Abstract
Introduction
Satellite cells are muscle resident stem cells and are responsible for muscle regeneration. In this study we investigate the involvement of PKCε during muscle stem cell differentiation in vitro and in vivo. Here, we describe the identification of a previously unrecognized role for the PKCε – HMGA1 signaling axis in myoblast differentiation and regeneration processes.
Methods
PKCε expression was modulated in the C2C12 cell line and primary murine satellite cells in vitro, as well as in an in vivo model of muscle regeneration. Immunohistochemistry and immunofluorescence, RT-PCR and shRNA silencing techniques were used to determine the role of PKCε and HMGA1 in myogenic differentiation.
Results
PKCε expression increases and subsequently re-localizes to the nucleus during skeletal muscle cell differentiation. In the nucleus, PKCε blocks Hmga1 expression to promote Myogenin and Mrf4 accumulation and myoblast formation. Following in vivo muscle injury, PKCε accumulates in regenerating, centrally-nucleated myofibers. Pharmacological inhibition of PKCε impairs the expression of two crucial markers of muscle differentiation, namely MyoD and Myogenin, during injury induced muscle regeneration.
Conclusion
This work identifies the PKCε – HMGA1 signaling axis as a positive regulator of skeletal muscle differentiation.
Keywords: PKCε, HMGA1, C2C12, satellite cells, skeletal muscle differentiation
Introduction
Adult skeletal muscle homeostasis as well as myofiber repair are maintained by a small subset of muscle stem/progenitor cells called Myosatellites or Satellite Cells (SCs). SCs reside between the sarcolemma and the basal membrane of skeletal muscle fibers and are able to give rise to additional SCs or differentiate into mature skeletal muscle cells to form new fibers [1, 2]. The members of the MyoD family (Myod, Myf5, Myogenin and Mrf4) are basic helix–loop–helix (bHLH) transcription factors that are critical molecular mediators of skeletal muscle differentiation [3]. Myod and Myf5 are considered promote the early stages of differentiation regulating skeletal muscle cell commitment, proliferation and cell cycle withdrawal of SCs [4], whereas Myogenin and Mrf4 mediate the processes of late muscle cell differentiation, promoting the formation and the final maturation of myotubes [5, 6].
High mobility group (HMG) proteins are non-histone chromatin associated proteins that indirectly modulate the transcription of their targets by altering higher order chromatin structure. HMGA1 is expressed in embryonic and undifferentiated cells, but is largely absent in adult organs [7]. HMGA1 down-regulation in C2C12 cell line is required to initiate the skeletal muscle differentiation program allowing the expression of the MyoD family myogenic factors [8]. However, little is known about the regulatory mechanisms that influence HMGA1 expression during myogenic differentiation.
The ε isoform of the PKC family (PKCε) is a serine-threonine kinase that is expressed in a wide variety of tissues including the hematopoietic system, intestine, brain, skin, liver, adipose tissue, kidney as well as cardiac and skeletal muscle. In many of these, PKCε regulates tissue homeostasis by regulating cell death and differentiation [9-14]. It is known that the θ isoform of the PKC family promotes the fusion of myoblasts and regulates the expression of caveolin-3 and β1D integrin [15]. Of note, it has also been demonstrated that PKCε expression increases during insulin-induced myogenic differentiation of the C2C12 cells [16].
In this study we investigated the functional role of PKCε in skeletal muscle cell differentiation as well as a potential role of PKCε as an upstream suppressor of Hmga1. We found that inhibition of PKCε prevents myogenic differentiation of C2C12 and primary SCs, whereas its overexpression accelerates cell differentiation. In vivo, PKCε inhibition results in impaired muscle regeneration and reduced expression of Myogenin and Mrf4. Mechanistically, we show that PKCε down-regulates Hmga1 expression, which consequently leads to the increase expression of myogenic differentiation genes. Finally, we demonstrate PKCε inhibition obstructs the process of injury-induced muscle regeneration in vivo.
Materials and methods
Mice
The experimental procedures were conducted according to the “Guide for the Care and Use of Laboratory Animals” (Directive 2010/63/EU of the European Parliament).
All the procedures described in this study were also approved by the Local Animal Research Ethics Committee of Ferrara (C.E.A.S.A) and Parma.
Cardiotoxin injury and immunohistochemistry
Acute injury was induced by intramuscular injection of Cardiotoxin (10 μM) in the tibialis muscle of CD1 adult mice [17]. In the case of PKCε –active peptides treatment, εV1-2 or ψεRACK (100 nM) were injected together with cardiotoxin. To study the regenerative process, mice were euthanized for histological analysis 3 and 7 days after injury. Muscle samples were fixed with 4% paraformaldehyde and embedded in paraffin. Sections (4 μm) were blocked with goat serum and incubated with primary anti PKCε antibody (Novus Biological NBP1-30126). Detection was performed using Vectastain elite ABC kit (Vector Laboratories) and nuclei were counterstained with haematoxylin [18].
Cell cultures
Mouse myoblast C2C12 cell line and primary SC were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with heat-inactivated 10% fetal bovine serum (FBS), 2mM glutamine and 1% antibiotics (Growth Medium, GM). Cells were maintained in a humidified 5% CO2 atmosphere at 37°C. When the cell cultures reached 80% confluence, GM was substituted with DMEM supplemented with 2% horse serum (Differentiation Medium, DM) to induce myogenic differentiation. Each experiment was performed in triplicate.
Satellite cells isolation
SCs were isolated from hindlimb muscles of 2 days old CD1 mice. Briefly, muscles were incubated with collagenase/dispase solution (Roche, Basel, Switzerland) 4 times for 15 minutes at 37°C in agitation. Cell suspension was filtered with 40 μm nylon cell strainer and processed with Feeder Removal Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). This immunomagnetic separation kit allows depletion of mouse fibroblasts from muscle digestion and ensures higher levels of SC purity than the “pre-plating SC isolation” method [17]. The SC obtained were seeded at a density of 1.25 × 105/cm2 in collagen-coated culture dishes and grown in fibroblast-conditioned GM medium (fcGM). fcGM was obtained diluting (1:1 ratio) the filtered supernatant of primary cultures of mouse fibroblasts with fresh GM medium.
