Abstract
Soluble guanylate cyclase (sGC) is a receptor for nitric oxide (NO). Binding of NO to ferrous (Fe2+) heme increases its catalytic activity, leading to the production of cGMP from GTP. Hydrogen sulfide (H2S) is a signalling molecule that exerts both direct and indirect anti-oxidant effects. In the present, study we aimed to determine whether H2S could regulate sGC redox state and affect its responsiveness to NO-releasing agents and sGC activators. Using cultured rat aortic smooth muscle cells, we observed that treatment with H2S augmented the response to the NO donor DEA/NO, while attenuating the response to the heme-independent activator BAY58-2667 that targets oxidized sGC. Similarly, overexpression of H2S-synthesizing enzyme cystathionine-γ lyase reduced the ability of BAY58-2667 to promote cGMP accumulation. In experiments with phenylephrine-constricted mouse aortic rings, treatment with rotenone (a compound that increases ROS production), caused a rightward shift of the DEA/NO concentration-response curve, an effect partially restored by H2S. When rings were pre-treated with H2S, the concentration-response curve to BAY 58-2667 shifted to the right. Using purified recombinant human sGC, we observed that treatment with H2S converted ferric to ferrous sGC enhancing NO-donor-stimulated sGC activity and reducing BAY 58-2667-triggered cGMP formation. The study identified an additional mechanism of cross-talk between the NO and H2S pathways at the level of redox regulation of sGC. Our results provide evidence that H2S reduces sGC heme Fe, thus, facilitating NO-mediated cellular signaling events.
Keywords: H2S, Nitric oxide, cGMP, ROS, sGC activators
1. INTRODUCTION
Nitric oxide (NO) is a signaling molecule that affects diverse physiological and pathophysiological processes in practically all systems and organs [1, 2]. In the cardiovascular system, NO regulates vascular tone, inhibits platelet aggregation, promotes angiogenesis, modulates inflammatory responses and exerts cardioprotective effects [3]. Although NO is capable of reacting with a plethora of molecular targets, the response to low physiological levels of NO is predominantly mediated by the heterodimeric soluble guanylyl cyclase (sGC) [4]. sGC is a heme protein with high affinity and specificity towards NO [5–7] that converts GTP to the second messenger cGMP; sGC activity is increased several hundred fold upon binding of NO [6].
Maintenance of sGC heme moiety in the ferrous state is essential for the sGC NO sensing function [8]. Oxidation of sGC heme by specific agents [9] or oxidative stress induced by various pathological conditions [10] renders sGC insensitive to normal levels of NO and attenuates NO/cGMP signaling. Moreover, it has been demonstrated that sGC with oxidized heme has a propensity to lose the heme moiety [11, 12]. The pool of ferric or heme-free sGC is much more susceptible to degradation [12, 13]. About fifteen years ago, NO-independent sGC activators and stimulators were discovered [14, 15]; these agents enhance sGC activity in a heme-dependent or -independent manner. sGC activators are unique for their ability to increase the catalytic activity of heme-free/oxidized sGC. sGC stimulators have already been granted approval for human use for certain types of pulmonary hypertension [16].
For many decades, H2S was considered a toxic gas that penetrates cells by simple diffusion [17]. Following the discovery that mammalian cells are capable of producing H2S, this molecule underwent a dramatic metamorphosis from a dangerous pollutant to a biologically relevant molecule, reminiscent of the transformation of NO [18, 19]. H2S is now accepted as a signaling molecule with important roles in physiology and disease[20–22]. H2S triggers many of the same responses as NO in the cardiovascular system; it reduces blood pressure, promotes angiogenesis and limits infarct size following ischemia/reperfusion injury [21, 23]. Although distinct effectors have been identified for H2S and NO, both agents are capable of enhancing cGMP levels. Much like NO donors, exposure of cells or tissues to H2S donors leads to intracellular cGMP accumulation [24, 25]. However, unlike NO which stimulates sGC, H2S raises cGMP by preventing its breakdown [26], by enhancing endothelial NO synthase (eNOS) activity [25, 27] as well as by liberating NO from stable biological stores of NO [28]. The interdependence of NO and H2S signaling is an area of intense investigation, with several groups aiming to unravel its importance for normal cell function, as well as various pathophysiological states.
Our previous studies demonstrated that H2S does not directly affect the activity of ferrous sGC [25]. In the current study, we investigated whether H2S affects the function of sGC carrying oxidized ferric heme. We report that H2S reduces ferric sGC heme into a ferrous state, which is accompanied by the restoration of NO activation of purified and cellular sGC, and the recovery of NO-dependent vasodilation. Conversely, H2S-dependent reduction of sGC heme diminishes the response to ferric-sGC by BAY58-2667; this later observation has significant implications for the pharmacology of sGC activators, especially in light of the translational efforts of this class of agents.
2. Materials and Methods
2.1 Reagents
Cell culture media and serum were obtained from Life Technologies GIBCO-BRL (Paisley, UK). All cell culture plastic ware was purchased from Corning-Costar Inc. (Corning, NY). DC Protein assay kit; penicillin and streptomycin were purchased from Applichem (Darmstadt, Germany). BAY 58-2667 was obtained from Adipogen AG(Switzerland). HiTrap desalting columns were purchased from GE Healthcare Bio-Sciences (Pittsburgh, PA). The cGMP EIA kit was obtained from Enzo Life Sciences (Farmingdale, NY). Guanosine-5’-[(α,β)-methyleno]triphosphate (GpCpp) was purchased from Jena Bioscience (Jena, Germany). Sodium hydrosulfide (NaHS) and sodium sulfide (Na2S), DEA/NO, sodium nitroprusside (SNP), ODQ, rotenone, phenylephrine, protease/phosphatase inhibitors and all other chemicals used in solutions and buffers were purchased from Sigma-Aldrich Co. LLC (St Louis, MO).
