This study explores the relationship between a novel assessment of vascular health, passive leg movement (PLM), and an established measure, flow-mediated dilation (FMD), to aid in the interpretation and implementation of the novel technique. Overall, positive relationships between PLM and FMD support PLM as a relevant gauge of vascular health.
Keywords: endothelium, nitric oxide, cardiovascular disease, aging
Abstract
The vasodilatory response to passive leg movement (PLM) appears to provide a novel, noninvasive assessment of vascular function. However, PLM has yet to be compared with the established noninvasive assessment of vascular health, flow-mediated dilation (FMD). Therefore, as an initial evaluation of the construct validity of PLM and upright seated and supine PLM as well as brachial (BA) and superficial femoral (SFA) artery FMDs were performed in 10 young (22 ± 1) and 30 old (73 ± 2) subjects. During upright seated PLM, the peak change in leg blood flow (ΔLBF) and leg vascular conductance (ΔLVC) was significantly correlated with BA (r = 0.57 and r = 0.66) and SFA (r = 0.44 and r = 0.41, ΔLBF and ΔLVC, respectively) FMD. Furthermore, although the relationships were not as strong, the supine PLM response was also significantly correlated with BA (r = 0.38 and r = 0.35) and SFA (r = 0.39 and r = 0.35, ΔLBF and ΔLVC, respectively) FMD. Examination of the young and old separately, however, revealed that significant relationships persisted in both groups only for the upright seated PLM response and BA FMD (young: r = 0.73 and r = 0.77; old: r = 0.35 and r = 0.45, ΔLBF and ΔLVC, respectively). Normalizing FMD for shear rate during PLM abrogated all significant relationships between the PLM and FMD response, suggesting a role for nitric oxide (NO) in these associations. Collectively, these data indicate that PLM, particularly upright seated PLM, likely provides an index of vascular health analogous to the traditional FMD test. Given the relative ease of PLM implementation, these data have important positive implications for PLM as a clinical vascular health assessment.
NEW & NOTEWORTHY
This study explores the relationship between a novel assessment of vascular health, passive leg movement (PLM), and an established measure, flow-mediated dilation (FMD), to aid in the interpretation and implementation of the novel technique. Overall, positive relationships between PLM and FMD support PLM as a relevant gauge of vascular health.
the endothelium plays an important role in the regulation of vasomotor tone, as well as the inhibition of platelet aggregation and thrombus formation, primarily by the release of nitric oxide (NO) (20). Therefore, the degradation of endothelial function, largely determined by attenuated NO bioavailability, is an antecedent to overt cardiovascular disease (CVD), and the assessment of endothelial health is important for the evaluation of CVD risk (15, 19, 24, 47). Indeed, invasive assessments, such as intracoronary infusions of vasoactive drugs, have long been recognized as valuable tools to assess vascular health and assess cardiovascular risk (2, 15, 19). However, more recently, the prognostic value of noninvasive assessments of endothelial function, such as flow-mediated dilation (FMD) testing, has gained popularity due to the minimal patient burden as well as the potential feasibility in clinical settings (2, 13, 22, 35, 37).
The FMD test utilizes Doppler ultrasound to measure the change in conduit artery diameter in response to a reactive hyperemia induced by a brief period of vascular occlusion (4, 13). The magnitude of brachial artery (BA) FMD was originally thought to be predominantly NO mediated (17) and has been documented to be related to the change in coronary artery diameter in response to acetylcholine (ACh) administration (2, 21). Although the dominant role of NO has more recently been called into question (10, 46), the BA FMD test appears to be an important, independent predictor of CVD risk (29–31), which may not be contingent on the ability of FMD to assess NO bioavailability (10). In addition, FMD has been utilized extensively in research to demonstrate vascular dysfunction in numerous clinical populations (16, 40), as well as with normal, healthy aging (5, 43). However, the somewhat complex methodology and analysis of the FMD test have precluded widespread clinical adoption.
Passive leg movement (PLM) provides a novel approach to evaluate vascular function by inducing a transient movement-induced hyperemia without the increase in metabolism associated with active exercise (42). In healthy humans, PLM, performed in both the upright seated and supine postures, elicits a brief hyperemic response, and the magnitude of hyperemia is attenuated in populations previously recognized to exhibit endothelial dysfunction (23, 41). Furthermore, the hyperemic and vasodilatory responses to PLM appear to be largely NO mediated, especially in the upright seated as nitric oxide synthase (NOS) inhibition diminishes these responses by up to ∼90% in the young (25, 36, 37). The NO dependence of PLM also appears to depend on posture, with a greater contribution of NO to the hyperemic response (more of a reduction with NOS inhibition) observed in the upright seated posture compared with that observed in the supine posture (12). Of note, in contrast to FMD, PLM does not rely on precise measurements of arterial diameter, as the common femoral artery does not dilate even during active exercise. PLM relies on the relatively simple assessment of the change in blood velocity in the easily imaged femoral artery to determine leg blood flow (LBF) and, when combined with the measurement of mean arterial pressure (MAP), leg vascular conductance (LVC). This reduces the technical requirements of PLM in terms of data collection and analysis. Collectively, the ability of PLM to assess NO bioavailability and vascular health, and the relative ease of implementation, make PLM an attractive clinical option, but the construct validity of PLM has yet to be assessed by a direct comparison with the prognostic FMD test.
Therefore, the purpose of this study was to evaluate the construct validity of PLM as a clinically relevant measure of vascular health by comparing vascular function as assessed by PLM, in the upright seated and supine postures, with that measured by the traditional FMD. As aging can affect vascular function, the relationship between PLM and FMD was evaluated in a combined group of young and old subjects, as well as in the young and old groups alone. We tested the overall hypothesis that vascular function as assessed by PLM and FMD would be related. Furthermore, because of the greater reliance of the upright PLM response on NO (12), we hypothesized that the strongest relationships would be observed between the upright seated PLM response and FMD. Finally, because PLM is evaluated in the leg, and evidence exists for limb-specific differences in vascular function (26, 34, 44, 45), we also sought to determine the potential impact of the anatomical location of the FMD [BA vs. superficial femoral artery (SFA)] on the relationship between the PLM response and FMD.
METHODS
Subjects.
