Abstract
We have identified Severe Combined Immunodeficiency (SCID) in a line of Yorkshire pigs at Iowa State University. These SCID pigs lack B-cells and T-cells, but possess Natural Killer (NK) cells. This SCID phenotype is caused by recessive mutations in the Artemis gene. Interestingly, two human tumor cell lines, PANC-1 and A375-SM, survived after injection into these SCID pigs, but, as we demonstrate here, these cells, as well as K562 tumor cells, can be lysed in vitro by NK cells from SCID and non-SCID pigs. NK cells from both SCID and non-SCID pigs required activation in vitro with either recombinant human IL-2 or the combination of recombinant porcine IL-12 and IL-18 to kill tumor targets. We also showed that SCID NK cells could be activated to produce perforin, and perforin production was greatly enhanced in NK cells from both SCID and non-SCID pigs after IL-2 cytokine treatment. While CD16+, CD172− NK cells constituted an average of only 4% in non-SCID pigs, NK cells averaged 27% of the peripheral blood mononuclear cell population in SCID pigs. We found no significant differences in killing activity per NK cell between SCID and non-SCID pigs. We conclude that survival of human cancer cells in these SCID pigs is not due to an intrinsic defect in NK cell killing ability.
Keywords: natural killer, NK Cells, SCID, Pig, Innate Immunity, Artemis, Perforin
Introduction
Severe combined immunodeficiency (SCID) can be caused by genetic defects in over 30 genes (Cossu, 2010). Several mouse lines have been developed through genetic modification to model known SCID phenotypes in humans (Xiao et al., 2008). In addition, SCID-type defects have been identified to occur spontaneously in several animal species, including the dog (Meek et al., 2001), mouse (Barthels, 2013), and horse (Perryman, 2004).
Recently, a novel SCID phenotype was serendipitously discovered in a line of Yorkshire pigs that was selected for increased feed efficiency at Iowa State University (Cai et al., 2008) during a large-scale Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) challenge study (Cino-Ozuna et al., 2012). Upon necropsy of piglets that died unexpectedly early in the study, abnormal lymph node and thymus structures as well as very low antibody titers were detected, indicating a SCID-like phenotype. A genome-wide association analysis of 172 pigs within the selection line pedigree that was segregating the SCID phenotype identified a 5.6 Mb region on Sus scrofa chromosome 10, which contains the Artemis (DCLRE1C) gene (Waide et al., 2015). Defects in Artemis are known to specifically affect the mechanism of recombination of the T-Cell Receptor (TCR) and B-Cell Receptor (BCR) complexes (Cossu, 2010). The Artemis gene encodes an endonuclease that cleaves the hairpin loop created by the RAG1 and RAG2 proteins during somatic rearrangement of these two complexes (Schuetz et al., 2014). Due to the clear relevance of this gene for the SCID phenotype observed, molecular genetic analyses of this gene was performed, revealing two independent mutations in Artemis. Both alleles have a Mendelian recessive mode of inheritance and cause the SCID phenotype in either the homozygous or compound heterozygous state (Waide et al., 2015).
As seen in other species with a defect in Artemis (Schuetz et al., 2014), these pigs lack B-lymphocytes and T-lymphocytes but produce Natural Killer (NK) cells (Ewen et al. 2015; Waide et al., 2015). NK cells are innate lymphocytes cytolytic to cells not presenting ‘self’ Major Histocompatibility Complex I (MHC class I), including virally infected cells or tumor cells with down-regulated MHC class I (Vivier et al., 2011). NK cell lysis of target cells can be accomplished by exocytosis of granular proteins perforin, granzyme B, and other pro-apoptotic proteins (Maher et al, 2002). The NK cell recognizes surface changes in tumor or virally infected cells, binds via activating receptors (for review see Pegram et al., 2010), and releases granular contents including perforin, which form pores in the target cell. Perforin alone can result in osmotic lysis of the target cell. However, perforin–induced membrane changes can allow granzyme B, co-localized in the granule, to enter the target cell and induce programmed cell death or apoptosis. (Maher et al., 2002; Bolitho et al., 2007). Resting murine NK cells have very low levels of perforin and granzyme B, but exposure to an activating signal increases the production of each protein (Fehniger et al., 2007). NK cells in mice deficient for granzyme B and perforin have minimal cytotoxic activity, even if activated by cytokines in vitro (Fehniger et al., 2007). Activation of porcine NK cells has been demonstrated using numerous cytokines, including recombinant human IL-2 and IFN-α (Mori et al., 1998). Activation of NK cells is also well documented with IL-15, IL-12, and IL-18 (Fehniger et al., 2007) (Pintarič et al., 2008).
As an animal model for human research, the SCID pig may be more advantageous than other species, as the domestic pig is more physiologically and immunologically similar to humans (Meurens et al., 2012) and has an “immunome” with greater homology to humans than mice (Dawson et al., 2013). Basel et al. (2012) showed that the SCID pigs did not reject two human tumor cell lines, PANC-1 (pancreatic carcinoma) and A375SM (melanoma). As these immune-compromised pigs possessed phenotypically identifiable NK cells, yet failed to reject cancer target cells, the authors concluded that the NK cells are not functional due to lack of appropriate cytokine stimulation from absent T-cells. However, no studies have directly measured intrinsic killing activity of NK cell from the Artemis-mutated SCID pigs.
The presence or absence of functional NK cells in SCID patients can impact the success of clinical procedures, such as bone marrow transplantation, and likelihood of Graft versus Host Disease (Hassan et al., 2014). Since SCID models with detectable NK cells have been predicted (Lunn et al., 1995) or reported (Buckley et al., 1997) to be functionally impaired or operative, it is important to determine the functionality of the NK cells within the porcine Artemis SCID model. This information will be relevant to the utilization of the SCID pig model in preclinical studies. This paper addresses the role of NK cells in SCID pigs by defining their cytolytic activity, responses to cytokine activation in vitro, and production of perforin.
