasks. Here, we define five different forms (Figure 1), which can be broadly divided into symmetric and non-symmetric coordination patterns [14], [15]. Symmetric coordination patterns require that each hand perform the same activity and are made up of in-phase, antiphase, or complex phasing movements. In-phase movements require simultaneous mirror-image spatial and temporal movement of each arm, such as opening a drawer or carrying a tray. Antiphase movements require temporal alternation of spatially symmetric movements of each arm, such as walking or using a steering wheel when driving. Complex phasing includes spatially similar movements with irregular timing (such as 2:1 movement repetition or out-of-phase rhythm), unequal amplitudes, or unequal forces. Functionally, these patterns are found mostly in music, such as playing the drums or piano. Non-symmetric coordination patterns, on the other hand, have different spatial, timing, and force requirements of each arm. These movements may reflect independent goals for each arm, such as holding and steadying a cup while opening a door, or complementary goals of each arm, such as cutting meat using a knife and fork in each hand. During complementary movements, task functions of each hand typically involve the dominant hand acting as the manipulator and the non-dominant hand acting as the supporter or stabilizer [14]. However, the majority of bilateral coordination investigations focus on symmetric tasks [16]–[18].
Figure 1.
Forms of Bilateral Coordination
Motor performance deficits of symmetric tasks
In general, performance accuracy in maintaining stable coordination between the arms is more affected in older compared to young adults, particularly as the complexity of coordination demands increase. Symmetric bilateral task demands are typically broken down to include movement frequency (speed), phase, force, and amplitude (distance). Phase refers to the relative timing of one arm with respect to the other arm whereby in-phase is simultaneous, antiphase is alternating, and complex phasing is irregular. Stability is an indicator of accuracy and refers to the ability to accurately maintain spatial and phase requirements as movement speed increases. Phase demands also interact with movement speed in that phase transitions between unstable and stable coordination states are modulated by movement frequency [19]. For example, as movement speed increases, antiphase movements switch to in-phase movements and this transition occurs at lower movement speeds as the complexity of the phasing between the hands increases. Although the investigation of symmetric movements within the context of these tasks demands may not appear functionally relevant, they represent important components of bilateral coordination.
With respect to movement frequency, declines in the ability to maintain a target pace have been identified beyond a simple slowing in movement execution with age. In a comparison between unilateral and bilateral in-phase and complex phasing reaches to near and far targets, older adults demonstrated significantly increased movement time for all tasks and greater asynchrony while initiating the bilateral tasks compared to young adults [20]. This age-related difference becomes more apparent during antiphase tasks as speed increases. In a comparison of in-phase and antiphase tasks (shoulder abduction/external rotation, adduction/internal rotation) performed at varying movement speeds, older adults performed similarly to young adults during in-phase coordination at all speeds and antiphase coordination at slow speeds [21]. However, during faster movement speeds, only older adults failed to maintain the stable antiphase movement pattern [21]. The effects on antiphase and complex phasing coordination have also been illustrated through the influence of force output demands. In addition to a reduction of handgrip maximum voluntary contraction (MVC), older adults take significantly longer to alternate grip forces between the two hands at multiple equal and unequal percentages of their MVC [22]. The above evidence illustrates an age-associated reduction in the stability of phasing accuracy with increased task demands, such as faster movement speeds and increased force demands.
The ability to maintain phase stability during symmetrical movements reveals further bilateral coordination deficits with age. Summers and colleagues [23] compared the performance of young and older adults during continuous and intermittent bimanual circle-drawing. Using their index fingers, participants continuously traced the circumference of circle templates for the continuous task and paused after the completion of each circle for the more complex intermittent task. Both tasks were performed during in-phase (left hand clockwise, right hand anti-clockwise) and antiphase (both hands anti-clockwise) coordination modes at both preferred and fast (50% greater than preferred) movement frequencies. For continuous circle drawing, older adults demonstrated performance similar to young adults for both coordination modes and movement frequencies. For intermittent circle drawing, older adults performed similarly to young adults during the in-phase coordination mode, but demonstrated greater temporal and spatial error during the antiphase mode, regardless of frequency [23]. Temprado and colleagues [24] investigated the effects of aging on phase transition stability for in-phase and antiphase coordination patterns (forearm pronation) by manipulating movement frequency. Overall, as movement frequency increased, the number of phase transitions and movement variability away from the target frequency increased and the time-to-transition between phases decreased. Compared to young adults, older adults demonstrated phase transitions from antiphase to in-phase at lower frequency thresholds, more transitions with less time-to-transition at all frequency levels, and greater movement variability at the highest frequency [24]. In general, greater age-related changes in phasing accuracy are observed during greater temporal demands.
Motor performance deficits of non-symmetric tasks
Non-symmetric coordination tasks are more complex and require the largest processing demands compared to symmetrical coordination modes. During these movements, interference between the arms occurs when each arm produces movements with different amplitudes, directions, frequencies, or forces, requiring higher levels of motor skill and flexibility [25], [26]. Despite their functional relevance, there are no direct investigations of age-related changes to non-symmetric coordination patterns. Nevertheless, changes to motor lateralization might provide some insight into possible deficits in complementary non-symmetric bilateral tasks.
