Whether tumor necrosis factor-α (TNF) modulates cardiac stem cell (CSC) function is unknown. We show that TNF, primarily via TNF receptor-1, inhibits cardiomyogenic commitment of CSCs, and channels an alternate neuroadrenergic-like fate via both receptors. This suggests that TNF diminishes the efficacy of cardiac repair and enhances local adrenergic activation.
Keywords: cardiac stem cell, tumor necrosis factor, TNF receptor, cell differentiation
Abstract
Despite expansion of resident cardiac stem cells (CSCs; c-kit+Lin−) after myocardial infarction, endogenous repair processes are insufficient to prevent adverse cardiac remodeling and heart failure (HF). This suggests that the microenvironment in post-ischemic and failing hearts compromises CSC regenerative potential. Inflammatory cytokines, such as tumor necrosis factor-α (TNF), are increased after infarction and in HF; whether they modulate CSC function is unknown. As the effects of TNF are specific to its two receptors (TNFRs), we tested the hypothesis that TNF differentially modulates CSC function in a TNFR-specific manner. CSCs were isolated from wild-type (WT), TNFR1−/−, and TNFR2−/− adult mouse hearts, expanded and evaluated for cell competence and differentiation in vitro in the absence and presence of TNF. Our results indicate that TNF signaling in murine CSCs is constitutively related primarily to TNFR1, with TNFR2 inducible after stress. TNFR1 signaling modestly diminished CSC proliferation, but, along with TNFR2, augmented CSC resistance to oxidant stress. Deficiency of either TNFR1 or TNFR2 did not impact CSC telomerase activity. Importantly, TNF, primarily via TNFR1, inhibited cardiomyogenic commitment during CSC differentiation, and instead promoted smooth muscle and endothelial fates. Moreover, TNF, via both TNFR1 and TNFR2, channeled an alternate CSC neuroadrenergic-like fate (capable of catecholamine synthesis) during differentiation. Our results suggest that elevated TNF in the heart restrains cardiomyocyte differentiation of resident CSCs and may enhance adrenergic activation, both effects that would reduce the effectiveness of endogenous cardiac repair and the response to exogenous stem cell therapy, while promoting adverse cardiac remodeling.
Listen to this article's corresponding podcast at http://ajpheart.podbean.com/e/tnf-and-cardiac-stem-cell-differentiation/.
NEW & NOTEWORTHY
Whether tumor necrosis factor-α (TNF) modulates cardiac stem cell (CSC) function is unknown. We show that TNF, primarily via TNF receptor-1, inhibits cardiomyogenic commitment of CSCs, and channels an alternate neuroadrenergic-like fate via both receptors. This suggests that TNF diminishes the efficacy of cardiac repair and enhances local adrenergic activation.
recent evidence suggests that the mammalian heart can undergo cardiomyocyte renewal from resident cardiac stem cells (CSCs) and/or proliferating cardiomyocytes (42). Although reports are variable, endogenous cardiomyocyte renewal rates are considered to be limited (∼1% per year) (7, 38, 42). Additionally, while renewal rates increase after cardiac stress (6, 22, 38), this increase is insufficient to repair the heart after myocardial infarction (MI) or injury (42). Prior studies have identified in the adult heart a population of cells, termed cardiac stem cells (CSCs), which express the stem cell marker c-kit without expressing markers of hematopoietic lineage (i.e., c-kit+Lin− CSCs) and that exhibit evidence of cardiomyogenic specification in vitro (5, 39). Autologous transplantation of expanded c-kit+ CSCs improves cardiac function and remodeling in rodent models of both acute injury and chronic heart failure (HF) (3, 5, 10, 15, 21, 41). Nonetheless, the mechanisms underlying the salutary effects of cell transplantation on cardiac function in ischemic HF remain unclear. Although it has been assumed that adoptively transferred CSCs transdifferentiate into cardiac cells, several animal studies have demonstrated low rates of transplanted CSC persistence, engraftment, and cardiomyocyte differentiation (21, 24, 28, 37, 41). Moreover, chronically failing human hearts exhibit a nearly fourfold increase in c-kit+ CSC abundance compared with nonfailing hearts (26); despite this, the failing heart does not regenerate structure or function. This suggests that factors in the cardiac microenvironment may compromise CSC differentiation and regenerative potential in HF. Identifying such mediators would be of critical importance to enhancing both endogenous healing and the therapeutic response to exogenous cell therapy in ischemic cardiomyopathy.
Given that pathological inflammation is a hallmark of HF (29), we postulated that inflammatory cytokines may inhibit CSC differentiation and function in the failing heart. Tumor necrosis factor-α (TNF) in particular serves as a foundation cytokine that regulates the expression of other proinflammatory mediators such as interleukin (IL)-1β and IL-6 via nuclear factor (NF)-κB (1). The effects of TNF on pathological remodeling and proinflammatory responses in HF are complex and receptor (R)-specific: TNFR1 exacerbates, whereas TNFR2 alleviates, these events (17, 19, 32). However, whether and how TNF modulates CSC function and differentiation, and whether dichotomous TNFR-specific effects also extend to CSCs, is unknown. Here, we tested the hypothesis that TNF differentially modulates in vitro CSC function and differentiation in a TNFR-specific manner.
METHODS
CSC Isolation and Characterization
All use of animals was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and/or the University of Louisville, and were compliant with the NIH Guide for the Care and Use of Laboratory Animals (DHHS publication No. 85-23, revised 1996). The following mouse strains (from Jackson Laboratories) were used: C57BL/6 wild-type (WT, no. 000664), green fluorescent protein (GFP) transgenic (Tg) mice (GFP expressed under direction of the human ubiquitin C promoter, no. 007076), TNFR1−/− mice (no. 002818), and TNFR2−/− mice (no. 002620). CSCs were isolated from adult male mouse hearts using primary explant cell outgrowth cultures magnetically sorted for c-kit+Lin− cells as described previously (27, 45). In brief, heart cell outgrowths were initially expanded in Ham's F12 Kaighn's modification culture medium (Fisher-HyClone) supplemented with 0.2 mM l-glutathione, 5 mU/ml erythropoietin, 10 ng/ml leukemia inhibitory factor (LIF), 10 ng/ml basic fibroblast growth factor (bFGF), 5% horse serum, 10% fetal bovine serum, and 1% penicillin-streptomycin. Lineage positive cells were depleted from the cultures using magnetic microbeads (Miltenyi Biotec) conjugated to a panel of antibodies against hematopoietic lineage markers (Lin: CD5, CD45R, Cd11b, Gr-1, 7-4, TER-119). The remaining Lin− cells were then expanded and further magnetically sorted using a specific anti-c-kit antibody (Santa Cruz) and consequently enriched for c-kit+ cells. The enriched CSCs were seeded in the above culture medium and were used at cell passages <10 for all the experiments outlined.
Flow Cytometry
For flow cytometric analyses, CSCs were incubated for 1 h on ice and stained with a cocktail of fluorophore-labeled specific antibodies against the lineage markers above (biotin-conjugated monoclonal antibodies with Cy5 labeled streptavidin secondary antibody, Miltenyi Biotec), c-kit (SouthernBiotech), Sca-1 and CD34 (eBioscience), DDR2 (fibroblast marker, Santa Cruz), and CD31 (BD Biosciences). Data were acquired on an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software.
Immunoblotting and Electrophoretic Mobility Shift Assay (EMSA)
Isolation of total, cytosolic, and nuclear proteins, SDS-PAGE and immunoblotting were performed using standard protocols as previously described (19, 20). Primary antibodies for immunoblotting were against p38 mitogen-activated protein kinase (MAPK) and c-Jun NH2-terminal kinase (JNK) (Cell Signaling); and NF-κB p65/p50, inhibitor of κBα (IκBα), α-tubulin, TNFR1, TNFR2, GFP, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and Lamin A (Santa Cruz Biotechnology). NF-κB DNA binding activity and subunit composition were analyzed by EMSA and supershift analysis as described previously (19, 20).
