Abstract
OBJECTIVE
Ferumoxytol is increasingly reported as an alternative to gadolinium-based contrast agents for MR angiography (MRA), particularly for patients with renal failure. This article summarizes more than 3 years of clinical experience with ferumoxytol-enhanced MRA for a range of indications and anatomic regions.
CONCLUSION
Ferumoxytol-enhanced MRA has many advantages including that it is safe for patients with renal failure and provides a lengthy plateau of vascular signal as a blood pool agent that allows longer navigated MRA sequences.
Keywords: 4D flow, contrast agents, ferumoxytol, inflammation, MR angiography (MRA), navigated MRA, ultrasmall superparamagnetic iron oxide (USPIO)
Ferumoxytol is an ultrasmall superparamagnetic iron oxide (USPIO) particle that was first investigated as an MRI contrast agent more than a decade ago [1]. It was approved by the U.S. Food and Drug Administration as an IV treatment of iron-deficiency anemia in patients with chronic kidney disease [2, 3]. Ferumoxytol is an attractive alternative to standard gadolinium-based MRI contrast agents that carry potential risks if given to patients with renal failure [4–6]. Another unique feature of ferumoxytol as a contrast agent is that, because of its size and carbohydrate coating, it has a prolonged intravascular residence time of more than 12 hours [7, 8]. This extended plateau of increased vascular signal can be used for much longer imaging acquisitions than are possible with extracellular gadolinium-based agents, allowing improved performance of navigated MRI sequences, venous imaging, and the option for repeat imaging without the need for additional contrast material.
Ferumoxytol can be administered as a bolus injection, allowing both first-pass arterial and blood pool imaging [7, 9]. It has been broadly applied in both pediatric and adult patients for intracranial, chest, and abdominal vascular MRI [10–14]. In addition to its role as a positive contrast vascular imaging agent, ferumoxytol has a unique property as a USPIO: It is phagocytosed by macrophages after a delay [15]. This macrophage-selective feature of ferumoxytol allows the identification of pathologic inflammation, which can be applied to vessel wall imaging [16]. The goal of this article is to summarize more than 3 years of clinical experience with ferumoxytol-enhanced MR angiography (MRA) at our institution for a range of applications including standard clinical indications (i.e., pulmonary embolism, aorta, coronary and peripheral vascular imaging) and advanced research topics (i.e., 4D flow imaging and imaging to detect vascular inflammation).
Subjects and Imaging Techniques
This study was approved by the institutional review board at our facility and is compliant with HIPAA. A total of 102 patients underwent MRA with ferumoxytol (Feraheme, AMAG Pharmaceuticals) between December 2010 and January 2015. Informed consent for the use of ferumoxytol was acquired for all examinations, and medical records were reviewed to rule out iron overload and other potential contraindications for MRI or for USPIO administration.
The clinical indications for the examinations were evaluation of abdominal aortic aneurysms (AAAs) (30 patients, 29.4%), thoracoabdominal aortic aneurysms (three patients, 2.9%), pulmonary embolism (eight patients, 7.8%), aortic dissection (two patients, 2.0%), intracranial aneurysms (two patients, 2.0%), arteriovenous fistulas (15 patients, 14.7%), coronary arteries (four patients, 3.9%), carotid arteries (11 patients, 10.8%), and lower extremity vasculature (27 patients, 26.5%). The mean age of the study group was 70.7 ± 10.5 (SD) years (range, 47–95 years); there were 100 male and two female participants. All patients with the exception of those studied for AAA and pulmonary embolism had impaired renal function and received ferumoxytol to avoid risks of gadolinium-related toxicity. Patients were carefully monitored during and after the injection of ferumoxytol because the reported incidence of anaphylaxis is higher with ferumoxytol than that with gadolinium-based contrast agents [3]. There were no adverse events in any of the 102 patients studied.
All examinations were performed on a 3-T unit (Magnetom Skyra, Siemens Health-care). Brain studies used a 20-channel head and neck coil, body studies used a combination of a 24-channel spine matrix coil and an 18-channel phased-array body flex coil, and lower extremity studies were performed with a 36-channel peripheral angiography coil. Ferumoxytol was packaged in a 17-mL vial containing 510 mg (concentration of 30 mg/mL). Patients received doses of ferumoxytol ranging from 1.25 to 2 mg/kg for studies of the aorta and doses of from 1 to 1.6 mg/kg for all other studies. The bolus was administered by diluting ferumoxytol at a ratio of 1 part ferumoxytol to 4 parts sterile normal saline and then injecting 20–30 mL of ferumoxytol IV at a rate of 2 mL/s followed by a saline flush. The optimal bolus timing was performed using a TWIST (time-resolved angiography with interleaved stochastic trajectories [Siemens Healthcare]) sequence with the injection of 2 mL of contrast material.