Results
Peripheral blood mononuclear cells (PBMCs) from SCID and non-SCID piglet littermates were first tested for their ability to recognize and kill several types of human tumor targets, including those previously shown to survive in SCID pigs (Basel et al. 2012): A375SM (melanoma); PANC-1 (pancreatic carcinoma); as well as K562 (chronic myelogenous leukemia) cells, a standard target cell for human and porcine NK cell research. Cells were plated at three different effector to target (E:T) ratios (5:1, 10:1, 20:1) with effectors of PBMCs (used as a source of NK cells) and the three cancer cell type targets. Percent Cytotoxicity in PBMCs required cytokine activation (human recombinant IL-2) to kill tumor cells in vitro, as no lysis of any of the three cell types tested was observed for cells treated with medium alone at any E:T ratio. The difference in killing at every E:T ratio for cells treated with IL-2 versus medium alone was significant (p-value < 0.0001) (Figure 1). Activated PBMCs had similar levels of cytotoxicity for all cancer target cells across all E:T ratios, and percent cytotoxicity was not significantly different between cancer target cell types (p-value = 0.065).
Figure 1. Human cancer cell lines are killed by IL-2 activated porcine NK cells.
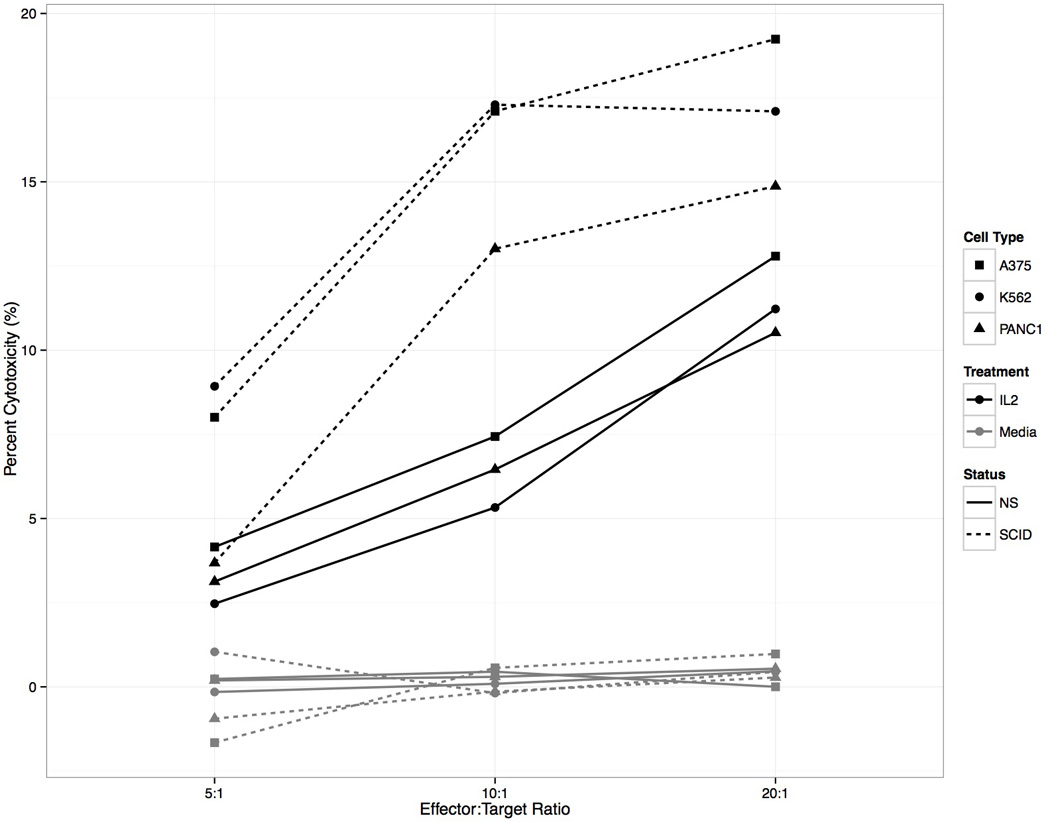
PBMCs from SCID and non-SCID piglets incubated in the presence or absence of recombinant human IL-2 (rh IL-2) and tested for cytotoxicity against A375-SM, K562, or PANC-1 human cell lines. Percent Cytotoxicity was reported for 5:1, 10:1, and 20:1 effector:target ratios. SCID PBMCs had the highest percent cytotoxicity (dotted line) compared to non-SCID (NS) littermate cells (solid line). PBMCs not treated (activated) with IL-2, but rather treated with media alone, did not lyse target cells from any of the tumor lines (gray lines).
Flow cytometric analysis of PBMC’s was used in combination with these assays to correlate NK cell activity directly to NK cell effector concentration. We defined NK cells as PBMCs that were CD16+(FcγR) and CD172− (CD172; monocyte/granulocyte). Flow cytometric analysis demonstrated SCID pigs had higher concentrations of NK cells (percent NK) among isolated PBMCs (26.6% [SCID] compared to 4.0% [non-SCID]) (p-value < 0.0001) across all days (representative data is shown in Figures 2a (SCID) and 2b (non-SCID littermate)). By normalizing the cytotoxicity data based on the percent NK cells in the PBMC preparation for each sample, intrinsic killing ability was reported as the number of tumor targets killed per NK cell (Figure 2c) and found to be comparable in SCID and non-SCID littermates. Due to limitations in blood volume that can be collected from young animals, subsequent experiments were performed using only the K562 target cell line, which demonstrated similar trends and percent cytotoxicity to all cancer cell types.
