Abstract
Growth delay is common in children with chronic kidney disease (CKD), often associated with poor quality of life. The role of anemia in uremic growth delay is poorly understood. Here we describe an induction of uremic growth retardation by a 0.2% adenine diet in wild-type (WT) and hepcidin gene (Hamp) knockout (KO) mice, compared with their respective littermates fed a regular diet. Experiments were started at weaning (3 wk). After 8 wk, blood was collected and mice were euthanized. Adenine-fed WT mice developed CKD (blood urea nitrogen 82.8 ± 11.6 mg/dl and creatinine 0.57 ± 0.07 mg/dl) and were 2.1 cm shorter compared with WT controls. WT adenine-fed mice were anemic and had low serum iron, elevated Hamp, and elevated IL6 and TNF-α. WT adenine-fed mice had advanced mineral bone disease (serum phosphorus 16.9 ± 3.1 mg/dl and FGF23 204.0 ± 115.0 ng/ml) with loss of cortical and trabecular bone volume seen on microcomputed tomography. Hamp disruption rescued the anemia phenotype resulting in improved growth rate in mice with CKD, thus providing direct experimental evidence of the relationship between Hamp pathway and growth impairment in CKD. Hamp disruption ameliorated CKD-induced growth hormone-insulin-like growth factor 1 axis derangements and growth plate alterations. Disruption of Hamp did not mitigate the development of uremia, inflammation, and mineral and bone disease in this model. Taken together, these results indicate that an adenine diet can be successfully used to study growth in mice with CKD. Hepcidin appears to be related to pathways of growth retardation in CKD suggesting that investigation of hepcidin-lowering therapies in juvenile CKD is warranted.
Keywords: chronic kidney disease, adenine, growth delay, hepcidin, anemia
chronic kidney disease (CKD) in children has significant morbidity and mortality (34, 64), especially in the setting of severe growth failure (29). Growth failure is a unique feature of pediatric CKD that has lacked a convenient mouse model. The pathogenesis of growth delay in children with CKD is thought to be multifactorial. Malnutrition, disruption of growth hormone (GH)-insulin-like growth factor 1 (IGF1) axis, mineral and bone disorder (MBD), anemia, metabolic acidosis, and chronic inflammation are all believed to contribute to growth delay in pediatric/juvenile CKD (33). However, with the exception of the GH/IGF-1 axis, no convincing clinical or experimental data exist to support the putative role of these comorbidities in the development of uremic growth failure.
The role of anemia in growth delay of children with CKD is controversial. While earlier clinical reports appeared to support a relationship between anemia and poor growth in children with CKD (52), more recent investigations failed to confirm the association between growth and anemia (44) or growth and adherence to anemia treatments (5). To the best of our knowledge, no experimental studies have reported whether correction of anemia in juvenile CKD would improve linear growth.
The hepatic hormone hepcidin (Hamp), the master regulator of iron metabolism (39), is linked to several energy metabolic pathways (23) and is now recognized as an important player in CKD pathogenesis. Serum hepcidin is elevated in adults (60) and children (9) with CKD, contributing to the development of anemia through reduction of iron availability from diet and body stores. A recent report indicated that serum hepcidin concentrations correlated inversely with growth in peritoneal dialysis-dependent children with end-stage renal disease (ESRD) (14). Human hepcidin overexpression in mice caused an iron-deficient phenotype, including stunted growth (36). However, the role of hepcidin in growth delay caused by juvenile CKD remains largely unclear. Hepcidin has been recently proposed as a promising therapeutic target in CKD. In a rat model of adenine-induced CKD, the small molecule bone morphogenic protein (BMP) inhibitor LDN-193189, a hepcidin-lowering agent, mobilized iron for incorporation into red blood cells and increased the hemoglobin content of reticulocytes (55). Lack of hepcidin partially protects from the inflammatory insult and ensuing anemia in a mouse model of anemia of inflammation triggered by heat killed Brucella abortus (24). Antihepcidin antibody treatment was shown to modulate iron metabolism and was effective in that model (48).
A high adenine diet has been shown to induce CKD in adult mice (57) and juvenile rats (17) via the development of tubulointerstitial nephropathy. The mechanism of renal injury is related to poor solubility of the adenine metabolite 2,8-dihydroxyadenine in the urine and its precipitation in renal tubules (12). Renal insufficiency due to 2,8-dihydroxiadenine nephrolithiasis has been observed in humans with adenine phosphoribosyltransferase deficiency (46). No systemic effects of the standard high adenine diet, other than those mediated by kidney injury, have been reported. To induce renal damage, adenine has to be converted to 2,8-dihydroxiadenine in the kidney. While other tissues may convert adenine into 2,8-dihydroxyadenine, this metabolite is degraded by extrarenal cells at a rate sufficient to prevent accumulation of significant quantities of 2,8-dihydroxyadenine in the circulation (42). In the study reported here, we describe a model of CKD in growing mice that closely resembles CKD in children. The CKD was induced by administration of a 0.2% adenine diet. This model was then utilized to ascertain the effects of Hamp deletion on the CKD phenotype.
MATERIALS AND METHODS
Animals and experimental design.
The animal protocol was approved by the Institutional Animal Care and Use Committee of Weill Cornell Medicine and complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Mice were housed in standard cages in a temperature-controlled environment (70–74°F) and maintained on a 12:12-h light-dark cycle. They had free access to food and water. At weaning (21 days of age) in-house bred male C57BL/J6 wild-type (WT) and Hamp-knockout (HKO) mice were randomly assigned to four groups (5–10 mice per group): WT controls, HKO controls [CKD(−)], WT with CKD, and HKO with CKD [CKD(+)]. CKD was induced by an adenine high phosphorus diet [0.2% adenine, 0.9% phosphorus; Harlan/Envigo Teklad, Madison, WI (adenine diet)]. Eight weeks later, mice were euthanized by Isoflurane overdose, followed by cervical dislocation. Linear growth was tracked by weekly measurements of the nose (snout) to tail tip length of Isoflurane anesthetized animals. Half of this length was used to calculate body mass index (BMI). Body weight of anesthesized animals was measured using a calibrated electronic scale (Ohaus ABSCL Compact Scale, CL201).
Blood biochemistry and complete blood count.
Blood was drawn at euthanasia via retro-orbital puncture. Serum blood urea nitrogen (BUN), creatinine, and phosphorus were measured on a Beckman Coulter AU 680 chemistry analyzer. Serum iron was measured using colorimetric assay (Pointe Scientific, Canton, MI). ELISA was used to measure serum hepcidin (Intrinsic Lifesciences, La Jolla, CA), FGF23 (EMD Millipore, St. Charles, MO), erythropoietin, GH, and IGF1 (R&D Systems, Minneapolis, MN). Serum cytokines (IL-6, TNF-α, and KC-GRO) were determined using a V-Plex kit from Meso Scale Discovery (Rockville, MD) on the SI2400 Multiplex Analyzer. Complete blood counts (CBCs) were performed using the IDEXX Procyte DX hematology analyzer.
mRNA expression.
Following euthanasia, kidneys and livers were removed, flash-frozen in liquid nitrogen, and stored at −80°C until analysis. Total cellular RNAs from frozen tissues were purified using PureLink RNA Mini Kit (ThermoFisher Scientific, Waltham, MA) following the manufacturer's instruction. The reverse transcription of RNA to cDNA was carried out using High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific). Real-time qPCR analyses of target gene expression were performed using the TaqMan Gene Expression Assay Mix and TaqMan Gene Expression Master Mix (ThermoFisher Scientific) in an ABI 7900 HT Fast Real-Time PCR System according to the manufacturer's protocol. All primers and probes for the target genes were obtained from ThermoFisher Scientific and mouse Actb was used as an internal control (Table 1). The amplification efficiencies of all target genes and internal control gene were above 90% as stated by the manufacturer (ThermoFisher Scientific), which were further validated by using a five-log range of dilution of a pooled mouse sample. The expression of the control gene was not significantly different among the groups. Data were analyzed by the comparative computed tomography (CT) method (50). Results are presented in arbitrary units, with one unit being the mean relative mRNA level in the WT CKD(−) control mice.
Table 1.
TaqMan gene expression assays-on-demand identifiers
| Genes | TaqMan probes ID |
|---|---|
| Target genes | |
| Ghr | Mm00439093_m1 |
| Igf1 | Mm00439561_m1 |
| Col1a1 | Mm00801666_g1 |
| Fn1 | Mm01256744_m1 |
| Socs2 | Mm00850544_g1 |
| Internal control gene | |
| Actb | Mm02619580_g1 |
Western blotting.
Liver tissues were treated with lysis buffer and protein concentrations were determined using BCA protein assay kit (Thermo Scientific). Tissue homogenates were loaded on to a XCell SureLock Midi-Cell Electrophoresis System. Proteins were electroblotted onto an immunoblot PVDF membrane (Bio-Rad). After transfer, nonspecific binding was prevented with 5% skimmed milk TBS-Tween. After blocking, the membrane was incubated with anti-GH receptor antibody (R&D Systems). Chemiluminescent bands were visualized using enhanced chemiluminescence solution (Thermo Scientific). Quantification of western blots was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Kidney histology.