CSC Proliferation
CSC proliferation was evaluated by a colorimetric dehydrogenase enzyme-based MTS assay (CellTiter 96 Aqueous, Promega). WT, TNFR1−/−, and TNFR2−/− CSCs were cultured in 96-well plates in culture medium devoid of growth factors but containing 10% FBS and antibiotics. For both assays, CSCs were seeded in a twofold serial dilution starting from 4,000 cells per well in triplicate. Following 48 h of culture, CSCs were incubated for 3 h with the MTS reagent. The number of viable cells in culture was determined by absorbance of the generated formazan product at 490 nm, measured using a microplate reader (BioTek Epoch). Five independent experiments were performed, each in triplicate.
Oxidative Stress-Induced CSC Cytotoxicity
Hydrogen peroxide (H2O2)-induced CSC cytotoxicity was indexed by lactate dehydrogenase (LDH) activity in the culture supernatant using the colorimetric LDH cytotoxicity assay kit (Cayman Chemical). WT, TNFR1−/−, and TNFR2−/− CSCs were seeded in a 24-well plate (9,600 cells/well) in CSC culture medium. After 48 h of culture, the medium was replaced with serum-free F12 medium, and the cells were further incubated for ∼20 h prior to treatment with 500 μM H2O2 for 3 or 6 h. The culture supernatants were then collected and analyzed for LDH activity following the manufacturer's instructions. Briefly, culture supernatant (100 μl; in triplicate) was added to each well of a 96-well ELISA plate and allowed to react with 100 μl of LDH substrate containing solution provided in the assay kit. Following incubation, absorbance was read at 490 nm, with the intensity of color being directly proportional to the amount of LDH in the supernatant. To determine LDH activity (μU), the recorded absorbance from H2O2-treated CSCs was extrapolated from a standard curve generated using known LDH standards.
CSC Telomerase Activity
To assess propensity for cell senescence, CSC telomerase activity was quantified using an RT-PCR based in vitro assay (TRAPeze RT Telomerase Detection Kit, EMD Millipore). CSC cell pellets (1 × 106 cells) were lysed in CHAPS buffer. Following protein quantification in the cleared CSC lysates, 1 μg of total lysate in 2 μl of CHAPS buffer was assayed for telomerase activity as per the manufacturer's instructions. In the first step of the reaction, telomerase in the lysate adds several telomeric repeats (GGTTAG) onto the 3′ end of a synthetic substrate oligonucleotide (TS) provided in the kit. In the second step, the extended products so generated are amplified using Titanium Taq-Polymerase (BD Clontech) and the supplied fluorescein-labeled Amplifluor primers using RT-PCR. The fluorescence is directly proportional to the amount of telomeric repeat added-TS products generated. The quantity of extended telomerase substrate was obtained by converting the RT-PCR CT values from a standard curve generated using known attomole quantities of the TSR8 control template. Results are expressed as percent telomerase activity compared with WT CSCs.
CSC Differentiation
CSCs (passages 4–9) were differentiated in vitro using the 28-day azacytidine-transforming growth factor (TGF)-β-ascorbic acid cardiomyocyte differentiation protocol of Smits et al. (40), with some modifications. Using this protocol, CSCs were differentiated in the absence or presence of TNF (20 ng/ml) in basal CSC culture media (containing only 10% FBS and antibiotics, but devoid of growth factors). Following the initial azacytidine treatment, TNF in the culture media was replenished during media changes (every 3–4 days) until the end of the experiment. For differentiation controls, CSCs were cultured in complete CSC culture media until nearly confluent (>90%). CSCs were cultured for 28 days, at which time supernatants were collected and stored at −80°C for determination of catecholamine levels. The cells were used for RNA isolation to evaluate lineage-specific gene expression using real-time (RT) PCR.
RT-PCR Analysis
Quantitative RT-PCR to evaluate mRNA expression was performed as described previously (19, 20, 23, 43). Briefly, TRIzol (Life Technologies) extracted total RNA was quantified using a NanoDrop 1000 spectrophotometer (Thermo Scientific). Total RNA (500 ng) was subjected to cDNA synthesis using the High Efficiency cDNA Synthesis Kit (Invitrogen). The levels of various mRNA transcripts were determined using Fast SYBR Green (Life Technologies) and gene-specific forward and reverse primer sets (Table 1) on a ViiA 7 RT-PCR instrument (Life Technologies). GAPDH or β-actin expression was used to normalize mRNA expression levels using the ΔΔCT comparative method.
Table 1.
Forward and reverse primers used for RT-PCR
| Gene Name | Gene ID | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| β-MHC (Myh7) | 140781 | TCCTCACATCTTCTCCATCTCTGA | TGGACTGATTCTCCCGATCTG |
| Mef2c | 17260 | TTTGGTGTACAGCAATCCTGTCA | TGGGCCAGTGGCAGAAGA |
| Flk-1 (Kdr) | 16542 | CACCCCAGATCGGTGAGAAA | TCTGCATGGTCCCATACTGGTA |
| Nkx2.5 | 18091 | CTCCGCCAACAGCAACTTC | GGACTCTGCACGGTGTTCAA |
| Tyrosine Hydroxylase (TH) | 21823 | CGAGCTGCTGGGACACGTA | CTGGGAGAACTGGGCAAATG |
| GAP43 | 14432 | CTGAGGAGGAGAAAGACGCTGTA | TCCTGTCGGGCACTTTCC |
| Nestin | 18008 | AGCCATTGTGGTCTACGGAAGT | TCCACACACCCCAGTGGTT |
| β-III Tubulin | 22152 | TCACGCAGCAGATGTTCGAT | GTGGCGCGGGTCACA |
| α-SMA (Acta2) | 11475 | CCTGACGCTGAAGTATCCGATAG | TTTTCCATGTCGTCCCAGTTG |
| β-Actin (Actb) | 11461 | CGATGCCCTGAGGCTCTTT | TGGATGCCACAGGATTCCA |
| GAPDH | 14433 | GCGGCACGTCAGATCCA | GCGGCACGTCAGATCCA |
Catecholamine Assay
Levels of epinephrine and norepinephrine secreted by CSCs during in vitro differentiation were quantified using a competitive ELISA-based catecholamine assay (Rocky Mountain Diagnostics) as per the manufacturer's instructions. Briefly, epinephrine and norepinephrine in culture supernatants were extracted using affinity gel, followed by their acylation and enzymatic conversion. These derivatives were then added to a microtiter plate containing a bound antigen, which then competes for binding to specific peroxidase labeled antisera. After removal of free antigen and antigen-antiserum complexes by washing, bound antibody was detected upon addition of TMB substrate and monitoring the reaction at 450 nm. Quantitative catecholamine concentrations were obtained by interpolating the absorbance with a reference curve generated using known standard concentrations.
Immunostaining and Confocal Microscopy
Following differentiation, CSCs were fixed with freshly prepared 2% paraformaldehyde for 30 min at room temperature (RT) and then treated with 0.01% saponin for 10 min to permeabilize the cells. Following several PBS (containing calcium and magnesium) washes, cells were blocked using blocking buffer (PBS containing 0.01% saponin and 1% BSA) for 10 min at RT. Fixed and permeabilized cells were incubated with primary antibodies from Santa Cruz Biotech against α-sarcomeric actin (1:200 dilution), GFP (1:500 dilution), and tyrosine hydroxylase (1:200 dilution) in blocking buffer for 1 h at RT. After several washes with cold PBS, the cells were incubated with the appropriate fluorescent-labeled secondary antibodies (1:1,000 dilution; Molecular Probes) for 30 min in the dark at RT. Cells were washed again followed by addition of DAPI (300 nM; Invitrogen) to counterstain nuclei. The stained cells were analyzed with a Nikon-A1 confocal microscope and the images were acquired using a 60× objective.