Figure 2. (a,b,c) Representative flow cytometric analysis of PBMCs used for normalization of data presented in Figure 1.
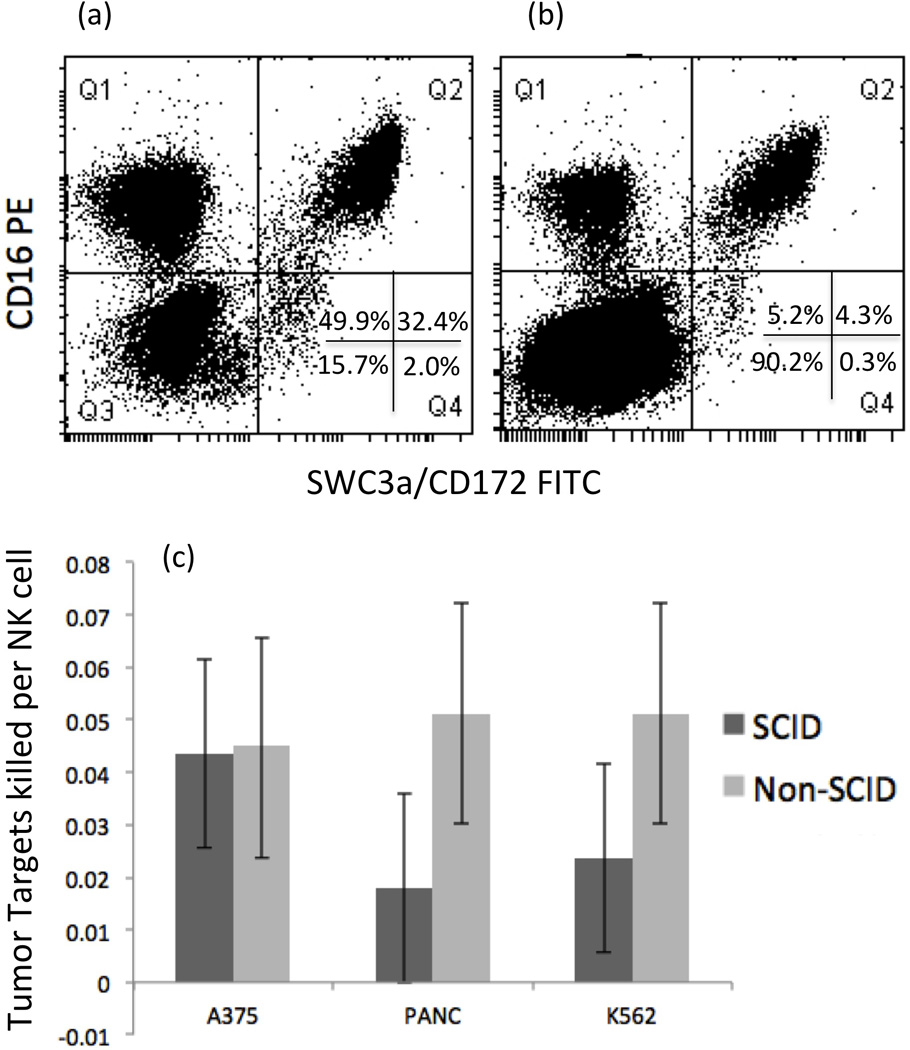
Percentages for CD16/CD172(SWC3a) populations shown in lower right hand quadrant (Q4). Data shown in (a) is a representative example of a SCID pig; 49.9% CD16+/ CD172− NK cells, and 32.4% CD16+/CD172+ monocytes. Data shown in (b) is typical of a non-SCID pig; 5.2% CD16+/ CD172− NK cells and 4.3% CD16+/CD172+ monocyte. The ratio of tumor target cell killed per NK cell is depicted in (c) where SCIDs and non-SCIDs can be compared directly. Error bars represent standard errors.
To test for differences in development of cytolytic activity of NK cells from SCID pigs, we isolated PBMCs from peripheral blood of SCID and non-SCID littermates at two time points in the first two weeks post-farrow (approximately 4 and 11 days of age; early) and twice in the second two weeks (approximately 22 and 31 days of age; late) (Figures 3b and 4b). Time points were chosen based on reported differences in normal porcine NK cell activity before and after the first two weeks of life (Yang et al., 1986). In contrast to the work by Yang et al. (1986), we did not find significant differences in percent cytotoxicity between early and late time points for normal piglet samples activated with IL-2 (p-value= 0.65; Figure 3b), nor for samples activated with IL-12 and IL-18 (p-value= 0.06; Figure 4b). Overall, cytotoxicity of PBMCs from SCID animals was statistically higher than that of PBMCs from non-SCID littermates at all E:T ratios (p-value < 0.01) (Figure 3a). This was also true for PBMCs from SCID piglets compared to PBMCs from non-SCID piglets both before and after the first two weeks of life (p-values < 0.01 for data at the 10:1 E:T ratio) (Figure 3b). By accounting for the percent NK cells in the PBMC fractions, we were able to calculate the number of NK cells present per well and analyze tumor targets killed per NK cell. We thus demonstrated that the intrinsic killing activity of NK cells was not significantly different between SCID and non-SCID animals (Figure 3c). Averaged across all time points and over the 5:1, 10:1, 20:1 E:T ratios, the number of tumor targets lysed per NK cell was similar in SCID animals (0.36 ± 0.086) and non-SCID animals (0.33 ± 0.089) (p-value= 0.72) (Figure 3c).
Figure 3. NK cells from both SCID and non-SCID pigs can kill human cancer cells when activated with IL-2.