Kidneys were fixed in 10% neutral buffered formalin for 24 h and subsequently paraffin embedded. Sections were cut 5-μm thick and stained with hematoxylin-eosin (H&E) to evaluate morphology and architecture of tubules, interstitium and glomeruli, and stained with Masson's trichrome to demonstrate collagen matrix accumulation. A single blinded comparative pathologist performed fibrosis quantification by counting the area of the images stained blue. For each kidney, four images measuring each 566,940 μm2 were acquired from randomly selected areas of the renal cortex. Images were then analyzed using the ImageJ 1.51d software (National Institutes of Health) with the Colour Deconvolution 1.7 plugin (45). The mean positive staining area for each kidney was calculated from the values obtained from the four images. Selection of the threshold was performed by the pathologist to ensure that collagen was defined as positive and all other elements, including tubular casts and brush borders were defined as negative.
Growth plate histology and morphometry.
The left hindlimbs were removed from all groups of mice, cleaned of muscle and skin, and preserved in 10% neutral buffered formalin. Following fixation, the tibias were decalcified in 10% EDTA containing 0.05 M Tris buffer, pH 7.4 for 10 days. Decalcified tissues were embedded in paraffin, sectioned at 7 μm along the long axis of the bone and stained using hematoxylin and eosin (H&E) and the safranin O method for proteoglycans. With the use of Bioquant Osteo II software (Bioquant Image Analysis, Nashville, TN), the height of each growth plate was measured by collecting 15–30 measurements along the middle region of each plate. The curved ends of the growth plates, due to the curvature of the growing bony ends, were not included in these analyses.
Micro-CT analysis of bone.
Femurs removed after euthanasia were dissected free of soft tissue and placed in 70% ethanol. A distal segment of the femurs (1.35 mm in length starting 100 μm from the growth plate) and a 1.4 mm segment of the mid-diaphysis were used, respectively, for trabecular and cortical bone microcomputed tomography (micro-CT) analysis on a Scanco μCT 35 system (Scanco Medical, Brüttisellen, Switzerland). Voxel size of 6 μm, 55 KVp, 0.36° rotation step (180° angular range), and a 400-ms exposure per view were used for the scans. The Scanco μCT software (HP, DECwindows Motif 1.6) was used for three-dimensional (3D) reconstruction and viewing of images. 3D reconstructions were performed automatically with a user-defined beam hardening correction factor. Mineral density calibrations were done based on preset by Scanco algorithms for 55 KVp during the reconstructions. Evaluation of the 3D reconstructed volumes was performed using the Scanco morphometry and densitometry software for open VMS on a Hewlett-Packard RAID server. After 3D reconstruction, volumes were segmented using a global threshold of 0.4 g/cc. Histomorphometric indexes were reported using nomenclature recommended by the American Society of Bone and Mineral Research (19).
Statistical analysis.
Statistical significance of the differences between two groups was determined with an unpaired two-sided Student's t-test. One-way ANOVA was used for the ANOVA among four groups, with Bonferroni's post hoc multiple comparisons test if variance was significant. Plot error bars depict SD, and data are reported as means ± SD. A two-sided P value was considered significant when P < 0.05.
RESULTS
Adenine diet induces chronic kidney disease and growth retardation in developing mice.
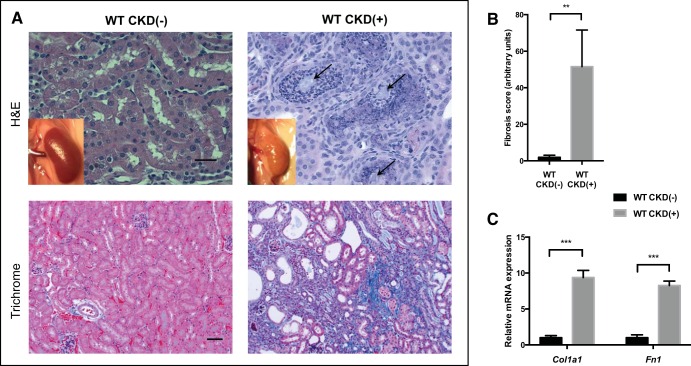
After receiving the adenine diet for 8 wk, WT mice showed significant elevation of BUN and serum creatinine compared with control mice, as shown in Table 2. Normalized to body weight, serum creatinine was significantly higher (5-fold) in adenine-fed mice compared with control mice (50.3 ± 4.6 vs. 10.0 ± 2.9 mg·dl−1·kg−1, respectively, P < 0.001). CKD(+) WT mice were hyperphosphatemic and had elevated FGF23, low serum iron, and elevated serum hepcidin, all significantly different from the CKD(−) WT controls (Table 2). Kidney histology revealed typical features of adenine-induced tubulointerstitial nephropathy, including crystal formation, severe tubulointerstitial inflammation (Fig. 1, A, top right), and interstitial fibrosis (Fig. 1, A, bottom right, and B). Profibrotic genes Col1a1 and Fn1 mRNA expression was shown to be 9- and 8-fold higher, respectively, in the kidneys of adenine-fed mice, compared with controls (Fig. 1C).
Table 2.
Renal function and other biochemical serum parameters in wild-type mice fed 0.2% adenine diet and control diet
| Parameters | Control Diet (n = 6) | Adenine Diet (n = 6) | P Value |
|---|---|---|---|
| BUN, mg/dl | 31.8 ± 3.9 | 82.8 ± 11.6 | <0.001 |
| Creatinine, mg/dl | 0.25 ± 0.07 | 0.57 ± 0.07 | <0.001 |
| Creatinine/body weight, mg·dl−1·kg−1 | 10.0 ± 2.9 | 50.3 ± 4.6 | <0.001 |
| Phosphorus, mg/dl | 6.8 ± 2.6 | 17.0 ± 3.0 | 0.001 |
| FGF23, ng/ml | 0.334 ± 0.078 | 204.0 ± 115.0 | 0.003 |
| Growth hormone, ng/ml | 1.78 ± 2.28 | 19.37 ± 5.43 | <0.001 |
| IGF1, ng/ml | 225.9 ± 40.7 | 327.5 ± 37.7 | 0.002 |
| Iron, μg/dl | 257.8 ± 64.5 | 155.0 ± 12.3 | 0.02 |
| Hepcidin, ng/ml | 183.1 ± 69.3 | 563.0 ± 237.0 | 0.005 |
| Erythropoietin, pg/ml | 92.5 ± 38.4 | 235.7 ± 102.9 | 0.01 |
Values are means ± SD. BUN, blood urea nitrogen. FGF23, fibroblast growth factor 23; IGF1, insulin-like growth factor 1.
Fig. 1.
Tubulointerstitial inflammation, crystalline deposits, and interstitial fibrosis in the kidneys of developing adenine-fed mice. A: macroscopic appearance (insets) and histology of the kidneys. Control kidney appears normal, whereas kidney of an adenine-fed mouse is pale, with a rough tuberous surface. Hematoxilin and eosin (H&E) staining (top, ×40 magnification) demonstrated crystalline deposits (arrows) and severe tubulointerstitial inflammation in the kidneys of WT (wild-type) mice with chronic kidney disease [CKD(+)]. Mason trichrome staining (botttom, ×20 magnification) revealed advanced interstitial fibrosis, with tubular loss and atrophy in the kidneys of adenine-fed CKD(+) mice. Scale bars: 20 μm, top; 50 μm, bottom. B: fibrosis quantification with image deconvolution technique. Adenine fed mice had 40-fold more Mason trichrome blue stained collagen compared with control mice (4 mice per group). C: collagen type I-α1 (Col1a1) relative mRNA expression was 9-fold higher, and fibronectin 1 (Fn1) mRNA 8-fold higher in the kidneys of adenine-fed mice compared with controls. Three kidney samples per group were used for PCR. Error bars represent SD. **P < 0.01; ***P < 0.001.
Micro-CT analysis of the femurs demonstrated reduced amounts of bone with a decreased bone volume fraction (BV/TV) in the bone cortex and increased cortical porosity in CKD(+) WT mice compared with CKD(−) WT mice. BV/TV was also decreased in trabecular bone. The decreased BV/TV was caused by a decrease in the number of trabeculae and their thickness, which also yielded an increase in trabecular separation. The decreased connectivity density confirmed this effect (Table 3).
Table 3.
Adenine-induced CKD results in cortical and trabecular bone loss in young mice
| Parameters | Control (n = 7) | CKD (n = 7) | P Value |
|---|---|---|---|
| Cortical bone | |||
| TV, mm3 | 1.86 ± 0.51 | 1.64 ± 0.38 | 0.38 |
| BV, mm3 | 0.81 ± 0.22 | 0.46 ± 0.12 | 0.003 |
| BV/TV, % | 43.3 ± 2.8 | 28.0 ± 1.7 | <0.001 |
| Ct.Po, % | 7.28 ± 1.2 | 11.8 ± 1.17 | <0.001 |
| Ct.Th, mcm | 0.17 ± 0.02 | 0.10 ± 0.007 | <0.001 |
| pMOI, mm4 | 0.37 ± 0.08 | 0.19 ± 0.03 | <0.001 |
| Trabecular bone | |||
| TV, mm3 | 2.61 ± 0.23 | 2.58 ± 0.08 | 0.72 |
| BV, mm3 | 0.44 ± 0.11 | 0.25 ± 0.04 | 0.002 |
| BV/TV, % | 16.6 ± 4.0 | 10.0 ± 1.0 | 0.002 |
| Tb.Th, mm | 0.039 ± 0.005 | 0.030 ± 0.002 | 0.001 |
| Tb.N, 1/mm | 5.68 ± 0.26 | 4.38 ± 0.29 | <0.001 |
| Tb.Sp, mm | 0.17 ± 0.01 | 0.22 ± 0.02 | <0.001 |
| Bs/BV | 68.1 ± 8.4 | 80.9 ± 7.3 | 0.01 |
| Conn.D, 1/mm3 | 387.1 ± 31.5 | 215.3 ± 23.9 | <0.001 |
Values are means ± SD. CKD, chronic kidney disease; TV, total volume; BV, bone volume; Ct.Po, cortical porosity; Ct.Th, cortical thickness; pMOI, polar moment of inertia; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation; Bs/BV, bone-specific surface; Conn.D, connectivity/density.