2.2 Ex vivo studies
All animal procedures were in compliance with the European Community guidelines for the use of experimental animals and approved by the Committee Centro Servizi Veterinari of the University of Naples “Federico II”; institutional regulations do not require the use of animal protocol numbers for approved protocols. Animals (C57Bl mice) were sacrificed with CO2 and thoracic aortas were rapidly harvested, dissected, and cleaned of adherent connective and fat tissue. Rings of about 1 mm length were denuded of the endothelium, cut and placed in organ baths (2.5 ml) filled with oxygenated (95% O2 −5% CO2) Krebs solution maintained at 37°C. The rings were connected to an isometric transducer (type 7006, Ugo Basile, Comerio, Italy) and changes in tension were recorded continuously with a computerized system (Data Capsule 17400, Ugo Basile, Comerio, Italy). The composition of the Krebs solution was as follow (mM): NaCl 118, KCl 4.7, MgCl2 1.2, KH2PO4 1.2, CaCl2 2.5, NaHCO3 25, and glucose 10.1. The rings were stretched until a resting tension of 1.5 g was reached and allowed to equilibrate for at least 45 min, during which time tension was adjusted, as necessary, to 1.5g and bathing solution was periodically changed. In each experiment, rings were first challenged with PE (1 µM) until the responses were reproducible. The rings were then washed and contracted with PE (1 µM) and, once a plateau was reached, a cumulative concentration-response curve of the DEA/NO or BAY 58-2667 were performed. Some rings were pretreated with rotenone (10 µM) for 15min with or without Na2S (50 µM). After the 15min pre-incubation time, cumulative concentration-response curves were performed.
2.3 Cell culture
Rat aortic smooth muscle cells (RASMC) were isolated from 12- to 14-wk-old male Wistar rats, five rats per isolation, as previously described. Animals were anesthetized with pentobarbital sodium (40 mg/kg ip). Once fully anesthetized as judged by the lack of reaction to a noxious stimulus, animals were exsanguinated; thoracic aortas were then removed. More than 95% of cells isolated stained positive for smooth muscle α-actin. Cells between passages 2 and 5 were used for all experiments. RASMC were routinely cultured in DMEM containing 4.5 g/l glucose and supplemented with 10% fetal bovine serum and antibiotics.
2.4 cGMP enzyme immunoassay
Cells were grown to confluence and were washed twice with phosphate buffered saline and then incubated in HBSS in the presence of isobutyl methyl xanthine (IBMX;1mM) for 5 min. Cells were then treated with vehicle, rotenone (10µM), Na2S (50µM) or a Na2S/rotenone combination for 30min. Fifteen minutes after the exposure to DEA/NO (1µM) or BAY 58-2667 (1µM), media were aspirated and 200 µl of 0.1 N HCl were added into each well to extract cGMP. After 30 min, HCl extracts were collected and centrifuged at 600g for 10 min to remove debris. The supernatants were directly analyzed for cGMP by enzyme immunoassay.
2.5 sGC expression and purification
Human ferrous sGC was purified from Sf9 cells infected with baculoviruses expressing α1 and β1 sGC subunits following the procedures described previously[29]. Purified ferrous sGC was stored at −80°C in 50 mM TEA pH7.4 and 1 mM DTT, 1 mM EDTA and 1 mM EGTA until further use. To prepare ferric sGC, the sample of ferrous sGC was passed through a HiTrap desalting column to remove DTT. sGC in thiol-free buffer was then titrated with increasing concentrations of ODQ (0.1–1 µM) until sGC heme moiety was fully oxidized, as demonstrated by the conversion of the Soret band from 432 nm to 393 nm. The preparation was then passed through the desalting column again to remove ODQ. This preparation of ferric sGC was used for spectroscopic studies and activity measurements.
2.6 Reduction of ferric sGC by hydrogen sulfide
The reduction of ferric sGC by H2S was monitored using the UV-VIS spectrophotometer. For titration of NaHS 3 µM ferric sGC was supplemented with increasing concentrations of NaHS (0.01– 1000 µM) and the specrum was recorded 30 seconds after the addition of NaHS. To monitor the dynamic of sGC reduction by NaHS, ~1.5 µM preparation of ferric sGC was supplemented with 200 µM NaHS and the spectral changes were recorded in a kinetic mode of the instrument every 60 second. The apparent rate of reduction was calculated based on the data fitting using the first order reaction algorithm (UV-Vis ChemStation software, Agilent Technologies).
2.7 Assay of sGC activity in vitro
Enzymatic activity was assayed by the formation of [32P] cGMP from α[32P]GTP at 37°C as described previously[30]. In brief, the reaction was initiated adding 1 mM GTP/ α[32P]GTP to 0.1 µg sGC (ferric or ferrous) in 25 mM TEA, pH 7.5, 1 mg/ml BSA, 1 mM cGMP, 3 mM MgCl2, 0.05 mg/ml creatine phosphokinase and 5 mM creatine phosphate. To measure the response of ferric or ferrous sGC to sodium nitroprusside (SNP) or BAY58-2667, the activators were added together with GTP. After 30min the reaction was stopped by zinc acetate followed by precipitation of unreacted GTP by Zn carbonate. cGMP was separated from the remaining GTP by alumina chromatography and the level of synthesized cGMP was quantified based on Cherenkov radiation.
2.8 Data analysis
Data are expressed as means ± SEM. Statistical comparisons between groups were performed using ANOVA followed by a posthoc test or Student’s t-test, as appropriate. Differences were considered significant when P<0.05. GraphPad Prism software (version 4.02, GraphPad Software, San Diego, CA) was used for all the statistical analysis.
