Abstract
Numerous compounds stimulate rodent β-cell proliferation; however, translating these findings to human β-cells remains a challenge. To examine human β-cell proliferation in response to such compounds, we developed a medium-throughput in vitro method of quantifying adult human β-cell proliferation markers. This method is based on high-content imaging of dispersed islet cells seeded in 384-well plates and automated cell counting that identifies fluorescently labeled β-cells with high specificity using both nuclear and cytoplasmic markers. β-Cells from each donor were assessed for their function and ability to enter the cell cycle by cotransduction with adenoviruses encoding cell cycle regulators cdk6 and cyclin D3. Using this approach, we tested 12 previously identified mitogens, including neurotransmitters, hormones, growth factors, and molecules, involved in adenosine and Tgf-1β signaling. Each compound was tested in a wide concentration range either in the presence of basal (5 mM) or high (11 mM) glucose. Treatment with the control compound harmine, a Dyrk1a inhibitor, led to a significant increase in Ki-67+ β-cells, whereas treatment with other compounds had limited to no effect on human β-cell proliferation. This new scalable approach reduces the time and effort required for sensitive and specific evaluation of human β-cell proliferation, thus allowing for increased testing of candidate human β-cell mitogens.
Keywords: human islet, β-cell proliferation
loss of pancreatic β-cells occurs in both type 1 diabetes mellitus, characterized by autoimmune destruction of β-cells, and type 2 diabetes mellitus in which β-cell dysfunction and deficiency occur in the context of peripheral insulin resistance. One strategy to restore β-cell mass has been focused on the discovery of molecules capable of inducing β-cell proliferation.
Studies of the human pancreas have found that β-cell proliferation peaks neonatally at ∼1–3% and then sharply declines after the first year of life eventually approaching near 0% and remains at this low level throughout adulthood (17, 21, 24, 27). The extremely low proliferation rate in adult human β-cells suggests that, even though these cells retain expression of factors required for cell cycle entry, the progression of the cell cycle is halted by cell cycle inhibitors (11, 14, 15). A recent study by Robitaille et al. identified p18 and p21 as cell cycle inhibitors required to maintain β-cell quiescence and demonstrated that silencing these inhibitors facilitates cell cycle entry in human β-cells (32). Furthermore, several studies have established that overexpressing cyclins and/or cyclin-dependent kinases (CDKs) can induce adult human β-cell proliferation and lead to an increased number of β-cells (13, 16, 37, 39). These findings suggest that factors or compounds capable of overcoming cell cycle inhibition could reactivate adult human β-cell replication.
The majority of studies focused on understanding and identifying regulators of β-cell regeneration have been performed in rodent models, and more recently some high-throughput screens have been used with the goal of finding potential human β-cell mitogens (1, 2, 34, 42, 45). Unfortunately, even though several factors that induce robust proliferation of rodent β-cells have been identified, including signaling proteins, hormones, growth factors, neurotransmitters, and small molecules, most have either not been tested on human β-cells or are unable to induce adult human β-cell proliferation (1, 2, 4, 10, 18–20, 23, 34, 45, 47). Because of species-specific differences in β-cell proliferative potential, it is critical that mitogenic factors identified in other systems be tested in human β-cells to establish their therapeutic potential (5, 12). Although some progress has been made in developing methods for culturing dispersed human islet cells for screening potential mitogens, several challenges remain. 1) Near negligible levels of basal human β-cell proliferation make it difficult to develop methods sensitive enough to detect changes in proliferation. Even at its peak neonatally, human β-cell proliferation only reaches ∼2%, and this physiological limit has been proposed as a maximum target for therapeutic interventions (44). However, identifying compounds able to stimulate β-cell proliferation from near zero to no more than 2% requires protocols with high sensitivity and specificity for the detection of proliferating β-cells. 2) Suitable human β-cell lines are not available, requiring the use of islets from human donors. However, the functional variability due to both intrinsic (e.g., genetics, race, age, body mass index) and extrinsic (e.g., isolation protocol, time in culture) factors makes it difficult to consistently reproduce results from a small sample size (22, 42). Previous work by our group and others has demonstrated the importance of prescreening human islets for their quality to minimize extrinsic differences between preparations, thereby maximizing experimental reproducibility (22, 25). 3) Confounding artifacts in islet cell culture, including close apposition of non-β-endocrine cells as well as highly proliferative fibroblasts, make it difficult to achieve the sensitivity required to automate analysis (2, 32, 33, 42).
To address these challenges, we developed a scalable method for testing potential adult human β-cell mitogens in vitro that 1) provides for quality control of human β-cells from different donors by minimizing extrinsic variability and increasing sensitivity, 2) increases specificity by using both cytoplasmic and nuclear markers for identification of proliferating β-cells, 3) automates both plate imaging and proliferation analysis, and 4) produces results comparable with the conventional method of manually counting proliferating β-cells. In this report the term “proliferation” is used advisedly to connote cells immunolabeled with markers of proliferation (i.e., Ki-67). We recognize that this marker is not a direct measure of proliferation as defined by an increase in β-cell number. Here our purpose was to develop a method to identify molecules and biologics that stimulate cell cycle entry in human β-cells and therefore merit further validation as candidate mitogens capable of inducing authentic human β-cell proliferation. This new screening system provides a robust and reliable framework for this type of evaluation of the mitogenic effects tested compounds have on primary human β-cells.
MATERIALS AND METHODS
Human islet purification and perifusion.
Human islets from 12 donors (9 male, 3 female) with an average age of 40 yr (range 19–53) and average body mass index of 31.0 (range 23.8–40.2) were obtained through the Integrated Islet Distribution Program (iidp.coh.org) and delivered in CMRL islet media (CMRL medium with 25% human serum albumin, 4.8 μM vitamin E, and 8.2 mM vitamin B3) or Standard Prodo Islet Media (Prodo Laboratories, Irvine, CA). To maximize islet quality, upon arrival islets were handpicked to near 100% purity. Islet function was assessed in a dynamic cell perifusion system for insulin secretory response to determine islet quality as previously described (12, 22) (Fig. 1A). Briefly, 60 purified size-matched islets were placed in the perifusion chamber at 37°C and sequentially perifused with 5 mM glucose, 16.7 mM glucose, and 16.7 mM glucose + 3-isobutyl,1-methylxanthine (IBMX) medium at a perifusate flow rate of 1 ml/min. Effluent was collected at 3-min intervals using an automatic fraction collector. Insulin concentration in each fraction was measured by radioimmunoassay (RI-13K; Millipore, Billerica, MA) (22).