The data presented in this study are from a retrospective analysis of vascular function data from young and older healthy males (to eliminate variance due to sex) recently collected in the Utah Vascular Research Laboratory (UVRL). Specifically, we selected all data in which upright seated and supine PLM, as well as BA and SFA FMD, had been assessed in the same subjects. A total of 40 male subjects, 10 young (<25 yr) and 30 old (>65 yr), individuals were identified according to these criteria. All subjects were healthy and normally active. Subjects diagnosed with cardiovascular or metabolic disease, as well as other potentially confounding conditions such as hypertension or lung disease, were excluded from this analysis. The subject characteristics are documented in Table 1. Protocols were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs (VA) Center and written, informed consent was obtained from all subjects before their participation.
Table 1.
Subject characteristics
| Young | Old | |
|---|---|---|
| Age, yr | 22 ± 1 | 73 ± 2* |
| Height, cm | 177 ± 2 | 176 ± 2 |
| Weight, kg | 76 ± 3 | 74 ± 4 |
| BMI, kg/m2 | 24 ± 1 | 24 ± 1 |
| MAP, mmHg | 86 ± 1 | 94 ± 4* |
| Resting LBF, ml/min | 363 ± 40 | 290 ± 35 |
| Thigh volume, dl | 72 ± 3 | 65 ± 2 |
| Glucose, mg/dl | 70 ± 3 | 78 ± 3 |
| Cholesterol, mg/dl | 169 ± 14 | 186 ± 8 |
| Triglycerides, mg/dl | 91 ± 8 | 78 ± 2 |
| HDL, mg/dl | 48 ± 3 | 53 ± 3 |
| LDL, mg/dl | 116 ± 7 | 115 ± 5 |
| Hemoglobin, g/dl | 15.6 ± 0.3 | 15.4 ± 0.3 |
| BA diameter, mm | 5.82 ± 0.6 | 5.3 ± 0.2 |
| SFA diameter, mm | 8.04 ± 0.8 | 7.4 ± 0.2 |
Data are presented as mean ± SE.
BMI, body mass index; MAP, mean arterial pressure; LBF, leg blood flow; HDL, high-density lipoproteins; LDL, low-density lipoproteins; BA, brachial artery; SFA, superficial femoral artery. *P < 0.05, significantly different from the young.
General procedures.
On the day of each vascular function assessment, subjects reported to the laboratory having fasted for 8 h and were without caffeine for the previous 24 h. In addition, subjects were asked to refrain from any daily antioxidants and/or multivitamins for at least 3 days before data collection and did not participate in any strenuous physical activity for at least 24 h before data collection. Subjects were also refrained from taking any vasoactive prescription medications for 24 h before the vascular assessments. The BA and SFA FMDs were evaluated during one visit and upright seated and supine PLM tests were performed on another, in a counterbalanced fashion. All PLM and FMD testing for a given subject was performed within approximately a 1-mo period.
Flow-mediated dilation.
Details of the FMD procedure have been described previously and were performed in accordance with current recommendations (13). Briefly, subjects rested in the supine posture for ∼20 min before the start of data collection and remained in this position throughout the entire protocol. For the BA FMD, a blood pressure cuff was placed on the right arm, proximal to the elbow, distal to the placement of the ultrasound Doppler probe on the BA. The BA was insonated approximately midway between the antecubital and axillary regions. For SFA FMDs, the cuff was placed ∼15 cm below the inguinal ligament, and the SFA was scanned in the proximal portion of the thigh, at least 5 cm distal to the bifurcation and proximal to the cuff (18, 26, 34). Both BA and SFA diameter and blood velocity (Vmean) measurements were obtained continuously at rest for 30 s before inflation of the blood pressure cuff (to 250 mmHg for 5 min) and for 2 min after cuff deflation (Logiq 7, GE Medical Systems, Milwaukee, WI). Vmean (angle-corrected, and intensity weighted area under the curve) was automatically calculated using commercially available software (Logic 7). End-diastolic, ECG R-wave gated images were collected via video output from the Logiq 7 for blinded offline analysis of BA and SFA vasodilation using automated edge-detection software (Medical Imaging Applications, Coralville, IA). FMD was quantified as the maximal percentage change in BA and SFA diameter after cuff release. Shear rate was calculated as follows: shear rate (s−1) = 8Vmean/arterial diameter. Cumulative area under the curve (AUC) values for shear rate were integrated with the trapezoidal rule and calculated as follows: Σ{yi[x(i+1) − xi] + (1/2)[y(i+1) − yi][x(i+1) − xi]}, where y is shear rate and x is time. Normalized FMD was calculated by dividing FMD (percentage) by the cumulative shear rate area under the curve until the time of peak BA or SFA vasodilation. Reactive hyperemia was also calculated for 2 min following cuff release during the BA FMD and SFA FMD by: Vmeanπ(BA diameter/2)2 × 60, where blood flow is in milliliters per minute, and peak reactive hyperemia following cuff release was determined. BA and SFA FMDs were performed on the same day, in a randomized and counterbalanced order.
Passive leg movement.
PLM was performed as previously described (11, 22, 23, 37). Briefly, subjects rested in the upright seated or supine posture (for upright seated and supine PLM, respectively) for ∼20 min before the start of data collection and remained in this position throughout the PLM assessment in that posture. The PLM protocol consisted of a 1-min baseline data acquisition followed by a 2-min bout of passive leg flexion and extension. Passive exercise was achieved by a member of the research team moving the subject's lower leg through a range of motion, defined by 90 and 180° knee joint angles, at a rate of 1 Hz (while the contralateral leg remained fully extended and supported). Real-time feedback to the investigator was provided by a digital goniometer to ensure a consistent range of motion and a metronome was used to maintain the cadence. Before the start and throughout the protocol subjects were encouraged to remain passive and resist any urge to assist with leg movement. To avoid a startle reflex and active resistance to the passive movement, subjects were made aware that passive movement would take place sometime in the next minute, but, to minimize the chance of an anticipatory response, they were not informed exactly when movement would initiate. In those subjects who were initially unable to remain passive during PLM (<5% of subjects), additional practice PLM trials were employed. For the PLM procedure in general, if an individual is unable to remain passive after additional practice trials, their data are excluded from analysis. The upright seated and supine PLM were performed on the same day, in a randomized and counterbalanced order.
