Abstract
Recent studies suggested a direct link between circadian rhythms and regulation of sodium excretion. Endothelin-1 (ET-1) regulates sodium balance by promoting natriuresis through the endothelin B receptor (ETB) in response to increased salt in the diet, but the effect that the time of day has on this natriuretic response is not known. Therefore, this study was designed to test the hypothesis that ETB receptor activation contributes to the diurnal control of sodium excretion and that sex differences contribute to this control as well. Twelve-hour urine collections were used to measure sodium excretion. On day 3 of the experiment, a NaCl load (900 μeq) was given by oral gavage either at Zeitgeber time [ZT] 0 (inactive period) or ZT12 (active period) to examine the natriuretic response to the acute salt load. Male and female ETB-deficient (ETB def) rats showed an impaired natriuretic response to a salt load at ZT0 compared with their respective transgenic controls (Tg cont). Male ETB def rats showed a delayed natriuretic response to a salt load given at ZT12 compared with male Tg cont, a contrast to the prompt response shown by female ETB def rats. Treatment with ABT-627, an ETA receptor antagonist, improved the natriuretic response seen within the first 12 h of a ZT0 salt load in both sexes. These findings demonstrate that diurnal excretion of an acute salt load 1) requires ET-1 and the ETB receptor, 2) is more evident in male vs. female rats, and 3) is opposed by the ETA receptor.
Keywords: ETB receptors, ETA receptors, circadian rhythm, kidney, male and female, rats
despite advancements in understanding how circadian rhythms affect cardiovascular health, the impact of salt on sodium excretion and blood pressure at different times of the day is an area that requires more information. Sodium excretion throughout the day typically follows a diurnal rhythm, with levels higher during the active period and lower during the inactive period and are independent of food and water intake (24, 25). Diurnal rhythms in renal functions such as water and electrolyte excretion are necessary to maintain body homeostasis and good health. Disruptions in daily rhythmic activity, such as shift work or sleep disorders, can lead to an increased risk of a range of diseases such as diabetes, chronic kidney disease, heart failure, and sleep apnea (1).
Men in the United States have a higher likelihood of developing hypertension than premenopausal women. In numerous animal models with salt sensitivity, such as those with angiotensin II- induced hypertension (21, 31) and DOCA-salt hypertension (33), females exhibited a protective effect against the rise in blood pressure. There are several factors that may contribute to this sex difference, one of which is the ability of the kidney to handle sodium. It is important to note that females are able to excrete a similar amount of sodium as males but at a lower arterial pressure (19).
Endothelin-1 (ET-1) is an important regulator of blood pressure through the maintenance of sodium homeostasis, via mechanisms that vary between sexes (23). Through the actions of its two receptors, ETA and ETB, ET-1 is able to control sodium excretion. In general, these receptors have opposing actions throughout the body. Through the ETB receptor, ET-1 promotes sodium excretion in response to increased salt intake (11). The abundance of ETB receptors in the renal medulla of the collecting duct allows the kidney to promote natriuresis in response to increased salt intake from the diet. Studies have previously shown that defects in ETB receptor function lead to salt-sensitive hypertension (9, 27). In addition, knockout of ET-1 or ETB receptor genes specifically in the collecting duct causes sodium retention and hypertension (2, 11), emphasizing the importance of this system for maintaining sodium balance. Our laboratory and others have previously reported on the sex differences in ETB receptor function (18, 20, 21) and the increased ratio of ETB/ETA receptors in the renal medulla of females compared with males (17). We have also shown that the ETA receptor contributes to the natriuretic response to ET-1 in female ETB-deficient (ETB def) rats (26), consistent with a more efficient ability of females to excrete sodium compared with males. While the ETB receptor is important in promoting natriuresis, whether the time of day has any effect on the natriuretic response to the salt load is unknown for either males or females. Furthermore, the role of the ETA receptor in this response is unclear due to its opposing antinatriuretic and possibly natriuretic actions.
The current study was designed to test the hypothesis that ETB receptor activation contributes to the diurnal control of sodium excretion. ETB-deficient rats and their controls were given an acute salt load (900 μeq) and had their urine collected in 12-h intervals over 5½ days to determine the natriuretic response to the salt load. To determine whether there are sex differences in diurnal control of sodium excretion, we used male and female ETB-deficient rats. ETA receptor function is unopposed in ETB receptor deficiency, so we tested the role of the ETA receptor in the acute natriuretic response to a salt load in both sexes through administration of the ETA receptor antagonist ABT-627.
