Abstract
The peroxisome proliferator-activated receptor (PPAR) family of nuclear receptors is central to the pathophysiology and treatment of metabolic disease through the receptors' ability to regulate the expression of genes involved in glucose homeostasis, adipogenesis, and lipid metabolism. However, the mechanism by which PPAR is regulated remains incompletely understood. We generated a transgenic mouse strain (ZFP-TG) that overexpressed Zfp407 primarily in muscle and heart. Transcriptome analysis by RNA-Seq identified 1,300 differentially expressed genes in the muscle of ZFP-TG mice, among which PPAR target genes were significantly enriched. Among the physiologically important PPARγ target genes, Glucose transporter (Glut)-4 mRNA and protein levels were increased in heart and muscle. The increase in Glut4 and other transcriptional effects of Zfp407 overexpression together decreased body weight and lowered plasma glucose, insulin, and HOMA-IR scores relative to control littermates. When placed on high-fat diet, ZFP-TG mice remained more glucose tolerant than their wild-type counterparts. Cell-based assays demonstrated that Zfp407 synergistically increased the transcriptional activity of all PPAR subtypes, PPARα, PPARγ, and PPARδ. The increased PPAR activity was not associated with increased PPAR mRNA or protein levels, suggesting that Zfp407 posttranslationally regulates PPAR activity. Collectively, these results demonstrate that Zfp407 overexpression improved glucose homeostasis. Thus, Zfp407 represents a new drug target for treating metabolic disease.
type 2 diabetes affects nearly 300 million individuals worldwide (30). Complications of type 2 diabetes include cardiovascular disease, neuropathy, nephropathy, and retinopathy, among others. Central to the pathophysiology of type 2 diabetes is the insulin resistance of peripheral tissues, including adipose, liver, and muscle, that is defined by decreased insulin-stimulated glucose uptake. These changes are also associated with altered levels of cytokine and fatty acid release and elevated inflammation (7, 29).
Zinc finger protein 407 (Zfp407) was identified as a regulator of insulin-stimulated glucose uptake during a siRNA screen performed in 3T3-L1 adipocytes (10). Zfp407 is predicted to encode a 246-kDa protein with 24 zinc finger domains. Zfp407 knockdown in adipocytes reduced insulin-stimulated glucose uptake and decreased expression of peroxisome proliferator-activated receptor (PPAR)-γ target genes, including the glucose transporter GLUT4 (10). The PPAR family of nuclear hormone receptors, which includes PPARα, PPARγ, and PPARδ, is central to the pathophysiology and pharmacological treatment of insulin resistance (20). Genetic variation in all three PPAR family members is associated with altered insulin sensitivity in humans, demonstrating their importance in regulating glucose homeostasis (2, 6, 11, 38).
PPARγ expression is highest in adipocytes, where it controls adipogenesis and lipid homeostasis (1). PPARγ functions in other tissues by enhancing anti-immune responses and lipid metabolism in macrophages, increasing lipid storage in liver, and enhancing glucose-stimulated insulin secretion in pancreatic β-cells (1). PPARα is expressed highest in the liver and promotes fatty acid oxidation (35). PPARα regulates energy store management in the liver during fasting (21). PPARδ, which is widely expressed, regulates fatty acid catabolism and energy homeostasis in adipose tissue and muscle and suppresses macrophage-derived inflammation (5).
While PPAR family members each have unique expression patterns, all 3 are expressed together in skeletal muscle tissue (9). Muscle is the major contributor of postprandial glucose uptake and accounts for nearly 91% of all glucose uptake (12). PPAR activity in muscle is necessary for maintaining glucose homeostasis, as muscle-specific PPARγ deficiency in mice leads to insulin resistance (18, 27). Additionally, PPARγ or PPARδ overexpression in muscle improves whole body insulin sensitivity (3, 39). Furthermore, PPARα muscle-specific overexpressing mice were glucose intolerant but still protected from diet-induced obesity (15). It is clear that in skeletal muscle each PPAR family member has nonredundant contributions to maintaining whole body glucose homeostasis (3, 15, 18, 27, 39).
Zfp407 positively regulates the transcription of PPARγ target genes in the 3T3-L1 adipocyte cell line (10). However, the in vivo and tissue-specific effects of Zfp407 on organismal physiology and pan-PPAR signaling have not been tested. Thus we generated a new transgenic mouse strain that primarily overexpresses Zfp407 in muscle to test whether Zfp407 can modulate the PPAR family of nuclear receptors in vivo and improve whole body glucose homeostasis.
MATERIALS AND METHODS
Cell culture.
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum, l-glutamine-pen-strep, and 0.05% Trypsin-EDTA, were obtained from Life Technologies (Carlsbad, CA). 293T cells were cultured in DMEM with 10% FBS and 1× l-glutamine-pen-strep.
Mice.
Zfp407 transgenic mice were generated on a C57BL/6J background at the Case Transgenic and Targeting Core with a linearized mouse DNA fragment encoding a Myc/DDK-tagged Zfp407 protein under the control of a CMV promoter. Mice were housed in ventilated racks with access to food and water ad libitum and maintained at 21°C on a 12:12-h light-dark cycle. All mice were cared for as described under the Guide for the Care and Use of Animals, 8th edition (2011), and all experiments were approved by Institutional Animal Care and Use Committee at Case Western Reserve University and carried out in an American Association for the Accreditation of Laboratory Animal Care approved facility. Mice were fed standard chow diet LabDiet 5010 unless indicated otherwise (PMI Nutrition International, St. Louis, MO). For dietary studies, mice were fed either a high-fat/high-sucrose diet (HFD) with 58% of kcal from fat or a nutritionally balanced control diet (CD) with 10.5% of kcal from fat [D12331 (HFD) and D12328 (CD); Research Diets, New Brunswick, NJ]. Wild-type (nontransgenic) C57BL/6J littermates were used as controls for all mouse experiments. For metabolic studies, mice were fasted for 16 h overnight and blood glucose levels measured via retro orbital bleeds. Whole blood was collected by cardiac puncture using BDmicrotainer tubes with K2 EDTA. Body composition was determined noninvasively using an echo-MRI 1100 (33). For tissue collection, mice were anesthetized with isofluorane and euthanized by cervical dislocation at 9 AM (ZT3). Tissues were either snap-frozen in liquid nitrogen or placed in RNAlater (Thermo Fisher Scientific, Waltham, MA).
Glucose and insulin tolerance test.
Following 86 days on the HFD or CD, mice were fasted overnight for 16 h, blood samples were collected by tail vein nick, and glucose levels were measured with a handheld glucometer at baseline (time 0) and following intraperitoneal injection of dextrose (2 g/kg body wt) or insulin (1 U/kg body weight) dissolved in water or PBS, respectively.
RNA analysis.
