Abstract
Acute kidney injury (AKI) is a common and often devastating condition among hospitalized patients and is associated with markedly increased hospital length of stay, mortality, and cost. The pathogenesis of AKI is complex, but animal models support an important role for catalytic iron in causing AKI. Catalytic iron, also known as labile iron, is a transitional pool of non-transferrin-bound iron that is readily available to participate in redox cycling. Initial findings related to catalytic iron and animal models of kidney injury have only recently been extended to human AKI. In this review, we discuss the role of catalytic iron in human AKI, focusing on recent translational studies in humans, assay considerations, and potential therapeutic targets for future interventional studies.
Keywords: labile iron, AKI, non-transferrin-bound iron, HO-1, ferritin
acute kidney injury (aki) is a common and often devastating condition associated with markedly increased hospital length of stay, mortality, and cost (10, 53, 54). An important role for iron in the pathogenesis of AKI has long been appreciated. Iron, the most abundant transitional metal in the body, has the capacity to readily accept and donate electrons, cycling reversibly between ferrous (Fe2+) and ferric (Fe3+) forms. This property, which is essential for its functions as a critical component of cytochromes, oxygen-binding molecules (e.g., hemoglobin), and many enzymes, also makes iron dangerous in excess because it can catalyze the formation of free radicals, which can damage macromolecular components of cells.
Catalytic iron, also known as labile iron, is a transitional pool of extracellular and intracellular iron that is not transferrin bound, but rather loosely bound to albumin or low-molecular-weight metal complexing groups, such as citrate, acetate, malate, phosphate, and adenine nucleotides (17, 25, 51). Although plasma catalytic iron is only a small portion of total body iron (Fig. 1), it represents a pool of “free iron” that is readily available to participate in redox cycling. By facilitating the conversion of hydrogen peroxide to free-radical ions, a process known as the Fenton reaction, catalytic iron causes oxidative damage to cellular membranes, proteins, and DNA (23, 28). In this review, we discuss the role of catalytic iron in AKI, focusing on recent translational studies in humans, assay considerations, and potential therapeutic targets for future studies.
Fig. 1.
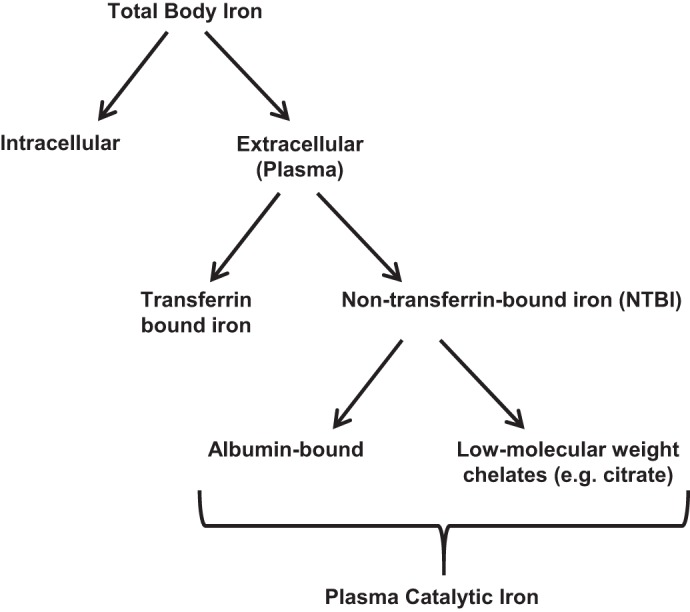
Distribution of cellular and extracellular iron. Extracellular (plasma) iron represents a small portion (∼3 mg) of total body iron (∼3,000–5,000 mg), the vast majority of which is intracellular (e.g., stored in ferritin, hemoglobin, and other cellular iron-containing proteins). Under normal physiological conditions, plasma iron is transferrin bound. In some disorders, a small portion of plasma iron is non-transferrin-bound iron (NTBI). The latter is either bound to albumin or to low-molecular-weight chelates, such as citrate. A portion of circulating NTBI is readily available to participate in redox cycling and is referred to as plasma catalytic iron or labile plasma iron (LPI).