RNA extraction and quantitative RT-PCR
Total RNA was extracted using the RNeasy mini kit (Qiagen) according to the manufacturer’s instructions. 1 μg of total RNA was reverse transcribed using ImProm-II™ Reverse Transcription System (Promega, Fitchburg, WI) in a final volume of 20 μl. Quantitative real-time PCR assay of mouse differentiation myogenic markers was performed using Syber Green method.
Myod primers: fw 5’-TTC TTC ACC ACA CCT CTG ACA -3’ rev 5’-GCC GTG AGA GTC GTC TTA ACT T -3’ Mrf4 (Myf6) primers: fw 5’ –GAG ATT CTG CGG AGT GCC AT -3’ rev 5’- TTC TTG CTT GGG TTT GTA GC-3’ Myogenin primers: fw 5’- ATC CAG TAC ATT GAG CGC CT-3’ rev 5’-GCA AAT GAT CTC CTG GGT TG -3’ Myf5 primers: fw 5’- TGA GGG AAC AGG TGG AGA AC -3’ rev 5’ – AGC TGG ACA CGG AGC TTT TA -3’ Pkcε (prkce) primers: fw 5’- ATG TGT GCA ATG GGC GCA AG -3’ rev 5’- CGA GAG ATC GAT GAT CAC GT -3’ Hmga1 primers: fw 5’-CAA GCA GCC TCC GGT GAG -3’ rev 5’- TGT GGT GAC TTT CCG GGT CTT G -3’
Mouse beta-glucoronidase (Gusb), known to be a good internal control to study mRNA expression in muscular derived cell lines [19] was used to normalize all results. Gusb primers: fw 5’ – CCG CTG AGA GTA ATC GGA AAC – 3’ rev 5’- TCT CGC AAA ATA AAG GCC G -3’
Polymerase chain reactions were made by StepOne Real-Time PCR System (Applied Biosystems) and GoTaq ® qPCR Master Mix (Promega). For each well, the 20 μl reaction medium contained: 10 μl of 2X GoTaq ® qPCR Master Mix (with SYBR Green), 100 nM each forward and reverse primer, 7.6 μl of RNase-free water and 2 μl cDNA template 1:5. The cycling conditions were: 95°C for 20s followed by 40 cycles of 95°C for 3s and 60°C for 30s. Real-Time RT-PCR products were confirmed by the analysis of melting curves.
Immunofluorescence
Immunofluorescence was performed as previously described [20]. Briefly, cells were grown in 48 wells dishes containing a cover slide. At the indicated time points, cells were washed in PBS and fixed with 4% paraformaldehyde in PBS for 10 minutes at room temperature and stored in PBS at 4°C. Samples were permeabilized 3 times with 1% BSA, 0.2% Triton X-100 in PBS for 5 minutes at room temperature. Then, cells were incubated in 10% goat serum in PBS for 1 hour at room temperature to saturate non-specific binding sites. Samples were incubated for 1.5 hours with primary antibody diluted 1:200 in 1% goat serum in PBS. PKCε and myosin were detected by anti-PKCε rabbit serum (Novus Biologicals, Littleton, CO NBP1-30126) and anti-Myosin Heavy Chain (MHC) monoclonal antibody (clone MF-20; Developmental Study Hybridoma Bank), respectively. Cells were washed in PBS and then incubated with secondary antibody (Alexa Fluor 488 Donkey anti-mouse IgG and Alexa Fluor 594 anti-rabbit Donkey IgG) 1:1000 for 1 hour at room temperature. Nuclei were counterstained with DAPI; fluorescence was observed with a Nikon Eclipse 80i (Tokyo, Japan) fluorescent microscope (Nikon Plan). Images were acquired by Nikon Camera DS-JMC and analysed by Nis element F2.30 (Nikon, Japan). Myogenic differentiation levels were analyzed by fusion index (number of nuclei in the myotubes/total number of nuclei). For each sample at least 500 nuclei were counted and reported values are means of 3 independent experiments ± standard deviation. Fusion index analysis is reported as percentage (0% = no detectable fusion event among MYOSIN+ cell). *p<0,05 Anova-Dunnett test vs control cells.
Cellular fractions separation and Western Blot analysis
5×106 cells were treated with NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce), used according to manifacturer’s protocol. For Western Blot analysis, samples were resuspended in lysis buffer (50 mM Tris-HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM phenylmethylsulfonyl fluoride; 1 mM Na3VO4; 1 mM NaF) and 30 μg of total proteins were loaded on 10% SDS-polyacrylamide gels. Nitrocellulose membranes were incubated with the specific primary antibody (dilutions and buffers were as indicated by manufacturer) anti-PKCε (Merck Millipore, Darmstadt, Germany 06-991), anti-HSP70 (Sigma-Aldrich, St. Louis, MO, H5147), anti- α-tubulin (Sigma-Aldrich, St. Louis, MO), anti-insulin receptor β chain (IRβ, (Cell Signaling, Danvers, MA, #3025), anti-Myogenin (Santa Cruz, Dallas, TE sc-12732), anti-myoD (Santa Cruz sc-32758), anti GAPDH (Merk Millipore MAB374) anti-HMGA1 (Abcam, Cambridge, UK ab4078), then washed and incubated with 1:5000 peroxidase-conjugated anti-rabbit or with 1:2000 peroxidase conjugated anti-mouse IgG (Pierce). Signals were revealed by ECL Supersignal West Pico Chemiluminescent Substrate detection system (Pierce).
Cell transfection
PKCε expression levels were up-regulated in C2C12 cells by the transfection of 3 μg of murine GFP-PKCε plasmid and of GFP-K522M mutated PKCε control plasmid (kindly provided by Prof. Peter Parker, Cancer Research Institute, UK) [21] using the Superfect Transfection reagent (Qiagen, Hilden, Germany). Hmga1 silencing was obtained by transfection of 100 nM specific siRNAs or control siRNA (Ambion, Austin, TX). The siRNAs from Ambion are identified by the following catalog numers: ID S67596 and ID S67598. In addition, PKCε activity was pharmacologically modulated by the εV1-2 (CEAVSLKPT) and ψεRACK (CHDAPIGYD) peptides, conjugated to TAT47-57 (CYGRKKRRQRRR) by a cysteine disulfide bound [22]. Briefly, εV1-2 is a specific PKCε inhibitor designed from the C2 region of PKCε protein that acts as a binding competitor between PKCε and its anchoring protein εRACK. Instead, ψεRACK is a PKCε allosteric activator derived from the C2 region sequence, implicated in auto inhibitory intramolecular interactions. Peptides are high specific for PKCε and they don’t interact with other PKC isozymes [23]. Peptides regulate both the enzymatic function and the localization of PKCε through the subcellular compartments. C2C12 cells and SC were incubated with DM and treated with 1μM of peptides every 24 hours for 48 or 72 hours.
