Abstract
Lower urinary tract (LUT) symptoms (LUTS), including frequency, urgency, incomplete voiding, and slow stream, are common in both men and women with advancing age. The most common cause for LUTS in aging men is benign prostatic hyperplasia. Some studies have also revealed an inverse association of serum testosterone levels with LUTS; however, the underlying mechanisms by which gonadal hormones affect the LUT have not been clarified. In the present study, we examined the effect of orchiectomy and testosterone replacement on LUT function in adult male Sprague-Dawley rats. Six weeks after bilateral orchiectomy or sham operations and 3 wk after injection of long-acting testosterone undecanoate (100 mg/kg im), transvesical cystometry and external urethral sphincter electromyogram (EUS EMG) recordings were performed under urethane anesthesia. The micturition reflex was elicited in both sham and orchiectomized animals. In orchiectomized rats, volume threshold for inducing micturition decreased by 47.6%; however, contraction amplitude, duration, and voiding efficiency were similar in sham and orchiectomized rats. The active period during EUS EMG bursting was lengthened during micturition in orchiectomized animals. Testosterone treatment, which normalized plasma testosterone levels, reversed these changes but also increased the duration of EUS EMG bursting. Orchiectomy also reduced mean voiding flow rate estimated from the duration of EUS EMG bursting, an effect that was not reversed by testosterone. The results indicate that orchiectomy affects both the active and passive properties of the bladder and urethra, and that many, but not all, of the changes can be reversed by testosterone.
Keywords: orchiectomy, androgen, external urethral sphincter, electromyogram, lower urinary tract symptoms
lower urinary tract (lut) symptoms (LUTS), including frequency, urgency, incomplete voiding, hesitancy, slow stream, nocturia, and urine retention, are highly prevalent in both men and women and increase with advancing age. The most common cause for LUTS in aging men is benign prostatic hyperplasia.
Biologically active free testosterone levels are known to decline with age by 2–3% annually in men over 40 yr old (2), which may result in andropause symptoms called late-onset hypogonadism syndrome (LOHS) (11, 25, 29). Therefore, aging men with LOHS may exhibit anatomic and physiological changes in the LUT that induce symptoms similar to LUTS (23, 25). Trifiro et al. (30) identified inverse associations of serum testosterone with LUTS in men (30). Kalinchenko et al. (12) and Karazindiyanoglu and Cayan (13) reported that testosterone replacement improves lower urinary function by increasing bladder capacity and compliance and by decreasing detrusor pressure at maximal flow in men with LOHS.
Experiments that have been conducted on rats to examine the mechanisms underlying the effects of testosterone on the LUT have unfortunately yielded variable results. Maggi et al. (21) reported that testosterone treatment for 10 days, which increased prostate weight, had little effect on bladder weight, bladder capacity, or amplitude of bladder contractions in urethane-anesthetized rats, but induced detrusor instability and markedly increased residual volume, indicating infravesical outflow obstruction. On the other hand, cystometry in unanesthetized rats revealed that testosterone treatment for 2 wk increased micturition pressure, bladder capacity, residual volume, and micturition volume (22). Because these effects could not be correlated with changes in bladder or urethral excitatory innervation, as revealed by responses of smooth muscle to excitatory drugs or electrical field stimulation, it was concluded that the excitatory effects of testosterone could be mediated in part by an action on the micturition reflex pathway at spinal or supraspinal levels.
On the other hand, recent experiments (27) in ketamine-anesthetized rats 60 days after orchiectomy (ORX) suggest that testosterone has inhibitory effects on bladder activity. ORX reduced bladder capacity, and testosterone treatment reversed this change. A similar effect of testosterone replacement therapy to increase bladder capacity has been observed in rabbits after ORX (4). The differences in results may be due to different concentrations of testosterone used in the experiments; i.e., supraphysiological levels produced by administration of hormone in control animals vs. replacement therapy after ORX to restore normal hormonal levels. In addition, effects of supraphysiological levels on the urethral outlet to induce urinary retention could indirectly influence bladder function.
In the present study, we used cystometry in combination with external urethral sphincter (EUS) electromyography (EMG) to examine the influence of ORX and testosterone replacement on LUT function in adult Sprague-Dawley rats. The most sensitive cystometric parameters to changes in hormone levels were the bladder volume threshold (VT) for inducing reflex voiding and estimated voiding flow rate (VFR), suggesting that male hormones act at multiple sites and/or mechanisms to regulate urine storage and voiding function.
MATERIALS AND METHODS
Experimental protocols.
Adult male Sprague-Dawley rats (weight 300–350 g, ∼9 wk old) were housed in a room controlled for temperature, humidity, and with lights on from 7:00 AM to 7:00 PM. ORX or sham operations were performed on 54 animals, and experiments were conducted either 6 or 12 wk postsurgery. The 6-wk group of animals was divided into three experimental subgroups (12 rats/subgroup): 1) sham operation control rats, in which the testicles were exposed but not removed; 2) bilateral orchiectomized rats, treated with a single injection of vehicle at 3 wk post-ORX; and 3) bilateral orchiectomized rats, which received at 3 wk post-ORX a single dose of testosterone undecanoate (100 mg/kg im, Nebido, Bayer Schering Pharma, Berlin, Germany). This treatment is effective in inducing physiological testosterone levels in orchiectomized rats for a minimum of 4 wk (3); the animals underwent testing 3 wk posttreatment (6 wk postsurgery).
