Abstract
Extracellular vesicles (EV) are endogenously produced, membrane-bound vesicles that contain various molecules. Depending on their size and origins, EVs are classified into apoptotic bodies, microvesicles, and exosomes. A fundamental function of EVs is to mediate intercellular communication. In kidneys, recent research has begun to suggest a role of EVs, especially exosomes, in cell-cell communication by transferring proteins, mRNAs, and microRNAs to recipient cells as nanovectors. EVs may mediate the cross talk between various cell types within kidneys for the maintenance of tissue homeostasis. They may also mediate the cross talk between kidneys and other organs under physiological and pathological conditions. EVs have been implicated in the pathogenesis of both acute kidney injury and chronic kidney diseases, including renal fibrosis, end-stage renal disease, glomerular diseases, and diabetic nephropathy. The release of EVs with specific molecular contents into urine and plasma may be useful biomarkers for kidney disease. In addition, EVs produced by cultured cells may have therapeutic effects for these diseases. However, the role of EVs in kidney diseases is largely unclear, and the mechanism underlying EV production and secretion remains elusive. In this review, we introduce the basics of EVs and then analyze the present information about the involvement, diagnostic value, and therapeutic potential of EVs in major kidney diseases.
Keywords: exosome, biomarker, therapy, acute kidney injury, chronic kidney disease
extracellular vesicles (EVs) are membrane-bound vesicles produced and released by a cell that contain various molecules, such as proteins, lipids, DNA, mRNA, and microRNAs. Recent research has demonstrated an emerging role of EVs in mediating cell-cell or intercellular communication (13, 40). By this function, EVs act as a highly conserved mechanism for signal transmission between cells in diverse biological and physiological processes. As such, changes and dysfunction in EVs may be associated with the development of diseases, and the molecules or molecular signature of EVs may serve as noninvasive diagnostic biomarkers of diseases. Moreover, the unique biological activity of EVs has displayed potential benefit for the correction of cellular dysfunction and, in turn, the therapy of diseases (21). EVs have also been considered to be ideal nanovectors for biodelivery, specifically for drug delivery in clinical application (22, 60). In kidneys, renal EVs are produced and secreted by kidney cells and have been implicated in renal function and diseases (40). In this review, we aim to provide an overview of the basic science of EVs and analysis of the available information regarding the involvement of EVs in major kidney diseases.
EVs
Three main types of EVs have been described, including apoptotic bodies, microvesicles or microparticles, and exosomes (62, 66). They are distinguished from each other by their origin, size, and content (Table 1). In general, the sizes of apoptotic bodies, microvesicles, and exosome are >1,000, 100-1,000, and 40–150 nm in diameter, respectively. In terms of origin, microvesicles and apoptotic bodies are derived directly from the plasma membrane, whereas exosomes originate from the endosome. Endosomes form the multivesicular body (MVB) and subsequently fuse with the plasma membrane to release exosomes (58). EVs contain proteins, lipids, and RNAs, whereas apoptotic bodies may also contain DNA fragments. Some common proteins identified in EVs include TSG101, tetraspanins (CD9, CD63), heat shock proteins, annexins, flotillin, Alix from MVB, and membrane fusion protein GTPases. EVs also contain lipids, such as cholesterol and sphingomyelin, and RNAs, such as mRNA and microRNA (33). EV release is either spontaneous or induced, depending on cell type and functional status. It has been reported that EV production and release depends on the activation of specific cell surface receptors and a number of enzymes (18, 32). For exosomes, the endosomal sorting complex required for transport (ESCRT) involved in MVB formation is the main regulatory system, whereas the other regulatory molecules, such as Rab GTPaze, contribute as well (27, 34).
Table 1.
Main types of EVs and their properties
| Exosomes | Microvesicles | Apoptotic Bodies | |
|---|---|---|---|
| Size | 40–150 nm | 100–1,000 nm | >1,000 nm |
| Origin | Late endosome (multivesicular body) | Plasma membrane | Apoptotic cell (plasma membrane) |
| Main functions | Transmission of proteins and RNAs | Transmission of RNAs and DNAs | Not clearly defined |
| Proteins | ESCRT components, adhesion molecules (tetraspanins:CD63,CD9), membrane transport/fusion protein (flotillins, annexins), antigen presentation (MHC I), signal transduction, enzymes, cytoskeletal proteins, other cytosolic proteins (TSG101), heat shock protein (HSP 70) | Integrins, flotillins, selectins, CD40, metalloproteinases | Annexin, histones |
| Lipids | Lysobisphosphatidic acid, cholesterol, ceramide, sphingomyelin, and low concentration of phosphatidylserine | High amount of cholesterol, sphingomyelin, ceramide, high concentration of phosphatidylserine | High concentration of phosphatidylserine |
| Nucleic acids | mRNA and miRNA | mRNA and miRNA | mRNA, miRNA, fragments of DNA |
| Morphology | Homogeneous cup shape | Heterogeneous irregular | Heterogeneous irregular |
| Isolation method | Immunoprecipitation (commercial Kit), ultracentrifugation (100,000–200,000 g), ultracentrifugation with density gradient | Ultracentrifugation (10,000–60,000 g) | No standardized protocol |
| References | (35, 41, 46) | (39, 41, 65) | (31) |
EV, extracellular vesicles; ESCRT, endosomal sorting complex required for transport.