Statistical Analysis
Data are shown as means ± SE. Statistical differences between experimental groups were analyzed using the Student's t-test or ANOVA (one- or two-way), as appropriate. A P value < 0.05 was considered statistically significant.
RESULTS
Characterization of Adult Murine CSCs
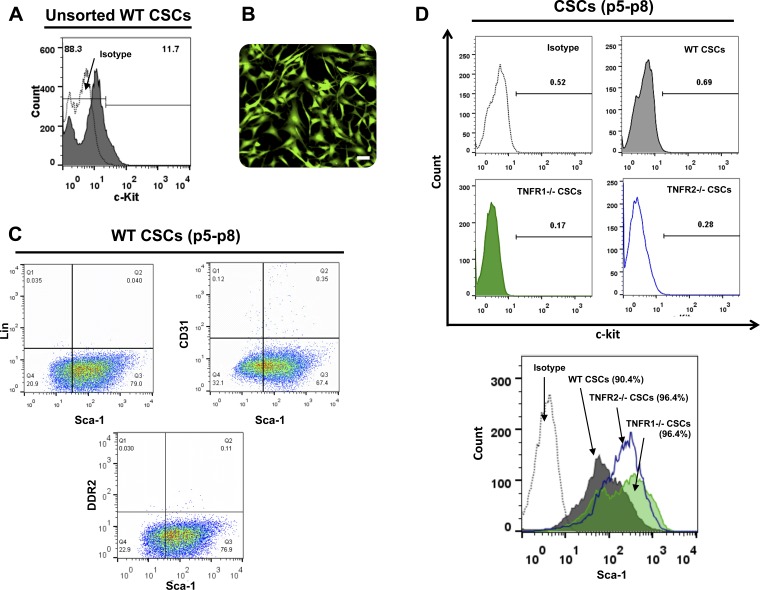
CSCs were isolated from adult mouse hearts by explant culture. Flow cytometric analysis of initial unsorted cells emigrating from the explants revealed ∼11–12% c-kit expressing CSCs within this heterogeneous cell population (Fig. 1A). c-kit+Lin− CSCs were enriched from these initial cultures using magnetic immunobeads. Following enrichment, CSC surface marker expression was evaluated by flow cytometry during subsequent expansion and passage (P4–P9). Figure 1B depicts representative epifluorescence images of expanded GFP Tg CSCs in culture showing typical stellate and spindle-shaped cell morphology. Flow cytometry revealed that passaged WT CSCs reliably expressed Sca-1 (∼70–80%), but were Lin− and also negative for the fibroblast marker DDR2 and the endothelial cell marker CD31 (Fig. 1C). Interestingly, as reported previously (45), cultured WT, TNFR1−/−, and TNFR2−/− murine CSCs did not retain c-kit expression upon passage, as assessed by flow cytometry (Fig. 1D), as well as by immunoblotting and RT-PCR (data not shown), but did maintain Sca-1 expression. This loss of c-kit expression stands in contrast to CSCs derived from other species (e.g., rats, humans) that stably retain c-kit expression in culture (4, 5).
Fig. 1.
Characterization of murine resident cardiac stem cells (CSCs). A: representative flow cytometric plot depicting c-kit expression in unsorted CSCs isolated from adult WT mouse hearts. B: representative fluorescent image of magnetically sorted, c-kit enriched GFP Tg CSCs isolated from adult mouse hearts; scale bar, 50 μm. C: representative flow cytometric plots for surface marker expression in minimally expanded wild-type (WT) CSCs at passages 5–8 (p5–p8). Markers shown are lineage (Lin), CD31 (endothelial marker), and DDR2 (fibroblast marker). D: representative flow cytometry histograms for c-kit and Sca-1 expression in WT, TNFR1−/−, and TNFR2−/− CSCs at passages 5–8 and corresponding isotype control antibody labeling.
CSC TNF Signaling Is Functionally Intact and Primarily Linked to TNFR1 at Baseline
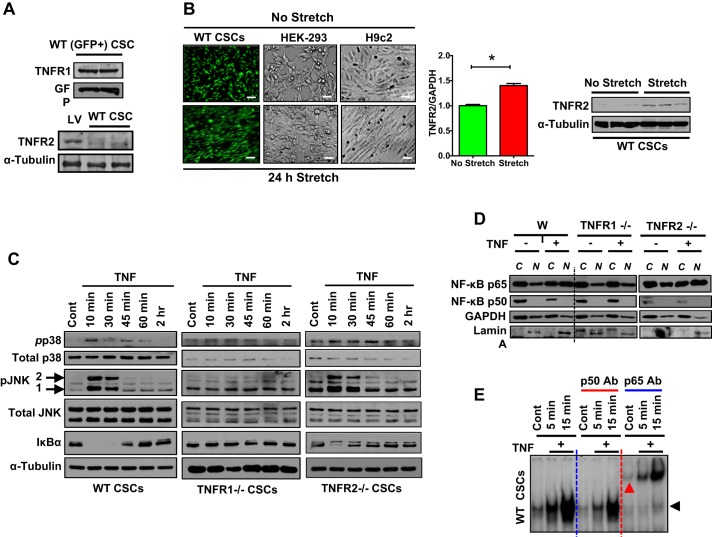
Total cell lysates were harvested from GFP Tg CSCs and analyzed by immunoblotting for TNFR protein expression. As shown in Fig. 2A, naive murine CSCs constitutively expressed TNFR1 whereas TNFR2 expression was low compared with its expression in mouse left ventricle (positive control). Although nearly undetectable at baseline, TNFR2 expression in CSCs was to some degree inducible. When subjected to 24 h of mechanical stretch (sine-wave pattern at 60 cycles/min) in vitro using a Flexercell compression system (Flexcell International), CSCs reproducibly aligned in parallel along the planes of applied stretch [similar to H9c2 cardiomyocytes, but in contrast to human embryonic kidney HEK-293 cells that did not align with stretch (Fig. 2B)]. In conjunction with cell alignment, mechanical stretch augmented TNFR2 expression in CSCs, as evaluated by both RT-PCR and immunoblotting compared with unstretched control CSCs.
Fig. 2.
TNF-TNFR signaling axis in murine CSCs. A: TNFR immunoblots in WT (or GFP Tg) murine CSCs. GFP and α-tubulin expression were used as loading controls. B, left: representative fluorescent or bright field images of GFP Tg CSCs, HEK-293 cells, and H9c2 myoblasts in either static cultures (no stretch) or immediately following 24 h mechanical stretch; scale bars, 100 μm. B, middle: TNFR2 gene expression in WT CSCs following 24 h of culture under static or stretch conditions; n = 6/group, *P < 0.05. B, right: TNFR2 protein expression by immunoblotting in unstretched and stretched (24 h) WT CSCs; α-tubulin was used as loading control. C: representative immunoblots for the TNF signaling effectors total/phospho- p38 MAPK, total/phospho-JNK, and IκBα in WT, TNFR1−/−, and TNFR2−/− CSCs treated with TNF 20 ng/ml for the indicated times. D: representative immunoblots for NF-κB subunits p65 and p50 in cytosolic (C) and nuclear (N) extracts from CSCs with or without 30 min TNF exposure; C loading control GAPDH; N loading control Lamin A. E: EMSA for NF-κB DNA-binding activity. NF-κB subunit composition was assessed by supershift assay using antibodies specific for p50 and p65. Black arrowhead depicts shifts while red arrowhead depicts p65 antibody-induced supershift in DNA binding.