In the 12-wk group of animals, testosterone treatment was not evaluated. These animals were divided into only two experimental subgroups: 1) sham operation control rats, which were studied 12 wk postsurgery; and 2) bilateral orchiectomized rats studied 12 wk postsurgery.
Physiological investigation.
The experiments were performed under urethane anesthesia (1.1 g/kg sc). Fluids were administered via a femoral vein catheter, and body temperature was maintained between 36 and 38°C with a heating lamp. The urinary bladder was exposed via a midline abdominal incision, and the rostral half of the pubic symphysis was removed to expose the midurethra and EUS. Fine insulated silver wire electrodes (0.05 mm diameter) with exposed tips were inserted into the EUS on the lateral sides of the midurethra. EUS EMG activity was displayed on an oscilloscope and a paper recorder along with bladder pressure and also recorded on a computer. EUS EMG was attributed to striated muscle because the activity was eliminated after neuromuscular blockade with pancuronium bromide (5). A polyethylene tube 60 (1.0 mm inner diameter and 1.5 mm outer diameter) was inserted through the bladder dome into the bladder lumen and tied in place, and the abdominal wall was closed. The polyethylene tube was in turn connected via a three-way stopcock to an infusion pump and a pressure transducer, and the system was filled with physiological saline. Urodynamic examination usually began 3–4 h after the induction of anesthesia. After the bladder was emptied, transvesical cystometry with urethra open was performed at an infusion rate of 0.123 ml/min with saline at room temperature. Fluid evacuation during micturition was videotaped to determine beginning and end of voiding.
The infusion pump was turned off after induction of a voiding contraction, and residual volume was measured by empting the bladder by pressure on the abdominal wall. Various parameters were measured: 1) VT, the volume of saline sufficient to induce bladder contractions exceeding a pressure of 15 cmH2O; 2) baseline pressure (BP), the intravesical pressure following voiding; 3) pressure threshold (PT), the intravesical pressure inducing a voiding bladder contraction; 4) contraction amplitude (CA), the maximal intravesical pressure during voiding; 5) contraction duration (CD), duration of a voiding contraction; 6) postvoiding residual volume (PVR), the volume of saline withdrawn from the bladder after voiding; 7) voiding efficiency (VE), expressed as a percentage using the formula: VE = voided volume (VV) (VT − PVR)/VT × 100; 8) bladder compliance (BC), estimated by the formula: VT/(PT − BP); and 9) VFR, estimated by the formula: VT − PVR/bursting duration (BD).
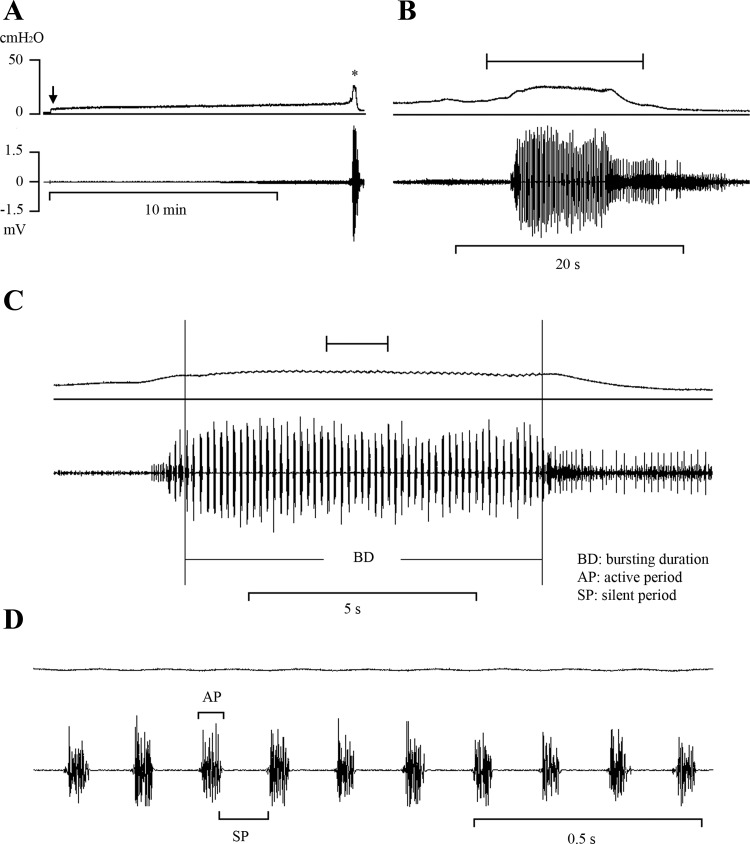
The EUS EMG activity analysis was blinded to the status of the rat. As described in an earlier paper (8), various EUS EMG/cystometrogram (CMG) parameters were measured, including average BD, silent period (SP), active period (AP) (Fig. 1), total silent period (TSP) during each voiding, CD, and the ratio of BD to CD expressed as a percentage. As shown in Fig. 1, the bursting period was analyzed during the time when the EMG was completely quiescent during the interval between bursts. This occurred when intravesical pressure began to decline during voiding. Before this time, some phasic EMG activity could be detected, but an SP was not obvious.
Fig. 1.