Isolation of EVs
EVs are released by most cell types and are present in various body fluids, including urine, serum, and saliva (43). The isolation method is a key to EV research and clinical application. Presently, several EV isolation methods have been described and commonly used. Traditionally, EVs are isolated and purified by differential centrifugation (64); the differences in isolation of main types of EVs by centrifugation are summarized in Table 1. In the last few years, commercial kits for EV isolation became available, which are mainly based on sucrose or iodixanol density gradient to obtain EVs at relatively higher yields than differential centrifugation alone (61). More recently, a polyethylene glycol-based method for enrichment of EVs, called Extra PEG, has been described (54). Apparently, Extra PEG may provide a more convenient method because it reduces the requirement of ultracentrifugation, but the merits and pitfalls of this method remain to be verified. Very few studies have isolated EVs from kidney tissues. Nonetheless, Borges et al. (7) reported the increase of exosomes from kidney tissues following unilateral urinary obstruction. In this study, exosomes were isolated from kidney cortex by mechanical sectioning and enzymatic digestion with collagenase and trypsin, followed by ultracentrifugation of the supernatant (7).
EVs in Kidneys
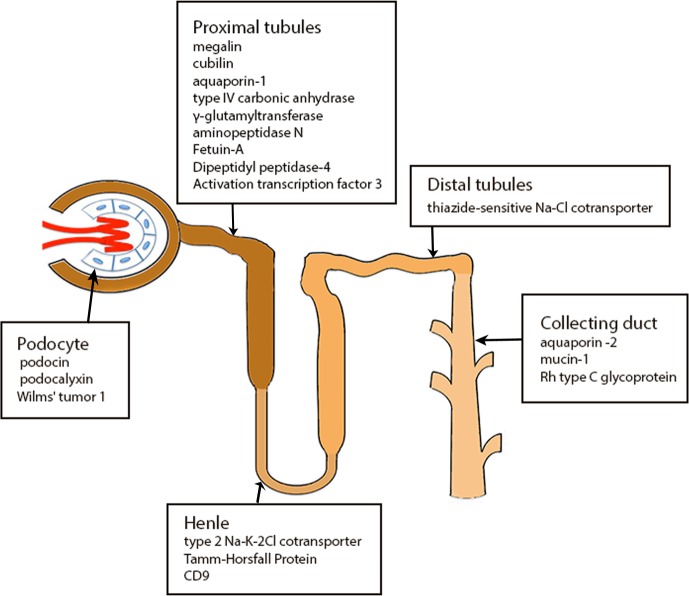
Recent research has implicated EVs in renal physiology and the pathogenesis of major kidney diseases. Almost all cell types in kidneys produce and secret EVs. As summarized in Fig. 1, exosomal proteins may originate from all segments of the nephron, including glomerular podocytes, proximal tubules, thick ascending limb of Henle, distal tubule, and the collecting duct (50). Further studies (25, 50) demonstrated that, because of the glomerular structure of mechanical and charge barriers, circulating EVs from serum cannot cross the nephron at least under physiological conditions, suggesting that urinary EVs (uEVs) are mainly derived from renal cells.
Fig. 1.
Proteins originated from different cells type of the nephron transported via extracellular vesicles.
EVs in kidneys also function as an important vector for intercellular communication. Early work by Brown et al. (8) showed that the proteins of proximal tubule cells may be transported to downstream collecting duct cells. This concept has been verified by recent studies. For example, Street and colleagues (57) suggested that collecting duct epithelial cells may release exosomes that contain Aquaporin-2 (AQP2) and that this release is physiologically regulated. Exosomes can transfer functional AQP2 between cells, representing a novel mechanism for cell-to-cell communication within the kidney (57). Indeed, a number of proteins from various segments of the nephron may be released into urine or transported via EVs to other cells in kidneys (Fig. 1).
EVs as Diagnostic Biomarkers for Kidney Diseases
Acute kidney injury.
As indicated above, proteomics analysis of urinary exosomes has confirmed that exosomal proteins may be originated from all segments of the nephron. Importantly, the content of exosomes may change in response to various pathophysiological conditions. As such, exosomal proteins may be biomarkers for specific diseases. In this regard, fetuin-A and AQP1 protein level in urinary exosomes have been identified as potential biomarkers for acute kidney injury (AKI). During renal ischemia/reperfusion (I/R) in rats, AQP1 protein in urine exosomes decreased significantly. Remarkably, similar decreases were detected in patients after renal allograft transplantation, suggesting that urinary exosomal AQP1 may be a biomarker for AKI induced by renal I/R (56). For fetuin-A, a marked increase was detected in urinary exosomes in both animal models and human patients of AKI; moreover, the increase of fetuin-A occurred before the increase of serum creatinine (69). In addition, Zhou et. al. (67) detected an increased level of activating transcription factor 3 (ATF3) in urinary exosomes (but not in whole urine) in both cisplatin and I/R -induced AKI in mice. Again, this increase started before serum creatinine, indicating that ATF3 may be a biomarker for early diagnosis of AKI. In support of this possibility, a clinical study showed that urinary exosomes containing ATF3 mRNA were 60-fold higher in patients with AKI than that of normal controls (15).