3. RESULTS
3.1 H2S reverses the effects of sGC heme oxidation in cells
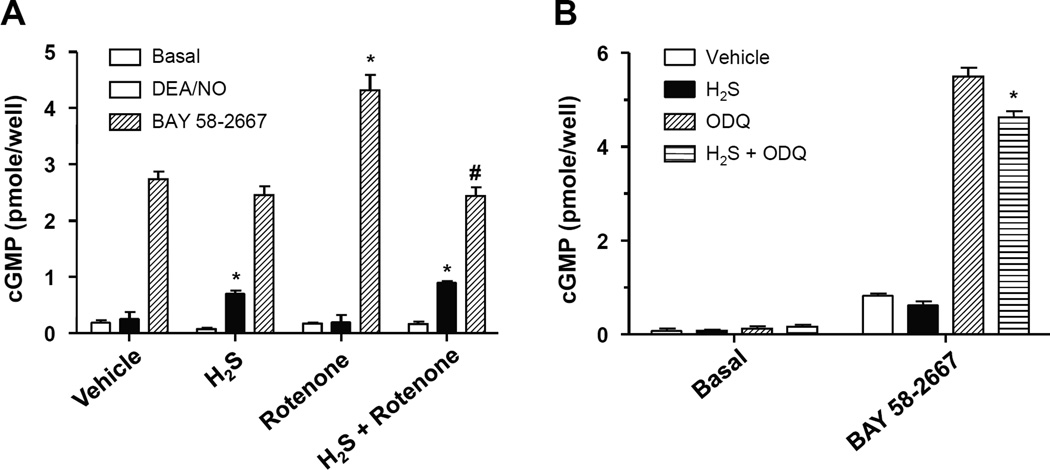
Reactive oxygen and nitrogen species have a potential to oxidize the heme moiety of sGC and negatively affect NO/cGMP signaling [14]. We investigated the ability of hydrogen sulfide to restore sGC altered responses caused by oxidative stress. To generate a persistent, low-to-intermediate level oxidative stress in rat aortic smooth muscle cells we used rotenone (10µM) to inhibit the electron flow of the mitochondrial respiratory chain. Exposure to rotenone enhanced BAY58-2667-dependent cGMP accumulation in RASMC (Fig. 1A). To investigate if hydrogen sulfide could reverse the oxidation of sGC heme caused by rotenone, cells were co-treated with an H2S-yielding salt. As shown in Figure 1A, such treatment suppressed the enhanced ability of BAY58-2667 to increase cGMP levels in the presence of rotenone. To probe the reactivity of sGC towards NO, a concentration of the NO donor DEA/NO was chosen that did not increase cGMP levels. Exposure of cells to H2S, unmasked the cGMP-stimulating capacity of DEA/NO. This observation is consistent with the ability of H2S to decrease the content of oxidized cellular ferric sGC and increase the amount of sGC that could be activated by NO (ferrous sGC). In a different experimental setting, oxidation of sGC heme was achieved by administration of ODQ, a specific sGC heme oxidant. Even with this strong sGC oxidizing agent, H2S diminished the fraction of ferric sGC, as attested by diminished cGMP synthesis in response to BAY58-2667 (Fig. 1B).
Figure 1. H2S reverses the effects of rotenone and ODQ on cGMP accumulation.
(A): Rat aortic smooth muscle cells were incubated with vehicle, rotenone (10µM), H2S (Na2S, 50µM) or a combination of H2S and rotenone for 30min. Cells were then incubated with DAE/NO (1µM) or BAY 58-2667 (10µM) for 15min in the presence of IBMX (1mM). Cellular cGMP was extracted with 0.1N HCl and measured by EIA; n= 4;* P<0.05 vs respective vehicle group; # p<0.05 vs respective rotenone group. (B): Rat aortic smooth muscle cells were incubated with vehicle, ODQ (0.1 µM), H2S (Na2S, 50µM) or a combination of H2S and ODQ for 30min. Cells were then incubated with BAY 58-2667 (10µM) for 15min in the presence of IBMX (1mM). Cellular cGMP was extracted with 0.1N HCl and measured * P < 0.05 vs ODQ.
3.2 H2S-generating cystathionine γ-lyase diminishes the content of ferric heme sGC
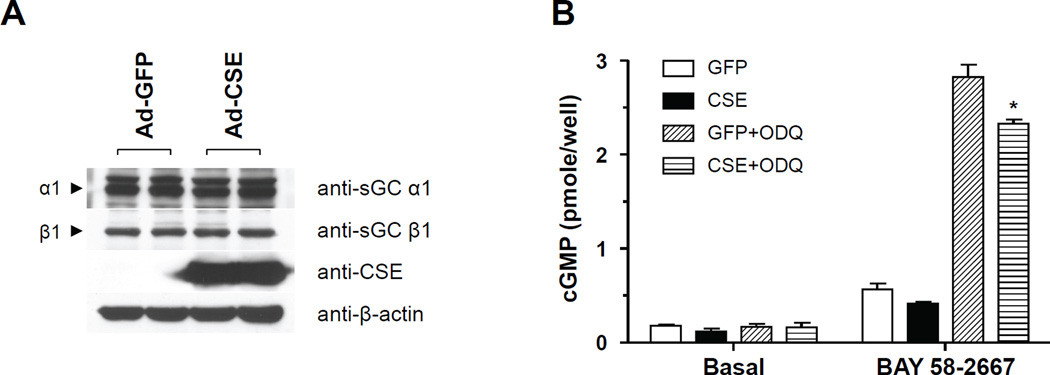
Cystathionine γ-lyase (CSE) is an enzymes of the transulfuration pathway that generates hydrogen sulfide [19]. To test whether elevated endogenous H2S affects sGC function we used RASMC and infected them with an adenovirus expressing CSE or green fluorescent protein (GFP) as control. CSE overexpression did not alter sGC α1 or β1 levels (Fig. 2A). We observed that CSE over expression resulted in reduced cGMP synthesis in RASMC in response to BAY58-2667 treated with ODQ (Fig. 2B). The findings with endogenously generated H2S replicate the observations made with exogenously added H2S and are consistent with decreased cellular content of sGC with ferric heme.
Figure 2. CSE over expression attenuates sGC activator (BAY 58-2667)-induced cGMP accumulation.
(A) Representative western blot from smooth muscle cells infected with CSE adenovirus. (B) Cells were infected with a GFP or a CSE expressing adenovirus (10 m.o.i). Forty eight hours later cells washed and incubated with ODQ (50nM) and then exposed to BAY 58-2667 (1µM). Cellular cGMP was extracted with 0.1N HCl and measured by EIA; n= 4;* P<0.05 *vs ODQ.