Fundamental investigations of motor control have focused on lateralized function for over a century [27]–[30]. Earlier studies suggested that the preferred hand moves faster, is more accurate at faster speeds, and more consistent in force production [27], [31]–[33]. More recently, the view of a dominant left hemisphere specialization of motor function has shifted to the idea that the left and right hemisphere are specialized for different aspects of motor control [34], [35]. More specifically, this dynamic-dominance hypothesis posits that a) the left hemisphere is specialized for predictive mechanisms, specifying efficient and smooth trajectories under stable environmental conditions, and b) the right hemisphere is specialized for impedance control that is robust to unstable environmental conditions. These hemisphere specializations are believed to provide the basis for behavioral observations of complementary roles during bimanual coordination of the right (dominant) arm for reaching and manipulating, and the left (non-dominant) arm for stabilizing [36], [37]. Hand dominance has been shown to influence bilateral coordination in young adults, such that individuals with a reduced lateral preference demonstrate a bilateral performance advantage [38] and that arm specializations interfere with the performance of symmetrical tasks [39].
Current evidence both supports and challenges the idea that motor lateralization changes with age. Many studies demonstrate age-related reductions in motor lateralization, including attenuation of hand dominance with a shift towards ambidexterity [40], reduced laterality during imagined (mental) actions [41], and reduced asymmetry during multidirectional reaching movements [42]. In contrast, others have failed to identify age-related changes in hand laterality when tasks were broken down into movement stages of preparation and execution [43]. A simple unimanual pointing task using a pre-cueing paradigm [44] illustrated a right (dominant) hand advantage for execution speed and left (non-dominant) hand advantage for preparation speed across young, middle age, and older individuals, suggesting that motor lateralization may be preserved with aging [43]. The transfer effect of sensorimotor skills from the trained arm to the untrained arm (i.e. interlimb transfer), which is commonly found in young adults, also demonstrates conflicting results. While one study identified an age-related reduction in interlimb transfer of visuomotor adaptation during unimanual reaching [45], another illustrated a preservation of interlimb transfer during a multidirectional drawing task [46]. Despite conflicting results of these preceding studies, it is important to note that the evidence is based solely on unimanual motor tasks. Therefore, it is not yet clear whether or how age-related changes in motor lateralization will affect bilateral coordination tasks. Further, due to changes at the cortical level, motor lateralization may differentially influence bilateral coordination in older compared to young adults. For example, age related reductions in white matter (WM) volume and unilateral motor function illustrated reduced structural evidence of motor lateralization, possibly due to neural dedifferentiation [47].
Change in cognitive contributions
Evidence that bilateral coordination deficits are most evident during increased task demands may reflect the increased cognitive processing involved in such tasks. In other words, increases in movement frequency or phasing requirements seem to reach a threshold by which age-related cognitive changes cannot sustain accurate performance [19], [20], [23]. Bangert and colleagues found that older adults demonstrated the greatest impairment during antiphase compared to in-phase repetitive finger tapping and performance of this condition correlated with self-reported executive dysfunction [48]. These findings align with the idea that the increased cognitive demands of bilateral coordination [49] may magnify age-related bilateral coordination differences due to the known cognitive declines with aging [48]. In this respect, the link between bilateral deficits and dementia and Alzheimer’s is not surprising [8], [9]. Declines in complex bilateral hand movements (e.g. Purdue Pegboard assembly test) have been shown to reliably differentiate individuals with mild cognitive impairment and individuals with Alzheimer’s disease from those with typical age-related declines [9], [10].
Changes in sensory contributions
The accuracy of bilateral coordination is largely dependent on perceptual information [25],[15]. In view of declines in peripheral sensory afferent and cognitive processing, age-related deficits in sensory integration may likely contribute to bilateral coordination deficits.
With regard to vision, an age effect to bilateral performance is dependent on the amount and type of available visual information. To assess the influence of visual information on force adaptation, performance of a complex phasing force coordination task (varying force contributions of a sum force by each index finger), was compared across age groups for task conditions with high and low visual information levels [50]. Performance error and variability improved with higher visual information levels for both age groups, suggesting that increased reliance on visual information can compensate for age-related declines in bilateral performance. However, the accuracy of force adaptation was reduced for older adults during task conditions with low visual information and greater force differences between the hands, illustrating a limit to the age-related compensation. How visual attention is directed also affects bilateral coordination [51]. Performance of a bimanual tracking task under different visual conditions (central, peripheral, full, or no vision) of either voluntary or passive movements illustrated similar general effects of visual and task condition of both age groups, although older adults were either more impaired or adapted less with different visual information. Specifically, conditions with central visual attention to the active limb impaired performance in both groups, but more so in the older adults, while central vision to the passive limb improved performance in both groups, but less so in older adults. However, it should be noted that the reliance on proprioceptive information likely changed under different visual conditions as well, which has previously been shown to contribute to these age differences in bilateral perception [52].
A recent study utilized both behavioral and neural methods to investigate how visual and auditory sensory information can improve motor performance of in-phase and antiphase bilateral finger tapping. Performance was more accurate and less variable during conditions with only auditory and auditory plus visual information compared to only vision or no added sensory information. Electroencephalography measures illustrated higher attentional and sensorimotor activation in older adults and similar perceptual activations in both young and older adults underlying the auditory stimulation advantage for improving bilateral coordination [53]. These results indicate that the auditory integration is retained in older adults, which support the use of auditory cues as an adjunct to traditional and novel rehabilitation protocols aimed at improving bilateral motor training.
