This study is the first to demonstrate that loss of a muscle-specific chromatin-binding protein, Smyd1, is sufficient to induce cardiac hypertrophy and failure. Moreover, the findings demonstrate that augmentation of Smyd1 levels can block hypertrophy in cell models. These studies may support novel strategies for cardiac-targeted epigenetic therapy.
Keywords: Smyd1, epigenetics, heart failure, cardiac hypertrophy, histone methyltransferase
Abstract
All terminally differentiated organs face two challenges, maintaining their cellular identity and restricting organ size. The molecular mechanisms responsible for these decisions are of critical importance to organismal development, and perturbations in their normal balance can lead to disease. A hallmark of heart failure, a condition affecting millions of people worldwide, is hypertrophic growth of cardiomyocytes. The various forms of heart failure in human and animal models share conserved transcriptome remodeling events that lead to expression of genes normally silenced in the healthy adult heart. However, the chromatin remodeling events that maintain cell and organ size are incompletely understood; insights into these mechanisms could provide new targets for heart failure therapy. Using a quantitative proteomics approach to identify muscle-specific chromatin regulators in a mouse model of hypertrophy and heart failure, we identified upregulation of the histone methyltransferase Smyd1 during disease. Inducible loss-of-function studies in vivo demonstrate that Smyd1 is responsible for restricting growth in the adult heart, with its absence leading to cellular hypertrophy, organ remodeling, and fulminate heart failure. Molecular studies reveal Smyd1 to be a muscle-specific regulator of gene expression and indicate that Smyd1 modulates expression of gene isoforms whose expression is associated with cardiac pathology. Importantly, activation of Smyd1 can prevent pathological cell growth. These findings have basic implications for our understanding of cardiac pathologies and open new avenues to the treatment of cardiac hypertrophy and failure by modulating Smyd1.
NEW & NOTEWORTHY
This study is the first to demonstrate that loss of a muscle-specific chromatin-binding protein, Smyd1, is sufficient to induce cardiac hypertrophy and failure. Moreover, the findings demonstrate that augmentation of Smyd1 levels can block hypertrophy in cell models. These studies may support novel strategies for cardiac-targeted epigenetic therapy.
heart failure is a particularly nefarious result of many different forms of cardiovascular disease and has a massive human health burden; >5 million Americans have heart failure (a number projected to increase to >8 million by 2030) with ∼825,000 new cases annually. Diabetes mellitus, hypertension, heart attacks, and atherosclerosis all predispose patients to heart failure, and, although pharmacological treatments are available, outcomes are dismal with a 50% mortality rate 5 yr after diagnosis (13). Clearly, novel approaches for treatment of heart failure are desperately needed.
During development, the mammalian heart expands the cardiac myocyte pool through hyperplasia. Soon after birth, however, myocytes (the contractile unit of the heart) undergo mitosis without cytokinesis and then exit the cell cycle (39). Like most terminally differentiated cells, adult cardiac myocytes lack the ability to proliferate and, in response to environmental stress, adapt via hypertrophic growth (9, 47). This hypertrophic response can be initially beneficial to maintain the workload of the heart following injuries such as myocardial infarction, but animal models and human studies have consistently shown that hypertrophic growth in the heart is a precursor to cellular and organ failure (18). It has been well established that heart failure involves activation of a transcriptional network that is conserved across animal models and humans. Specifically, genes associated with normal muscle development, but silenced in the healthy adult heart, become reactivated (40). Development and disease are regulated by a host of myocyte-specific transcription factors that regulate cardiac genes (37, 44). Manipulating this network, therefore, has significant therapeutic potential; however, the molecular entities regulating these genomic targets at the level of chromatin have been elusive.
Recent studies have provided new insights into the epigenetic cues that determine distinct developmental transitions from pluripotency to mature cardiac myocyte (38, 49), but the landscape in the adult heart is more complex. Previous studies have demonstrated that histone deacetylases play a powerful role in controlling cardiac growth (36, 52). Subsequent studies on chromatin-modifying enzymes have implicated specific classes and modifications of histone deacetylases (3, 23), acetyltransferases (50), chromatin-remodeling complexes (15), and histone mark readers (4) in heart failure. A fundamental gap in our understanding of the mammalian heart relates to how cardiac phenotype is established and maintained according to cardiac myocyte-specific chromatin modifiers. The absence of such insights also limits therapeutic targeting of cardiac chromatin, insofar as the heart is comprised of many distinct cell types, most if not all of which express the epigenetic modifiers explored in these previous studies.
To identify chromatin modifiers responsible for maintenance of the adult cardiac myocyte transcriptome (and phenotype), we used quantitative proteomics to measure proteins associated with chromatin in the mouse heart and to detect changes occurring following heart failure induced by pressure overload. We then filtered the proteins according to cell type specificity and alteration with disease, examining only those that increased with heart failure. Among proteins in this group, only one was muscle specific, the SET and MYND domain containing histone methyltransferase, Smyd1. Previous studies on Smyd1 (also known as Bop) have demonstrated its role in early cardiac development (14), but its role in the adult heart was unknown. We generated mice with Smyd1 specifically deleted in an inducible manner in the adult cardiac myocyte. These animals develop heart failure following activation of a gene expression profile normally repressed by Smyd1 and associated with cardiac remodeling and dysfunction. We demonstrate that Smyd1 acts in part via transcriptional repression and can prevent cardiac hypertrophy at the cellular level. These findings implicate Smyd1 histone methyltransferase as a novel muscle-specific molecular target for heart failure.
METHODS
Cardiac-specific inducible Smyd1 knockout mouse.
All protocols involving animals conform to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UCLA Animal Research Committee. The endogenous Smyd1 sequence was obtained from a Lambda FixII Vector 129SV Mouse Genomic Library (Stratagene). LoxP sites were introduced, flanking the second and third exons of smyd1 followed by a neomycin cassette flanked by a third LoxP site, using a targeting construct designed in the Osdupdel vector (gift from William A. Kuziel). This targeting vector was electroporated into 129S6 embryonic stem (ES) cells, and surviving clones, selected with gancyclovir and G418, were screened for homologous recombination by Southern analysis by digestion with either Bgl II and Sal I or Bgl II and Kpn I and hybridization with probe 2 and probe 3, respectively. ES clones showing correct targeting in both arms were injected into C57BL/6 blastocysts to create chimeric mice, which were then mated to C57BL/6 females to create a germline knockin (Smyd1KI). Smyd1KI mice, B6.129 hybrids, were crossed to ubiquitously expressing EIIa-Cre mice, B6.FVB hybrids, and progeny were screened for deletion of the neomycin cassette by Southern analysis and backcrossed to remove EIIa-Cre. Resulting Smyd1flox mice, lacking EIIa-Cre, were crossed with α-MHC-MerCreMer mice purchased from Jackson Laboratory (cat. no. 005657). To induce recombination, mice were fed a diet containing tamoxifen, 0.4 mg/g of chow diet (Harlan, cat. no. TD.07262). All mice used in this study were males 8–10 wk of age upon beginning tamoxifen chow feeding. To examine DNA synthesis and cell cycle progression, mice were injected with three doses of 5-bromo-1-(2-deoxy-β-d-ribofuranosyl) uracil (BrdU; 50 mg/kg body wt) at 12-h intervals and killed 2–4 h after the last injection.
Echocardiography.
Echocardiography (ECHO) was performed on a Vevo 2100 (Visual Sonics) to determine cardiac parameters in live mice as described (11, 32), including the following indices: left ventricular size (end-diastolic and end-systolic dimension), wall thickness (intraventricular septum and posterior wall thickness), ventricular mass, and ejection fraction.
Histology.
At the time of death, mice were anesthetized, and the heart was arrested in diastole with an intracardiac injection of 2,3-butanedione monoxime (10 mM). Whole hearts were rapidly excised from the animals fixed in 4% paraformaldehyde and then embedded in paraffin. Hearts were then sectioned at 4 μm and placed on slides. Small intestine was also harvested as a control for BrdU incorporation studies, as this tissue has a high cellular turnover rate.