METHODS
Experimental Animals
Experiments were performed using male and female transgenic control (Tg cont) and ETB-deficient (ETB def) rats (Wistar-Kyoto genetic background) obtained from our in-house breeding colony at the University of Alabama at Birmingham. Both Tg cont and ETB def rats carry the transgene for the ETB receptor driven by the dopamine-β-hydroxylase promoter that rescues ETB-deficient rats from a lethal phenotype by expressing a functional ETB receptor in adrenergic tissues (10). However, these rats lack functional ETB receptors in nonadrenergic tissue such as the vascular endothelium and renal tubular epithelium. Animals were housed under conditions of constant temperature and humidity with a 12:12-h light-dark cycle. Experimental protocols and animal care methods were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental Protocol
To determine whether the diurnal variation of natriuresis is blunted in ETB-deficient rats, we gave male Tg cont and ETB def rats a 900-μeq salt load in 1 ml H2O by oral gavage in a >1- to <2-s bolus, either at the beginning of their active [dark cycle; Zeitgeber time (ZT; ZT12)] period or their inactive (light cycle; ZT0) period. After the equilibration period, the experiment started at the beginning of the active period (day 0, ZT12). We monitored food and water intake and collected urine in 12-h intervals. On day 3 of the experiment, rats were divided into two groups. The first group was given an acute NaCl load (900 μeq Na+ in 1 ml H2O) by oral gavage at ZT12, while the second group was given the salt load at ZT0. Measurements for urine volume, food, and water were continued for 2 additional days. Urine was analyzed for electrolyte content with an EasyLyte Na/K/Cl/Li analyzer (Medica, Bedford, MA). After the final collection period (5½ days: 11 consecutive 12-h time points), rats were returned to regular cages for 3–4 days before the protocol was repeated for a follow-up experiment. For the second week of experiments, the time of day for acute salt loading was reversed for each individual rat, meaning that all rats that received an acute salt load at ZT0 or ZT12 in the first week then alternatively received a salt load at ZT12 or ZT0. To determine whether there were any possible sex differences in this acute natriuretic response, the experiment was repeated in female Tg cont and ETB def rats. To assess the role of the ETA receptor in the diurnal natriuretic response to a salt load, separate groups of rats received the ETA-selective antagonist ABT-627 (5 mg·kg−1·day−1; Abbott Laboratories) in the drinking water (27) with standard chow beginning at the time they were placed in metabolic cages, and throughout the time while subjected to the identical acute salt-loading protocol. The acute salt load was given 4–5 days after the ABT-627 treatment began, so the rats are expected to be back in sodium balance at the time of the acute salt load.
All rats were placed in metabolic cages 2 days before the start of the experiment to allow for acclimatization to the cage. Following this equilibration period, individually synced telemetry receivers were placed on the side of the cage to simultaneously monitor blood pressure while urine was being collected. Rats were maintained on standard chow containing 0.3% Na+ and tap water throughout the study.
Telemetry Blood Pressure Measurements
As previously described (27), 24-h ambulatory blood pressure measurements were made using PA-C40 transmitters (Data Sciences International, Duluth, MN). In brief, rats were anesthetized with 2–3% isoflurane, followed by exposure of the abdominal aorta by midline incision and brief occlusion of the aorta. The transmitter catheter was inserted into the distal portion of the aorta and held in place using tissue glue (Vetbond, 3M, St. Paul, MN). The transmitter body was sutured to the abdominal wall along the incision line as the incision was closed. Wound clips were used to close the skin. Rats were allowed to recover for at least 1 wk after surgery before engaging in any experimental protocols. Mean arterial pressure (MAP) was recorded at 10-s intervals every 10 min throughout the study.
Statistical Analysis
All data are expressed as means ± SE. Statistical analyses of the change in urinary sodium excretion (ΔUNaV) and mean arterial pressure (ΔMAP) in male and female Tg control and ETB def animals were made by two-way ANOVA, followed by Bonferroni post hoc tests. ΔUNaV and ΔMAP were calculated by taking the average of the baseline values for a specific time of day and subtracting that value from the respective postload value. Results with P < 0.05 were considered statistically significant.