High-resolution 3D MRA examinations of all patients were performed with a 1.3-mm isotropic spatial resolution, standard imaging parameters, and a scanning time of approximately 1 minute. Additional navigated MRA sequences including inversion recovery FLASH imaging were performed during the equilibrium phase of contrast distribution for studies of the coronary arteries, pulmonary arteries, and thoracic aorta. ECG gating was also applied to studies of the coronary arteries and thoracic aorta. Vessel wall imaging for the evaluation of the macrophage-specific properties of ferumoxytol after a delay was performed with unenhanced and 3- to 5-day contrast-enhanced 3D imaging using a high-resolution (1.3-mm isotropic) turbo spin-echo sequence with a variable flip angle. Multiecho T2*-weighted imaging was also acquired for the quantitative assessment of ferumoxytol uptake in the vessel wall. Four-dimensional flow was acquired for selected aorta cases during the equilibrium phase with ECG gating, but not respiratory gating; a 2.2-mm isotropic spatial resolution; a temporal resolution of approximately 60 ms; and a total scanning time of 10–15 minutes.
Clinical Applications
Aorta
Cross-sectional imaging has become central to the identification and management of aortic disease [17, 18]. Because of its speed and accessibility for unstable patients, CT angiography (CTA) is typically elected for evaluation of acute disease, and MRI is more commonly used for surveillance imaging [19]. In this setting, MRI has the advantage of significantly reducing radiation exposure and eliminating the use of iodinated contrast material over what may be years of follow-up imaging (Appendix 1).
Typically MRA is performed with gadolinium-based contrast agents, but these agents are contraindicated in patients with renal failure. A second issue with standard gadolinium agents is that they are extracellular and have a relatively fast decline of vascular signal. This fast decline of vascular signal has increasingly become an issue for imaging motion-prone regions of the aorta such as the aortic root, which is crucial for planning of transcatheter aortic valve replacement [20]. High-resolution 3D imaging is required with cardiac gating, but scanning times are too long for conventional breath-hold approaches [21]. Longer sequences with respiratory navigation offer a solution but require the prolonged vascular signal that ferumoxytol supplies as a blood pool contrast agent (Fig. 1). The signal loss caused by the T2* effect of ferumoxytol at high concentrations has been discussed as a potential problem for first-pass MRA, but we have not found signal loss to be a problem at the blood pool concentrations used. Additionally, ferumoxytol greatly facilitates the use of advanced functional imaging approaches for evaluating the aorta, and these approaches are increasingly being explored, including imaging of hemodynamics and inflammation [22]. This issue will be discussed further in the section titled “Additional Vascular Applications.”
Fig. 1.
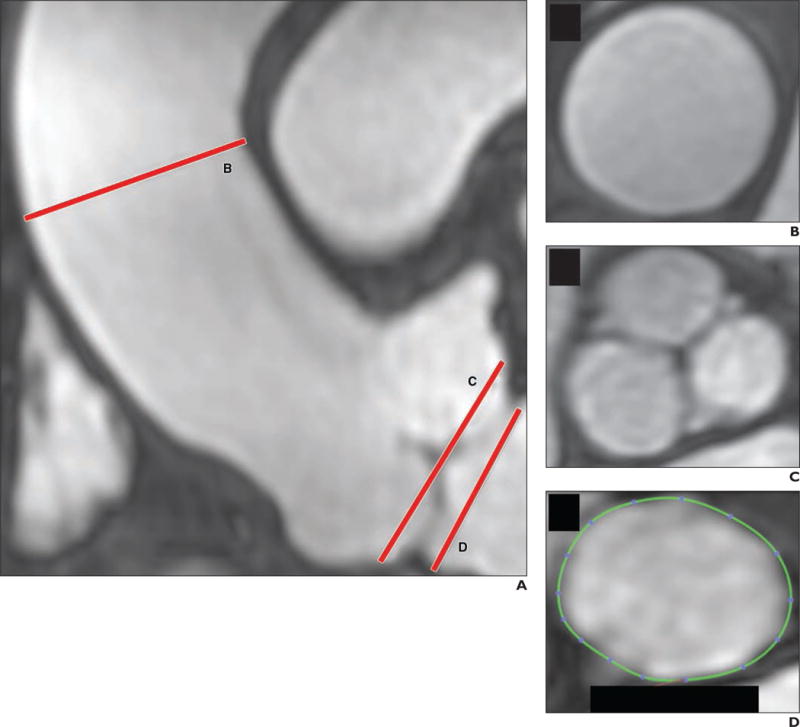
Respiratory navigation and cardiac-gating performed during lengthy equilibrium state, reached after administration of ferumoxytol, allow high-resolution volumetric MR angiography (MRA) of aortic root with excellent vascular signal. This acquisition with navigated inversion recovery FLASH took approximately 9 minutes. Patient is 63-year-old man who underwent MRA for evaluation of aorta.
A, Coronal MRA image shows tubular ascending aorta (line B), sinuses of Valsalva (line C), and aortic annulus (line D).
B and C, Cross-sectional MRA images show that visualization of tubular ascending aorta (B) and sinuses of Valsalva (C) is possible.
D, MRA image shows that area of aortic annulus can be precisely measured; this measurement is key for planning transcatheter aortic valve replacement.
Coronary Arteries
Free-breathing 3D coronary angiography has been the standard approach for imaging the coronary arteries with MRI. The main limitations of this technique are related to the long scanning time and lower temporal resolution when compared with coronary CTA. The use of blood pool contrast agents has the potential to increase the signal-to-noise ratio (SNR), which allows increased spatial resolution and shorter scanning times. Initial experiences with human studies showed improved image quality, with better delineation between the coronary vessels and the myocardium and better visualization of the distal branches [23, 24]. A small clinical study has also shown increased diagnostic accuracy of USPIO-enhanced coronary MRA in comparison with unenhanced techniques for the identification of significant coronary stenosis on invasive coronary angiography [25]. We have found that ferumoxytol-enhanced MRA affords excellent signal for longer respiratory-navigated whole-heart sequences (Fig. 2).