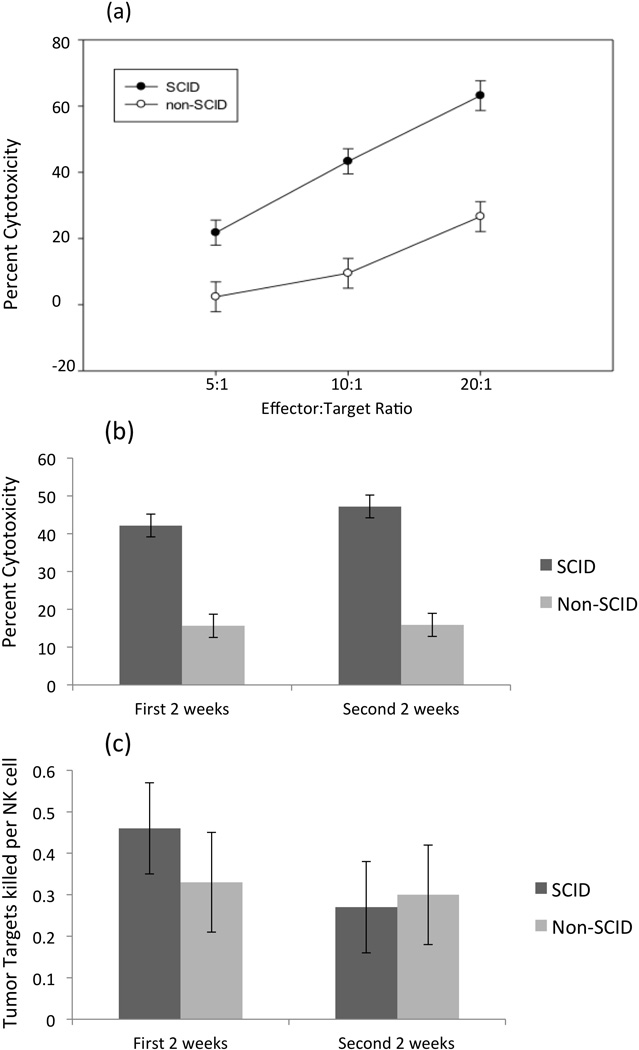
NK cell populations in isolated PBMCs from SCID and non-SCID piglets were used in killing assays with K562 cells. (a) Killing of K562 cells by PBMCs isolated from SCID pigs and non-SCID pigs and incubated with rhIL-2 at 5:1, 10:1, & 20:1 effector-to-target ratios. Each effector-to-target ratio was averaged for SCID and non-SCID animals across all days (4–31 days post farrowing). (b) Average percent cytotoxicity of cells from SCID and non-SCID animals at the 10:1 effector-to-target ratio. The first two week time point includes time points from days 4–11, and the second two week time point includes days 18–31 (c) Number of K562 tumor targets killed per NK cell is based on total effectors and targets in a well when activated with IL-2. Ratio is averaged across all effector-to-target ratios. Error bars represent standard errors.
Figure 4. NK cells from both SCID and non-SCID pigs can kill human cancer cells when activated with a combination of IL-12 and IL-18.
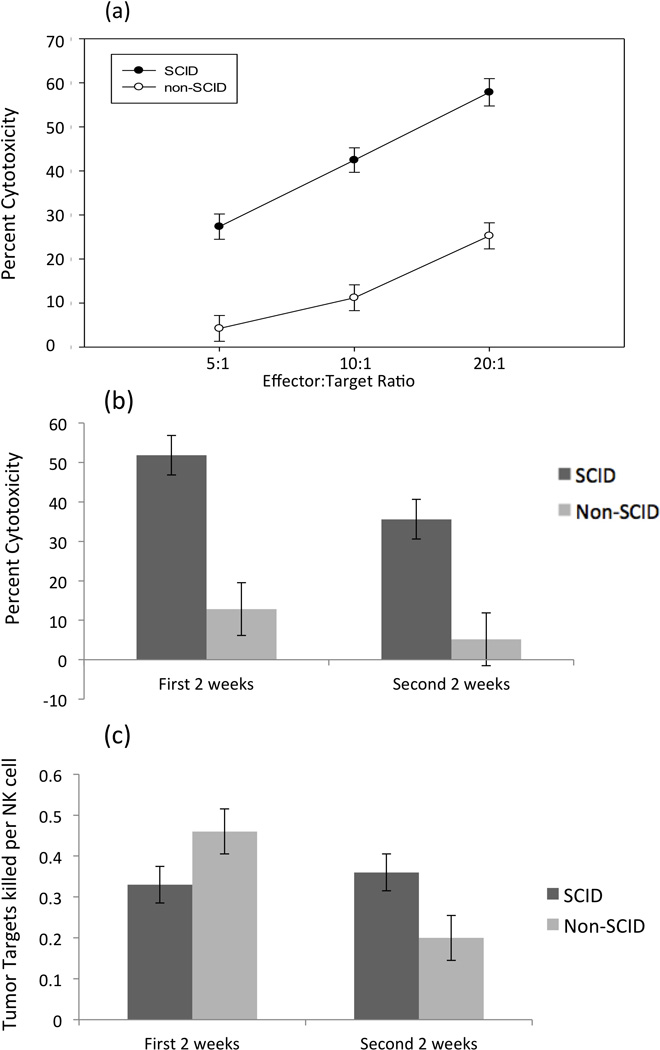
(a) Killing of K562 cells by PBMCs isolated from SCID pigs and non-SCID pigs and incubated with IL-12 and IL-18 across effector-to-target ratios. Each effector-to-target ratio was averaged for SCID and non-SCID animals across all days (8–31 days post farrowing). (b) Percent cytotoxicity is averaged for SCID and non-SCID animals at the 10:1 effector-to-target ratio. The first two week time point includes bleeds from days 4–11, and the second two week time point includes days 18–31 (c) Ratio of K562 tumor targets killed per NK cell based on total effectors and targets in a well when activated with combined IL-12 and IL-18. Ratio is averaged across 5:1, 10:1, 20:1 effector-to-target ratios. Error bars represent standard errors.