Catalytic Iron in Animal Models of AKI
Two broad lines of evidence support a role for catalytic iron in the pathogenesis of AKI: catalytic iron levels are increased in the kidneys of animals with AKI, and iron chelators are protective (50). Specifically, catalytic iron has been implicated in AKI resulting from a wide range of insults, including ischemia-reperfusion injury (2), aminoglycosides (55), cisplatin (4), rhabdomyolysis (3), hemoglobinuria (43), and iodinated radiocontrast (24). In many of these models, pretreatment with an iron chelator prevents or attenuates AKI (4, 44, 55, 56). Until recently, the relevance of these findings to human AKI was unknown.
Catalytic Iron in Human AKI
Unlike animal models of kidney injury, the study of catalytic iron in human AKI has largely relied on measurement of catalytic iron levels in blood samples (methodology discussed below), since kidney tissue is unavailable in most patients with AKI. Robust animal data demonstrating a causal role for catalytic iron in AKI suggest that elevated plasma catalytic iron levels could serve as an important biomarker and potential mediator of AKI in humans. This hypothesis was recently tested in a series of prospective cohort studies that evaluated plasma and serum catalytic iron levels in three commonly encountered AKI clinical settings: cardiac surgery-associated AKI, critical illness-associated AKI, and contrast-induced AKI.
Cardiac surgery-associated AKI.
Leaf et al. (32) measured plasma catalytic iron levels, along with plasma free hemoglobin, total iron, transferrin, and ferritin, in 250 adult patients who underwent cardiac surgery with cardiopulmonary bypass (CPB). Each of these plasma iron markers was measured preoperatively, at the end of CPB, and on postoperative days 1 and 3. Plasma catalytic iron levels, but not other iron markers, rose significantly at the end of CPB (Fig. 2A) and were directly associated with CPB time and number of packed red blood cell (pRBC) transfusions. In multivariate analyses adjusted for age and preoperative estimated glomerular filtration rate, patients in the highest compared with lowest quartile of catalytic iron on postoperative day 1 had a greater odds of in-hospital death or need for renal replacement therapy (death/RRT), AKI, and several other adverse events postoperatively (Fig. 2B).
Fig. 2.
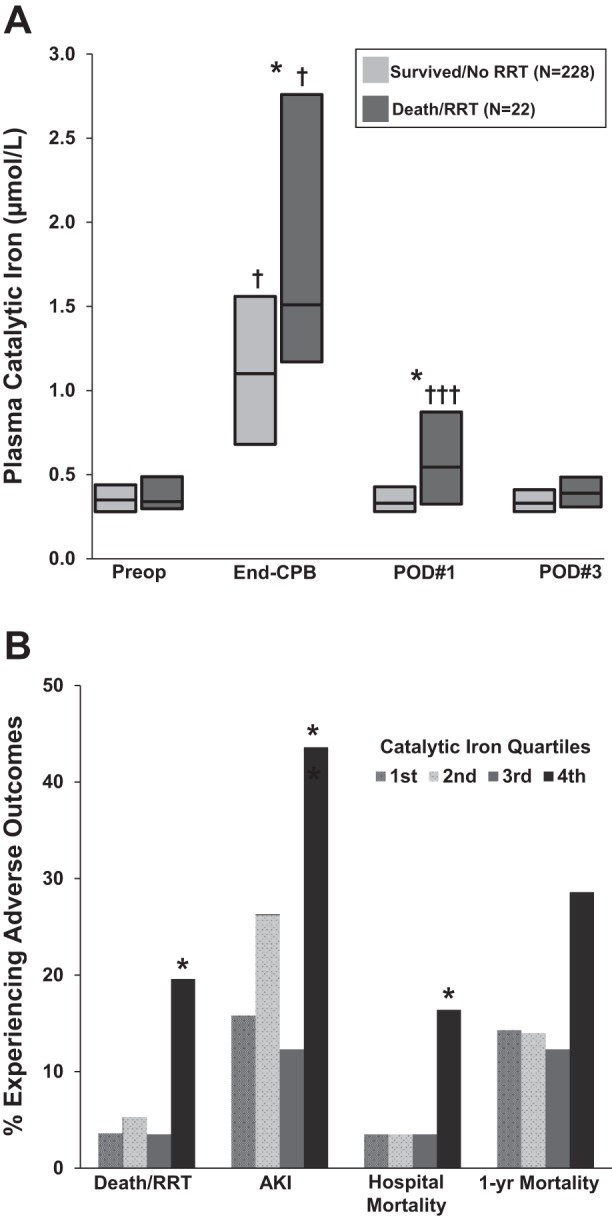
Plasma catalytic iron levels and adverse outcomes following cardiac surgery. A: plasma catalytic iron levels are significantly higher at the end of cardiopulmonary bypass (CPB) compared with preoperative (Preop) levels. †P < 0.001 and †††P < 0.05 for within-group comparison to preoperative levels. Furthermore, catalytic iron levels at the end of CPB and on postoperative day 1 (POD#1) are higher in patients who develop in-hospital death or need for renal replacement therapy (death/RRT). *P < 0.01 for between-group comparisons. Bars represent median (25th–75th interquartile range). B: patients with catalytic iron levels in the highest compared with lowest quartile on POD#1 have a greater risk of death/RRT, AKI, and in-hospital mortality after adjusting for age and preoperative estimated glomerular filtration rate. *P < 0.05. [Adapted from Leaf et al. (32) with permission.]