We next evaluated whether CSCs have a functional TNFR signaling axis. WT, TNFR1−/−, and TNFR2−/− CSCs were serum-starved overnight and then treated with TNF [20 ng/ml, a concentration we have previously shown (19) to robustly activate TNF signaling in cardiomyocytes] for the times indicated in Fig. 2. Following TNF stimulation, total CSC protein lysates were analyzed for activation of p38 MAPK, JNK, and NF-κB, the main downstream signaling effectors of TNF. Soluble TNF induced a time-dependent and circumscribed activation of both p38 and JNK in WT and TNFR2−/− CSCs (Fig. 2C) when analyzed by immunoblotting. In contrast, TNFR1−/− CSCs exhibited no change in p38 and JNK activation. Similarly, TNF also induced NF-κB activation and p65 subunit nuclear translocation in WT and TNFR2−/− CSCs, but not in TNFR1−/− CSCs (Fig. 2, C–E). NF-κB activity was evaluated indirectly by immunoblotting for IκBα, and directly via assessment of nuclear translocation of p65 and p50 subunits by immunoblotting and EMSA. As shown in Fig. 2C, IκBα was significantly degraded (indicative of NF-κB activation) as early as 10 min following TNF stimulation in WT and TNFR2−/− CSCs, whereas its expression was unchanged in TNFR1−/− CSCs. Moreover, TNF induced nuclear translocation of the NF-kB p65 subunit in WT and TNFR2−/− CSCs, but not in TNFR1−/− CSCs (in contrast, the p50 subunit was not impacted in any group; Fig. 2D). EMSA and supershift analysis confirmed that TNF-induced NF-κB activity in CSCs comprised primarily the p65 subunit (Fig. 2E). Taken together, these data establish that TNF signaling is intact in CSCs and primarily related to TNFR1, which is constitutively expressed. In contrast, TNFR2 expression is modest and induced only upon stress. Hence, the impact of TNF on CSC function, at least under unstressed conditions, would relate predominantly to TNFR1.
TNFR1 Modestly Diminishes CSC Proliferation and Does Not Impact Replicative Senescence
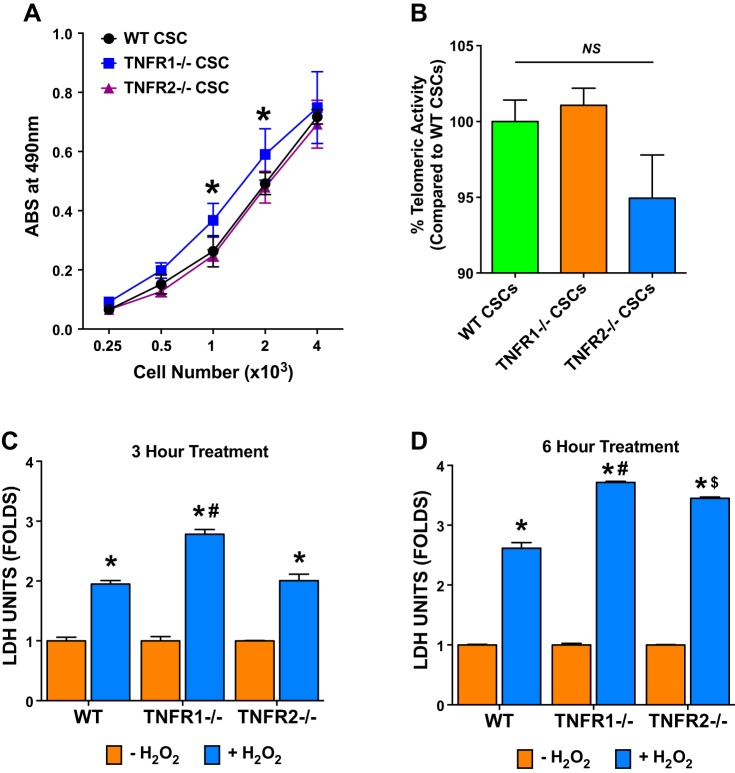
WT, TNFR1−/−, and TNFR2−/− CSCs were plated in a 96-well tissue culture plate at various cell densities (250–4,000 cells/well) and cultured for 48 h in CSC culture medium devoid of growth factors. CSCs were then exposed to MTS reagent for an additional 3 h to allow for incorporation of the reagent in viable proliferating cells. As shown in Fig. 3A, the proliferation assay indicated a cell density-dependent increase in cell proliferation in WT, TNFR1−/−, and TNFR2−/− CSCs. However, TNFR1−/− CSCs exhibited a small but significant increase in proliferation compared with both WT and TNFR2−/− CSCs at intermediate cell densities (1 and 2 × 103 cells/well), suggesting that TNFR1 signaling restrains CSC proliferation.
Fig. 3.
CSC proliferation, replicative senescence, and response to oxidative stress. A: WT, TNFR1−/−, and TNFR2−/− CSC proliferation as assessed using an MTS-based assay at varying initial cell densities as indicated; data are from five independent experiments; *P < 0.05 vs. WT and TNFR2−/− CSCs at respective cell densities. B: replicative senescence of WT, TNFR1−/−, and TNFR2−/− CSCs as evaluated by an RT-PCR based telomerase activity assay; data are from three independent experiments each done in triplicate. WT CSC telomerase activity was considered 100%. C and D: WT, TNFR1−/−, and TNFR2−/− CSC cell death in response to hydrogen peroxide (H2O2; 500 μM) exposure for either 3 (C) or 6 h (D) as evaluated by lactate dehydrogenase (LDH) release into the culture media. LDH units (μU) are presented as fold compared with medium alone (−H2O2) group. n = 3/group; *P < 0.05 vs. respective −H2O2 group; #P < 0.05 vs. WT and TNFR2−/− CSC + H2O2 groups; $P < 0.05 vs. WT CSC + H2O2 group.
An important functional attribute of stem cells that frequently divide is reduced replicative senescence and resistance to telomere shortening, as reflected by increased telomerase activity. WT, TNFR1−/−, and TNFR2−/− CSCs were evaluated for telomerase activity using an RT-PCR-based telomerase assay. CSC-telomerase activity was calculated by extrapolating the real-time PCR cycle threshold (cT) values on a standard curve generated using a control template (TSR8) with known concentration. As shown in Fig. 3B, all three CSC types exhibited similar telomerase activities, with a nonsignificant trend toward reduced telomerase activity in TNFR2−/− CSCs compared with WT and TNFR1−/− CSCs. Hence, deficiency of either TNFR did not impact CSC propensity toward senescence.
TNFR1, and to a Lesser Extent TNFR2, Augments CSC Resistance to Oxidant Stress
In vitro CSC resistance to H2O2-induced cytotoxicity was indexed by LDH release into the culture medium. Oxidative stress-mediated cell death was measured after 3 and 6 h of H2O2 exposure (500 μmol/l) following 48 h of CSC culture. As shown in Fig. 3, C and D, H2O2 induced significant CSC cell death and LDH release into conditioned media compared with untreated controls at 3 and 6 h. TNFR1−/− CSCs exhibited greater LDH release compared with both WT and TNFR2−/− CSCs at both 3 and 6 h, and TNFR2−/− CSCs exhibited greater LDH release over WT at 6 h. Hence, both TNFR1 and TNFR2 signaling confer CSC resistance to oxidant stress-induced cytotoxicity, with more pronounced effects related to TNFR1. These results have important implications for CSC survival in the harsh and pro-oxidative microenvironments that occur in the post-infarct and failing heart.
TNF Modulates CSC Differentiation by Promoting an Alternative Neuroadrenergic-like Fate, and TNFR1-Dependent Suppression of Cardiomyogenic Fate
Murine CSC differentiation was evaluated in vitro using the protocol of Smits et al. (40), shown to induce human CSC differentiation into cardiomyocytes with high efficiency (>80%). To evaluate the effects of TNF, CSCs (WT, TNFR1−/−, and TNFR2−/−) were differentiated in the absence or presence of TNF (20 ng/ml) for the entire 28-day differentiation protocol. Cell morphology and gene expression programs were subsequently evaluated.