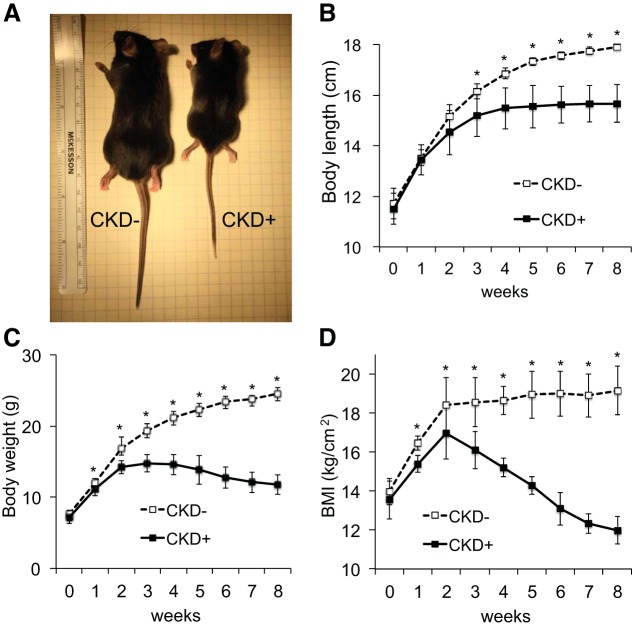
Linear growth was significantly retarded in the juvenile WT adenine-fed mice compared with their littermate controls fed a regular diet (Fig. 2). The differences became significant after 3 wk of the experimental diet and continued to escalate during the remainder of the experimental period. After 3 wk, control animals continued to grow; however, mice with adenine-induced CKD demonstrated growth stunting from week 4 onwards (Fig. 2, A and B). As a result, adenine-fed mice were, on average, 2.1 cm shorter than controls at the end of the experiment (15.7 ± 0.7 vs. 17.9 ± 0.1 cm. respectively, P < 0.001). The adenine-fed group also had a slower rate of weight gain and after 3 wk on the experimental diet mice began losing weight. At the end of the experiment, mice with CKD showed, on average, a 52.2% weight deficit, compared with controls (Fig. 2C). After an initial brief increase in BMI, mice with CKD quickly showed a drastic reduction in BMI, which, by 6 wk, fell below the mean level of baseline BMI (Fig. 2D). At euthanasia, adenine-fed mice had a 60% deficit in BMI, compared with controls.
Fig. 2.
Growth rate in juvenile adenine-induced chronic kidney disease (CKD). Body length and weight were measured at the beginning of experiment and then weekly for 8 wk in mice fed 0.2% adenine diet (n = 9) and their littermates fed the control diet (n = 9). Body length was measured as a nose (snout) to tail tip length of the Isoflurane anesthetized animals. Half of this length was used to calculate body mass index (BMI) as weight (kg)/[length (cm)]2. A: representative images of the littermates fed control diet (left) and adenine diet (right). Adenine-fed mice [CKD(+)] were, on average, 2.1 cm shorter than controls [CKD(−)] at the end of the experiment. B: body length over the course of the experimental period. The differences in body length became significant after 3 wk of the experimental diet and continued to escalate during the remainder of the experimental period. While control animals continued to grow, mice with adenine-induced CKD demonstrated growth stunting from week 4 onwards. C: body weight. CKD(+) group had slower rate of weight gain and mice began losing weight by 3 wk of the experiment. D: BMI. After an initial brief increase in BMI, mice with CKD developed drastic reduction of BMI which by 6 wk fell below the baseline BMI. Error bars represent SD. *P < 0.05.
The adenine diet was well tolerated by juvenile mice and there were no deaths before scheduled euthanasia. The mice did exhibit somewhat decreased motor activity over the last 2 wk on the protocol and a slightly limping gait was observed at that time; however, this did not limit the ability of mice to easily reach food and water. More frequent bedding changes were occasionally required for adenine-fed mice due to severe polyuria, which is a well-recognized phenomenon in the adenine model of CKD (65).
The effect of the adenine diet on changes in the growth hormone/insulin-like growth factor 1 (GH/IGF1) axis was also assessed. Examination of the liver for transcriptional levels of genes of interest shown in Table 1 was carried out by real-time PCR. Expression of growth hormone receptor (Ghr) and insulin-like growth factor 1 (Igf1) mRNA in the liver was significantly reduced in mice with CKD compared with controls, as well as liver GHR protein level, while serum levels of GH and IGF1 (Table 2) were increased, as described in the next section.
Hamp disruption reduces uremic growth retardation.
Based on reported links among growth and BMI, adiposity, iron metabolism, and hepcidin in children and adolescents (2, 7, 14), we hypothesized that elevated hepcidin might contribute to growth retardation in CKD. To test the hypothesis we induced CKD in HKO mice using the adenine diet. The protocol was identical to the one used for WT mice. The relative effects of the adenine diet on growth parameters in WT and HKO mice are shown in Fig. 3.
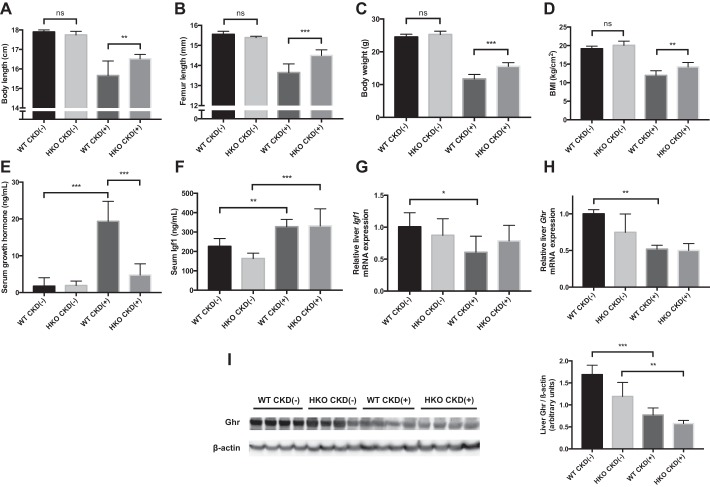
Fig. 3.
Relative effects of adenine diet on growth parameters in wild-type (WT) and HKO mice. Growth parameters were measured in four groups of mice: CKD(−) WT controls (n = 9), CKD(−)HKO controls (n = 5), CKD(+) WT (n = 9), and CKD(+) HKO (n = 6). A: body length at euthanasia in four groups of mice was measured as a nose to tail tip length. No significant differences in body length were observed between control WT and control HKO mice fed a regular diet. In contrast, the body length of CKD(+) WT mice was significantly shorter than the body length of CKD(+) HKO mice (15.7 ± 0.7 vs. 16.5 ± 0.2 cm respectively, P = 0.01). B: femur length at euthanasia in four groups of mice was measured radiographically at the time of micro-computed tomography. The control CKD(−)HKO group had slightly shorter femurs than control CKD(−)WT mice, while in the CKD(+) groups, HKO mice had significantly longer femurs than the WT mice (14.5 ± 0.3 vs. 13.6 ± 0.4 mm, respectively, P < 0.001). C: body weight at euthanasia was not different between controls. In contrast, CKD(+) HKO mice had increased body weight compared with CKD(+) WT controls. D: BMI was not different between control groups (bars at left) but CKD(+) HKO mice had higher BMI than CKD(+) WT. E: serum growth hormone (Gh) concentration was 10-fold elevated in CKD(+) WT mice compared with CKD(−)WT controls; no statistically significant differences in Gh concentration between CKD(+) HKO and CKD(−)HKO groups were found (P = 0.75). F: serum IGF1 concentration was elevated in both CKD(+) groups compared with their respective CKD(−)controls. G: in contrast to serum changes, liver mRNA expression of Igf1 was significantly reduced in the WT mice with CKD compared with WT controls. No differences in liver Igf1 mRNA expression between CKD(−)HKO and CKD(+) HKO groups were seen. H: liver mRNA expression of growth hormone receptor (Ghr) was significantly reduced in CKD(+) WT mice compared with CKD(−)WT controls. In HKO mice the differences did not reach statistical significance (P = 0.3). I: Western blot analysis demonstrated decreased GHR protein concentration in the liver of CKD(+) mice compared with CKD(−) controls, more so for the WT group. Four liver samples per group were used for PCR and western blot. Error bars represent SD. * P < 0.05; **P < 0.01; *** P < 0.001.