Changes in cortical contributions
The neural activity of the motor system is comprised of task-specific combinations of excitatory and inhibitory influences within the motor network [54]. In general, older adults recruit increased brain areas [55]–[57] and demonstrate more pronounced interactions between brain regions to perform cognitive and motor tasks [58], [59]. Similar to the age-related behavioral changes in bilateral coordination, the most substantial neural changes have been identified during tasks with increased spatiotemporal differences between the arms. In young adults, interhemispheric inhibitory interactions are essential for preventing interference from the opposite hemisphere during bilateral tasks [57], [60]. As outlined below, structural and functional changes of these interhemispheric connections in older adults may, in some cases, provide a compensatory mechanism to maintain bilateral control with age.
Increased reliance on interhemispheric connections to complete bilateral tasks occurs via the corpus callosum (CC) [61]. Several studies have demonstrated a relationship between age-related structural changes of the CC and bilateral coordination across a variety of tasks and temporal coordination demands. One recent study [62], investigated the relationship between age-related changes in CC structural integrity and performance accuracy of in-phase and antiphase bilateral finger tapping tasks [48]. Overall, older adults demonstrated smaller anterior CC and reduced WM integrity compared to young adults [62]. Surprisingly, for tasks with the largest interhemispheric processing demands, larger CC size and superior WM integrity related to worse motor performance in young adults, while the integrity of the same areas related to better performance within the older adults. Similarly, a follow-up study found that better performance of bilateral force production tasks related to better CC integrity in older adults [63]. CC integrity, in turn, was related to interhemispheric inhibitory (IHI) connections, which were reduced in older adults. Further, disinhibitory IHI’s between the two primary motor cortices (M1) assessed at both rest and during bilateral tasks were found to predict better bilateral task performance in older adults [63], [64]. These results are thought to reflect the altered interhemispheric interactions that are generally observed with aging such that young adults utilize IHI [65], while older adults utilize interhemispheric facilitation during bilateral task performance [59], [62].
Additional support for age-related interactions between neural structure and bimanual function was demonstrated by a comparison of the microstructural organization and integrity of seven CC sub-regions with behavioral tests of bimanual function [66]. Although performance was impaired for all bimanual tasks in older adults, an association between the behavioral outcomes and neural structure was identified. In general, greater WM integrity of the CC occipital region related to better bimanual fine motor skills and greater WM integrity of the premotor, primary motor and sensory CC regions related to better performance on bilateral finger tapping, choice reaction, and complex visuomotor tracking tasks. In contrast, the relationship between WM integrity and performance was reduced in young adults, suggesting that the relationship between age-related changes to the CC and bilateral coordination are task specific and likely occur within the cortical regions connected to these CC pathways.
Other investigations have focused on age-related changes in brain functional connectivity as they relate to bilateral performance. For example, performance of a complex bimanual tracking task [67] was compared to changes in resting state functional connectivity of the sensorimotor network [68]. Older adults demonstrated increased sensorimotor resting state functional connectivity, in which reductions in bimanual performance strongly related to increases in premotor functional connectivity [68]. Kiyami and colleagues [69] assessed differences in functional connectivity of the sensorimotor network during in-phase and antiphase bilateral finger tapping. In young adults, inter- and intra-hemispheric task-specific connectivity is modulated by left dorsal premotor area (PMd) during in-phase and antiphase tasks. Older adults, in contrast, demonstrated reduced task specific interhemispheric connections of left PMd during both tasks, while intrahemispheric connectivity from left PMd to left M1 was increased during the in-phase task [69]. Given the role of the PMd for motor planning and monitoring, these findings also suggest that older adults may have deficits in these processing stages, rather than motor execution. This altered functional specialization of the motor network may also provide a mechanism to explain the age-related motor lateralization reductions discussed earlier.
Age-related declines in bilateral coordination are likely modulated by a combination of these structural and functional cortical changes. One recent study used structural, neurophysiological, and behavioral measures to assess interactions between structure (WM microstructure) and function (interhemispheric interaction) leading to age-related declines in bilateral control [64]. In contrast to the functional connectivity studies discussed above, the results of this study suggested that PMd function was maintained with aging. Instead, older adults demonstrated a declined ability to modulate IHI between prefrontal areas and M1 during tasks with more complex phasing [64], providing further support for the link to cognitive declines with aging [48]. Finally, older adults demonstrated an association between brain structure, neurophysiologic function, and bilateral performance while only neurophysiological function related to performance in young adults. Therefore, as the authors postulated, declines in WM integrity may lead to the altered interhemispheric interactions that are responsible for age-related bilateral coordination deficits [64].
Altogether, the above evidence is in agreement that changes in the structure and function of interhemispheric connections predominantly contribute to age-related changes in bilateral upper extremity coordination. However, it is unclear whether this change is due to connections between premotor or cognitive areas, or a combination of both. Moreover, bilateral tasks requiring simultaneous actions of lateralized motor behaviors, are likely represented by a more complex modulation of intra- and inter- hemispheric connectivity [70], which have yet to be identified.
Motor Learning Implications for Rehabilitation
Given the age-related bilateral coordination deficits and evidence in support of bilateral rehabilitation [71]–[74], it is important to examine age-related changes in bilateral motor learning to assess whether the deficits acquired by age, injury or insult can be ameliorated.