Measurements of arterial blood velocity and vessel diameter were performed during the PLM protocols, with the Logic 7 ultrasound system and a linear array transducer operating at an imaging frequency of 9 MHz. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area. Second-by-second blood velocities were obtained using the same transducer with a Doppler frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel based on real-time ultrasound visualization. Arterial diameter and mean blood velocity (Vmean) was measured and second-by-second LBF in the femoral artery was calculated as: blood flow = Vmeanπ(vessel diameter/2)2 × 60, where blood flow is in milliliters per minute. Beat-by-beat MAP signals were recorded by finger photoplethysmography with a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands) using commercially available data acquisition software (AcqKnowledge, Biopac Systems). LVC was calculated by dividing second-by-second LBF by beat-by-beat MAP for the first 60 s of PLM, and 12-s averages of LBF by the corresponding 12 s averages of MAP for the last 60 s of the PLM bout. ΔLBF and ΔLVC were calculated by subtracting baseline values from the peak (3-s average) LBF and LVC values, respectively, during PLM. In addition, cumulative AUC values for LBF (LBF AUC) and LVC (LVC AUC) were integrated with the trapezoidal rule and calculated as follows: Σ{yi[x(i+1) − xi] + (1/2)[y(i+1) − yi][x(i+1) − xi]}, where y represents LBF or LVC for LBF or LVC AUC, respectively, and x is time, for the first 60 s of PLM. Baseline LBF and LVC are set to 0 for these calculations such that the calculated AUC is the AUC of the PLM response above baseline, to account for differences in resting LBF or LVC. Of note, based on our experience with PLM, ΔLBF and ΔLVC appear to more reproducible then LBF and LVC AUC. Consequently, although both delta peak and AUC values are reported, the focus has been placed on ΔLBF and ΔLVC, and these data are presented in the figures.
Statistical analysis.
Student's independent samples t-tests were used to evaluate the impact of age on the assessed indices of vascular function. Correlations between relevant variables were evaluated using Pearson correlation coefficients. Statistical significance was set at α = 0.05 for all tests. Because these analyses were largely exploratory, this α-value was not corrected for multiple comparisons. All group data are expressed as mean ± SE.
RESULTS
Impact of age on vascular function.
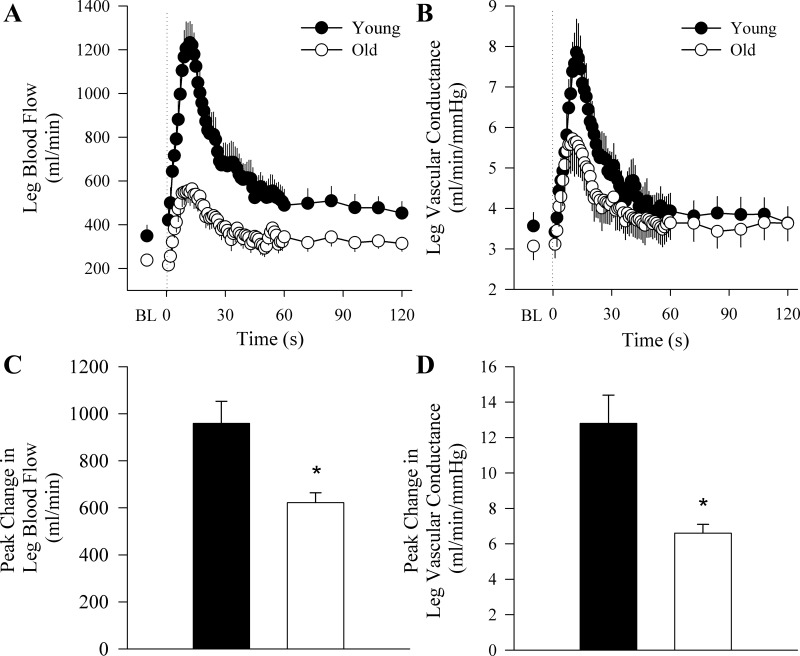
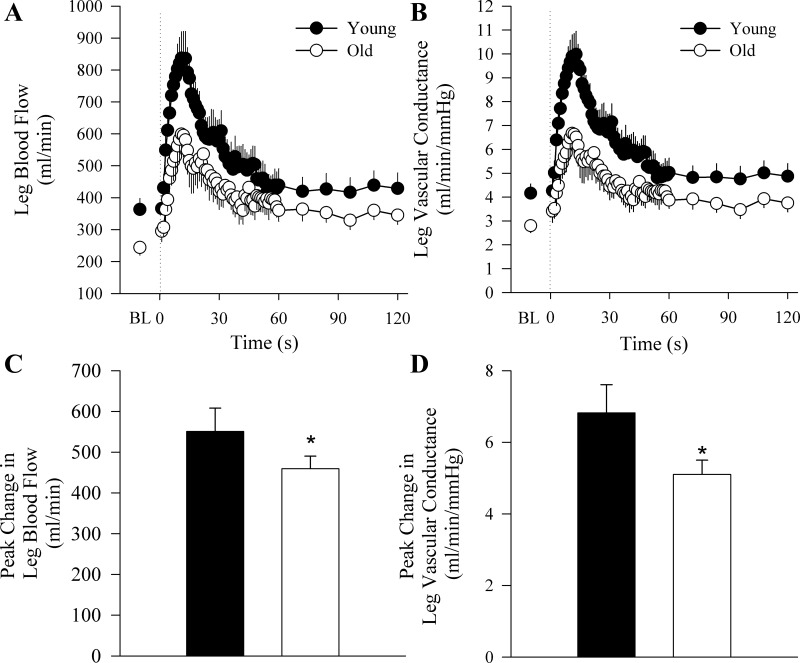
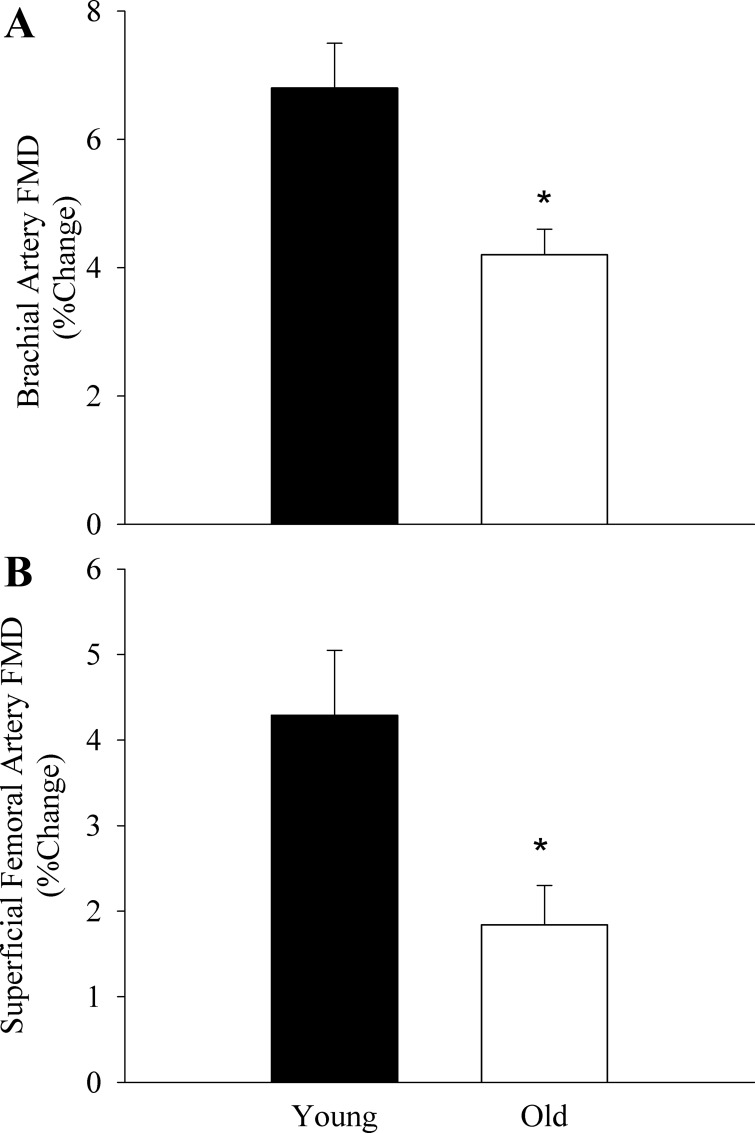