3.3 Effects of H2S on NO and BAY 58-2667-induced vasorelaxation
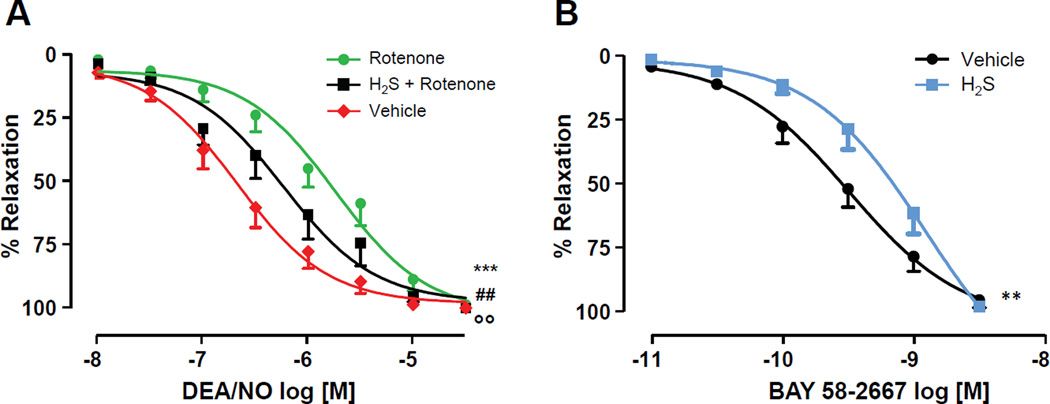
sGC plays a key role in mediating NO-dependent vasodilation[6]. Previous studies demonstrated that oxidative stress and various pathological conditions may increase the pool of ferric sGC or heme-deficient sGC in blood vessels and lead to impaired NO response in vasculature. Therefore, we tested whether H2S-dependent reduction of sGC heme translates into improved NO/sGC signaling in conditions of oxidative stress. We first evaluated the vasoreactivity of control and rotenone-treated mouse thoracic aorta. As shown in Fig. 3A, rotenone-pretreated aortic segments exhibited a significant reduction in the sensitivity to NO with a rightward shift in the concentration-response curve been observed. The apparent EC50 for DEA/NO increased from 149.9±1.30 nM to 2.00±1.29 µM, for control and rotenone-treated vessels, respectively. However, when H2S was administered during the last 10 minutes of rotenone treatment, a substantial restoration of NO-dependent vasodilation was observed. It should be noted that rotenone, H2S or their simultaneous addition did not affect the ability of the tissue to contract in response to phenylephrine. Additional experiments were performed to confirm that H2S affects the fraction of ferric sGC in the isolated aorta. Vessels were exposed to H2S and then relaxation was triggered by BAY58-2667. As demonstrated in Fig. 3B, vessels treated with H2S were less responsive to BAY58-2667 than control vessels, suggesting that in the presence of H2S lower level of sGC carrying ferric heme exist.
Figure 3. H2S partially rescues rotenone-induced reduction of DEA/NO vasorelaxation, while it inhibits BAY 58-2667-triggered vasorelaxation.
Phenylephrine-constricted aortic rings were pre-treated with rotenone (10µM) or H2S+rotenone for 15 min. The sulfide salt Na2S (50µM) was used as a source of H2S. After incubation, cumulative concentration-response curves to DEA/NO (A, P< 0.05 *** rotenone vs vehicle; ## H2S + rotenone vs vehicle; & H2S + rotenone vs rotenone) or BAY 58-2667 (B, ** P<0.05 H2S vs vehicle) were performed.
3.4 H2S promotes restoration of ferrous sGC
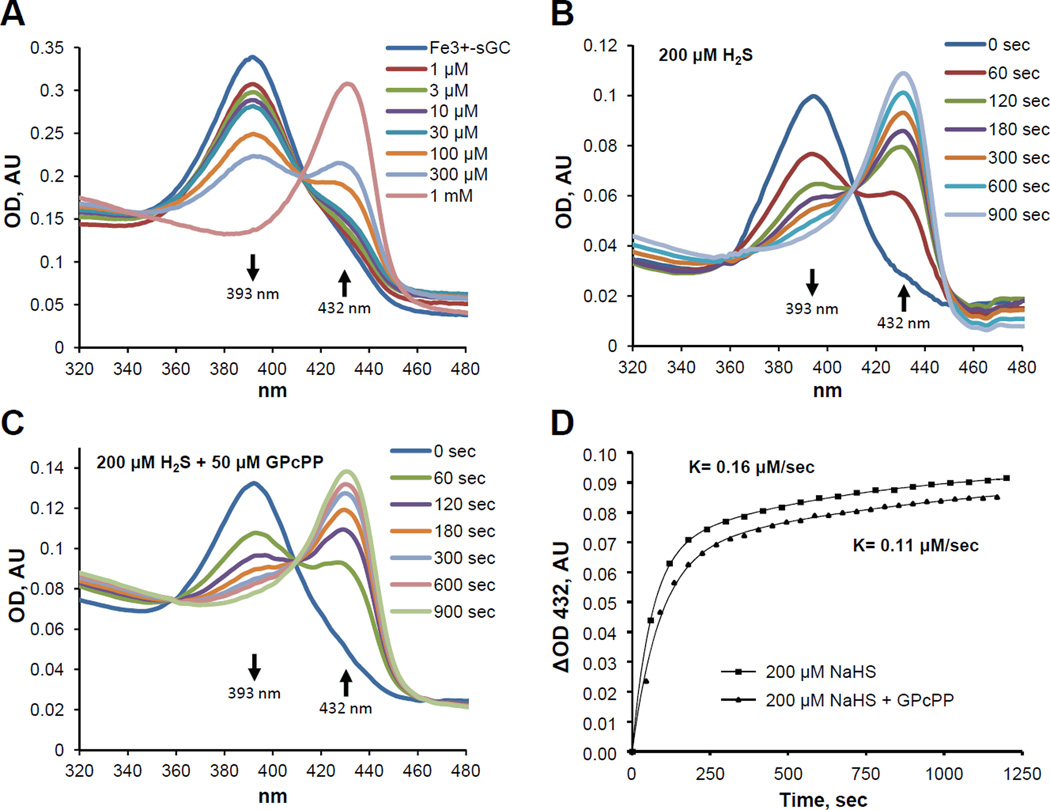
To study the mechanism of altered responsiveness to NO and sGC activators in the presence of H2S, we evaluated if H2S directly affects the heme status and/or activity of purified ferric sGC. Therefore, we tested if addition of H2S can change the redox status of sGC heme by monitoring the UV-Vis absorbance of sGC heme. We observed that H2S induced a concentration- and time-dependent conversion of 393 nm Soret band of ferric sGC into the 432 nm Soret band of ferrous sGC (Fig. 4), directly demonstrating that H2S reduces sGC heme moiety. Previous studies demonstrated that the dynamics of sGC response to NO and CO gaseous ligands is different in presence of GTP substrate. Therefore, we evaluated if GTP affects the dynamics of sGC reduction by H2S. We determined that the addition of GpCpp (a non-hydrolyzable GTP analog) does not alter the overall process of sGC heme reduction (Fig. 4C). However, a slight decrease in the apparent reduction rate from 0.16 ± 0.03 to 0.11 ± 0.04 µM/sec was observed (Fig. 4D).