Fig. 2.
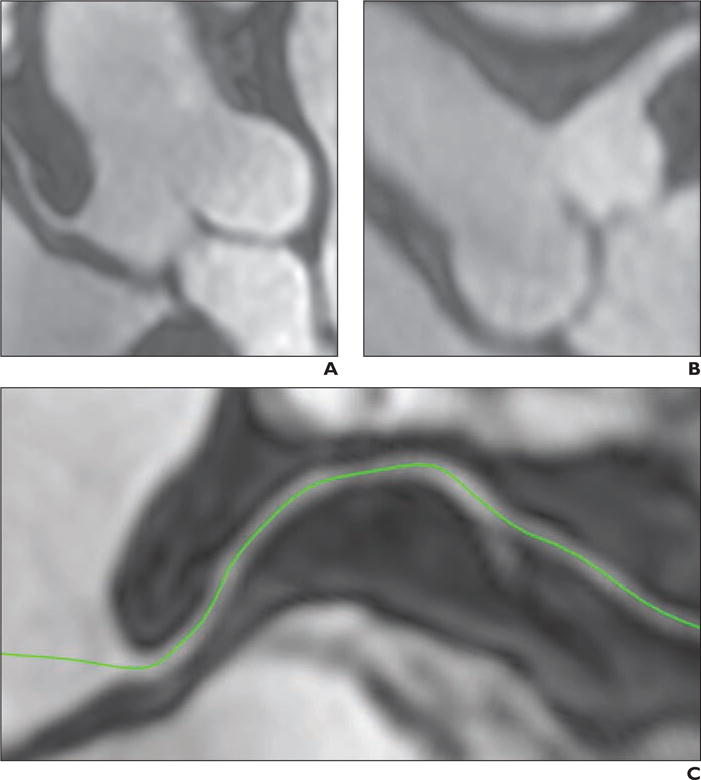
Free-breathing 3D whole-heart angiography performed using ferumoxytol-enhanced MR angiography (MRA) allows excellent visualization of coronary arteries. Patient is 63-year-old man who underwent MRA for evaluation of coronary arteries.
A and B, MRA images show right coronary artery origin (A) and left main coronary artery (B).
C, Multiplanar reformation MRA image of right coronary artery (green).
Coronary imaging with USPIOs has also been the subject of research studies for the identification of vulnerable plaque. Kooi et al. [26] show increased accumulation of ferumoxytol by signal drop on T2*-weighted imaging in coronary plaques after 24 hours. They speculated that the T2* signal drop could represent inflammatory activity and that this characteristic of ferumoxytol may be used to identify vulnerable plaques [26].
Pulmonary Artery
Pulmonary CTA has become the practical reference standard for the imaging and management of suspected pulmonary embolism [27]. Nevertheless, there are limitations to using CTA. The foremost limitation is the use of iodinated contrast material, which cannot be administered to patients with a glomerular filtration rate of less than 30 mL/min/1.73 m2 due to nephrotoxicity. In patients on dialysis, the administration of iodinated contrast material can ablate residual renal function, resulting in decreased quality of life and increased patient morbidity [28]. Second, the use of pulmonary CTA is associated with significant radiation exposure, which is particularly problematic in younger female patients [29]. Last, CTA can be limited because of poor contrast bolus timing and artifacts caused by patient motion.
Ferumoxytol-enhanced MRA offers a solution to each of these problems associated with CTA. Typically MRA is not used in the setting of patients with renal failure because of the association of nephrogenic systemic fibrosis, but ferumoxytol is safe for use in these patients [4, 30]. MRA in general has no associated ionizing radiation and so is preferable in younger patients [31]. Finally, because ferumoxytol is a blood pool agent, it allows additional imaging after first-pass arterial imaging. A repeat breath-hold angiographic image can simply be obtained after a delay if there is respiratory artifact seen on the first-pass acquisition (Fig. 3). Additionally, the prolonged steady-state arterial enhancement of ferumoxytol allows the application of novel free-breathing approaches, which is appealing for imaging patients who cannot hold their breath [32]. For example, self-navigated respiratory-compensated techniques can minimize respiratory motion during longer free-breathing acquisitions [33] (Fig. 4).
Fig. 3.
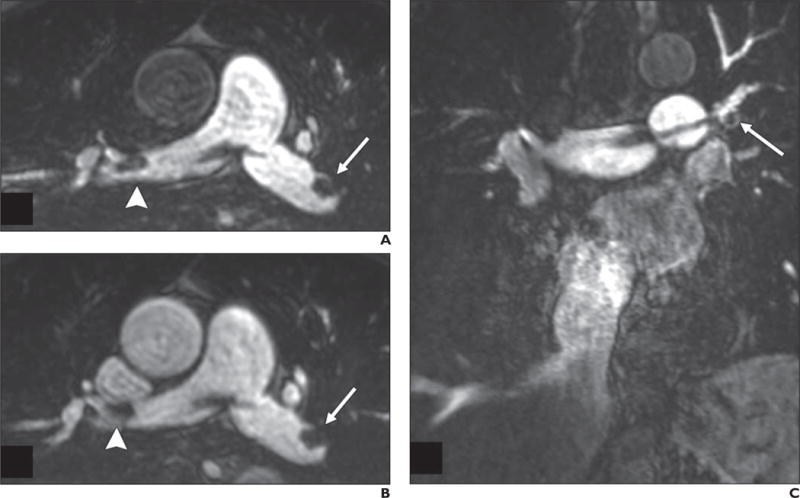
Ferumoxytol-enhanced MR angiography (MRA) of 69-year-old man with saddle pulmonary embolism.