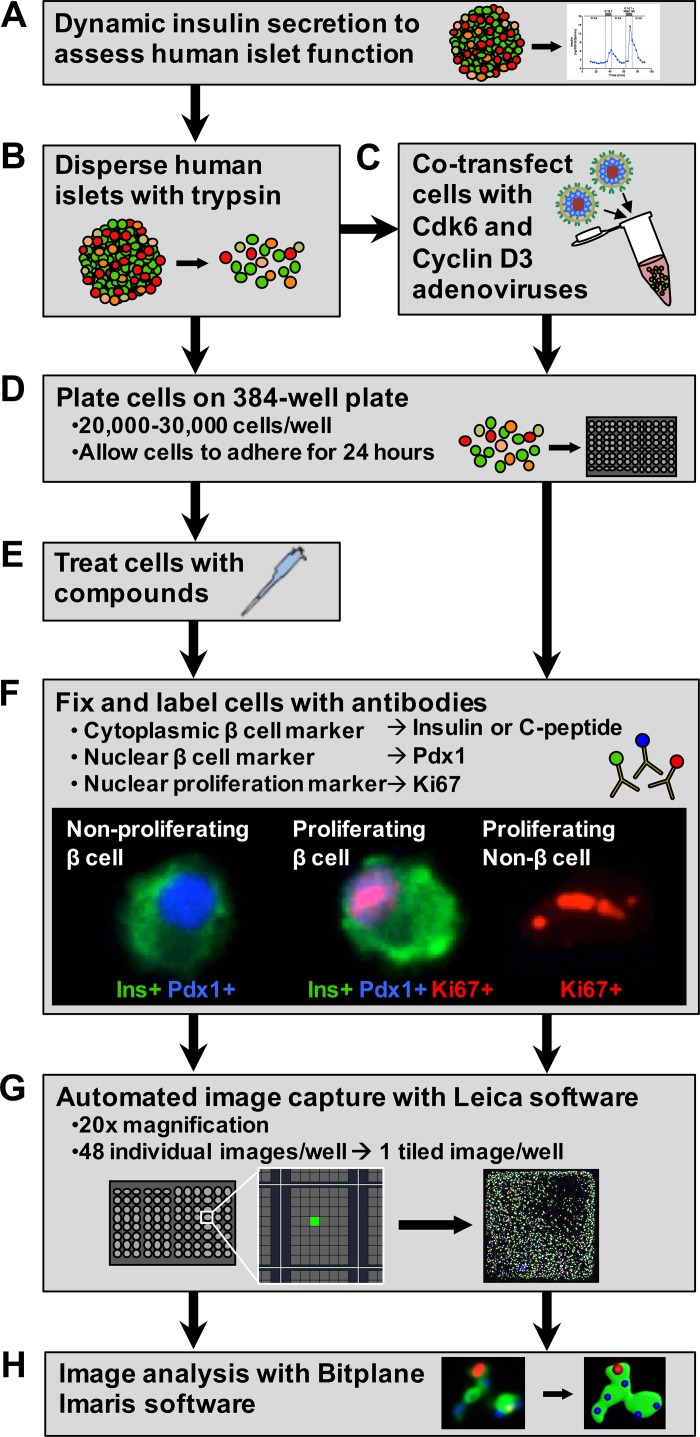
Fig. 1.
Workflow for semiautomated evaluation of human β-cell proliferation. A and B: human islets from each donor were evaluated for β-cell function (A) and then dispersed into a single cell suspension (B). C and D: an aliquot of dispersed cells from each donor was cotransfected with cdk6 and cyclin D3 adenoviruses to evaluate their proliferative potential (C) before being plated on 384-well collagen I-coated plates (D) to evaluate β-cell proliferative potential. E: nontransfected cells were also plated, allowed to adhere for 24 h, and then treated with compounds for 72 h. F: following the treatment period, all cells were fixed and labeled with antibodies to identify β-cells using both cytoplasmic (insulin or C-peptide) and nuclear (Pdx1) markers and to identify proliferating cells (Ki-67). G: cells were imaged using Leica LAS AF Matrix Developer software, which automates the capture of 48 images/well at ×20 magnification and tiles them to produce a single merged image of each well. H: these images were then analyzed for β-cell proliferation using Bitplane Imaris software.
Human islet dispersion, transduction, and culture.
Purified human islets were washed three times with 2 mM EDTA in 10 mM PBS and dispersed in a single cell suspension by incubating with 0.025% trypsin in 10 mM PBS at room temperature for 12–15 min with constant gentle pipetting (Fig. 1B). Trypsin was quenched with RPMI culture medium (RPMI medium with 10% FBS and 1% penicillin-streptomyocin) at basal glucose (5 mM), and then islet cells were washed two times with the same media. Cells were resuspended in RPMI culture medium and then counted using a hemocytometer. All subsequent transductions, plating, and compound treatments were performed at both basal (5 mM) and high (11 mM) glucose concentrations to determine whether glucose had an effect on β-cell proliferation at baseline or in response to cyclin D3 and cdk6 overexpression or treatment with potential β-cell mitogens.
As an additional quality control measure, before plating, an aliquot of islet cells from each donor was cotransduced with adenoviruses encoding cell cycle regulators cyclin D3 and cdk6 (100–250 multiplicity of infection) to induce cell cycling and evaluate the proliferative potential of β-cells from each donor (Fig. 1C). Cells were incubated with viruses in RPMI culture medium at both basal (5 mM) and high (11 mM) glucose levels without FBS for 1 h at 37°C. Adenoviruses were prepared as described previously (14). After 1 h, the transduction was terminated by adding RPMI culture medium.
Both transduced and nontransduced islet cells were then plated on collagen I-coated 384-well plates (BD Biosciences, San Jose, CA) at 20,000–30,000 cells/well (Fig. 1D). During initial method development, we tested other plate coatings, including poly-d-lysine and extracellular matrix from HTB-9 human bladder carcinoma cells and determined that there was no significant difference in β-cell attachment or proliferation between the coatings, as others have also noted (data not shown) (3, 32). Thus, moving forward we used commercially available collagen I-coated plates in all of our experiments. All plated cells were cultured at 37°C and allowed to adhere for 24 h before compound treatment.
Compound preparation and islet cell treatment.
Plated cells were exposed to the compounds listed in Table 1, with each treatment group repeated in triplicate or quadruplicate on islet preparations from three to six different donors (1, 4, 6–8, 10, 19, 23, 26, 28–31, 35, 38, 40, 41, 43, 46, 47). Compounds were prepared in RPMI culture medium at basal (5 mM) and high (11 mM) glucose from stock concentrations as described in Table 2. We used harmine as a control compound because recent work by Wang et. al. demonstrated that it stimulates human β-cell proliferation both in vitro and in vivo and leads to increased β-cell numbers, while avoiding both DNA damage and β-cell death (43). After islet cells had adhered for 24 h, the culture medium was replaced with medium containing compounds or controls, and cells remained in culture for another 72 h (Fig. 1E). RPMI culture medium without compounds at basal (5 mM) and high (11 mM) glucose was used to treat both control and transduced islet cells, with vehicle controls prepared at dilutions equal to those of their most concentrated respective compound solution (see Table 2).
Table 1.