RESULTS
Diurnal Natriuretic Response to an Acute Salt Load in Male and Female Rats
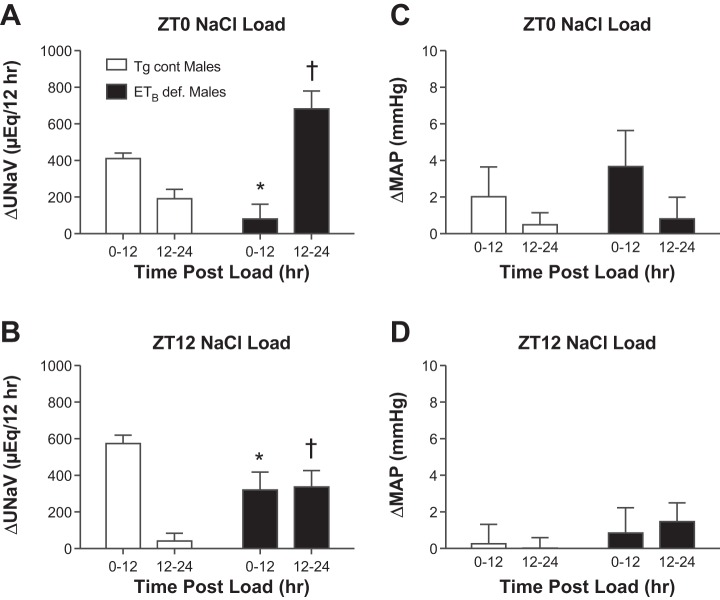
For males given a salt load at ZT0, Tg cont rats excreted the majority of their salt load within the first 12 h postload (Fig. 1A). ETB def rats, however, showed an impaired acute natriuretic response after the ZT0 salt load, excreting almost the entire salt load in the second 12-h period postload. Calculation of the change in sodium excretion for the entire 24-h period following the acute salt load revealed no significant differences in sodium excretion between genotypes (602 ± 62 vs. 763 ± 131 μeq Na in Tg cont vs. ETB def males, respectively). Male Tg cont rats given a salt load at ZT12 excreted the majority of the salt load in the first 12 h after the load was given (Fig. 1B). In ETB def male rats, there was a delayed response to the ZT12 salt load, excreting less sodium in the first 12 h after the load was given and more in the second 12-h period compared with controls. This delay was not as pronounced as what was seen in ETB def rats given a salt load at ZT0. These changes occurred without any significant difference in MAP between genotypes (Fig. 1, C and D). Again, the change in the 24-h excretion rate was not different between Tg cont and ETB def male rats follow the salt load at ZT12 (615 ± 43 vs. 656 ± 81 μeq Na, respectively).
Fig. 1.
A: effect of an inactive period (ZT0) salt load on the rate of sodium excretion in males; n = 6–7 rats/group. PGenotype = not significant (NS). PTime < 0.05. PGenotype*Time < 0.05. *P < 0.05 vs. transgenic control (Tg cont) 0–12. †P < 0.05 vs. Tg cont 12–24. B: effect of an active period (ZT12) salt load on the rate of sodium excretion in males; n = 5–6 rats/group. *P < 0.05 vs. Tg cont 0–12. †P < 0.05 vs. 12–24 Tg cont. PGenotype = NS. PTime < 0.05. PGenotype*Time < 0.05 and effect of a salt load at ZT0 (C) and ZT12 (D) on change in mean arterial pressure (MAP) in males; n = 5–6 rats/group. C and D: PGenotype, PTime, and PGenotype*Time = NS.
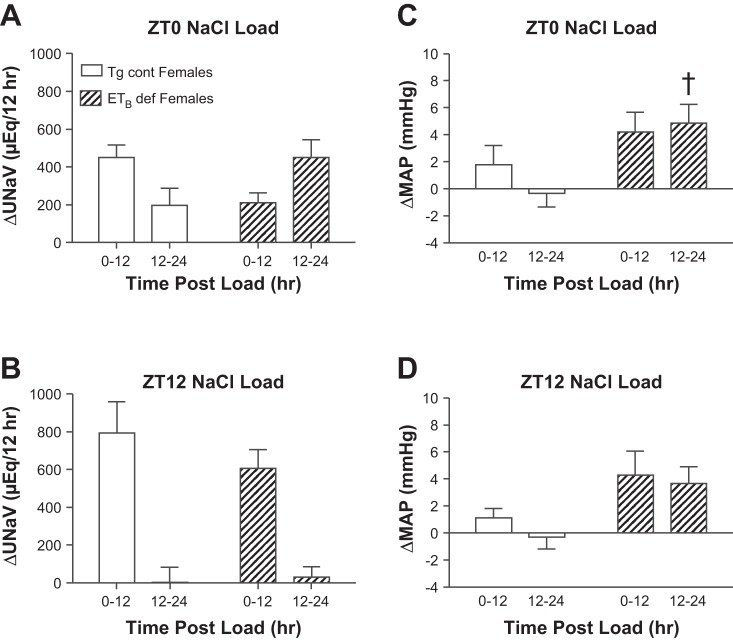
After given a salt load at ZT0, Tg cont females excreted the majority of the salt within the first 12 h (Fig. 2A). ETB def rats showed a delayed response, excreting about two-thirds of the salt load in the second 12-h period after the load was given, resulting in a significant interaction as determined by two-way ANOVA (PGenotype*Time < 0.05). After given a ZT12 salt load, Tg cont females excreted all of the excess salt in the first 12 h postload. ETB def females showed the same pattern of natriuresis, excreting the majority of their salt load within the first 12 h as well (Fig. 2B). These differences in natriuretic patterns between genotypes were seen without a significance difference in MAP (Fig. 2, C and D) with no significant interaction between genotype and time. The 24-h excretion rate of sodium was not significantly different between Tg cont and ETB def females following a salt load given at either ZT0 (648 ± 84 vs. 662 ± 103 μeq Na, respectively) or ZT12 (796 ± 234 vs. 637 ± 120 μeq Na, respectively). Taken together, these results suggest that a lack of ETB receptor function results in a delayed sodium excretion response to an acute salt load, and this impairment is more evident in male compared with female rats.
Fig. 2.