A–C, First-pass breath-hold MRA images (A and C) show clot (arrows, A and C; arrowhead, A) extending into right and left main pulmonary arteries. MRA image obtained at repeat imaging during equilibrium state (B) allows clear depiction of clot (arrow and arrowhead, B).
Fig. 4.
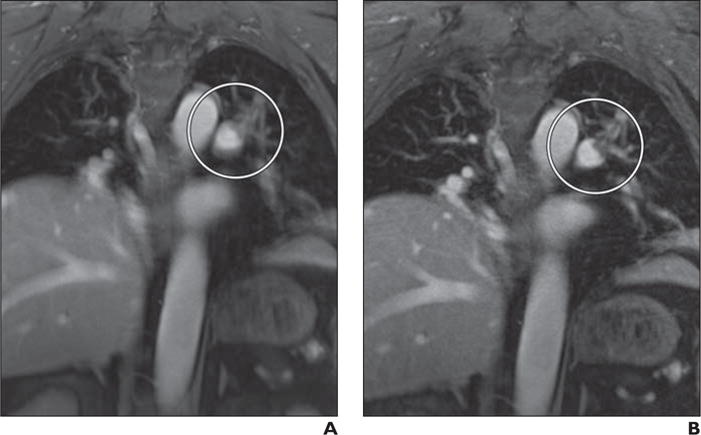
Steady-state MR angiography (MRA) images obtained during free breathing after injection of ferumoxytol. Patient is 69-year-old man with pulmonary embolism in left main and upper lobe segmental arteries.
A, MRA image obtained from 5-minute radial acquisition without use of respiratory compensation shows clot (circle).
B, MRA image obtained with respiratory compensation using self-navigation removes motion artifact to allow clearer depiction of embolism (circle) than A.
Intracranial Vasculature
Although both CTA and catheter angiography provide high-contrast and high-spatial-resolution images of the intracranial vasculature, MRA is an attractive option. Intracranial MRA is less susceptible to motion artifacts than chest or abdominal studies, permitting the acquisition of longer high-resolution imaging without gating. Calcification and bone are essentially invisible to most MRI methods and therefore do not obscure vessels either from blooming artifacts or in projection views, which are significant problems for CTA studies.
Untreated cerebral aneurysms require serial monitoring to assess stability over time. Noninvasive imaging without radiation is preferred for these serial studies. Time-of-flight MRA has excellent spatial resolution but suffers from saturation artifacts in regions of slowly recirculating flow—a common issue in cerebral aneurysms. Contrast-enhanced MRA is well suited to this application, and ferumoxytol is a particularly attractive option for imaging individuals who also have compromised renal function. Cerebral aneurysms are best visualized during the arterial phase of contrast passage, especially in the anterior circulation where the carotid siphon is surrounded by the cavernous plexus. This examination is best accomplished by injecting contrast material in a tight bolus. The semisynthetic carbohydrate coating of ferumoxytol permits this type of injection, whereas other USPIOs generally require a slow infusion.
Intracranial arteriovenous malformations can occupy a large territory and necessitate studies that provide uniform contrast filling, often whole-head coverage, and delineation of the entire vasculature—both arteries and veins. In this situation, steady-state imaging with ferumoxytol can be performed with an extended acquisition time and can provide excellent contrast-to-noise properties (Fig. 5).
Fig. 5.
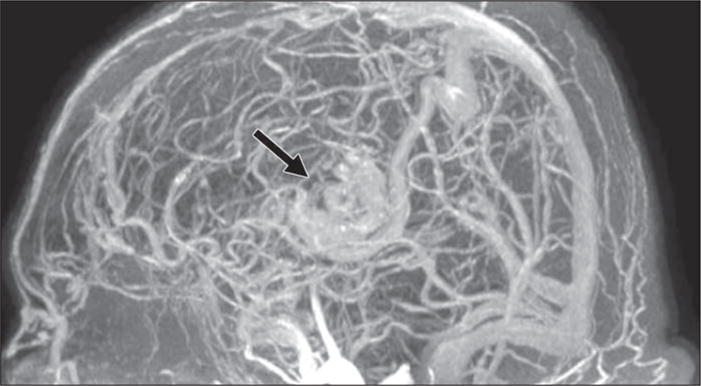
Ferumoxytol-enhanced MR angiography (MRA) performed during equilibrium state with whole-head coverage of 76-year-old man with large arteriovenous malformation (AVM). MRA image shows AVM (arrow) and reveals entire intracranial vasculature—both arteries and veins—with uniform contrast.