Function and reported effects of compounds tested on human islet cells
| Compound | Function | Reported Effects | Ref. No.* |
|---|---|---|---|
| DYRK family | |||
| Harmine | Dyrk1a inhibitor | Increases β-cell proliferation (mouse, rat, human) | 31, 43 |
| Decreases β-cell development (mouse) | |||
| Neurotransmitters | |||
| GABA | Inhibitory neurotransmitter | Increases β-cell proliferation (mouse, human) | 30, 35, 38 |
| Serotonin | Monoamine neurotransmitter | Regulates pregnancy-related β-cell expansion (mouse) | 23 |
| Adenosine signaling/metabolism | |||
| NECA | Adenosine receptor agonist | Increases β-cell proliferation (zebrafish, mouse) | 1 |
| UK-432097 | Selective A2A adenosine receptor agonist | Decreases inflammation and promotes wound healing | 26, 46 |
| A-134974 | Selective adenosine kinase inhibitor | Increases β-cell proliferation (zebrafish) | 1 |
| Hormones/growth factors | |||
| Prolactin | Lactogenic hormone | Regulates pregnancy-related β-cell expansion (rat, mouse) | 4, 19 |
| PDGF | Cell growth and division | Regulates age-dependent β-cell proliferation (mouse, human) | 8 |
| Erythropoietin | Erythropoiesis and angiogenesis | Increases β-cell proliferation (mouse) | 10 |
| Exendin-4 | GLP-1R agonist | Regulates β-cell mass (rat) | 29, 40, 47 |
| Increases β-cell proliferation (rat) | |||
| TGF-β superfamily | |||
| Myostatin | Growth differentiation factor | Regulates islet development and insulin sensitivity (mouse) | 6 |
| Activin A | Signaling and regulation of reproduction, development, and homeostasis | Regulates islet development and insulin secretion (mouse) | 6, 7 |
| Increases β-cell proliferation (rat) |
GABA, γ-aminobutyric acid; PDGF, platelet-derived growth factor; NECA, 5′-N-ethylcarboxamidoadenosine; GLP-1R glucagon-like peptide-1 receptor.
Because of space limitations, only selected publications evaluating these compounds are noted.
Table 2.
Preparation of compounds tested on human islet cells
| Compound | Stock Concentration | Solvent | Control Dilution | Concentrations Tested | Source | Catalog No. |
|---|---|---|---|---|---|---|
| Dyrk family | ||||||
| Harmine | 100 mM | DMSO | 1:100 | 1, 10 μM | Sigma | 286044 |
| Neurotransmitters | ||||||
| GABA | 100 mM | PBS | 1:20 | 100, 1,000, 2,500 μM | Sigma | A2129 |
| Serotonin | 10 mM | Water | 1:40 | 10, 100, 250 μM | Sigma | L510041 |
| Adenosine signaling/metabolism | ||||||
| NECA | 40 mM | DMSO | 1:100 | 1, 10, 100 μM | Tocaris | 1691 |
| UK-432097 | 1 mM | DMSO | 1:100 | 0.1, 1, 10 μM | Axon Medchem | 1193 |
| A-134974 | 10 mM | Water | 1:40 | 0.1, 1, 10 μM | Sigma | A2846 |
| Hormones/growth factors | ||||||
| Human prolactin | 0.1 mg/ml | 4 mM HCl/0.1% BSA | 1:40 | 100, 1,000, 2,500 ng/ml | R&D Systems | 682-PL-050 |
| PDGF | 0.025 mg/ml | PBS | 1:20 | 50, 500, 1,250 ng/ml | Sigma | P3076 |
| Erythropoietin | 500 U/ml | PBS/0.1% BSA | 1:20 | 1, 10, 20 U/ml | R&D Systems | 287-TC-500 |
| Exendin-4 | 10 μM | PBS | 1:20 | 10 nM | California Peptide | 507–77 |
| TGF-β superfamily | ||||||
| Myostatin | 100 μg/ml | 4 mM HCl/0.1% BSA | 1:40 | 500, 1,000, 2,500 ng/ml | R&D Systems | 788-G8-010 |
| Activin A | 50 μg/ml | PBS/0.1% BSA | 1:20 | 10, 100, 1,000 ng/ml | R&D Systems | 338-AC-010 |
Immunocytochemistry.
Following treatment, the culture medium was removed, and wells were rinsed one time with 10 mM PBS before cells were fixed with paraformaldehyde and immunolabeled for β-cell and proliferation markers directly in the wells (Fig. 1F). Cells were fixed with fresh 4% paraformaldehyde in 10 mM PBS for 30 min at 4°C and then washed with 10 mM PBS for 20 min at room temperature before permeabilization with 0.2% Triton X-100 in 10 mM PBS for 15 min at room temperature. Wells were then blocked with 5% normal donkey serum for 30 min at room temperature before labeling.
Dispersed endocrine cells in culture form dense monolayer clusters, making it difficult to unambiguously assign a cytoplasmic stain to a specific nucleus in the cluster (2). Another aspect of human islet cell culture that can complicate accurate β-cell identification is the presence of rapidly proliferating fibroblastic cells, which are often closely associated with endocrine cell clusters (2, 42). Because baseline proliferation of adult human β-cells is so low, misidentification of even a small percentage of these non-β-cells will confuse the results and make it difficult to detect differences in β-cell proliferation caused by compounds of interest. It is also possible that dedifferentiation or loss of β-cell identity may occur during culture, since some β-cell markers are not stable in culture (33). To minimize errors from these confounding artifacts of islet cell culture, we used both cytoplasmic insulin (1:500; Dako, Cambridge, MA) or C-peptide (1:100; Developmental Studies Hybridoma Bank, Iowa City, IA) and nuclear Pdx1 (1:10,000; gift from Dr. Chris Wright, Vanderbilt University, Nashville, TN) to identify β-cells for quantification in addition to Ki-67 (1:500; Abcam, Cambridge, UK) as a proliferation marker (Fig. 1F). To avoid confusion, in the remainder of the text, we only use insulin (rather than insulin or C-peptide) to refer to the β-cell-specific cytoplasmic marker used.
Following permeabilization, cells were labeled with primary antibodies (insulin or C-peptide, Pdx1, and Ki-67) prepared in fresh antibody buffer (10 mM PBS with 1% BSA and 0.1% Triton X-100) overnight at 4°C. Cells were washed with 0.1% Triton X-100 in 10 mM PBS for 30 min and then labeled with appropriate secondary antibodies (Jackson Immunoresearch, West Grove, PA) prepared in fresh antibody buffer for 1 h at room temperature. Cells were labeled with DAPI (1:50,000) for 10 min and washed with 0.1% Triton X-100 in 10 mM PBS for 15 min followed by a 15-min wash in 10 mM PBS all at room temperature. Fluoroshield Mounting Medium (Abcam) was added to each well, and the plates were either immediately imaged or stored at 4°C protected from light and dehydration.
Automated image acquisition.
Images were acquired with a Leica DMI 6000B fluorescence microscope equipped with a Leica DFC360FX digital camera, XY scanning stage, and Z autofocus function (Leica Microsystems, Wetzlar, Germany). To automate plate imaging, we set up a template matrix using the Multiple Mosaics application in the LAS AF MATRIX M3 Developer Suite (Leica Microsystems). This matrix was made up of one image mosaic per well that encompassed the entire area of the well. Each mosaic captured 48 images at ×20 magnification using autofocus to find the focal plane in every field and tiled those images to produce a single merged image of each well (Fig. 1G). Once established, this template matrix enabled the automation of this process, which yielded highly resolved images that were exported for analysis.