Heart sections were incubated with Texas red-X-conjugated wheat germ agglutinin (Invitrogen, 1:100) for 90 min and assessed for cell size. Hematoxylin and eosin (H and E) staining and Masson's trichrome (Sigma) staining were performed according to the manufacturer's protocols. Tissue sections were incubated with primary antibodies to BrdU (ab6326, Abcam; secondary: A11006, Invitrogen) and α-actinin (A7811, Sigma; secondary: A11004, Invitrogen) and mounted with Vectashield containing DAPI (H-1200, Vector Laboratories). Tissue sections were visualized and imaged on either a Nikon eclipse TE2000-U microscope with SPOT software (Diagnostic Instruments) or a Nikon A1R MP multiphoton confocal microscope.
Cell fractionation.
Murine heart tissue and isolated neonatal rat ventricular myocytes (NRVMs) were subject to cellular fractionation as previously described to examine compartmental-specific changes in protein abundance and localization (12, 33).
Microarrays.
For microarray analysis, heart tissue was removed from the left and right ventricles of Smyd1 knockout (KO) animals (or normal diet-fed control mice) 2 or 9 wk after tamoxifen treatment. Total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer's protocol. RNA was analyzed for genome-wide expression analysis using an Illumina mouse bead chip (Mouse Ref 8 v. 2.0). All total RNA samples were quantified using a Ribogreen fluorescent assay and normalized to 10 ng/μl before amplification. Amplified and labeled cRNA was produced from 100 ng of each sample using the Illumina-specific version of the Ambion TotalPrep 96 kit (cat. no. 4393543). After a second Ribogreen quantification and normalization step, amplified and labeled cRNA was hybridized overnight at 58°C to the expression arrays. Washing and signal development were performed with the aid of a SciGene model 650c microarray processor (LittleDipper). Chips were scanned on an Illumina iScan confocal scanner under standard parameters. Expression data was extracted and compiled using BeadStudio software (Illumina). Samples were analyzed in triplicate for each group, and data for each gene were averaged between the three samples. Data were subjected to background subtraction and quantile normalization.
Isolated rat cardiomyocytes.
NRVMs were obtained by enzymatic dissociation from 1-day-old litters and plated in DMEM media (Invitrogen, no. 11965) containing 1% penicillin, 1% streptomycin, 1% insulin-transferrin-sodium selenite supplement, and 10% fetal bovine serum for the first 24 h, after which the cells are cultured in serum-free media. NRVMs were treated with adenovirus expressing either FLAG-tagged mouse Smyd1(a), Smyd1(b), or an empty virus (control). To induce hypertrophy in isolated NRVMs, cells were treated with either isoproterenol (ISO, 1 μM) or phenylephrine (PE, 10 μM) for 48 h. For cell size analysis, NRVMs were fixed with paraformaldehyde and stained with Alexa Fluor 488 phalloidin (A12379, Invitrogen) according to the manufacturer's protocol and imaged on a Nikon eclipse TE2000-U microscope.
Electrophoresis and Western blotting.
Proteins were separated by standard SDS-PAGE using Laemmli buffer. For Western blotting, proteins were transferred to nitrocellulose, membranes blocked with milk, and protein signals detected by enzyme-linked chemiluminescence (GE Biosciences). Ponceau staining of membranes was used to confirm transfer and protein loading. Antibodies used in this study are as follows, including source and dilution: histone H3 (Abcam, ab1791; 1:10,000 dilution); histone H3-trimethylated-K9 (Abcam, ab8898; 1:500 dilution); histone H3-trimethylated K4 (Abcam, ab8580; 1:300 dilution); histone H4 (Abcam, ab10158; 1:1,000); histone H4-trimethylated K20 (Abcam, ab9053; 1:300 dilution); β-actin (Sigma, A1978; 1:1,000 dilution); Gapdh (Santa Cruz Biotechnology, sc-20357; 1:1,000 dilution); FLAG (Sigma, F1804; 1:1,000 dilution); Smyd1 (Abcam, 32482; 1:1,000 dilution); Smyd1 (Santa Cruz Biotechnology, sc-79080; 1:1,000 dilution); p53 (Santa Cruz Biotechnology, sc-1313; 1:500 dilution); Smyd2 (Abcam, ab38821; 1:1,000 dilution); HSP90 (Abcam, ab13494; 1:300 dilution); HSP90 (Cell Signaling, no. 4874; 1:1,000 dilution).
Quantitative real-time PCR analysis.
Total RNA was isolated from the left ventricle of the heart or from cultured NRVMs using TRIzol (Invitrogen) according to the manufacturer's protocol. Total RNA was transcribed using SuperScript First-Strand Synthesis system for RT-PCR (Invitrogen) according to the manufacturer's protocol to produce cDNA. cDNA transcripts were amplified on the iCycler iQ real-time PCR detection system with iQ SYBR Green Supermix (Bio-Rad). Expression levels were analyzed using the iQ5 Optical Systems software v2.0 and normalized against GAPDH by subtracting the mean cycle number for each experimental group from the mean cycle number for GAPDH from the same group. Fold change was calculated using the ΔΔCt method.
Luciferase reporter assay.
HeLa cells were grown in DMEM (Corning Cellgro) supplemented with 10% FBS and 1% penicillin/streptomycin on white 96-well plates. At 70% confluence, the cells were infected with either Ad-CMV-Null (empty virus) or Ad-Smyd1A-Flag adenovirus at a multiplicity of infection (MOI) of 100 in serum-free media. After 24 h, the cells were transfected with 75 ng of human TGF-β3, Nppa, negative control (scrambled), or positive control (actin) luciferase reporter construct (SwitchGear Genomics) using FuGene HD (SwitchGear Genomics) at a 6:1 ratio to DNA and incubated for 48 h. Luciferase activity was assayed using the LightSwitch Luciferase assay reagent (SwitchGear Genomics) according to the manufacturer's instructions and measured using a BioTek Synergy Neo HTS Multi-Mode microplate reader. This assay was also performed in NRVM cells; however, despite the detection of signal from the actin positive control construct, no signal was observed for TGF-β3 or Nppa. The promoter regions for these genes in human and rat contain significant sequence variability and may be the reason for the absence of signal in rat cells.
ChIP-PCR.
NRVMs (6 × 106) infected with adenovirus-expressing Smyd1a-FLAG or an empty vector control (in the presence or absence of PE treatment, 48 h) were fixed in 1% formaldehyde, lysed in membrane extraction buffer (Magnetic ChIP Kit), and sonicated using a Sonic Dismembrator (Fisher Scientific), leading to fragments between 300 and 1,000 bp (for endogenous ChIP experiments, wild-type or Smyd1 KO heart lysates were used). ChIP was performed using a commercially available Pierce Magnetic ChIP Kit (Thermo, 26157) according to manufacturer's instructions. DNA-bound protein was immunoprecipitated using an anti-FLAG (Sigma, F1804), anti-histone H3 trimethylated K4 (Abcam, ab8580), and anti-IgG (Santa Cruz Biotechnology, 2025) as a negative control. The DNA recovered was analyzed by quantitative real-time PCR using primer sets that amplified the promoter region of Tgf-β3 or atrial natriuretic factor (ANF) (Nppa). PCR was performed in duplicate with equal amounts of specific antibody-immunoprecipitated sample, control (IgG) and Input. Values were normalized to input measurements, and enrichment was calculated using the ΔΔCt method.
Bioinformatic analysis.
The heatmap in Fig. 5A was generated using the function “heatmap.2” from gplots package (2.11.3) in R (3.0.1). The distance/dissimilarity between genes was calculated using Euclidean distance, as default. To achieve a better separation between upregulated expression (red) and downregulated expression (green), the single linkage method was used as the agglomeration method for hierarchical clustering.