Short hairpin RNA (shRNA) cell infection
In some experiments we also used shRNA gene silencing to obtain a complete shut-down of PKCε expression. In this case we used a pLKO.1 lentiviral vector encoding shRNA against mouse PKCε (Open-Biosystem, Thermo Scientific,Waltham, MA). As control (shRNACTRL), we used the MISSION pLKO.1-puro Non- Target shRNA Control Plasmid, containing a shRNA insert that does not target any known genes from any species (Sigma-Aldrich, St. Louis, MO). The shRNA expressing viruses were produced in 293TL cells according to standard protocols. Mouse proliferating C2C12 cell line was infected with Pkcε shRNA or CTRL shRNA and then cultured in the presence of puromycin (2 μg/ml) to select infected, puromycin-resistant cells.
Statistical analysis
Data sets were examined by analysis of variance (ANOVA) for comparisons between multiple groups and Dunnett’s test for comparing a control group to all other groups (when necessary). A P value of less than 0.05 was considered statistically significant.
RESULTS
PKCε expression, activation and localization during C2C12 and primary satellite cell differentiation
To evaluate PKCε expression during myotube formation in vitro and ex vivo, C2C12 and SC cells, respectively, were cultured in low serum medium for one week. Quantitative real time PCR analyses at several time points during the differentiation process confirmed that the expression of the early myogenic differentiation markers (Myod and Myf5), progressively decreased during the differentiation of C2C12 and primary SCs. As previously described [24], the transcription factors of the middle and late phases of skeletal muscle differentiation, Myogenin and Mrf4 accumulated during myofibers formation (Figure S1 A-B). Both Pkcε mRNA and PKCε protein levels progressively increased as proliferating myoblasts transitioned into myotube formation (Figure 1 A-D).
Figure 1. PKCε expression and localization during C2C12 and primary SC differentiation.
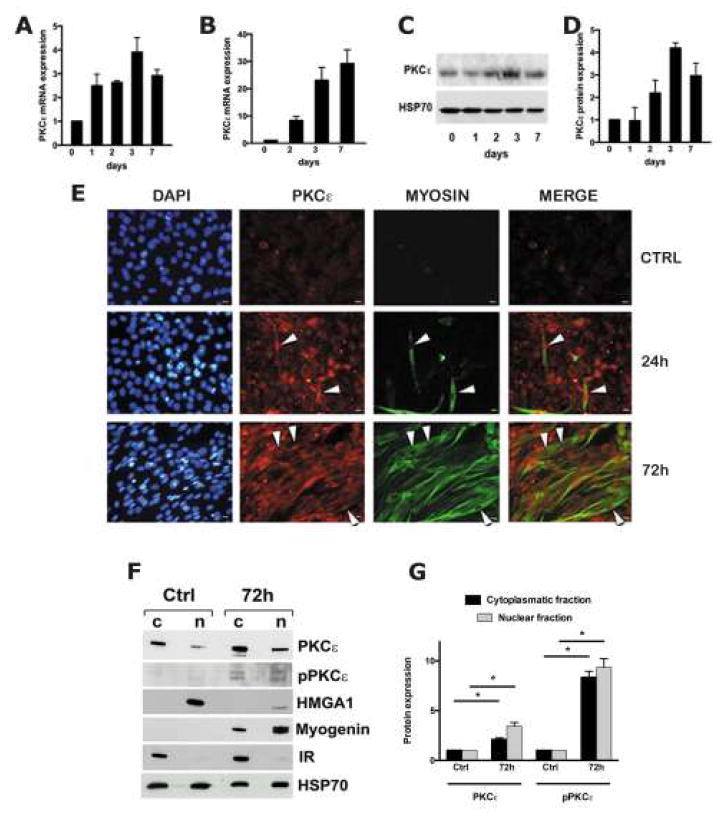
Panel A and B: PCR Real Time analysis of Pkcε mRNA during C2C12 (panel A) and SC cultures (panel B) differentiation, respectively. Results are representative of three independent experiments; values are reported as fold increase of control cell cultures (0 days) ± standard deviation. *p<0.05 Anova-Dunnett test (vs undifferentiated cells). Panel C: Western Blot analysis of PKCε protein expression levels during C2C12 cell differentiation; HSP70 was used as housekeeping protein. Panel D: Densitometry analysis of PKCε protein levels. HSP70 was used for normalization. Panel E: Immunofluorescence analysis: blue signal from nuclei obtained by DAPI staining in control (ctrl), in 24 hours (24h) differentiated- and in 72 hours (72h) differentiated cells; PKCε staining red; MYOSIN staining green colour. Arrow heads indicate cells with strong PKCε nuclear staining. Scale bar corresponds to 10 μm. Panel F: Western blot analysis of nuclear (n) and cytoplasmic (c) extracts from undifferentiated (Ctrl) and 72h differentiated C2C12 cells (72h); membranes were probed with anti-PKCε, anti phospho-PKCε (pPKCε), anti-HMGA1, anti-Myogenin and anti-HSP70 antibodies. Anti-Insulin Receptor (IR) antibody was used to exclude nuclear contamination by the cytoplasmic fraction. Panel G: Densitometry analysis of the PKCε expression levels. The values, normalized with respect to HSP70, are the mean of three independent experiments ± standard deviations (n=3). *p<0.05 Anova-Dunnett test (vs control cells).