Automated image analysis.
In our protocol, β-cell proliferation was defined as the percent of proliferating β-cells (insulin+Pdx1+Ki-67+) divided by total β-cells (insulin+Pdx1+). An average of 3,500 total β-cells (range 1,000–16,100) were counted per donor for each treatment condition. Prior approaches have quantified proliferating and total β-cells by manually counting β-cells in each image (43). We were able to automate this process using Surfaces and Spots functions in Imaris 7.6 software (Bitplane, Zurich, Switzerland) to identify β-cells by both cytoplasmic insulin and nuclear Pdx1, and proliferating cells by nuclear Ki-67 (Fig. 1H).
After loading an image in the Imaris software (Fig. 2A), we first defined the insulin+ area using the Surfaces function (Fig. 2B). The Spots function was then used to identify Pdx1+ nuclei. These Pdx1+ spots were then filtered by first masking the Pdx1 channel on the insulin surface created previously; second, creating spots on the masked Pdx1 channel; and finally, identifying the original Pdx1+ spots that colocalized with the masked Pdx1+ spots using the Colocalize Spots tool. These insulin+Pdx1+ spots represented the number of total β-cells in the well (Fig. 2C). This algorithm takes into account cases where the insulin signal at the nucleus is too weak to fall within the insulin+ Surface threshold, and rather than filling in the nuclear area with insulin+ Surface, the algorithm requires at least some insulin+ Surface overlap with Pdx1 staining for the cell to be counted as a β-cell, thus preventing surrounding insulin-negative cells from being falsely labeled as β-cells. Next, proliferating Ki-67+ nuclei were identified using the Spots function and colocalized with the insulin+Pdx1+ spots defined in the previous step using the Colocalize Spots tool. These insulin+Pdx1+Ki-67+ spots then represented the number of proliferating β-cells in the well (Fig. 2D). The parameters and thresholding for the Surfaces and Spots functions were guided by markup images throughout the process. These markups allowed us to visually determine whether we were identifying the cells in the image properly and ensure we did not under- or overestimate the Surfaces or Spots throughout the thresholding process. To guarantee a consistent and unbiased process, each marker/channel (insulin, Pdx1, Ki-67) was independently thresholded.
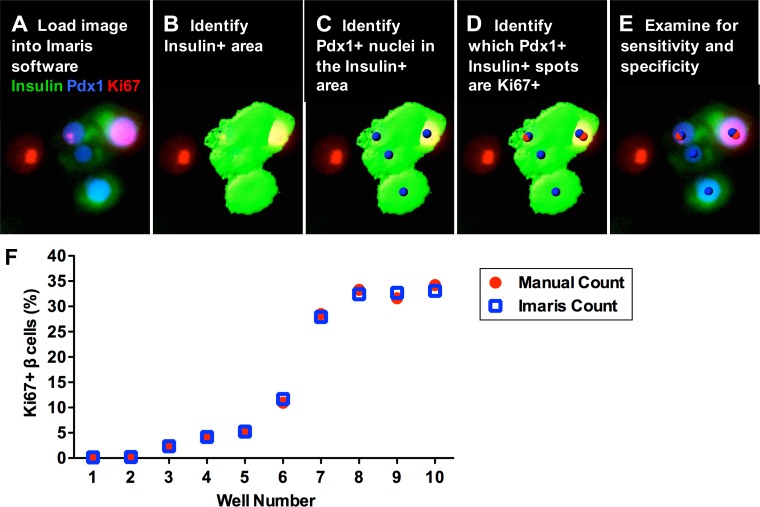
Fig. 2.
Validation of the Imaris analysis method for quantifying human β-cell proliferation. A and B: images were loaded in Imaris software (A), and then insulin+ area was identified using the Surfaces function (B). C: the Spots function was then used to identify Pdx1+ nuclei that colocalized with Pdx1+ staining masked by the insulin+ surface. These Pdx1+insulin+ spots represent the total number of β-cells in the well. D: Ki-67+ nuclei were then identified using the Spots function and colocalized with the Pdx1+insulin+ spots to determine the number of Pdx1+insulin+Ki-67+ proliferating β-cells. E: the image with Surface and Spot markups was then examined for sensitivity and specificity of the analysis. F: quantification of human β-cell proliferation using Imaris software was validated using images from 10 different wells with varying β-cell proliferation rates due to different levels of cyclin D3 and cdk6 [wells 1 and 2, no transduction; wells 3-10, transduced with 50–250 multiplicity of infection (MOI)] plated at basal (5 mM) glucose. Each well was evaluated by both manual counting and the Imaris algorithm (A-E) to determine the number of proliferating β-cells. The average coefficient of variation between manual and Imaris counts for each well was 3.2% (range 0.6–10.7%), indicating that there is no significant difference between manual and Imaris analysis methods.
Once the parameters and thresholding for the Surfaces and Spots functions had been established, the Batch Coordinator was used to process all images from the plate. Following analysis, images with Surface and Spot markups were examined for sensitivity and specificity and to ensure that no staining artifacts interfered with the analysis (Fig. 2E). For example, low sensitivity indicated that the thresholds for the Surfaces and/or Spots functions were set too high to detect all β-cells, and low specificity indicated that thresholds were set too low and were either misidentifying background staining or, in the case of the Surfaces function, were bleeding into unstained areas and incorrectly identifying non-β-cells as β-cells. If necessary, parameters and thresholds were adjusted, and images were reanalyzed.
RESULTS
Automated image analysis accurately measures human β-cell proliferation.
Before using this method to evaluate the effect of potential mitogens on β-cell proliferation, we sought to determine whether quantification of human adult β-cell proliferation markers using Imaris software was comparable to the conventional method of manually counting β-cells over a wide range of proliferation indexes. We performed this analysis validation using images from 10 different wells with varying levels of human adult β-cell proliferation, including untreated samples with extremely low levels of baseline proliferation to samples with a proliferation index over 30%, which was achieved by inducing different levels of cyclin D3 and cdk6 expression in dispersed islet cells. Each well was evaluated for β-cell proliferation markers both by manually counting each β-cell and by using the Imaris algorithm (Fig.2, A–E) to count total and proliferating β-cells (Fig. 2F). The average coefficient of variation between manual and Imaris counts for each well was 3.2 ± 1.0%, indicating no significant difference between manual and automated analysis methods and demonstrating that adult human β-cell proliferation can be accurately measured throughout a range of proliferation levels using this automated protocol.
Furthermore, to demonstrate a reduction in artifacts and errors in β-cell identification using our method, β-cells were identified in individual wells by the Imaris algorithm using both double insulin/Pdx1 and single insulin labeling (with DAPI as a nonspecific nuclear marker), and resulting cell counts were compared with those where β-cells were identified by manual counting. Double insulin/Pdx1 labeling led to significantly fewer false positives compared with the method using insulin labeling alone (7.7 ± 1.9 vs. 32.4 ± 2.2%, P < 0.01, n = 3 donors) while there was no significant difference in the number of false negatives between the two approaches (12.5 ± 3.4 vs. 13.7 ± 1.0%, P = 0.75, n = 3 donors). Therefore, the probability of β-cells being correctly identified by our double labeling method is 92.3 ± 1.9% compared with only 67.6 ± 2.2% using traditional single insulin labeling (P < 0.01, n = 3 donors).