Bladder (top traces) and EUS EMG activity (bottom traces) recorded during a continuous transvesical infusion CMG in a normal anesthetized rat. A: reflex bladder contraction, indicated by an abrupt, large increase in bladder pressure, was accompanied by large-amplitude EUS EMG activity. B: same recording indicated by asterisk in A shown at faster time scale. The period of the recording indicated by the bracket above the record in B is shown at a faster time scale in C. C: tonic EUS EMG activity precedes the large rise in intravesical pressure and shifts to a bursting pattern at the peak of bladder contraction before the onset of voiding. The period of the recording indicated by the bracket above the record in C is shown at a faster time scale in D. D: recordings in C shown at very fast time scale showing individual EUS EMG bursts composed of active (AP) and silent periods (SP, brackets) and the small fluctuations in intravesical pressure accompanying each burst. Vertical calibration, intravesical pressure (in cmH2O); horizontal calibration, time (in min or s). Arrow in A indicates the start of saline infusion.
In the absence of direct measurements of voiding flow, an estimate of mean VFR can be obtained using several methods (8), including dividing VV by the duration of voiding represented by either: 1) bladder CD, 2) the TSP of the EUS EMG, or 3) the period of EUS bursting (BD). Recent experiments by Fan et al. (9) in which urethral flow was directly monitored with a flow meter attached to the urethra in anesthetized male Sprague-Dawley rats revealed that voiding occurs during the period of EUS bursting. Thus VFR in the present experiments was estimated by dividing the VV by duration of voiding measured indirectly by duration of EUS bursting (BD).
At least three transvesical CMGs were obtained in each animal. All parameters were calculated with the aid of Acknowledge software (BIOPAC Systems). Computed data were compiled in spreadsheets and averaged using Excel (Microsoft). The Institutional Animal Care and Use Committee of Taichung Veterans General Hospital approved the experimental protocol.
ORX surgery.
Bilateral ORX using sterile procedures was performed under isoflurane anesthesia. Testicles were removed through a midline abdominal incision after transection of spermatic cords. In sham controls, the testicles were visualized but not removed. Animals were treated with ampicillin (50 mg/kg sc) for 2–3 days.
Testosterone and estradiol assay.
Six weeks post-ORX, rats were anesthetized with isoflurane. Blood samples were collected during the physiological studies, and the separation of serum was achieved by centrifugation. The samples were stored at −70°C until analysis. Biochemical analysis of testosterone and estradiol levels was performed by a chemiluminescence immunoassay using a Siemens ADVIA-Centaur Enhanced Assay System (Siemens). Hormone levels were not measured in rats 12 wk post-ORX. The chemiluminescence immunoassays had levels of detection values of 15 pg/dl for estradiol and 15 ng/dl for testosterone, and r2 values of 0.9987 and 0.9925, respectively. The intra-assay and interassay coefficients of E2 and testosterone were 11.1 and 2.0% and 4.7 and 4.7%, respectively.
Statistical analysis.
The results are given as means ± SE. Data were tested for normal distribution using Shapiro-Wilk test and found to be not normally distributed (P < 0.01). Thus associations between sham operations and ORX were assessed by the nonparametric Kruskal-Wallis test, followed by the Mann-Whitney U-test. Differences were considered statistically significant at P < 0.05.
RESULTS
Effect of ORX and testosterone replacement therapy on body weight, bladder weight, and hormone levels.
Compared with sham animals, ORX (6 wk duration) significantly reduced body weight by 8.6%, bladder weight by 11%, and plasma testosterone levels by 97%, but did not significantly change plasma estrogen levels (Table 1). Testosterone treatment for 3 wk starting 3 wk after ORX restored body weight and bladder weight and significantly increased plasma testosterone levels 11.7% above the levels in sham controls, but did not alter plasma estrogen levels (Table 1).
Table 1.
Body and bladder weight and serum testosterone and estradiol levels in rats after sham-op, ORX, and ORX with testosterone replacement
| n | Weight, g | Bladder, mg | T, ng/dl | E2, ng/dl | |
|---|---|---|---|---|---|
| Sham-op | 22 | 531.0 ± 28.8 | 117.5 ± 13.4 | 575.9 ± 337.6 | 1.62 ± 0.33 |
| ORX 6Wk | 20 | 485.5 ± 29.6 | 104.5 ± 11.6 | 19.1 ± 12.1 | 1.78 ± 0.89 |
| vs. Sham-op P value | 0.000* | 0.006* | 0.000* | 0.420 | |
| ORX +3Wk T | 13 | 504.7 ± 36.4 | 126.8 ± 15.7 | 643.1 ± 389.7 | 1.72 ± 0.34 |
| vs. Sham-op P value | 0.052 | 0.034* | 0.243 | 0.354 | |
| vs. ORX 6Wk P value | 0.250 | 0.000* | 0.000* | 0.928 |
Values are means ± SE; n, no. of rats.
T, testosterone; E2, estradiol; sham-op, sham-operated; ORX 6Wk, bilateral orchiectomy 6 wk before study; ORX +3Wk T, bilateral orchiectomy 6 wk and testosterone replacement 3 wk before study.
P < 0.05 indicates a statistically difference (by Mann-Whitney U-test).
Effect of ORX on bladder activity during transvesical CMGs.