Figure 4. H2S-dependent conversion of Fe3 to Fe2+ sGC is concentration- and time-dependent, and mildly affected by GTP substrate.
(A): UV-Vis spectra of the Soret region for ferric sGC (3 µM) in 50 mM TEA buffer (pH 7.4) were recorded 1 minute after the addition of the indicated amount of H2S in the form of a sulfide salt (NaHS). (AU, absorbance unit). (B): 3 µM ferric sGC was mixed with an equal volume of H2S (NaHS, 200 µM) in 50 mM TEA buffer (pH 7.4) and spectral changes were recorded for 20 minutes. (C): Spectral changes for ferric sGC supplemented with 50 µM GpCpp after the addition of H2S. (D): Time-dependent accumulation of 432 nm band in samples of ferric sGC alone or in the presence of 50 µM GPcPP in response to H2S.
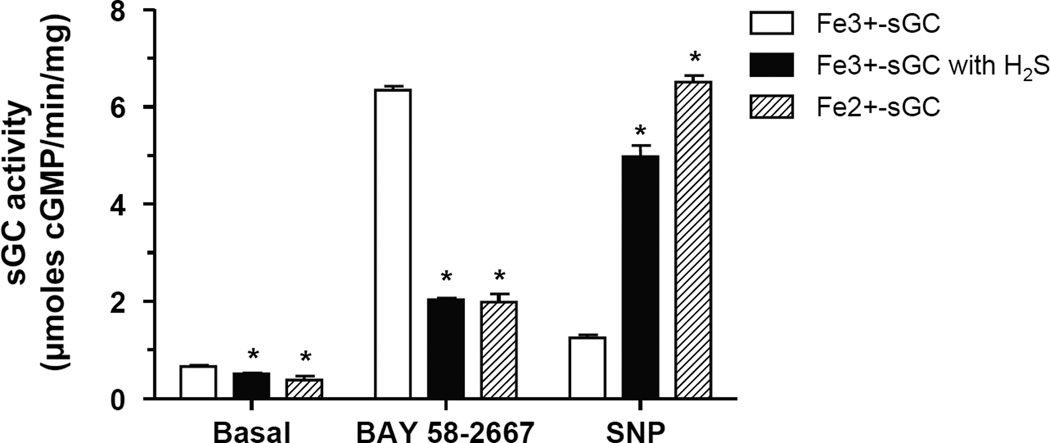
As expected, ferric heme sGC was non-responsive to the NO donor sodium nitroprusside, but was strongly activated by 100 nM BAY58-2667 (Fig. 5). On the contrary, ferrous sGC was strongly activated by sodium nitroprusside and had a moderate response to 100 nM BAY58-2667. In the presence of H2S, ferric sGC exhibited a diminished response to BAY58-2667 and a robust activation to a NO donor, closely resembling the properties of ferrous sGC.
Figure 5. H2S reduces Fe3+ heme to Fe2+ in sGC leading to changes in enzyme responsiveness to NO and BAY 58-2667.
Purified samples of ferric (Fe3+-sGC) or ferrous (Fe2+-sGC) sGC were incubated with or without H2S (NaHS, 25µM) and stimulated with sodium nitroprusside (SNP; 10 µM), or BAY 58-2667 (100 nM). The formation of [α-32P] cGMP from [α-32P] GTP was quantified; n=6, * P<0.01 vs ferric sGC without H2S.
4. DISCUSSION
The main finding of the present study is that H2S is capable of reducing the prosthetic heme group of sGC from Fe+3 to Fe+2, therefore increasing the NO-activatable pool of sGC in cells. The shift in sGC redox state caused by H2S has important implications not only for its endogenous ligand, but also impacts on the pharmacological activity of sGC activators.
We have previously shown that incubation of cells with sulfide salts increases cGMP accumulation in rat aortic smooth muscle cells; this effect is attributed to inhibition of phosphodiesterase [24, 26]. To rule out that any changes observed in cGMP levels in the current series of experiments might result from PDE inhibition, all of measurements were performed in the presence of the non-selective PDE inhibitor IBMX, at a concentration which inhibits PDE in rat smooth muscle cells[31, 32]. Indeed, exposure of cells to Na2S alone did not cause a change in cGMP levels in the presence of IBMX. Exposure of cells to 1µM DEA/NO failed to increase cGMP; however, when cells were incubated to H2S, 1µM DEA/NO promoted an increase in intracellular cGMP, presumably due to conversion of existing cellular ferric to ferrous sGC. On the other hand, when cells were exposed to 1µM BAY 58-2667 a sharp increase in cGMP levels we observed, confirming previous observations [33]. Rotenone, an agent that promotes ROS generation by inhibiting complex I of the respiratory chain [34], increased the response to BAY 58-2667 that selectively targets the oxidized-heme/free pool of sGC. Interestingly, this effect was reversed by incubating cells with a source of H2S. Sulfide salts (NaHS and Na2S) although widely used in the literature to deliver H2S to cells suffer a major drawback [35]; once dissolved in aqueous buffers instantly decompose yielding a burst on H2S. To deliver H2S in a physiologically-relevant manner, we infected cells with an adenovirus that over expresses CSE, a major H2S-gennerating enzyme in vascular tissue [17]. CSE-overexpressing cells displayed reduced responsiveness to BAY 58-2667. The above data taken together are in line with the notion that H2S shifts the sGC redox balance towards ferrous sGC.