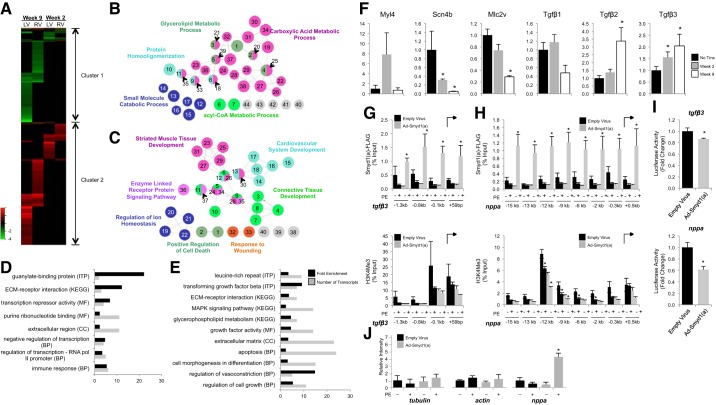
Fig. 5.
Transcriptome analyses reveal Smyd1 to be a transcriptional repressor of a core set of developmental genes. Transcriptome analyses were performed separately on right ventricles (RV) and left ventricles (LV) from mice with deletion of Smyd1 at 2 wk and 9 wk after removal from tamoxifen diet (controls were normal diet-fed littermates). A: all genes measured in both groups, RV and LV, are displayed in heat map format (red is upregulation, green downregulation, and black statistically unchanged) and clustered according to similar behavior. 2 clusters were defined as indicated, and the functionality of genes in those clusters was determined by gene ontology (GO) analysis. The networks for cluster 1 (B) and 2 (C) for the biological process ontology are shown. In the network figures, node size indicates the P value, and linkage between two terms indicates the relatedness, as determined by κ statistics. The color indicates functional groups with each group represented by their most significant leading term. All terms shown in the network image of cluster 1 are filtered at P < 0.005; those in cluster 2 are filtered at P < 0.0001. Bioinformatic analyses of transcripts with altered expression in the LV, at week 2 (D) and week 9 (E) after Tmx treatment, show significant enrichment in genes involved in extracellular matrix remodeling, fibrosis, and transcriptional repression. MF, molecular function; BP = biological process; CC, cellular component; KEGG, KEGG analysis; ITP, Interpro analysis. Microarray results for several of these transcripts were subsequently validated by RT-PCR (F: all those we attempted to validate are shown; n = 3–6/group; *P < 0.05 vs no tamoxifen group). Chromatin immunoprecipitation (ChIP) and qPCR for Smyd1 (using FLAG antibody) show enrichment in the promoter region of target genes tgfbeta3 (G) and nppa (H); however, no corresponding enrichment of histone H3 lysine K4 trimethylation was detected in these regions (bottom). *P ≤ 0.05. I: luciferase reporter assay using the tgfbeta3 and nppa promoters confirms that Smyd1 acts as a transcriptional repressor by inhibiting transcription of these genes; n = 6/group; *P < 0.05. J: as a negative control, Smyd1 is enriched by ChIP-PCR at neither β-tubulin nor β-actin using primers shown previously to target the regulatory regions upstream of these genes [−3 kb for β-tubulin (24), −73 bp for β-actin (29)].
Gene ontology (GO) analysis for cluster 1 and 2 was conducted using ClueGO (v2.1.0) (5), a plug-in of Cytoscape (3.0.1) (42). When we searched for enrichment of GO terms, right-sided hypergeometric test was used with Benjamini-Hochberg correction on P value. The threshold of P value for biological process terms is 0.005 for cluster 1 and 0.0001 for cluster 2 and for cellular component and molecular function terms is 0.05 for both clusters. GO terms were restricted to levels 3 to 8. GO annotation evidence under Inferred from Electronic Annotation (IEA) was excluded. The restriction threshold for GO term connection (κ score), which determines the association strength between the terms, was set to 0.4. GO term fusion function and grouping function were enabled to reduce term redundancy. The size of each term node represents its relative P value, and the color represents the group to which it belongs. The leading term of each GO term group was assigned based on the highest significance. GO, KEGG, and Interpro analysis of all changing transcripts was performed using the DAVID Bioinformatics Resource (v6.7) developed by the NIAID (NIH).
RESULTS
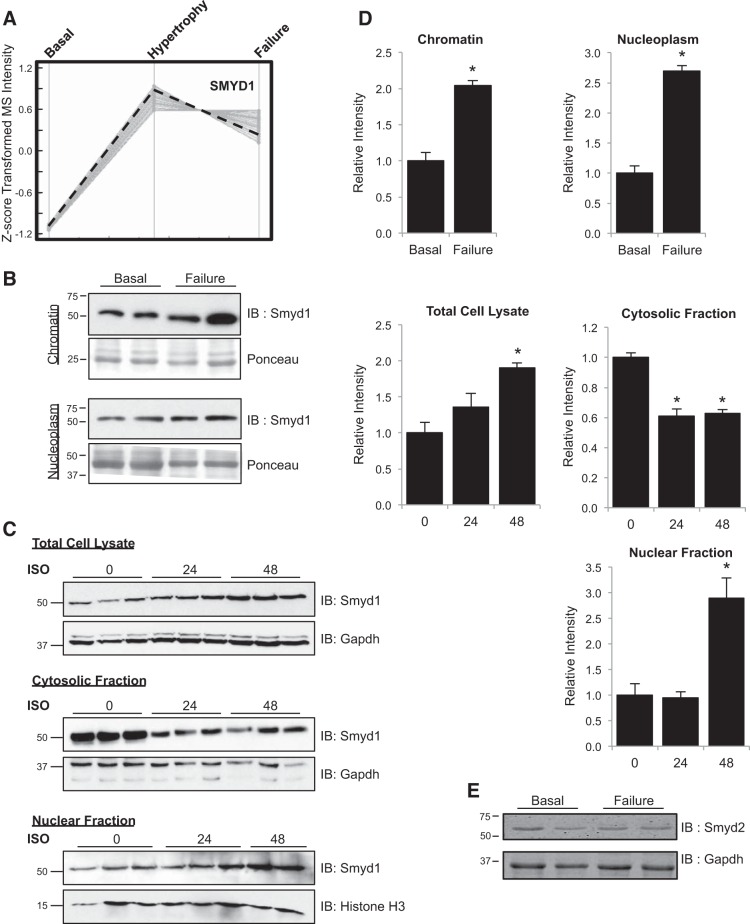
To identify novel regulators of heart failure, we carried out a quantitative proteomic analysis of cardiac chromatin. Adult male mice were subjected to transverse aortic constriction, and their cardiac function was monitored by echocardiography for a period of 2–6 wk. In this model, mice develop progressive cardiac disease that is hallmarked by two phases: a compensatory hypertrophy phase, in which the muscle increases in size while the function of the organ is preserved, and a failure phase, in which the left ventricular chamber size increases and function deteriorates. Using high-resolution mass spectrometry, we identified 176 proteins displaying altered association with chromatin as mice progressed into hypertrophy and failure (34). Unsupervised clustering of these proteins, following Z-score transformation of mass spectrometric intensities, revealed nine modules of proteins exhibiting distinct behaviors during pressure overload-induced heart failure. We performed GO analyses and literature searches on these proteins and identified the histone methyltransferase Smyd1 as a potential novel regulator of cardiac gene expression in the diseased heart. As detected in our mass spectrometry analysis, chromatin-bound Smyd1 is significantly increased during hypertrophy, and this increase is maintained in heart failure (Fig. 1A). Subsequent Western blotting confirmed that both nucleoplasmic- and chromatin-bound populations of Smyd1 increased in the hypertrophic and failing mouse heart [Fig. 1, B and D; pressure overload hypertrophy is associated with manifold changes in histone variant expression, but these do not include a change in the total abundance of histone H3, as detected by Western blotting (11)]. To investigate whether changes in Smyd1 levels were conserved across species and hypertrophic stimulus, we treated isolated NRVMs with ISO, a β-adrenergic receptor agonist that induces pathological cell growth. Similar to what was observed in mice after pressure overload, ISO [at a dose shown to induce myocyte hypertrophy (31)] increased total cellular Smyd1 expression in isolated cells (Fig. 1, C, top, and D). Analysis of subcellular compartments revealed that this increase occurred preferentially in the nuclear fraction and was associated with Smyd1 translocation from cytosol to nucleus and/or resulted from a change in protein stability in one of these compartments (Fig. 1C, middle and bottom, and 1D) although the data did not allow us to distinguish between these possibilities.