Human β-cells in purified islet preparations are functional and demonstrate proliferative potential.
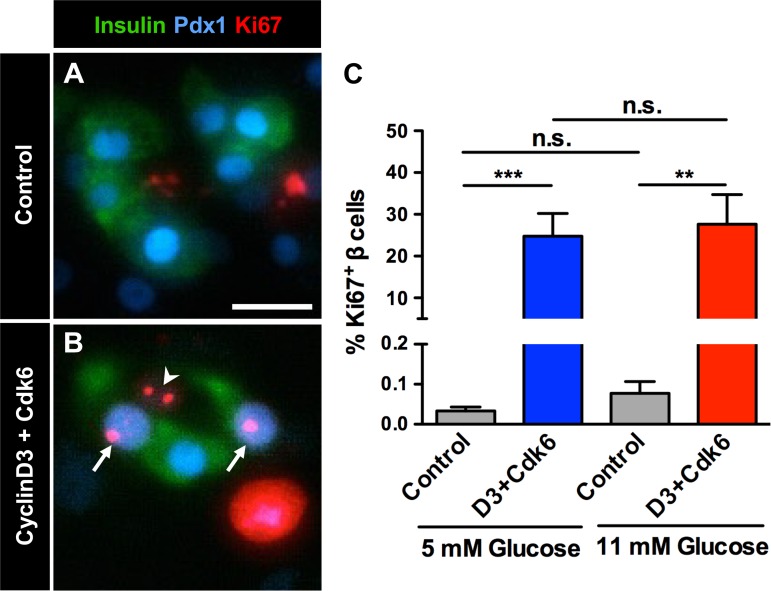
To minimize the effects of variability between human islet donors, all human islets were purified by handpicking and evaluated for β-cell function in a dynamic cell perifusion system (22). Human islet preparations used to test compounds were examined in a cell perifusion system and had normal basal insulin secretion at 5.6 mM glucose and an elevated insulin secretory response when stimulated with either 16.7 mM glucose (4.8 ± 1.2-fold above baseline) or 16.7 mM glucose + IBMX (10.8 ± 2.4-fold above baseline). Cell cycling was induced in dispersed islet cells from all donors by cotransduction with adenoviruses expressing cyclin D3 and cdk6, which significantly increased human β-cell proliferation at basal (5 mM) and high (11 mM) glucose (Fig. 3). Baseline β-cell proliferation at basal (5 mM) glucose was 0.03 ± 0.01%, which is comparable to reported proliferation indexes of adult human β-cells from autopsy samples, and increased to 24.5 ± 5.5% with transduction (Fig. 3C) (17, 21, 24, 27). Glucose concentration had no effect on human β-cell proliferation of either control or transduced β-cells (Fig. 3C).
Fig. 3.
Adenoviral expression of cyclin D3 and cdk6 induces human β-cell proliferation. Dispersed islet cells from all human donors were plated following dispersion in basal (5 mM) or high (11 mM) glucose. An aliquot of cells at each glucose level was cotransduced with adenoviruses encoding cell cycle regulators cyclin D3 and cdk6 (D3+Cdk6) before plating (100–250 MOI). A and B: labeling for cytoplasmic β-cell marker insulin (Ins, green), nuclear β-cell marker Pdx1 (blue), and proliferation marker Ki-67 (red) in control (A) and transduced (B) cells at 5 mM glucose. Arrows mark proliferating β-cells, and arrowhead marks a proliferating non-β-cell. Scale bar in A represents 20 μm and also applies to B. C: adenoviral expression of cyclin D3 and cdk6 induces human β-cell proliferation in cells from all donors at both basal (5 mM) and high (11 mM) glucose; n = 6–9 donors/treatment. **P < 0.01, 5 mM glucose control vs. D3+Cdk6. ***P < 0.001, 11 mM glucose control vs. D3+Cdk6. Comparisons between controls or transfected cells at 5 vs. 11 mM glucose were not statistically significant (ns).
Evaluation of potential adult human β-cell mitogens.
After validating the accuracy of our proliferation analysis, we wanted to determine whether this method could be used to effectively evaluate candidate compounds for their potential to stimulate cell cycle entry in human β-cells. We tested 13 compounds implicated in β-cell mass regulation or β-cell proliferation, including neurotransmitters, growth factors, hormones, proteins, and small molecules that modulate different signaling pathways (DYRK family, TGF-β superfamily, adenosine kinase pathway) (Table 1). All of these compounds were identified as stimuli of β-cell proliferation primarily in rodent or zebrafish models, but three of them, harmine, γ-aminobutyric acid (GABA), and platelet-derived growth factor (PDGF), had also been evaluated in human β-cells (8, 43).
Human islet cells were treated with these potential human β-cell mitogens at a range of concentrations at both basal (5 mM) and high (11 mM) glucose for a total of 66 different treatment conditions, each tested on islet cells from three to six different donors (see Table 2; Fig. 4). For these studies we obtained an average of 1,563 ± 325 islet cells/human islet, therefore requiring ∼13 human islets/well or 5,000 human islets/384-well plate to achieve a density of 20,000 islet cells/well. A previous study that seeded 8,000 islet cells/well found that they were only able to quantify 120 β-cells/well (1.2% of total islet cells plated/well), limiting their ability to detect small changes in β-cell proliferation (42). However, by plating at a higher density, we were able to quantify 1,235 ± 25 β-cells/well, representing 7.1 ± 1.2% of total islet cells plated/well, thereby allowing for more sensitivity in detecting small changes in human β-cell proliferation.
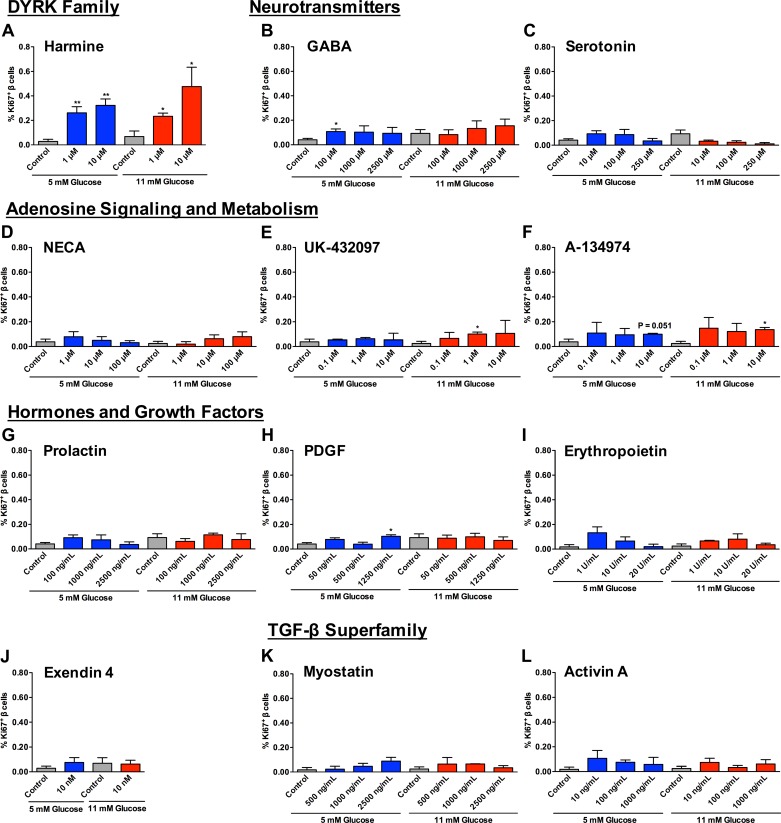
Fig. 4.