Peripheral Vasculature
Renal disease has a higher incidence in patients with peripheral vascular disease than in the general population. Consequently, ferumoxytol can play a vital role in evaluating the runoff vessels when there is no other viable alternative to obtain the needed coverage that extends from the abdominal aorta to the feet [34]. Imaging with ferumoxytol is performed using parameters similar to those for gadolinium-based studies and using acquisitions with overlapping paracoronal slabs at three stations: the abdomen, thighs, and calves (Fig. 6). After first-pass imaging, high-resolution datasets can be acquired at steady state. The close proximity of the veins and arteries throughout the lower extremities precludes arteries-only maximum intensity projections. Instead, vessels are best viewed in multiplanar reformations in either longitudinal planes or transverse planes. Arterial phase imaging with ferumoxytol is considered equivalent to imaging performed with gadolinium agents but offers the benefits of high-resolution imaging in the steady state [34].
Fig. 6.
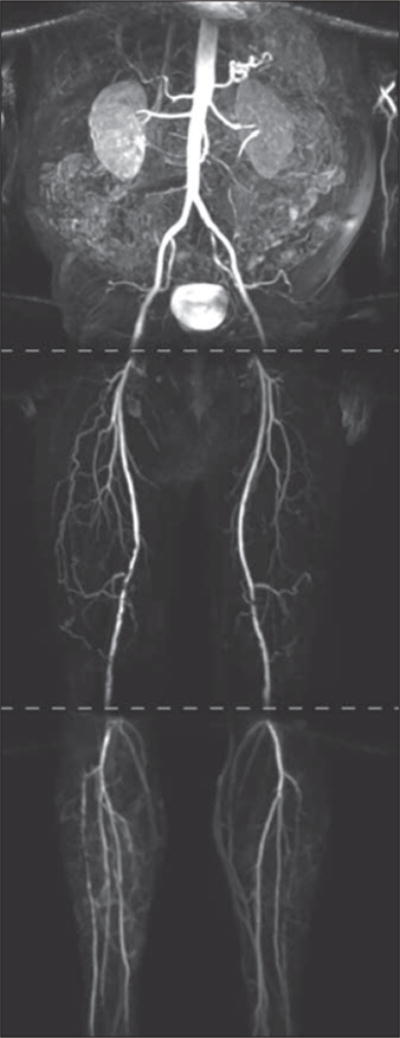
First-pass imaging of peripheral vasculature with ferumoxytol-enhanced MR angiography (MRA) using three-station runoff acquisition. Phased-array coils were used over abdomen and legs, and breath-holding was used for imaging abdominal station. Mask and subtraction images were acquired for each station. Patient is 68-year-old man who underwent MRA for evaluation of abdomen and lower extremities. Acquisition times were 11 seconds (abdomen), 11 seconds (thighs), and 14 seconds (calves) using acceleration with GRAPPA (generalized autocalibrating partial parallel acquisition) factor of 2 and elliptic centric k-space ordering.
Additional Vascular Applications
4D Flow Imaging
Phase-contrast MRI (PC-MRI) is a quantitative technique used to measure blood flow with gradients that induce phase shifts proportional to the velocity of flow. The phase of the MR signal can then be used to generate a velocity map. Common clinical applications of PC-MRI include single-plane assessment for quantification valvular regurgitation and cardiac shunt ratios. The technique can also be applied in 3D using time-resolved volumetric imaging and is commonly referred to as “4D flow” [35]. The resulting time-resolved images allow unique and powerful visualization of dynamic aortic blood flow, and many possible clinical applications are currently being explored [36]. Because 4D flow acquisitions can be time-consuming, acceleration techniques are commonly used. This acceleration, however, comes at the price of reduced SNR and consequently lower-fidelity velocity data.
Blood pool contrast agents with their relatively long plateau of vascular signal have proven beneficial for 4D flow acquisitions [37, 38]. Because the T1-shortening effect of ferumoxytol is similar to that of gadolinium-based blood pool agents, it results in increased vascular signal for MRA and 4D flow imaging. The plateau of vascular signal that ferumoxytol provides allows the collection of high-fidelity velocity data over longer 4D flow acquisitions in the 10- to 15-minute time frame (Fig. 7). Gadolinium-based blood pool agents such as gadofosveset trisodium also have prolonged vascular signal with marked T1 shortening for more than 1 hour [39], but these agents are contraindicated in patients with renal failure.
Fig. 7.
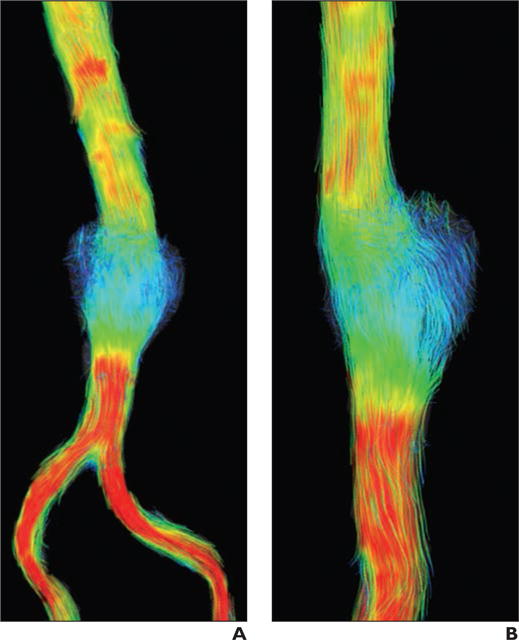
Small saccular abdominal aortic aneurysm (AAA) was imaged with ferumoxytol-enhanced MR angiography (MRA) during equilibrium state using 4D flow acquisition. Systolic flow is visualized with streamlines, which are representations of instantaneous velocity field that are color-coded according to speed of flow. Patient is 65-year-old man who underwent MRA for evaluation of AAA.