Although several studies have illustrated that motor learning is intact in older adults [75], recent efforts have sought to identify whether bilateral motor learning is maintained with age. Two investigations used a bimanual sequence learning task to assess motor learning differences between young and older adults [76], [77]. One used an implicit learning approach and illustrated no learning deficit in the older group [76], while the other used an explicit learning approach and found that older adults demonstrated reduced motor learning [77]. These results corroborate prior unilateral evidence that implicit learning is preserved with aging while explicit learning declines [78]. Switch cost times (change in response time when the sequence was switched between hands) were also examined [76], [77]. Older adults demonstrated greater switch cost times at baseline, which were thought to be reflective of reduced IHI or less asymmetric cortical activation for motor planning and execution. However, for one study, both young and older adults demonstrated no difference in switch costs between age-groups following training [77], suggesting that it may be possible to mitigate these deficits with training. Further investigation regarding the neural underpinnings of the divergent responses to different motor learning approaches is required.
Several factors influence motor learning, such as task structure, complexity, and difficulty [75], which have started to be addressed in the context of bilateral tasks. One of these variables is the so-called contextual interference effect. Blocked practice, in which a particular movement practiced over multiple repetitions, typically demonstrates greater initial performance changes, while random practice, in which different movement parameters or skills are practiced randomly, typically results in better skill retention [79]. Pauwels and colleagues compared the influence of age on the contextual interference effect by having subjects learn a bimanual dial rotation task [80]. Antiphase and complex symmetric movement patterns of this task were used as low and high task complexity variants. Results indicated that the effects of blocked and random practice schedules were the same for both age groups, as both groups demonstrated greater skill acquisition that was retained after one week with the random practice compared to the blocked practice schedule, independent of task complexity levels.
The extent to which motor learning of one task generalizes to the performance of another task provides an example of the effect of task context on motor learning. With respect to aging, the effects of bilateral ballistic training (rapid abductions of the index fingers) on subsequent unilateral training of the same task was assessed by comparing changes in bilateral and unilateral performance, corticospinal excitability, and intracortical inhibition [81]. Strong transfer of motor learning between bilateral and unilateral tasks was evident in both young and older adults with increased acceleration of muscle activation during unilateral tasks following bilateral training, though changes were greater for young adults. While unilateral training appeared to result in greater increases in corticospinal excitability, these increases were not significant. Bilateral training, in contrast, resulted in a bilateral release of corticospinal inhibition only for the older adults These results were thought to indicate that neural adaptations differ between unilateral and bilateral training and that bilateral training induces greater alterations to inhibitory circuits in older adults. Moreover, the results suggest that although training-induced gains were larger in young adults, the older adults retained similar between-task transfer affects.
Observed differences in motor learning between young and older adults may be biased based on the task-specific performance measures used to compare groups. For example, results of a study assessing differences in motor learning of a fine motor bilateral fingertip force task paralleled other studies discussed, with poorer absolute performance in older adults but similar amounts of motor learning as demonstrated by equal performance changes [82]. However, a more specific comparison of performance measures revealed the greatest improvement on force amplitude for older adults and on temporal precision for young adults, suggesting age-related differences in acquisition strategy. Similarly, a study of motor learning of an antiphase bilateral task with augmented visual feedback found that, in addition to reduced performance, older adults also had reduced improvement rate of performance changes [83]. At the same time, this study also manipulated visual (with vs. without) and proprioceptive (with vs. without vibratory stimuli of arm) sensory information. Movement patterns were more variable during task conditions without vision and with vibratory interference of proprioception, with greater performance deficits of the young adults. This indicates that motor learning may be preserved in older adults, though optimized differently. The aforementioned sensory influences to motor performance suggest that older adults may have adapted or compensated to changes in sensory function, such that they rely more on feedforward rather than feedback mechanisms [83]. While these changes may contribute to motor learning changes, it is also important to consider the effects of individual and combined sensory modalities to bilateral motor performance.
To summarize, it is important to consider age-specific opportunities to augment motor performance through bilateral motor learning, which is likely optimized differentially for older compared to young adults. Different performance changes following training suggest there is an advantage to utilizing implicit motor learning approaches, given that implicit learning appears to be preserved with aging while explicit learning is not. Moreover, additional research is needed to determine how to best ameliorate age-related declines in bilateral coordination.
Conclusions
Age-related deficits observed during bilateral tasks occur during more complex bilateral tasks when there is greater interference and differences between movement demands of each arm. Secondary changes that may underlie declines in bilateral control with aging are also observed in cognitive function and sensorimotor integration. Age-related changes to cortical contributions predominantly involve interhemispheric connections, which are important for performing more complex bilateral tasks with greater differences between each arm. Despite degradation of motor processes, some aspects of motor learning are preserved with aging, suggesting it may be possible to mitigate these motor declines through rehabilitation. Non-symmetric bilateral tasks have not been well characterized in non-disabled young or older adults and addressing this knowledge gap is a useful step for guiding advancements in bilateral rehabilitation in older and neurological populations [73]. Future research efforts should be directed not only on functional bilateral movements, but also on the age-related changes to the underlying systems contributing to these movements.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
As an inventor of a bilateral arm training technology, Jill Whitall anticipates the possibility of receiving income in the future from her institution (UMB) under its Intellectual Property Policy.