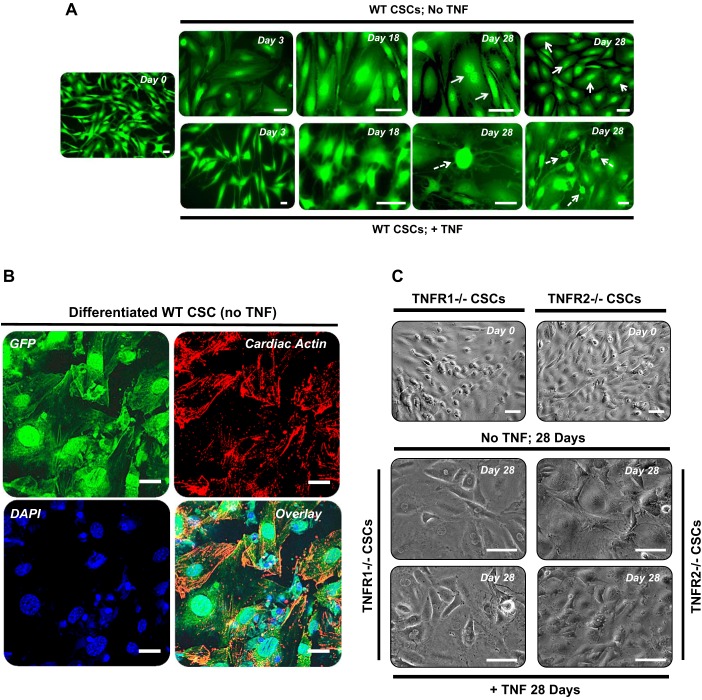
Cell morphology.
CSCs underwent significant morphological changes during the course of differentiation. Figure 4A depicts representative epifluorescence images of WT (GFP Tg) CSCs at days 0, 3, 18, and 28 with and without TNF treatment. During differentiation, WT CSCs progressively acquired a broad-based and flattened morphology, with many cells exhibiting striations and binucleation by the end of the differentiation protocol, which somewhat resembled neonatal cardiomyocytes (indicated by arrows). Figure 4B depicts coimmunostaining for GFP and cardiac sarcomeric actin in differentiated WT CSCs, further illustrating the striated sarcomeric pattern in these cells. In contrast, WT CSCs differentiated in the presence of exogenous TNF displayed a predominance of elongated and/or contracted morphology, with multiple cells harboring dendritic type projections emanating from centrally located, highly fluorescent cell bodies (broken arrows in Fig. 4A), reminiscent of a neuronal-like cell shape. Phase-contrast images of TNFR1−/− and TNFR2−/− CSCs (nonfluorescent cells) are shown in Fig. 4C. Both TNFR1−/− and TNFR2−/− CSCs exhibited flattened cell morphology at 28 days regardless of concomitant TNF exposure, suggesting that signaling via both receptors is necessary for the development of neuronal-like morphology.
Fig. 4.
TNF/TNFR signaling impacts CSC differentiation. CSCs were differentiated in vitro for 28 days as detailed in the text. A: representative fluorescent images of GFP Tg (WT) CSCs at days 0, 3, 18, and 28 of the protocol without or with TNF (20 ng/ml) in the differentiation media. The top panels (no TNF) demonstrate progressive development of broad-based CSC morphology, with many cells exhibiting striations and binucleation (solid arrows) by day 28, reminiscent of neonatal cardiomyocytes. The bottom panels (+TNF) demonstrate abundant cells with contracted or elongated morphology and dendritic or axonal type projections (broken arrows) more akin to neuronal cells. Scale bars, 50 μm. B: representative 60× confocal images of GFP Tg WT CSCs differentiated in vitro for 28 days and stained for GFP (green, top left), α-sarcomeric cardiac actin (red, top right), and DAPI (blue, bottom left). The overlaid image is shown in bottom right panel. Scale bars, 10 μm. C: phase-contrast images of TNFR1−/− and TNFR2−/− CSCs at days 0 and 28 of differentiation with or without TNF (20 ng/ml) in the differentiation medium; the flattened cell morphology exhibited is similar regardless of TNF exposure in both CSC groups. Scale bars, 50 μm.
Cardiomyogenic gene expression.
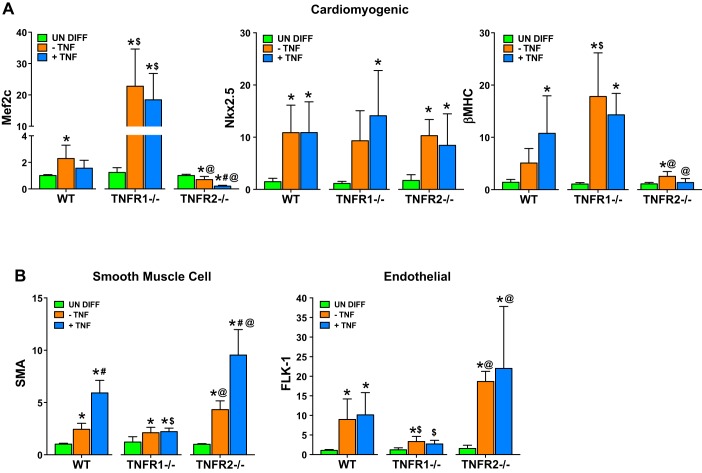
Total RNA isolated from differentiated CSCs and control undifferentiated CSCs was analyzed for expression of the cardiomyocyte genes myocyte enhancer factor 2c (Mef2c), Nkx2.5, and β-myosin heavy chain (β-MHC). WT CSCs differentiated either in the absence or presence of exogenous TNF exhibited significantly augmented cardiomyocyte gene expression, consistent with cardiomyogenic fate specification (Fig. 5A). Differentiated TNFR1−/− CSCs also demonstrated increased cardiomyocyte gene expression, but with significantly greater expression of Mef2c and β-MHC compared with WT CSCs, presumably representing divergent CSC responses to endogenously generated TNF ligand; none of these effects were significantly impacted by TNF. In contrast, differentiated TNFR2−/− CSCs (with unopposed TNFR1 signaling) exhibited Mef2c downregulation, and further suppression of both Mef2c and β-MHC expression with exogenous TNF exposure. There were no differences in Nkx2.5 expression in WT, TNFR1−/−, and TNFR2−/− CSCs, with or without exogenous TNF. These data suggest that TNF restrains cardiomyogenic gene program in CSCs primarily through TNFR1 signaling. Importantly, although differentiated CSCs expressed cardiomyogenic mRNA transcripts, GATA4 and Mef2c protein expression (via immunoblot analysis) was inconsistent and variable (data not shown). Differentiated CSCs also did not exhibit characteristic adult cardiomyocyte morphology or function. Hence, under these in vitro differentiation conditions, CSCs increased cardiomyogenic commitment (as assessed by gene expression and immunostaining), but remained at an immature and nonfunctional stage.
Fig. 5.
Impact of TNF on cell-specific gene programs during CSC differentiation. Real-time PCR analysis for cardiomyogenic (A) and smooth muscle and endothelial (B) gene expression in WT, TNFR1−/−, and TNFR2−/− CSCs after 28 days of CSC differentiation in the absence or presence of TNF (20 ng/ml). Results are from 3–5 independent experiments done in duplicate or triplicate. One-way ANOVA was used to analyze statistical differences within each CSC group, whereas Student's t-test was used to analyze differences specifically between −TNF and +TNF treated CSC subgroups. *P < 0.05 vs. respective undifferentiated (undiff) group; #P < 0.05 vs. respective −TNF group; $P < 0.05 vs. corresponding WT group; @P < 0.05 vs. corresponding TNFR1−/− group.