We then proceeded to test cytotoxicity of SCID and non-SCID NK cells when activated with cytokines that are produced by myeloid-derived cells. The combination of recombinant porcine IL-12 and recombinant porcine IL-18 (rpIL-12/rpIL-18) has been shown to activate porcine NK cells (Pintarič et al., 2008). We found that both SCID and non-SCID NK cells could be activated to lyse K562 targets with a combination of rpIL-12/rpIL-18 (Figure 4a). Similar to the results with IL-2 activation, total PBMCs from SCID pigs activated by rpIL-12/rpIL-18 demonstrated a higher percent cytotoxicity across most E:T ratios than PBMCs from non-SCID pigs (p-value (5:1) = 0.08, p-value (10:1) < 0.03, p-value (20:1) < 0.03)(Figure 4a). As seen with IL-2-stimulated cells, the higher cytotoxicity trend for SCID cells was demonstrated for each of the two-week periods (Figure 4b). However, when PBMC cytotoxicity was normalized based on the percent NK cells, there was no significant difference in cytotoxic capability between SCID and non-SCID NK cells activated with rpIL-12/rpIL-18 at either the early or late time points (p-values > 0.87; Figure 4c).
As another measure of function of SCID NK cells, we also examined the ability of cells from SCID and non-SCID animals to produce perforin in vitro in response to activation with recombinant human IL-2. PBMCs incubated with rh IL-2 for three days were stained for CD16 and CD172 as surface ‘NK’ cell markers (within PBMCs) and intracellular perforin. Results showed CD16+ CD172− NK cells from SCID or non-SCID pigs responded similarly to activation with increased production of intracellular perforin (Figure 5). In Figure 5a, we demonstrate that perforin production was increased in IL-2 activated SCID NK cells compared to non-activated NK cells, and in Figure 5b we demonstrate that perforin mean fluorescence response to activation was similar in cells from a non-SCID pig (Figure 5b).
Figure 5. Production of perforin by SCID and non-SCID CD16+ CD172− NK cells after activation with recombinant human IL-2.
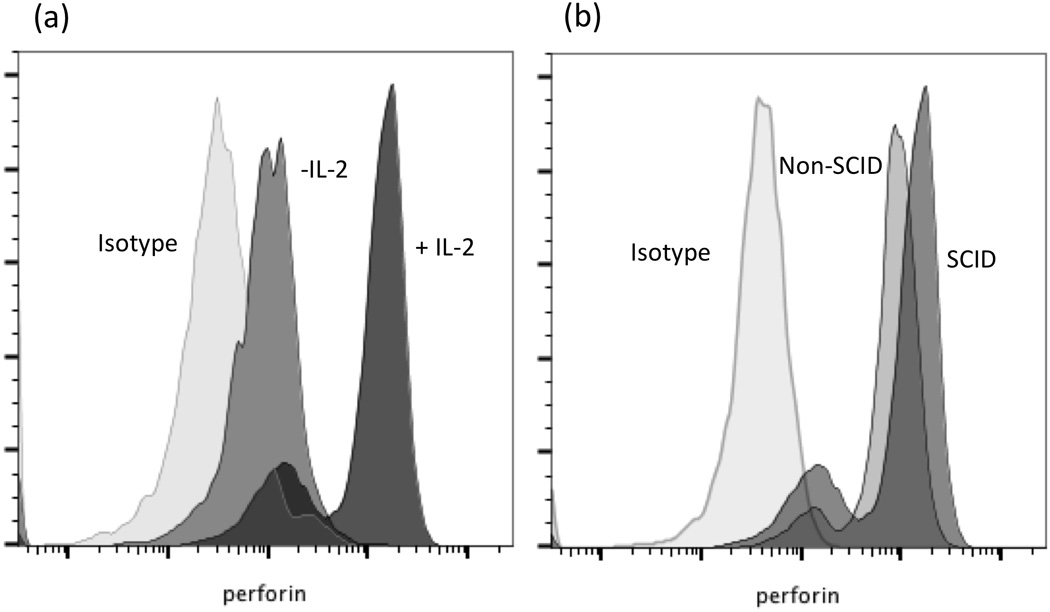
Perforin production by NK cells from a (a) SCID pig incubated without (IL2−) or with (+IL2) addition of rh IL-2 and (b) by non-SCID and SCID pig NK cells following 3 days of rhIL-2 stimulation. Non-specific isotype matched antibody was used a staining for the intracellular control.
Discussion
This study answers an important question raised by the results of the Basel et al. (2012); whether PANC-1 and A375SM cancer cell targets can be killed by suitably activated NK cells from SCID pigs. We demonstrated that these human cancer cells could be recognized and killed in vitro by activated NK cells from both non-SCID and SCID pigs. As humans and other species with naturally-occurring SCID have variation in the existence and functionality of NK cells (Buckley et al., 1997), it was important to determine the functional status of NK cells from the defective Artemis SCID pig. In the work presented herein, we have established that porcine NK cells from both normal and SCID pigs can be activated with cytokines, including IL-2, to functionally lyse a target cell. While IL-2 is a well-documented activating agent of NK cells (Pintarič et al., 2008), it is produced by T-cell lymphocytes, which are not detectable in the blood of our SCID model (Ewen et al., 2015). We then assessed the functionality of NK cells when activated with cytokines produced by myeloid-derived cells, such as macrophages and dendritic cells. Normal levels of both granulocytes and monocytes have been detected in un-stimulated SCID animals (Ewen et al., 2015) and are expected to be capable of producing IL-12 and IL-18. Further investigations will explore the cytokine producing capabilities and natural cytokine profile of the SCID pig. NK cells from SCID animals were able to kill tumor targets when activated with a combination of IL-12 and IL-18. As seen with IL-2 activation, there was no significant difference between the SCID and non-SCID NK cells in tumor targets killed per NK cell following exposure to IL-12 and IL-18 (Figure 3). We acknowledge that the CD16+, CD172− NK cell population in non-SCID pigs includes a minor proportion of CD16+, CD3+ T-like lymphocytes that may have cytolytic capacity (Denyer et al., 2006). In order to portray that the majority of killing by PBMCs from non-SCID pigs was due to conventional NK cells, we used flow cytometry to show that less than 2% of total PBMCs in non-SCIDs were CD16+, CD3+, CD172− (data not shown). Thus, it is unlikely that any cytotoxicity observed from this minor population of cells in non-SCID pigs impacts the main findings of cytotoxic capacity of SCID-derived NK cells. We also demonstrated that SCID-derived CD16+ CD172− NK cells responded to IL-2 activation to produce intracellular perforin, which was similar between cells isolated from SCID and non-SCID piglets. Thus, SCID NK cells appear to be intrinsically functional and capable of killing human cancer cells and of producing perforin in response to IL-2 activation in vitro.