Urinary neutrophil gelatinase-associated lipocalin (NGAL) is a recently identified molecular biomarker of AKI. Interestingly, recent work demonstrated that NGAL in urinary exosomes correlated with delayed graft function after kidney transplantation, suggesting that urinary exosomal NGAL may be an early biomarker for prognosis in postrenal transplantation condition (2). Thus urinary exosomes may be the sites of concentration of biomarkers of renal diseases, making it a potential source for further identification of sensitive biomarkers for AKI.
Renal fibrosis and end-stage renal disease.
In a clinical study, it was observed that the mRNA level of CD2AP, a podocyte marker, was reduced in uEVs from patients with chronic kidney disease (CKD). Importantly, this downregulation was correlated with renal function, level of proteinuria, and the severity of renal fibrosis, suggesting its biomarker potential (45). Further studies analyzed microRNAs in uEVs of patients with CKD. It was shown that exosomal miR-29 and miR-200 were significantly reduced in patients with CKD compared with controls, and notably the reduction correlated with renal function decline and the degree of tubular-interstitial fibrosis (44). In addition, osteoprotegerin, an inflammatory marker, was shown to be increased in uEVs of patients with CKD (6). These data suggest that EVs may reflect renal fibrosis as well as the inflammatory state within the renal microenvironment of patients with CKD.
In obstructive nephropathy, uEVs may be useful for evaluating the risk of developing renal dysfunction (59). uEVs in the patients affected by posterior urethral valves contained much higher levels of TGF-β1 and cell adhesion molecules than the control group. In addition, the level of the profibrotic factor TGF-β1 in uEVs showed a correlation with the glomerular filtration rate.
In end-stage renal disease (ESRD), circulating EVs (endothelial microparticles, EMPs) were reported to impair endothelial-dependent vasorelaxation, which may be associated with the decrease in endothelial nitric oxide release and endothelial function (3). A prospective study involving 81 patients on hemodialysis suggested that EMPs may be able to predict all-cause and cardiovascular mortality for patients with ESRD hemodialysis (47). If verified, this would help identifying patients who need more aggressive or intensive treatment. Other studies have reported that the level of circulating EVs derived from endothelial cells may correlate with arterial stiffness of patients on hemodialysis (20, 23). Moreover, it was demonstrated that the increased serum EMPs may be a reliable independent predictor of outcome in patients with ESRD(4).
Glomerular diseases.
EVs derived from glomeruli are constantly released into urine in the physiological state. As a result, changes of uEVs are considered to be a direct sign of glomerular diseases, including podocyte injury. In this aspect, Wilms's tumor 1 transcription factor (WT-1, a podocyte protein) in uEVs has been reported to correlate well with podocyte injury in both animal models and patients with chronic glomerular diseases (38). In animal models, WT-1 in uEVs is detected before obvious glomerular sclerosis. Clinical data further showed that WT-1 in urinary exosomes was presented in 9 out of 10 patients with focal segmental glomerulosclerosis (FSGS) but not in any of the 8 controls examined (67). Zhou et al. (68) also reported that urinary exosomal WT-1 was significantly increased in patients with FSGS compared with healthy controls or patients with steroid-sensitive nephrotic syndrome (SSNS). In addition, urinary exosomal WT-1 was significantly decreased in patients in remission for either FSGS or SSNS or following steroid treatment of patients with SSNS (68). These studies suggest that exosomal WT-1 may be a promising noninvasive biomarker that can detect early progression and treatment-induced regression of podocyte injury in FSGS or SSNS. However, the potential of exosomal WT-1 as a glomerulopathy biomarker has not been verified in pediatric patients with nephrotic syndrome. WT-1 levels in uEVs did not vary according to the responsiveness to the steroid therapy in pediatric patients (42). Moreover, exosomal WT-1 was detected only in 60% of 40 children with FSGS and SSNS, indicating that WT-1 in uEVs may not be a sensitive biomarker for pediatric FSGS and SSNS (42).
uEVs may be a useful diagnostic tool to help us differentiate early IgA nephropathy and thin basement membrane nephropathy in pediatric and adult patients with microscopic hematuria. In this regard, Moon et al. (49) identified four different biomarkers differently expressed in uEVs of these patients; levels of aminopeptidase N and vasorin precursor were higher in the thin basement membrane nephropathy group compared with the IgA nephropathy group, whereas α-1-antitrypsin and ceruloplasmin levels increased in the IgA group.