RNA was isolated using the PureLink RNA purification kit with TRIzol protocol (Thermo Fisher Scientific). Total RNA was reverse transcribed using the high-capacity cDNA reverse transcription kit without the RNase inhibitor (Applied Biosystems, Carlsbad, CA). Primer sequences for detecting endogenous (primers in the 3′-UTR), transgene (reverse primer in the myc tag) or total Zfp407 (primers in exon 2 and 3) mRNA were as follows: endogenous Zfp407 forward primer 5′-CCACG GAACT TTGTC GTGTT-3′, endogenous Zfp407 reverse primer 5′-TGCTC TATGG CACAG GTTCA-3′; transgene Zfp407 forward primer 5′-CACAG TGATC CAGAG CCAAA-3′, transgene Zfp407 reverse primer 5′-TTGCT GCCAG ATCCT CTTCT-3′; total Zfp407 forward primer 5′-GAGAG GAGAA CCAGG GCAAC-3′, total Zfp407 reverse primer 5′-CCTCA TCCCA AGGTG CCTTT-3′. All other primer sequences were published previously (10). Quantitative PCR (qPCR) reactions were performed with the power SYBR green PCR Master Mix and run on a Bio-Rad (Hercules, CA) CFX Connect Real Time System. Expression levels were calculated using the ΔΔCT method relative to the Arbp control gene. For RNA-Seq analysis, total RNA was isolated as described above with an additional on-column DNase treatment step. RNA quality was determined on the Agilent BioAnalyzer 2100, and all samples had an RNA integrity number score >9.5. Illumina TruSeq sequencing libraries were prepared at the CWRU genomics core. Samples were run on an Illumina HiSeq2500 with an average of 41,923,675 reads per sample (range: 32,620,433–51,759,718). Reads were aligned to the mouse genome (Ensembl m38.82) using TopHat version 2.1, SamTools version 1.2, and Bowtie version 2.2.6 (22, 24, 25). Gene expression count tables were generated using HTSeq version 0.6.1 (4) and analyzed for differential expression by DESeq2 version 1.10 (26). Heat maps were generated in R version 3.2.2 with the heatmap.2 function in the g plots package version 2.17. Gene ontology analysis was conducted at geneontology.org (17). Enriched KEGG pathways were identified using Molecular Signatures Database (34). A false discovery rate-adjusted P value of <0.05 was considered statistically significant for RNA-Seq analyses. The RNA-Seq expression profiling data are available in the Gene Expression Omnibus series GSE83541.
Western blotting.
Western blotting was performed and quantitated as described (10). Anti-GLUT4 (2213) and anti-PPARγ (2430 and 2443) antibodies were from Cell Signaling Technology (Danvers, MA). Anti-PPARδ (60193-1) and anti-PPARα (15540-1) were from Proteintech Group (Rosemont, IL). Anti-GAPDH (MA5-15738) was from Thermo Fischer Scientific. A custom anti-ZFP407 antibody was generated in rabbit against the COOH-terminal 149 amino acids of the mouse ZFP407 protein (Proteintech Group). Goat anti-rabbit (31460) and goat anti-mouse (31430) secondary antibodies were from Thermo Fisher Scientific.
Plasma metabolites.
Plasma insulin concentrations were determined using the Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem, Downers Grove, IL). β-Hyroxybutyrate, cholesterol, triglyceride, and nonesterified fatty acids were measured at Marshfield Laboratories (Marshfield, WI). HOMA-IR scores were calculated using the following equation: insulin (μU/ml) × glucose (mg/dl)/405.
Muscle triglyceride analysis.
Mouse skeletal muscle (biceps femoris) (70–100 mg) was saponified with an equal volume by weight of 3 M KOH-65% ethanol. The sample was incubated at 70°C for 1 h and then at room temperature for 24 h. The sample volume was adjusted to 300 μl of 50 mM Tris/100 mg of tissue used. Glycerol concentration was measured against glycerol standards using a commercially available triglyceride glycerol phosphate oxidase reagent kit (Pointe Scientific, Lincoln Park, MI).
PPAR luciferase reporter assay.
293T cells were transfected using Lipofectamine 3000 (Life Technologies). DNA plasmid constructs transfected encoded PPARγ (Addgene no. 8862) (37), PPARδ (36), PPARα (36), ZFP407 (MR214555; Origene Technologies, Rockville, MD), an empty vector control plasmid (pRK5-Myc), and the PPAR target gene luciferase reporter plasmid (Addgene no. 1015) (23); 490 ng/well of DNA from the above plasmids was transfected with 10 ng of pRL-SV40 encoding Renilla for normalization. Luciferase and Renilla were measured 24 h posttransfection with the Dual-Glo Luciferase Assay System (Promega, Madison, WI).
Histology.
Mouse skeletal muscle (biceps femoris) and heart tissues were fixed in 10% vol/vol neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin by the Case Tissue Resources Core. Succinate dehydrogenase staining (SDH) and α-glycerol-3-phosphate dehydrogenase (GPDHα) stains were performed on cryosections as described (13). Semiquantification of fiber intensity was performed on a blinded basis using a scale of 0–4 (0, no staining; 4, most intense staining) by two individual scorers (n = 3 samples/group with 5 random pictures taken per sample at ×200 magnification). Final counts were normalized and analyzed by Student’s t-test.
Genotyping and transgene copy number determination.
Transgenic Zfp407 mice were genotyped using a three-primer PCR reaction that amplifies a single 156-base pair DNA fragment in wild-type (WT) mice and an additional 396-base pair fragment in transgenic mice: forward primer, 5′-TCTGT GACCT CTGTG GCTTC-3′; reverse primer 1, 5′-AAGCA AACCA TAGGA CTTGG ACA-3′; reverse primer 2, 5′-GGCAC TCATA GGACT TGGAC A-3′. Copy number of the Zfp407 transgene was determined as described previously (19) using the following primer pair: exon 1 forward primer 5′-CCAAC CCACA GGCAC CCTGC-3′ and exon 1 reverse primer 5′-ACTCG GACGG TGTTG CTGCG-3′.
Statistics.
Data are shown as means ± SE unless indicated otherwise. Western blot, qPCR, and muscle triglyceride data were analyzed by two-tailed Student’s t-test. Glucose tolerance tests were analyzed by repeated-measures ANOVA followed by Bonferroni’s correction for multiple comparison. The physiological parameters described in Table 1 were analyzed by a two-way ANOVA for effects of genotype and sex. The physiological parameters described in Tables 2 and 3 were analyzed by a three-way ANOVA for effects of genotype, sex and diet. For all physiological parameters listed in Tables 1–3, a sample size of n ≥ 75 was considered large enough to assume normal distribution by histogram visualization. When the sample sizes were n < 75, a Shapiro-Wilk test for normal distribution was performed on the residuals of the ANOVA analysis. If a trait failed normality (P < 0.05), then values were reshaped by taking the natural log. The reshaped values all passed the Shapiro-Wilk test for normal distribution, and so ANOVA was performed on these values. P values <0.05 were considered statistically significant for all statistical tests.
Table 1.