H2S is known to have both direct and indirect anti-oxidant actions. H2S reacts with ROS and reactive nitrogen species, including hydrogen peroxide, and peroxynitrite, although with relatively modest rate constants [36]. In addition, H2S has been shown to up regulate the expression/activity antioxidant enzymes, to affect the expression of ROS generating enzymes and to increase glutathione levels [17, 19, 37]. The beneficial effect of H2S on NO signaling in our experiments that employed rotenone could result from neutralization of ROS, thus preventing oxidation of sGC heme. Another possible mechanism of beneficial effect of sulfides on cellular sGC activity under conditions of oxidative stress is the reaction of sulfide ion with oxidized cysteine residues. sGC has a large number of cysteine residues that are susceptible to modification by ROS. Proteomic analysis of sGC from cells that were chronically exposed to oxidative stress via an aldosterone-dependent mechanism demonstrated that the Cys122 residue of the β1 subunit undergoes several oxidative modifications [38]. This specific residue has been implicated in modulating the potency of NO activation of sGC [39]. Under physiologic pH, cellular sulfides are more likely to react with oxidized protein thiols, such as protein sulfenic acid, than intracellular glutathione [40]. Therefore, H2S-dependent restoration of NO-inducible cGMP synthesis in cells exposed to rotenone could in theory be, at least, in part explained by the reduction of sGC thiols. However, this is most likely not the case, as in the purified enzyme preparation H2S restored responsiveness of sGC exposed to the selective heme oxidant ODQ that has no effect on sGC thiols. To evaluate if H2S can directly target the prosthetic heme group of sGC, we used ODQ an agent that selectively oxidizes the heme moiety of sGC [9]. In line with its ability to convert ferrous to ferric sGC, ODQ-treated cells exhibited and increased cGMP response to BAY 58-2667. This effect of ODQ was attenuated by H2S, suggesting that H2S has direct effect on sGC heme.
To better characterize the effect of H2S on sGC redox state, we used human recombinant sGC. After purification sGC was converted to its ferric state by a reaction with ODQ; the single peak at 393nm confirmed the successful preparation of fully oxidized sGC. In agreement with the current literature [15, 41], the activity of sGC with ferric heme was unaffected by NO reflecting the low affinity of Fe+3 sGC for NO. At the same time, Fe+3 sGC responded well to BAY58-2667, an sGC activator that has a higher affinity to sGC with oxidized heme. The opposite was true of ferrous sGC, which demonstrated only a small change in activity in response to BAY 58-2667, but was readily activated by NO. Incubation of Fe+3 sGC with H2S, caused a concentration-dependent shift in the absorption maximum of heme from 393 nm, characteristic for ferric sGC heme to 432 nm, which is indicative of ferrous heme sGC. Full conversion from ferric to ferrous heme took 900 sec. The activity of Fe+3 sGC towards NO was restored when incubated with H2S. Overall, the observations made with the recombinant sGC are well in agreement with those made with the cultured smooth muscle cells, reinforcing the notion that H2S keeps sGC in a reduced, NO-responsive state.
Our observation that hydrogen sulfide reduces ferric state of sGC heme is in line with many observations reported for other heme-containing proteins [42]. Sulfide even at low concentrations can act as electron donor to the ferric derivative of cytochrome C oxidase and convert it into an active reduced form[43]. Similar heme-reducing activity of hydrogen sulfide was reported for the ferric catalase [44] and several peroxidases [45, 46]. Hemoglobin and myoglobin in ferric Fe3+ state bind H2S as heme ligand and are rapidly reduced to the deoxy Fe2+ and/or Fe2+-O2 derivative[47]. It should be noted that the reaction of H2S with oxygen-carrying hemoglobin and myoglobin results in the formation of sulfheme, a chlorine-type heme with a sulfur atom incorporated into one of the pyrrole rings. Generation of sulfheme was also described for catalase and lactorperoxidase[48]. However, in our spectral observations of the interaction between ferric or ferrous sGC with H2,S we observed no traces of the 620 nm optical band characteristic for sulfheme (data not shown). Therefore, we conclude that the only effect of H2S on sGC is the reduction of heme iron from ferric to ferrous.
Superoxide anion generation leads to NO scavenging and limits NO biological activity[2]. Incubation of vessels with the rotenone, caused a shift of the relaxation curve to DEA/NO to the right, due to production of ROS. The increase in EC50 for NO donors can be reversed by superoxide dismutase and other antioxidants. Incubation of vessels with H2S partially restored the DEA/NO response. As in this vessel preparation PDE was not inhibited, we cannot safely conclude that the shift of the DEA/NO concentration curve is exclusively due to reduction of sGC. However, this observation is well in accordance with our cell culture and purified enzyme experiments. In a different series of experiments, vessels were pre-incubated with H2S and then exposed to BAY 58-2667. sGC activator responses were shifted to the right, as a greater amount of sGC is expected to be in its reduced, BAY 58-2667-unresponsive form. These findings confirm and extend our observations in vitro and provide functional relevance for this H2S/NO interaction at the sGC level.
Several levels of H2S-NO cross-talk and interactions, ranging from direct chemical interactions to convergence onto downstream signaling pathways have been identified [49–52]. The direct reaction between H2S and NO yields nitroxyl that triggers vasodilation [51] that can activate sGC [53]. On the other hand, the nitrosopersulfide (SSNO−) formed following reaction of S-nitrosothiols with sulfide, liberates NO to activate cGMP [50]. H2S promotes phosphorylation of eNOS on Ser1177, leading to increased NO output [25, 27]. eNOS dimerization is essential for enzymatic activity; H2S preserves dimeric eNOS by persulfidation and inhibition of Cys443 nitrosation [54]. H2S has also been shown to inhibit phosphodiesterase (PDE) activity, with a 30-fold selectivity for PDE5[55]. Increased NO bioavailability is supported by H2S activation of xanthine oxidase to reduce nitrite in ischemic tissues [28]. All of these events culminate in activation of the cGMP/PKG cascade regulating cardiovascular function. As a consequence, vasorelaxation concentration-response curves to H2S are shifted to the right in eNOS KO mice, while no stimulation of angiogenesis and cardioprotection on response to H2S donors can be observed in mice lacking eNOS[25, 27, 56]. The ability of H2S to affect sGC responsiveness to NO demonstrated in the current report provides an additional level of cross-talk between NO and H2S.
In conclusion, we have demonstrated that sGC regulates sGC redox status, favoring the existence of ferrous sGC. Maintenance of physiological H2S levels would not only prevent NO destruction by enhancing anti-oxidant pathways, but would also preserve NO-responsiveness of sGC through the action of H2S on the heme moiety. Enhanced expression of H2S producing enzymes might counteract the oxidation of sGC encountered in disease states, while a decline in cellular H2S could make sGC refractory to normal levels of NO. Although discrete pools of sGC are known to exist (reduced and oxidized), the cellular constituents responsible regulating the balance between these two forms of sGC remain elusive [57, 58]. H2S being a freely diffusible small molecule would be well suited to serve as an endogenous sGC reducing agent. Our findings have important pharmacological implications as H2S donors which are currently in clinical development might represent a way to restore sGC responsiveness to NO. Further studies validating such interactions in vivo are required.