Proteinuria is still regarded as an easily accessible and valid marker in the evaluation of glomerular diseases, but it cannot discriminate the type of underlying pathogenesis (19). uEVs contain mRNA, microRNA, proteins, as well as surface receptors of specific cell types, providing the information about the types of glomerular impairment (49, 71). For example, Rood et al. (55) reported that lysosome membrane protein-2 was upregulated in uEVs obtained from patients with idiopathic membranous nephropathy compared with normal controls as well as uEVs of patients with idiopathic FSGS(55).
Diabetic kidney disease.
uEVs, especially urinary exosomes, have recently become the source for searching for novel and earlier biomarkers of diabetic kidney disease (DKD) or diabetic nephropathy. In an animal model of DKD, two exosomal proteins (Xaa-Pro dipeptidase and major urinary protein 1) were shown to be up- and downregulated, respectively (51). Zubiri et al. (71) further detected a group of altered proteins, including MLL3, AMBP, and VDAC1, in urinary exosomes of patients with DKD compared with healthy controls. Kalani et al. (38) recently demonstrated the presence of WT-1 in urinary exosomes of diabetic patients, and notably exosomal WT-1 increased when renal function worsened. In addition, patients with type 1 diabetes mellitus with proteinuria showed significantly higher WT-1 in urinary exosomes than those without proteinuria (38). These data suggest that the increase of WT-1 in urinary exosomes may serve as a biomarker of podocyte injury or malfunction in DKD. In further support, exosomal WT-1 was detected in all diabetic patients with proteinuria but only in half of those without proteinuria and only 1 of 25 healthy controls. Expression of WT-1 in uEVs also showed a significant correlation with the decrease of renal function (68). Apart from WT-1, other molecular markers of podocytes, such as podocalyxin and podoplanin, have been also detected in uEVs in DKD. For example, Burger et al. (11) detected increased levels of podocalyxin and podoplanin in microparticles from diabetic mice before albuminuria.
In addition to proteins, differential miRNA profiling in uEVs can be used to identify miRNAs that may differentiate patients with type 1 diabetes with or without DKD. Barutta et al. (5) recently detected 22 microRNAs with altered level in patients with microalbuminuria compared with patients without albuminuria. Among these microRNAs, miR-130a and miR-145 were significantly upregulated in patients with microalbuminuria, whereas miR-155 and miR-424 were markedly decreased (5). Consistently, diabetic animals showed a significant increase in urine exosomal miR-145. In cultured mesangial cells, high glucose treatment led to the release of miR-145-containing exosomes (5). Together, these data suggest that urinary exosomal miR-145 has a good potential to be a biomarker for DKD.
Other renal diseases.
In exosomes isolated from healthy volunteers' urine, Miranda and colleagues (48) successfully detected over 1,000 proteins with origins of different segments of the nephron. Notably, 34 of these proteins are known to be associated with kidney diseases. For example, Na-K-2Cl symport is associated with antenatal Bartter syndrome type 1, polycystin-1 (PC1) with autosomal dominant polycystic kidney disease (PKD) type 1, thiazide-sensitive Na-Cl cotransporter with Gitelman's syndrome, AQP2 with autosomal dominant and recessive nephrogenic diabetes. It remains elusive how these proteins are incorporated into urinary exosomes, but their presence provides a clue for detection of some congenital or hereditary diseases. For example, Na-K-Cl cotransporter, which is normally detected in urinary exosomes of healthy subjects, was founded to be absent in patients with Bartter syndrome type 1, a genetic disorder caused by a mutation of the gene encoding Na-K-Cl cotransporter (26). Likewise, Na-Cl cotransporter was not detectable in uEV from patients with Gittelman's syndrome (37).
uEVs of patients with PKD also have notable characteristics. It has been reported that cystin and ADP ribosylation factor-like 6 are abnormally expressed in uEVs of patients with PKD (30). Recently, Hogan et al. (30) further observed a significantly higher level of transmembrane protein-2 (TMEM2) in urinary exosomes from patients with PKD1 compared with healthy controls. Remarkably, the PC1-to-TMEM2 ratio was inversely correlated with kidney volume, suggesting that PC1-to-TMEM2 ratio may provide a novel technique to assess kidney volume as well as disease progression in patients with PKD (29).
EVs as Therapeutic Agents for Kidney Diseases
AKI.