Elizabeth Woytowicz and Kelly Westlake declare no conflicts of interest.
Contributor Information
Elizabeth Woytowicz, Email: ewoytowicz@som.umaryland.edu.
Jill Whitall, Email: jwhitall@som.umaryland.edu.
Kelly P. Westlake, Email: kwestlake@som.umaryland.edu.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of major importance
- 1.Ostwald SK, Snowdon DA, Rysavy SDM, Keenan NL, Kane RL. Manual Dexterity as a Correlate of Dependency in the Elderly. J Am Geriatr Soc. 1989 Oct;37(10):963–969. doi: 10.1111/j.1532-5415.1989.tb07282.x. [DOI] [PubMed] [Google Scholar]
- 2.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007 Feb;36(1):228–35. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 3••.Bailey RR, Klaesner JW, Lang CE. Quantifying Real-World Upper-Limb Activity in Nondisabled Adults and Adults With Chronic Stroke. Neurorehabil Neural Repair. 2015 Apr; doi: 10.1177/1545968315583720. Demonstrated that older adults rely primarily on bilateral movements thoughout the day. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilbreath SL, Heard RC. Frequency of hand use in healthy older persons. Aust J Physiother. 2005;51(2):119–122. doi: 10.1016/s0004-9514(05)70040-4. [DOI] [PubMed] [Google Scholar]
- 5.Hortobagyi T, Mizelle C, Beam S, DeVita P. Old Adults Perform Activities of Daily Living Near Their Maximal Capabilities. Journals Gerontol Ser A Biol Sci Med Sci. 2003 May;58(5):M453–M460. doi: 10.1093/gerona/58.5.m453. [DOI] [PubMed] [Google Scholar]
- 6.Seidler RD, Stelmach GE. Reduction in Sensorimotor Control With Age. Quest. 1995 Aug;47(3):386–394. [Google Scholar]
- 7.Onder G, Penninx BWJH, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2005 Jan;60(1):74–9. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 8.Verheij S, Muilwijk D, Pel JJM, van der Cammen TJM, Mattace-Raso FUS, van der Steen J. Visuomotor impairment in early-stage Alzheimer’s disease: changes in relative timing of eye and hand movements. J Alzheimers Dis. 2012 Jan;30(1):131–43. doi: 10.3233/JAD-2012-111883. [DOI] [PubMed] [Google Scholar]
- 9.Scherder E, Dekker W, Eggermont L. Higher-level hand motor function in aging and (preclinical) dementia: its relationship with (instrumental) activities of daily life--a mini-review. Gerontology. 2008 Jan;54(6):333–41. doi: 10.1159/000168203. [DOI] [PubMed] [Google Scholar]
- 10.Kluger A, Gianutsos JG, Golomb J, Ferris SH, George AE, Franssen E, Reisberg B. Patterns of Motor Impairment in Normal Aging, Mild Cognitive Decline, and Early Alzheimer’ Disease. Journals Gerontol Ser B Psychol Sci Soc Sci. 1997 Jan;52B(1):P28–P39. doi: 10.1093/geronb/52b.1.p28. [DOI] [PubMed] [Google Scholar]
- 11.Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010 Apr;34(5):721–33. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorond FA, Cruz-Almeida Y, Clark DJ, Viswanathan A, Scherzer CR, De Jager P, Csiszar A, Laurienti PJ, Hausdorff J, Chen WG, Ferrucci L, Rosano C, Studenski SA, Black SE, Lipsitz LA. Aging, the Central Nervous System, and Mobility in Older Adults: Neural Mechanisms of Mobility Impairment. J Gerontol A Biol Sci Med Sci. 2015 Sep;70(12):1526–32. doi: 10.1093/gerona/glv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayram MB, Siemionow V, Yue GH. Weakening of Corticomuscular Signal Coupling During Voluntary Motor Action in Aging. J Gerontol A Biol Sci Med Sci. 2015 Aug;70(8):1037–43. doi: 10.1093/gerona/glv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winstein C, Wing AM, Whitall J. Motor control and learning principles for rehabilitation of upper limb movements after brain injury. In: Grafman J, Robertsom LH, editors. Handbook of Neuropsychology. 2. 2003. pp. 77–137. [Google Scholar]
- 15.Hoyer EH, Bastian AJ. The effects of task demands on bimanual skill acquisition. Exp brain Res. 2013 Apr;226(2):193–208. doi: 10.1007/s00221-013-3425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelso JAS. Phase transitions and critical behavior in human bimanual coordination. Am J Physiol - Regul Integr Comp Physiol. 1984;246(6) doi: 10.1152/ajpregu.1984.246.6.R1000. [DOI] [PubMed] [Google Scholar]
- 17.Kelso JAS. Dynamic patterns: The self-organization of brain and behavior. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- 18.Swinnen SP. Intermanual coordination: from behavioral principles to neural-network interactions. Nat Rev Neurosci. 2002 May;3(5):348–359. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- 19.Schoner G, Haken H, Kelso JAS. A stochastic theory of phase transitions in human hand movement. Biol Cybern. 1986 Feb;53(4):247–257. doi: 10.1007/BF00336995. [DOI] [PubMed] [Google Scholar]
- 20.Stelmach GE, Amrhein PC, Goggin NL. Age differences in bimanual coordination. J Gerontol. 1988 Jan;43(1):P18–23. doi: 10.1093/geronj/43.1.p18. [DOI] [PubMed] [Google Scholar]
- 21.Wishart LR, Lee TD, Murdoch JE, Hodges NJ. Effects of Aging on Automatic and Effortful Processes in Bimanual Coordination. Journals Gerontol Ser B Psychol Sci Soc Sci. 2000 Mar;55(2):P85–P94. doi: 10.1093/geronb/55.2.p85. [DOI] [PubMed] [Google Scholar]