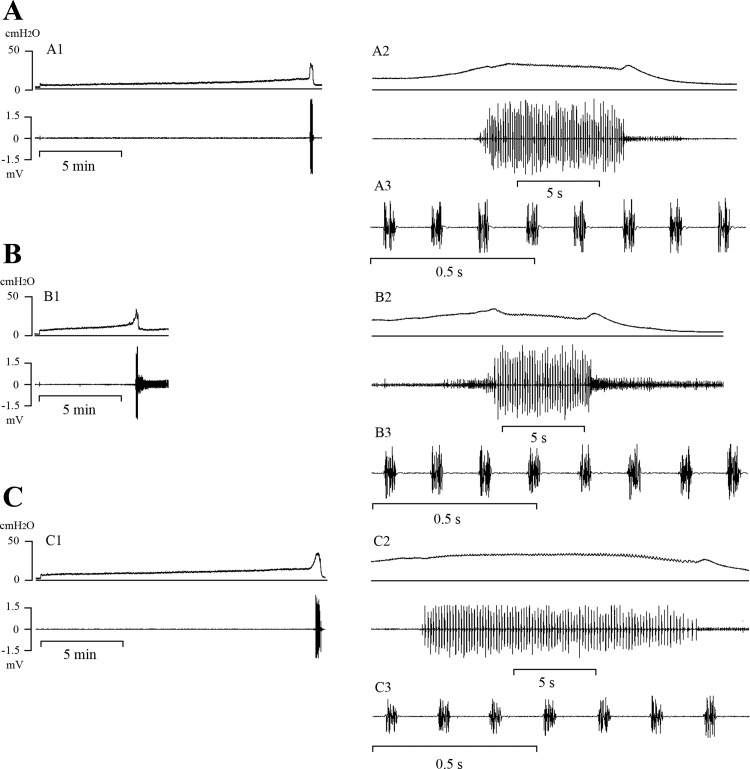
Various parameters of LUT function were assessed during a CMG with the urethral outlet open, to allow fluid to be evacuated during voiding. Micturition reflexes were elicited in all sham and orchiectomized animals 6 wk after the operation (Fig. 2). Compared with measurements in sham animals (Fig. 2A), ORX (Fig. 2B) increased intravesical BP by 44% (from 2.7 to 3.9 cmH2O) and increased the intravesical PT for initiating micturition by 43% (from 8.7 to 12.5 cmH2O), but decreased the VT for micturition by 48% (from 1.7 to 0.89 ml) (Fig. 2B) and decreased BC (from 0.29 to 0.1 ml/cmH2O). ORX did not change CA, CD, or VE. PVR was reduced (from 0.2 to 0.1 ml) but in proportion to the decrease in VT (Table 2).
Fig. 2.
Effects of orchiectomy (ORX) and testosterone replacement on intravesical pressure (top traces) and EUS EMG activity (bottom traces) during constant infusion cystometrograms in 3 rats (A–C) 6 wk after ORX. On the left (A1–C1), the records are at a slow time scale. Part of the same recording illustrating the period during voiding are shown at two faster time scales in the records on the right (A2–C3). Recordings were obtained in a sham-operated rat (A), an ORX rat (B), and after 3 wk of testosterone replacement in an ORX rat (C). Vertical calibration, intravesical pressure (in cmH2O); horizontal calibration, time (in min or s).
Table 2.
Effect of ORX and T replacement on cystometric parameters
| 6 Wk |
12 Wk |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham-op | ORX | P value | ORX +3Wk T | vs. Sham-op P value | vs. ORX P value | Sham-op | ORX | P value | |
| n | 12 | 13 | 11 | 9 | 9 | ||||
| VT, ml | 1.70 ± 0.46 | 0.89 ± 0.47 | 0.000* | 1.82 ± 0.44 | 0.740 | 0.000* | 2.28 ± 0.54 | 1.13 ± 0.40 | 0.000* |
| BP, cmH2O | 2.70 ± 0.73 | 3.89 ± 1.30 | 0.046* | 3.78 ± 0.82 | 0.002* | 0.531 | 3.58 ± 0.70 | 3.69 ± 0.58 | 0.863 |
| PT, cmH2O | 8.71 ± 1.45 | 12.52 ± 1.77 | 0.000* | 11.20 ± 1.73 | 0.003* | 0.733 | 10.72 ± 1.79 | 11.06 ± 2.19 | 0.863 |
| CA, cmH2O | 33.71 ± 7.17 | 32.83 ± 3.75 | 0.769 | 34.45 ± 4.18 | 0.525 | 0.303 | 33.58 ± 2.82 | 33.74 ± 4.73 | 1.000 |
| CD, min | 0.42 ± 0.11 | 0.40 ± 0.06 | 0.936 | 0.50 ± 0.11 | 0.059 | 0.026* | 0.51 ± 0.13 | 0.44 ± 0.11 | 0.258 |
| PVR, ml | 0.21 ± 0.12 | 0.10 ± 0.06 | 0.003* | 0.25 ± 0.11 | 0.449 | 0.001* | 0.35 ± 0.11 | 0.15 ± 0.05 | 0.000* |
| %VE | 87.51 ± 4.53 | 87.70 ± 3.67 | 0.894 | 86.38 ± 5.67 | 0.695 | 0.569 | 84.52 ± 3.87 | 85.88 ± 5.25 | 0.605 |
| BC, ml/H2O | 0.29 ± 0.09 | 0.10 ± 0.04 | 0.000* | 0.26 ± 0.08 | 0.260 | 0.000* | 0.35 ± 0.15 | 0.17 ± 0.09 | 0.008* |
| VFR, ml/s | 0.21 ± 0.08 | 0.12 ± 0.03 | 0.000* | 0.15 ± 0.05 | 0.016* | 0.277 | 0.21 ± 0.06 | 0.11 ± 0.03 | 0.002* |
Values are means ± SE; n, no. of rats. 6 Wk or 12 Wk ORX, bilateral orchiectomy 6 wk or 12 wk before study; ORX +3Wk T, bilateral orchiectomy 6 wk and testosterone replacement 3 wk before study.