Immunofluorescence microscopy was then applied to evaluate the subcellular localization of PKCε protein during the differentiation of C2C12 cell cultures. In undifferentiated C2C12 cells, PKCε levels were low with prevalent peri-nuclear staining (Figure 1E). During the first 24 hours of skeletal muscle differentiation, PKCε is preferentially localized inside the nucleus (arrow heads, middle panels of Figure 1E). PKCε then increases in both in the nucleus and cytoplasm at 72 hours (Figure 1E). The expression of the late muscle cell differentiation marker myosin was not detected in undifferentiated C2C12 cells, but progressively accumulated in the cytoplasm of forming myotubes (Figure 1E).
Consistent with the results obtained by immunofluorescence, cell fractionation of C2C12 cells revealed that the nuclear content of PKCε protein significantly increases 3 days after the induction of cell differentiation (Figure 1F-G). Interestingly, while PKCε is upregulated and activated (increase of phospho-PKCε levels), HMGA1 expression is concomitantly down-regulated (Figure 1F-G).
PKCε stimulates in vitro C2C12 and satellite cells differentiation via Myogenin and Mrf4 modulation
Given these data, we subsequently investigated how the induction of PKCε expression correlates with changes in the expression of myogenic genes during terminal muscle differentiation. To determine whether PKCε influences the expression of the myogenic transcription factors (Myod, Myf5, Myogenin and Mrf4), C2C12 cells were engineered to express either a wild type mouse PKCε-GFP fusion protein (PKCε-GFP) or a kinase-inactive fusion protein carrying a point mutation in the catalytic core of the enzyme (PKCεm-GFP). Transfection efficiency of both plasmids was comparable (40±3% for PKCε-GFP and 43±5% PKCεm-GFP; supplementary figure 2). Expression of PKCε-GFP, but not inactive PKCεm-GFP, significantly increased Mrf4 and Myogenin mRNA levels but didn’t significantly impact MyoD and Myf5 expression (Figure 2B). Similarly, C2C12 cells (Figure 2C) and primary SC cultures (Figure 2D) treated with ψεRACK PKCε activator showed increased Mrf4 and Myogenin mRNA expression levels, whereas the εV1-2 PKCε inhibitor yielded the opposite effect.
Figure 2. PKCε promotes myogenic differentiation through Myogenin and Mrf4 mRNA expression.
Panel A: Quantitative Real Time-PCR for Pkcε mRNA expression in C2C12 cell cultures transfected with wild type Pkcε(PKCε-GFP) or mutated Pkcε (PKCεm-GFP) compared with not transfected cells (-). Panel B: Quantitative Real Time-PCR for MyoD, Myf5, Mrf4 and Myogenin mRNAs (myog) in C2C12 cells transfected with wild type Pkcε (PKCε-GFP) or mutated Pkcε (PKCεm-GFP). Panel C-D: Quantitative Real Time-PCR for Mrf4 and Myogenin mRNAs (MYOG) in C2C12 (panel C) and SC cultures (Panel D) treated with 1 μM of PKCε specific activator and inhibitor peptides (ψεRACK and εV1-2, respectively). Housekeeping Gusb was used as reference gene. Values are reported as means of 3 independent experiments ± standard deviation. *p<0.05 Anova-Dunnett test vs untreated cells. Panels E-M: MYOSIN and Hoechst staining of 48h differentiated C2C12 cultures treated with peptides. (E) Hoechst staining of TAT treated cells; (F) Myosin (MHC) immunofluorescence of TAT treated cells, (G) merge of panels E-F. (H) Hoechst staining of εV1-2 treated cells; (I) Myosin (MHC) immunofluorescence of εV1-2 treated cells; (J) merge of panels H-I. (K) Hoechst staining of ψεRACK treated C2C12 , (L) Myosin (MHC) immunofluorescence of ψεRACK treated cells; (M) merge of panels K-L. Arrow heads indicate myotubes. Scale bar in M (100 μm) is the same for all the panels.
Fusion index analysis was performed on C2C12 cells treated with the εV1-2 PKCε inhibitor or ψε RACK activator to assess the extent by which PKCε inhibition impacts differentiation (Figure 2E-M). C2C12 cells exposed to the εV1-2 PKCε inhibitor showed a significant decrease in fusion index (20±15% vs 50±10% of TAT treated cells, p<0.05 Anova-Dunnett test vs TAT treated cells), while cells treated with the ψε RACK activator showed a significant increase in fusion index (85±12% vs 50±10% in TAT treated cells, p<0.05). These results, in combination with those of gene expression modulation experiments [16], reinforce a critical non-redundant role of nuclear PKCε in myogenic differentiation.
Hmga1 is down-modulated by PKCε during C2C12 cell differentiation
Consistent with previous studies, we observed a progressive decrease of Hmga1 expression (Figure 3A) in terminally differentiating C2C12 cell cultures [8]. Therefore, a potential relationship was investigated between PKCε and HMGA1 in proliferating C2C12 cells over-expressing PKCε. Expression of PKCε-GFP, but not of the inactive mutated PKCεm-GFP correlated with decreased expression of Hmga1 mRNA and protein as well as an accumulation of Myogenin in undifferentiated cells (Figure 3B-D). HMGA1 immunoprecipitation studies revealed that, although at low levels, endogenous PKCε form a complex with HMGA1. Furthermore, by overexpressing recombinant PKCε we observed the catalytically active form, but not the kinase dead version, co-precipitates with HMGA1, suggesting that kinase activity of PKCε is required for this interaction (Figure 3E).
Figure 3. HMGA1 is a target of PKCε during C2C12 cell differentiation.