Acknowledgments
This work has been co-financed by the European Union (European Social Fund – ESF) and Greek national funds through the Operational Program "Education and Lifelong Learning" of the National Strategic Reference Framework (NSRF) - Research Funding Program Aristeia 2011 (1436) to AP, by EU FP7 REGPOT CT-2011-285950 – “SEE-DRUG”, by the COST Action BM1005 (ENOG: European network on gasotransmitters) and by the National Institutes of Health (R01GM107846) to CS.
Footnotes
Author disclosure Statement: The authors report no conflicts of interest
References
- 1.Davis K, Martin E, Turko I, Murad F. Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol. 2001;41:203–236. doi: 10.1146/annurev.pharmtox.41.1.203. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991;21:361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 3.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garthwaite J. New insight into the functioning of nitric oxide-receptive guanylyl cyclase: physiological and pharmacological implications. Mol Cell Biochem. 2010;334:221–232. doi: 10.1007/s11010-009-0318-8. [DOI] [PubMed] [Google Scholar]
- 5.Boon EM, Marletta MA. Ligand discrimination in soluble guanylate cyclase and the H-NOX family of heme sensor proteins. Curr Opin Chem Biol. 2005;9:441–446. doi: 10.1016/j.cbpa.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Koesling D, Russwurm M, Mergia E, Mullershausen F, Friebe A. Nitric oxide-sensitive guanylyl cyclase: structure and regulation. Neurochem Int. 2004;45:813–819. doi: 10.1016/j.neuint.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Martin E, Berka V, Bogatenkova E, Murad F, Tsai A-L. Ligand Selectivity of Soluble Guanylyl Cyclase: Effect of the hydrogen-bonding tyrosine in the distal heme pocket on binding of oxygen, nitric odie and carbon monoxide. J Biol Chem. 2006;281:27836–27845. doi: 10.1074/jbc.M601078200. [DOI] [PubMed] [Google Scholar]
- 8.Derbyshire ER, Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem. 2012;81:533–559. doi: 10.1146/annurev-biochem-050410-100030. [DOI] [PubMed] [Google Scholar]
- 9.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 10.SP Stasch JP, Nedvetsky PI, Nedvetskaya TY, H S AK, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Müller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Müller-Esterl W, Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116:2552–2561. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz BG, Hu X, Brailey JL, Berry RE, Walker FA, Montfort WR. Oxidation and Loss of Heme in Soluble Guanylyl Cyclase from Manduca sexta. Biochemistry. 2011;50:5813–5815. doi: 10.1021/bi200794c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meurer S, Pioch S, Pabst T, Opitz N, Schmidt PM, Beckhaus T, Wagner K, Matt S, Gegenbauer K, Geschka S, Karas M, Stasch J-P, Schmidt HHHW, Müller-Esterl W. Nitric Oxide–Independent Vasodilator Rescues Heme-Oxidized Soluble Guanylate Cyclase From Proteasomal Degradation. Circ Res. 2009;105:33–41. doi: 10.1161/CIRCRESAHA.109.198234. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann LS, Schmidt PM, Keim Y, Schaefer S, Schmidt H, Stasch JP. Distinct molecular requirements for activation or stabilization of soluble guanylyl cyclase upon haem oxidation-induced degradation. Br J Pharmacol. 2009;157:781–795. doi: 10.1111/j.1476-5381.2009.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HHHW, Stasch J-P. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papapetropoulos A, Hobbs AJ, Topouzis S. Extending the translational potential of targeting NO/cGMP-regulated pathways in the CVS. Br J Pharmacol. 2015;172:1397–1414. doi: 10.1111/bph.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghofrani H-A, Galiè N, Grimminger F, Grünig E, Humbert M, Jing Z-C, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ. Riociguat for the Treatment of Pulmonary Arterial Hypertension. N Engl J Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 17.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 19.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 20.Paul BD, Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 21.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond) 2011;121:459–488. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Szabo C, Ichinose F, Ahmed A, Whiteman M, Papapetropoulos A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol Sci. 2015;36:568–578. doi: 10.1016/j.tips.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Zaid A, Giannogonas P, Cantalupo A, Dhayade S, Karalis KP, Wang R, Feil R, Cirino G. cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS One. 2012;7:e53319. doi: 10.1371/journal.pone.0053319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gerö D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 27.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci U S A. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG. Hydrogen Sulfide Stimulates Ischemic Vascular Remodeling Through Nitric Oxide Synthase and Nitrite Reduction Activity Regulating Hypoxia-Inducible Factor-1α and Vascular Endothelial Growth Factor–Dependent Angiogenesis. J Am Heart Assoc. 2012;1:e004093. doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin E, Berka V, Sharina I, Tsai A-L. Mechanism of Binding of NO to Soluble Guanylyl Cyclase: Implication for the Second NO Binding to the Heme Proximal Site. Biochemistry. 2012;51:2737–2746. doi: 10.1021/bi300105s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz G. General principles of assays for adenylate cyclase and guanylate cyclase activity. Methods Enzymol. 1974;38:115–125. doi: 10.1016/0076-6879(74)38018-4. [DOI] [PubMed] [Google Scholar]
- 31.Pan X, Arauz E, Krzanowski JJ, Fitzpatrick DF, Polson JB. Synergistic interactions between selective pharmacological inhibitors of phosphodiesterase isozyme families PDE III and PDE IV to attenuate proliferation of rat vascular smooth muscle cells. Biochem Pharmacol. 1994;48:827–835. doi: 10.1016/0006-2952(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 32.Papapetropoulos A, Marczin N, Snead MD, Cheng C, Milici A, Catravas JD. Smooth muscle cell responsiveness to nitrovasodilators in hypertensive and normotensive rats. Hypertension. 1994;23:476–484. doi: 10.1161/01.hyp.23.4.476. [DOI] [PubMed] [Google Scholar]