The old subjects exhibited attenuated vascular function as measured by the hyperemic and vasodilatory responses to upright seated (Fig. 1) and supine (Fig. 2) PLM as well as BA and SFA FMD (Fig. 3). When the PLM response was expressed as LBF and LVC AUC, the age-associated differences were still present in the upright seated posture (LBF: 375 ± 52 vs. 231 ± 24 ml/min, P = 0.007; LVC: 4.3 ± 0.9 vs. 2.4 ± 0.3 ml·min−1·mmHg−1, P = 0.007, for young and old, respectively) but not when supine (LBF: 205 ± 34 vs. 160 ± 20 ml/min, P = 0.26; LVC: 2.5 ± 0.9 vs. 1.7 ± 0.2 ml·min−1·mmHg−1, P = 0.1, for young and old, respectively). There were no differences in baseline BA or SFA diameter between young and old subjects (Table 1). Time to peak dilation was not different between young and old subjects for BA FMD (young: 60 ± 8 s, old: 69 ± 6 s, P = 0.4) or SFA FMD (young: 65 ± 12 s, old: 61 ± 7 s, P = 0.8). Furthermore, there were no differences in shear rate between young and old subjects for either BA (young: 26,072 ± 2,774 s−1 vs. old: 24,598 ± 3,193 s−1, P = 0.69) or SFA (young: 16,181 ± 2,623 s−1 vs. old: 12,920 ± 1,953 s−1, P = 0.39) FMD. There were also no differences in FMD normalized for shear rate between young and old subjects for BA (young: 0.25 ± 0.04%/s vs. old: 0.18 ± 0.03%/s, P = 0.09) or SFA FMD (young: 0.24 ± 0.06 vs. old: 0.33 ± 0.12 P = 0.59). Peak reactive hyperemia was attenuated in the old relative to the young during the BA FMD (young: 1,078 ± 239 ml/min vs. old: 625 ± 29 ml/min, P = 0.007) and the SFA FMD (young: 2,436 ± 494 ml/min vs. old: 874 ± 72 ml/min, P = 0.001).
Fig. 1.
Hyperemic response to upright seated passive leg movement (PLM). Group mean data illustrating the second-by-second hyperemic response for the first 60 s and 12-s averages for the following 60 s of PLM, expressed as leg blood flow (LBF; A) and leg vascular conductance (LVC; B) to PLM. The onset of PLM is demarcated by the dotted line. C and D: the peak change from baseline in LBF and LVC, respectively. Data are presented as mean ± SE. *P < 0.05, significantly different from the young.
Fig. 2.
Hyperemic response to supine passive leg movement (PLM). Group mean data illustrating the second-by-second hyperemic response for the first 60- and 12-s averages for the following 60 s of PLM, expressed as leg blood flow (LBF; A) and leg vascular conductance, (LVC; B) to PLM. The onset of PLM is demarcated by the dotted line. C and D: the peak change from baseline in LBF and LVC, respectively. Data are presented as mean ± SE. *P < 0.05, significantly different from the young.
Fig. 3.
Brachial (A) and superficial femoral artery (B) flow-mediated dilation (FMD). Data are expressed as percent change from baseline artery diameter. Data are presented as mean ± SE. *P < 0.05, significantly different from the young.
Upright seated PLM and FMD.