- 22.Lin C-H, Chou L-W, Wei S-H, Lieu F-K, Chiang S-L, Sung W-H. Influence of aging on bimanual coordination control. Exp Gerontol. 2014 May;53:40–7. doi: 10.1016/j.exger.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Summers JJ, Lewis J, Fujiyama H. Aging effects on event and emergent timing in bimanual coordination. Hum Mov Sci. 2010 Oct;29(5):820–30. doi: 10.1016/j.humov.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Temprado JJ, Vercruysse S, Salesse R, Berton E. A dynamic systems approach to the effects of aging on bimanual coordination. Gerontology. 2010 Jan;56(3):335–44. doi: 10.1159/000262445. [DOI] [PubMed] [Google Scholar]
- 25.Mechsner F, Kerzel D, Knoblich G, Prinz W. Perceptual basis of bimanual coordination. Nature. 2001 Nov;414(6859):69–73. doi: 10.1038/35102060. [DOI] [PubMed] [Google Scholar]
- 26.White O, Diedrichsen J. Responsibility assignment in redundant systems. Curr Biol. 2010 Jul;20(14):1290–5. doi: 10.1016/j.cub.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 27.Woodworth RS. Accuracy of voluntary movement. Psychol Rev. 1899;3:1–114. [Google Scholar]
- 28.Elliott D, Roy EA. Manual Asymmetries in Motor Performance. CRC Press; 1996. [Google Scholar]
- 29.McManus IC. Right- and left-hand skill: failure of the right shift model. Br J Psychol. 1985;76(Pt 1):1–34. doi: 10.1111/j.2044-8295.1985.tb01926.x. [DOI] [PubMed] [Google Scholar]
- 30.Sainburg RL, Kalakanis D. Differences in Control of Limb Dynamics During Dominant and Nondominant Arm Reaching. J Neurophysiol. 2000 May;83(5):2661–2675. doi: 10.1152/jn.2000.83.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters M. Why the preferred hand taps more quickly than the non-preferred hand: Three experiments on handedness. Can J Psychol Can Psychol. 1980 Feb;34(1):62–71. [Google Scholar]
- 32.Todor JI, Cisneros J. Accommodation to increased accuracy demands by the right and left hands. J Mot Behav. 1985 Sep;17(3):355–72. doi: 10.1080/00222895.1985.10735354. [DOI] [PubMed] [Google Scholar]
- 33.Carson RG, Chua R, Elliott D, Goodman D. The contribution of vision to asymmetries in manual aiming. Neuropsychologia. 1990 Jan;28(11):1215–20. doi: 10.1016/0028-3932(90)90056-t. [DOI] [PubMed] [Google Scholar]
- 34.Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev. 2005 Oct;33(4):206–13. doi: 10.1097/00003677-200510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Mutha PK, Haaland KY, Sainburg RL. Rethinking motor lateralization: specialized but complementary mechanisms for motor control of each arm. PLoS One. 2013 Jan;8(3):e58582. doi: 10.1371/journal.pone.0058582. Suggests that the view of motor lateralization has shifted from the dominant left hemisphere specialization of motor function to the theory that the left and right hemisphere are specialized for different aspects of motor control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guiard Y. Asymmetric Division of Labor in Human Skilled Bimanual Action. J Mot Behav. 1987 Aug; doi: 10.1080/00222895.1987.10735426. [DOI] [PubMed] [Google Scholar]
- 37.Peters M. Does handedness play a role in the coordination of bimanual movement? [Google Scholar]
- 38.Kourtis D, De Saedeleer L, Vingerhoets G. Handedness consistency influences bimanual coordination: a behavioural and electrophysiological investigation. Neuropsychologia. 2014 May;58:81–7. doi: 10.1016/j.neuropsychologia.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Dounskaia N, Nogueira KG, Swinnen SP, Drummond E. Limitations on coupling of bimanual movements caused by arm dominance: when the muscle homology principle fails. J Neurophysiol. 2010 Apr;103(4):2027–38. doi: 10.1152/jn.00778.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalisch T, Wilimzig C, Kleibel N, Tegenthoff M, Dinse HR. Age-related attenuation of dominant hand superiority. PLoS One. 2006 Jan;1(1):e90. doi: 10.1371/journal.pone.0000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paizis C, Skoura X, Personnier P, Papaxanthis C. Motor Asymmetry Attenuation in Older Adults during Imagined Arm Movements. Front Aging Neurosci. 2014 Jan;6:49. doi: 10.3389/fnagi.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Przybyla A, Haaland KY, Bagesteiro LB, Sainburg RL. Motor asymmetry reduction in older adults. Neurosci Lett. 2011 Feb;489(2):99–104. doi: 10.1016/j.neulet.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chua R, Pollock BJ, Elliott D, Swanson LR, Carnahan H. The influence of age on manual asymmetries in movement preparation and execution. Dev Neuropsychol. 1995 Jan;11(1):129–137. [Google Scholar]