Effect of a salt load at ZT0 (A) and ZT12 (B) on the rate of sodium excretion in females and effect of a salt load at ZT0 (C) and ZT12 (D) on the change in MAP in females; n = 8–10 rats/group. A: PGenotype = NS. PTime = NS. PGenotype*Time < 0.05. B: PGenotype = NS. PTime < 0.05. PGenotype*Time = NS. For C and D, PGenotype, PTime, and PGenotype*Time = NS.
Effect of ETA Receptor Blockade on the Diurnal Natriuretic Response
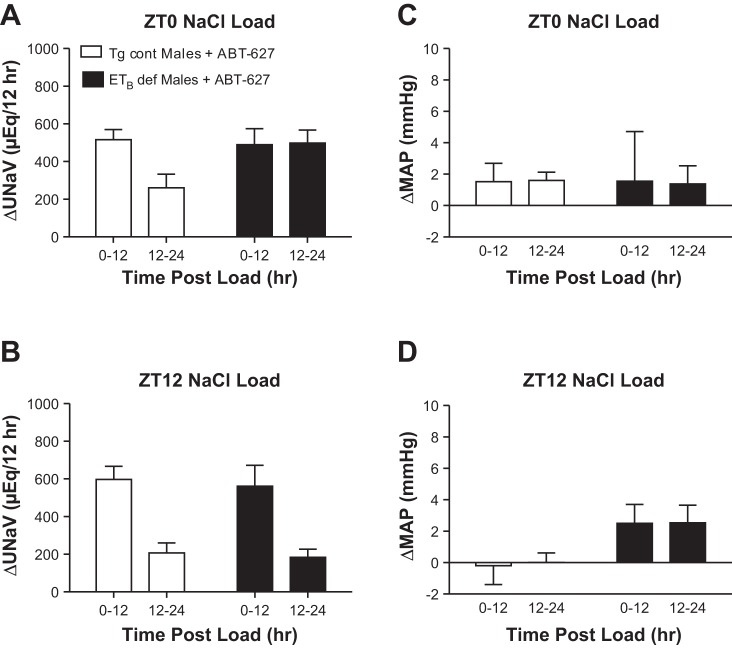
ABT-627 lowered the 24-h MAP in both Tg cont and ETB def male rats, but the decrease only reached significance in ETB def rats (Table 1). Treatment with ABT-627 did not produce a significant difference in ΔMAP between genotypes (Fig. 3, C and D). When given a salt load at ZT0, Tg cont males once again excreted the majority of the excess salt in the first 12 h (Fig. 3A). ETB def males treated with the ETA receptor antagonist and given a salt load at ZT0 excreted nearly half of the load within the first 12 h after the salt load was given. Although treatment with ABT-627 resulted in a delayed natriuretic response in ETB def males at ZT0 (Fig. 3A), the ETA receptor antagonist significantly improved the natriuretic response to an acute salt load compared with ETB def males given normal water [424 ± 41 vs. 50 ± 91 μeq Na/12 h in ETB def+ABT-627 (n = 6) and untreated ETB def (n = 5), respectively, P < 0.01]. For males given a salt load at ZT12, Tg cont males displayed a natriuretic pattern similar to males given normal water, excreting the majority of their salt load within the first 12 h postload (Fig. 3B). ETB def males given ABT-627 showed this pattern as well, excreting most of the excess salt in the first 12-h period, resulting in no significant interaction between genotype and time for a salt load at either ZT0 or ZT12. This was in contrast to the roughly 50-50 patterns of excretion in the first two postload periods displayed in ETB def males that were given normal water throughout the experiment (Fig. 1A). In animals treated with ABT-627, sodium excretion was not significantly different between genotypes following a salt load given at either ZT0 (777 ± 111 vs. 988 ± 111 μeq Na in Tg cont+ABT-627 vs. ETB def+ABT-627, respectively) or ZT12 (804 ± 92 vs. 745 ± 98 μeq Na in Tg cont+ABT-627 vs. ETB def+ABT-627, respectively).
Table 1.
Baseline values of mean arterial pressure (MAP) and urinary sodium excretion (UNaV) in male and female rats during the active and inactive periods
| Genotype/Treatment |
||||
|---|---|---|---|---|
| Tg cont | Tg cont+ABT-627 | ETB def | ETB def+ABT-627 | |
| Active period | ||||
| MAP, mmHg | ||||
| Males | 113 ± 3 | 105 ± 3 | 136 ± 3* | 116 ± 9† |
| Females | 112 ± 2 | 101 ± 1* | 132 ± 1* | 106 ± 4† |
| UNaV, μeq Na+ | ||||
| Males | 764 ± 38 | 841 ± 67 | 724 ± 56 | 731 ± 97 |
| Females | 960 ± 71 | 773 ± 57 | 902 ± 76 | 735 ± 58 |
| Inactive period | ||||
| MAP, mmHg | ||||
| Males | 112 ± 2 | 105 ± 3 | 135 ± 2* | 111 ± 5† |
| Females | 110 ± 2 | 99 ± 2* | 129 ± 2* | 99 ± 3*† |
| UNaV, μeq Na+ | ||||
| Males | 433 ± 35 | 613 ± 70 | 431 ± 32 | 580 ± 29 |
| Females | 554 ± 47 | 352 ± 21* | 634 ± 30 | 450 ± 31† |
Values are means ± SE (n = 4–10 rats/group). Tg cont, transgenic control rats; ETB def, ETB receptor deficient; NS, not significant. MAP values: Males, PGenotype/Treatment < 0.05. PTime = NS. PGenotype/Treatment*Time = NS. Females, PGenotype/Treatment < 0.05. PTime = 0.05. PGenotype/Treatment*Time = NS. UNaV values: Males, PGenotype/Treatment = NS. PTime < 0.05. PGenotype/Treatment*Time = NS. Females, PGenotype/Treatment < 0.05. PTime = 0.05. PGenotype/Treatment*Time = NS.