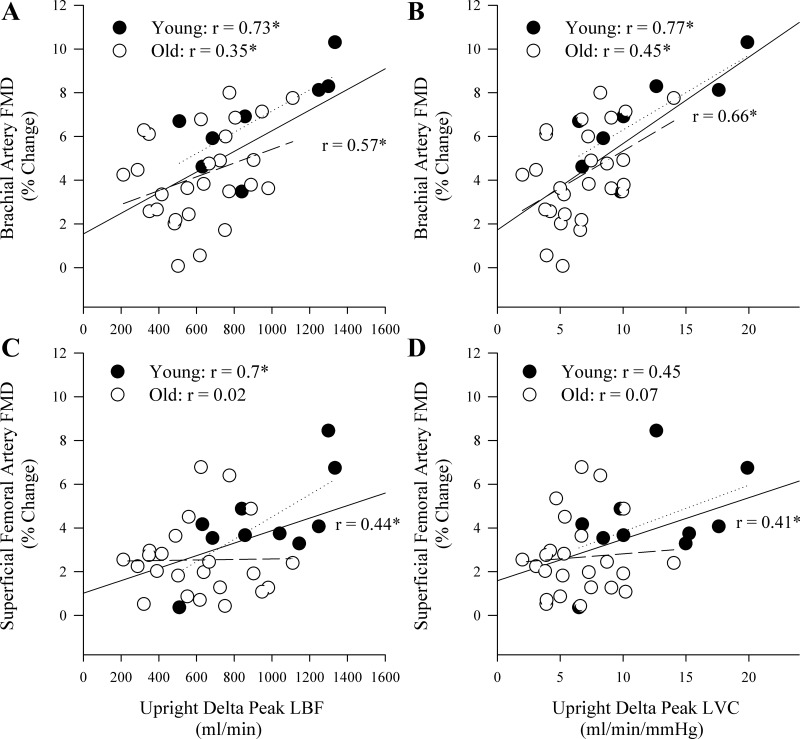
In the upright seated posture, there was a significant positive correlation between the PLM response and BA FMD, when the PLM response was expressed as ΔLBF and ΔLVC (Fig. 4, A and B) as well as LBF (r = 0.55) and LVC (r = 0.63) AUC. Significant positive relationships between ΔLBF and ΔLVC and BA FMD were also observed in both the young and old groups when each was examined in isolation (Fig. 4, A and B). Furthermore, LBF (young: r = 0.61, old: r = 0.41) and LVC (young: r = 0.88, old r = 0.41) AUC were significantly related to BA FMD. The upright seated PLM response was also significantly correlated with SFA FMD when the response was expressed as ΔLBF and ΔLVC (Fig. 4, C and D) as well as LBF (r = 0.38) and LVC (r = 0.33) AUC. When the young and old were examined separately, however, the only relationships that remained significant were those in the young group (Fig. 4, C and D). There were no significant relationships between the upright seated PLM response and peak reactive hyperemia during the BA FMD in the whole group (r = 0.2 and 0.3 for LBF and LVC, respectively), the young group (r = 0.12 and 0.17, for LBF and LVC, respectively), or the old group (r = 0.004 and 0.14, for LBF and LVC, respectively). The upright seated PLM response was significantly correlated with peak reactive hyperemia during the SFA FMD in the whole group (r = 0.39 and 0.49 for LBF and LVC, respectively), but not the young group (r = 0.09 and 0.22, for LBF and LVC, respectively), or the old group (r = 0.07 and 0.1, for LBF and LVC, respectively).
Fig. 4.
Correlations between the hyperemic response to upright seated passive leg movement (PLM) and flow-mediated dilation (FMD) assessed in the brachial and superficial femoral arteries. A and B: the relationships between the peak change in leg blood flow (LBF) and leg vascular conductance (LVC), respectively, during upright seated PLM and brachial artery FMD. C and D: the relationships between the peak change in LBF and LVC, respectively, during upright seated PLM and superficial femoral artery FMD. The solid lines represent the correlation for the whole group, the dotted lines represent the young group, and the dashed lines represent the old group. *P < 0.05.
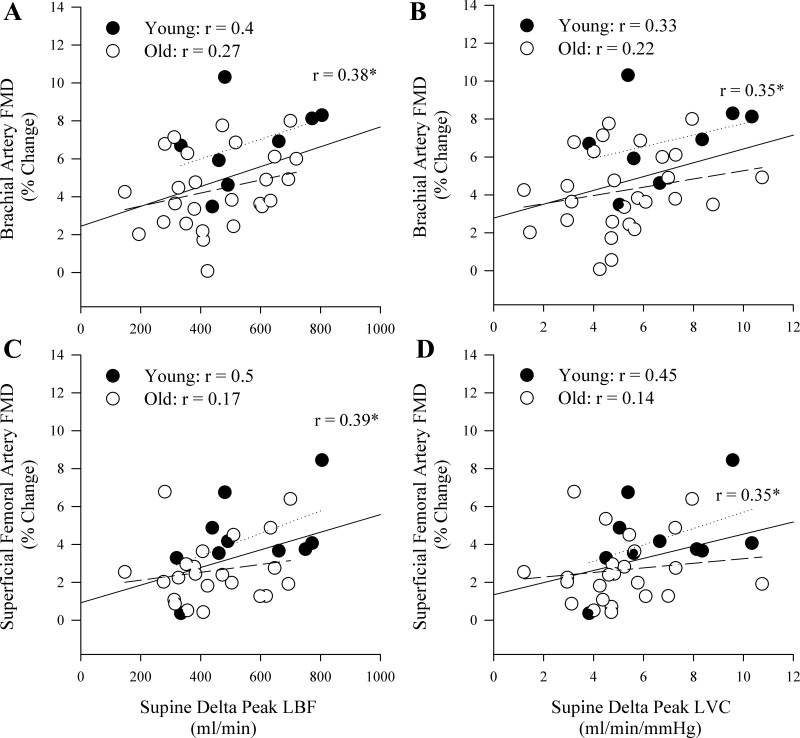
Supine PLM and FMD.
There was a significant correlation between the supine PLM response and BA FMD when the supine PLM response was expressed as ΔLBF and ΔLVC (Fig. 5, A and B) but not as LBF (r = 0.02) or LVC (r = 0.05) AUC. In addition, although there was evidence of positive relationships between BA FMD and the supine PLM response in the young and old groups examined in isolation, these relationships were not significant (Fig. 5, A and B). In the whole group, there was a significant positive relationship between the supine PLM response and SFA FMD when the PLM response was expressed as ΔLBF and ΔLVC (Fig. 5, C and D). However, the relationships between supine PLM and SFA FMD were not significant when the PLM response was expressed as LBF (r = 0.14) or LVC (r = 0.17) AUC. Furthermore, none of these relationships were significant in the young or old groups when examined separately (Fig. 5). There were no significant relationships between the supine PLM response and peak reactive hyperemia during the BA FMD in the whole group (r = 0.05 and 0.004 for LBF and LVC, respectively), the young group (r = 0.003 and 0.04, for LBF and LVC, respectively), or the old group (r = 0.08 and 0.17, for LBF and LVC, respectively). There were no significant relationships between the supine PLM response and peak reactive hyperemia during the SFA FMD in the whole group (r = 0.22 and 0.16 for LBF and LVC, respectively), the young group (r = 0.1 and 0.11, for LBF and LVC, respectively), or the old group (r = 0.17 and 0.12, for LBF and LVC, respectively) alone.
Fig. 5.
Correlations between the hyperemic response to supine passive leg movement (PLM) and flow-mediated dilation (FMD) assessed in the brachial and superficial femoral arteries. A and B: the relationships between the peak change in leg blood flow (LBF) and leg vascular conductance (LVC), respectively, during supine PLM and brachial artery FMD. C and D: the relationships between the peak change in LBF and LVC, respectively, during supine PLM and superficial femoral artery FMD. The solid lines represent the correlation for the whole group, the dotted lines represent the young group, and the dashed lines represent the old group. *P < 0.05.