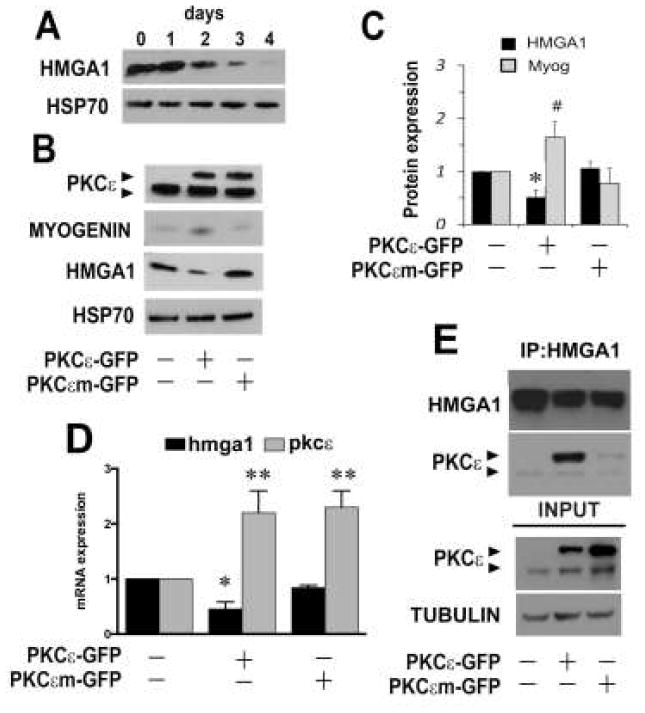
Panel A Western blot analysis of HMGA1 during C2C12 myogenic differentiation for 4 days. HSP70 was used for normalization. Panels B-C: Western blot analysis of PKCε, Myogenin, HMGA1, and HSP70 in undifferentiated C2C12 cell cultures treated with (+) vectors expressing wild type Pkcε (PKCε-GFP) or mutated Pkcε (PKCεm-GFP). A representative experiment of three replicates is shown. Panel C: Densitometry analysis of HMGA1 and Myogenin (Myog) protein expression in C2C12 cells transfected with wild type or mutated Pkcε. Values are means of 3 independent experiments ± standard deviation. HSP70 was used for normalization. *p<0.05 Anova-Dunnet test (vs untreated cells).
Panel D: Quantitative Real Time PCR analysis of Hmga1 in C2C12 cell cultures transfected with wild type Pkcε (PKCε-GFP) or mutated Pkcε (PKCεm-GFP) compared with not transfected cells (-). Panel E:Immunoprecipitation of HMGA1 in not transfected C2C12 cells (-), in C2C12 cells overexpressing the with wild form of PKCε-GFP fusion protein or the kinase-dead PKCεm-GFP. The immunoprecipitate was blotted with PKCε or HMGA1 antibodies (upper blots). Input lysates were blotted with PKCε and α-TUBULIN antibodies.
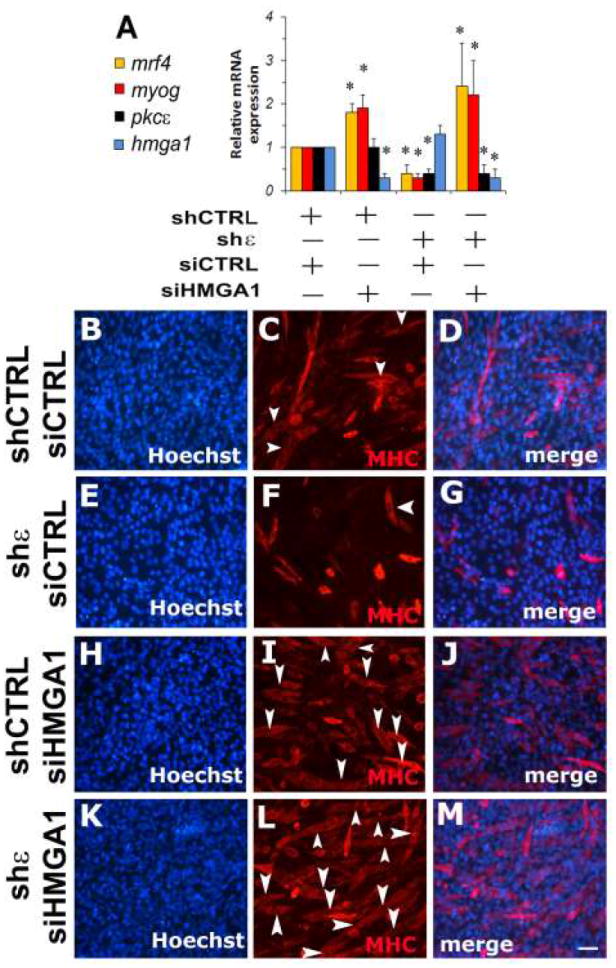
To examine how combined inhibition of Pkcε and Hmga1 affect Mrf4 and Myog gene expression during cell differentiation, C2C12 were transfected with Pkcε-targeting shRNA, Hmga1-specific siRNA or both. Reducing Hmga1 expression results in a significant increase of Myogenin and Mrf4 steady-state mRNA levels, whereas Pkcε inhibition significantly reduces Myogenin and Mrf4 expression (Figure 4A). Blocking Pkcε expression significantly impairs myotube formation (Figure 4E-G), determining a significant reduction of fusion index (10±3% vs 43±10% of shCTRL/siCTRL treated cells, p<0.05 Anova-Dunnett test vs shCTRL/siCTRL treated cells). Inhibition of Hmga1 expression leads to an increase in myotube formation (Figure 4H-J) and consequently of fusion index (62±8% vs 43±10% of shCTRL/siCTRL treated cells, p<0.05 Anova-Dunnett test vs shCTRL/siCTRL treated cells). Combined inhibition of Pkcε and Hmga1 expression significantly increases the expression of muscular differentiation markers (Figure 4A), the number of myotubes (Figure 4K-M) and fusion index (85±7% vs 43±10% of shCTRL/siCTRL treated cells, p<0.05 Anova-Dunnett test vs shCTRL/siCTRL treated cells), indicating that Hmga1 is a down-stream target of PKCε in the regulation of muscle cell differentiation program.
Figure 4. PKCε - HMGA1 axis promotes C2C12 cell differentiation.