Critical illness-associated AKI.
In a separate study conducted by the same group (33), plasma catalytic iron levels were measured on intensive care unit day 1 in 121 critically ill patients admitted to medical or surgical intensive care units. Again, higher plasma catalytic iron levels were associated with a greater risk of death/RRT, AKI, and other adverse events. These associations were independent of age, estimated glomerular filtration rate, and number of pRBC transfusions.
Contrast-induced AKI.
In the largest study of catalytic iron and human AKI conducted to date, Lele et al. (34) measured serum catalytic iron levels in 806 patients with acute coronary syndrome who underwent coronary angiography. Catalytic iron levels were measured at baseline and at 24 and 48 h after exposure to iodinated radiocontrast. Patients with vs. without contrast-induced AKI had higher catalytic iron levels at 24 and 48 h.
Measurement of Catalytic Iron in Human Blood Samples
Catalytic iron can be measured in plasma or serum by two different methods: the bleomycin-detectable catalytic iron (BDI) assay, and the labile plasma iron (LPI) assay. A summary of the characteristics of the two assays is shown in Table 1. The BDI assay relies on the principle that the antitumor antibiotic, bleomycin, degrades DNA in the presence of catalytic iron and reducing agent (ascorbate). The DNA degradation products react with thiobarbituric acid to form a pink chromogen, which is quantified spectrophotometrically (16, 22). The LPI assay relies on the conversion of the nonfluorescent oxidation-sensitive probe, dihydrorhodamine, into the fluorescent rhodamine, in the presence of catalytic iron and reducing agent (ascorbate). Comparison of the generated fluorescence in the presence or absence of an iron chelator (deferrioxamine or deferiprone) confers specificity for exchangeable or chelatable iron (9, 15).
Table 1.
Comparison of the bleomycin-detectable catalytic iron assay vs. the labile plasma iron assay
| BDI | LPI | |
|---|---|---|
| Reference assay | Halliwell et al. (22); Evans and Halliwell (16) | Esposito et al. (15) |
| Agents | Ascorbate, bleomycin, DNA, and thiobarbituric acid | Ascorbate and dihydrorhodamine |
| Method of detection | Colorimetric | Fluorescence |
| Lower limit of detection | Variable* | Variable‡ |
| Interassay CV | Variable† | Variable‡ |
| Commercially available | No | Yes (Aferrix Ltd., Tel-Aviv, Israel) |
| Used in clinical AKI studies | Yes (Refs. 32–34) | No |
| Used in clinical studies of iron-overload disorders | Yes (Refs. 8, 12) | Yes (Refs. 7, 9) |
BDI, bleomycin-detectable catalytic iron; LPI, labile plasma iron; CV, coefficient of variation.
Depending on the laboratory, with 0.03 μmol/l reported by Leaf et al. (32).
Depending on the laboratory, with <10% reported by Leaf et al. (32).
Depending on the laboratory or the commercial kit used (12).
In recent studies among patients undergoing hematopoetic stem cell transplantation, and among patients with iron-overload disorders, the BDI and LPI assays were compared and provided similar results (12, 27). Interestingly, in these studies both DBI and LPI were related to transferrin saturation (TSAT), with BDI and LPI mostly undetectable at TSAT < 80%. However, in patients undergoing cardiac surgery, as well as in critically ill patients, Leaf et al. detected elevated plasma concentrations of BDI at much lower TSATs (32, 33). It is unknown whether this is due to differences in the BDI vs. LPI assays, or differences in patient populations.