Human β-cell proliferation is induced by treatment with some, but not all, compounds tested. A: treatment of human islet cells with harmine at 1 and 10 μM in 5 or 11 mM glucose increased β-cell proliferation. **P < 0.01, 5 mM glucose control vs. 1 μM harmine or 10 μM harmine. *P < 0.01, 5 mM glucose control vs. 1 μM harmine or 10 μM harmine. B and C: neurotransmitters GABA (B) and serotonin (C) had a limited effect on β-cell proliferation, and only treatment with 100 μM GABA at 5 mM glucose was statistically significant *P < 0.05, 5 mM glucose control vs. 100 μM GABA. D–F: compounds involved in adenosine signaling and metabolism [NECA (D), UK-432097 (E), and A-134974 (F)] had a modest effect, with 1 μM UK-432097 and 10 μM A-134974 at 11 mM glucose causing a small but statistically significant increase in β-cell proliferation. *P < 0.05, 11 mM glucose control vs. 1 μM UK-432097 and control vs. 10 μM A-134974. G–J: hormones prolactin (G), PDGF (H), erythropoietin (I), and exendin-4 (J) had no effect on β-cell proliferation other than 1,250 ng/ml PDGF at 5 mM glucose, which caused a small but significant increase in proliferation. *P < 0.05, 5 mM glucose control vs. 1,250 ng/ml PDGF. K and L: treatment with members of the TGF-β superfamily myostatin (K) and activin A (L) had no significant effect on β-cell proliferation. Comparisons between controls or compound treatments at 5 vs. 11 mM glucose were not statistically significant in any compound or control tested, and there was no difference between vehicle controls (P = 0.13–0.62); n = 3–6 donors/treatment condition.
Treatment of human islet cells with the control compound harmine caused an increase in β-cell proliferation at 1 and 10 μM in both glucose concentrations (Fig. 4A). In fact, harmine was the only compound that significantly increased β-cell proliferation across all conditions tested. GABA, PDGF, UK-432097, and A-134974 each demonstrated a limited increase in β-cell proliferation in a single condition (Fig. 4, B, E, F, and H). Other compounds tested, including serotonin, myostatin, activin A, 5′-N-ethylcarboxamidoadenosine (NECA), prolactin, erythropoietin, and exendin-4, had minimal to no effect on human β-cell proliferation (Fig. 4). Glucose level did not have a significant effect on human β-cell proliferation in any treatment or control tested, and there was no significant difference between vehicle controls (Fig. 4).
DISCUSSION
As efforts to identify molecules that stimulate human β-cell proliferation and regeneration have increased over the last several years, the difficulty of translating findings from animal models to humans has highlighted the need for effective strategies to test new candidates in human systems. We developed a semiautomated method for evaluating adult human β-cell proliferation in vitro that provides a platform for testing proposed human β-cell mitogens and addresses some of the ongoing challenges of working with human islets in culture.
While working with primary human islets is an important step in translating findings from animal models into humans, variability between islet preparations due to both intrinsic and extrinsic factors can prove confounding (22, 25, 36). A previous study that measured human β-cell proliferation in vitro noted low reproducibility across donors, suggesting that these variations may influence the ability of a given islet preparation to respond to test compounds (42). Previous work by our group demonstrated the importance of prescreening human islet preparations for their quality to minimize extrinsic variability (22). In designing this screening method, we worked to minimize variability between islet preparations as much as possible based on these previous studies by our lab and others. In addition to performing functional assessment of human islets, as a measure of their quality, we handpicked all islets upon arrival to homogeneity to minimize the effect of impurities in culture and studied each treatment condition across multiple donors rather than across wells plated from the same donor. Because adult human β-cell proliferation was the parameter we were evaluating and baseline proliferation is low or negligible, in addition to assessing dynamic insulin secretion, we also assessed the proliferative potential of β-cells from each islet preparation by overexpressing cyclin D3 and cdk6 with adenovirusus, which induces cell cycling and has been shown to increase human β-cell numbers (39). Using this positive control, we demonstrated that β-cells from all islet preparations we used were capable of entering the cell cycle. Although variation between samples is an expected feature of primary cell preparations, by employing these quality control measures we ensured that the β-cells used to evaluate candidate molecules were functional and able to proliferate and that the candidate molecules tested were capable of promoting β-cell proliferation in multiple islet preparations from different donors to be identified as a β-cell mitogen.
We demonstrated the usefulness of our method in evaluating potential adult human β-cell mitogens by performing a screen of 13 compounds at different concentrations in the presence of basal and high glucose (66 treatment conditions). We wanted to ensure that we could detect human β-cell proliferation in response to treatment in addition to forced overexpression of cell cycle regulators, but, because no definitive adult human β-cell mitogen has been identified, we selected compounds to test based on their ability in previous studies to promote β-cell proliferation or regulate β-cell mass. Most of these compounds had only been tested previously in animal models, but three (harmine, GABA, PDGF) had been evaluated in human systems (8, 30, 43). Harmine has been shown to increase human β-cell numbers while avoiding DNA damage and β-cell death (43). Our screening system also demonstrated that harmine increases adult human β-cell proliferation. The degree of effect we observed was marginally less than that observed in the previous study, most likely due to the high degree of variability between islet preparations and our increased level of stringency in terms of β-cell detection using two markers instead of one, thereby minimizing false positives. The other compounds tested had limited to no effect. One reason human β-cells may fail to respond to these confirmed rodent β-cell mitogens is difference in receptor expression between rodents and humans, exemplified by a recent study showing that human β-cells lack prolactin receptors (9). Some of the compounds (GABA, PDGF, UK-432097, and A-134974) demonstrated marginal increases in β-cell proliferation in certain conditions that proved to be statistically significant; however, it is unclear whether these results are biologically meaningful. Because human β-cell proliferation peaks at around 2% during development, this proliferation rate has been proposed as a therapeutic target for drug development (44). Because of differences between human islet cells in vivo and in culture using various systems, in addition to inherent variability between donors, further studies will be needed to determine what level of proliferation achieved using our method should be set as a threshold for deciding which compounds should be developed further.