A and B, Frontal (A) and lateral (B) 4D flow images of AAA reveal relatively slow (blue) recirculating flow within belly of aneurysm.
Delayed Imaging
USPIOs such as ferumoxytol are taken up by and accumulate in macrophages within the first 24 hours after IV injection [15]. This macrophage-selective property allows imaging of vascular inflammation using delayed MRI. The signal characteristics of ferumoxytol depend on its concentration. At high concentrations, the reduced T2 and T2* relaxation effects predominate, leading to signal void on T2- or T2*-weighted images. At low concentrations, however, the reduced T1 relaxation effect leads to hyperintense signal on T1-weighted images; the relatively low blood pool concentration of ferumoxytol allows excellent positive contrast vascular imaging. By comparing the normalized signal intensity (using adjacent muscles as a reference) between unenhanced and ferumoxytol-enhanced images, quantification of the USPIO-induced signal intensity change is possible. Other approaches include qualitative assessment by the identified signal voids on T2- and T2*-weighted images and quantitative evaluation of the change in T2* values [40, 41]. One potential issue relating to the uptake of ferumoxytol by macrophages is that the resulting susceptibility artifact may preclude short-term follow-up with other diagnostic MRI sequences; this issue may be of particular concern for follow-up hepatic or oncologic imaging because ferumoxytol can persist in macrophages for up to 2 months [7].
Most of the clinical studies using the vascular macrophage-selective properties of ferumoxytol have focused on carotid plaques and AAA disease. Good correlation of ferumoxytol-enhanced MRA with histology-identified macrophages in carotid plaques and in vivo MRI-detected signal voids has been reported [26, 42]. USPIO uptake within carotid atheroma was found to be a good indicator of plaque vulnerability, which was related to recent stroke symptoms [43]. USPIO uptake has also been used to evaluate therapy response in asymptomatic patients. A significant reduction of inflammation was found with high-dose statin therapy compared with low-dose therapy during a 3-month period [44]. Imaging of inflammation associated with AAA disease is equally promising. Uptake is associated with an abundance of leukocytes in the intraluminal thrombus of AAA, and when uptake is detected, focal inflammation is linked to AAA growth rates that are threefold higher [16, 41]. We present a case of rapid AAA growth associated with focal signal dropout within the intraluminal thrombus on delayed ferumoxytol imaging (Fig. 8).
Fig. 8.
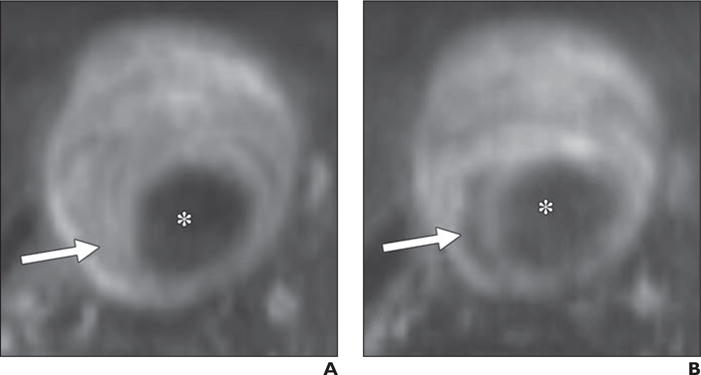
Three-dimensional black-blood fast spin-echo MRI applying variable refocusing flip angle trains with high isotropic resolution provides good visualization of intraluminal thrombus. Macrophage-selective properties of ferumoxytol allow identification of focal inflammation as shown by signal dropout with intraluminal thrombus on delayed imaging in 68-year-old man with rapidly growing (> 5 mm/y) abdominal aortic aneurysm (AAA).
A and B, Unenhanced (A) and delayed (3 days after ferumoxytol administration) ferumoxytol-enhanced (B) images show 4.9-cm AAA with substantial intraluminal thrombus (arrows), which is intrinsically bright on delayed image. Asterisks denote aortic lumen.
Conclusion
Ferumoxytol is a safe and effective alternative to gadolinium-based contrast agents for MRA. A principal advantage of ferumoxytol is that it is appropriate for use in patients with renal failure. As a blood pool agent, ferumoxytol also has the advantage of a long plateau of vascular signal. This characteristic can be exploited for repeat imaging, venous imaging, and better performance of longer navigated MRA sequences. These sequences may greatly benefit the imaging evaluation of key vascular regions including the aortic root, coronary arteries, and pulmonary arteries. Additionally, ferumoxytol has macrophage-selective properties on delayed imaging that potentially make it a powerful means of evaluating inflammation, which has increasingly been implicated in the progression of vascular disease.
Acknowledgments
Supported by the National Institutes of Health (NIH/NHLBI R01 HL114118 and R01 HL123759).
APPENDIX 1: Advantages of Ferumoxytol-Enhanced MR Angiography
Patient-Related Advantages
|
Imaging-Related Advantages
|
Footnotes
This is an ahead-of-print version of the article; the final version will appear in the September 2015 issue of the AJR.