P < 0.05 indicates a statistically difference (by Mann-Whitney U-test).
In the 12-wk ORX group, the percent changes in VT, PVR, and BC were similar in magnitude to those occurring in the 6-wk ORX group (Table 2). On the other hand, BP and PT, which were increased 6 wk after ORX, were not significantly changed by 12-wk ORX. However, it is noteworthy that BP and PT increase with age and were significantly larger in 12-wk than in 6-wk sham animals (Table 2).
Effect of ORX on EUS EMG activity.
During bladder filling, EUS EMG recordings in most animals exhibited consistent, low-amplitude, tonic EUS EMG activity before the onset of voiding (Fig. 1A); however, in some animals, this tonic activity increased gradually as the infusion volume approached the micturition VT (Fig. 1, A and B). During a bladder contraction and voiding, the EUS EMG activity markedly increased in amplitude and consisted of an initial period of tonic activity, followed by a bursting pattern of activity (Fig. 1C) characterized by clusters of high-frequency spikes (AP) separated by periods of quiescence (SP) (Fig. 1D). SP duration, TSP, BD, and the ratio of BD to CD (BD/CD) were not different in orchiectomized (Fig. 2, B2 and B3) and sham (Fig. 2, A2 and A3) animals; however, AP duration increased (range, 7–11% increase) in 6-wk and 12-wk orchiectomized animals (Table 3).
Table 3.
Effect of ORX and T replacement on EUS EMG activity
| 6 Wk |
12 Wk |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham-op | ORX | P value | ORX +3Wk T | vs. Sham-op P value | vs. ORX P value | Sham-op | ORX | P value | |
| n | 12 | 13 | 11 | 9 | 9 | ||||
| SP, s | 0.100 ± 0.009 | 0.099 ± 0.007 | 0.852 | 0.110 ± 0.012 | 0.044* | 0.018* | 0.101 ± 0.010 | 0.099 ± 0.007 | 0.605 |
| AP, s | 0.042 ± 0.005 | 0.046 ± 0.006 | 0.030* | 0.042 ± 0.005 | 0.608 | 0.150 | 0.040 ± 0.002 | 0.045 ± 0.004 | 0.008* |
| BD, s | 7.42 ± 2.16 | 6.66 ± 2.98 | 0.376 | 11.78 ± 4.13 | 0.009* | 0.004* | 9.63 ± 2.85 | 8.75 ± 2.29 | 0.666 |
| TSP, s | 5.25 ± 1.59 | 4.56 ± 2.07 | 0.347 | 8.53 ± 3.15 | 0.003* | 0.001* | 6.85 ± 1.97 | 6.00 ± 1.59 | 0.605 |
| %BD/CD | 31.32 ± 11.37 | 27.22 ± 8.94 | 0.538 | 39.73 ± 12.48 | 0.134 | 0.011* | 32.00 ± 5.76 | 34.26 ± 9.31 | 0.546 |
Values are means ± SE; n, no. of rats. 6 Wk or 12 Wk ORX, bilateral orchiectomy 6 wk or 12 wk before study; ORX +3Wk T, bilateral orchiectomy 6 wk and testosterone replacement 3 wk before study.
P < 0.05 indicates a statistically difference (by Mann-Whitney U-test).
Effect of testosterone replacement on bladder and EUS EMG activity 6 wk after ORX.
Transvesical CMGs were performed in the 6-wk orchiectomized animals at 3 wk after a single dose of testosterone undecanoate treatment (100 mg/kg im). The treatment restored plasma testosterone levels, as reported previously by Callies et al. (3), normalized body and bladder weight (Table 1), and increased VT (104%) (Fig. 2C1) to the level in sham-operated animals (Fig. 2A1). VT was not significantly different in sham and testosterone-treated orchiectomized animals (Table 2). Voiding volume increased in proportion to the increase in VT after testosterone replacement in orchiectomized animals, but VE was not altered (Table 2).
The SP component of the EUS EMG was significantly increased after testosterone replacement (Fig. 2C3, Table 3), and the small increase in the AP that occurred after ORX was eliminated (Table 3). BD, TSP, and BD/CD, which were not altered by ORX, were nevertheless significantly increased in orchiectomized animals by testosterone treatment in proportion to the increase in VT (Table 3). These parameters were also significantly larger after testosterone treatment compared with measurements in sham animals (Table 3).
Effect of ORX and testosterone replacement on estimated mean VFR.
Using the duration of EUS bursting as an indirect measure of the duration of voiding (9) and the direct measurement of VV, the mean VFR in sham control animals estimated by VV/BD was 0.21 ml/s. Although VE was unchanged after ORX, the estimated VFR (0.12 ml/s) was reduced by 42.8%. Testosterone replacement after ORX did not significantly reverse the decline in estimated VFR (0.15 ml/s). VFR was also significantly reduced in the 12-wk orchiectomized animals.