Use of an adult human islet cell culture system to assess human β-cell proliferation makes it possible to scale for rapid assessment of multiple compounds at different concentrations. However, as in any in vitro system, we acknowledge that some mitogenic agents may work through mechanisms not recreated in culture. The effects that substrate and the instability of β-cell markers have on the responsiveness of human β-cells to mitogenic stimuli in culture vs. in vivo are unknown, although we, like others, noticed no difference in proliferation index across the matrix substrates we tested (3, 32, 33). Dispersed human islet cells form a heterogeneous monolayer culture consisting of tight clusters of endocrine cells and rapidly proliferating fibroblastic cells. The close spatial association of these different cell types can make it extremely difficult to definitively identify β-cells using only a cytoplasmic marker and cause misidentification of proliferative fibroblastic cells as β-cells (2, 32, 42). These challenges become especially pronounced when automating image analysis and can lead to an artificial elevation of β-cell proliferation index, which may either mask the effect a treatment has on β-cell proliferation or incorrectly attribute an increase in proliferating non-β-cells to β-cells. Prior studies highlighting the difficulties in dealing with these artifacts of primary human islet cell culture guided our adoption of a dual-staining approach to allow for automation of the process while minimizing artifact by reducing errors in β-cell quantification. We used both cytoplasmic (insulin or C-peptide) and nuclear (Pdx1) markers to label β-cells and examined each image markup after analysis for any artifacts to ensure the high sensitivity and specificity of the algorithm. Because δ-cells also express Pdx1, cells that are Pdx1 positive and hormone negative could represent either β-cells that have lost insulin expression or δ-cells that have lost somatostatin expression. Although this dual-staining approach is unable to account for insulin-negative β-cells, the strength of this approach is that it successfully minimizes the misidentification of non-β-cells as β-cells. We validated our automated analysis by demonstrating that β-cell proliferation quantified by the algorithm was comparable to manually counting each β-cell to determine proliferation index.
Another limitation of studying human β-cell proliferation in vitro is the requirement of using surrogate proliferation markers because it is not feasible to definitively measure an increase in cell number due to the low replication rate in these cells. We used Ki-67 to identify proliferating cells in our culture system, which is expressed in cells that have entered the cell cycle; however, the method we describe here can also be adapted to substitute other proliferation markers (e.g., bromodeoxyuridine) (32). While it would be ideal to be able to directly identify an increase in human β-cell number, the purpose of this study was to develop a method to screen for molecules that may induce proliferation and which therefore merit further validation as mitogenic agonists.
High-throughput screening platforms are being developed, with exciting potential to drive the discovery of β-cell mitogens and cell cycle regulators for targeting (1, 2, 32, 42). Confirming discoveries made using these platforms in primary adult human β-cells remains critical to determine which compounds have therapeutic potential in human diabetes. We were able to reduce the effects of variation between islet preparations and developed an automated and scalable method for evaluating adult human β-cell proliferation in vitro. This method provides sufficient sensitivity to detect changes in human β-cell proliferation, which occur on a much smaller scale than in other organisms, and reliably detects changes in β-cell proliferation across multiple human islet preparations, which allows for timely and efficient evaluation of hundreds of compounds at multiple concentrations and/or in combination moving forward.
GRANTS
This work was supported by grants from the Department of Veterans Affairs (BX000666), the National Institute of Diabetes and Digestive and Kidney Diseases (DK72473, DK89572, DK89538, DK92758, DK97829, DK94199, DK104211, DK66636, DK63439, F30-DK097921, DK055023), the National Institute of General Medical Sciences (T32 GM07347), the Juvenile Diabetes Research Foundation (17-2013-321, 17-2013-324, 17-2012-36, 1-2011-603), the American Diabetes Association (7-12-BS-046), and by the Vanderbilt Diabetes Research and Training Center (DK-20593; Islet Procurement and Analysis and Hormone Assay and Analytical Services Cores).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.I.A., R.A., N.F.-T., P.W., A.F.S., M.B., and A.C.P. conception and design of research; K.I.A. and M.B. performed experiments; K.I.A. and J.B. analyzed data; K.I.A., R.A., M.B., and A.C.P. interpreted results of experiments; K.I.A. prepared figures; K.I.A. drafted manuscript; K.I.A., R.A., N.F.-T., A.F.S., M.B., and A.C.P. edited and revised manuscript; K.I.A., R.A., J.B., N.F.-T., P.W., A.F.S., M.B., and A.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Didier Stainier (Max Planck Institute for Heart and Lung Research) and Olov Andersson (Karolinska Institutet) for helpful discussions and collaboration regarding adenosine signaling compounds. We also thank Dr. Chris Wright (Vanderbilt University) for the generous gift of the Pdx1 antibody used in these studies.
REFERENCES
- 1.Andersson O, Adams BA, Yoo D, Ellis GC, Gut P, Anderson RM, German MS, Stainier DYR. Adenosine signaling promotes regeneration of pancreatic β cells in vivo. Cell Metab 15: 885–894, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annes JP, Ryu JH, Lam K, Carolan PJ, Utz K, Hollister-Lock J, Arvanites AC, Rubin LL, Weir G, Melton DA. Adenosine kinase inhibition selectively promotes rodent and porcine islet β-cell replication. Proc Natl Acad Sci USA 109: 3915–3920, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beattie GM, Cirulli V, Lopez AD, Hayek A. Ex vivo expansion of human pancreatic endocrine cells. J Clin Endocrinol Metab 82: 1852–1856, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Brelje TC, Stout LE, Bhagroo NV, Sorenson RL. Distinctive roles for prolactin and growth hormone in the activation of signal transducer and activator of transcription 5 in pancreatic islets of langerhans. Endocrinology 145: 4162–4175, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53: 1087–1097, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Brown ML, Schneyer AL. Emerging roles for the TGFbeta family in pancreatic beta-cell homeostasis. Trends Endocrinol Metab 21: 441–448, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brun T, Franklin I, St-Onge L, Biason-Lauber A, Schoenle EJ, Wollheim CB, Gauthier BR. The diabetes-linked transcription factor PAX4 promotes β-cell proliferation and survival in rat and human islets. J Cell Biol 167: 1123–1135, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, Schorle H, Sage J, Kim SK. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature 478: 349–355, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Kleinberger JW, Takane KK, Salim F, Fiaschi-Taesch N, Pappas K, Parsons R, Jiang J, Zhang Y, Liu H, Wang P, Bender AS, Frank SJ, Stewart AF. Augmented Stat5 Signaling Bypasses Multiple Impediments to Lactogen-Mediated Proliferation in Human β-Cells. Diabetes 64: 3784–3797, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi D, Schroer SA, Lu SY, Wang L, Wu X, Liu Y, Zhang Y, Gaisano HY, Wagner KU, Wu H, Retnakaran R, Woo M. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J Exp Med 207: 2831–2842, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocaña A, Vasavada R, Stewart AF. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev 27: 356–370, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Dai C, Brissova M, Hang Y, Thompson C, Poffenberger G, Shostak A, Chen Z, Stein R, Powers AC. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia 55: 707–718, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, Harb G, Selk K, Cozar-Castellano I, Stewart AF. Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes 58: 882–893, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiaschi-Taesch NM, Kleinberger JW, Salim FG, Troxell R, Wills R, Tanwir M, Casinelli G, Cox AE, Takane KK, Scott DK, Stewart AF. Human pancreatic β-cell G1/S molecule cell cycle atlas. Diabetes 62: 2450–2459, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiaschi-Taesch NM, Kleinberger JW, Salim FG, Troxell R, Wills R, Tanwir M, Casinelli G, Cox AE, Takane KK, Srinivas H, Scott DK, Stewart AF. Cytoplasmic-nuclear trafficking of G1/S cell cycle molecules and adult human β-cell replication: a revised model of human β-cell G1/S control. Diabetes 62: 2460–2470, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiaschi-Taesch NM, Salim F, Kleinberger J, Troxell R, Cozar-Castellano I, Selk K, Cherok E, Takane KK, Scott DK, Stewart AF. Induction of human beta-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes 59: 1926–1936, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 97: 3197–3206, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guthalu Kondegowda N, Joshi-Gokhale S, Harb G, Williams K, Zhang XY, Takane KK, Zhang P, Scott DK, Stewart AF, Garcia-Ocaña A, Vasavada RC. Parathyroid hormone-related protein enhances human β-cell proliferation and function with associated induction of cyclin-dependent kinase 2 and cyclin E expression. Diabetes 59: 3131–3138, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 150: 1618–1626, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Jiao Y, Le Lay J, Yu M, Naji A, Kaestner KH. Elevated mouse hepatic betatrophin expression does not increase human β-cell replication in the transplant setting. Diabetes 63: 1283–1288, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49: 1325–1333, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Kayton NS, Poffenberger G, Henske J, Dai C, Thompson C, Aramandla R, Shostak A, Nicholson W, Brissova M, Bush WS, Powers AC. Human islet preparations distributed for research exhibit a variety of insulin-secretory profiles. Am J Physiol Endocrinol Metab 308: E592–E602, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, Yang K, Honig G, van der Hart M, Kishimoto N, Wang J, Yagihashi S, Tecott LH, Watada H, German MS. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 16: 804–808, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köhler CU, Olewinski M, Tannapfel A, Schmidt WE, Fritsch H, Meier JJ. Cell cycle control of β-cell replication in the prenatal and postnatal human pancreas. Am J Physiol Endocrinol Metab 300: E221–E230, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Lyon J, Manning Fox JE, Spigelman AF, Kim R, Smith N, O'Gorman D, Kin T, Shapiro AMJ, Rajotte RV, MacDonald PE. Research-focused isolation of human islets from donors with and without diabetes at the Alberta Diabetes Institute IsletCore. Endocrinology 157: 560–569, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Mantell S, Jones R, Trevethick M. Design and application of locally delivered agonists of the adenosine A(2A) receptor. Expert Rev Clin Pharmacol 3: 55–72, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57: 1584–1594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee A, Sidis Y, Mahan A, Raher MJ, Xia Y, Rosen ED, Bloch KD, Thomas MK, Schneyer AL. FSTL3 deletion reveals roles for TGF-beta family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci USA 104: 1348–1353, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perfetti R, Merkel P. Glucagon-like peptide-1: a major regulator of pancreatic beta-cell function. Eur J Endocrinol 143: 717–725, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Purwana I, Zheng J, Li X, Deurloo M, Son DO, Zhang Z, Liang C, Shen E, Tadkase A, Feng ZP, Li Y, Hasilo C, Paraskevas S, Bortell R, Greiner DL, Atkinson M, Prud'homme GJ, Wang Q. GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes 63: 4197–4205, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Rachdi L, Kariyawasam D, Guez F, Aïello V, Arbonés ML, Janel N, Delabar JM, Polak M, Scharfmann R. Dyrk1a haploinsufficiency induces diabetes in mice through decreased pancreatic beta cell mass. Diabetologia 57: 960–969, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Robitaille K, Rourke JL, McBane JE, Fu A, Baird S, Du Q, Kin T, Shapiro AMJ, Screaton RA. High-throughput functional genomics identifies regulators of primary human beta cell proliferation. J Biol Chem 291: 4614–4625, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes 57: 1575–1583, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Shen W, Tremblay MS, Deshmukh VA, Wang W, Filippi CM, Harb G, Zhang YQ, Kamireddy A, Baaten JE, Jin Q, Wu T, Swoboda JG, Cho CY, Li J, Laffitte BA, McNamara P, Glynne R, Wu X, Herman AE, Schultz PG. Small-molecule inducer of β cell proliferation identified by high-throughput screening. J Am Chem Soc 135: 1669–1672, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, Li Y, Zhang N, Chakrabarti R, Ng T, Jin T, Zhang H, Lu WY, Feng ZP, Prud'homme GJ, Wang Q. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci USA 108: 11692–11697, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan BA, Hollister-Lock J, Bonner-Weir S, Weir GC. Reduced Ki67 staining in the postmortem state calls into question past conclusions about the lack of turnover of adult human β-cells. Diabetes 64: 1698–1702, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takane KK, Kleinberger JW, Salim FG, Fiaschi-Taesch NM, Stewart AF. Regulated and reversible induction of adult human β-cell replication. Diabetes 61: 418–424, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian J, Dang H, Chen Z, Guan A, Jin Y, Atkinson MA, Kaufman DL. γ-Aminobutyric acid regulates both the survival and replication of human β-cells. Diabetes 62: 3760–3765, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiwari S, Roel C, Wills R, Casinelli G, Tanwir M, Takane KK, Fiaschi-Taesch NM. Early and Late G1/S cyclins and cdks act complementarily to enhance authentic human β-cell proliferation and expansion. Diabetes 64: 3485–3498, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tourrel C, Bailbé D, Meile MJ, Kergoat M, Portha B. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes 50: 1562–1570, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Tsuji N, Ninov N, Delawary M, Osman S, Roh AS, Gut P, Stainier DYR. Whole organism high content screening identifies stimulators of pancreatic Beta-cell proliferation. PLoS ONE 9: e104112, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walpita D, Hasaka T, Spoonamore J, Vetere A, Takane KK, Fomina-Yadlin D, Fiaschi-Taesch N, Shamji A, Clemons PA, Stewart AF, Schreiber SL, Wagner BK. A human islet cell culture system for high-throughput screening. J Biomol Screen 17: 509–518, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang P, Alvarez-Perez JC, Felsenfeld DP, Liu H, Sivendran S, Bender A, Kumar A, Sanchez R, Scott DK, Garcia-Ocaña A, Stewart AF. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med 21: 383–388, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang P, Fiaschi-Taesch NM, Vasavada RC, Scott DK, Garcia-Ocaña A, Stewart AF. Diabetes mellitus-advances and challenges in human β-cell proliferation. Nat Rev Endocrinol 11: 201–212, 2015. [DOI] [PubMed] [Google Scholar]
- 45.Wang W, Walker JR, Wang X, Tremblay MS, Lee JW, Wu X, Schultz PG. Identification of small-molecule inducers of pancreatic beta-cell expansion. Proc Natl Acad Sci USA 106: 1427–1432, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC. Structure of an agonist-bound human A2A adenosine receptor. Science 332: 322–327, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48: 2270–2276, 1999. [DOI] [PubMed] [Google Scholar]