Smooth muscle and endothelial cell differentiation.
CSCs are reportedly capable of differentiating into endothelial cells, smooth muscle cells (SMCs), and cardiomyocytes (5, 13). Therefore, we also measured SMC (α-smooth muscle actin, SMA) and endothelial cell (Flk1/VEGFR2) genes (44). As shown in Fig. 5B, compared with undifferentiated CSCs, differentiated WT, TNFR1−/−, and TNFR2−/− CSCs all had augmented SMA expression, consistent with SMC differentiation; however, exogenous TNF further increased SMA expression only in WT and TNFR2−/− CSCs, but not in TNFR1−/− CSCs. Differentiated CSCs also exhibited upregulation of Flk-1 gene expression, without significant change in response to exogenous TNF. Importantly, however, Flk-1 expression was markedly reduced in TNFR1−/− CSCs compared with either WT or TNFR2−/− CSCs, suggesting divergent responses to endogenously generated TNF ligand during CSC differentiation. These data indicate that TNFR1 signaling in CSCs, rather than supporting cardiomyogenic fate, instead promotes SMC and endothelial cell commitment.
An alternate neuroadrenergic-like fate of CSCs.
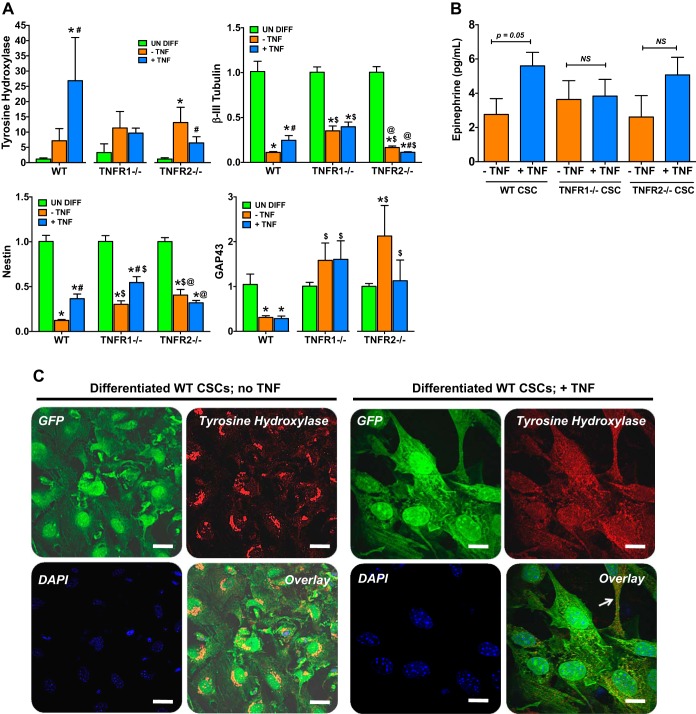
As described above, exogenous TNF exposure during CSC differentiation variably induced morphological changes resembling neuronal cells, with dendritic type cellular projections. Therefore, we evaluated whether TNF induced neuroadrenergic-type features in CSCs. As seen in Fig. 6A, mRNA expression of tyrosine hydroxylase (TH), which catalyzes the rate-limiting step of catecholamine biosynthesis in adrenergic neurons (11), increased in all three CSC groups (and significantly so in TNFR2−/− CSCs) during differentiation. Notably, concomitant exogenous TNF treatment dramatically increased TH expression (∼25-fold over undifferentiated cells) in WT CSCs. This upregulation of TH in response to TNF was prevented in TNFR1−/− CSCs and suppressed in TNFR2−/− CSCs. In contrast, neuronal marker genes—nestin, β-III tubulin, and growth-associated protein 43 (GAP43)—were all significantly downregulated in CSCs during differentiation, with blunting of β-III tubulin and nestin downregulation (but not GAP43) upon TNF treatment. Blunting of β-III tubulin and nestin downregulation during CSC differentiation was also observed in both TNFR1−/− and TNFR2−/− CSCs, with relative upregulation of GAP43, suggesting responses to endogenously generated TNF ligand that promote neuronal gene expression via both TNF receptors. Exogenous TNF treatment induced variable responses in TNFR−/− CSCs: TNF further blunted nestin downregulation in TNFR1−/− CSCs, further suppressed β-III tubulin expression in TNFR2−/− CSCs, and did not significantly change GAP43 upregulation. This variability of response may be a reflection of heterogeneity of CSCs in culture with regard to stages of cell cycle and differentiation.
Fig. 6.
TNF promotes an alternate neuroadrenergic fate in CSCs. A: real-time PCR analysis of neuroadrenergic and neuronal genes in WT, TNFR1−/−, and TNFR2−/− CSCs after 28 days of differentiation in the absence or presence of TNF (20 ng/ml). Results are from 3–5 independent experiments done in duplicate or triplicate. *P < 0.05 vs. respective undifferentiated (undiff) group; #P < 0.05 vs. respective −TNF group; $P < 0.05 vs. corresponding WT group; @P < 0.05 vs. corresponding TNFR1−/− group. B: quantification of epinephrine levels by ELISA in the culture supernatant from differentiated WT, TNFR1−/−, and TNFR2−/− CSCs. Results are from 3 independent experiments. C: representative 60× confocal images of GFP Tg WT CSCs differentiated in vitro for 28 days in the absence or presence of 20 ng/ml TNF, and stained for GFP (green), tyrosine hydroxylase (red), and DAPI (blue). Overlaid images are shown in the bottom right panels of each group. The arrow indicates GFP and tyrosine hydroxylase costaining in a dendritic-type projection from the differentiated CSC. Scale bars, 10 μm.
To complement the gene expression studies, we next indexed CSC biosynthetic capacity via quantitation of secreted epinephrine in culture supernatants. As seen in Fig. 6B, WT CSCs secreted significantly greater amounts of epinephrine upon exogenous TNF exposure during differentiation. TNF-induced augmentation of epinephrine was absent in TNFR1−/− CSCs. In TNFR2−/− CSCs, epinephrine levels tended to increase in response to TNF, but did not reach statistical significance. Figure 6C demonstrates TH immunostaining in WT CSCs differentiated without (left) and with (right) concomitant TNF. CSCs without TNF treatment revealed a localized perinuclear TH distribution; however, in TNF-treated CSCs, the TH staining pattern was diffuse and clearly distributed in the dendritic-type projections (arrow). This diffuse staining pattern was not seen in differentiated TNFR1−/− and TNFR2−/− CSCs exposed to exogenous TNF (data not shown). Taken together, the data suggest that TNF, via signaling from both TNFRs, can modulate CSC differentiation toward an immature neuroadrenergic-like fate capable of producing catecholamines. Hence, resident CSCs may potentially serve as a heretofore-unrecognized source of local cardiac adrenergic activation in diseases with chronic TNF elevation, such as HF.
DISCUSSION
In this study, we established that adult murine CSCs possess a functional TNF signaling axis that impacts several aspects of CSC competence and differentiation (Fig. 7). Specifically: 1) TNF signaling in CSCs is predominantly related to TNFR1 under homeostatic conditions, with TNFR2 inducible after stress; 2) TNFR1 signaling modestly diminishes CSC proliferative capacity, but augments resistance to oxidant stress (along with TNFR2, to a lesser extent); 3) TNF, primarily via TNFR1, inhibits the cardiomyogenic gene program during CSC differentiation, and instead promotes smooth muscle and endothelial cell fates; and 4) TNF, via both TNFR1 and TNFR2, can channel an alternate neuroadrenergic-like fate for CSCs during differentiation. Deficiency of either TNFR1 or TNFR2 did not impact CSC telomerase activity and the propensity to senescence. These findings carry important pathophysiological and translational implications for both stem cell-dependent endogenous cardiac repair and cell therapy in conditions such as myocardial infarction and chronic HF.