Fig. 1.
Identification of Smyd1 as a novel participant in pathological cardiac hypertrophy and failure. A: among hundreds of chromatin-bound proteins quantified during pressure overload-induced hypertrophy and heart failure, Smyd1 abundance increased during compensatory hypertrophy and failure, as measured by mass spectrometry-based peptide abundance from cardiac chromatin. This trend was confirmed by Western blotting (B); we also observed an increase in nucleoplasmic (i.e., nuclear localized, not bound to chromatin) Smyd1 during heart failure. IB, immunoblot. C: to determine whether activation of Smyd1 was conserved across species and stimulus, we examined expression and localization following isoproterenol (ISO; 1 μM for times indicated) treatment of ventricular myocytes. D: quantitation of Western blots from B and C. E: Smyd2 expression is unchanged in the setting of pressure overload-induced heart failure; n = 4–6 per group for all Westerns; bars are SE, *P < 0.05.
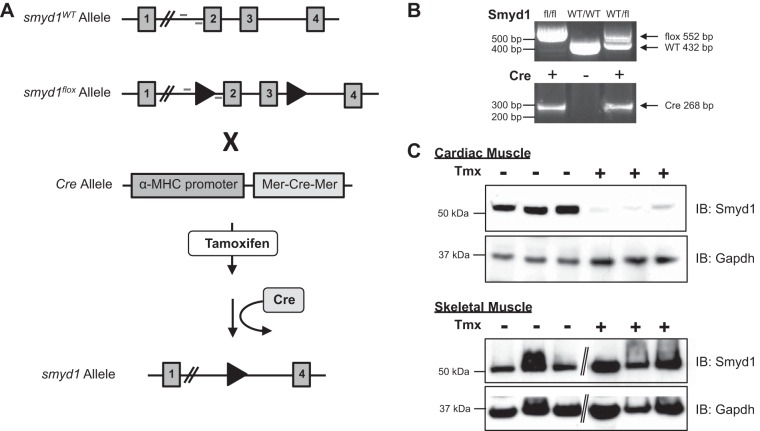
To investigate the role of Smyd1 in the adult heart, we next took a genetic approach. Inducible, myocyte-specific smyd1-null mice were generated by first engineering a smyd1 floxed mouse with the second and third exons targeted for deletion. These mice were bred to homozygosity, after which they grew and reproduced normally with no obvious phenotype. The smyd1flox/flox mice were then crossed with transgenic mice expressing the α-myosin heavy chain promoter-driven Cre gene flanked by two modified estrogen receptors. As described (43), this mouse expresses the resultant “MerCreMer” protein only in the heart. Upon treatment with the estrogen analog tamoxifen, this protein translocates to the nucleus and executes loxp-targeted recombination activity. The experimental scheme for these mice is shown in Fig. 2A and confirmed by PCR in Fig. 2B. Resulting MerCreMer-smyd1flox/flox mice grew and reproduced normally in the absence of tamoxifen. Treatment of the mice with tamoxifen for 3 wk, followed by 1 wk on a normal diet, led to robust loss of Smyd1 at the protein level in the heart, while skeletal muscle Smyd1 was left unaffected (Fig. 2C).
Fig. 2.
Generation of cardiac-specific, tamoxifen-inducible smyd1-null mice. A: positional cloning was used to insert loxp sites flanking exons 2 and 3 of smyd1 to produce the target allele. These mice were bred to homozygosity and then crossed with transgenic mice expressing the Cre recombinase positioned between two copies of the tamoxifen-responsive modified estrogen receptor and driven by the α-myosin heavy chain (MHC) promoter (so called Mer-Cre-Mer mice). B: PCR genotyping confirmed genetic manipulation (location of primers are indicated in A). Resulting mice express Mer-Cre-Mer in adult cardiomyocytes, and, upon treatment with tamoxifen (Tmx), this protein excises the floxed region of the mutant smyd1 allele. Mice fed a tamoxifen diet for 3 wk followed by 1 wk on a normal diet demonstrate near complete loss of Smyd1 protein specifically in the heart; Smyd1 in skeletal muscle is unaffected (C). Diagonal lines indicate removal of gel lanes. WT, wild-type.
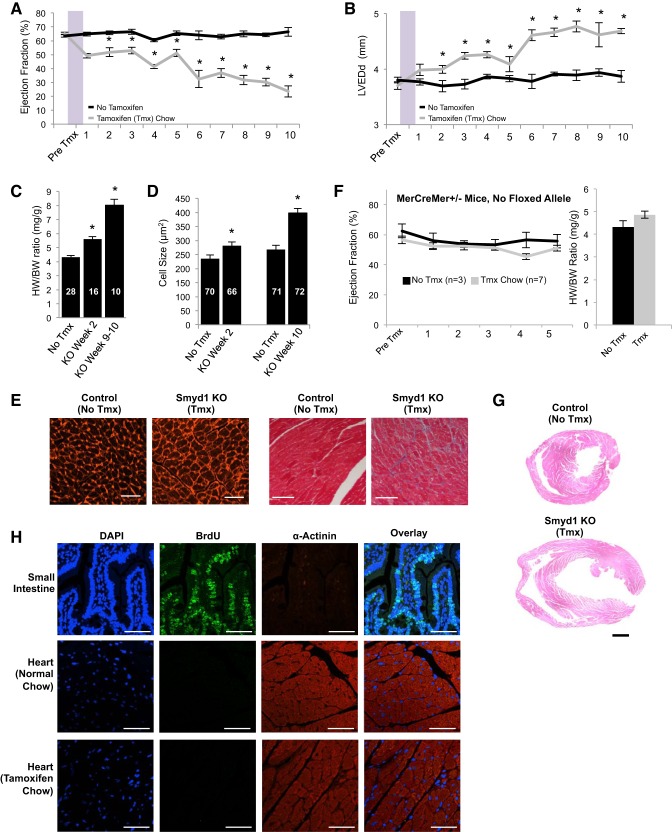
Loss of Smyd1 in the adult heart induced fulminant heart failure, as proven by rapid deterioration of ejection fraction (Fig. 3A) and dilation of the left ventricle (Fig. 3B). Postmortem analyses revealed significant increases in heart weight:body weight ratio as early as 2 wk (the earliest time point examined) after halting tamoxifen treatment. This index of cardiac pathology had nearly doubled by the 9–10-wk time point (Fig. 3C) and was driven by a concomitant increase in cardiomyocyte size, as measured by wheat germ agglutinin staining (Fig. 3, D and E, left). Fibrosis also resulted in the wake of Smyd1 depletion (Fig. 3E, right). Because others have reported a transient cardiomyopathy in MerCreMer mice in the absence of a floxed allele (22), we monitored cardiac function in a cohort of these mice in our study, observing no heart failure and cardiac hypertrophy phenotypes (Fig. 3F) following an identical treatment protocol used to generate the Smyd1-deficient animals.
Fig. 3.