No significant differences in body length were observed between CKD(−) WT and CKD(−) HKO control mice fed a regular diet. In contrast, the body length of CKD(+) WT mice was significantly shorter than of CKD(+) HKO mice (15.7 ± 0.7 vs. 16.5 ± 0.2 cm, respectively, P = 0.01), as shown in Fig. 3A. The cumulative deficiency in body length attainment in CKD(+) WT mice compared with CKD(−) WT mice was improved by 37% in HKO group. The ameliorative effect of hepcidin on linear growth was further confirmed by radiographic measurements of femur length. As shown in Fig. 3B, the CKD(−) HKO group had slightly shorter femurs than CKD(−) WT mice, while in the CKD(+) groups, HKO mice had significantly longer femurs than WT mice (14.5 ± 0.3 vs. 13.6 ± 0.4 mm, respectively, P < 0.001).
Body weight and BMI were also not significantly different between HKO and WT control mice. Although CKD(+) HKO did show weight and BMI loss while on the adenine diet compared with CKD(−) HKO mice, the changes were smaller than described above for WT mice. As a result, CKD(+) HKO mice had higher body weight (P < 0.001) and BMI (P = 0.004), compared with CKD(+) WT mice (Fig. 3, C and D). Muscle wasting was also observed in the CKD(+) WT mice as reflected by the significantly (P = 0.006) lower weight of the gastrocnemius muscle in CKD(+) WT group than in CKD(−) WT controls, when normalized to BMI. However, no significant differences in normalized gastrocnemius muscle weight were observed between CKD(+) HKO and CKD(−) HKO groups (P = 0.5).
Serum GH level was significantly increased in CKD(+) WT mice compared with CKD(−) WT controls, while the difference in serum GH levels between CKD(+) HKO and CKD(−) HKO groups was not significant (Fig. 3E). Serum IGF1 was also increased in CKD(+) groups compared with CKD(−) controls (Fig. 3F). Expression of the growth hormone receptor (Ghr) and insulin-like growth factor 1 (Igf1) mRNA in the liver was significantly reduced in CKD(+) WT mice compared with CKD(−) WT controls (Fig. 3, G and H). The level of Ghr protein was also reduced in the liver of CKD(+) mice compared with CKD(−) controls (Fig. 3I). In contrast, among CKD(+) HKO mice, no significant differences in Ghr and Igf1 liver mRNA was found compared with CKD(−) HKO controls (Fig. 3, G and H). The difference in Ghr protein levels between HKO CKD(+) vs. HKO CKD(−) groups was smaller than the respective difference between WT CKD(+) and WT CKD(−) groups (Fig. 3I). Therefore, it appears that Hamp deletion mitigated the alterations in GH/IGF1 axis induced by CKD compared with effects on WT mice treated with adenine.
Hamp disruption ameliorates alterations in the epiphyseal growth plate of mice with CKD.
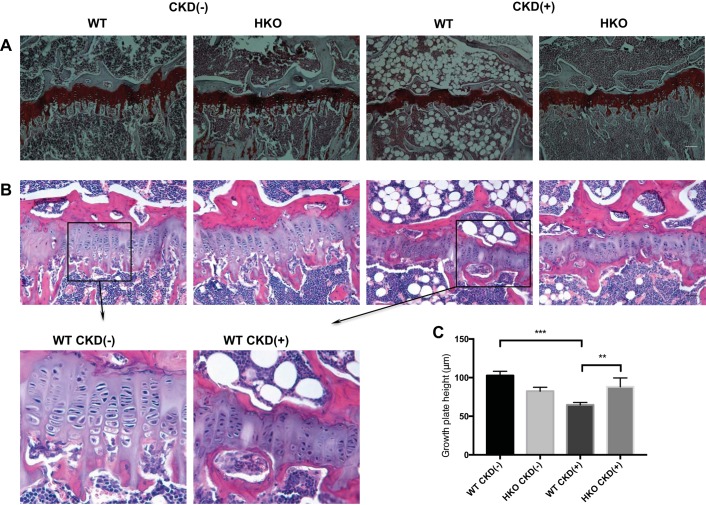
Histology of tibias revealed thinning of the growth plate in the CKD(+) WT mice compared with CKD(−) WT mice. The resting zone was especially diminished in the CKD(+) WT growth plates. Chondrocyte columns in CKD(+) WT appeared shortened and disorganized and had smaller chondrocytes. The conversion of cartilage to bone was significantly reduced in CKD(+) growth plates (Fig. 4A). Extensive bone marrow adiposity was noted in the CKD(+) WT tibias (Fig. 4, A and B).
Fig. 4.
Hamp disruption ameliorated metaphyseal growth plate alterations caused by adenine-induced CKD. A: safranin O staining of the proximal tibia growth plates in 4 groups of mice; scale bar = 100 μm. B: H&E staining of the proximal tibia growth plates in 4 groups of mice; scale bar = 50 μm. C: growth place height measurements in 4 groups of mice; n = 4 per group; the height of each growth plate was measured by collecting 15–30 measurements along the middle region of each plate. The average measurement coefficient of variation within mice was 14.0% (range 10.4 to 20.7%). Error bars represent between mice SD. **P < 0.01; *** P < 0.001.
The histology of CKD(−) HKO growth plates was similar to CKD(−) WT, and no pathologic features were identified. Tibia growth plates of CKD(+) HKO mice, while showing some degree of decreased conversion of cartilage to bone, demonstrated improved height of the growth plates, compared with CKD(+) WT (Fig. 4, A and B). Quantification of the growth plate height confirmed these observations (Fig. 4D): CKD(+) WT growth plates were significantly thinner than CKD(−) WT (64.7 ± 3.2 vs. 102.6 ± 5.5 μm, respectively, P < 0.001), while CKD(+) HKO growth plate height (83.0 μm) was greater than CKD(+) WT (P = 0.003) and not significantly different from CKD(−) HKO (P = 0.9).
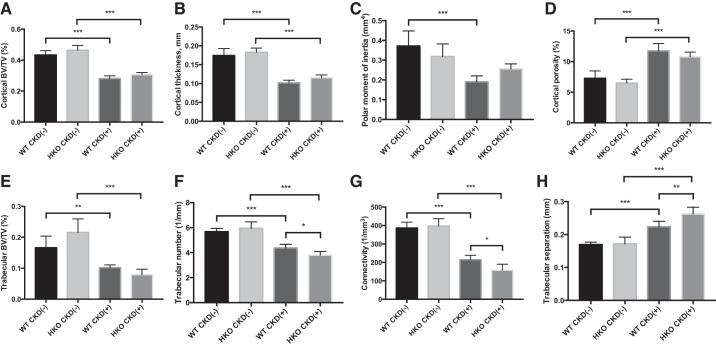
Hamp disruption does not improve bone quality (assessed by micro-CT) in young adenine-fed mice.
Consistent with smaller bone size (Fig. 3B), mice with CKD had cortical bone volume loss (Fig. 5A) and reduced cortical thickness (Fig. 5B), compared with to controls. As a result, the femurs of juvenile mice with CKD had lower polar moment of inertia (Fig. 5C). Cortical porosity was significantly increased in juvenile mice with CKD (Fig. 5D), indicating intrinsic loss and/or decreased formation of cortical bone, independent of the differences in bone size between mice with and without CKD. Cortical bone characteristics were not significantly different between WT and HKO mice. Thus Hamp deletion did not prevent the changes in cortical bone quality induced by CKD.
Fig. 5.
Bone parameters in WT and HKO mice with adenine-induced CKD and controls at the conclusion of the experiment. Cortical bone volume fraction (BV/TV; A), cortical thickness (B), and polar moment of inertia (C) were all significantly reduced in CKD(+) groups, more so in the CKD(+) WT group (likely due to reduced bone size). D: cortical porosity was increased in both CKD(+) groups compared with their respective controls. No significant differences between CKD(+) WT and CKD(+) HKO groups were observed. Trabecular bone volume fraction (BV/TV; E), trabecular number (F), and connectivity (G) were all significantly reduced in CKD(+) groups, more so in the CKD(+) HKO group, which was further supported by the respective increase in trabecular separation (H). Cortical bone scanning was performed on 1.4-mm segments of the femur mid-diaphysis. Trabecular bone was analyzed within the distal segments of the femurs (100 μm from the growth plate). Femurs of n = 7 mice per group were analyzed. Error bars represent SD. *P < 0.05; ** P < 0.01; *** P < 0.001.
Trabecular bone volume was significantly reduced in the CKD groups (Fig. 5E). This reduction was greater in HKO [60.2% reduction in CKD(−) vs. CKD(+)] compared with the change in WT mice (38.5% reduction). Trabecular bone volume loss was secondary to reduced trabecular number (Fig. 5F), lower connectivity (Fig. 5G), and increased trabecular separation (Fig. 5H) in mice with CKD. This loss was greater in HKO mice compared with WT mice. However, control CKD(−) HKO mice showed slightly lower trabecular number and connectivity than control WT mice. Therefore, analysis of trabecular bone micro-CT indicates that Hamp disruption did not improve and possibly even slightly worsened the effect of CKD on the trabecular bone. Overall, while the adenine diet induced a severe CKD-MBD phenotype in juvenile mice, it does not appear that Hamp disruption led to any improvement in bone parameters that we measured.