References
- 1.Prince MR, Zhang HL, Chabra SG, Jacobs P, Wang Y. A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast agent for cardiovascular MRI. J X Ray Sci Technol. 2003;11:231–240. [PubMed] [Google Scholar]
- 2.Spinowitz BS, Kausz AT, Baptista J, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol. 2010;85:315–319. doi: 10.1002/ajh.21656. [DOI] [PubMed] [Google Scholar]
- 4.Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [Erratum in Nephrol Dial Transplant 2006; 21:1745] [DOI] [PubMed] [Google Scholar]
- 5.Neuwelt EA, Hamilton BE, Varallyay CG, et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009;75:465–474. doi: 10.1038/ki.2008.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hope TA, Herfkens RJ, Denianke KS, LeBoit PE, Hung YY, Weil E. Nephrogenic systemic fibrosis in patients with chronic kidney disease who received gadopentetate dimeglumine. Invest Radiol. 2009;44:135–139. doi: 10.1097/RLI.0b013e31819343ba. [DOI] [PubMed] [Google Scholar]
- 7.Ittrich H, Peldschus K, Raabe N, Kaul M, Adam G. Superparamagnetic iron oxide nanoparticles in biomedicine: applications and developments in diagnostics and therapy. Rofo. 2013;185:1149–1166. doi: 10.1055/s-0033-1335438. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein JS, Varallyay CG, Dosa E, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies—a review. J Cereb Blood Flow Metab. 2010;30:15–35. doi: 10.1038/jcbfm.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuwelt EA, Varallyay CG, Manninger S, et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery. 2007;60:601–611. doi: 10.1227/01.NEU.0000255350.71700.37. discussion, 611–612. [DOI] [PubMed] [Google Scholar]
- 10.Stillman AE, Wilke N, Li D, Haacke M, McLachlan S. Ultrasmall superparamagnetic iron oxide to enhance MRA of the renal and coronary arteries: studies in human patients. J Comput Assist Tomogr. 1996;20:51–55. doi: 10.1097/00004728-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Ruangwattanapaisarn N, Hsiao A, Vasanawala SS. Ferumoxytol as an off-label contrast agent in body 3T MR angiography: a pilot study in children. Pediatr Radiol. 2014 Nov 27; doi: 10.1007/s00247-014-3226-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayak AB, Luhar A, Hanudel M, et al. High-resolution, whole-body vascular imaging with ferumoxytol as an alternative to gadolinium agents in a pediatric chronic kidney disease cohort. Pediatr Nephrol. 2015;30:515–521. doi: 10.1007/s00467-014-2953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ersoy H, Jacobs P, Kent CK, Prince MR. Blood pool MR angiography of aortic stent-graft endoleak. AJR. 2004;182:1181–1186. doi: 10.2214/ajr.182.5.1821181. [DOI] [PubMed] [Google Scholar]
- 14.Dósa E, Tuladhar S, Muldoon LL, Hamilton BE, Rooney WD, Neuwelt EA. MRI using ferumoxytol improves the visualization of central nervous system vascular malformations. Stroke. 2011;42:1581–1588. doi: 10.1161/STROKEAHA.110.607994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasan DM, Mahaney KB, Magnotta VA, et al. Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: a pilot study. Arterioscler Thromb Vasc Biol. 2012;32:1032–1038. doi: 10.1161/ATVBAHA.111.239871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nchimi A, Defawe O, Brisbois D, et al. MR imaging of iron phagocytosis in intraluminal thrombi of abdominal aortic aneurysms in humans. Radiology. 2010;254:973–981. doi: 10.1148/radiol.09090657. [DOI] [PubMed] [Google Scholar]
- 17.Clouse WD, Hallett JW, Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ., 3rd Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA. 1998;280:1926–1929. doi: 10.1001/jama.280.22.1926. [DOI] [PubMed] [Google Scholar]
- 18.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–828. doi: 10.1161/01.CIR.0000154569.08857.7A. [DOI] [PubMed] [Google Scholar]
- 19.Litmanovich D, Bankier AA, Cantin L, Raptopoulos V, Boiselle PM. CT and MRI in diseases of the aorta. AJR. 2009;193:928–940. doi: 10.2214/AJR.08.2166. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour A, Ismail TF, Moat N, et al. Multimodality imaging in transcatheter aortic valve implantation and post-procedural aortic regurgitation: comparison among cardiovascular magnetic resonance, cardiac computed tomography, and echocardiography. J Am Coll Cardiol. 2011;58:2165–2173. doi: 10.1016/j.jacc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Stuber M, Botnar RM, Danias PG, et al. Contrast agent-enhanced, free-breathing, three-dimensional coronary magnetic resonance angiography. J Magn Reson Imaging. 1999;10:790–799. doi: 10.1002/(sici)1522-2586(199911)10:5<790::aid-jmri25>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 22.Hope MD, Hope TA. Functional and molecular imaging techniques in aortic aneurysm disease. Curr Opin Cardiol. 2013;28:609–618. doi: 10.1097/HCO.0b013e3283644beb. [DOI] [PubMed] [Google Scholar]