Loss of Smyd1 in the adult heart induces progressive hypertrophy and failure at the organ and cell level. Cardiac function in smyd1flox/floxCre+/− mice was measured by echocardiography (ECHO). A baseline reading was taken in each mouse before 3 wk on tamoxifen or regular diet. After 1 wk back on regular diet in the experimental group, ECHO measurements were made on a weekly basis in both groups. We observed a progressive decline in left ventricular ejection fraction (A) coupled with a progressive increase in left ventricular end-diastolic dimension (LVEDd) (B) as a result of smyd1 deletion (n = 37 control mice and 36 tamoxifen=treated mice for A and B; *P < 0.05 vs. regular diet at same time point). Loss of Smyd1 also induced an increase in muscle mass at the whole organ (as measured by heart weight:body weight ratio, HW/BW, *P < 1E-5; C) and individual cardiac cell level as measured following wheat germ agglutinin staining (D and E, left, 10 wk after tmx; bar = 50 μm), concomitant with an increase in fibrotic deposition as measured by trichrome staining (E, right, 8 wk after tmx; bar = 100 μm); n values for HW/BW and cell size are indicated, the former indicating number of animals and the latter indicating number of cells (from a total of 4 animals per group). KO, knockout. F: MerCreMer-smyd1wt/wt mice (i.e., mice with no floxed alleles) develop neither cardiac dysfunction nor hypertrophy when administered the same tamoxifen protocol. G: marked chamber dilation is observed in Smyd1-deficient mice at 8–10 wk after return to normal chow. H: MerCreMer-smyd1flox/flox mice were injected with 5-bromo-1-(2-deoxy-β-d-ribofuranosyl) (BrdU) to label actively replicating cells. Small intestine positive control shows strong BrdU incorporation. We observe incorporation of BrdU in neither the hearts of MerCreMer-smyd1flox/flox mice fed a normal diet (middle) nor those with the same genotype on a tamoxifen diet for 10 wk (bottom; bar = 50 μm).
We attempted to carry out knockdown studies targeting Smyd1 in neonatal cardiac myocytes but were unsuccessful; doses of siRNA sufficient to knockdown Smyd1 induced significant cell death (data not shown). We speculate this finding reflects a differential role of the protein in neonatal development; indeed it is in agreement with the fact that the original Smyd1 germline knockout animals were lethal at the embryo stage (14). The progressive growth of the heart is also evident by H and E staining of tissue sections at 9 wk (Fig. 3G) after tamoxifen administration. Heart rate was unaffected by loss of smyd1 (data not shown). To investigate whether deletion of smyd1 led to myocyte proliferation, mice were administered BrdU, which is incorporated into DNA during mitosis. We observed no BrdU-positive myocytes in MerCreMer-smyd1flox/flox mice on normal or tamoxifen diet (Fig. 3H), suggesting that cellular proliferation was not a significant contributor to the increase in muscle mass observed following loss of smyd1. We have followed the MerCreMer-smyd1flox/flox mice out to 10 wk after return to normal chow and observed no increase in mortality compared with either tamoxifen-treated MerCreMer mice with no floxed allele or untreated mice (that is, no mice died in any of these groups up until that time point). In separate experiments (data not shown), we observed that subjecting the Smyd1-depleted mice to pressure overload led to a more precipitous decline in cardiac function compared with either Smyd1 depletion or aortic banding alone although further investigation will be necessary to determine how the stress of Smyd1 loss interacts with the pathophysiological response to aortic banding.
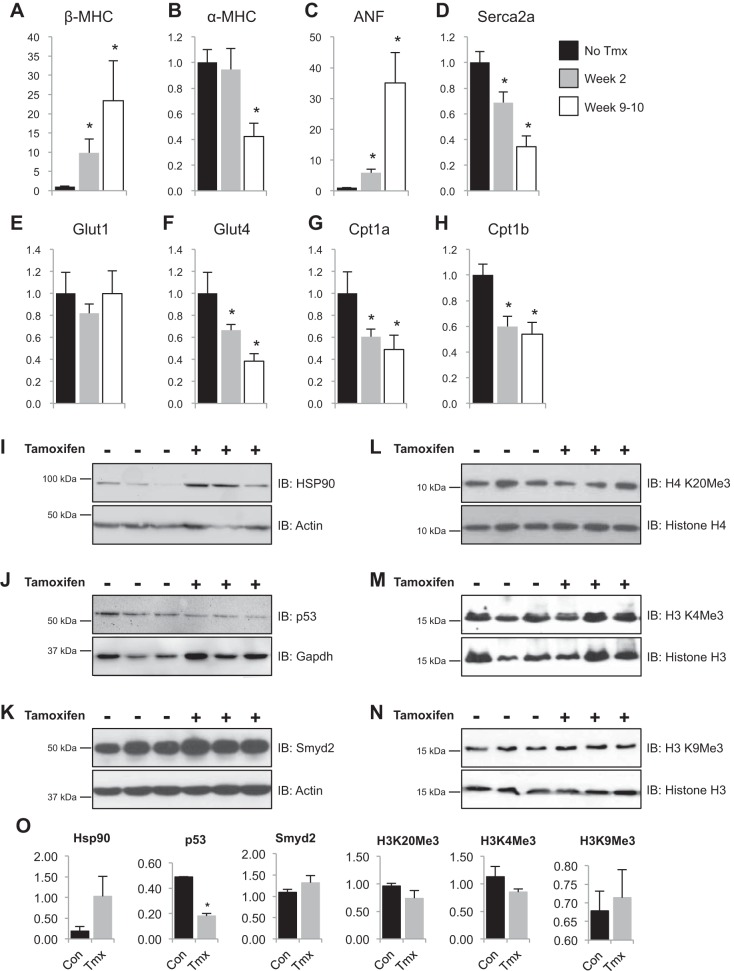
Cardiac hypertrophy has been reported to involve a shift in gene expression wherein the diseased adult heart converts to a transcriptome reminiscent of the fetal myocardium (40). The mechanisms controlling this transition at the level of chromatin are unknown. Because Smyd1 was found to be upregulated in heart failure (Fig. 1), we reasoned that this protein may be an endogenous inhibitor of this disease-associated gene expression program. To test this hypothesis, we investigated expression of pathological genes in smyd1-null hearts. RT-PCR measurements of transcript abundance for α/β-myosin heavy chains, atrial natriuretic factor, sarcoplasmic reticulum Ca2+ ATPase, glucose transporters (type 1 and 4), and carnitine palmitoyltransferase (type 1a and 1b) revealed significant changes following loss of smyd1 (Fig. 4, A–H). With the exception of Glut1, these changes were progressive and mirrored the deterioration of cardiac function in these mice (Fig. 4). We next examined signaling molecules implicated in Smyd1 function, including the Smyd1 chaperone HSP90 [Fig. 4I (2, 48)], which was increased, and the Smyd1 interactor p53 [Fig. 4J (2, 21)], which was decreased, in the smyd1-null heart. Although ostensibly incapable of functional compensation at the cell and organ level, loss of smyd1 led to a modest increase in expression from the closely related family member, Smyd2 [Fig. 4K; note that Smyd2 is dispensable for cardiac development and mature function (8), and pressure overload did not induce changes in its abundance in the wild-type heart as shown in Fig. 1E]. To investigate whether loss of smyd1 affects global levels of histone posttranslational modifications, we performed Western blotting for well-characterized histone methylation events in total cell lysates. Interestingly, loss of smyd1 had no effect on global levels of the heterochromatin mark, H4K20Me3 (Fig. 4L), the euchromatic mark, H3K4Me3 (Fig. 4M), or the constitutive heterochromatic mark, H3K9Me3 (Fig. 4N).
Fig. 4.
Smyd1 is a previously unknown regulator of a conserved transcriptional program underlying heart failure. A–H: fetal genes were measured by RT-PCR and values expressed as fold change relative to mice not fed tamoxifen. *P < 0.05; n = 5–8 for no tmx, n = 6 for post tmx 2 wk, and n = 7 for post tmx 9–10 wk. ANF, atrial natriuretic factor. I–N: as determined by Western blotting, loss of Smyd1 increased expression of Hsp90, decreased expression of p53, resulted in an upregulation of Smyd2, and had no effect on global levels of histone H4 (K20) and H3 (K4 and K9) trimethylation. O: quantitation of I–N; *P = 0.002.