DISCUSSION
Cystometry and EUS EMG in urethane-anesthetized adult Sprague-Dawley rats revealed that ORX of 6-wk duration selectively changed several storage and voiding functions of the LUT, including VT, BC, PT, BP, EUS EMG AP, and VFR, but did not change others, such as BD, CA, VE, CD, and EUS EMG SP. The changes are attributable to effects on activity of both the urethra and the bladder. Some of the changes were completely reversed by 3 wk of testosterone replacement therapy, whereas others were not reversed, indicating that ORX affects the LUT by multiple mechanisms, some of which may not be due to a reduction in testosterone levels or alternatively may not be reversed quickly by testosterone replacement. An analysis of the role of testosterone is further complicated by the finding that certain parameters of LUT function that were not altered by ORX were nevertheless affected by testosterone replacement. This result raises the possibility that the physiological effects of endogenous testosterone may differ in some instances from the pharmacological effects of exogenously administered testosterone.
The relationship between VE and VFR in untreated and testosterone-treated orchiectomized animals was also unusual. It might be expected that VE would correlate with VFR. However, after ORX, VE was not changed (87%), but VFR decreased by 42.8%. One question that might be raised about this lack of correlation is that VFR was not measured directly, but instead was estimated from measurements of VV and the duration of EUS EMG bursting (BD), which represents the duration of voiding in anesthetized male rats (9). Thus VFR was calculated by the formula VT − PVR/BD, in which VT − PVR provides a measurement of VV. In orchiectomized animals, VT and PVR were decreased in parallel, and VV was decreased. However, BD was not changed; thus estimated VFR decreased. After testosterone replacement, the changes in VT and PVR were reversed, but BD markedly increased. Thus estimated VFR remained below control. One interpretation of these results is that ORX reduces VT and in turn VV, but increases urethral outlet resistance, thereby reducing flow. However, even with the slower flow, VE is unaffected because flow is maintained until the bladder is 85–90% empty. Although the mechanisms that control BD and the duration of voiding are unknown, it is clear that testosterone replacement therapy does increase BD.
The decrease in the VT for initiating micturition without a change in the magnitude of micturition pressure indicates that ORX has a selective facilitatory effect on the sensory limb of the bladder reflex pathway to lower the threshold for activating the central micturition switching circuit, but does not affect the efferent (motor) limb of the reflex, which controls the amplitude of the micturition contraction. On the other hand, the reduction in VFR without a change in voiding pressure suggests that ORX disrupts the coordination between bladder and urethral outlet during voiding and must increase outlet resistance. However, this occurred without a marked change in the pattern of EUS bursting and, therefore, must be due to the following: 1) a change in the passive properties of the urethra, 2) a change in urethral smooth muscle activity, or 3) a reduction in the magnitude of the EUS contractions. In male rats, suppressing EUS contractions with a neuromuscular blocking agent suppresses voiding (9). Studies in rats also revealed that ORX of 8 wk duration reduced the density of striated muscle in the EUS and increased the density of collagen fibers (19). This change would be expected to increase the passive resistance of the urethral outlet and also reduce the efficiency of the EUS pumping action that contributes to flow (9). Degeneration of the striated muscle might not be rapidly reversed by testosterone administration, thus providing a possible explanation for the failure of testosterone replacement to normalize VFR.
An alteration in urethral smooth muscle function after ORX could be due to an increase in collagen and reduced elasticity, because a similar effect has been observed in bladder smooth muscle in castrated rats (10). This effect could explain the decrease in BC and increase in basal bladder pressure observed during cystometry in orchiectomized animals (Table 2). Tek et al. (27) reported a smaller (17%), nonsignificant reduction in BC 8 wk after ORX in ketamine-anesthetized Sprague-Dawley rats. Failure of the urethral smooth muscle to relax could also contribute to the apparent higher urethral outlet resistance and decreased VFR during voiding, because nitric oxide synthase production of nitric oxide, which induces urethral relaxation, is androgen dependent (28). Therefore, urethral smooth muscle relaxation, as well as EUS striated muscle contractions during voiding, might be reduced in castrated animals and contribute to reduced VFR.
An analysis of the effects of 8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT), a 5-HT1A agonist, on voiding in anesthetized male rats (9) has also provided evidence that urethral smooth activity can contribute to a reduced VFR during voiding. 8-OH-DPAT produced some effects that resembled those produced by ORX, including a reduced VT and a prolongation in the voiding period reflected by an increase in BD. It is noteworthy that, despite the prominent decrease in VFR, VE was increased in the 8-OH-DPAT-treated rats, in part by the prolonged period of voiding at a lower flow rate. Because 8-OH-DPAT acts centrally to enhance bladder and EUS activity, the mechanisms for increasing voiding duration while reducing VT are most reasonably attributed to a facilitation of the reflex control of the bladder and urethra. By analogy, it is also reasonable to speculate that the effect of ORX to maintain voiding duration while reducing VT is due to an action on the central nervous system.
ORX also produced a small but statistically significant increase (10%) in the AP of EUS EMG bursting during voiding. Because peak urethral flow occurs during the AP (9), this effect would be expected to enhance flow and partially counteract the changes of ORX that reduced flow. The change in AP duration was reversed by testosterone treatment, which also increased the EUS EMG SP and TSP parameters that were not affected by ORX.