The effect of normalizing FMD for shear.
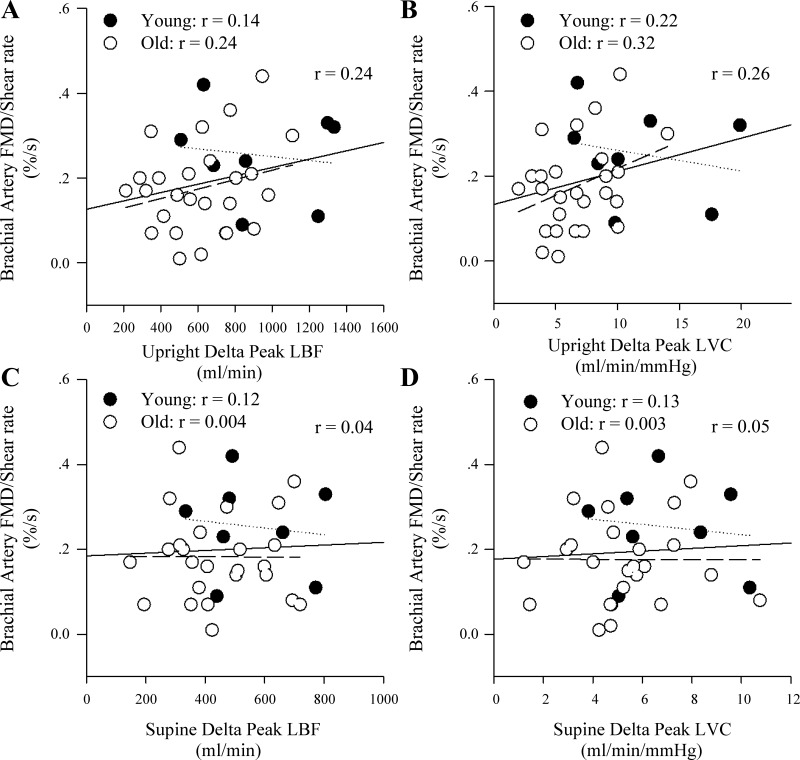
Normalization of FMD reduced the strength of all relationships between the indexes of upright seated and supine PLM-induced hyperemia and vasodilation for both BA (Fig. 6, A–D) and SFA (r = 0.05–0.2) FMD such that all variables were no longer significantly related.
Fig. 6.
Correlations between the hyperemic response to upright seated and supine passive leg movement (PLM) and flow-mediated dilation (FMD) assessed in the brachial artery normalized for shear rate. A and B: the relationships between the peak change in leg blood flow (LBF) and leg vascular conductance (LVC), respectively, during upright seated PLM and brachial artery FMD normalized for shear rate. C and D: the relationships between the peak change in LBF and LVC, respectively, during supine PLM and brachial artery FMD normalized for shear rate. The solid lines represent the correlation for the whole group, the dotted lines represent the young group, and the dashed lines represent the old group.
DISCUSSION
This study sought to perform an initial evaluation of the construct validity of PLM as a clinically relevant assessment of vascular health. As FMD has been determined to be predictive of CVD risk, this was achieved by comparing vascular function as assessed by PLM, in the upright seated and supine postures, with the traditional FMD in a combined group of young and old subjects, as well as in the young and old groups alone. In the current subjects, both the PLM and FMD assessments revealed the anticipated age-related reduction in vascular function and, in the whole group, there were significant relationships between the upright seated and supine PLM responses and both BA and SFA FMD. The strongest relationship for the whole group, however, was observed between the upright seated PLM response, documented to be the most NO dependent of the two postures (12), and BA FMD. Furthermore, the relationships between the PLM response in the upright seated posture and BA FMD were the only relationships to remain significant when evaluated in the young and old groups alone. In addition, normalizing FMD for shear rate, and thus, essentially, accounting for the NO stimulus for the FMD response, abrogated all of the previously significant relationships between ΔLBF and ΔLVC during PLM and FMD, emphasizing the potential importance of NO in these associations. Collectively, these data indicate that PLM, particularly upright seated PLM, likely provides a prognostic index of vascular health analogous to the traditional FMD test. Given the relative ease of PLM implementation compared with FMD testing, these data have important implications for the potential clinical use of PLM to assess vascular health.
PLM and BA FMD.
The change in BA diameter evoked by the BA FMD test has been documented to be related to the change in coronary artery diameter following intracoronary ACh infusion (2, 21). These data have led to the contention that BA FMD can be used to assess systemic vascular function, and more importantly, BA FMD can be used to noninvasively assess coronary vascular health and gauge CVD risk (10, 31, 39). Consequently, the BA FMD test has been used extensively to determine age- and disease-related changes in vascular function and is arguably the most widely utilized assessment of endothelial-dependent dilation in humans (4, 5, 16, 41, 43). Thus comparing the PLM response with the more traditional BA FMD has important implications for advancing the clinical relevance of PLM. This study revealed that the hyperemic responses to both upright seated and supine PLM were significantly correlated with BA FMD (Figs. 4, A and B, and 5, A and B). These data suggest that the magnitude of the hyperemic response to PLM, whether assessed as LBF or LVC, likely reflects systemic vascular health and therefore potentially provides a global index of CVD risk analogous to BA FMD. Of importance, the LBF response to PLM is obtained by measuring blood velocity in the easily imaged common femoral artery, which does not dilate during exercise, whereas the FMD test relies upon the measurement of small changes in arterial diameter, and therefore requires high-resolution imaging by a skilled sonographer and specific analysis software. Therefore, although an additional investigator is required to move the leg and the subject must be able to remain relaxed, the PLM test may provide prognostic information regarding vascular health and CVD risk, while forgoing many of the technical requirements that have likely precluded the clinical adoption of FMD testing.
NO, BA FMD, and PLM posture.