Metabolic trait data in 5-wk-old ZFP-TG and WT mice
| 2-Way ANOVA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex (Trait) | WT | SE | n | ZFP-TG | SE | n | Genotype | Sex | Interaction |
| Males | |||||||||
| Body weight, g | 20.39 | 0.22 | 66 | 18.29 | 0.33 | 60 | 2.30e-09 | <2.2e-16 | NS |
| Length, mm | 86.54 | 0.68 | 35 | 86.04 | 0.86 | 23 | 0.04645 | 7.68e-05 | NS |
| Body mass index, kg/m2 | 2.71 | 0.38 | 29 | 2.60 | 0.22 | 27 | NS | 1.30e-11 | NS |
| Fasting glucose, mg/dl | 103.57 | 3.59 | 30 | 83.22 | 4.60 | 23 | 1.02e-05 | 4.22e-05 | NS |
| Fasting insulin, ng/ml | 0.41 | 0.07 | 19 | 0.14 | 0.02 | 9 | 0.000623 | NS | NS |
| HOMA-IR | 3.07 | 0.68 | 19 | 0.83 | 0.15 | 9 | 1.87e-05 | NS | NS |
| Plasma β-hydroxybutyrate, mg/dl | 14.35 | 1.36 | 11 | 19.90 | 1.28 | 9 | 0.0002121 | NS | NS |
| Plasma cholesterol, mg/dl | 100.73 | 3.21 | 11 | 98.00 | 3.98 | 9 | NS | 1.28e-07 | NS |
| Plasma nonesterified fatty acids, mg/dl | 0.92 | 0.08 | 11 | 0.97 | 0.07 | 9 | NS | 0.02075 | NS |
| Plasma triglycerides, mg/dl | 140.18 | 15.85 | 11 | 149.89 | 12.78 | 9 | NS | 8.01e-05 | NS |
| Muscle triglycerides, mg/g tissue | 9.44 | 1.64 | 5 | 9.46 | 1.51 | 5 | NS | NS | NS |
| Females | |||||||||
| Body weight, g | 16.70 | 0.18 | 48 | 15.56 | 0.25 | 41 | 2.30e-09 | <2.2e-16 | NS |
| Length, mm | 84.48 | 0.48 | 27 | 82.05 | 0.60 | 19 | 0.04645 | 7.68e-05 | NS |
| Body mass index, kg/m2 | 2.36 | 0.39 | 25 | 2.30 | 1.04 | 17 | NS | 1.30e-11 | NS |
| Fasting glucose, mg/dl | 86.00 | 4.10 | 28 | 64.06 | 4.81 | 17 | 1.02e-05 | 4.22e-05 | NS |
| Fasting insulin, ng/ml | 0.39 | 0.06 | 16 | 0.23 | 0.07 | 11 | 0.000623 | NS | NS |
| HOMA-IR | 2.46 | 0.38 | 16 | 0.98 | 0.27 | 11 | 1.87e-05 | NS | NS |
| Plasma β-hydroxybutyrate, mg/dl | 16.46 | 1.46 | 10 | 23.25 | 1.91 | 10 | 0.0002121 | NS | NS |
| Plasma cholesterol, mg/dl | 71.90 | 4.82 | 10 | 71.00 | 4.92 | 10 | NS | 1.28e-07 | NS |
| Plasma nonesterified fatty acids, mg/dl | 1.46 | 0.30 | 10 | 1.46 | 0.25 | 10 | NS | 0.02075 | NS |
| Plasma triglycerides, mg/dl | 85.80 | 6.80 | 10 | 98.80 | 8.87 | 10 | NS | 8.01e-05 | NS |
| Muscle triglycerides, mg/g tissue | 10.80 | 1.29 | 5 | 11.70 | 1.84 | 5 | NS | NS | NS |
WT, wild type; NS, not significant; HOMA-IR, homeostatic model assessment of insulin resistance.
Table 2.
Metabolic trait data for CD- or HFD-fed 135-day-old ZFP-TG and WT male mice
| CD |
HFD |
3-Way ANOVA (P Value) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | WT | SE | n | ZFP-TG | SE | n | WT | SE | n | ZFP-TG | SE | n | Genotype | Sex | Diet | Interactions |
| Body Weight, g | 29.09 | 0.50 | 18 | 25.80 | 0.53 | 14 | 38.63 | 1.69 | 20 | 32.89 | 1.41 | 16 | 3.81e-06 | <2.2e-16 | 9.08e-13 | Sex/diet, P = 0.005 |
| Total weight gained, g | 8.53 | 0.51 | 16 | 7.01 | 0.64 | 11 | 18.36 | 1.42 | 20 | 14.66 | 1.08 | 16 | 1.80e-03 | 4.11e-14 | 3.72e-16 | Sex/diet, P < 0.001 |
| %Weight gained | 41.87 | 2.87 | 16 | 39.83 | 5.02 | 11 | 89.78 | 6.13 | 20 | 82.47 | 7.03 | 16 | NS | 1.10e-08 | 1.73e-15 | Sex/diet, P = 0.006 |
| %Fat mass | 17.91 | 0.92 | 14 | 17.62 | 1.08 | 10 | 26.20 | 1.56 | 20 | 23.27 | 1.75 | 16 | NS | 0.001773 | 3.62e-07 | NS |
| %Lean mass | 82.09 | 0.92 | 14 | 82.38 | 1.08 | 10 | 73.80 | 1.56 | 20 | 76.73 | 1.75 | 16 | NS | 0.01034 | 6.13e-08 | NS |
| Perigonadal fat mass, g | 0.27 | 0.04 | 14 | 0.24 | 0.05 | 10 | 0.95 | 0.12 | 20 | 0.71 | 0.13 | 14 | 0.034161 | 3.77e-08 | 7.08e-08 | Sex/diet, P = 0.003 |
| Inguinal fat mass, g | 0.16 | 0.03 | 14 | 0.15 | 0.02 | 10 | 0.60 | 0.08 | 20 | 0.43 | 0.07 | 14 | 0.032657 | 5.23e-06 | 8.42e-09 | Sex/diet, P = 0.008 |
| Length, mm | 98.94 | 0.62 | 19 | 95.93 | 0.89 | 14 | 102.00 | 0.57 | 16 | 100.86 | 1.14 | 14 | 0.005326 | 0.002286 | 5.20e-08 | Genotype/diet, P = 0.03 |
| Body mass index, kg/m2 | 3.00 | 0.63 | 18 | 2.86 | 0.84 | 16 | 3.71 | 1.40 | 20 | 3.20 | 0.91 | 15 | 5.87e-06 | <2.2e-16 | 4.46e-09 | Genotype/diet, P = 0.03 |
| Fasting glucose, mg/dl | 96.71 | 4.98 | 18 | 84.29 | 5.45 | 14 | 145.85 | 8.51 | 20 | 132.69 | 8.55 | 16 | 2.25e-02 | 0.01764 | 1.21e-09 | Genotype/diet, P = 0.03 |
| Fasting insulin, ng/ml | 0.34 | 0.06 | 8 | 0.23 | 0.06 | 7 | 0.44 | 0.11 | 10 | 0.35 | 0.07 | 9 | NS | NS | NS | NS |
| HOMA-IR | 2.10 | 0.41 | 8 | 1.54 | 0.51 | 7 | 3.69 | 0.75 | 10 | 3.44 | 0.88 | 9 | NS | 0.01524 | 0.03014 | NS |
| GTT AUC | 38270 | 2719 | 8 | 32,610 | 1851 | 7 | 43551 | 2636 | 10 | 34,917 | 2,343 | 9 | 0.0008729 | 8.31e-13 | 0.0078087 | NS |
| Plasma β-hydroxybutyrate, mg/dl | 21.01 | 0.97 | 9 | 17.21 | 1.69 | 9 | 13.56 | 1.57 | 10 | 12.50 | 1.52 | 9 | NS | 0.011259 | 0.002899 | NS |
| Plasma cholesterol, mg/dl | 121.56 | 5.58 | 9 | 117.22 | 10.00 | 9 | 145.70 | 14.37 | 10 | 122.33 | 10.70 | 9 | 0.03804 | 2.24e-05 | NS | NS |
| Plasma nonesterified fatty acids, mg/dl | 138.00 | 9.99 | 9 | 113.44 | 10.19 | 9 | 117.40 | 11.84 | 10 | 97.33 | 7.37 | 9 | NS | NS | NS | NS |
| Plasma triglycerides, mg/dl | 1.07 | 0.03 | 9 | 1.11 | 0.06 | 9 | 0.90 | 0.05 | 10 | 1.09 | 0.12 | 8 | 0.008887 | 2.06e-05 | 0.024818 | NS |
CD, control diet; HFD, high-fat diet; GTT AUC, glucose tolerance test area under the curve.
Table 3.