- 23.Prompona M, Cyran C, Nikolaou K, Bauner K, Reiser M, Huber A. Contrast-enhanced whole-heart MR coronary angiography at 3.0 T using the intravascular contrast agent gadofosveset. Invest Radiol. 2009;44:369–374. doi: 10.1097/rli.0b013e3181a40d1d. [DOI] [PubMed] [Google Scholar]
- 24.Wagner M, Rösler R, Lembcke A, et al. Whole-heart coronary magnetic resonance angiography at 1.5 Tesla: does a blood-pool contrast agent improve diagnostic accuracy? Invest Radiol. 2011;46:152–159. doi: 10.1097/RLI.0b013e3181fac6ef. [DOI] [PubMed] [Google Scholar]
- 25.Wagner M, Wagner S, Schnorr J, et al. Coronary MR angiography using citrate-coated very small superparamagnetic iron oxide particles as blood-pool contrast agent: initial experience in humans. J Magn Reson Imaging. 2011;34:816–823. doi: 10.1002/jmri.22683. [DOI] [PubMed] [Google Scholar]
- 26.Kooi ME, Cappendijk VC, Cleutjens KB, et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 27.Quiroz R, Kucher N, Zou KH, et al. Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. JAMA. 2005;293:2012–2017. doi: 10.1001/jama.293.16.2012. [DOI] [PubMed] [Google Scholar]
- 28.Morcos SK, Thomsen HS, European Society of Urogenital Radiology European Society of Urogenital Radiology guidelines on administering contrast media. Abdom Imaging. 2003;28:187–190. doi: 10.1007/s00261-001-0186-5. [DOI] [PubMed] [Google Scholar]
- 29.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Tutton S, Vu AT, et al. First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. J Magn Reson Imaging. 2005;21:46–52. doi: 10.1002/jmri.20235. [DOI] [PubMed] [Google Scholar]
- 31.Schiebler ML, Nagle SK, Francois CJ, et al. Effectiveness of MR angiography for the primary diagnosis of acute pulmonary embolism: clinical outcomes at 3 months and 1 year. J Magn Reson Imaging. 2013;38:914–925. doi: 10.1002/jmri.24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahlström KH, Johansson LO, Rodenburg JB, Ragnarsson AS, Akeson P, Börseth A. Pulmonary MR angiography with ultrasmall superparamagnetic iron oxide particles as a blood pool agent and a navigator echo for respiratory gating: pilot study. Radiology. 1999;211:865–869. doi: 10.1148/radiology.211.3.r99jn10865. [DOI] [PubMed] [Google Scholar]
- 33.Lin W, Guo J, Rosen MA, Song HK. Respiratory motion-compensated radial dynamic contrast-enhanced (DCE)-MRI of chest and abdominal lesions. Magn Reson Med. 2008;60:1135–1146. doi: 10.1002/mrm.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker JP, Nosova E, Sigovan M, et al. Ferumoxytol-enhanced magnetic resonance angiography is a feasible method for the clinical evaluation of lower extremity arterial disease. Ann Vasc Surg. 2015;29:63–68. doi: 10.1016/j.avsg.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. J Magn Reson Imaging. 2012;36:1015–1036. doi: 10.1002/jmri.23632. [DOI] [PubMed] [Google Scholar]
- 36.Burris NS, Hope MD. 4D flow MRI applications for aortic disease. Magn Reson Imaging Clin N Am. 2015;23:15–23. doi: 10.1016/j.mric.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsiao A, Lustig M, Alley MT, Murphy MJ, Vasanawala SS. Evaluation of valvular insufficiency and shunts with parallel-imaging compressed-sensing 4D phase-contrast MR imaging with stereoscopic 3D velocity-fusion volume-rendered visualization. Radiology. 2012;265:87–95. doi: 10.1148/radiol.12120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bock J, Frydrychowicz A, Stalder AF, et al. 4D phase contrast MRI at 3 T: effect of standard and blood-pool contrast agents on SNR, PC-MRA, and blood flow visualization. Magn Reson Med. 2010;63:330–338. doi: 10.1002/mrm.22199. [DOI] [PubMed] [Google Scholar]
- 39.Lauffer RB, Parmelee DJ, Dunham SU, et al. MS-325: albumin-targeted contrast agent for MR angiography. Radiology. 1998;207:529–538. doi: 10.1148/radiology.207.2.9577506. [DOI] [PubMed] [Google Scholar]
- 40.Trivedi RA, U-King-Im JM, Graves MJ, et al. In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke. 2004;35:1631–1635. doi: 10.1161/01.STR.0000131268.50418.b7. [DOI] [PubMed] [Google Scholar]
- 41.Richards JM, Semple SI, MacGillivray TJ, et al. Abdominal aortic aneurysm growth predicted by uptake of ultrasmall superparamagnetic particles of iron oxide: a pilot study. Circ Cardiovasc Imaging. 2011;4:274–281. doi: 10.1161/CIRCIMAGING.110.959866. [DOI] [PubMed] [Google Scholar]
- 42.Trivedi RA, Mallawarachi C, U-King-Im JM, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–1606. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 43.Howarth SP, Tang TY, Trivedi R, et al. Utility of USPIO-enhanced MR imaging to identify inflammation and the fibrous cap: a comparison of symptomatic and asymptomatic individuals. Eur J Radiol. 2009;70:555–560. doi: 10.1016/j.ejrad.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 44.Tang TY, Howarth SP, Miller SR, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study: evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]