P < 0.05 vs. Tg cont and †P < 0.05 vs. ETB def within each sex.
Fig. 3.
Effect of a salt load at ZT0 (A) and ZT12 (B) on the rate of sodium excretion in males treated with ABT-627 and effect of a salt load at ZT0 (C) and ZT12 (D) on change in MAP in males treated with ABT-627; n = 6–7 rats/group. A: PGenotype, PTime, and PGenotype*Time = NS. B: PGenotype = NS. PTime < 0.05. PGenotype*Time = NS. C and D: PGenotype, PTime, and PGenotype*Time = NS.
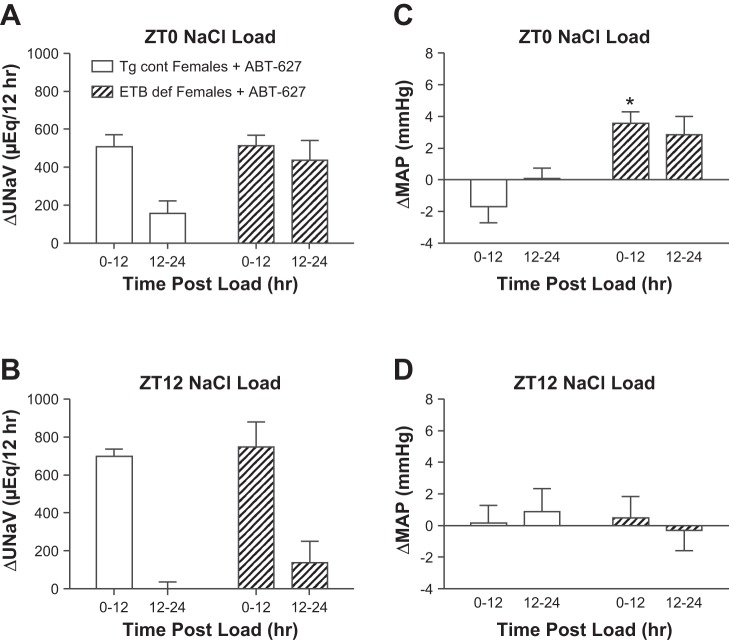
These acute salt-loading experiments involving ABT-627 treatment were also carried out in female rats. When given a salt load at ZT0, Tg cont females treated with ABT-627 excreted the majority of their salt load in the first 12 h postload (Fig. 4A). ETB def females treated with an ETA antagonist excreted more than half of their salt load within the first 12 h postload. In addition, ETB def females excreted significantly more salt in the second 12-h period following a ZT0 salt load than ABT-627-treated Tg cont females, but the total 24-h sodium excretion was not significantly different between groups (665 ± 62 vs. 950 ± 139 μeq Na in Tg cont+ABT-627 vs. ETB def+ABT-627, respectively). Although the ETA antagonist augmented the natriuretic response to a salt load given at ZT0 in ETB def females compared with females given normal water (Fig. 2A), the difference was not statistically significant. After being given a salt load at ZT12, Tg cont females excreted all of the excess salt within the first 12 h of the load being given (Fig. 4B). ETB def females showed a similar pattern, excreting the majority of the load in the first 12 h (Fig. 4B). This resulted in 24-h sodium excretion totals that were not significantly different from each other (687 ± 75 vs. 885 ± 183 μeq Na in Tg cont+ABT-627 vs. ETB def+ABT-627, respectively). There were no significant interactions between genotype and time for rats given a ZT0 or ZT12 salt load. Neither values for the Tg cont and ETB def rats treated with ABT-627 were statistically different from the groups that were given normal water (Fig. 2). ABT-627 significantly lowered MAP in both Tg cont and ETB def females (Table 1). This decrease in pressure produced a statistically significant difference in ΔMAP for females given a salt load at ZT0 (Fig. 4C), while it did not result in a difference in ΔMAP between genotypes given a salt load at ZT12 (Fig. 4D).
Fig. 4.