The mechanisms underlying the effects of ORX and testosterone replacement are potentially complex because androgen receptors are widely expressed in the body. For example, the decrease in bladder capacity after ORX in rats could be mediated by changes in the bladder and/or in neural control at sites in the peripheral or central nervous system. Androgen and estrogen receptors have been identified in the bladder smooth muscle, urothelium, striated muscle of the urethra, in the neurons in the pelvic autonomic ganglia (14–16, 18, 24), and in primary sensory neurons located in the L6-S1 dorsal root ganglia of male rats (17). Castrated rats exhibit atrophy of the urothelium and thinning of bladder smooth muscle, coupled with an increase in collagen fibers (1, 27). The effects on smooth muscle and collagen were reversed by treatment with testosterone (1, 27), which also improved bladder capacity (27). Tek et al. (27) concluded that testosterone replacement therapy in late-onset hypogonadal men with urogenital dysfunction might improve bladder function by increasing smooth muscle function. However, another study reported a significant decrease in elastic fibers in the bladders of castrated male rats that was reversed by testosterone therapy, but no change in collagen fibers or smooth muscle (10).
Castration downregulates the expression of β1- and β2-adrenergic receptors in rat heart, but upregulates expression of β3-adrenergic receptors. Testosterone replacement reverses the effect of castration on β2- and β3-receptors (26). Thus changes in β-adrenergic receptor expression in the bladder and alteration in sympathetic nerve inhibition of bladder activity could contribute to the changes in urine storage after ORX.
It is noteworthy that the one dose of testosterone undecanoate (100 mg/kg) produced normal but not supraphysiological plasma concentrations of testosterone. However, the treatment significantly increased some EUS EMG parameters (SP, BD, TSP, BD/CD) that were not affected by ORX, indicating that the latter parameters were not sensitive to the decrease in physiological concentrations of the hormone after ORX but were sensitive to the concentrations of testosterone produced by exogenous administration. Although these changes in EUS EMG parameters might be expected to influence voiding, VE was not changed indicating that 1) the changes were not of sufficient magnitude to affect voiding; 2) voiding in untreated animals was already very efficient (i.e., 87%) and could not be increased further; or 3) as discussed above, some changes negated the effects of other changes.
Our results are different than the results of previous studies in which testosterone proprionate was administered subcutaneously every day for 2 wk in a dose of 3 mg/kg to awake (22) or urethane-anesthetized (21) normal male Sprague-Dawley rats. In the latter studies, the treatment increased micturition pressure, bladder capacity, PVR, micturition volume, and induced bladder overactivity that was evident as nonvoiding contractions during bladder filling. Because testosterone treatment did not alter responses of bladder smooth muscle to electrical field stimulation or to cholinergic or purinergic agonists, it was concluded that the treatment altered the properties of the bladder afferent neurons or the micturition reflex circuitry in the central nervous system. Plasma levels of testosterone were not reported in these studies; however, based on other experiments in which plasma levels of testosterone were measured in orchiectomized rats after administration of a single 100 mg/kg dose of testosterone proprionate (4), it seems likely that the 3 mg/kg daily dose produced supraphysiological concentrations of testosterone that stimulated the neural control of the bladder. Our studies, which used a slow release formulation of testosterone, which normalizes plasma testosterone levels, indicate that physiological concentrations of the hormone promote bladder storage function but do not induce bladder overactivity.
A comparison of the effects of gonadectomy and hormone replacement therapy on LUT function in urethane-anesthetized rats reveals some prominent sex differences. Our previous studies (6) showed that a major effect of ovariectomy in female rats is a reduction in VE due to shortening of the SP of EUS EMG bursting during voiding, and that these effects are reversed by treatment with estradiol or propylpyrazole triol, an estrogen receptor-α agonist (7). Furthermore, ovariectomy did not alter VT, but treatment of ovariectomized rats with either estradiol or with a combination of propylpyrazole triol and diarylpropionitrile, an estrogen receptor-β agonist, increased VT and also decreased the duration of the AP of EUS EMG bursting activity. ORX produced the opposite effects: a reduction in VT without a change in VE. It should be noted, however, that the effects of ovariectomy on VT and voiding frequency may vary with the experimental conditions, because there are also reports of increased voiding frequency in awake rats after ovariectomy (20).
Conclusion.
The major effect of chronic hormone deprivation on male rat LUT function is a decrease in bladder capacity with no change in the amplitude of reflex bladder contractions or VE, suggesting that castration affects the afferent limb but not the efferent limb of the micturition reflex. However, ORX decreased VFR that is likely due to changes in the urethral outlet. Testosterone replacement reversed the change in VT, suggesting that the peripheral or central neural pathways responsible for micturition are modulated by gonadal hormones.
GRANTS
This study was supported by grants from Ministry of Science and Technology (MOST 103–2314-B-075A-009-MY2), Taichung Veterans General Hospital (TCVGH-1025003C), Taiwan, ROC, and from the US National Institute of Diabetes and Digestive and Kidney Diseases(NIH PO1-DK-093424).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.-L.C. conception and design of research; C.-L.C. performed experiments; C.-L.C. analyzed data; C.-L.C. interpreted results of experiments; C.-L.C. prepared figures; C.-L.C. and W.C.d.G. drafted manuscript; C.-L.C. and W.C.d.G. edited and revised manuscript; C.-L.C. approved final version of manuscript.