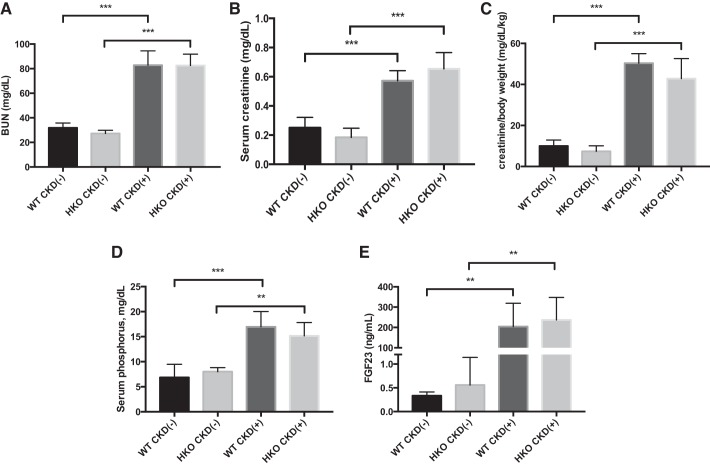
Hamp disruption does not mitigate severity of adenine-induced uremia in young mice.
Both CKD(+) WT and CKD(+) HKO mice in our study exhibited significantly elevated BUN (Fig. 6A) and serum creatinine (Fig. 6B) levels compared with their respective CKD(−) controls. The differences in serum creatinine were larger when normalized to body weight (Fig. 6C). There were, however, no significant differences in BUN and creatinine between WT and HKO juvenile mice fed the adenine diet. These findings indicate that Hamp deletion did not significantly affect the severity of uremia in juvenile mice with CKD and therefore growth differences between WT and HKO mice with CKD cannot be attributed to differences in the severity of uremia in this model.
Fig. 6.
Effects of adenine-induced CKD on renal function and mineral metabolism in WT and HKO mice. A: blood urea nitrogen (BUN) was 2.5-fold elevated in CKD(+) groups compared with CKD(−)controls. No differences between CKD(+) WT vs. CKD(+) HKO groups were seen. CKD(−) WT controls were also not different from CKD(−) HKO controls. B: serum creatinine was 2.5 elevated in CKD(+) groups compared with CKD(−) controls. There were no significant differences in serum creatinine between WT and HKO juvenile mice fed the adenine diet. C: serum creatinine normalized to body weight was 5-fold higher in CKD(+) adenine-fed groups compared with CKD(−) control groups. D: serum phosphorus. Both CKD(+) WT and CKD(+) HKO groups had severe hyperphosphatemia. No significant differences between CKD(+) WT and CKD(+) HKO groups were observed. E: Fibroblast growth factor 23 (FGF23) was elevated in CKD(+) WT and CKD(+) HKO groups compared with their respective controls. No significant differences between CKD(+) WT and CKD(+) HKO groups were observed. Error bars represent SD. *P < 0.05; **P < 0.01; *** P < 0.001.
Control groups had normal serum phosphorus and revealed no significant differences between CKD(−) WT and CKD(−) HKO mice. The adenine diet induced an approximately twofold increase in serum phosphorus in both WT and HKO mice (Fig. 6D) with no differences between the two CKD(+) groups. Serum FGF-23 levels were elevated in both CKD(+) WT and CKD(+) HKO mice compared with their respective controls. However, no differences in serum FGF-23 levels between the CKD(+) WT and CKD(+) HKO groups were observed (Fig. 6E). These findings suggest that Hamp deletion did not improve mineral metabolism in juvenile mice with adenine nephropathy and thus observed growth differences must have a different basis.
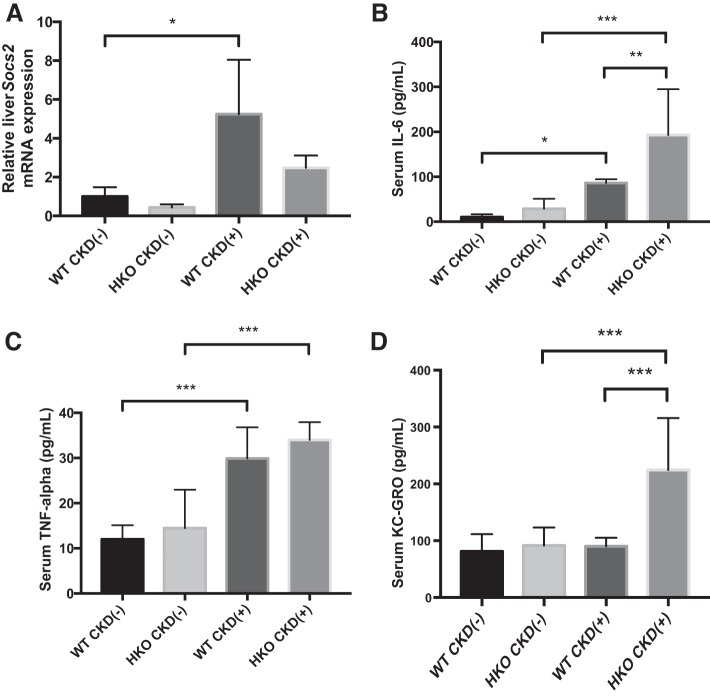
Hamp disruption does not mitigate systemic inflammation in mice with adenine-induced CKD.
We then proceeded to examine the role of Hamp disruption in modulation of systemic inflammation to determine its potential contribution to the improved growth observed in HKO mice. None of the inflammatory markers examined differed between CKD(−) WT and CKD(−) HKO control groups (Fig. 7). Inflammatory changes were observed in both CKD(+) WT and CKD(+) HKO mice compared with their respective CKD(−) controls. Increased mRNA expression of Socs2 was evident in the livers of CKD(+) mice as shown in Fig. 7A. However, the relative change of Socs2 expression in CKD(+) WT compared with CKD(−) WT (5.2-fold) was similar to the relative change in CKD(+) HKO compared with CKD(−) HKO (5.7-fold). Both CKD(+) WT and CKD(+) HKO groups had significantly elevated serum IL-6 (Fig. 7B) and TNF-α levels (Fig. 7C). IL-6 level was higher in CKD(+) HKO group than in CKD(+) WT group but when normalized to respective controls, the difference was not significant. The neutrophilic chemokine C-X-C motif ligand 1 (CXCL1/KC-GRO) was highly elevated in CKD(+) HKO, but unchanged in the CKD(+) WT group (Fig. 7D). Thus Hamp disruption did not improve the inflammatory milieu in mice with adenine-induced CKD, suggesting that inflammatory differences cannot be a basis for better growth of the CKD(+) HKO mice.
Fig. 7.
Effect of adenine-induced CKD and Hamp disruption on systemic inflammation. A: liver mRNA expression of suppressor of cytokine signaling 2 (Socs2) was 5-fold elevated in both CKD(+) groups, WT and HKO, compared with their respective controls. Four liver samples per group were used for PCR. B: serum IL-6 levels were significantly elevated in both CKD(+) groups, WT and HKO, compared with their respective controls, more so in the CKD(+) HKO group. There were no significant differences between WT controls and HKO controls. C: TNF-alpha levels were significantly elevated in both CKD(+) groups, compared with their respective controls. There were no significant differences between WT CKD(−) and HKO CKD(−) controls. D: neutrophilic chemokine (C-X-C motif) ligand 1 (KC-GRO) was highly elevated in CKD(+) HKO, but not in the CKD(+) WT group of mice. Cytokines were measured in n = 5–9 serum samples per group. Error bars represent SD. *P < 0.05; **P < 0.01; ***P < 0.001.
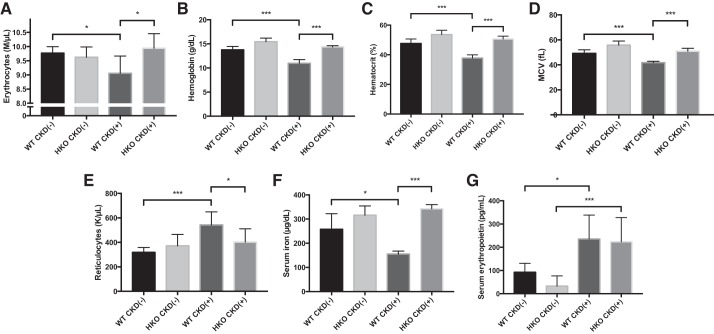
Hamp disruption prevents development of CKD-induced anemia in growing mice.
We examined erythroid parameters in the peripheral blood of four groups of mice to determine if CKD-induced anemia was present in this model, and if so, whether anemia severity would be mitigated by Hamp disruption, which could be the basis of the improved linear growth observed in CKD(+) HKO mice, compared with the CKD(+) WT group. The effects of adenine induced CKD in WT and HKO mice on hematologic parameters are shown in Fig. 8.
Fig. 8.
Adenine diet induces anemia and iron deficiency, ameliorated by Hamp disruption. Blood samples were obtained at euthanasia and complete blood counts (CBC) were analyzed in 4 groups of mice: CKD(−)WT controls (n = 6), CKD(−) HKO controls (n = 6), CKD(+) WT (n = 6), and CKD(+) HKO (n = 5). Red blood cells (A), hemoglobin (B), hematocrit (C), and mean corpuscular volume (MCV; D) were all significantly reduced in the CKD(+) WT mice compared with CKD(−) WT controls. HKO CKD(−) control mice had slightly higher red blood cell indices than WT CKD(−) control mice, with the exception of the red blood cell counts. In CKD(+) HKO, there was either no (for red blood cell count and hematocrit) or minimal (for hemoglobin and MCV) reduction in erythroid parameters compared with HKO controls. As a result, all red blood cell parameters in CKD(+) HKO were not different from CKD(−) WT controls. E: the reticulocyte counts were elevated by 70.2% in the CKD(+) WT group compared with WT controls. Reticulocyte counts in CKD(+) HKO mice were not significantly different from CKD(−) HKO controls. F: serum iron concentration was significantly reduced in CKD(+) WT mice compared with CKD(−) WT controls. In CKD(+) HKO group serum iron was not different from CKD(−) HKO controls. G: serum erythropoietin levels were equally elevated in both CKD(+) groups compared with their respective controls. Error bars represent SD. *P < 0.05; ***P < 0.001.