Effect of a salt load at ZT0 (A) and ZT12 (B) on the rate of sodium excretion in females treated with ABT-627 and effect of a salt load at ZT0 (C) and ZT12 (D) on change in MAP in females treated with ABT-627; n = 4–6 rats/group. A and B: PGenotype = NS. PTime < 0.05. PGenotype*Time = NS. C: PGenotype = 0.05. PTime = NS. PGenotype*Time = NS 4D: PGenotype, PTime, and PGenotype*Time = NS. †P < 0.05 vs. Tg cont 12–24. *P < 0.05 vs. 0–12 Tg cont.
DISCUSSION
The major finding of this study is that a lack of ETB receptor function results in a delayed natriuretic response to an acute salt load, which is dependent on the time of day the load is given. Despite the slowed rate of natriuresis, there appeared to be a sex difference in the rate of sodium excretion, as females displayed a more efficient pattern of natriuresis in response to an acute salt load compared with males. Furthermore, it appears that both ET receptors play a role in the acute natriuretic response to a salt load. The ETB receptor promotes the proper excretion of the acute salt load, while the ETA receptor contributes to the impaired response to the salt load, as evidenced by an improved natriuretic response after administration of the ETA receptor antagonist ABT-627. These results were found to be independent of major changes in blood pressure, consistent with previous findings that the ET system works to excrete excess salt through a direct effect on tubular reabsorption independently of pressure-natriuresis. While the molecular mechanism behind the diurnal variation in sodium excretion needs to be examined further in future studies, the current study demonstrates the importance of the endothelin system in the time-of-day maintenance of sodium homeostasis.
The finding that a lack of ETB receptor function results in a delayed acute natriuretic response to a salt load that is time of day dependent (Figs. 1 and 2) lends credence to the hypothesis that ET-1 is involved in the circadian regulation of sodium balance. There are many redundant systems that facilitate the excretion of sodium (7, 28) as evidenced by the changes in 24-h sodium excretion being similar between strains (i.e., the sodium load will eventually be excreted). These systems are needed to excrete a sodium load in rats lacking an ETB receptor, regardless of when the acute salt load was given.
Stow et al. (30) has shown previously that the core circadian clock gene Per1 negatively regulates several genes involved in sodium transport, including Edn1, which encodes ET-1. In that study, they found that a loss of Per1 from the system led to increased renal ET-1 levels (30). Furthermore, preliminary data from our laboratory suggest that ET-1 activates ETB receptors in the inner medulla of the kidney in a salt-dependent manner to regulate the circadian pattern of the core clock gene Bmal1 and promote sodium excretion (Speed, JS unpublished observations). This observation supports the established role of ET-1 in regulating sodium transport through its interactions with the epithelial sodium channel (ENaC) in the collecting duct (4). The high density of ETB receptors in this region allow for the inhibition of ENaC upon activation of these receptors, inhibiting sodium reabsorption, and promoting sodium excretion (3, 32, 34). Given that Per1 has been shown in numerous studies to also regulate the expression of ENaC (13, 14, 30), the interaction between ET-1 and the renal circadian clock system could provide a basis for exploring the mechanisms behind how natriuresis is regulated at different times of the day.
The result that female ETB deficient rats appear to have a more efficient time-of-day-dependent pattern of natriuresis than males suggests a sex difference in the ability of ET-1 to regulate sodium balance. A major reason for this difference in sodium excretion may be attributed to the levels of sex hormones. Estrogen and progesterone have been shown to regulate ENaC expression (5, 8). In addition, our laboratory has shown that ovariectomy abolishes the sex difference seen in UNaV in female ETB-deficient rats and their transgenic controls (26). Hartley and Forsling (15) previously showed that the levels of sodium excretion change over the course of the estrous cycle in the rat. In the current study, we did not measure circulating levels of sex hormones, but we presume they were within normal limits. To counter any possible effects in sodium excretion due to the estrous cycle, the female rats were evenly staggered into and out of their metabolic cages on sequential days for our acute salt-loading experiments, as opposed to all of them being placed in the cages on the same day. After the ΔUNaV was calculated, the amount of variability in our resulting data was small (Figs. 2 and 4), indicating that the estrous cycle did not have a major influence on our results. From our studies, females appear to be less dependent on the protective, natriuretic effects of the ETB receptor than males.