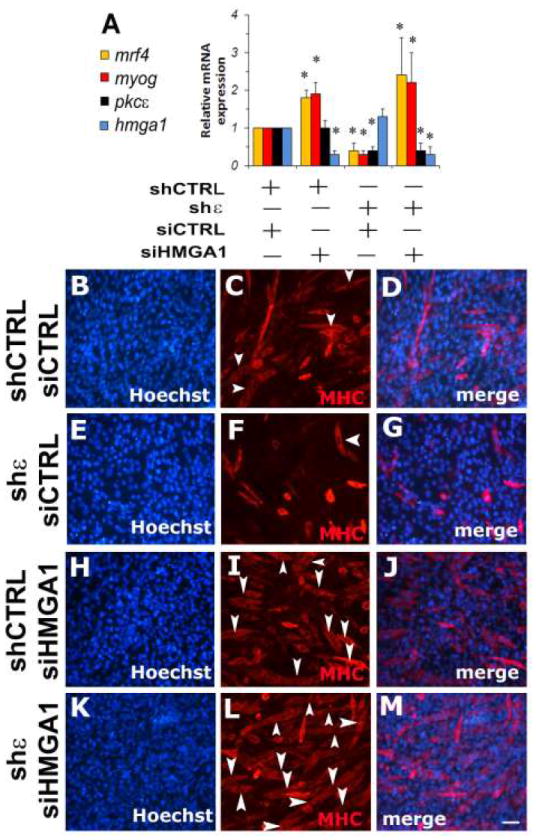
Panel A: Quantitative Real Time-PCR for Mrf4 mRNA expression (mrf4), Myogenin (myog), Pkcε and Hmga1 in C2C12 cell cultures infected with PKCε specific shRNA (shε) or control shRNA (shCTRL). After selection with puromycin (2μg/ml), infected cells were transfected with Hmga1 specific siRNAs (siHMGA1) or control siRNA (siCTRL) and then induced to differentiate for 2 days. Values are means of 3 independent experiments ± standard deviation. *p<0,05 by Anova-Dunnett test of Mrf4 and Myogenin expression (vs control cell cultures), respectively.
Panels B-M: MYOSIN and Hoechst staining of 48h differentiated C2C12 cultures after silencing of Pkcε (panels E-G and K-M) or Hmga1 (panels H-J and K-M). Panels B-D: control C2C12 cultures infected with control shRNA and, after puromycin selection, transfected with control siRNA (shCTRL siCTRL); (B) Hoechst staining, (C) Myosin immunofluorescence, (D) merge of B-C. Panels E-G: C2C12 cultures infected with Pkcε shRNA and, after puromycin selection, transfected with control siRNA (shε siCTRL); (E) Hoechst staining, (F) Myosin immunofluorescence, (G) merge of E-F. Panels H-J: C2C12 cultures infected with control shRNA and, after puromycin selection, transfected with Hmga1 siRNA (shCTRL siHMGA1); (H) Hoechst staining; (I) Myosin immunofluorescence; (J) merge of panels H-I. Panels K-M: C2C12 cultures infected with Pkcε shRNA and, after puromycin selection, transfected with Hmga1 siRNA (shε siHMGA1); (K) Hoechst staining; (L) Myosin immunofluorescence; (M) merge of K-L. Arrow heads indicate myotubes. Scale bar in M (100 μm) is the same for all the panels.
In vivo induction of Pkcε during muscle regeneration
To extend these initial observations, the impact of modulating Pkcε expression skeletal muscle repair and regeneration in vivo was assessed. To induce muscle injury and stimulate repair mechanisms, Cardiotoxin (CTX) was injected into mouse tibialis muscles. Western blot analyses of bulk muscle tissue revealed that PKCε sharply increases at day 3 post-CTX injection and continues to increase for at least 7 days following injury (Figure 5A and 5B). Histo-pathological analysis showed that the up-regulation of PKCε expression is most prominent in the fibers located at the site of injury, including the new regenerating fibers (centrally-nucleated fibers) (Figure 5C). Mouse tibialis muscles were then injected with CTX in combination with the PKCε inhibitor peptide (εV1-2), the PKCε activator peptide (ψεRACK) or control. Administration of εV1-2 inhibitor significantly inhibits CTX-induced PKCε phosphorylation (Figure 5D) and leads to a significant decrease in the levels of Myogenin and MyoD (Figure 5D and 5E). The non-redundant role of PKCε on muscle regeneration in vivo was also observed by morphological analysis of peptide treated tibialis muscles (supplementary figure 3).
Figure 5. PKCε is up-regulated during in vivo skeletal muscle regeneration.

Panel A: Western blot analysis of protein extracts from regenerating tibialis muscle at 3 and 7 days after cardiotoxin induced injury in CD1 adult mice. The blot was incubated with anti-PKCε, anti-Myogenin and anti-HSP70 antibodies. Panel B: Densitometry analysis of PKCε protein levels. Values, normalized by HSP70 expression levels, are mean of 3 independent experiments ± standard deviations (n=3). Panel C: Immunohistochemical detection of PKCε and haematoxilin/eosin (H/E) staining of serial muscle section of CD1 untreated adult mice (control) and treated with CTX (3 and 7 days). Centro-nucleated regenerating fibers expressing PKCε are indicated (arrow heads). Scale bar corresponds to 40 μm and it is the same for all panels. Panel D: p-PKCε, Myogenin and MYOD western blot analysis of protein extracts from regenerating tibialis muscles at 7 days after cardiotoxin (CTX), cardiotoxin with εV1-2 (CTX εV1-2) and cardiotoxin with ψεRACK (CTX ψεRACK) injection. GAPDH was used as loading control. Panel E: Densitometry analysis of p-PKCε, Myogenin and MYOD expression levels. The values, normalized respect to GAPDH, are mean of 3 independent experiments ± standard deviations. *p<;0.05 Anova-Dunnett test of PKCε expression vs untreated muscle; # p≤ 0.05 and § p≤0.03 Anova-Dunnett test (vs CTX treated muscle).
DISCUSSION
During muscle development, myoblasts fuse together to form muscle fibers. Once the muscle is built, postnatal muscle growth and regeneration is maintained by the subset of muscle stem/progenitor cells called satellite cells (SC). Recent studies have raised the possibility that PKC family members play a crucial role in muscle differentiation [15, 16]. In the context of myogenic differentiation of C2C12 cell line and SC primary cells, our present data show that PKCε, belonging to the novel group of the serine-threonine kinase C family, is activated and up-regulated during muscle stem cell differentiation. Interestingly, the active form of PKCε, phosphorylated on Serine 729, increases during differentiation and is preferentially located in the nucleus (Figure 2). Previous studies have shown that the nuclear translocation of PKCε occurs through F-Actin as a possible transporter of phospho-PKCε [25]. Our data seem to be different from what Gaboardi et al. previously described. In their article, using an insulin- induced model of C2C12, they demonstrated that PKCε is mostly localized in cytoplasm, nearby the Golgi membrane. The discrepancy with our data can be explained in part by the use of different protocols and reagents.