Similarly to the effects reported for adult rodents fed a high adenine diet (55) and children with CKD (9), WT mice with adenine-induced CKD developed anemia in our study, characterized by significantly reduced red blood cell numbers (7.3% drop) (Fig. 8A). Hemoglobin levels dropped by 20.2% to 10.9 ± 0.7 g/dl in CKD(+) WT from 13.8 ± 0.7 g/dl in CKD(−) WT mice (P < 0.001) as shown in Fig. 8B, and the hematocrit dropped by 20.7 to 37.8 ± 2.1% in CKD(+) WT from 47.6 ± 3.0% in CKD(−) WT mice (P < 0.001) as shown in Fig. 8C. Mean corpuscular volume of red blood cells also declined significantly as shown in Fig. 8D. The reticulocyte count was appropriately elevated by 70.2% in the CKD(+) WT group (Fig. 8E) and the percentage was also increased (by 84.0%). Serum iron levels in CKD(+) WT mice were reduced compared with CKD(−) WT controls (Fig. 8F). The serum erythropoietin level was approximately twofold elevated in CKD(+) WT mice compared with CKD(−) WT controls (P = 0.01; Fig. 8G). Thus the adenine diet reliably induced the renal anemia phenotype in WT mice.
HKO control mice, as expected, had slightly higher red blood cell indexes than WT control mice, with the exception of the red blood cell count (Fig. 8). Induction of CKD in HKO mice resulted in the same direction of change in RBC indexes as in WT mice, but the magnitude was lower. Hemoglobin was reduced by 5.8% in CKD(+) HKO compared with CKD(−) HKO mice, which was more than threefold less than after adenine treatment of WT mice (Fig. 8B). Similarly, the hematocrit was reduced by 4.6% in CKD(+) HKO mice by adenine, which was 4.5-fold less compared with CKD(+) WT mice (Fig. 8C). Consistent with these findings, CKD(+) HKO mice did not show a statistically higher reticulocyte count (Fig. 8E). Serum iron levels in CKD(+) HKO were not significantly different from CKD(−) HKO controls (P = 0.25, Fig. 8F). Serum erythropoietin was increased in CKD(+) HKO mice compared with CKD(−) HKO controls but not different from the CKD(+) WT group (Fig. 8G).
Because no statistically significant differences in any hematologic parameters or iron status were observed between CKD(+) HKO mice and CKD(−) WT control mice, these studies demonstrate that Hamp disruption effectively rescued and normalized the anemia phenotype in the adenine-induced CKD model that we present here.
DISCUSSION
In this paper we describe, for the first time, a model of uremic growth retardation in developing mice induced by an adenine diet. This mouse model allowed us to utilize a targeted gene knockout approach to demonstrate the involvement of the hepcidin (Hamp) pathway in the growth retardation in CKD.
Hepcidin, a master regulator of iron homeostasis, plays a major role in the development of anemia in CKD (67). In our study, induction of CKD by adenine diet in HKO mice resulted in the development of CKD, similar to that in the WT group. However, HKO mice with adenine-induced CKD did not develop anemia or iron deficiency within the experimental period. This was in contrast to the WT group, in which the adenine diet induced anemia, low serum iron, and hepcidin activation. To the best of our knowledge, this is the first time that the effect of Hamp KO on anemia in CKD is described. In our study, Hamp KO not only rescued the anemia phenotype in juvenile mice with adenine-induced CKD, but also improved their linear growth. This effect of Hamp disruption may be either direct, or mediated by increased iron availability, or a combination of the two mechanisms. Future studies will be required to answer the question of whether lack of hepcidin could contribute to the improvement of linear growth, anemia, and other CKD complications in an iron-independent fashion. It will be also important to confirm the relationship between serum hepcidin and growth in a large clinical cohort of children with CKD. It remains to be answered whether modifying the other components of anemia pathogenesis in juvenile CKD would have an impact on the linear growth.
This study revealed highly significant growth and BMI differences between adenine-fed and control mice with CKD thus providing a useful mouse model of growth retardation and cachexia in juvenile CKD. Malnutrition is a typical feature of pediatric CKD (10), likely contributing to growth stunting (8). Cachexia is a major problem in both children (1) and adults (41) with CKD that is associated with high morbidity (27) and mortality (31). Adult male C57BL/6J mice with CKD were reported to lose ∼25% of their initial body weight over the 6 wk of adenine diet (47). In the 5/6 nephrectomy model, loss of lean body mass was also demonstrated (16). In our experiments, mice with adenine-induced CKD had initially slower weight gain than controls followed by weight loss. Absolute changes in body weight may be difficult to interpret in growing animals in isolation from the analysis of linear growth. Therefore, in addition to body weight we analyzed the dynamics of BMI. At the end of experimental period, BMI fell below the level seen before introduction of the adenine diet at weaning. Gastrocnemius muscle weight-to-BMI ratio was significantly lower in adenine-fed mice compared with controls, suggesting a particular lack of proper gain and/or loss of the muscle mass in this model.
Disturbance of hypothalamus-liver-bone GH/IGF1 axis has been implicated in the growth delay seen in children with CKD (61). Recombinant human GH is the only therapy proven to improve growth in these children (5). Expression of the Igf1 mRNA, growth hormone receptor (Ghr) mRNA and Ghr protein in the liver was reduced in mice with CKD in this study, contrasting with elevated serum levels of GH and IGF1. This indicates that the GH/IGF1 axis was altered in our model, resembling changes described in the 5/6 nephrectomized rats (58) and children with CKD (49). Consistent with the reports on the use of adenine diet in adult rodents (17, 47, 66), our study demonstrated the development of such major CKD complications as anemia, iron deficiency, inflammation, and MBD. Profound osteopenia in juvenile mice with adenine-induced CKD was evidenced in micro-CTs of both cortical and trabecular bone.
The longitudinal growth delay seen in adenine-fed mice in this study was associated with major alterations in the epiphyseal growth plates, partially rescued by Hamp disruption. We found that growth plate height was reduced in CKD(+) WT mice compared with CKD(−) WT controls. However, no significant differences in growth plate height were found between CKD(+) HKO and CKD(−) HKO mice. The growth plate in CKD has been studied primarily in rat models, and various alterations of growth plate morphology in uremic rats have been described (18, 25). While some reports indicated widening of the growth plate in 5/6 nephrectomized uremic rats, mainly on the level of the hypertrophic zone (59), others found thinning of the growth plates (17). These discrepancies may be related to the differences in experimental design (CKD duration, age of animals etc.), as well as to species- and strain-specific characteristics. Analysis of growth plate at various time points throughout the experimental period was beyond the scope of our study but would be of a great interest to perform in the future using the current model. Thinning of the growth plate in CKD(+) WT mice in our study may indicate an acceleration of age-related bone changes in mice with CKD, as supported also by the profound bone marrow adiposity (53). Our growth plate findings provide an initial proof of principal evidence that the adenine-based mouse model of CKD has a growth plate phenotype in developing mice that can be used in future studies.
Clinical data suggest that CKD-MBD may contribute to growth delay in children with CKD (62). The relationship between anemia and CKD-MBD is also well established (40, 43). Furthermore, recent data indicate a relationship between hepcidin and mineral metabolism in CKD (13). One could hypothesize that Hamp disruption would improve mineral metabolism and bone quality in juvenile CKD and thus contribute to improved growth. However, we did not observe any differences in serum phosphorus and FGF-23 between CKD(+) HKO and CKD(+) WT mice. Furthermore, bone micro-CT examination did not reveal any improvement in the trabecular architecture of CKD(+) HKO mice compared with CKD(+) WT. While some of the cortical parameters showed improvement in CKD(+) HKO, this was likely related to the overall increase in bone size. The severity of uremia was similar between the CKD(+) WT and CKD(+) HKO groups.
The optimal hemoglobin target for patients with CKD and ESRD has been the subject of long-standing debate in the nephrology community (26). Investigation of bone outcomes has rarely been incorporated into clinical trials of anemia management in CKD. Trabecular bone loss has been recently described in a mouse model of polycythemia vera (37), which is characterized by chronic erythroid expansion and fragile bones. Given that trabecular bone loss in CKD(+) HKO mice was more severe than in CKD(+) WT mice in our study, it would be of interest to further explore the correlation between hematologic variables, treatment modalities (iron, erythropoiesis-stimulating agents), and markers of bone quality in human CKD.
Hepcidin, iron metabolism, anemia, and inflammation are interrelated (24, 28). Systemic inflammation is common in CKD (4) and it can inhibit linear growth in juvenile animals (21) and children (38). The relationship between iron and inflammation in CKD is not entirely clear. While some studies revealed proinflammatory effects of iron administration in adults with CKD (3), in dialysis patients intravenous iron administration was found to decrease inflammation (63). In our study, Hamp disruption did not improve any of the inflammatory markers measured and worsened some of them. Our results also suggest that the degree of iron overload in 11-wk-old CKD(−) HKO mice was probably not sufficient to induce significant systemic inflammation. With regard to the linear growth improvement observed in CKD(+) HKO mice, our data indicate that this was not promoted by the inflammatory changes, because such changes went mainly in the opposite direction to those expected to improve growth.