Metabolic trait data for CD- or HFD-fed 135-day old ZFP-TG and WT female mice.
| CD |
HFD |
3-Way ANOVA (P Value) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | WT | SE | n | ZFP-TG | SE | n | WT | SE | n | ZFP-TG | SE | n | Genotype | Sex | Diet | Interactions |
| Body weight, g | 22.33 | 0.67 | 14 | 19.58 | 0.50 | 11 | 25.47 | 0.91 | 10 | 23.70 | 0.91 | 13 | 3.81e-06 | <2.2e-16 | 9.08e-13 | Sex/diet P = 0.005 |
| Total weight gained, g | 4.70 | 0.46 | 10 | 4.21 | 0.42 | 11 | 8.86 | 0.61 | 13 | 8.01 | 0.65 | 13 | 1.80e-03 | 4.11e-14 | 3.72e-16 | Sex/diet P < 0.001 |
| %Weight gained | 30.42 | 3.54 | 9 | 27.69 | 3.58 | 10 | 53.12 | 3.80 | 13 | 51.93 | 4.65 | 13 | NS | 1.10e-08 | 1.73e-15 | Sex/diet P = 0.006 |
| %Fat mass | 16.67 | 0.38 | 10 | 16.45 | 0.72 | 10 | 21.48 | 1.46 | 10 | 19.38 | 1.69 | 11 | NS | 0.001773 | 3.62e-07 | NS |
| %Lean mass | 83.33 | 0.38 | 10 | 83.55 | 0.72 | 10 | 77.53 | 1.62 | 10 | 79.23 | 1.72 | 11 | NS | 0.01034 | 6.13e-08 | NS |
| Perigonadal fat mass, g | 0.11 | 0.01 | 10 | 0.10 | 0.01 | 10 | 0.24 | 0.06 | 9 | 0.22 | 0.06 | 10 | 0.034161 | 3.77e-08 | 7.08e-08 | Sex/diet P = 0.003 |
| Inguinal fat mass, g | 0.11 | 0.01 | 10 | 0.10 | 0.01 | 10 | 0.23 | 0.04 | 9 | 0.21 | 0.04 | 10 | 0.032657 | 5.23e-06 | 8.42e-09 | Sex/diet P = 0.008 |
| Length, mm | 97.74 | 0.99 | 19 | 94.27 | 0.73 | 11 | 99.00 | 0.60 | 14 | 98.88 | 11.60 | 16 | 0.005326 | 0.002286 | 5.20e-08 | Genotype/diet P = 0.03 |
| Body mass index, kg/m2 | 2.31 | 0.53 | 11 | 2.24 | 0.77 | 11 | 2.69 | 0.76 | 14 | 2.43 | 0.68 | 16 | 5.87e-06 | <2.2e-16 | 4.46e-09 | Genotype/diet P = 0.03 |
| Fasting glucose, mg/dl | 104.71 | 7.66 | 14 | 74.00 | 6.43 | 11 | 113.62 | 7.95 | 13 | 114.57 | 0.65 | 14 | 2.25e-02 | 0.01764 | 1.21e-09 | Genotype/diet P = 0.03 |
| Fasting insulin, ng/ml | 0.26 | 0.06 | 9 | 0.31 | 0.07 | 7 | 0.27 | 0.05 | 9 | 0.22 | 0.06 | 8 | NS | NS | NS | NS |
| HOMA-IR | 1.79 | 0.42 | 9 | 1.65 | 0.34 | 7 | 2.14 | 0.43 | 9 | 1.47 | 0.40 | 8 | NS | 0.01524 | 0.03014 | NS |
| GTT AUC | 24,995 | 937 | 9 | 22,145 | 1,312 | 7 | 29,168 | 2,467 | 9 | 25,336 | 803 | 8 | 0.0008729 | 8.31e-13 | 0.0078087 | NS |
| Plasma β-hydroxybutyrate, mg/dl | 20.23 | 2.23 | 9 | 19.65 | 2.03 | 11 | 17.72 | 1.51 | 10 | 18.90 | 1.62 | 11 | NS | 0.011259 | 0.002899 | NS |
| Plasma cholesterol, mg/dl | 95.44 | 5.06 | 9 | 96.36 | 4.93 | 11 | 109.60 | 7.92 | 10 | 90.00 | 8.29 | 11 | 0.03804 | 2.24e-05 | NS | NS |
| Plasma nonesterified fatty acids, mg/dl | 96.78 | 6.67 | 9 | 91.64 | 3.62 | 11 | 90.70 | 6.89 | 10 | 84.36 | 7.21 | 11 | NS | NS | NS | NS |
| Plasma triglycerides, mg/dl | 0.98 | 0.08 | 9 | 1.26 | 0.19 | 11 | 1.23 | 0.33 | 10 | 0.95 | 0.10 | 10 | 0.008887 | 2.06e-05 | 0.024818 | NS |
| Muscle triglycerides, mg/g tissue | ND | ND | 12.18 | 2.26 | 4 | 15.47 | 2.10 | 5 | NS | ND | ND | ND | ||||
ND, not determined.
RESULTS
ZFP-TG mice overexpress Zfp407 in heart and muscle.
A new strain of transgenic mice (ZFP-TG) that carry a transgene encoding a Myc/DDK-tagged Zfp407 protein under the control of a CMV promoter was generated (Fig. 1A). The transgene was injected into fertilized one-cell embryos from the strain C57BL/6J, upon which it was randomly inserted into the genome. ZFP-TG mice have 11.5 ± 1.32 exogenous copies of the Zfp407 cDNA in addition to the two endogenous copies of the gene. ZFP-TG mice were viable with no obvious morphological defects, although a slight deficiency of female ZFP-TG hemizygous mice was observed relative to the expected number of transgenic offspring in a back-cross (Chi-square: females, P < 0.05; males, P > 0.1). Endogenous Zfp407 mRNA levels were unchanged between ZFP-TG and WT mice in all tissues, whereas most tissues examined in ZFP-TG did express the transgene at varying levels, with the highest expression occurring in heart and muscle (Fig. 1, B and C). Total Zfp407 mRNA expression was increased significantly in gonadal and subcutaneous adipose tissue, brain, and kidney (Fig. 1D). Additionally, the highest expression levels occurred in the heart and muscle with a 3.0- and 19.3-fold increase, respectively (Fig. 1D). Corresponding 4.0- and 67-fold increases in Zfp407 protein levels were detected in heart and muscle, respectively, whereas no other tissue had significantly increased levels of Zfp407 protein (Fig. 2). Therefore, Zfp407 is overexpressed primarily in heart and skeletal muscle, although there may be additional small increases in Zfp407 protein levels in tissues such as brain and kidney, and we cannot exclude the possibility of Zfp407 overexpression in other cell and tissue types not tested.
Fig. 1.
Zinc finger protein 407 (Zfp407) mRNA overexpression in ZFP-TG mice. A: transgene construct with mouse Zfp407 cDNA tagged with Myc and DDK. B–D: endogenous (B), transgenic (C), and total Zfp407 mRNA expression (D) were measured by quantitative PCR and normalized to levels of Arbp in wild-type (WT) and ZFP-TG (TG) mice (n = 4–10 mice/group). *P < 0.05; **P < 0.01; ***P < 0.001. CMV, cytomegalovirus promoter; polyA, human growth hormone polyA signal; SQ, subcutaneous.
Fig. 2.
Zfp407 protein is overexpressed in heart and muscle of ZFP-TG mice. ZFP407 and GAPDH protein expression were measured by Western blot in WT and ZFP-TG (TG) mice (n = 4–14 mice/group). **P < 0.01; ***P < 0.001.