- 44.Rosenbaum DA. Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen. 1980 Dec;109(4):444–74. doi: 10.1037//0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Przybyla A, Wuebbenhorst K, Haaland KY, Sainburg RL. Aging reduces asymmetries in interlimb transfer of visuomotor adaptation. Exp brain Res. 2011 Apr;210(2):283–90. doi: 10.1007/s00221-011-2631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan Z, Van Gemmert AWA. The effects of aging on the asymmetry of inter-limb transfer in a visuomotor task. Exp brain Res. 2013 Sep;229(4):621–33. doi: 10.1007/s00221-013-3625-y. [DOI] [PubMed] [Google Scholar]
- 47.Koppelmans V, Hirsiger S, érillat SM, Jäncke L, Seidler RD. Cerebellar gray and white matter volume and their relation with age and manual motor performance in healthy older adults. Hum Brain Mapp. 2015 Jun;36(6):2352–63. doi: 10.1002/hbm.22775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bangert AS, Reuter-Lorenz PA, Walsh CM, Schachter AB, Seidler RD. Bimanual coordination and aging: neurobehavioral implications. Neuropsychologia. 2010 Mar;48(4):1165–70. doi: 10.1016/j.neuropsychologia.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swinnen SP, Wenderoth N. Two hands, one brain: cognitive neuroscience of bimanual skill. Trends Cogn Sci. 2004 Jan;8(1):18–25. doi: 10.1016/j.tics.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Hu X, Newell KM. Aging, visual information, and adaptation to task asymmetry in bimanual force coordination. J Appl Physiol. 2011 Dec;111(6):1671–80. doi: 10.1152/japplphysiol.00760.2011. [DOI] [PubMed] [Google Scholar]
- 51.Boisgontier M, Van Halewyck F, Corporaal S, Willacker L, van den Bergh V, Beets I, Levin O, Swinnen S. Vision of the active limb impairs bimanual motor tracking in young and older adults. Front Aging Neurosci. 2014 Nov;6:320. doi: 10.3389/fnagi.2014.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boisgontier MP, Swinnen SP. Age-related deficit in a bimanual joint position matching task is amplitude dependent. Front Aging Neurosci. 2015 Jan;7:162. doi: 10.3389/fnagi.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blais M, Martin E, Albaret J-M, Tallet J. Preservation of perceptual integration improves temporal stability of bimanual coordination in the elderly: an evidence of age-related brain plasticity. Behav Brain Res. 2014 Dec;275:34–42. doi: 10.1016/j.bbr.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 54.Capaday C. The integrated nature of motor cortical function. Neuroscientist. 2004 Jun;10(3):207–20. doi: 10.1177/107385403262109. [DOI] [PubMed] [Google Scholar]
- 55.Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002 Feb;58(4):630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- 56.Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci. 2005 Jul;25(29):6787–96. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goble DJ, Coxon JP, Van Impe A, De Vos J, Wenderoth N, Swinnen SP. The neural control of bimanual movements in the elderly: Brain regions exhibiting age-related increases in activity, frequency-induced neural modulation, and task-specific compensatory recruitment. Hum Brain Mapp. 2010 Aug;31(8):1281–95. doi: 10.1002/hbm.20943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev. 2006 Aug;5(3):239–54. doi: 10.1016/j.arr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 59••.Heitger MH, Goble DJ, Dhollander T, Dupont P, Caeyenberghs K, Leemans A, Sunaert S, Swinnen SP. Bimanual motor coordination in older adults is associated with increased functional brain connectivity--a graph-theoretical analysis. PLoS One. 2013 Jan;8(4):e62133. doi: 10.1371/journal.pone.0062133. Suggests that the altered interhemispheric interactions that are generally observed with aging such that young adults utilize inter-hemispheric inhibitory connections (IHI), while older adults utilize inter-hemispheric facilitation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.émy FR, Wenderoth N, Lipkens K, Swinnen SP. Acquisition of a new bimanual coordination pattern modulates the cerebral activations elicited by an intrinsic pattern: an fMRI study. Cortex. 2008 May;44(5):482–93. doi: 10.1016/j.cortex.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Zaidel E, Iacoboni M. The Parallel Brain: The Cognitive Neuroscience of the Corpus Callosum. MIT Press; 2003. [Google Scholar]
- 62.Fling BW, Walsh CM, Bangert AS, Reuter-Lorenz PA, Welsh RC, Seidler RD. Differential callosal contributions to bimanual control in young and older adults. J Cogn Neurosci. 2011 Sep;23(9):2171–85. doi: 10.1162/jocn.2010.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fling BW, Seidler RD. Fundamental differences in callosal structure, neurophysiologic function, and bimanual control in young and older adults. Cereb Cortex. 2012 Dec;22(11):2643–52. doi: 10.1093/cercor/bhr349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Fujiyama H, Van Soom J, Rens G, Gooijers J, Leunissen I, Levin O, Swinnen SP. Age-Related Changes in Frontal Network Structural and Functional Connectivity in Relation to Bimanual Movement Control. J Neurosci. 