Sample size and ethical limitations prevent many important questions related to growth delay in children with CKD from being directly investigated in clinical settings (17). Until recently, experimental approaches to CKD-induced growth failure were limited to use of the 5/6 nephrectomy rat model (15). General limitations of that model have included high animal mortality, variability in the degree of uremia, acute renal failure in the postsurgical period, and technical complexity leading to issues of reproducibility with the model. These limitations are naturally magnified when surgery must be performed on a growing juvenile rat, rendering use in juvenile mice impractical. Furthermore, growth delay in 5/6 nephrectomized juvenile rats has been attributed primarily to the initial growth arrest postsurgery, as shown by the subsequent recovery of normal growth velocity (35). While 5/6 nephrectomy is generally regarded as a model of glomerulosclerosis due to hyperfiltration injury (32), the majority of pediatric cases of CKD and ESRD are related to congenital abnormalities of kidney and urinary tract with predominantly tubular injury and relative preservation of glomeruli until late stages of the disease (51). Therefore, the tubulointerstitial kidney injury in adenine-induced nephropathy represents a significant advantage with respect to modeling many aspects of pediatric CKD.
Because of developmental differences between growing mice and children, such as the shorter and accelerated early life in mice, it is difficult to determine the ideal experimental timeframe to model human growth in mice (20). This is further complicated due to the delayed puberty frequently seen in children with advanced CKD (30). Therefore, ideally the mouse model of uremic growth delay should match the timespan, not only of childhood, but also adolescence and young adulthood. As mice attain sexual maturity on average at postnatal day 70 (10-wk) (56), we designed our experiments to cover that time period plus one additional wk of mouse “young adulthood” (weaning at 3-wk plus 8 wk of adenine diet equaling 11-wk, postnatal day 77) as the endpoint in this study. Similar to children, adenine-fed juvenile mice exhibited anatomic signs of delayed puberty in this study. In adult mice, a 0.2% adenine diet was reported to reliably induce uremia in 6 wk (47).
Our study provides new insight into the role of hepcidin in CKD and opens new avenues for therapeutic interventions. Recombinant human growth hormone therapy usually effectively improves growth in children with CKD (22), but their final adult height still remains suboptimal, leading to decreased quality of life and issues with social adaptation (6). Better control of major CKD complications, such as anemia, mineral and bone disease, and acidosis has been thought to offer means for improving growth in pediatric CKD (33). However, adherence to many of these specific treatments, except for growth hormone, has not led to improved growth in North American patients (5). Furthermore, erythropoietin-resistance has been increasingly found to be a cause of poor anemia control (11). Emerging alternative treatments for anemia in CKD include anti-hepcidin interventions (54). Our study suggests that hepcidin-targeted therapies may not only contribute to the control of anemia of CKD but may also improve linear growth and nutritional status in these children. Further studies are needed to investigate whether reduction of hepcidin activity may have a proinflammatory effect and lead to trabecular bone loss in CKD. Moreover, it will be important to evaluate how reduction of hepcidin affects iron absorption, recycling, and erythropoiesis compared with various forms of iron supplementation.
In conclusion, the adenine-based dietary mouse model of CKD in developing mice provides a useful and reliable tool to study uremic growth delay and related problems relevant to pediatric CKD. Induction of CKD in age-matched HKO mice revealed that Hamp disruption prevented the development of anemia. Furthermore, Hamp disruption improved linear growth and BMI in mice with CKD, opening a new approach for in depth investigation of anti-hepcidin therapies in CKD targeting control of anemia, growth, and nutritional status outcomes.
GRANTS
This work was supported in part by the National Institutes of Health/National Center for Advancing Translational Sciences Grant UL1TR00457 awarded to the Clinical and Translational Science Center, Weill Cornell Medicine (to O. Akchurin and Y.-S. Zhu), and National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-095112 (to S. Rivella) and R01-DK-57661 (to M. E. Choi).
DISCLOSURES
S. Rivella is a consultant or receives funds from Ionis and Novartis Pharmaceuticals, Merganser Biotech, Bayer Healthcare, and Medgenics. None of these relationships influenced the analysis of the results presented in this manuscript.
AUTHOR CONTRIBUTIONS
O.A., S.C.-R., M.E.C., A.L.B., and S.R. conception and design of research; O.A., A.S., S.B.D., Y.-S.Z., and E.P. performed experiments; O.A., A.S., S.B.D., Y.-S.Z., and E.P. analyzed data; O.A., A.S., S.B.D., Y.-S.Z., S.C.-R., M.E.C., A.L.B., and S.R. interpreted results of experiments; O.A. and A.S. prepared figures; O.A. drafted manuscript; O.A., A.S., S.B.D., Y.-S.Z., S.C.-R., M.E.C., A.L.B., and S.R. edited and revised manuscript; O.A., A.S., S.B.D., Y.-S.Z., S.C.-R., M.E.C., A.L.B., and S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The results of this study were partially presented at the American Society for Bone and Mineral Research annual meeting in Seattle, WA (October 2015) and at the American Society of Nephrology Kidney Week in San Diego, CA (November 2015). The analyses of serum hepcidin, FGF23, GH, IGF1, erythropoietin, cytokines, and target gene expression in kidney and liver were carried out in the General and Molecular Core Laboratory of Clinical and Translational Science Center, Weill Cornell Medicine. We thank Adrian Spitzer for critical review of the manuscript. We appreciate the help of Lyudmila Lukashova with micro-CT, Sara Gardenghi with animal experiments, and Sébastien Monette with image analysis.
REFERENCES
- 1.Abraham AG, Mak RH, Mitsnefes M, White C, Moxey-Mims M, Warady B, Furth SL. Protein energy wasting in children with chronic kidney disease. Pediatr Nephrol 29: 1231–1238, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aeberli I, Hurrell RF, Zimmermann MB. Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight children. Int J Obes 33: 1111–1117, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R. Proinflammatory effects of iron sucrose in chronic kidney disease. Kidney Int 69: 1259–1263, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif 39: 84–92, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Akchurin OM, Schneider MF, Mulqueen L, Brooks ER, Langman CB, Greenbaum LA, Furth SL, Moxey-Mims M, Warady BA, Kaskel FJ, Skversky AL. Medication adherence and growth in children with CKD. Clin J Am Soc Nephrol 9: 1519–1525, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Uzri A, Matheson M, Gipson DS, Mendley SR, Hooper SR, Yadin O, Rozansky DJ, Moxey-Mims M, Furth SL, Warady BA, Gerson AC. The impact of short stature on health-related quality of life in children with chronic kidney disease. J Pediatr 163: 736–741 e731, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amato A, Santoro N, Calabro P, Grandone A, Swinkels DW, Perrone L, del Giudice EM. Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. Int J Obes 34: 1772–1774, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Arnold WC, Danford D, Holliday MA. Effects of caloric supplementation on growth in children with uremia. Kidney Int 24: 205–209, 1983. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson MA, Kim JY, Roy CN, Warady BA, White CT, Furth SL. Hepcidin and risk of anemia in CKD: a cross-sectional and longitudinal analysis in the CKiD cohort. Pediatr Nephrol 30: 635–643, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayestaran FW, Schneider MF, Kaskel FJ, Srivaths PR, Seo-Mayer PW, Moxey-Mims M, Furth SL, Warady BA, Greenbaum LA. Perceived appetite and clinical outcomes in children with chronic kidney disease. Pediatr Nephrol 31: 1121–1127, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bamgbola OF. Pattern of resistance to erythropoietin-stimulating agents in chronic kidney disease. Kidney Int 80: 464–474, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Bendich A, Brown GB, Philips FS, Thiersch JB. The direct oxidation of adenine in vivo. J Biol Chem 183: 267–277, 1950. [Google Scholar]
- 13.Carvalho C, Isakova T, Collerone G, Olbina G, Wolf M, Westerman M, Gutierrez OM. Hepcidin and disordered mineral metabolism in chronic kidney disease. Clin Nephrol 76: 90–98, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Casimiro de Almeida J, Lou-Meda R, Olbert M, Seifert M, Weiss G, Wiegerinck ET, Swinkels DW, Solomons NW, Schumann K. The growth attainment, hematological, iron status and inflammatory profile of Guatemalan juvenile end-stage renal disease patients. PLoS One 10: e0140062, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chantler C, Lieberman E, Holliday MA. A rat model for the study of growth failure in uremia. Pediatr Res 8: 109–113, 1974. [DOI] [PubMed] [Google Scholar]
- 16.Cheung WW, Ding W, Gunta SS, Gu Y, Tabakman R, Klapper LN, Gertler A, Mak RH. A pegylated leptin antagonist ameliorates CKD-associated cachexia in mice. J Am Soc Nephrol 25: 119–128, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claramunt D, Gil-Pena H, Fuente R, Garcia-Lopez E, Loredo V, Hernandez-Frias O, Ordonez FA, Rodriguez-Suarez J, Santos F. Chronic kidney disease induced by adenine: a suitable model of growth retardation in uremia. Am J Physiol Renal Physiol 309: F57–F62, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Cobo A, Carbajo E, Santos F, Garcia E, Lopez JM. Morphometry of uremic rat growth plate. Miner Electrolyte Metab 22: 192–195, 1996. [PubMed] [Google Scholar]