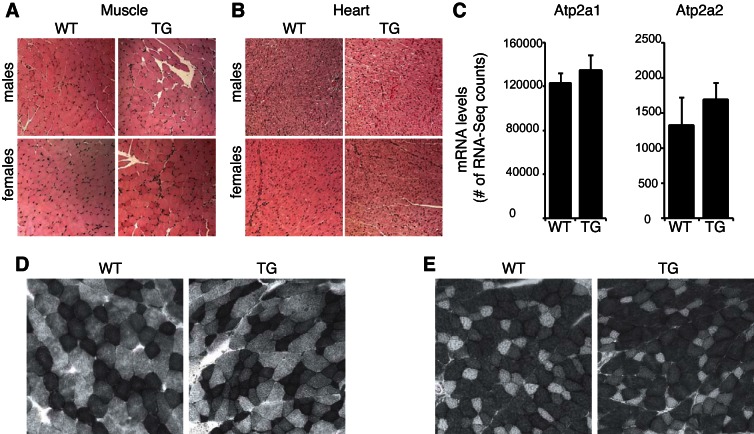
To determine whether increased ZFP407 protein levels affected heart or muscle gross morphology, sections of these tissues from male and female mice were stained by hematoxylin and eosin and blindly analyzed by a pathologist. No structural differences were detected, and there were no overt changes in inflammatory cell infiltration between WT and ZFP-TG tissue (Fig. 3, A and B). Gene expression-based markers of slow-twitch (Atp2a1) and fast-twitch (Atp2a2) fibers did not differ between ZFP-TG and control strains (Fig. 3C). Furthermore, SDH (more oxidative, slow-twitch fibers) and GPDHα (more glycolytic, fast-twitch fibers) staining intensities did not differ between WT and ZFP-TG muscle tissue (Fig. 3, D and E), suggesting that there was no difference in the distribution of fast- and slow-twitch muscle fibers.
Fig. 3.
No morphological differences in muscle and heart tissue between ZFP-TG and WT mice. A and B: muscle (A) and heart tissue (B) from ZFP-TG (TG) and WT mice stained with hemotoxylin and eosin (n = 4 mice/group). Total magnification, ×200. C: gene expression of Atp2a1 (fast-twitch muscle fiber marker) and Atp2a2 (slow-twitch muscle fiber marker) in muscle from TG and WT male mice (n = 5/group). D and E: representative succinate dehydrogenase (D) and α-glycerol-3-phosphate dehydrogenase staining (E) from muscle of ZFP-TG and WT male mice (n = 3/group).
Transcriptome changes in ZFP-TG muscle.
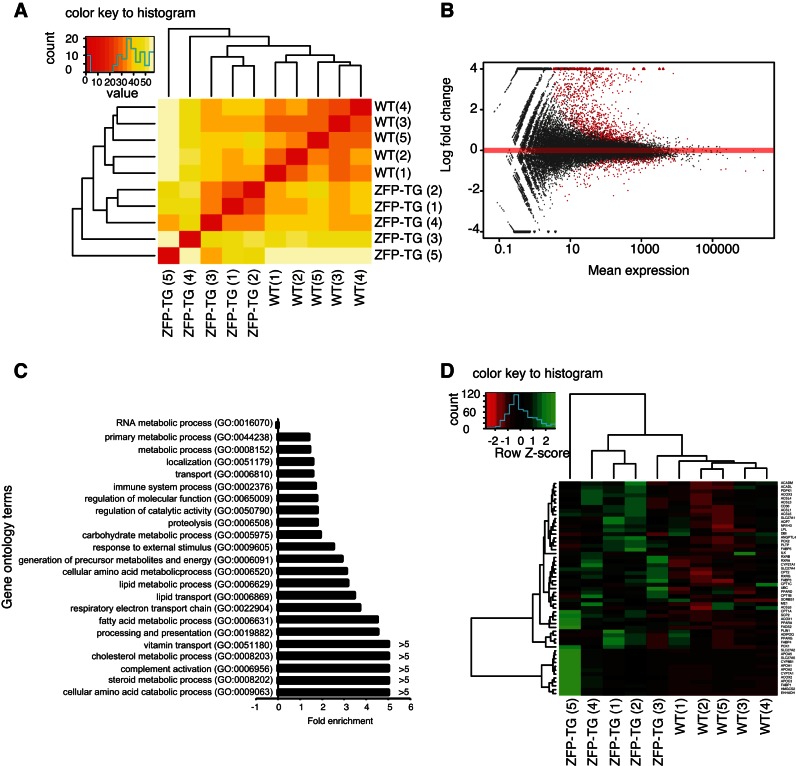
Whole transcriptome analysis of muscle tissue was undertaken to identify downstream target genes regulated by Zfp407. Hierarchical clustering of the muscle transcriptome of ZFP-TG and control mice showed distinct patterns of gene expression, demonstrating widespread transcriptional changes induced by Zfp407 overexpression (Fig. 4A). There were 1,300 genes differentially expressed between ZFP-TG and control mice, including 901 upregulated and 299 downregulated (Fig. 4B and Supplemental Table S1; Supplemental Material for this article can be found on the AJP-Endocrinology and Metabolism web site). Zfp407 was the most statistically significantly upregulated gene, with a 21-fold increase in ZFP-TG muscle (Supplemental Table S1).
Fig. 4.
ZFP407 overexpression alters the muscle transcriptome. A and B: gene expression analysis in muscle from ZFP-TG and WT mice (n = 5/group) analyzed by hierarchical clustering of the transcriptome in ZFP-TG and WT mice (A) and volcano plot of gene expression (B), with differentially expressed genes indicated in red. C: fold enrichment of gene ontology terms among differentially expressed genes between ZFP-TG and WT mice. D: heat map of gene expression for the peroxisome proliferator-activated receptor (PPAR) KEGG pathway (03320). ZFP-TG 1–5 and WT 1–5 indicate individual biological replicates. Red, decreased relative expression; green, increased relative expression.
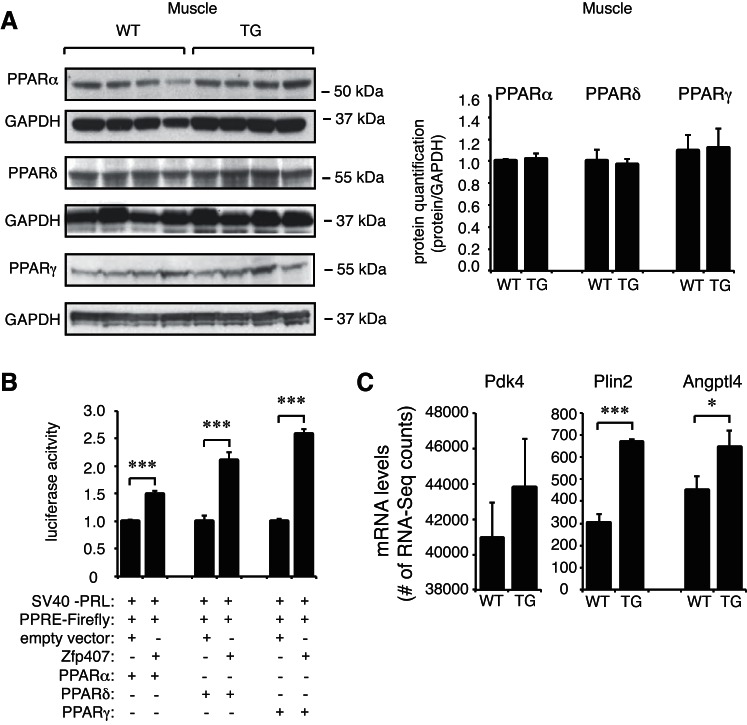
Gene ontology analysis of differentially expressed genes identified a number of significantly upregulated pathways, including cholesterol, steroid and fatty acid metabolic processes, antigen processing and presentation, and complement activation (Fig. 4C). Analysis of KEGG pathways among the differentially expressed genes demonstrated that genes within the PPAR signaling pathways were significantly enriched (P = 4.0 × 10−6; Table 4). As PPAR pathway genes were significantly enriched among the differentially expressed genes, and Zfp407 was previously implicated in PPAR signaling (10), we examined the expression of all genes within the PPAR KEGG pathway. PPAR pathway genes were consistently upregulated in the ZFP-TG mice relative to controls (Fig. 4D). Among the key PPAR target genes in the muscle, Plin2 and Angptl4 expression was significantly upregulated by Zfp407 overexpression, whereas Pdk4 demonstrated a trend toward increased expression in the muscle of ZFP-TG mice (Fig. 5C) (8, 32, 40). However, levels of PPARα, PPARγ, and PPARδ mRNA and protein did not differ between ZFP-TG and WT mice (Figs. 4B and 5A).