2016 Feb;36(6):1808–1822. doi: 10.1523/JNEUROSCI.3355-15.2016. Demonstrates that age-related declines in bilateral coordination are likely modulated by a combination of structural (WM integrity) and functional (interhemispheric connectivity) cortical changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maki Y, Wong KFK, Sugiura M, Ozaki T, Sadato N. Asymmetric control mechanisms of bimanual coordination: an application of directed connectivity analysis to kinematic and functional MRI data. Neuroimage. 2008 Oct;42(4):1295–304. doi: 10.1016/j.neuroimage.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 66.Serbruyns L, Gooijers J, Caeyenberghs K, Meesen RL, Cuypers K, Sisti HM, Leemans A, Swinnen SP. Bimanual motor deficits in older adults predicted by diffusion tensor imaging metrics of corpus callosum subregions. Brain Struct Funct. 2015 Jan;220(1):273–90. doi: 10.1007/s00429-013-0654-z. [DOI] [PubMed] [Google Scholar]
- 67.Sisti HM, Geurts M, Clerckx R, Gooijers J, Coxon JP, Heitger MH, Caeyenberghs K, Beets IAM, Serbruyns L, Swinnen SP. Testing multiple coordination constraints with a novel bimanual visuomotor task. PLoS One. 2011 Jan;6(8):e23619. doi: 10.1371/journal.pone.0023619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solesio-Jofre E, Serbruyns L, Woolley DG, Mantini D, Beets IAM, Swinnen SP. Aging effects on the resting state motor network and interlimb coordination. Hum Brain Mapp. 2014 Aug;35(8):3945–61. doi: 10.1002/hbm.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiyama S, Kunimi M, Iidaka T, Nakai T. Distant functional connectivity for bimanual finger coordination declines with aging: an fMRI and SEM exploration. Front Hum Neurosci. 2014 Jan;8:251. doi: 10.3389/fnhum.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujiyama H, Van Soom J, Rens G, Cuypers K, Heise K-F, Levin O, Swinnen SP. Performing two different actions simultaneously: The critical role of interhemispheric interactions during the preparation of bimanual movement. Cortex. 2016 Apr;77:141–154. doi: 10.1016/j.cortex.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: A rehabilitation approach for chronic stroke. Prog Neurobiol. 2005 Apr;75(5):309–20. doi: 10.1016/j.pneurobio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 72.McCombe Waller S, Whitall J. Bilateral arm training: why and who benefits? NeuroRehabilitation. 2008 Jan;23(1):29–41. [PMC free article] [PubMed] [Google Scholar]
- 73.Sainburg R, Good D, Przybyla A. Bilateral Synergy: A Framework for Post-Stroke Rehabilitation. J Neurol Transl Neurosci. 2013 Oct;1(3) [PMC free article] [PubMed] [Google Scholar]
- 74.Whitall J, Waller SM, Silver KHC, Macko RF. Repetitive Bilateral Arm Training With Rhythmic Auditory Cueing Improves Motor Function in Chronic Hemiparetic Stroke. Stroke. 2000 Oct;31(10):2390–2395. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 75.Voelcker-Rehage C. Motor-skill learning in older adults—a review of studies on age-related differences. Eur Rev Aging Phys Act. 2008 Jan;5(1):5–16. [Google Scholar]
- 76.Bhakuni R, Mutha PK. Learning of bimanual motor sequences in normal aging. Front Aging Neurosci. 2015 Jan;7:76. doi: 10.3389/fnagi.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoff M, Trapp S, Kaminski E, Sehm B, Steele CJ, Villringer A, Ragert P. Switching between hands in a serial reaction time task: a comparison between young and old adults. Front Aging Neurosci. 2015 Jan;7:176. doi: 10.3389/fnagi.2015.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verneau M, van der Kamp J, Savelsbergh GJP, de Looze MP. Age and Time Effects on Implicit and Explicit Learning. Exp Aging Res. 2014 Aug;40(4):477–511. doi: 10.1080/0361073X.2014.926778. [DOI] [PubMed] [Google Scholar]
- 79.Pauwels L, Swinnen SP, Beets IAM. Contextual interference in complex bimanual skill learning leads to better skill persistence. PLoS One. 2014 Jan;9(6):e100906. doi: 10.1371/journal.pone.0100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pauwels L, Vancleef K, Swinnen SP, Beets IAM. Challenge to promote change: both young and older adults benefit from contextual interference. Front Aging Neurosci. 2015 Jan;7:157. doi: 10.3389/fnagi.2015.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hinder MR, Carroll TJ, Summers JJ. Transfer of ballistic motor skill between bilateral and unilateral contexts in young and older adults: neural adaptations and behavioral implications. J Neurophysiol. 2013 Jun;109(12):2963–71. doi: 10.1152/jn.00535.2012. [DOI] [PubMed] [Google Scholar]
- 82.Vieluf S, Godde B, Reuter E-M, Temprado J-J, Voelcker-Rehage C. Practice effects in bimanual force control: does age matter? J Mot Behav. 2015 Jan;47(1):57–72. doi: 10.1080/00222895.2014.981499. [DOI] [PubMed] [Google Scholar]
- 83.Swinnen SP. Age-related deficits in motor learning and differences in feedback processing during the production of a bimanual coordination pattern. Cogn Neuropsychol. 1998 Jul;15(5):439–466. doi: 10.1080/026432998381104. [DOI] [PubMed] [Google Scholar]