To further investigate the signaling pathways and specific gene expression events regulated by Smyd1, we took an unbiased transcriptome analysis approach. Hearts were harvested at an early (2 wk) and late (9 wk) time point after tamoxifen administration, and right and left ventricles were collected. These time points correspond to mild and severe cardiac dysfunction, respectively, following loss of smyd1 in these animals. mRNA was purified, and expression microarrays were conducted on these samples, with the age-matched normal diet-fed animal serving as a time- and chamber-specific control (4 animals were examined in each group). This dataset is a resource for understanding molecular targets of Smyd1 but also an important global measurement of the transcriptome remodeling that occurs during the transition of the heart into hypertrophy and on to failure.
Before focusing on individual genes, however, we wanted to determine the global role of Smyd1 to influence gene expression. To do this, all transcriptomic data (including early and late time points, right and left ventricles) were plotting as a heat map and clustered in an unsupervised manner. The result (Fig. 5A) displays upregulated (red areas, so called cluster 2) and downregulated (green areas, cluster 1) transcripts. We next performed GO and KEGG analysis on these two major clusters (Fig. 5, B and C) to generate network maps of key processes regulated by Smyd1. Network nodes are sized according to P value and colored according to the most significantly enriched term in each group. There are two salient observations: first, downregulated transcripts are enriched for genes involved in metabolic processes (Fig. 5B); second, upregulated genes in the knockout are enriched for developmental and muscle growth genes (Fig. 5C), providing unbiased evidence in support of the conclusion that Smyd1 is a global repressor of development and growth in the adult heart.
We next examined each of the microarray datasets individually using GO and KEGG (followed by Interpro domain analyses for the implicated pathways), revealing the pathways implicated in a chamber-specific manner over time, as loss of smyd1 induces cardiac hypertrophy and failure. Bioinformatic analyses revealed a significant enrichment in molecules involved in transcriptional repression, muscle development, growth, TGF-β signaling, and extracellular matrix (Fig. 5, D and E), supporting our histological observation of increased collagen deposition in the smyd1-depleted myocardium (Fig. 3E). Indeed, RT-PCR was used to confirm changes in expression of several of the genes measured by microarray, including three members of the TGF-β family (Fig. 5F), further validating the role of Smyd1 to repress growth signaling and gene expression in the normal heart.
To identify gene targets directly regulated by Smyd1 and to further investigate the mechanism by which Smyd1 controls gene expression, we examined the tgfbeta3 and nppa loci using ChIP-PCR. Previous studies had shown that sequences within 1 kb 5′ of the transcriptional start sites of tgfbeta3 (28) and nppa (10) were essential for transcription. Consistent with these observations, we observed significant enrichment of Smyd1 binding upstream of both the tgfbeta3 and nppa transcriptional start sites (Fig. 5, G and H, top), demonstrating direct recruitment of Smyd1 to their promoter regions. It should be noted that we also performed ChIP further 5′ of these regions because previous work had identified critical control regions substantially upstream of the transcriptional start site for tgfbeta3 (28) and particularly for nppa (19). Contrary to our initial hypothesis, Smyd1 binding did not correlate with enrichment of histone H3 lysine 4 trimethylation across the region (Fig. 5, G and H, bottom). However, luciferase analyses confirmed that Smyd1 binding to these regions repressed transcription of tgfbeta3 and nppa (Fig. 5I). To determine whether Smyd1 modulates these genes in the heart, we investigated localization of Smyd1, as well as multiple histone marks, to the transcription start site region in tgfbeta3 and nppa using ChIP-PCR. Loss of Smyd1 was also associated with a decrease in multiple histone marks, concomitant with a decrease in total histone H3, in the promoters of these genes (data not shown), which we interpret as evidence of general nucleosome depletion in the promoter regions, possibly as part of transcriptional activation, although future studies will be required to test this conjecture. Last, ChIP-PCR against genes not targeted by Smyd1 [using primers (24, 29) validated to target regulatory regions of these genes from previous publications] shows a lack of enrichment at beta-actin and beta-tubulin, supporting specificity in targeting.
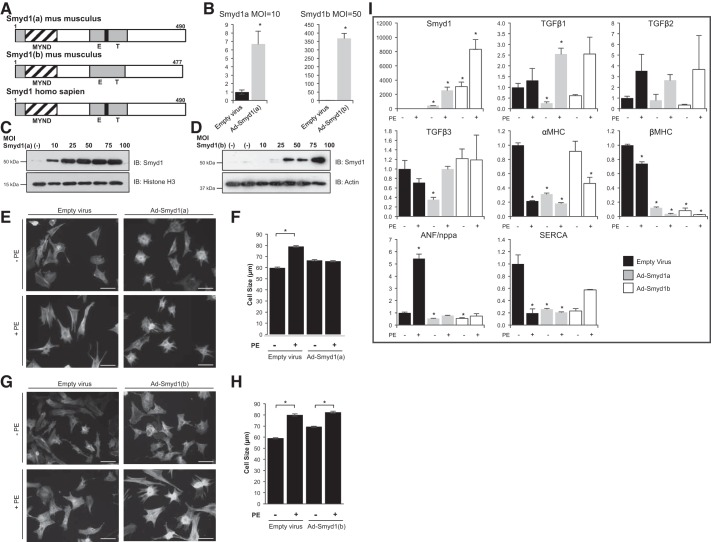
Because the absence of Symd1 induced rapid heart failure in the adult mouse, we last sought to determine whether overexpression of Smyd1 would be sufficient to prevent hypertrophy. There are two isoforms of Smyd1 in the mouse heart; Smyd1a differs from Smyd1b by the presence of a 13-amino-acid region in the E-T domain of the former, a feature that is conserved in the single isoform of Smyd1 in humans (Fig. 6A). Adenoviral-mediated overexpression of Smyd1a or Smyd1b was successful (at varying MOI) to increase Smyd1 protein levels (Fig. 6, B–D). We detected no effect of this overexpression on basal cell phenotype in isolated cardiac myocytes. However, overexpression of Smyd1a (Fig. 6, E and F), but not Smyd1b (Fig. 6, G and H), was sufficient to block PE-induced hypertrophy. Overexpression of Smyd1a or Smyd1b also altered the expression of some genes often associated with cardiac pathology (Fig. 6I). For example, basal levels of SERCA and α/β-myosin heavy chain (MHC) were downregulated (empty virus infection blocked the PE-induced upregulation of β-MHC we observe in untransfected cells), and the increase in ANF induced by PE was blocked. In agreement with the actions of TGF-β3 as a potential downstream target of Smyd1, the basal expression of this gene (along with TGF-β1) was diminished after Smyd1 activation. These findings indicate that, although only Smyd1a was effective to prevent hypertrophy in isolated cardiac myocytes, both isoforms can regulate gene expression, and their actions impact both basal and agonist-induced transcript abundance.
Fig. 6.
Overexpression of Smyd1 attenuates cellular hypertrophy in vivo. A: there are 2 splice variants of Smyd1 in mouse heart, differing by a 13-amino-acid sequence present in Smyd1a and absent in Smyd1b, but conserved in the single human Smyd1 transcript. B–D: adenoviral infection of myocytes led to robust Smyd1 expression, as confirmed by RT-PCR and Western blotting. MOI, multiplicity of infection. E and F: overexpression of Smyd1a had no effect on basal cell size but prevented phenylephrine (PE)-induced myocyte hypertrophy (phalloidin staining; scale bar = 100 μm; quantified from ∼100 cells per group in 3 independent experiments; *P < 0.001). G and H: overexpression of Smyd1b required higher MOI and had no effect on PE-induced cell growth (quantified as described in F). I: RT-PCR analysis of fetal genes and Smyd1 targets in neonatal rat ventricular myocytes after adenovirus infection in the presence and absence of PE. *P ≤ 0.05 compared with empty virus control without PE.