These results indicate that the survival of human cancer cells in SCID pigs shown by Basel et al. (2012) is not due to an intrinsic inability to respond to cytokines, nor to a defect in the ability to lyse the tumor target cells. Based on these results, two possibilities can be presented for the survival of human tumor cells in the SCID pig model; 1) SCID NK cells are not activated in vivo, or 2) migration of NK cells to injected target cells is impaired. Our current hypothesis is that SCID NK cells are not being activated in vivo, due to a deficit of IL-2 due to the absence of T lymphocytes. In order to test this hypothesis it will be necessary to assay activation and migration of circulating NK cells in SCID pigs through direct administration of cytokines (IL2 or IL12/18) or molecules known to activate NK cells in vivo (Duluc et al., 2009), though that was outside the scope of this work.
A large animal model that lacks major components of the immune system is valuable for studying specific immune cell subsets, response to disease, therapeutic strategies for cancer, and stem cell transplantation (Huang et al., 2014). Thus, in addition to improving our understanding of the relationships between the innate and adaptive immune systems in porcine immunity, our characterization of NK cell functionality in this swine SCID model will further develop it as a large animal model in preclinical testing of human stem cell therapeutics and specific treatments for Artemis SCID patients.
Methods
Creation and care of SCID piglets
Six litters were generated from matings between carrier females and a compound heterozygous SCID affected boar that was rescued by littermate bone marrow transfer (BMT). One week before farrowing, pregnant sows were washed and transferred from the Iowa State University (ISU) Lauren Christian Swine Research Center to an environmentally-controlled, clean room at the ISU College of Veterinary Medicine. In total, 33 piglets were processed within 24 hours of farrowing and tissue samples were collected for identification of the SCID affected animals. The SCID genotype was determined through a PCR test (Waide et al., 2015); 18 SCID and 15 non-SCID pigs were used in these experiments. All animal handling protocols and standard operating procedures (SOPs) were approved by and met all IACUC requirements.
Cytotoxicity Assay
Maintenance of Tumor Target Cell Lines
Frozen aliquots of PANC-1 and A375SM were obtained from D. Troyer (Kansas State University, Manhattan, KS) and K562 cells from ATCC. Cells were thawed, washed, and grown in complete RPMI media (RPMI-1640 supplemented with L-glutamine (2 mM), gentamicin (50 ug/mL), Hepes (10 mM) (all from Life Technologies) and heat-inactivated fetal bovine serum (10%; FBS; Atlanta Biologicals, Inc, Flowery Branch, GA) at 37°C with 7% CO2 with subculture every 2–3 days to maintain cells in log growth phrase.
Isolation of PBMCs
Data was produced using SCID and non-SCID littermates from the litters described above. Heparinized blood (3–8 mL) was collected into vacutainer tubes (BD #367874 Becton Dickinson and Company, Franklin Lakes, NJ). Blood was diluted in Hanks’ Balanced Saline Solution (HBSS; Life Technologies, Grand Island, NY) and PBMCs collected using Ficoll-paque Plus (GE Healthcare Bio-Sciences Corp, Piscataway, NJ). Mononuclear cell layers were washed twice with HBSS and resuspended in complete RPMI. An aliquot of cells was taken to the ISU Flow Cytometry Facility and enumerated using a cell viability kit to determine live cell counts (BD #349480). Cell suspensions were diluted with complete RPMI and added to 96-well low evaporation culture plates (Costar, Cambridge, MA). Recombinant human (rh) IL-2 (Sigma-Aldrich-Cat#17908) or recombinant porcine IL-12 and IL-18 (rpIL-12/IL-18; 912-PL/588-PL; R&D Systems, Minneapolis, MN) was used at a concentration of 2 ng/well, or complete RPMI alone was added to appropriate wells. PBMC suspensions were diluted to 2 × 106 cell/mL suspensions and were plated to create 5:1, 10:1, and 20:1 effector to target ratios. Plates were incubated overnight at 37°C with 7% CO2.
Chromium Labeling of Targets and Application to PBMCs
Following overnight incubation of PBMCs prepared as described above, tumor target cell lines (A375SM, PANC-1, or K562 cells) were washed with RPMI and labeled with 200uCi chromium (Cr51) for 80 minutes with gentle agitation every 20 minutes. Complete media was used to wash cells and remove extracellular Cr51. Tumor cell suspensions were diluted to 2×105 cells/mL and 1×104 cells in 50uL was added to each well containing PBMCs or control media. Controls of spontaneous and total release wells were also prepared. Plates were incubated for 6 hours at 37°C.