The popularity of the BA FMD test is largely due to assertion that FMD serves as a “bioassay” for NO. Indeed, although the exact contribution of NO to BA FMD is debatable (9, 38, 46), the preponderance of evidence suggests that when a BA FMD test is performed following current guidelines (13) (e.g., cuff distal to the probe, 5 min of occlusion, etc.), NO likely does play a significant role, with estimates of BA FMD NO dependence ranging from 30 to 90% (10, 46). The hyperemic and vasodilatory responses to PLM also appear to be largely NO mediated, as NOS inhibition diminishes these responses by up to ∼90% (25, 36, 37). However, the contribution of NO to the hyperemic response appears to depend on posture (37). Specifically, Groot et al. (12) utilized NOS inhibition during both upright seated and supine PLM and determined that NO was responsible for ∼50% of the ΔLBF and ΔLVC while in the upright seated posture but only ∼30% of the ΔLBF and ΔLVC in the supine posture. Furthermore, Mortensen et al. (25) observed a positive relationship between the hyperemic response to ACh infusion in the leg and the upright seated PLM response (r = 0.75 and 0.83 for LBF and LVC AUC, respectively). The upright posture increases perfusion pressure in the femoral artery, the driving force for LBF, which likely augments LBF and thus NO release by the endothelium and NO-dependent vasodilation (12). Thus NO is an important component of the response to both the upright seated and supine PLM response, but NO appears to play a greater role during upright seated PLM.
Interestingly, while there were significant positive relationships between the PLM response in both postures and BA FMD in the current study, the relationships were strongest during upright seated PLM (Figs. 4, A and B, and 5, A and B). In addition, when the relationships were evaluated in young and old subjects alone, the only relationships that remained significant in both the young and old groups were those between the PLM response in the upright seated posture and BA FMD (Fig. 4, A and B). These findings were likely influenced by the relative role of NO during upright seated vs. supine PLM. Specifically, the greater reliance on NO for vasodilation in the upright posture during PLM (12) likely enhances the fidelity of PLM to determine differences in NO-dependent vascular function.
The finding that normalizing BA FMD for shear rate attenuated the strength of the relationships between PLM and FMD such that they were no longer significant (Fig. 6, A–D) also bolsters the concept that NO bioavailability is a mediator of the relationship between the PLM response and BA FMD. Specifically, increased shear stress on the endothelium following cuff release is considered to be a prominent stimulus for NO release and subsequent endothelium-dependent dilation during the FMD test (13). Thus normalization of the change in arterial diameter during the FMD test to shear rate, although certainly not perfect (3, 6, 14, 33), represents a method by which to account for differences in dilatory stimulus and potentially NO release by the endothelium. Therefore, comparing FMD normalized for shear stress, and thus essentially removing the stimulus for NO release during FMD testing, with the PLM response (Fig. 6, A–D), represents a method to evaluate the role of NO as a mediator of the relationship between the two assessments of vascular function. Collectively, these data lend credence to the role of NO as a mediator of the relationship between PLM and FMD and support the concept that the upright seated PLM response provides a more sensitive index of NO bioavailability (12). Thus, compared with supine PLM, the greater contribution of NO to the upright seated PLM response and the more robust relationships between the upright seated PLM response and BA FMD in the whole group, as well as in the young and old examined separately, support upright seated PLM as the most informative PLM approach to gauge vascular function and possibly cardiovascular health.
PLM and SFA FMD.
While BA FMD has been suggested to provide an indication of systemic NO bioavailability and vascular health (2), and therefore potentially be reflective of vascular function in other vascular beds, there is accumulating evidence of heterogeneous vascular function in the arms and legs of humans (26, 34, 44, 45). Additionally, the lower limbs may be at a greater predisposition for age-related decrements in vascular function (27). Thus FMD was performed in the SFA in addition to the BA in this study to investigate the potential influence of limb-specific differences in vascular function on the information acquired from PLM. Interestingly, SFA FMD was significantly correlated with BA FMD (r = 0.47) as well as the PLM response in the upright seated and supine postures (Figs. 4, C and D, and 5, C and D). Somewhat surprisingly, these data potentially suggest homogenous vascular function in the upper and lower limbs, and the ability to extrapolate information regarding systemic vascular health from assessments in different vascular beds. However, it should be noted that the strength of the relationship between the same test (FMD) performed in two anatomical locations (BA and SFA) suggests that only ∼22% of the variation in one test is explained by the other. Furthermore, the relationships between the PLM response and SFA FMD did not persist in the young and old groups examined alone, apart from in the young during upright PLM (Figs. 4, C and D, and 5, C and D). Thus it is difficult to arrive at a definitive conclusion regarding homogenous or heterogeneous vascular function across limbs from these data. Regardless, it should be highlighted that PLM is performed in the lower limb and is able to determine age-related differences in vascular function in this important vascular bed (Figs. 1 and 2). Additionally, the data from the current study suggest that the PLM response is reflective of systemic vascular health, as the PLM response was most robustly related to BA FMD, which, in contrast to the SFA FMD, has been documented to be predictive of CVD risk.
Experimental considerations.
The intent of the current study was to further the clinical utility of PLM to assess vascular health. As such, we compared the PLM response with the results of FMD testing, which is a clearly established and widely used technique to assess vascular function. However, as discussed by Flammer et al. (8), to truly establish a clinically relevant assessment of vascular health, whether the test can improve CVD and mortality risk stratification, and whether the response to the test reflects coronary endothelial function, among other factors, must be determined. In addition, the role of NO in the FMD response is certainly debatable (9, 38) and the FMD test is predominantly an assessment of conduit artery function, whereas the PLM response is largely determined by dilation of resistance vessels distal to the common femoral artery. Of note, the PLM response in the upright seated and supine postures was also compared with peak reactive hyperemia following cuff release during the FMD tests, which, for the BA FMD, has also been shown to predict CV events (1, 28), and might be expected to more closely reflect the PLM response given that peak reactive hyperemia and the PLM response are both largely determined by dilation of resistance vessels (vs. conduit arteries). However, the only significant relationships that were observed were between the upright PLM response, expressed as LBF and LVC, and peak reactive hyperemia during the SFA FMD when the entire group was examined. The relatively less robust relationship between PLM and reactive hyperemia may be explained by the modest role of NO in postocclusion reactive hyperemia (7, 18, 32, 46). Therefore, overall, the data for PLM in relation to FMD presented in the current study should be considered only as initial evidence for the construct validity of PLM as an assessment of vascular health and comparison of the PLM response to other more NO-dependent assessments of vascular function and/or comparison to other assessments of resistance vessel function are important. It may also be advantageous to use electromyogram recordings to ensure quiescent leg muscles during the PLM test, but it must also be recognized that even passive movement can result in an increase in the EMG signal, albeit this may be predominantly noise, making such an approach practically less useful. Future studies should include both men and women to increase the generalizability of the findings. However, the current, initial findings indicate that larger clinical trials to assess the capability of PLM to improve CVD risk assessment and reflect coronary health are warranted.