In the nucleus, PKCε is able to mediate the phosphorylation of many targets and alter their activation, subcellular localization or degradation [26, 27]. We have observed that Hmga1 is a possible target of nuclear PKCε in muscle cell differentiation. HMGA proteins are non-histone architectural elements of chromatin that dynamically modulate DNA-linked processes. These proteins are expressed in embryonic stem cells and in proliferating cells but are not detectable in fully differentiated cells [28]. Li et al. demonstrated that Hmga2 is important for myoblast proliferation and early myogenesis [29]. Also the Hmga1 isoform is known to be involved in muscle differentiation. Notably, Brocher et al. [8] have shown that Hmga1 down-regulation during the early phases of myogenesis is important for inducing the expression of myogenic markers, MyoD and Myogenin. Less is known about the signaling pathway that is involved in Hmga1 regulation during myogenesis. Here, for the first time, we show that PKCε alters Hmga1 expression during in vitro and ex vivo skeletal muscle differentiation. Specifically, we have found that siRNA-mediated inhibition of Hmga1 leads to increased expression of Myogenin and Mrf4 mRNA. We have also observed that the levels of nuclear PKCε expression increase in the nucleus upon differentiation and that inhibition of PKCε diminishes Myogenin and Mrf4 expression as well as myotube formation. Of note, the inhibition of muscle cell differentiation generated by shRNA Pkcε silencing could be completely abrogated by the simultaneous inhibition of Hmga1 expression. As skeletal muscle cell differentiation needs Hmga1 shut down to progress, we suggest that the nuclear translocation of activated PKCε is critical for Hmga1 inhibition and SC differentiation.
Our data together with Gogoi et al. observations [30] demonstrate that HMGA1, phosphorylated by PKCε, may reside longer in the heterochromatin preferentially interacting with positively charged histones.
Since PKCε promotes myogenic differentiation in vitro and ex vivo, which is a crucial phase of skeletal muscle regeneration, we studied the involvement of this kinase in a model of CTX - induced muscle repair in mice. We found that PKCε is up-regulated 7 days after injury, preferentially localizing at regenerating centrally-nucleated fibers. To pursue a better understanding of the PKCε involvement in muscle regeneration, we injected (intra-muscular) CTX- treated animals with a specific PKCε inhibitor peptide (εV1-2) to block PKCε activation and translocation. The consistent decrease of both Myogenin and Myod expression upon PKCε inhibition supports that PKCε contributes to the muscle regeneration process in vivo. The PKCε activator peptide, ψεRACK, did not enhance PKCε phosphorylation or the expression of either Myod or Myogenin induced by CTX. We infer that this observation is likely due to PKCε activation reaching a plateau level in the injured muscle.
Overall, this study provides the first evidence for a role of the PKCε-HMGA1 axis in skeletal muscle differentiation and regeneration.
Supplementary Material
Acknowledgments
We are grateful to Vincenzo Palermo and Luciana Cerasuolo for technical support.
FUNDING
This work was supported by FIRB-accordi di programma 2010 CUP D91J10000100001 to M.V. (IT-Ministry of the University and Scientific and Technological Research/Ministry of Education, University and Research, MIUR) and Regione Emilia-Romagna Area 1 – Strategic Program 2010-2012 code PRUa1RI-2012-006 to P.M. D.D.M.: PhD fellow was supported by Cariparma Foundation.
Footnotes
Disclosures
No conflicts of interest are declared by the author(s)
References
- 1.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tajbakhsh S. Skeletal muscle stem cells in developmental versus regenerative myogenesis. J Intern Med. 2009;266:372–389. doi: 10.1111/j.1365-2796.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 3.Pownall ME, Gustafsson MK, Emerson CP., Jr Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol. 2002;18:747–783. doi: 10.1146/annurev.cellbio.18.012502.105758. [DOI] [PubMed] [Google Scholar]
- 4.Ishibashi J, Perry RL, Asakura A, Rudnicki MA. MyoD induces myogenic differentiation through cooperation of its NH2- and COOH-terminal regions. J Cell Biol. 2005;171:471–482. doi: 10.1083/jcb.200502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassar-Duchossoy L, Gayraud-Morel B, Gomès D, Rocancourt D, Buckingham M, Shinin V, Tajbakhsh S. Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature. 2004;431:466–471. doi: 10.1038/nature02876. [DOI] [PubMed] [Google Scholar]
- 6.Venuti JM, Morris JH, Vivian JL, Olson EN, Klein WH. Myogenin is required for late but not early aspects of myogenesis during mouse development. J Cell Biol. 1995;128:563–576. doi: 10.1083/jcb.128.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozturk N, Singh I, Mehta A, Braun T, Barreto G. HMGA proteins as modulators of chromatin structure during transcriptional activation. Front Cell Dev Biol. 2014 Mar 6;2:5. doi: 10.3389/fcell.2014.00005. eCollection 2014. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brocher J, Vogel B, Hock R. HMGA1 down-regulation is crucial for chromatin composition and a gene expression profile permitting myogenic differentiation. BMC Cell Biol. 2010;11:64. doi: 10.1186/1471-2121-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton PM, Messing RO. The substrates and binding partners of protein kinase C epsilon. Biochem J. 2010;427:189–196. doi: 10.1042/BJ20091302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gobbi G, Di Marcantonio D, Micheloni C, Carubbi C, Galli D, Vaccarezza M, Bucci G, Vitale M, Mirandola P. TRAIL up-regulation must be accompanied by a reciprocal PKCε down-regulation during differentiation of colonic epithelial cell: implications for colorectal cancer cell differentiation. J Cell Physiol. 2012;227:630–638. doi: 10.1002/jcp.22765. [DOI] [PubMed] [Google Scholar]