- 19.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28: 2–17, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci 152: 244–248, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Farquharson C, Ahmed SF. Inflammation and linear bone growth: the inhibitory role of SOCS2 on GH/IGF-1 signaling. Pediatr Nephrol 28: 547–556, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Fine RN, Kohaut EC, Brown D, Perlman AJ. Growth after recombinant human growth hormone treatment in children with chronic renal failure: report of a multicenter randomized double-blind placebo-controlled study. Genentech Cooperative Study Group. J Pediatr 124: 374–382, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Li Z, Gabrielsen JS, Simcox JA, Lee SH, Jones D, Cooksey B, Stoddard G, Cefalu WT, McClain DA. Adipocyte iron regulates leptin and food intake. J Clin Invest 125: 3681–3691, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardenghi S, Renaud TM, Meloni A, Casu C, Crielaard BJ, Bystrom LM, Greenberg-Kushnir N, Sasu BJ, Cooke KS, Rivella S. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood 123: 1137–1145, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna JD, Santos F, Foreman JW, Chan JC, Han VK. Insulin-like growth factor-I gene expression in the tibial epiphyseal growth plate of growth hormone-treated uremic rats. Kidney Int 47: 1374–1382, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Horl WH. Anaemia management and mortality risk in chronic kidney disease. Nat Rev Nephrol 9: 291–301, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi H, Kanda E, Mandai S, Akazawa M, Iimori S, Oi K, Naito S, Noda Y, Toda T, Tamura T, Sasaki S, Sohara E, Okado T, Rai T, Uchida S. Combination of low body mass index and serum albumin level is associated with chronic kidney disease progression: the chronic kidney disease-research of outcomes in treatment and epidemiology (CKD-ROUTE) study. Clin Exp Nephrol 2016 Feb 26. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.Kim A, Fung E, Parikh SG, Valore EV, Gabayan V, Nemeth E, Ganz T. A mouse model of anemia of inflammation: complex pathogenesis with partial dependence on hepcidin. Blood 123: 1129–1136, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ku E, Fine RN, Hsu CY, McCulloch C, Glidden DV, Grimes B, Johansen KL. Height at first RRT and mortality in children. Clin J Am Soc Nephrol 11: 832–839, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lane PH. Puberty and chronic kidney disease. Adv Chronic Kidney Dis 12: 372–377, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol 25: 2088–2096, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma LJ, Fogo AB. Model of robust induction of glomerulosclerosis in mice: importance of genetic background. Kidney Int 64: 350–355, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Mahan JD, Warady BA. Assessment and treatment of short stature in pediatric patients with chronic kidney disease: a consensus statement. Pediatr Nephrol 21: 917–930, 2006. [DOI] [PubMed] [Google Scholar]
- 34.McDonald SP, Craig JC. Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Molinos I, Santos F, Carbajo-Perez E, Garcia E, Rodriguez J, Garcia-Alvarez O, Gil H, Ordonez FA, Loredo V, Mallada L. Catch-up growth follows an abnormal pattern in experimental renal insufficiency and growth hormone treatment normalizes it. Kidney Int 70: 1955–1961, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA 99: 4596–4601, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oikonomidou PR, Casu C, Yang Z, Crielaard B, Shim JH, Rivella S, Vogiatzi MG. Polycythemia is associated with bone loss and reduced osteoblast activity in mice. Osteoporos Int 27: 1559–1568, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padeh S, Pinhas-Hamiel O, Zimmermann-Sloutskis D, Berkun Y. Children with oligoarticular juvenile idiopathic arthritis are at considerable risk for growth retardation. J Pediatr 159: 832–837, e831–832, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806–7810, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Patel NM, Gutierrez OM, Andress DL, Coyne DW, Levin A, Wolf M. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int 77: 715–720, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Pereira RA, Cordeiro AC, Avesani CM, Carrero JJ, Lindholm B, Amparo FC, Amodeo C, Cuppari L, Kamimura MA. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant 30: 1718–1725, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Philips FS, Thiersch JB, Bendich A. Adenine intoxication in relation to in vivo formation and deposition of 2,8-dioxyadenine in renal tubules. J Pharmacol Exp Ther 104: 20–30, 1952. [PubMed] [Google Scholar]
- 43.Rao DS, Shih MS, Mohini R. Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N Engl J Med 328: 171–175, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Rodig NM, McDermott KC, Schneider MF, Hotchkiss HM, Yadin O, Seikaly MG, Furth SL, Warady BA. Growth in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children Study. Pediatr Nephrol 29: 1987–1995, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23: 291–299, 2001. [PubMed] [Google Scholar]
- 46.Runolfsdottir HL, Palsson R, Agustsdottir IM, Indridason OS, Edvardsson VO. Kidney disease in adenine phosphoribosyltransferase deficiency. Am J Kidney Dis 67: 431–438, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santana AC, Degaspari S, Catanozi S, Delle H, de Sa Lima L, Silva C, Blanco P, Solez K, Scavone C, Noronha IL. Thalidomide suppresses inflammation in adenine-induced CKD with uraemia in mice. Nephrol Dial Transpl 28: 1140–1149, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, Winters A, Juan T, Li H, Begley CG, Molineux G. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood 115: 3616–3624, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Schaefer F, Veldhuis JD, Stanhope R, Jones J, Scharer K. Alterations in growth hormone secretion and clearance in peripubertal boys with chronic renal failure and after renal transplantation. Cooperative Study Group of Pubertal development in Chronic Renal Failure. J Clin Endocrinol Metab 78: 1298–1306, 1994. [DOI] [PubMed] [Google Scholar]
- 50.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Seikaly MG, Ho PL, Emmett L, Fine RN, Tejani A. Chronic renal insufficiency in children: the 2001 Annual Report of the NAPRTCS. Pediatr Nephrol 18: 796–804, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Seikaly MG, Salhab N, Gipson D, Yiu V, Stablein D. Stature in children with chronic kidney disease: analysis of NAPRTCS database. Pediatr Nephrol 21: 793–799, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Singh L, Brennan TA, Russell E, Kim JH, Chen Q, Brad Johnson F, Pignolo RJ. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone 85: 29–36, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun CC, Vaja V, Babitt JL, Lin HY. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am J Hematol 87: 392–400, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun CC, Vaja V, Chen S, Theurl I, Stepanek A, Brown DE, Cappellini MD, Weiss G, Hong CC, Lin HY, Babitt JL. A hepcidin lowering agent mobilizes iron for incorporation into red blood cells in an adenine-induced kidney disease model of anemia in rats. Nephrol Dial Transpl 28: 1733–1743, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taft RA, Davisson M, Wiles MV. Know thy mouse. Trends Genet 22: 649–653, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Tamura M, Aizawa R, Hori M, Ozaki H. Progressive renal dysfunction and macrophage infiltration in interstitial fibrosis in an adenine-induced tubulointerstitial nephritis mouse model. Histochem Cell Biol 131: 483–490, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Tonshoff B, Powell DR, Zhao D, Durham SK, Coleman ME, Domene HM, Blum WF, Baxter RC, Moore LC, Kaskel FJ. Decreased hepatic insulin-like growth factor (IGF)-I and increased IGF binding protein-1 and -2 gene expression in experimental uremia. Endocrinology 138: 938–946, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Troib A, Guterman M, Rabkin R, Landau D, Segev Y. Endurance exercise and growth hormone improve bone formation in young and growth-retarded chronic kidney disease rats. Nephrol Dial Transpl 31: 1270–1279, 2015. [DOI] [PubMed] [Google Scholar]
- 60.Troutt JS, Butterfield AM, Konrad RJ. Hepcidin-25 concentrations are markedly increased in patients with chronic kidney disease and are inversely correlated with estimated glomerular filtration rates. J Clin Lab Anal 27: 504–510, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulinski T, Mohan S, Kiepe D, Blum WF, Wingen AM, Mehls O, Tonshoff B. Serum insulin-like growth factor binding protein (IGFBP)-4 and IGFBP-5 in children with chronic renal failure: relationship to growth and glomerular filtration rate. The European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood German Study Group for Growth Hormone Treatment in Chronic Renal Failure. Pediatr Nephrol 14: 589–597, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Waller SC, Ridout D, Cantor T, Rees L. Parathyroid hormone and growth in children with chronic renal failure. Kidney Int 67: 2338–2345, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Weiss G, Meusburger E, Radacher G, Garimorth K, Neyer U, Mayer G. Effect of iron treatment on circulating cytokine levels in ESRD patients receiving recombinant human erythropoietin. Kidney Int 64: 572–578, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Wong H, Mylrea K, Feber J, Drukker A, Filler G. Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int 70: 585–590, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Yokozawa T, Zheng PD, Oura H. Biochemical features induced by adenine feeding in rats. Polyuria, electrolyte disorders, and 2,8-dihydroxyadenine deposits. J Nutr Sci Vitaminol 30: 245–254, 1984. [DOI] [PubMed] [Google Scholar]
- 66.Yokozawa T, Zheng PD, Oura H, Koizumi F. Animal model of adenine-induced chronic renal failure in rats. Nephron 44: 230–234, 1986. [DOI] [PubMed] [Google Scholar]
- 67.Zaritsky J, Young B, Wang HJ, Westerman M, Olbina G, Nemeth E, Ganz T, Rivera S, Nissenson AR, Salusky IB. Hepcidin–a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol 4: 1051–1056, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]