The ETB receptor has a range of functions that includes effects on tubular ENaC activity, endothelial-dependent vasodilation, and, importantly, clearance of ET-1 from the circulation and protection against overstimulation of the ETA receptor. To address the question of whether the ETA receptor contributes to the impaired diurnal natriuretic response in rats lacking functional ETB receptors, we treated ETB-deficient rats with the ETA antagonist ABT-627 throughout the course of the experiment. The finding that ETA receptor blockade improves the natriuretic response to a salt load in both males and females (Figs. 3 and 4) suggests that overactivation of the ETA receptor, at least in part, factors into the delayed natriuretic response to a salt load that is observed. This result was found to be in contrast to a prior study from our laboratory in which treatment with ABT-627 inhibited natriuresis, showing that the ETA receptor contributed to the natriuretic response induced by direct intramedullary administration of ET-1 (26). However, the current result is in line with conventional thinking concerning ETA receptors at the whole animal level. While ETB receptors are highly expressed in the endothelial cells and tubules of the renal medulla, ETA receptors are primarily expressed on vascular smooth muscle cells and participate primarily in vasoconstriction (22). Iglarz et al. (16) showed in a prior study that ETA antagonism improves flow-dependent dilation in the vasculature of the spontaneously hypertensive rat model. Future studies in which an ETA antagonist can bypass the systemic vasculature via intramedullary infusion of the drug would allow us to determine the effect of a kidney-specific ETA receptor on the natriuretic response to increased salt intake. A previous study by Ge et al. (12) has shown the effects of a collecting duct-specific knockout of ETA receptors (ETA KO) on renal function. However, the mice produced in that study had their ETA receptors knocked out from birth, possibly resulting in morphological differences in function vs. a treatment involving chronic ETA receptor blockade. Additional studies from this same laboratory showed that fluid retention produced by ETA blockade is dependent upon renal tubular ETA actions. Clearly, there are complex, contrasting actions of the ETA receptor that require further investigation.
Although baseline MAP was significantly higher in male ETB def rats compared with their transgenic controls (Table 1), there was little to no effect on MAP between genotypes in response to the acute salt load (Fig. 1, C and D), suggesting that a change in MAP does not account for the differential natriuretic response. Of note, neither Tg cont nor ETB def rats displayed a very prominent diurnal rhythm in MAP while in metabolic cages although we have reported clear circadian blood pressure rhythms in previous studies (29). The reason for the lack of a MAP rhythm in the current experiments is not clear but could be related to the stress of being in metabolic cages. Nonetheless, changes in MAP cannot explain the differences in the response to the acute salt load.
The ETA receptor antagonist has also been shown to lower blood pressure as our laboratory has previously reported (27); however, despite operating at lower arterial pressures, there were no significant changes in MAP following the acute salt load. It is important to note that recent findings by Dhaun et al. (6) showed that blood pressure rhythms were improved after a chronic treatment with an ETA receptor antagonist, which may be related to an improved ability to excrete salt throughout the day. Unlike the many humans with salt sensitivity, our ETB def model has a robust diurnal rhythm (27). It will be important to explore the ETB receptor function in humans with a nondipping phenotype.
In our studies, the acute salt load is roughly equivalent to doubling the daily amount of salt intake. The salt load may not have been high enough or prolonged enough to trigger salt-dependent hypertension in this strain of rat. Under a typical high-salt diet (4% NaCl), a ETB def rat receives ∼10 times the daily intake of salt, which has been shown to lead to high blood pressure (27, 29). In addition, these rats were only given one acute dose of salt, whereas most studies involving a high-salt diet give the food chronically. Salt can have a significant influence on blood pressure regulation, but there are multiple factors beyond sodium handling that may contribute to its regulation as well.
Perspectives
As more knowledge about circadian rhythms and how they impact sodium balance and blood pressure has been acquired over the years, more attention has become focused on understanding the mechanisms that allow for the regulation of the processes at different times of the day. Individuals that have impaired circadian rhythms have an increased risk of cardiovascular events, so the importance of understanding our eating habits can have an effect on how well the kidney is able to restore water and electrolyte balance in the body. In addition, sex differences in sodium handling and blood pressure present another factor that must be considered as we try to understand how eating behaviors can affect our cardiovascular health. Work in recent years has started to examine the interaction between endothelin and the renal circadian clock, but more research needs to be done to determine the functional significance of their interaction with regard to sodium balance. Knowing how ET-1 is involved in regulating sodium balance at different times of the day can open up new therapeutic and/or lifestyle changes by adding more focus on when patients are treated (i.e., chronotherapy) and where specifically to target (i.e., ETA receptor antagonist in the kidney) to provide a more efficient approach to the treatment of hypertension.
GRANTS
This work was supported by National Institutes of Health Grants P01 HL69999, P01 HL95499, K99 HL127178, and T32 DK007545.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.G.J. and D.M.P. provided conception and design of research; J.G.J. and C.J. performed experiments; J.G.J. analyzed data; J.G.J., J.S.S., and D.M.P. interpreted results of experiments; J.G.J. and D.M.P. prepared figures; J.G.J. drafted manuscript; J.G.J., J.S.S., C.J., and D.M.P. edited and revised manuscript; J.G.J., J.S.S., C.J., and D.M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Hiram Ocasio for technical assistance and Jourdan A. Mason for assistance with the experiments.