REFERENCES
- 1.Abdel-Hamid AA, Ali EM. Effect of testosterone therapy on the urinary bladder in experimental hypogonadism of rats. J Mol Histol 46: 263–272, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Beutel ME, Wiltink J, Hauck EW, Auch D, Behre HM, Brahler E, Weidner W. Correlations between hormones, physical, and affective parameters in aging urologic outpatients. Eur Urol 47: 749–755, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Callies F, Kollenkirchen U, von zur Muhlen C, Tomaszewski M, Beer S, Allolio B. Testosterone undecanoate: a useful tool for testosterone administration in rats. Exp Clin Endocrinol Diabetes 111: 203–208, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Celayir S. Effects of different sex hormones on male rabbit urodynamics: an experimental study. Horm Res 60: 215–220, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Cheng CL, Chai CY, de Groat WC. Detrusor-sphincter dyssynergia induced by cold stimulation of the urinary bladder of rats. Am J Physiol Regul Integr Comp Physiol 272: R1271–R1282, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CL, de Groat WC. Effect of ovariectomy on external urethral sphincter activity in anesthetized female rats. J Urol 186: 334–340, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng CL, de Groat WC. Effects of agonists for estrogen receptor alpha and beta on ovariectomy-induced lower urinary tract dysfunction in the rat. Am J Physiol Renal Physiol 306: F181–F187, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol 187: 445–454, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Fan WJ, Chen SC, Hsieh TH, Lai CH, Lin YS, Peng CW, Kou YR. Influence of serotonergic mechanisms on the urine flow rate in male rats. Am J Physiol Regul Integr Comp Physiol 307: R1239–R1250, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Gallo CB, Miranda AF, Felix-Patricio B, Ramos CF, Cardoso LE, Costa WS, Sampaio FJ. Effects of castration and hormone replacement in the urinary bladder of rats: structural, ultrastructural, and biochemical analysis. J Androl 33: 684–690, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86: 724–731, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Kalinchenko S, Vishnevskiy EL, Koval AN, Mskhalaya GJ, Saad F. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male 11: 57–61, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Karazindiyanoglu S, Cayan S. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male 11: 146–149, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Keast JR. The autonomic nerve supply of male sex organs–an important target of circulating androgens. Behav Brain Res 105: 81–92, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Keast JR. Effects of testosterone on pelvic autonomic pathways: progress and pitfalls. J Auton Nerv Syst 79: 67–73, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Keast JR. Plasticity of pelvic autonomic ganglia and urogenital innervation. Int Rev Cytol 248: 141–208, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Keast JR, Gleeson RJ. Androgen receptor immunoreactivity is present in primary sensory neurons of male rats. Neuroreport 9: 4137–4140, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Keast JR, Saunders RJ. Testosterone has potent, selective effects on the morphology of pelvic autonomic neurons which control the bladder, lower bowel and internal reproductive organs of the male rat. Neuroscience 85: 543–556, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Kracochansky M, Reis LO, Lorenzetti F, Ortiz V, Dambros M. Impact of castration with or without alpha-tocopherol supplementation on the urethral sphincter of rats. Int Braz J Urol 38: 277–283, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Kullmann FA, Limberg BJ, Artim DE, Shah M, Downs TR, Contract D, Wos J, Rosenbaum JS, de Groat WC. Effects of beta3-adrenergic receptor activation on rat urinary bladder hyperactivity induced by ovariectomy. J Pharmacol Exp Ther 330: 704–717, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maggi CA, Manzini S, Giuliani S, Meli A. Infravesical outflow obstruction in rats: a comparison of two models. Gen Pharmacol 20: 345–349, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Pandita RK, Persson K, Hedlund P, Andersson KE. Testosterone-induced prostatic growth in the rat causes bladder overactivity unrelated to detrusor hypertrophy. Prostate 35: 102–108, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Pradidarcheep W. Lower urinary tract symptoms and its potential relation with late-onset hypogonadism. Aging Male 11: 51–55, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Salmi S, Santti R, Gustafsson JA, Makela S. Co-localization of androgen receptor with estrogen receptor beta in the lower urinary tract of the male rat. J Urol 166: 674–677, 2001. [PubMed] [Google Scholar]
- 25.Shigehara K, Namiki M. Late-onset hypogonadism syndrome and lower urinary tract symptoms. Korean J Urol 52: 657–663, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun J, Fu L, Tang X, Han Y, Ma D, Cao J, Kang N, Ji H. Testosterone modulation of cardiac beta-adrenergic signals in a rat model of heart failure. Gen Comp Endocrinol 172: 518–525, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Tek M, Balli E, Cimen B, Efesoy O, Oguz I, Cayan S. The effect of testosterone replacement therapy on bladder functions and histology in orchiectomized mature male rats. Urology 75: 886–890, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Traish AM, Kim NN, Goldstein I, Moreland RB. Alpha-adrenergic receptors in the penis: identification, characterization, and physiological function. J Androl 20: 671–682, 1999. [PubMed] [Google Scholar]
- 29.Travison TG, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab 92: 549–555, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Trifiro MD, Parsons JK, Palazzi-Churas K, Bergstrom J, Lakin C, Barrett-Connor E. Serum sex hormones and the 20-year risk of lower urinary tract symptoms in community-dwelling older men. BJU Int 105: 1554–1559, 2010. [DOI] [PubMed] [Google Scholar]