Therapeutic Targets
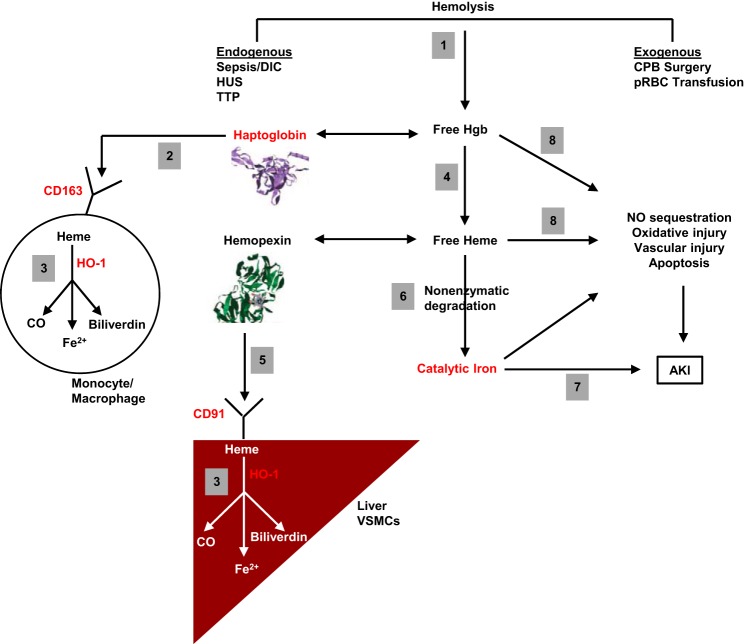
A schematic of the proposed pathophysiology of iron-associated AKI is provided in Fig. 3. Notably, the complex nature of iron homeostasis provides a variety of opportunities for pharmacological intervention. Targets that are particularly attractive for further study include iron chelators, haptoglobin, ferritin, and inducers of heme oxygenase-1 (HO-1) and the CD163/CD91 scavenger receptors.
Fig. 3.
Proposed pathophysiological mechanisms of catalytic iron-induced AKI. 1: Hemolysis, which can occur through endogenous or exogenous processes, results in the release of free hemoglobin (Hgb) into the plasma. 2: Free Hgb is bound by haptoglobin, and the complex is taken up by monocytes and macrophages via the scavenger receptor, CD163. CD163 can also facilitate receptor-mediated endocytosis of free Hgb, even in the absence of haptoglobin (48). 3: Once internalized, Hgb is broken down into heme, which is degraded further by heme oxygenase-1 (HO-1) into carbon monoxide (CO), biliverdin, and Fe2+. The latter is oxidized into Fe3+ and sequestered by ferritin, which is upregulated by HO-1 (41). 4: Free Hgb not sequestered by haptoglobin may be oxidized into free heme in the circulation. 5: Free heme is sequestered by hemopexin and taken up by hepatocytes, vascular smooth muscle cell (VSMCs), and other cell types via the scavenger receptor, CD91, also known as low density lipoprotein receptor-related protein 1 (LRP1). 6: When the ability of haptoglobin and hemopexin to scavenge free Hgb and free heme is overwhelmed, respectively, nonenzymatic degradation (i.e., in the absence of HO-1) may release catalytic iron from heme. 7: This catalytic iron catalyzes the formation of free radicals, which can damage macromolecular components of cells, resulting in AKI (5). 8: Even in the absence of catalytic iron generation, free Hgb and free heme may contribute to AKI by a variety of mechanisms, including nitric oxide (NO) sequestration, which results in vasoconstriction (14), oxidant-mediated cellular damage (46), vascular injury (30), and induction of apoptosis in the presence of other cytotoxic agonists, such as tumor necrosis factor (TNF) (49). Key targets for potential therapeutic intervention are colored in red. CPB, cardiopulmonary bypass; DIC, disseminated intravascular coagulation; HUS, hemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura.
Iron chelation.