- 11.Mirandola P, Gobbi G, Ponti C, Sponzilli I, Cocco L, Vitale M. PKCepsilon controls protection against TRAIL in erythroid progenitors. Blood. 2006;107:508–513. doi: 10.1182/blood-2005-07-2676. [DOI] [PubMed] [Google Scholar]
- 12.Gobbi G, Mirandola P, Carubbi C, Micheloni C, Malinverno C, Lunghi P, Bonati A, Vitale M. Phorbol ester-induced PKCepsilon down-modulation sensitizes AML cells to TRAIL-induced apoptosis and cell differentiation. Blood. 2009;113:3080–3087. doi: 10.1182/blood-2008-03-143784. [DOI] [PubMed] [Google Scholar]
- 13.Gobbi G, Mirandola P, Sponzilli I, Micheloni C, Malinverno C, Cocco L, Vitale M. Timing and expression level of protein kinase C epsilon regulate the megakaryocytic differentiation of human CD34 cells. Stem Cells. 2007;25:2322–2329. doi: 10.1634/stemcells.2006-0839. [DOI] [PubMed] [Google Scholar]
- 14.Gobbi G, Mirandola P, Carubbi C, Masselli E, Sykes SM, Ferraro F, Nouvenne A, Thon JN, Italiano JE, Jr, Vitale M. Proplatelet generation in the mouse requires PKCε-dependent RhoA inhibition. Blood. 2013;122:1305–11. doi: 10.1182/blood-2013-04-490599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madaro L, Marrocco V, Fiore P, Aulino P, Smeriglio P, Adamo S, Molinaro M, Bouché M. PKCθ signaling is required for myoblast fusion by regulating the expression of caveolin-3 and β1D integrin upstream focal adhesion kinase. Mol Biol Cell. 2011;22:1409–1419. doi: 10.1091/mbc.E10-10-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaboardi GC, Ramazzotti G, Bavelloni A, Piazzi M, Fiume R, Billi AM, Matteucci A, Faenza I, Cocco L. A role for PKCepsilon during C2C12 myogenic differentiation. Cell Signal. 2010;22:629–635. doi: 10.1016/j.cellsig.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Ceccarelli G, Benedetti L, Galli D, Prè G, Silvani G, Crosetto N, Magenes G, Cusella De Angelis MG. Low-amplitude high frequency vibration down-regulates myostatin and atrogin-1 expression, two components of the atrophy pathway in muscle cells. J Tissue Eng Regen Med. 2014;8:396–406. doi: 10.1002/term.1533. [DOI] [PubMed] [Google Scholar]
- 18.Galli D, Carubbi C, Masselli E, Corradi D, Dei Cas A, Nouvenne A, Bucci G, Arcari ML, Mirandola P, Vitale M, Gobbi G. PKCε is a negative regulator of PVAT-derived vessel formation. Exp Cell Res. 2015 Jan 15;330:277–86. doi: 10.1016/j.yexcr.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura M, Nikawa T, Kawano Y, Nakayama M, Ikeda M. Effects of dimethyl sulfoxide and dexamethasone on mRNA expression of housekeeping genes in cultures of C2C12 myotubes. Biochem Biophys Res Commun. 2008;367:603–8. doi: 10.1016/j.bbrc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Galli D, Gobbi G, Carrubbi C, Di Marcantonio D, Benedetti L, De Angelis MG, Meschi T, Vaccarezza M, Sampaolesi M, Mirandola P, Vitale M. The role of PKCε-dependent signaling for cardiac differentiation. Histochem Cell Biol. 2013;139:35–46. doi: 10.1007/s00418-012-1022-4. [DOI] [PubMed] [Google Scholar]
- 21.Ivaska J, Whelan RD, Watson R, Parker PJ. PKC epsilon controls the traffic of beta1 integrins in motile cells. EMBO J. 2002;21:3608–3619. doi: 10.1093/emboj/cdf371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandman R, Disatnik MH, Churchill E, Mochly-Rosen D. Peptides derived from the C2 domain of protein kinase C epsilon (epsilon PKC) modulate epsilon PKC activity and identify potential protein-protein interaction surfaces. J Biol Chem. 2007;282:4113–4123. doi: 10.1074/jbc.M608521200. [DOI] [PubMed] [Google Scholar]
- 23.Begley R, Liron T, Baryza J, Mochly-Rosen D. Biodistribution of intracellularly acting peptides conjugated reversibly to Tat. Biochem Biophys Res Commun. 2004;318:949–54. doi: 10.1016/j.bbrc.2004.04.121. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa M, Mizofuchi H, Kobayashi Y, Tsuzuki G, Yamamoto M, Wada S, Kamemura K. Terminal differentiation program of skeletal myogenesis is negatively regulated by O-GlcNAc glycosylation. Biochim Biophys Acta. 2012;1820:24–32. doi: 10.1016/j.bbagen.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Dasgupta S, Bhattacharya S, Maitra S, Pal D, Majumdar SS, Datta A, Bhattacharya S. Mechanism of lipid induced insulin resistance: activated PKCε is a key regulator. Biochim Biophys Acta. 2011;1812:495–506. doi: 10.1016/j.bbadis.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Gupta P, Ho PC, Huq MD, Khan AA, Tsai NP, Wei LN. PKCepsilon stimulated arginine methylation of RIP140 for its nuclear-cytoplasmic export in adipocyte differentiation. PLoS One. 2008;3:e2658. doi: 10.1371/journal.pone.0002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dey D, Bhattacharya A, Roy S, Bhattacharya S. Fatty acid represses insulin receptor gene expression by impairing HMGA1 through protein kinase Cepsilon. Biochem Biophys Res Commun. 2007;357:474–9. doi: 10.1016/j.bbrc.2007.03.183. [DOI] [PubMed] [Google Scholar]
- 28.Catez F, Hock R. Binding and interplay of HMG proteins on chromatin: Lessons from live cell imaging. Biochim Biophys Acta. 2010;1799:15–27. doi: 10.1016/j.bbagrm.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Gilbert JA, Zhang Y, Zhang M, Qiu Q, Ramanujan K, Shavlakadze T, Eash JK, Scaramozza A, Goddeeris MM, Kirsch DG, Campbell KP, Brack AS, Glass DJ. An HMGA2-IGF2BP2 axis regulates myoblast proliferation and myogenesis. Dev Cell. 2012;23:1176–88. doi: 10.1016/j.devcel.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gogoi B, Chatterjee P, Mukherjee S, Buragohain AK, Bhattacharya S, Dasgupta S. A polyphenol rescues lipid induced insulin resistance in skeletal muscle cells and adipocytes. Biochem Biophys Res Commun. 2014;452:382–8. doi: 10.1016/j.bbrc.2014.08.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