DISCUSSION
During normal development, a series of epigenetic transitions converts multipotent progenitor cells into the various lineages of the heart, including cardiac myocytes. Soon after birth in mammals, cardiomyocytes become binucleated and stop dividing. From this point forward, their response to injury or stress is (almost always) to either die or increase in size. Although the latter can be beneficial to maintain the function of the organ, cellular hypertrophy—and the concomitant conversion to a pathologic (and perhaps more developmentally primitive) transcriptome and proteome that accompanies it—ultimately leads to heart failure. In the present study, we provide in vivo evidence that addresses key elements of this mystery of cardiac function. How does the healthy adult heart keep developmental genes silenced? Our data demonstrate that loss of Smyd1 in the adult heart removes a repression on such genes, leading to extensive transcriptome remodeling and precipitous heart failure. In the normal heart, Smyd1 is upregulated during the hypertrophic phase of disease, probably as part of an endogenous defense mechanism that is ultimately overwhelmed when the heart transitions to failure.
Previous investigations have shown unequivocally that chromatin modification can induce hypertrophic growth, as well as restrict it, including in physiological and pathological conditions (3, 4, 15, 23, 36, 50). One study showed that the histone methyltransferase JMJD2A is a positive regulator of hypertrophy, with overexpression leading to exacerbated response to hypertrophy and knockout protecting against it (53). However, this protein is not cardiac specific, likely explaining the lack of a phenotype in these JMJD2A transgenic or knockout animals at baseline. Despite this exciting finding, until now no study to our knowledge has demonstrated a muscle-specific chromatin protein that restricts adult cardiac growth. Our results are a step toward reconciling cardiac-specific gene expression with the cell type-independent, genome-wide actions of many chromatin modifiers. How do cardiac transcription factors, active in the adult heart, avoid spurious transcription of developmentally silenced genes? Our data demonstrate that this occurs partly through the actions of Smyd1, which we observe to be an endogenous inhibitor of a panel of pathological and developmental genes in the adult myocardium.
There are five Smyd family members expressed in mammals, but only Smyd1 is restricted to striated muscle. The human smyd1 gene produces a single transcript, which shares this striated muscle specificity with two of the three murine smyd1 transcripts, Smyd1a and b (the third, murine Smyd1c, being restricted to T cells). Previous studies have shown Smyd1 to be necessary for early heart morphogenesis (14) and to be transcriptionally regulated by serum response factor and myogenin (26). Other investigations have examined Smyd isoforms in striated muscle, demonstrating a role for zebrafish Smyd1b in skeletal and cardiac myofibril assembly (27, 46). In our study, Smyd1b in the mouse had no effect on hypertrophic cell growth (Fig. 6, G and H), whereas the Smyd1a isoform prevented pathological changes in myocytes exposed to PE (Fig. 6, E and F). In addition to these novel phenotypic findings regarding the ability of Smyd1 to inhibit disease progression, this study supports a paradigm shift in the basic biological function of Smyd1. Smyd family members were originally thought to activate gene transcription through methylation of histone H3 lysine K4; however, to date this enzymatic activity has only been reported to occur in vitro. Conversely, the notion of Smyd1 as a transcriptional repressor was first suggested by Gottlieb et al. (14), without direct in vivo evidence for this action in adult myocardium. The present study demonstrates the in vivo activity of Smyd1 as a transcriptional repressor of a host of genes (Fig. 5, A–C). Furthermore, we show by ChIP and luciferase reporter assay that Smyd1 binds directly to the promoter regions of tgfβ3 and nppa/ANF and inhibits their expression. These findings were not associated with significant changes in H3K4Me3 globally or at targeted loci we evaluated, calling into question the model in which Smyd1 targets this histone modification in vivo.
Disease models in vivo and in cell culture have complementary strengths and weaknesses for analyzing gene expression. In Smyd1-deficient hearts, we observed a progressive reprogramming of fetal genes, with β-MHC and ANF increased and α-MHC and SERCA2A decreased. These changes were consistent with the expected trends for these genes during heart failure. However, unlike α-MHC and ANF, neither β-MHC nor SERCA2A responded as the anticipated trend in the Smyd1 overexpression scenario. There are several possible explanations for these observations. For example, the in vivo situation may result from, not only the direct actions of Smyd1, but also indirect actions that require more time to develop to the observed endpoint of heart failure. Also, the overexpression experiment that led to elevated levels of Smyd1 may not be optimal in that we are attempting to modify baseline gene expression in addition to agonist-induced changes. Future studies using transgenic and/or viral strategies to modulate Smyd1 levels in the heart may be required to fully address the regulation of these specific gene targets.
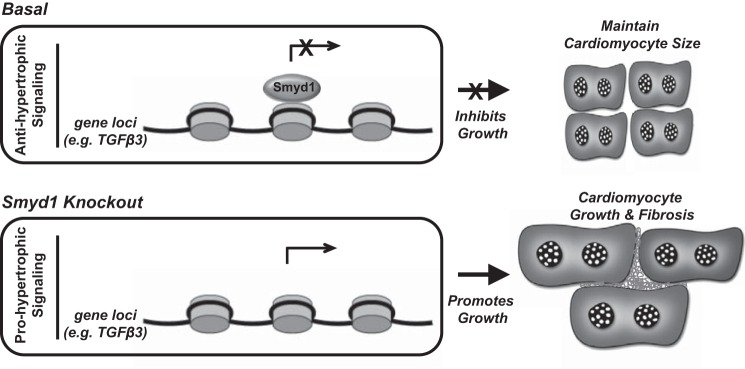
Because of the known role of Smyd proteins to regulate sarcomere function in vivo (48), we sought to examine nonnuclear populations of Smyd1 during disease. Our findings indicate that changes in nuclear Smyd1 in the setting of heart failure involve a concomitant decrease in cytosolic Smyd1 (which under basal conditions is comparable to nuclear Smyd1); this occurs along with an increase in total cellular Smyd1 protein levels. Furthermore, and also relevant to the actions of Smyd1 outside of the nucleus, loss of Smyd1 led to increased extracellular fibrosis, which could be in part due to the actions of Smyd1 at the membrane, in addition to transcriptional actions of this protein (Fig. 7). In contrast, overexpression experiments failed to alter the general myofibrillar architecture while being sufficient to block agonist-induced hypertrophy. Therefore, further work is required to distinguish genomic from nongenomic actions of Smyd1, given the convincing data from previous studies implicating this family of proteins in cytoplasmic and membrane, i.e., nonhistone, targets. Indeed on the basis of our work with histone posttranslational modifications in this study, we think it highly likely that many of the targets of Smyd1 are unidentified residues on histones and/or unknown proteins in the nucleus and beyond.
Fig. 7.
Model for functions of Smyd1 in the adult myocardium. Under basal conditions, Smyd1 functions as a transcriptional repressor to inhibit cell growth and maintain cardiomyocyte size. Loss of Smyd1 in the adult myocardium leads to prohypertrophic signaling resulting in myocyte growth, fibrosis, and functional decline.
It is interesting to speculate on the binding partners with which Smyd1 interacts to achieve specificity in its intracellular tasks. Previous investigations demonstrated roles for p53 and Hsp90 in Smyd family function (20, 48), in addition to being independently implicated in cardiac hypertrophy (25, 41), and indeed these proteins underwent altered expression following Smyd1 depletion in our study. Furthermore, we directly tested the hypothesis that global histone H3K4 trimethylation levels would be altered in the knockout mice; they were not, contrary to our expectation. We did, however, see alteration in levels of H3K4Me3 around key cardiac genes TGFβ and ANF following Smyd1 overexpression, suggesting that there is focal regulation of histone marks. In our view, the most likely explanation for these observations is that global levels of chromatin accessibility are modulated by a cadre of proteins, rather than a single enzyme or pathway, and, when Smyd1 is depleted, other chromatin modifiers are engaged to maintain a modicum of control on gene expression. Our observation that transcriptome remodeling after Smyd1 depletion leads to massive changes in cardiac gene expression, but not wholesale transcriptional chaos (for instance, permanently silenced noncardiac genes are not activated en masse), supports a buffering capacity in the epigenomic machinery that preserves the cardiac phenotype.