Iron chelators form a complex with iron and thereby promote its excretion through the urinary or biliary pathways. In various experimental models of AKI, pretreatment with the iron chelator, deferoxamine, protects animals against a wide variety of nephrotoxic insults (4, 44, 55, 56). Furthermore, administration of apotransferrin or neutrophil gelatinase-associated lipocalin, both endogenous iron-binding molecules, protects against kidney injury in animal models of renal ischemia-reperfusion injury (13, 36, 39). However, only a few small studies have attempted to extend these findings from animal AKI models to humans.
Menasche et al. (38) evaluated the effects of intravenous deferoxamine vs. placebo in 24 adult patients undergoing cardiac surgery with CPB. Although the study was underpowered to detect differences in rates of AKI between the two groups, isolated neutrophils from patients treated with deferoxamine produced fewer superoxide radicals than those from control patients. In a follow-up study by the same group, deferoxamine-treated patients undergoing cardiac surgery had lower plasma lipid peroxidation parameters than the control group, suggesting a potentially beneficial effect on iron-mediated oxidative stress (37).
Paraskevaidis and colleagues (45) randomly assigned 45 patients undergoing coronary artery bypass grafting to receive an 8-h intravenous infusion of deferoxamine, beginning immediately after induction of anesthesia, vs. placebo. Compared with placebo, deferoxamine-treated patients had lower peak levels of oxygen free-radical production, as well as higher left ventricular ejection fraction at 12 mo follow-up. Rates of AKI were similar between the two groups (45).
Lastly, Fraga et al. (18) randomly assigned 30 critically ill patients with sustained hypotension to receive combination therapy with deferoxamine and N-acetylcysteine vs. placebo. While the incidence of AKI was similar between the two groups, deferoxamine/N-acetylcysteine-treated patients had lower serum creatinine levels at hospital discharge compared with controls. A larger follow-up study by the same group was recently completed (NCT00870883), and results are awaited.
While these pilot studies appear promising, additional well-designed and adequately powered studies of iron chelation for the prevention and/or treatment of AKI are needed. One of the critical considerations for such studies will be the choice of iron chelator. The parenteral iron chelator, deferoxamine, was the drug used in most of the preclinical AKI models and has been used in humans for nearly half a century for the treatment of chronic iron overload disorders. In contrast, two oral iron chelators, deferasirox and deferiprone, became available more recently. Deferasirox and deferiprone have lower iron-binding affinities than deferoxamine (22.5 and 19.9 pM, respectively, compared with 26.6 pM), but their greater lipid solubility and enhanced intracellular penetrance suggest greater potential for chelation of cytosolic iron compared with deferoxamine. However, deferasirox has been associated with AKI (26), whereas deferiprone is excreted primarily via the urinary rather than the biliary pathway, potentially limiting its efficacy in patients with impaired renal function.
Haptoglobin.
By increasing the efficiency of free hemoglobin sequestration, administration of haptoglobin could attenuate hemoglobin/iron-mediated nephrotoxicity in clinical settings associated with hemolysis (e.g., cardiac surgery). This strategy was effective in preventing hemoglobin-induced AKI in guinea pigs (1), as well as pRBC transfusion-induced AKI in a murine model (21). In humans, therapeutic administration of a plasma-derived haptoglobin product developed by Benesis in Japan was evaluated in several small studies with mixed results (47). However, this product is not currently available.
Ferritin.
Ferritin is a key protein that has the capacity to sequester large amounts of intracellular iron and thereby protects against oxidant-mediated cellular damage (5). Recent animal studies have highlighted the importance of renal proximal tubular heavy chain ferritin expression in regulating renal inflammation and AKI (6, 57). Additionally, it has long been recognized that the renoprotective effects of HO-1, a key cytoprotective protein, may be largely attributable to upregulation of ferritin (41). In humans, there is conflicting epidemiological data on the role of ferritin in AKI. A pilot study of 30 patients found that low preoperative serum ferritin levels were associated with a greater incidence of AKI following CPB surgery (11). However, a larger subsequent study (n = 120) failed to confirm this association (52). Whether pharmacological targeting of ferritin could enhance the efficiency of catalytic iron sequestion and thereby improve renal outcomes remains to be studied.
HO-1.