- 33.Rekowski MvW, Kumar V, Zhou Z, Moschner J, Marazioti A, Bantzi M, Spyroulias GA, van den Akker F, Giannis A, Papapetropoulos A. Insights into soluble guanylyl cyclase activation derived from improved heme-mimetics. J Med Chem. 2013;56:8948–8952. doi: 10.1021/jm400539d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial Complex I Inhibitor Rotenone Induces Apoptosis through Enhancing Mitochondrial Reactive Oxygen Species Production. J Biol Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 35.Papapetropoulos A, Whiteman M, Cirino G. Pharmacological tools for hydrogen sulphide research: a brief, introductory guide for beginners. Br J Pharmacol. 2015;172:1633–1637. doi: 10.1111/bph.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carballal S, Trujillo M, Cuevasanta E, Bartesaghi S, Möller MN, Folkes LK, García-Bereguiaín MA, Gutiérrez-Merino C, Wardman P, Denicola A, Radi R, Alvarez B. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic Biol Med. 2011;50:196–205. doi: 10.1016/j.freeradbiomed.2010.10.705. [DOI] [PubMed] [Google Scholar]
- 37.Ju Y, Zhang W, Pei Y, Yang G. H2S signaling in redox regulation of cellular functions. Can J Physiol Pharmacol. 2013;91:8–14. doi: 10.1139/cjpp-2012-0293. [DOI] [PubMed] [Google Scholar]
- 38.Maron BA, Zhang YY, Handy DE, Beuve A, Tang SS, Loscalzo J, Leopold JA. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem. 2009;284:7665–7672. doi: 10.1074/jbc.M809460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci U S A. 2007;104:12312–12317. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagy P, Winterbourn CC. Redox chemistry of biological thiols. Adv Mol Toxicol. 2010;4:182–222. [Google Scholar]
- 41.Stasch JP, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol. 2009:277–308. doi: 10.1007/978-3-540-68964-5_13. [DOI] [PubMed] [Google Scholar]
- 42.Nagy P. Mechanistic chemical perspective of hydrogen sulfide signaling. Methods Enzymol. 2015;554:3–29. doi: 10.1016/bs.mie.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 43.Collman JP, Ghosh S, Dey A, Decreau RA. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc Natl Acad Sci U S A. 2009;106:22090–22095. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicholls P. The action of anions on catalase peroxide compounds. Biochem J. 1961;81:365–374. doi: 10.1042/bj0810365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura S, Nakamura M, Yamazaki I, Morrison M. Reactions of ferryl lactoperoxidase (compound II) with sulfide and sulfhydryl compounds. J Biol Chem. 1984;259:7080–7085. [PubMed] [Google Scholar]
- 46.Palinkas Z, Furtmuller PG, Nagy A, Jakopitsch C, Pirker KF, Magierowski M, Jasnos K, Wallace JL, Obinger C, Nagy P. Interactions of hydrogen sulfide with myeloperoxidase. Br J Pharmacol. 2014;172:1516–1532. doi: 10.1111/bph.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pietri R, Roman-Morales E, Lopez-Garriga J. Hydrogen sulfide and hemeproteins: knowledge and mysteries. Antioxid Redox Signal. 2011;15:393–404. doi: 10.1089/ars.2010.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rios-Gonzalez BB, Roman-Morales EM, Pietri R, Lopez-Garriga J. Hydrogen sulfide activation in hemeproteins: the sulfheme scenario. J Inorg Biochem. 2014;133:78–86. doi: 10.1016/j.jinorgbio.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bibli S-I, Yang G, Zhou Z, Wang R, Topouzis S, Papapetropoulos A. Role of cGMP in hydrogen sulfide signaling. Nitric Oxide. 2015;46:7–13. doi: 10.1016/j.niox.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Cortese-Krott MM, Kuhnle GGC, Dyson A, Fernandez BO, Grman M, DuMond JF, Barrow MP, McLeod G, Nakagawa H, Ondrias K, Nagy P, King SB, Saavedra JE, Keefer LK, Singer M, Kelm M, Butler AR, Feelisch M. Key bioactive reaction products of the NO/H(2)S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci U S A. 2015;112:E4651–E4660. doi: 10.1073/pnas.1509277112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eberhardt M, Dux M, Namer B, Miljkovic J, Cordasic N, Will C, Kichko TI, de la Roche J, Fischer M, Suárez SA, Bikiel D, Dorsch K, Leffler A, Babes A, Lampert A, Lennerz JK, Jacobi J, Martí MA, Doctorovich F, Högestätt ED, Zygmunt PM, Ivanovic-Burmazovic I, Messlinger K, Reeh P, Filipovic MR. H(2)S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO–TRPA1–CGRP signalling pathway. Nat Commun. 2014;5:4381. doi: 10.1038/ncomms5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolluru GK, Shen X, Kevil CG. A tale of two gases: NO and H(2)S, foes or friends for life? Redox Biol. 2013;1:313–318. doi: 10.1016/j.redox.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller TW, Cherney MM, Lee AJ, Francoleon NE, Farmer PJ, King SB, Hobbs AJ, Miranda KM, Burstyn JN, Fukuto JM. The Effects of Nitroxyl (HNO) on Soluble Guanylate Cyclase Activity: Interaction at the ferous heme and cysteine thiols. J Biol Chem. 2009;284:21788–21796. doi: 10.1074/jbc.M109.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altaany Z, Ju Y, Yang G, Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal. 2014;7:ra87. doi: 10.1126/scisignal.2005478. [DOI] [PubMed] [Google Scholar]
- 55.Panopoulos P, Yang G, Asimakopoulou A, Topouzis S, Wang R, Szabo C, Papapetropoulos A. Selectivity of hydrogen sulfide towards cyclic nucleotide phosphodiesterases. Nitric Oxide. 2015;47:S39. [Google Scholar]
- 56.Bibli S-I, Andreadou I, Chatzianastasiou A, Tzimas C, Sanoudou D, Kranias E, Brouckaert P, Coletta C, Szabo C, Kremastinos DT, Iliodromitis EK, Papapetropoulos A. Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway. Cardiovasc Res. 2015;106:432–442. doi: 10.1093/cvr/cvv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Follmann M, Griebenow N, Hahn MG, Hartung I, Mais F-J, Mittendorf J, Schäfer M, Schirok H, Stasch J-P, Stoll F, Straub A. The Chemistry and Biology of Soluble Guanylate Cyclase Stimulators and Activators. Angew Chem Int Ed Engl. 2013;52:9442–9462. doi: 10.1002/anie.201302588. [DOI] [PubMed] [Google Scholar]
- 58.Stasch J-P, Pacher P, Evgenov OV. Soluble Guanylate Cyclase as an Emerging Therapeutic Target in Cardiopulmonary Disease. Circulation. 2011;123:2263–2273. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]