Table 4.
Zfp407 differentially altered KEGG pathways
| Term | Genes | % | P Value | Fold Enrichment | Bonferroni |
|---|---|---|---|---|---|
| Complement and coagulation cascades | 32 | 3.8 | 2.20e-20 | 7.8 | 3.60e-18 |
| Retinol metabolism | 22 | 2.6 | 2.90e-11 | 5.9 | 4.90e-09 |
| Drug metabolism | 21 | 2.5 | 1.60e-09 | 5.1 | 2.70e-07 |
| Drug metabolism | 16 | 1.9 | 1.80e-08 | 6.1 | 3.00e-06 |
| Steroid hormone biosynthesis | 15 | 1.8 | 5.80e-08 | 6.1 | 9.50e-06 |
| Metabolism of xenobiotics by cytochrome P450 | 17 | 2.0 | 3.10e-07 | 4.7 | 5.10e-05 |
| Linoleic acid metabolism | 14 | 1.7 | 6.10e-07 | 5.5 | 1.00e-04 |
| Arachidonic acid metabolism | 18 | 2.1 | 1.70e-06 | 4.0 | 2.80e-04 |
| PPAR signaling pathway | 17 | 2.0 | 4.10e-06 | 3.9 | 6.70e-04 |
| Antigen processing and presentation | 18 | 2.1 | 6.50e-06 | 3.6 | 1.10e-03 |
| Ascorbate and aldarate metabolism | 8 | 0.9 | 6.00e-06 | 9.7 | 1.00e-03 |
| Primary bile acid biosynthesis | 8 | 0.9 | 6.00e-06 | 9.7 | 1.00e-03 |
| Glycine, serine and threonine metabolism | 10 | 1.2 | 3.60e-05 | 5.7 | 5.90e-03 |
| CAM | 21 | 2.5 | 2.40e-04 | 2.5 | 4.00e-02 |
| Androgen and estrogen metabolism | 9 | 1.1 | 3.10e-04 | 5.0 | 4.90e-02 |
Zfp407, zinc finger protein 407; CAM, cell adhesion molecules; PPAR, peroxisome proliferator-activated receptor.
Fig. 5.
Zfp407 is a pan-PPAR activator. A: PPARα, PPARδ, and PPARγ protein levels in muscle of ZFP-TG (TG) and WT mice (n = 4 mice/group). B: a PPAR consensus reporter plasmid was transfected into 293T cells with the following vectors as indicated: pRK5-myc (empty vector control) or cDNA expression vectors encoding Zfp407, PPARα, PPARδ, or PPARγ. C: Pdk4, Plin2, and Angptl4 mRNA levels as examined by RNA-Seq (n = 5 mice/group). *P < 0.05; ***P < 0.001. PPRE, PPAR response element.
Other KEGG pathways that were enriched included retinol, drug and linoleic acid metabolism, and the complement and coagulation cascades (Table 4). The differentially expressed genes in these KEGG pathways were typically expressed specifically in the liver but were consistently activated in the muscle by Zfp407 overexpression. Among these liver-specific genes were hepatic nuclear factor-4α (HNF-4α) and 61 HNF-4α target genes (Table 5). This suggests that Zfp407 may drive expression of this pathway, which is critical for gene expression programming of hepatocytes (28, 31).
Table 5.
Gene set enrichment analysis of Zfp407 differentially altered gene sets
| Gene Set Name (No. of Genes), k | Description | No. of Genes in Overlap, k | P Value | FDR Q Value |
|---|---|---|---|---|
| HSIAO_LIVER_SPECIFIC_G ENES (244) | Liver selective genes | 108 | 1.16e-129 | 7.52e-126 |
| MODULE_23 (565) | Genes in the cancer module 23 | 117 | 4.45e-96 | 1.44e-92 |
| GNF2_HPX (135) | Neighborhood of HPX | 68 | 1.91e-86 | 4.12e-83 |
| MODULE_55 (834) | Genes in the cancer module 55 | 124 | 1.12e-83 | 1.82e-80 |
| MODULE_88 (838) | Genes in the cancer module 88 | 123 | 2.69e-82 | 3.48e-79 |
| GNF2_HPN (133) | Neighborhood of HPN | 65 | 1.70e-81 | 1.83e-78 |
| CAR_HPX (73) | Neighborhood of HPX | 51 | 9.12e-76 | 8.42e-73 |
| GNF2_LCAT (124) | Neighborhood of LCAT | 59 | 4.19e-73 | 3.39e-70 |
| MODULE_24 (453) | Genes in the cancer module 24 | 90 | 6.75e-72 | 4.85e-69 |
| OHGUCHI_LIVER_HNF4A_TARGETS_DN (149) | Genes down-regulated in liver samples of liver-specific knockout of HNF-4α (GeneID = 3172). | 61 | 1.85e-70 | 1.20e-67 |
FDR, flase discovery rate; LCAT, lecithin:cholesterol acyltransferase; HNF-4α, hepatic nuclear factor-4α.
Zfp407 positively regulates pan-PPAR activity.
Whereas Zfp407 positively regulates the activity of PPARγ (10), it was not known whether this is also true for PPARα or PPARδ or whether this regulation would be mediated via the canonical PPAR response element (PPRE). To test whether Zfp407 controls the activity of PPARα and PPARδ, Zfp407 was coexpressed with these PPARs and the PPRE-containing PPAR luciferase reporter plasmid. Co-overexpression of PPARα and PPARδ with Zfp407 resulted in a 1.5 ± 0.1- and 2.1 ± 0.2-fold increase, respectively, in luciferase activity relative to each PPAR alone (Fig. 5B). Thus, Zfp407 positively regulates PPRE-dependent expression with all three PPARs.
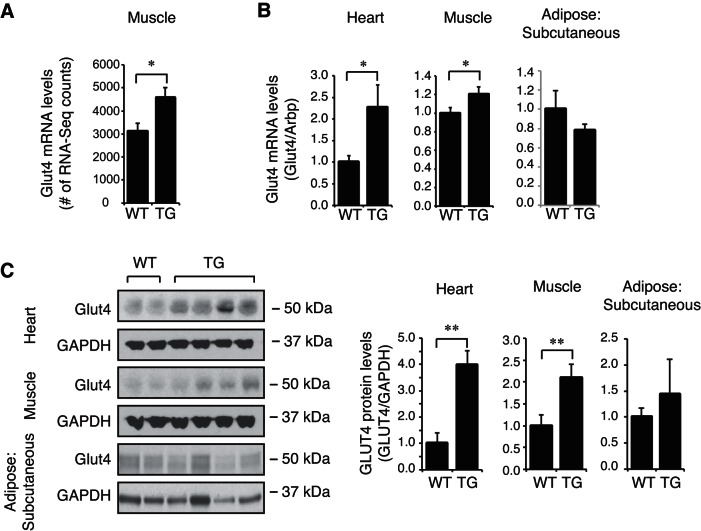
Zfp407 overexpression increased Glut4 levels.
A significant increase in Glut4 mRNA in ZFP-TG muscle was detected by both RNA-Seq and qPCR (Fig. 6, A and B). Glut4 protein levels were also increased in the heart and muscle of ZFP-TG mice, where Zfp407 protein levels were increased, but not in subcutaneous adipose tissue, where Zfp407 protein levels were unchanged (Fig. 6C). Thus, the increase in Glut4 expression due to Zfp407 overexpression is likely cell autonomous.