Harvesting of Plates
Triton-X 100 (10%) was added to total release wells and pipetted up and down. Plates were centrifuged at 200 rpm for 5 min. An equal volume of supernatant was collected from each well into individual counting tubes. Radioactivity of supernatants was determined using a gamma-counter (Gamma trac 1191, TM Analytic Inc., ElkGrove Village, IL). Results were reported as counts per minute (CPM). Percent cytotoxicity was averaged across triplicates, and calculated as:
Flow cytometry
Staining for CD16, CD172
We enumerated NK cells as PBMCs with the following phenotype: CD16+, CD172 (SWC3a)-. Isolated PBMCs (as described above) were diluted in Phosphate Buffered Saline (PBS)- Sodium Azide (0.05%) solution (PBS-Azide) and incubated with pre-titered antibodies in an ice water bath for 45 minutes. Primary antibodies included a cocktail of mouse anti-pig CD172− (SWC3a; IgG2b, BD Biosciences-Cat #553640), mouse anti-pig CD16 (IgG1, AbD Serotec-Cat# MCA1971) and heat-inactivated swine serum for blocking. After washing with PBS-Azide, a cocktail of secondary antibodies was added and incubated in an ice bath for an additional 45 minutes. The secondary antibodies used were goat anti-mouse IgG2b- FITC (Southern Biotech-Cat#1090-02) and goat anti-mouse IgG1- PE (Southern Biotech-Cat#1072-09). Samples were washed with PBS, fixed with PBS/2% formaldehyde solution and submitted within 7 days of fixation to the ISU Flow Cytometry Facility for analysis on a BD Biosciences FACSCanto II (San Jose, CA).
Intracellular staining for perforin
PBMCs were isolated (as reported above) and plated at a concentration of 2 × 105 cells per well in a 96-well plate in the presence and absence of rhIL-2 and complete RPMI media. Cells were incubated for 3 days at 37 °C. A protein transport inhibitor (BD GogliPlug, Cat# 555029) was added 6 hours prior to staining (1 uL per 106 cells). An equal volume of supernatant was collected from each well into counting tubes (two wells per flow tube). Extracellular proteins (CD16, CD172) were stained as described above. Cells were then fixed and permeabilized with BD Cytofix/Cytoperm kit as directed by the manufacturer (Cat# 555028). Directly conjugated PerCP-Cy5.5 primary antibody targeted against perforin (BD Pharmingen Cat# 563762) or an isotype control (BD Pharmingen Cat# 558304) was added to samples and incubated on ice in the dark for 30 min. Cells were washed with BD Perm wash buffer (according to kit instructions) and stored until flow cytometric acquisition.
Flow cytometry data was analyzed and figures were created with BD FACSDiva Software (5-3-6) and Flowjo (V.10.1).
Statistical Analyses
Data generated on SCID and non-SCID pigs was analyzed with a mixed model in SAS 9.2 (SAS Institute Inc., Cary, NC). For analysis of the effect of IL-2 stimulation of NK cell cytotoxicity against the three tumor targets shown in Figure 1, a mixed model was run with fixed effects of treatment (IL-2 presence or absence), dilution, SCID status, and target cell type; animal was fit as a random effect. For later Figures, phenotype responses of percent cytotoxicity or number of tumor targets killed per NK cell were analyzed with a repeated measures (by animal) mixed model with fixed effects of litter, time point, dilution, and SCID status. Significance was set at a p-value ≤ 0.05.
Acknowledgments
This work was funded by Iowa State University Office of Vice President for Research, and grant 1R24OD019813-01 from NIH. EJP gratefully acknowledges support from a USDA National Needs Fellowship Grant (2012-38420-19286). We thank the Lauren Christian Swine Breeding farm staff and Iowa State University Laboratory Animal Research staff for excellent professional care of the SCID pigs. We thank Dr. Deryl Troyer for the A375-SM and PANC-1 cell lines, and give tremendous thanks to Dr. Martine Schroyen, the ISU Cytometry Flow Facility, and Dr. Shawn Rigby for all the help and advice. We also thank Sam Humphrey for assistance with flow cytometric software.
References
- Basel MT, Balivada S, Beck AP, Kerrigan MA, Pyle MM, Dekkers JC, Wyatt CR, Rowland RR, Anderson DE, Bossmann SH, et al. Human xenografts are not rejected in a naturally occurring immunodeficient porcine line: a human tumor model in pigs. Biores Open Access. 2012;1:63–68. doi: 10.1089/biores.2012.9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthels C, Puchalka J, Racek T, Klein C, Brocker T. Novel Spontaneous Deletion of Artemis Exons 10 and 11 in Mice Leads to T- and B-Cell Deficiency. In: Moser M, editor. PLoS ONE. 9. Vol. 8. 2013. p. e74838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolitho P, Voskoboinik I, Trapani JA, Smyth MJ. Apoptosis induced by the lymphocyte effector molecule perforin. Curr Opin Immunol. 2007;19(3):339–347. doi: 10.1016/j.coi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Buckley R, Schiff R, Schiff S, Market L, Williams L, Harville T, Roberts J, Puck J. Human severe combined immunodeficiency: Genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130:378–387. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- Cai W, Kaiser MS, Dekkers JC. Genetic analysis of longitudinal measurements of performance traits in selection lines for residual feed intake in Yorkshire swine. J Anim Sci. 2011;89:1270–1280. doi: 10.2527/jas.2010-3107. [DOI] [PubMed] [Google Scholar]
- Cino-Ozuna AG, Rowland RRR, Nietfeld JC, Kerrigan MA, Dekkers JCM, Wyatt CR. Preliminary findings of a previously unrecognized porcine primary immunodeficiency disorder. Vet Pathol. 2012;50:144–146. doi: 10.1177/0300985812457790. [DOI] [PubMed] [Google Scholar]