Besides being potential biomarkers for AKI diagnosis and prognosis, EVs have shown promising beneficial effects on tissue repair and regeneration during AKI (Table 2). Microvesicles derived from bone marrow, mesenchymal, or endothelial progenitor stem cells could protect against various types of AKI and promote kidney repair or recovery (9, 10, 24). Exosomes from mesenchymal stem cells could also protect rats against gentamicin-induced kidney injury (53). Zhou and colleagues (70) further demonstrated that exosomes derived from human umbilical cord mesenchymal stem cells (hucMSCs) could alleviate AKI induced by cisplatin in rats. Mechanistically, hucMSC-derived exosomes reduced cisplatin-induced oxidative stress and apoptosis in vivo and facilitated renal epithelial cell proliferation in vitro (70). Gatti et al. (24) have reported that administration of microvesicles released from human adult mesenchymal stem cells, immediately after renal I/R, could protect rats from AKI by inhibiting apoptosis and stimulating tubular epithelial cell proliferation. Functionally, the rats treated with microvesicles had significantly lower serum creatinine and BUN than those injected with vehicle alone (24). Similarly, in mice with ischemic AKI model, administration of exosomes derived from human endothelial colony-forming cells at the time of reperfusion significantly attenuated the increases in plasma creatinine, tubular necrosis, macrophage infiltration, oxidative stress, and apoptosis in kidney tissues in mice (12). Beneficial effects of EVs derived from human liver stem cells have also been reported in glycerol-induced AKI in mice (28).
Table 2.
Therapeutic test of EVs in kidney disease models
| EV Origin | Type | Disease Model | Result | Ref. |
|---|---|---|---|---|
| Mesenchymal stem cells | Exosome-like microvesicles | Gentamicin-mediated nephrotoxicity in rats | Inhibited Cr increase, necrosis, apoptosis, and increased cell proliferation | (53) |
| Mesenchymal stem cell | Microvesicles | Glycerol-induced AKI in SCID mice | Accelerated the morphological and functional recovery | (10) |
| Human adult mesenchymal stem cells | Microvesicles | AKI induced by ischemia-reperfusion injury (IRI) in rats | Inhibited apoptosis and stimulated tubular epithelial cell proliferation | (24) |
| Mesenchymal stem cells | Microvesicles | Lethal cisplatin-induced AKI of SCID mice | Ameliorated renal function and morphology and improved survival | (9) |
| Human umbilical cord mesenchymal stem cells | Exosomes | Cisplatin-induced AKI in Rat | Ameliorated oxidative stress and cell apoptosis, promoted cell proliferation | (70) |
| Human endothelial colony-forming cells | Exosomes | A mice model of ischemic AKI | Attenuated Cr, tubular necrosis, macrophage infiltration, oxidative stress, and apoptosis | (12) |
| Human liver stem cells | Extracellular vesicles | Mice AKI induced by intramuscle glycerol injection | Ameliorated renal function and morphology | (28) |
| Mesenchymal stromal cell vesicles | Extracellular vesicles | Glycerol-induced AKI in severe combined immunodeficient mice | Induced morphological and functional recovery in AKI | (17) |
| Tubular epithelial cells under hypoxia | Exosomes | Fibroblasts | Fibrosis protein expression increased | (7) |
| Endothelial progenitor cells | Extracellular vesicles | Experimental anti-Thy1.1 glomerulonephritis in rats | Inhibited antibody- and complement-mediated injury of mesangial cells | (14) |
| Human urine-derived stem cells | Exosomes | Type 1 diabetic nephropathy | Reduced the urinary microalbumin excretion, prevented apoptosis, increased glomerular endothelial cell proliferation | (36) |
AKI, acute kidney injury.
Mechanistically, several studies attributed the protective effect of EVs on kidney diseases mostly to their RNA content, especially microRNAs (16, 63). In this regard, Collino and colleagues (17) reported that EVs derived from the cells with silenced Drosha, a key enzyme for microRNA biogenesis, were ineffective in promoting morphological and functional recovery in AKI. RNA sequencing analysis further showed that differentially expressed kidney genes after injury were restored by treatment with wide-type cell-derived EVs, but not with Drosha-knockdown cell vesicles. In gene ontology analysis, those genes were associated with fatty acid metabolism, inflammation, matrix-receptor interaction, and cell adhesion (17).
Of note, EVs are not always beneficial to kidneys, as they can also aggravate the progression of diseases. This notion is supported by that observation that, in renal I/R, EVs from proximal tubular cells may induce fibroblast activation and expansion to promote interstitial fibrosis and tissue deterioration (52).
CKD.
As presented above, there is intensive research on the potential of EVs as biomarkers for CKD. In contrast, very limited is known about the therapeutic effect of EVs in CKD. In this regard, Gatti et al. (24) reported that microvesicles derived from human adult mesenchymal stem cells, not only protected against ischemic AKI, but also alleviated its progression to CKD. Of note, in this study, the effect of microvesicles on AKI to CKD transition was most likely due to the amelioration of initial AKI. Mechanistically, the beneficial effect of microvesicles was diminished after pretreatment with RNases, suggesting a critical role of RNAs. As a significant extension of this observation, ischemically injured renal tubule epithelial cells were shown to release exosomes to activate fibroblasts via TGF-β mRNA, contributing to the development of renal fibrosis in post-AKI kidneys (7). However, it is unclear how the production and release of exosomes are regulated in injured tubular cells. Nonetheless, exosomes may be a brand new candidate for tubulointerstitial communication in renal disease progression.