REFERENCES
- 1.Agarwal R. Regulation of circadian blood pressure: from mice to astronauts. Curr Opin Nephrol Hypertens 19: 51–58, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest 114: 504–511, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol 302: C188–C194, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang CT, Sun CY, Pong CY, Chen YC, Lin GP, Chang TC, Wu MS. Interaction of estrogen and progesterone in the regulation of sodium channels in collecting tubular cells. Chang Gung Med J 30: 305–312, 2007. [PubMed] [Google Scholar]
- 6.Dhaun N, Moorhouse R, MacIntyre IM, Melville V, Oosthuyzen W, Kimmitt RA, Brown KE, Kennedy ED, Goddard J, Webb DJ. Diurnal variation in blood pressure and arterial stiffness in chronic kidney disease: the role of endothelin-1. Hypertension 64: 296–304, 2014. [DOI] [PubMed] [Google Scholar]
- 7.DiBona GF. Interaction of stress and dietary NaCl intake in hypertension: renal neural mechanisms. Compr Physiol 3: 1741–1748, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Gambling L, Dunford S, Wilson CA, McArdle HJ, Baines DL. Estrogen and progesterone regulate alpha, beta, and gammaENaC subunit mRNA levels in female rat kidney. Kidney Int 65: 1774–1781, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 105: 925–933, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest 102: 1092–1101, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol 291: F1274–F1280, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Ge Y, Stricklett PK, Hughes AK, Yanagisawa M, Kohan DE. Collecting duct-specific knockout of the endothelin A receptor alters renal vasopressin responsiveness, but not sodium excretion or blood pressure. Am J Physiol Renal Physiol 289: F692–F698, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Gumz ML, Cheng KY, Lynch IJ, Stow LR, Greenlee MM, Cain BD, Wingo CS. Regulation of alphaENaC expression by the circadian clock protein Period 1 in mpkCCD(c14) cells. Biochim Biophys Acta 1799: 622–629, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119: 2423–2434, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartley DE, Forsling ML. Renal response to arginine vasopressin during the oestrous cycle in the rat: comparison of glucose and saline infusion using physiological doses of vasopressin. Exp Physiol 87: 9–15, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Iglarz M, Matrougui K, Levy BI, Henrion D. Chronic blockade of endothelin ETA receptors improves flow dependent dilation in resistance arteries of hypertensive rats. Cardiovasc Res 39: 657–664, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Jin C, Speed JS, Hyndman KA, O'Connor PM, Pollock DM. Sex differences in ET-1 receptor expression and Ca2+ signaling in the IMCD. Am J Physiol Renal Physiol 305: F1099–F1104, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawanishi H, Hasegawa Y, Nakano D, Ohkita M, Takaoka M, Ohno Y, Matsumura Y. Involvement of the endothelin ETB receptor in gender differences in deoxycorticosterone acetate-salt-induced hypertension. Clin Exp Pharmacol Physiol 34: 280–285, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Khraibi AA, Liang M, Berndt TJ. Role of gender on renal interstitial hydrostatic pressure and sodium excretion in rats. Am J Hypertens 14: 893–896, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Kittikulsuth W, Looney SW, Pollock DM. Endothelin ETB receptors contribute to sex differences in blood pressure elevation in angiotensin II hypertensive rats on a high-salt diet. Clin Exp Pharmacol Physiol 40: 362–370, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kittikulsuth W, Pollock JS, Pollock DM. Sex differences in renal medullary endothelin receptor function in angiotensin II hypertensive rats. Hypertension 58: 212–218, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohan DE, Inscho EW, Wesson D, Pollock DM. Physiology of endothelin and the kidney. Compr Physiol 1: 883–919, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills JN, Stanbury SW. Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. J Physiol 117: 22–37, 1952. [PMC free article] [PubMed] [Google Scholar]
- 25.Moore-Ede MC, Herd JA. Renal electrolyte circadian rhythms: independence from feeding and activity patterns. Am J Physiol Renal Fluid Electrolyte Physiol 232: F128–F135, 1977. [DOI] [PubMed] [Google Scholar]
- 26.Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension 53: 324–330, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–F150, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Reinhardt HW, Seeliger E. Toward an integrative concept of control of total body sodium. News Physiol Sci 15: 319–325, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Speed JS, D'Angelo G, Wach PA, Sullivan JC, Pollock JS, Pollock DM. High salt diet increases the pressor response to stress in female, but not male ETB-receptor-deficient rats. Physiol Rep 3: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, Cain BD, Wingo CS, Gumz ML. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension 59: 1151–1156, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1–7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension 56: 658–666, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Unique endothelin receptor binding in kidneys of ETB receptor deficient rats. Am J Physiol Regul Integr Comp Physiol 284: R674–R681, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Tostes Passaglia RC, David FL, Fortes ZB, Nigro D, Scivoletto R, Catelli De Carvalho MH. Deoxycorticosterone acetate-salt hypertensive rats display gender-related differences in ETB receptor-mediated vascular responses. Br J Pharmacol 130: 1092–1098., 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yukimura T, Notoya M, Mizojiri K, Mizuhira V, Matsuura T, Ebara T, Miura K, Kim S, Iwao H, Song K. High resolution localization of endothelin receptors in rat renal medulla. Kidney Int 50: 135–147, 1996. [DOI] [PubMed] [Google Scholar]