Fig. 6.
Zfp407 overexpression increases glucose transporter 4 (Glut4) expression. Glut4 mRNA levels as examined by RNA-Seq (n = 5 mice/group; A), Glut4 mRNA levels as examined by qPCR (n = 6–12 mice/group; B), and Glut4 protein levels (n = 6–12 mice/group) in muscle, heart, and subcutaneous adipose tissue of ZFP-TG (TG) and WT mice (C). *P < 0.05; **P < 0.01.
Improved glucose homeostasis in ZFP-TG mice.
Two-way ANOVA analysis of metabolic data collected at 5 wk of age for both male and female chow-fed ZFP-TG and littermate nontransgenic control mice demonstrated that Zfp407 overexpression effects body weight, body length (nose to tail), fasting glucose, fasting insulin, and HOMA-IR (Table 1). Male and female ZFP-TG mice weighed less and were smaller (body length) than their WT littermates (Table 1). Both male and female ZFP-TG mice had decreased fasting plasma glucose levels, lower fasting plasma insulin levels, and lower HOMA-IR scores (Table 1). Collectively, this metabolic data demonstrates that ZFP407 overexpression improves fasting markers of glucose homeostasis. Cholesterol, triglycerides, and nonesterified fatty acids (free fatty acids) were unchanged in the plasma of ZFP-TG mice. However, two-way ANOVA demonstrated an effect of Zfp407 overexpression on plasma β-hydroxybutyrate (representative of ketone bodies) levels, which were increased in both male and female ZFP-TG mice relative to controls (Table 1). The increase in plasma β-hydroxybutyrate levels can potentially be due to increased adipose lipolysis associated with increased fatty acid uptake by the liver or due to physiological changes in the muscle.
Improved glucose tolerance in HFD-fed ZFP-TG mice.
Three-way ANOVA analysis of metabolic data collected following 100 days of CD or HFD feeding in male and female ZFP-TG and control mice demonstrated that Zfp407 overexpression affects final body weight and total weight gained but not the percent weight gained. Whereas ZFP-TG mice weighed less than their WT littermates, their percent body weight gained during CD or HFD feeding did not differ (Table 2). Additionally, plasma cholesterol levels were decreased in both male and female HFD-fed ZFP-TG mice. Muscle triglyceride levels did not differ between WT or ZFP-TG HFD-fed female mice (Table 2).
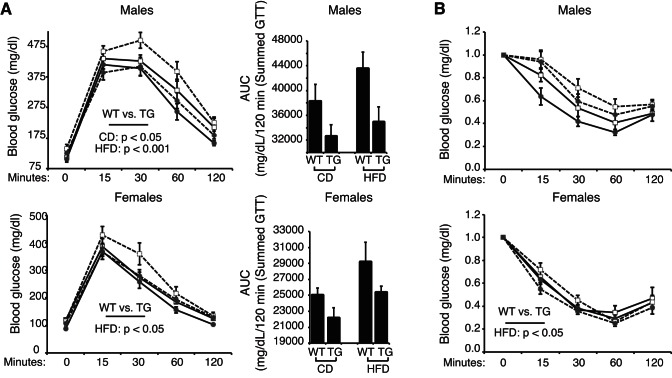
Fasting blood glucose levels were also lower in male and female ZFP-TG mice, however, there was no effect of Zfp407 overexpression on insulin levels or HOMA-IR scores (Table 2). Nonetheless, CD-fed male ZFP-TG mice were more glucose tolerant than WT littermates, as were both male and female ZFP-TG mice fed the HFD for 100 days (Fig. 7). Remarkably, the HFD-fed ZFP-TG mice remained as glucose tolerant as the CD-fed littermates, whereas WT mice fed the HFD became insulin resistant (Fig. 7). These data indicate that Zfp407 overexpression improves glucose homeostasis under obesogenic conditions.
Fig. 7.
Zfp407 overexpression improves glucose homeostasis of high-fat/high-sucrose diet (HFD)-fed mice. WT or ZFP-TG (TG) mice were fed control diet (CD) or HFD beginning at 5 wk of age. After 86 days of CD or HFD feeding, glucose tolerance tests were performed and area under the curve was calculated (A), and insulin tolerance tests were performed (B); n = 7–20/group). Solid line, CD; dashed line, HFD; □, WT; ●, ZFP-TG.
DISCUSSION
We describe a new transgenic mouse strain that overexpresses Zfp407, resulting in improved whole body glucose homeostasis. The improved metabolic profile is associated with the first demonstration that Zfp407 broadly regulates the expression of PPAR target genes in vivo, although the physiological phenotype associated with Zfp407 overexpression differs from other models of increased PPAR activity in the muscle. Muscle-specific PPARγ overexpression increased insulin sensitivity due to endogenous activation of adiponectin. This was associated with a higher percentage of oxidative muscle fibers but no change in body weight (3). PPARδ muscle-specific overexpression also increased the proportion of slow-twitch type I (oxidative) muscle fibers compared with fast-twitch (glycolytic) fibers, resulting in enhanced exercise endurance (39). The PPARδ transgenic mice were also protected from diet-induced obesity and were insulin sensitive relative to control mice (39). PPARα muscle-specific overexpression increased fatty acid oxidation rates and protected from diet-induced obesity, but these mice remained glucose intolerant (15). Taken together, the differences and similarities observed between each of the PPAR-overexpressing mice and our Zfp407-overexpressing mouse suggests that although Zfp407 does regulate the activity of the PPAR proteins, the combinatorial effect of pan-PPAR activation by Zfp407 or potentially other molecular pathways likely underlies the improvements in glucose homeostasis rather than the specific activation of a single PPAR family member.
Zfp407 overexpression was also sufficient to induce HNF-4α expression in the muscle, where it is not typically expressed, and with it the widespread activation of many liver-specific genes. This presumably nonphysiological induction of HNF-4α and its target genes demonstrates that Zfp407 overexpression alone is sufficient to induce a broad liver-specific transcriptional program within the context of a muscle cell, thus providing insight into its potential downstream target genes. Like other nuclear superfamily proteins such as PPARγ and retinoid X receptor-α (RXRα), HNF-4α binds to the classical DR1-binding motif (14). It is interesting to note that expression of the PPARs is not upregulated by Zfp407, whereas HNF-4α was upregulated at the mRNA level, and yet both HNF-4α and PPARs activate gene expression by heterodimerizing with RXRα and binding to the DR1 consensus sites. Thus, although we hypothesize that Zfp407 enhanced PPAR target expression through posttranslational effects on the PPARs, it is not clear as to whether Zfp407 has similar posttranslational effects on other nuclear receptors such as HNF-4α.