Conclusion and clinical implications.
Collectively, these data suggest that PLM, particularly when performed in the upright seated posture, provides an index of vascular function analogous to the traditional FMD test. It should be emphasized that similar relationships between the PLM response and FMD were observed for LBF and LVC. Therefore, the relatively simple measurement of the change in blood velocity in the common femoral artery during upright seated PLM to determine LBF, even without assessing changes in MAP, likely reflects systemic vascular health and NO bioavailability. These findings have important implications for the clinical relevance of PLM and the potential use of this method to assess CVD risk with aging and disease.
GRANTS
The study was supported by National Heart, Lung, and Blood Institute Grants P01-HL-09830, HL-103786, and HL-116579 and VA Merit Grant E6910R.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.J.R., M.A.H.W., and R.S.R. conception and design of research; M.J.R., H.J.G., and R.S.G. performed experiments; M.J.R., H.J.G., and R.S.G. analyzed data; M.J.R., H.J.G., M.A.H.W., and R.S.R. interpreted results of experiments; M.J.R. prepared Figs.; M.J.R. drafted manuscript; M.J.R., H.J.G., R.S.G., M.A.H.W., and R.S.R. edited and revised manuscript; M.J.R., H.J.G., R.S.G., M.A.H.W., and R.S.R. approved final version of manuscript.
REFERENCES
- 1.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation 123: 163–169, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Austin C. Commentaries on viewpoint: pick your Poiseuille: normalizing the shear stimulus in studies of flow-mediated dilation. J Appl Physiol 107: 1361; author reply 1366, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. [DOI] [PubMed] [Google Scholar]
- 6.de Groot P, Hopman MT. Commentaries on viewpoint: pick your Poiseuille: normalizing the shear stimulus in studies of flow-mediated dilation. J Appl Physiol 107: 1363; author reply 1366, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol 81: 1807–1814, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation 126: 753–767, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green D. Point: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1233–1234; discussion 1237–1238, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Richardson RS. Perfusion pressure and movement-induced hyperemia: evidence of limited vascular function and vasodilatory reserve with age. Am J Physiol Heart Circ Physiol 304: H610–H619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groot JH, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE, Bledsoe A, Richardson RS. The role of nitric oxide in passive leg movement-induced vasodilation with age: Insight from alterations in femoral perfusion pressure. J Physiol 593: 3917–3928, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayman MA, Wray DW, Richardson RS. Commentaries on viewpoint: pick your Poiseuille: normalizing the shear stimulus in studies of flow-mediated dilation. J Appl Physiol 107: 1363–1364; author reply 1366, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Ives SJ, Harris RA, Witman MA, Fjeldstad AS, Garten RS, McDaniel J, Wray DW, Richardson RS. Vascular dysfunction and chronic obstructive pulmonary disease: the role of redox balance. Hypertension 63: 459–467, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 586: 1137–1145, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind L, Berglund L, Larsson A, Sundstrom J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation 123: 1545–1551, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd-Jones DM, Bloch KD. The vascular biology of nitric oxide and its role in atherogenesis. Annu Rev Med 47: 365–375, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo S, Matsumoto T, Takashima H, Ohira N, Yamane T, Yasuda Y, Tarutani Y, Horie M. The relationship between flow-mediated brachial artery vasodilation and coronary vasomotor responses to bradykinin: comparison with those to acetylcholine. J Cardiovasc Pharmacol 44: 164–170, 2004. [DOI] [PubMed] [Google Scholar]
- 22.McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol 108: 76–84, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest 127: 2254–2263, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol 590: 4391–4400, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol 103: 843–851, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol 105: 1661–1670, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Philpott AC, Lonn E, Title LM, Verma S, Buithieu J, Charbonneau F, Anderson TJ. Comparison of new measures of vascular function to flow mediated dilatation as a measure of cardiovascular risk factors. Am J Cardiol 103: 1610–1615, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 51: 997–1002, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 134: 52–58, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Shechter M, Shechter A, Koren-Morag N, Feinberg MS, Hiersch L. Usefulness of brachial artery flow-mediated dilation to predict long-term cardiovascular events in subjects without heart disease. Am J Cardiol 113: 162–167, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Tagawa T, Imaizumi T, Endo T, Shiramoto M, Harasawa Y, Takeshita A. Role of nitric oxide in reactive hyperemia in human forearm vessels. Circulation 90: 2285–2290, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka H, DeVan AE, Barnes JN. Commentaries on viewpoint: pick your Poiseuille: normalizing the shear stimulus in studies of flow-mediated dilation. J Appl Physiol 107: 1361–1362; author reply 1366, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Thijssen DH, Rowley N, Padilla J, Simmons GH, Laughlin MH, Whyte G, Cable NT, Green DJ. Relationship between upper and lower limb conduit artery vasodilator function in humans. J Appl Physiol 111: 244–250, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tousoulis D, Antoniades C, Stefanadis C. Evaluating endothelial function in humans: a guide to invasive and non-invasive techniques. Heart 91: 553–558, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Morgan DE, Gmelch BS, Bledsoe A, Richardson RS. Passive leg movement and nitric oxide-mediated vascular function: the impact of age. Am J Physiol Heart Circ Physiol 308: H672–H679, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tschakovsky ME, Pyke KE. Counterpoint: Flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1235–1237; discussion 1237–1238, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Vita JA, Keaney JF Jr. Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Witman MA, Fjeldstad AS, McDaniel J, Ives SJ, Zhao J, Barrett-O'Keefe Z, Nativi JN, Stehlik J, Wray DW, Richardson RS. Vascular function and the role of oxidative stress in heart failure, heart transplant, and beyond. Hypertension 60: 659–668, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witman MA, Ives SJ, Trinity JD, Groot HJ, Stehlik J, Richardson RS. Heart failure and movement-induced hemodynamics: partitioning the impact of central and peripheral dysfunction. Int J Cardiol 178C: 232–238, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053–1060, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O'Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wray DW, Richardson RS. Aging, exercise, and limb vascular heterogeneity in humans. Med Sci Sports Exerc 38: 1804–1810, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Heterogeneous limb vascular responsiveness to shear stimuli during dynamic exercise in humans. J Appl Physiol 99: 81–86, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Wray DW, Witman MA, Ives SJ, McDaniel J, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Does brachial artery flow-mediated vasodilation provide a bioassay for NO? Hypertension 62: 345–351, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007. [DOI] [PubMed] [Google Scholar]