HO-1 is the rate-limiting enzyme in the breakdown of heme into carbon monoxide, iron, and biliverdin. A wealth of data from animal models support its relevance as a cytoprotective enzyme in AKI (40). Additionally, a recent study found that common genetic polymorphisms in the HO-1 gene promoter, which are known to be associated with HO-1 expression and activity, are associated with postoperative AKI in patients undergoing cardiac surgery (31). Thus development of novel therapeutic agents that induce renal and extrarenal (e.g., monocyte) HO-1 expression as a strategy for AKI prevention has been a topic of great interest (35). Unfortunately, most of the known inducers of HO-1 (e.g., free heme, endotoxin, H2O2) are compounds that may be unsafe for administration to humans.
CD163/CD91.
Finally, targeted upregulation of the scavenger receptors, CD163 and CD91, could increase the efficiency of hemoglobin and heme removal from the circulation, respectively, and thereby attenuate heme/iron-mediated AKI. Glucocorticoids are known inducers of CD163 cell surface expression on monocytes (20) and CD91 expression on macrophages (42). Interestingly, a recent study found that glucocorticoids may be beneficial in preventing severe AKI among patients undergoing cardiac surgery (29). However, since this was a post hoc study, the findings should be interpreted with caution; moreover, a more recent study found no benefit of glucocorticoids in the prevention of cardiac surgery-associated AKI (19).
In conclusion, a wealth of data on catalytic iron in animal models of AKI have only recently begun to be translated to human AKI. Additional studies are needed to establish whether plasma catalytic iron may serve as a useful biomarker of AKI in humans. Moreover, adequately powered clinical trials are needed to assess whether interventions that target iron regulatory pathways may improve clinical outcomes in human AKI.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant K23DK106448 (to D. E. Leaf).
DISCLOSURES
D. W. Swinkels is an employee of Radboudumc that offers high-quality NTBI and LPI assays on a fee-for-service basis (http://www.radboud-ironcenter.com/diagnostics/ntbi-lpi).
AUTHOR CONTRIBUTIONS
D.E.L. conception and design of research; D.E.L. and D.W.S. analyzed data; D.E.L. prepared figures; D.E.L. drafted manuscript; D.E.L. and D.W.S. edited and revised manuscript; D.E.L. and D.W.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Z. Ioav Cabantchik for advice on the section on measurement of catalytic iron in human blood samples.
REFERENCES
- 1.Baek JH, D'Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 122: 1444–1458, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baliga R, Ueda N, Shah SV. Increase in bleomycin-detectable iron in ischaemia/reperfusion injury to rat kidneys. Biochem J 291: 901–905, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baliga R, Zhang Z, Baliga M, Shah SV. Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int 49: 362–369, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int 53: 394–401, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem 267: 18148–18153, 1992. [PubMed] [Google Scholar]
- 6.Bolisetty S, Zarjou A, Hull TD, Traylor AM, Perianayagam A, Joseph R, Kamal AI, Arosio P, Soares MP, Jeney V, Balla J, George JF, Agarwal A. Macrophage and epithelial cell H-ferritin expression regulates renal inflammation. Kidney Int 88: 95–108, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brissot P, Ropert M, Le Lan C, Loreal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 1820: 403–410, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Burkitt MJ, Milne L, Raafat A. A simple, highly sensitive and improved method for the measurement of bleomycin-detectable iron: the “catalytic iron index” and its value in the assessment of iron status in haemochromatosis. Clin Sci (Lond) 100: 239–247, 2001. [PubMed] [Google Scholar]
- 9.Cabantchik ZI. Labile iron in cells and body fluids: physiology, pathology, and pharmacology. Front Pharmacol 5: 45, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Davis CL, Kausz AT, Zager RA, Kharasch ED, Cochran RP. Acute renal failure after cardiopulmonary bypass in related to decreased serum ferritin levels. J Am Soc Nephrol 10: 2396–2402, 1999. [DOI] [PubMed] [Google Scholar]