The overexpression of Zfp407 in ZFP-TG mice appeared to be restricted to the cardiac and skeletal muscle (Fig. 2). The CMV promoter used to drive Zfp407 overexpression typically drives expression in all tissues, although levels can vary greatly between tissue type (16). Therefore, it remains possible that the phenotypic outcomes observed may be due to Zfp407 overexpression in tissues or cells beyond just muscle and heart. Based on the metabolic improvements of ZFP-TG mice, it will be of interest to test additional Zfp407 transgenic mouse models utilizing more highly tissue-specific promoters. It is also important to note that the effects of Zfp407 overexpression could be supraphysiological in nature; nonetheless, the fact that Zfp407 overexpression in muscle tissue results in a reciprocal effect on PPAR target gene expression relative to the effects of Zfp407 inhibition in cultured adipocytes (10) suggests that Zfp407 controls pan-PPAR signaling in multiple tissues. Taken together, these results suggest that Zfp407 regulates the transcription of multiple pathways, including the PPAR and HNF-4α pathways, among others.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-099533 and DK-084079 to D. A. Buchner and DK-107535 to D. Bridges. D. Bridges was also supported by funds from the Memphis Research Consortium. Additionally, this work was supported by American Diabetes Association Grant 1-16-PDF-018, which was awarded to A. Charrier.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C. and D.A.B. conception and design of research; A.C., L.W., E.J.S., S.V.G., C.-w.K., C.M.C., and D.B. performed experiments; A.C., S.V.G., and D.A.B. analyzed data; A.C., S.V.G., and D.A.B. interpreted results of experiments; A.C. prepared figures; A.C. drafted manuscript; A.C., E.J.S., C.-w.K., C.M.C., D.B., and D.A.B. edited and revised manuscript; A.C., L.W., E.J.S., S.V.G., C.-w.K., C.M.C., D.B., and D.A.B. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Ron Conlon and the Case Western Reserve University Transgenic and Targeting Facility for generating the ZFP-TG strain, Drs. Noa Noy (Cleveland Clinic Lerner Research Institute) and Hung-Ying Kao (Case Western Reserve University) for providing plasmids, and Dr. Yuying Jiang (Case Western Reserve University) for assistance in tissue pathology analysis.
Present address of D. Bridges: Department of Nutritional Sciences, University of Michigan School of Public Health, Ann Arbor, MI 48109 (e-mail: davebrid@umich.edu).
REFERENCES
- 1.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 19: 557–566, 2013. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, Tuomi T, Gaudet D, Hudson TJ, Daly M, Groop L, Lander ES. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 26: 76–80, 2000. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 3.Amin RH, Mathews ST, Camp HS, Ding L, Leff T. Selective activation of PPARγ in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am J Physiol Endocrinol Metab 298: E28–E37, 2010. doi: 10.1152/ajpendo.00446.2009. [DOI] [PubMed] [Google Scholar]
- 4.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169, 2015. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest 116: 590–597, 2006. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402: 880–883, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Blüher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes 117: 241–250, 2009. doi: 10.1055/s-0029-1192044. [DOI] [PubMed] [Google Scholar]
- 8.Bosma M, Hesselink MK, Sparks LM, Timmers S, Ferraz MJ, Mattijssen F, van Beurden D, Schaart G, de Baets MH, Verheyen FK, Kersten S, Schrauwen P. Perilipin 2 improves insulin sensitivity in skeletal muscle despite elevated intramuscular lipid levels. Diabetes 61: 2679–2690, 2012. doi: 10.2337/db11-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 137: 354–366, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Buchner DA, Charrier A, Srinivasan E, Wang L, Paulsen MT, Ljungman M, Bridges D, Saltiel AR. Zinc finger protein 407 (ZFP407) regulates insulin-stimulated glucose uptake and glucose transporter 4 (Glut4) mRNA. J Biol Chem 290: 6376–6386, 2015. doi: 10.1074/jbc.M114.623736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claussnitzer M, Dankel SN, Klocke B, Grallert H, Glunk V, Berulava T, Lee H, Oskolkov N, Fadista J, Ehlers K, Wahl S, Hoffmann C, Qian K, Rönn T, Riess H, Müller-Nurasyid M, Bretschneider N, Schroeder T, Skurk T, Horsthemke B; DIAGRAM+Consortium, Spieler D, Klingenspor M, Seifert M, Kern MJ, Mejhert N, Dahlman I, Hansson O, Hauck SM, Blüher M, Arner P, Groop L, Illig T, Suhre K, Hsu YH, Mellgren G, Hauner H, Laumen H. Leveraging cross-species transcription factor binding site patterns: from diabetes risk loci to disease mechanisms. Cell 156: 343–358, 2014. doi: 10.1016/j.cell.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest 68: 1468–1474, 1981. doi: 10.1172/JCI110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn SE, Michel RN. Coordinated expression of myosin heavy chain isoforms and metabolic enzymes within overloaded rat muscle fibers. Am J Physiol 273: C371–C383, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Fang B, Mane-Padros D, Bolotin E, Jiang T, Sladek FM. Identification of a binding motif specific to HNF4 by comparative analysis of multiple nuclear receptors. Nucleic Acids Res 40: 5343–5356, 2012. doi: 10.1093/nar/gks190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP. A potential link between muscle peroxisome proliferator- activated receptor-alpha signaling and obesity-related diabetes. Cell Metab 1: 133–144, 2005. doi: 10.1016/j.cmet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Furth PA, Hennighausen L, Baker C, Beatty B, Woychick R. The variability in activity of the universally expressed human cytomegalovirus immediate early gene 1 enhancer/promoter in transgenic mice. Nucleic Acids Res 19: 6205–6208, 1991. doi: 10.1093/nar/19.22.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gene Ontology Consortium Gene Ontology Consortium: going forward. Nucleic Acids Res 43: D1049–D1056, 2015. doi: 10.1093/nar/gku1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med 9: 1491–1497, 2003. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- 19.Hill-Baskin AE, Markiewski MM, Buchner DA, Shao H, DeSantis D, Hsiao G, Subramaniam S, Berger NA, Croniger C, Lambris JD, Nadeau JH. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum Mol Genet 18: 2975–2988, 2009. doi: 10.1093/hmg/ddp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature 405: 421–424, 2000. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 21.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest 103: 1489–1498, 1999. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36, 2013. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc Natl Acad Sci USA 95: 4333–4337, 1998. doi: 10.1073/pnas.95.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359, 2012. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R; 1000 Genome Project Data Processing Subgroup . The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079, 2009. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ, Spiegelman BM, Kahn CR. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest 112: 608–618, 2003. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA. Control of pancreas and liver gene expression by HNF transcription factors. Science 303: 1378–1381, 2004. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saltiel AR. Insulin resistance in the defense against obesity. Cell Metab 15: 798–804, 2012. doi: 10.1016/j.cmet.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 4–14, 2010. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell 18: 175–189, 2010. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Staiger H, Haas C, Machann J, Werner R, Weisser M, Schick F, Machicao F, Stefan N, Fritsche A, Häring HU. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes 58: 579–589, 2009. doi: 10.2337/db07-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephenson EJ, Ragauskas A, Jaligama S, Redd JR, Parvathareddy J, Peloquin MJ, Saravia J, Han JC, Cormier SA, Bridges D. Exposure to environmentally persistent free radicals during gestation lowers energy expenditure and impairs skeletal muscle mitochondrial function in adult mice. Am J Physiol Endocrinol Metab 310: E1003–E1015, 2016. doi: 10.1152/ajpendo.00521.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugden MC, Bulmer K, Gibbons GF, Knight BL, Holness MJ. Peroxisome-proliferator-activated receptor-alpha (PPARalpha) deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Biochem J 364: 361–368, 2002. doi: 10.1042/bj20011699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan NS, Shaw NS, Vinckenbosch N, Liu P, Yasmin R, Desvergne B, Wahli W, Noy N. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol 22: 5114–5127, 2002. doi: 10.1128/MCB.22.14.5114-5127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 8: 1224–1234, 1994. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 38.Vänttinen M, Nuutila P, Kuulasmaa T, Pihlajamäki J, Hällsten K, Virtanen KA, Lautamäki R, Peltoniemi P, Takala T, Viljanen AP, Knuuti J, Laakso M. Single nucleotide polymorphisms in the peroxisome proliferator-activated receptor delta gene are associated with skeletal muscle glucose uptake. Diabetes 54: 3587–3591, 2005. doi: 10.2337/diabetes.54.12.3587. [DOI] [PubMed] [Google Scholar]
- 39.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2: e294, 2004. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes 48: 1593–1599, 1999. doi: 10.2337/diabetes.48.8.1593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.