In the anti-Thy1.1-induced model of glomerulonephritis, EVs derived from endothelial progenitor cells (EPCs) showed significant beneficial effects (14). These included the alleviation of mesangial cell activation, leukocyte infiltration, and apoptosis, associated with the reduction of proteinuria. Serum complement hemolytic activity and renal function were also increased by EVs of EPCs in this model. Mechanistically, the effect of EVs may be related to its content of mRNAs coding for antiapoptotic factors and the complement inhibitors (14).
The latest work by Jiang et al. (36) examined the effect of exosomes derived from urine-derived stem cells on DKD. They showed that intravenous injections of exosomes reduced urine volume, microalbuminuria, and podocyte and tubular epithelial cell apoptosis in streptozotocin-induced diabetic rats. In addition, the exosomes increased the proliferation of glomerular endothelial cells (36). Thus exosomes from urine-derived stem cells may have therapeutic effects on DKD.
Conclusions and Perspectives
Research of EVs, especially exosomes, is presently one of the most exciting areas in cell biology. In the kidney field, most previous studies focused on the biomarker potential of EVs, particularly those detected in urine. Nonetheless, the therapeutic effect of EVs has been recognized. The most promising source of therapeutic EVs appears to be the mesenchymal stem cell of different origins (52), whereas exosomes from epithelial cells cultured in conditioned media also showed some effect (7). In addition, EVs may be used to deliver therapeutic molecules, such as proteins, mRNAs, siRNAs, and microRNAs (1).
Despite these studies and potentials, the investigation of EVs in kidney diseases has just begun its journey. There are numerous questions to be raised and answered. EVs are roughly classified mainly according to their origins and sizes, but apparently even the exosomes derived from the same cell may not have the same contents. Also it is unclear how they are produced and released under normal physiological condition and how they are changed under pathological or disease conditions. After their release, where do they go, and what are their specific recipient cells? In recipient cells, what are the main molecular contents that account for their cell biological effects? Although the biomarker potential of uEVs has been suggested, it has to be verified by large clinical studies. In addition, the therapeutic efficacy of EVs needs to be further examined in experimental models and then hopefully translated to the bed side. As an emerging frontier in regenerative medicine, the research of EVs needs to identify the most suitable cell type(s) and the most efficient condition for the production of beneficial EVs. It is also necessary to elucidate where and how the EVs travel and work in the recipient cells in diseased organs. Addressing these and other related questions would lead to an in-depth understanding of the cell biology of EVs and their clinical potentials, including those for diagnosis and treatment of various kidney diseases.
GRANTS
This work was supported in part by grants from National Natural Science Foundation of China (81430017) and the National Institutes of Health and Department of Veterans Administration (US).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.Z., X.Z., H.Z., Q.Y., Y.L., and Z.D. conception and design of research; W.Z. and X.Z. performed experiments; W.Z., X.Z., and Z.D. analyzed data; W.Z., X.Z., and Z.D. interpreted results of experiments; W.Z. and X.Z. prepared figures; W.Z. and X.Z. drafted manuscript; W.Z., X.Z., H.Z., Q.Y., Y.L., and Z.D. edited and revised manuscript; W.Z., X.Z., H.Z., Q.Y., Y.L., and Z.D. approved final version of manuscript.
REFERENCES
- 1.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29: 341–345, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez S, Suazo C, Boltansky A, Ursu M, Carvajal D, Innocenti G, Vukusich A, Hurtado M, Villanueva S, Carreno JE, Rogelio A, Irarrazabal CE. Urinary exosomes as a source of kidney dysfunction biomarker in renal transplantation. Transpl Proc 45: 3719–3723, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol 16: 3381–3388, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Amabile N, Guerin AP, Tedgui A, Boulanger CM, London GM. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: A pilot study. Nephrol Dial Transplant 27: 1873–1880, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Barutta F, Tricarico M, Corbelli A, Annaratone L, Pinach S, Grimaldi S, Bruno G, Cimino D, Taverna D, Deregibus MC, Rastaldi MP, Perin PC, Gruden G. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One 8: e73798, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benito-Martin A, Ucero AC, Zubiri I, Posada-Ayala M, Fernandez-Fernandez B, Cannata-Ortiz P, Sanchez-Nino MD, Ruiz-Ortega M, Egido J, Alvarez-Llamas G, Ortiz A. Osteoprotegerin in exosome-like vesicles from human cultured tubular cells and urine. PLoS One 8: e72387, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borges FT, Melo SA, Ozdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH 2nd, LeBleu VS, Kalluri R. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 24: 385–392, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown D, Verbavatz JM, Valenti G, Lui B, Sabolic I. Localization of the CHIP28 water channel in reabsorptive segments of the rat male reproductive tract. Eur J Cell Biol 61: 264–273, 1993. [PubMed] [Google Scholar]