- 12.de Swart L, Hendriks JC, van der Vorm LN, Cabantchik ZI, Evans PJ, Hod EA, Brittenham GM, Furman Y, Wojczyk B, Janssen MC, Porter JB, Mattijssen VE, Biemond BJ, MacKenzie MA, Origa R, Galanello R, Hider RC, Swinkels DW. Second international round robin for the quantification of serum non-transferrin-bound iron and labile plasma iron in patients with iron-overload disorders. Haematologica 101: 38–45, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vries B, Walter SJ, von Bonsdorff L, Wolfs TG, van Heurn LW, Parkkinen J, Buurman WA. Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia-reperfusion injury. Transplantation 77: 669–675, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol 16: 672–676, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood 102: 2670–2677, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Evans PJ, Halliwell B. Measurement of iron and copper in biological systems: bleomycin and copper-phenanthroline assays. Methods Enzymol 233: 82–92, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Evans RW, Rafique R, Zarea A, Rapisarda C, Cammack R, Evans PJ, Porter JB, Hider RC. Nature of non-transferrin-bound iron: studies on iron citrate complexes and thalassemic sera. J Biol Inorg Chem 13: 57–74, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Fraga CM, Tomasi CD, Biff D, Topanotti MF, Felisberto F, Vuolo F, Petronilho F, Dal-Pizzol F, Ritter C. The effects of N-acetylcysteine and deferoxamine on plasma cytokine and oxidative damage parameters in critically ill patients with prolonged hypotension: a randomized controlled trial. J Clin Pharmacol 52: 1365–1372, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Garg AX, Whitlock RP. Effect of methylprednisolone on acute kidney injury in patients undergoing cardiac surgery with cardiopulmonary bypass. In: Proceedings of the High-Impact Clinical Trials, American Society of Nephrology Kidney Week, San Diego, CA, November 7, 2015. Washington, DC: American Society of Nephrology, 2015. [Google Scholar]
- 20.Goldstein JI, Goldstein KA, Wardwell K, Fahrner SL, Goonan KE, Cheney MD, Yeager MP, Guyre PM. Increase in plasma and surface CD163 levels in patients undergoing coronary artery bypass graft surgery. Atherosclerosis 170: 325–332, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Graw JA, Rosales IA, Liu YL, Sabbisetti VS, Riley FE, Rechester O, Bloch KD, Warren HS, Bonventre JV, Zapol WM. Haptoglobin therapy prevents kidney injury in stored blood resuscitation of murine hemorrhagic shock (Abstract). Blood 124: 761, 2014.24957145 [Google Scholar]
- 22.Halliwell B, Aruoma OI, Mufti G, Bomford A. Bleomycin-detectable iron in serum from leukaemic patients before and after chemotherapy. Therapeutic implications for treatment with oxidant-generating drugs. FEBS Lett 241: 202–204, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186: 1–85, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Hanss BG, Valencia SH, Shah SV, Vari RC. The iron chelator deferoxamine prevents contrast media induced acute renal failure in the rabbit (Abstract). J Am Soc Nephrol 1: 612, 1990. [Google Scholar]
- 25.Hider RC, Silva AM, Podinovskaia M, Ma Y. Monitoring the efficiency of iron chelation therapy: the potential of nontransferrin-bound iron. Ann N Y Acad Sci 1202: 94–99, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Huang WF, Chou HC, Tsai YW, Hsiao FY. Safety of deferasirox: a retrospective cohort study on the risks of gastrointestinal, liver and renal events. Pharmacoepidemiol Drug Saf 23: 1176–1182, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Itkonen O, Vaahtera L, Parkkinen J. Comparison of bleomycin-detectable iron and labile plasma iron assays. Clin Chem 59: 1271–1273, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Jacob AK, Hotchkiss RS, DeMeester SL, Hiramatsu M, Karl IE, Swanson PE, Cobb JP, Buchman TG. Endothelial cell apoptosis is accelerated by inorganic iron and heat via an oxygen radical dependent mechanism. Surgery 122: 243–254, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Jacob KA, Leaf DE, Dieleman JM, van Dijk D, Nierich AP, Rosseel PM, van der Maaten JM, Hofland J, Diephuis JC, de Lange F, Boer C, Kluin J, Waikar SS; Dexamethasone for Cardiac Surgery Study Group. Intraoperative high-dose dexamethasone and severe AKI after cardiac surgery. J Am Soc Nephrol 26: 2947–2951, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood 100: 879–887, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Leaf DE, Body SC, Muehlschlegel JD, McMahon GM, Pichtner P, Collard CD, Shernan SK, Fox AA, Waikar SS. Length polymorphisms in heme oxygenase-1 and AKI after cardiac surgery. J Am Soc Nephrol. [Epub before print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Rawn JD, Frendl G, Waikar SS. Increased plasma catalytic iron in patients may mediate acute kidney injury and death following cardiac surgery. Kidney Int 87: 1046–1054, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Waikar SS. Plasma catalytic iron, AKI, and death among critically ill patients. Clin J Am Soc Nephrol 9: 1849–1856, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lele SS, Mukhopadhyay BN, Mardikar MM, Patel TA, Vasavada AK, Banker DN, Kapasi KD, Chauhan VC, Chawla KC, Raju SR, Hiremath SS, Chinchole SS, Rajapurkar MM. Impact of catalytic iron on mortality in patients with acute coronary syndrome exposed to iodinated radiocontrast-the ISCOM study. Am Heart J 165: 744–751, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Lever JM, Boddu R, George JF, Agarwal A. Heme oxygenase-1 in kidney health and disease. Antioxid Redox Signal 25: 165–183, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martines AM, Masereeuw R, Tjalsma H, Hoenderop JG, Wetzels JF, Swinkels DW. Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat Rev Nephrol 9: 385–398, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Menasche P, Antebi H, Alcindor LG, Teiger E, Perez G, Giudicelli Y, Nordmann R, Piwnica A. Iron chelation by deferoxamine inhibits lipid peroxidation during cardiopulmonary bypass in humans. Circulation 82, Suppl: IV390–IV396, 1990. [PubMed] [Google Scholar]
- 38.Menasche P, Pasquier C, Bellucci S, Lorente P, Jaillon P, Piwnica A. Deferoxamine reduces neutrophil-mediated free radical production during cardiopulmonary bypass in man. J Thorac Cardiovasc Surg 96: 582–589, 1988. [PubMed] [Google Scholar]
- 39.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 15: 3073–3082, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Nath KA. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens 23: 17–24, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 90: 267–270, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nilsson A, Vesterlund L, Oldenborg PA. Macrophage expression of LRP1, a receptor for apoptotic cells and unopsonized erythrocytes, can be regulated by glucocorticoids. Biochem Biophys Res Commun 417: 1304–1309, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Paller MS. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol Renal Fluid Electrolyte Physiol 255: F539–F544, 1988. [DOI] [PubMed] [Google Scholar]
- 44.Paller MS, Hedlund BE. Role of iron in postischemic renal injury in the rat. Kidney Int 34: 474–480, 1988. [DOI] [PubMed] [Google Scholar]
- 45.Paraskevaidis IA, Iliodromitis EK, Vlahakos D, Tsiapras DP, Nikolaidis A, Marathias A, Michalis A, Kremastinos DT. Deferoxamine infusion during coronary artery bypass grafting ameliorates lipid peroxidation and protects the myocardium against reperfusion injury: immediate and long-term significance. Eur Heart J 26: 263–270, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Ponka P. Cell biology of heme. Am J Med Sci 318: 241–256, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121: 1276–1284, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaer DJ, Schaer CA, Buehler PW, Boykins RA, Schoedon G, Alayash AI, Schaffner A. Cd163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood 107: 373–380, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Seixas E, Gozzelino R, Chora A, Ferreira A, Silva G, Larsen R, Rebelo S, Penido C, Smith NR, Coutinho A, Soares MP. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc Natl Acad Sci U S A 106: 15837–15842, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah SV, Rajapurkar MM, Baliga R. The role of catalytic iron in acute kidney injury. Clin J Am Soc Nephrol 6: 2329–2331, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Silva AM, Hider RC. Influence of non-enzymatic post-translation modifications on the ability of human serum albumin to bind iron. Implications for non-transferrin-bound iron speciation. Biochim Biophys Acta 1794: 1449–1458, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Tuttle KR, Worrall NK, Dahlstrom LR, Nandagopal R, Kausz AT, Davis CL. Predictors of ARF after cardiac surgical procedures. Am J Kidney Dis 41: 76–83, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Waikar SS, Liu KD, Chertow GM. The incidence and prognostic significance of acute kidney injury. Curr Opin Nephrol Hypertens 16: 227–236, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker PD, Shah SV. Evidence suggesting a role for hydroxyl radical in gentamicin-induced acute renal failure in rats. J Clin Invest 81: 334–341, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zager RA. Combined mannitol and deferoxamine therapy for myohemoglobinuric renal injury and oxidant tubular stress. Mechanistic and therapeutic implications. J Clin Invest 90: 711–719, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, Balla J, Verlander J, Darshan D, Kuhn LC, Agarwal A. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123: 4423–4434, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]