Fig. 7.
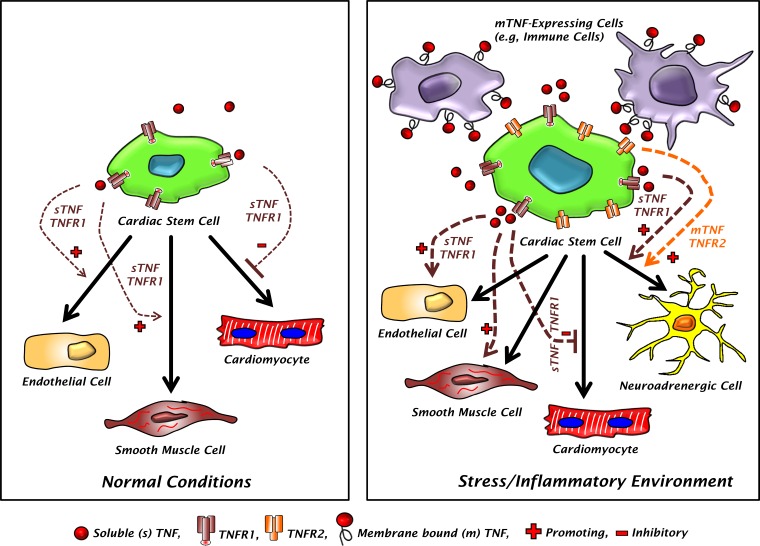
Model of the effects of TNF on CSC cell-fate commitment. CSCs are thought capable of endothelial cell (EC), smooth muscle cell (SMC), and cardiomyocyte differentiation. Under normal baseline conditions (left), CSCs primarily express TNFR1 whereas TNF levels are low and primarily comprise the soluble form (sTNF). The resulting low level of sTNF-TNFR1 interaction results in very modest effects on CSC commitment, inhibiting cardiomyocyte differentiation while promoting EC and SMC fate. In contrast, during inflammatory and stress states (right), CSCs express both TNFR1 and TNFR2, and there are much higher levels of both sTNF and membrane bound TNF (mTNF) on the surface of mTNF-expressing cells. This yields much higher degrees of both sTNF-TNFR1 and mTNF-TNFR2 (and -TNFR1) interaction resulting in much more pronounced inhibition of cardiomyocyte fate, enhancement of EC and SMC fate, and channeling of an alternative CSC neuroadrenergic fate.
TNF mediates its effects through two transmembrane receptors (TNFR1 and TNFR2) that display ligand selectivity: soluble TNF (sTNF) more effectively binds TNFR1, whereas membrane-bound TNF (mTNF) is the primary ligand for TNFR2 (1, 18). While TNFR1 is ubiquitously expressed on all nucleated cells including cardiomyocytes, TNFR2 expression is found primarily on endothelial and immune cells (1). Cardiomyocytes do not constitutively express TNFR2 to a significant degree; however, TNFR2 is induced in response to stress and TNF stimulation (2, 19). Here, we also demonstrated that resident CSCs primarily express TNFR1 at baseline, but that TNFR2 is inducible with mechanical stress and TNF exposure. Hence, under homeostatic conditions, TNF signaling in CSCs would be expected to occur primarily via TNFR1 and sTNF. On the other hand, under pathological conditions of augmented cardiac mechanical load, inflammatory activation, and tissue oxidant stress, such as occurs after MI and during pathological left ventricular (LV) remodeling in HF, TNF signaling may involve both TNFR1 and TNFR2 via both sTNF and mTNF, which may be expressed on infiltrating immune cells. This supports the paradigm of modulation of CSC function by direct interaction between CSCs, TNF, and inflammatory cells in cardiac pathology. Moreover, the two TNFRs directly influence CSC biology: TNFR1 modestly suppresses CSC proliferation, while both TNFR1 and TNFR2 signaling confer resistance to oxidant stress. The latter suggests that the TNFRs activate key prosurvival downstream effector(s) in response to oxidant stress-induced autocrine/paracrine TNF secretion and subsequent receptor ligation. Taken together, these findings implicate levels of inflammatory activation in general, and of TNF in particular, in the heart as critical determinants of resident CSC function.
One paradox regarding the reduced capacity of the heart for endogenous repair is that adverse remodeling occurs despite expansion of c-kit+ CSCs after myocardial infarction (16) and a sustained increase in c-kit+ progenitor abundance in the chronically failing heart (26). These observations suggest that factors in the cardiac microenvironment are inhibiting cardiomyocyte differentiation of these progenitor cells, thereby limiting the efficacy of endogenous cardiac repair. Our results indicate that TNF, primarily via TNFR1, inhibits the cardiomyogenic gene program during in vitro CSC differentiation and instead channels cells toward smooth muscle and endothelial cell fates. Given that heightened inflammation and TNF elaboration characterize both acute MI and chronic HF (19, 20, 29, 34), these findings implicate TNF, TNFR1, and tissue inflammation as potential factors that limit proper and/or efficient endogenous myocardial repair.
Indeed, we (19) and others (25, 32, 36) have established divergent roles for the TNFRs in modulating pathological LV remodeling, with particularly detrimental effects of TNFR1 signaling (and beneficial effects of TNFR2 signaling) in sustaining inflammation and apoptosis in the remodeling heart. The current study extends these results, implicating TNF elaboration and TNFR1-dependent suppression of resident CSC cardiomyocyte differentiation as additional factors exacerbating pathological remodeling, and further suggests that TNFR1-specific inhibition may offer a therapeutic strategy for the augmentation of endogenous cardiac repair. Moreover, clinical trials of exogenous stem cell therapy have necessarily administered progenitor cells into harsh proinflammatory and pro-oxidant tissue microenvironments after myocardial infarction or in chronic HF (31, 37), in which levels of proinflammatory cytokines such as sTNF and infiltration of mTNF-expressing immune cells are excessive. Therefore, our results also raise the consideration that circumscribed inhibition of TNF and/or TNFR1 following stem cell delivery and engraftment may improve the cardiomyogenic potential of these precursors, and thereby lead to more effective long-term cardiac repair and regeneration.
A key and novel finding of our study is the observation that TNF, via both TNFR1 and TNFR2, can promote an alternative neuroadrenergic-like fate in CSCs. This was evidenced by TNF-dependent induction of neuronal-type morphology in CSCs during cell differentiation, with concomitant upregulation of the neuroadrenergic marker TH and epinephrine secretion; all of these effects required the presence of both TNFR1 and TNFR2 on CSCs. An important limitation of our work is that these studies were performed exclusively in vitro, and as such, may not adequately represent the complex cell-level events occurring in vivo during cardiac pathology. Nonetheless, it is of interest that prior work has demonstrated that TNF modulates growth and differentiation of neural progenitor cells (8), and promotes a neural fate in human bone marrow mesenchymal stem cells after prolonged exposure (12).
While requiring further verification and validation in vivo, these new findings in CSCs raise the intriguing possibility of a heretofore-unrecognized source of adrenergic activation in HF. It is well established that heightened activation of the sympathetic nervous system (SNS) promotes LV remodeling in HF (9, 30). Human failing hearts have interstitial concentrations of norepinephrine that exceed levels cytotoxic to cardiac myocytes in culture (9), and exhibit significantly augmented spillover and release of both norepinephrine and epinephrine (14), observations that have been ascribed to increased sympathetic nerve outflow to the heart. Our results suggest that CSCs, pathologically activated by TNF in HF, may potentially serve as a nonneuronal, local myocardial source of catecholamines. We have previously demonstrated that in vivo beta-adrenergic stimulation can induce TNF expression in the failing heart (17, 33, 35). The current study suggests that the reverse relationship may also hold true, and that TNF can induce adrenergic activity via an unappreciated CSC plasticity toward a neuroadrenergic-like fate during conditions of inflammation. In this regard, circumscribed TNF blockade after stem cell therapy may also enhance responses to CSC transplantation by reducing subsequent CSC neuroadrenergic-like differentiation in the heart.