- Cossu F. Genetics of SCID. Ital J Pediatr. 2010;36(76) doi: 10.1186/1824-7288-36-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HD, Loveland JE, Pascal G, et al. Structural and functional annotation of the porcine immunome. BMC Genomics. 2013;14:332. doi: 10.1186/1471-2164-14-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer MS, Wileman TE, Stirling CMA, Zuber B, Takamatsu H. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of Natural Killer, Cytotoxic T, Natural Killer T and MHC unrestricted cytotoxic T-cells. Vet Immunol Immunopathol. 2006;110:279–292. doi: 10.1016/j.vetimm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Duluc D, Tan F, Scotet M, Blanchard S, Frémaux I, Garo E, Horvat B, Eid P, Delneste Y, Jeannin P. PolyI:C plus IL-2 or IL-12 induce IFN-γ production by human NK cells via autocrine IFN-β. Eur. J. Immunology. 2009;39:2877–2884. doi: 10.1002/eji.200838610. [DOI] [PubMed] [Google Scholar]
- Ewen CL, Cino-Ozuna AG, He H, Kerrigan MA, Dekkers JCM, Tuggle CK, Rowland RRR, Wyatt CR. Analysis of blood leukocytes in a naturally occurring immunodeficiency of pigs shows the defect is localized to B and T cells. Vet Immunol Immunopathol. 2014;162:174–179. doi: 10.1016/j.vetimm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;(6):798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Hassan A, Lee P, Maggina P, Xu JH, Moreira D, Slatter M, et al. Host Natural Killer Immunity Is a Key Indicator of Permissiveness for Donor Cell Engraftment in Patients with Severe Combined Immunodeficiency. J Allergy Clin Immunol. 2014;133:1660–1666. doi: 10.1016/j.jaci.2014.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Guo X, Fan N, Song J, Zhao B, Ouyang Z, Liu Z, Zhao Y, Yan Q, Yi X, Schambach A, Frampton J, Esteban MA, Yang D, Yang H, Liangxue L. RAG1/2 Knockout Pigs with Severe Combined Immunodeficiency. J Immunol. 2014;193:1496–1503. doi: 10.4049/jimmunol.1400915. [DOI] [PubMed] [Google Scholar]
- Leber R, Wiler R, Perryman LE, Meek K. Equine SCID: Mechanistic analysis and comparison with murine SCID. Vet Immunol Immunopathol. 1998;65:1–9. doi: 10.1016/s0165-2427(98)00174-3. [DOI] [PubMed] [Google Scholar]
- Lunn DP, McClure JT, Schobert CS, Holmes MA. Abnormal patterns of equine leucocyte differentiation antigen expression in severe combined immunodeficiency foals suggests the phenotype of normal equine natural killer cells. Immunol. 1995;84:495–499. [PMC free article] [PubMed] [Google Scholar]
- Maher KJ, Klimas NG, Hurwitz B, Schiff R, Fletcher MA. Quantitative Fluorescence Measures for Determination of Intracellular Perforin Content. Clinical and Diagnostic Laboratory Immunology. 2002;9(6):1248–1252. doi: 10.1128/CDLI.9.6.1248-1252.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek K, Kienker L, Dallas C, Wang W, Dark MJ, Venta PJ, Huie ML, Hirschhorn R, Bell T. SCID in Jack Russell terriers: a new animal model of DNA-PKcs deficiency. J Immunol. 2001;167:2142–2150. doi: 10.4049/jimmunol.167.4.2142. [DOI] [PubMed] [Google Scholar]
- Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. 2011;20(1):50–57. doi: 10.1016/j.tim.2011.11.002. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Jewett A, Cavalcanti M, Murakami-Mori K, Nakamura S, Bonavida B. Differential regulation of human NK cell-associated gene expression following activation by IL-2, IFN-a and PMA/ionomycin. International Journal of Onocolgy. 1998;12:1165–1170. doi: 10.3892/ijo.12.5.1165. [DOI] [PubMed] [Google Scholar]
- Perryman LE. Molecular pathology of severe combined immunodeficiency in mice, horses, and dogs. Vet Pathol. 2004;41:95–100. doi: 10.1354/vp.41-2-95. [DOI] [PubMed] [Google Scholar]
- Pregram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immun and Cell Biol. 2010;89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- Pintarič M, Gerner W, Saalmüller A. Synergistic effects of IL-2, IL-12 and IL-18 on cytolytic activity, perforin expression and IFN-γ production of porcine natural killer cells. Vet Immunol Immunopathol. 2008;121:68–82. doi: 10.1016/j.vetimm.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Schuetz C, Neven B, Dvorak CC, Leroy S, Ege MJ, Pannicke U, et al. SCID patients with ARTEMIS vs RAG deficiencies following HCT: increased risk of late toxicity in ARTEMIS-deficient SCID. Blood. 2014;123:281–289. doi: 10.1182/blood-2013-01-476432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waide EH, Dekkers JCM, Ross JW, Rowland RRR, Wyatt CR, Ewen C, Thekkoot DM, Boddicker NJ, Tuggle CK. Not all SCID pigs are created equally: Two independent mutations in Artemis gene found to cause Severe Combined Immunodeficiency (SCID) in pigs. Journal of Immunology. 2015 doi: 10.4049/jimmunol.1501132. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or Adaptive Immunity? The example of Natural Killer Cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Dunn E, Singh K, Khan IS, Yannone SM, Cowan MJ. A non-leaky Artemis-deficient mouse that accurately models the human severe combined immune deficiency phenotype, including resistance to hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:1–11. doi: 10.1016/j.bbmt.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WC, Schultz RD. Ontogeny of natural killer cell activity and antibody dependent cell mediated cytotoxicity in pigs. Dev Comp Immunol. 1986;10:405–418. doi: 10.1016/0145-305x(86)90030-3. [DOI] [PubMed] [Google Scholar]