- 9.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 7: e33115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20: 1053–1067, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger D, Thibodeau JF, Holterman CE, Burns KD, Touyz RM, Kennedy CR. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J Am Soc Nephrol 25: 1401–1407, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger D, Vinas JL, Akbari S, Dehak H, Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS, Burns KD. Human endothelial colony-forming cells protect against acute kidney injury: Role of exosomes. Am J Pathol 185: 2309–2323, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78: 838–848, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Cantaluppi V, Medica D, Mannari C, Stiaccini G, Figliolini F, Dellepiane S, Quercia AD, Migliori M, Panichi V, Giovannini L, Bruno S, Tetta C, Biancone L, Camussi G. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol Dial Transplant 30: 410–422, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Chen HH, Lai PF, Lan YF, Cheng CF, Zhong WB, Lin YF, Chen TW, Lin H. Exosomal ATF3 RNA attenuates pro-inflammatory gene MCP-1 transcription in renal ischemia-reperfusion. J Cell Physiol 229: 1202–1211, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res 38: 215–224, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, Pomatto M, Oliviero S, Tetta C, Quesenberry PJ, Camussi G. AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying microRNAs. J Am Soc Nephrol 26: 2349–2360, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255–289, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Cravedi P, Remuzzi G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br J Clin Pharmacol 76: 516–523, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dursun I, Poyrazoglu HM, Gunduz Z, Ulger H, Yykylmaz A, Dusunsel R, Patyroglu T, Gurgoze M. The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrol Dial Transplant 24: 2511–2518, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Erdbrugger U, Le TH. Extracellular vesicles in renal diseases: More than novel biomarkers? J Am Soc Nephrol 27: 12–26, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fais S, Logozzi M, Lugini L, Federici C, Azzarito T, Zarovni N, Chiesi A. Exosomes: The ideal nanovectors for biodelivery. Biol Chem 394: 1–15, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, Brunet P, Dignat-George F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost 4: 566–573, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant 26: 1474–1483, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Gildea JJ, Seaton JE, Victor KG, Reyes CM, Bigler Wang D, Pettigrew AC, Courtner CE, Shah N, Tran HT, Van Sciver RE, Carlson JM, Felder RA. Exosomal transfer from human renal proximal tubule cells to distal tubule and collecting duct cells. Clin Biochem 47: 89–94, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, Wang NS, Knepper MA. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 20: 363–379, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell 21: 77–91, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Herrera Sanchez MB, Bruno S, Grange C, Tapparo M, Cantaluppi V, Tetta C, Camussi G. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res Ther 5: 124, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogan MC, Bakeberg JL, Gainullin VG, Irazabal MV, Harmon AJ, Lieske JC, Charlesworth MC, Johnson KL, Madden BJ, Zenka RM, McCormick DJ, Sundsbak JL, Heyer CM, Torres VE, Harris PC, Ward CJ. Identification of biomarkers for PKD1 using urinary exosomes. J Am Soc Nephrol 26: 1661–1670, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, Charlesworth MC, Torres VE, LaRusso NF, Harris PC, Ward CJ. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 20: 278–288, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 104: 2761–2766, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Inal JM, Kosgodage U, Azam S, Stratton D, Antwi-Baffour S, Lange S. Blood/plasma secretome and microvesicles. Biochim Biophys Acta 1834: 2317–2325, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Iraci N, Leonardi T, Gessler F, Vega B, Pluchino S. Focus on extracellular vesicles: Physiological role and signalling properties of extracellular membrane vesicles. Int J Mol Sci 17: 171, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jae N, McEwan DG, Manavski Y, Boon RA, Dimmeler S. Rab7a and Rab27b control secretion of endothelial microRNA through extracellular vesicles. FEBS Lett 589: 3182–3188, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Jayachandran M, Miller VM, Heit JA, Owen WG. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J Immunol Methods 375: 207–214, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC, Fan Y, Wang Y, Wang NS. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther 7: 24, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joo KW, Lee JW, Jang HR, Heo NJ, Jeon US, Oh YK, Lim CS, Na KY, Kim J, Cheong HI, Han JS. Reduced urinary excretion of thiazide-sensitive Na-Cl cotransporter in Gitelman syndrome: Preliminary data. Am J Kidney Dis 50: 765–773, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Kalani A, Mohan A, Godbole MM, Bhatia E, Gupta A, Sharma RK, Tiwari S. Wilm's tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS One 8: e60177, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koniusz S, Andrzejewska A, Muraca M, Srivastava AK, Janowski M, Lukomska B. Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front Cell Neurosci 10: 109, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krause M, Samoylenko A, Vainio SJ. Exosomes as renal inductive signals in health and disease, and their application as diagnostic markers and therapeutic agents. Front Cell Dev Biol 3: 65, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: New players in metabolic and cardiovascular disease. J Endocrinol 228: R57–R71, 2016. [DOI] [PubMed] [Google Scholar]