In summary, we have shown that adult murine CSCs possess a functional TNF signaling axis related primarily to TNFR1 at baseline, but to both TNFR1 and TNFR2 under conditions of stress, and that TNF impacts multiple aspects of CSC competence and differentiation. Most notably, TNF inhibits, primarily via TNFR1, the development of a cardiomyogenic gene program during CSC differentiation, and instead upregulates smooth muscle and endothelial cell gene expression. Additionally, via both TNFR1 and TNFR2, TNF promotes a neuroadrenergic-like differentiation fate for CSCs. These findings suggest that a proinflammatory milieu with elevated TNF levels in the heart restrains cardiomyocyte differentiation of resident CSCs and may actually enhance adrenergic activation, both effects that would reduce the effectiveness of endogenous cardiac repair and the response to exogenous stem cell therapy, while promoting adverse cardiac remodeling. These findings require validation in vivo, but suggest that TNF and/or TNFR1 blockade may be useful as a useful therapeutic adjunct for improving responses to CSC cell therapy.
GRANTS
This work was supported by National Institutes of Health Grants HL-78825 (to R. Bolli, S. D. Prabhu, A. Bhatnagar, and S. P. Jones), RR-024489 (to A. Bhatnagar and S. P. Jones), HL-113530 (to R. Bolli), and HL-99014 (to S. D. Prabhu), and a Veterans Affairs Merit Award (to S. D. Prabhu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.H., S.P.J., A.B., R.B., and S.D.P. conception and design of research; T.H., Y.X., M.A.I., and Q.L. performed experiments; T.H., Y.X., M.A.I., Q.L., and S.D.P. analyzed data; T.H., Y.X., M.A.I., Q.L., S.P.J., A.B., R.B., and S.D.P. interpreted results of experiments; T.H., Y.X., M.A.I., and S.D.P. prepared figures; T.H. and S.D.P. drafted manuscript; T.H., Y.X., M.A.I., Q.L., S.P.J., A.B., R.B., and S.D.P. approved final version of manuscript; S.P.J., A.B., R.B., and S.D.P. edited and revised manuscript.
REFERENCES
- 1.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3: 745–756, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Al-Lamki RS, Brookes AP, Wang J, Reid MJ, Parameshwar J, Goddard MJ, Tellides G, Wan T, Min W, Pober JS, Bradley JR. TNF receptors differentially signal and are differentially expressed and regulated in the human heart. Am J Transplant 9: 2679–2696, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angert D, Berretta RM, Kubo H, Zhang H, Chen X, Wang W, Ogorek B, Barbe M, Houser SR. Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circ Res 108: 1226–1237, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA 104: 14068–14073, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 763–776, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med 344: 1750–1757, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science 324: 98–102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. Tumor necrosis factor-α modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells 26: 2361–2371, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Bristow MR. β-Adrenergic receptor blockade in chronic heart failure. Circulation 101: 558–569, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA 102: 3766–3771, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem 91: 1025–1043, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Egea V, von Baumgarten L, Schichor C, Berninger B, Popp T, Neth P, Goldbrunner R, Kienast Y, Winkler F, Jochum M, Ries C. TNF-alpha respecifies human mesenchymal stem cells to a neural fate and promotes migration toward experimental glioma. Cell Death Differ 18: 853–863, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfo M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154: 827–842, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Esler M, Kaye D. Measurement of sympathetic nervous system activity in heart failure: the role of norepinephrine kinetics. Heart Fail Rev 5: 17–25, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation 120: 2077–2087, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fransioli J, Bailey B, Gude NA, Cottage CT, Muraski JA, Emmanuel G, Wu W, Alvarez R, Rubio M, Ottolenghi S, Schaefer E, Sussman MA. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells 26: 1315–1324, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garlie JB, Hamid T, Gu Y, Ismahil MA, Chandrasekar B, Prabhu SD. Tumor necrosis factor receptor 2 signaling limits β-adrenergic receptor-mediated cardiac hypertrophy in vivo. Basic Res Cardiol 106: 1193–1205, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 83: 793–802, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-κB and inflammatory activation. Circulation 119: 1386–1397, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte NF-κB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc Res 89: 129–138, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLos One 9: e96725, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med 13: 970–974, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res 114: 266–282, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keith MC, Bolli R. “String theory” of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circ Res 116: 1216–1230, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishore R, Tkebuchava T, Sasi SP, Silver M, Gilbert HY, Yoon YS, Park HY, Thorne T, Losordo DW, Goukassian DA. Tumor necrosis factor-α signaling via TNFR1/p55 is deleterious whereas TNFR2/p75 signaling is protective in adult infarct myocardium. Adv Exp Med Biol 691: 433–448, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, Margulies KB. Increased cardiac myocyte progenitors in failing human hearts. Circulation 118: 649–657, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Guo Y, Ou Q, Chen N, Wu WJ, Yuan F, O'Brien E, Wang T, Luo L, Hunt GN, Zhu X, Bolli R. Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res Cardiol 106: 849–864, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marban L, Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 125: 100–112, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 91: 988–998, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 111: 2837–2849, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Mohsin S, Siddiqi S, Collins B, Sussman MA. Empowering adult stem cells for myocardial regeneration. Circ Res 109: 1415–1428, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monden Y, Kubota T, Inoue T, Tsutsumi T, Kawano S, Ide T, Tsutsui H, Sunagawa K. Tumor necrosis factor-α is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am J Physiol Heart Circ Physiol 293: H743–H753, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Murray DR, Prabhu SD, Chandrasekar B. Chronic β-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation 101: 2338–2341, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S. Cytokine gene expression after myocardial infarction in rat hearts: possible implication in left ventricular remodeling. Circulation 98: 149–156, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Prabhu SD, Chandrasekar B, Murray DR, Freeman GL. Beta-adrenergic blockade in developing heart failure: effects on myocardial inflammatory cytokines, nitric oxide, and remodeling. Circulation 101: 2103–2109, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Ramani R, Mathier M, Wang P, Gibson G, Togel S, Dawson J, Bauer A, Alber S, Watkins SC, McTiernan CF, Feldman AM. Inhibition of tumor necrosis factor receptor-1-mediated pathways has beneficial effects in a murine model of postischemic remodeling. Am J Physiol Heart Circ Physiol 287: H1369–H1377, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 113: 810–834, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493: 433–436, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith AJ, Lewis FC, Aquila I, Waring CD, Nocera A, Agosti V, Nadal-Ginard B, Torella D, Ellison GM. Isolation and characterization of resident endogenous c-Kit+ cardiac stem cells from the adult mouse and rat heart. Nat Protoc 9: 1662–1681, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Smits AM, van Vliet P, Metz CH, Korfage T, Sluijter JP, Doevendans PA, Goumans MJ. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc 4: 232–243, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 121: 293–305, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Berlo JH, Molkentin JD. An emerging consensus on cardiac regeneration. Nat Med 20: 1386–1393, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, Ford R, Spinale FG, Riggs DW, Srivastava S, Bhatnagar A, Bolli R, Prabhu SD. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation 121: 1912–1925, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 118: 489–498, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Zafir A, Readnower R, Long BW, McCracken J, Aird A, Alvarez A, Cummins TD, Li Q, Hill BG, Bhatnagar A, Prabhu SD, Bolli R, Jones SP. Protein O-GlcNAcylation is a novel cytoprotective signal in cardiac stem cells. Stem Cells 31: 765–775, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]