- 42.Lee H, Han KH, Lee SE, Kim SH, Kang HG, Cheong HI. Urinary exosomal WT1 in childhood nephrotic syndrome. Pediatr Nephrol 27: 317–320, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles 4: 29509, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lv LL, Cao YH, Ni HF, Xu M, Liu D, Liu H, Chen PS, Liu BC. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol 305: F1220–F1227, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Lv LL, Cao YH, Pan MM, Liu H, Tang RN, Ma KL, Chen PS, Liu BC. CD2AP mRNA in urinary exosome as biomarker of kidney disease. Clin Chim Acta 428: 26–31, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res 40: D1241–D1244, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merino A, Portoles J, Selgas R, Ojeda R, Buendia P, Ocana J, Bajo MA, del Peso G, Carracedo J, Ramirez R, Martin-Malo A, Aljama P. Effect of different dialysis modalities on microinflammatory status and endothelial damage. Clin J Am Soc Nephrol 5: 227–234, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda KC, Bond DT, McKee M, Skog J, Paunescu TG, Da Silva N, Brown D, Russo LM. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int 78: 191–199, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moon PG, Lee JE, You S, Kim TK, Cho JH, Kim IS, Kwon TH, Kim CD, Park SH, Hwang D, Kim YL, Baek MC. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics 11: 2459–2475, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101: 13368–13373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raimondo F, Corbetta S, Morosi L, Chinello C, Gianazza E, Castoldi G, Di Gioia C, Bombardi C, Stella A, Battaglia C, Bianchi C, Magni F, Pitto M. Urinary exosomes and diabetic nephropathy: A proteomic approach. Mol Bio 9: 1139–1146, 2013. [DOI] [PubMed] [Google Scholar]
- 52.Ranghino A, Dimuccio V, Papadimitriou E, Bussolati B. Extracellular vesicles in the urine: Markers and mediators of tissue damage and regeneration. Clin Kidney J 8: 23–30, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reis LA, Borges FT, Simoes MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One 7: e44092, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rider MA, Hurwitz SN, Meckes DG Jr. ExtraPEG: A polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep 6: 23978, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rood IM, Merchant ML, Wilkey DW, Zhang T, Zabrouskov V, van der Vlag J, Dijkman HB, Willemsen BK, Wetzels JF, Klein JB, Deegens JK. Increased expression of lysosome membrane protein 2 in glomeruli of patients with idiopathic membranous nephropathy. Proteomics 15: 3722–3730, 2015. [DOI] [PubMed] [Google Scholar]
- 56.Sonoda H, Yokota-Ikeda N, Oshikawa S, Kanno Y, Yoshinaga K, Uchida K, Ueda Y, Kimiya K, Uezono S, Ueda A, Ito K, Ikeda M. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 297: F1006–F1016, 2009. [DOI] [PubMed] [Google Scholar]
- 57.Street JM, Birkhoff W, Menzies RI, Webb DJ, Bailey MA, Dear JW. Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J Physiol 589: 6119–6127, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tkach M, Thery C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 164: 1226–1232, 2016. [DOI] [PubMed] [Google Scholar]
- 59.Trnka P, Ivanova L, Hiatt MJ, Matsell DG. Urinary biomarkers in obstructive nephropathy. Clin J Am Soc Nephrol 7: 1567–1575, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 61.van der Pol E, Boing AN, Gool EL, Nieuwland R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost 14: 48–56, 2016. [DOI] [PubMed] [Google Scholar]
- 62.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64: 676–705, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, Brennan EP, Wilkinson-Berka JL, Wise AF, Ricardo SD. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther 24: 1290–1301, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-'t Hoen EN, Piper MG, Sivaraman S, Skog J, Thery C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: Toward clinical application. J Clin Invest 126: 1152–1162, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience 65: 783–797, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou H, Cheruvanky A, Hu X, Matsumoto T, Hiramatsu N, Cho ME, Berger A, Leelahavanichkul A, Doi K, Chawla LS, Illei GG, Kopp JB, Balow JE, Austin HA 3rd, Yuen PS, Star RA. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int 74: 613–621, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou H, Kajiyama H, Tsuji T, Hu X, Leelahavanichkul A, Vento S, Frank R, Kopp JB, Trachtman H, Star RA, Yuen PS. Urinary exosomal Wilms' tumor-1 as a potential biomarker for podocyte injury. Am J Physiol Renal Physiol 305: F553–F559, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou H, Pisitkun T, Aponte A, Yuen PS, Hoffert JD, Yasuda H, Hu X, Chawla L, Shen RF, Knepper MA, Star RA. Exosomal Fetuin-A identified by proteomics: A novel urinary biomarker for detecting acute kidney injury. Kidney Int 70: 1847–1857, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 4: 34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zubiri I, Posada-Ayala M, Sanz-Maroto A, Calvo E, Martin-Lorenzo M, Gonzalez-Calero L, de la Cuesta F, Lopez JA, Fernandez-Fernandez B, Ortiz A, Vivanco F, Alvarez-Llamas G. Diabetic nephropathy induces changes in the proteome of human urinary exosomes as revealed by label-free comparative analysis. J Proteomics 96: 92–102, 2014. [DOI] [PubMed] [Google Scholar]