A recent emergence (16, 17, 38, 49) of studies examining chromatin marks and transcription factors in cell systems and using recombinant proteins notwithstanding, genome-wide positioning of cardiac transcription factors in vivo, using ChIP-seq approaches, have been lacking. Indeed we were unsuccessful in our attempts to optimize immunoprecipitation of endogenous Smyd1 to sufficient levels to enable DNA sequencing, probably attributable to limitations of the available reagents and abundance and localization of endogenous Smyd1 (as discussed elsewhere, the protein partitions between nucleus and cytosol in cardiomyocytes).
Studies from human patients with heart failure indicate that significant transcriptome and phenotypic remodeling is possible even in the sick heart (30) through interventions such as ventricular assist devices (7, 51), which mechanically unload the ventricle. Indeed mechanical unloading of the heart in humans has been shown to return endogenous Smyd1 expression to basal levels following its upregulation in the diseased human heart (6). These observations provide rationale for strategies to globally remodel chromatin, but a limitation has been that this remodeling must be done in a cell type-specific manner, to avoid unwanted side effects and to improve efficacy. The identification of a muscle-specific histone modifier that represses endogenous cardiac growth provides an exciting molecular target to overcome this limitation. Recent studies have indicated that Smyd1 plays an important role in other types of striated muscle; its absence during development (Myf5-driven) undermined myoblast differentiation (35), whereas disruption in the committed myoblast lineage (Myf6-driven) led to fast-twitch skeletal muscle weakness, hypotrophy (in contrast to the hypertrophy in the heart), and myofibrillar disarray, among other subcellular abnormalities (45). Nevertheless, given the strong sequence conservation between mouse and human Smyd1 (94.49% similarity at the amino acid level; Fig. 6A) and the fact that Smyd1 is upregulated in human heart failure (1, 6), it is an exciting indication that this pathway may be operative in humans. The present study examines the molecular basis for how Smyd1 functions in vivo and provides compelling evidence that it may serve as a novel, muscle-specific therapeutic target.
GRANTS
This study was supported by NIH grants HL-105699 (T. Vondriska), HL-115238 (T. Vondriska), and HL-107674 (S. Franklin) and funds from the Department of Anesthesiology at UCLA to S. Franklin and T. Vondriska. H. Chen was the recipient of an American Heart Association Predoctoral Fellowship. S. Jiang was the recipient of the Chinese Council Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.F., H.O.T., and T.M.V. conception and design of research; S.F., T.K., T.L.R., M.R.G., T.T., M.R.M., R.G., S.J., and S.R. performed experiments; S.F., T.K., M.R.G., H.C., Y.W., and T.M.V. analyzed data; S.F., T.K., M.R.G., H.C., Y.W., H.O.T., and T.M.V. interpreted results of experiments; S.F., M.R.G., H.C., and T.M.V. prepared figures; S.F. and T.M.V. drafted manuscript; S.F. and T.M.V. edited and revised manuscript; S.F., T.K., T.L.R., M.R.G., H.C., T.T., M.R.M., R.G., S.J., S.R., Y.W., H.O.T., and T.M.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank members of the Vondriska and Wang laboratories, Dr. W. Robb MacLellan, and Dr. Stephen Smale for helpful discussions and advice.
REFERENCES
- 1.Abaci N, Gulec C, Bayrak F, Komurcu Bayrak E, Kahveci G, Erginel Unaltuna N. The variations of BOP gene in hypertrophic cardiomyopathy. Anadolu Kardiyol Derg 10: 303–309, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Farha M, Lambert JP, Al-Madhoun AS, Elisma F, Skerjanc IS, Figeys D. The tale of two domains: Proteomics and genomics analysis of SMYD2, a new histone methyltransferase. Mol Cell Proteomics 7: 560–572, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell 133: 978–993, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Anand P, Brown JD, Lin CY, Qi J, Zhang R, Artero PC, Alaiti MA, Bullard J, Alazem K, Margulies KB, Cappola TP, Lemieux M, Plutzky J, Bradner JE, Haldar SM. BET bromodomains mediate transcriptional pause release in heart failure. Cell 154: 569–582, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pages F, Trajanoski Z, Galon J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25: 1091–1093, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borlak J, Thum T. Hallmarks of ion channel gene expression in end-stage heart failure. FASEB J 17: 1592–1608, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Burkhoff D, Klotz S, Mancini DM. LVAD-induced reverse remodeling: Basic and clinical implications for myocardial recovery. J Card Fail 12: 227–239, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Diehl F, Brown MA, van Amerongen MJ, Novoyatleva T, Wietelmann A, Harriss J, Ferrazzi F, Bottger T, Harvey RP, Tucker PW, Engel FB. Cardiac deletion of Smyd2 is dispensable for mouse heart development. PLoS One 5: e9748, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorn GW 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest 115: 527–537, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durocher D, Nemer M. Combinatorial interactions regulating cardiac transcription. Dev Genet 22: 250–262, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Franklin S, Chen H, Mitchell-Jordan S, Ren S, Wang Y, Vondriska TM. Quantitative analysis of the chromatin proteome in disease reveals remodeling principles and identifies high mobility group protein B2 as a regulator of hypertrophic growth. Mol Cell Proteomics 11: M111.014258, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin S, Zhang MJ, Chen H, Paulsson AK, Mitchell-Jordan SA, Li Y, Ping P, Vondriska TM. Specialized compartments of cardiac nuclei exhibit distinct proteomic anatomy. Mol Cell Proteomics 10: M110.000703, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2014 update: A report from the American Heart Association. Circulation 129: e28–e292, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb PD, Pierce SA, Sims RJ, Yamagishi H, Weihe EK, Harriss JV, Maika SD, Kuziel WA, King HL, Olson EN, Nakagawa O, Srivastava D. Bop encodes a muscle-restricted protein containing MYND and SET domains and is essential for cardiac differentiation and morphogenesis. Nat Genet 31: 25–32, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Hang CT, Yang J, Han P, Cheng HL, Shang C, Ashley E, Zhou B, Chang CP. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature 466: 62–67, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He A, Gu F, Hu Y, Ma Q, Ye LY, Akiyama JA, Visel A, Pennacchio LA, Pu WT. Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat Commun 5: 4907, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He A, Kong SW, Ma Q, Pu WT. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc Natl Acad Sci USA 108: 5632–5637, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med 358: 1370–1380, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Houweling AC, van Borren MM, Moorman AF, Christoffels VM. Expression and regulation of the atrial natriuretic factor encoding gene Nppa during development and disease. Cardiovasc Res 67: 583–593, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Perez-Burgos L, Placek BJ, Sengupta R, Richter M, Dorsey JA, Kubicek S, Opravil S, Jenuwein T, Berger SL. Repression of p53 activity by Smyd2-mediated methylation. Nature 444: 629–632, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Sirinupong N, Brunzelle J, Yang Z. Crystal structures of histone and p53 methyltransferase SmyD2 reveal a conformational flexibility of the autoinhibitory C-terminal domain. PLoS One 6: e21640, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res 105: 12–15, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kook H, Lepore JJ, Gitler AD, Lu MM, Wing-Man Yung W, Mackay J, Zhou R, Ferrari V, Gruber P, Epstein JA. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein. Hop J Clin Invest 112: 863–871, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48: 303–314, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Lee KH, Jang Y, Chung JH. Heat shock protein 90 regulates IkappaB kinase complex and NF-kappaB activation in angiotensin II-induced cardiac cell hypertrophy. Exp Mol Med 42: 703–711, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li D, Niu Z, Yu W, Qian Y, Wang Q, Li Q, Yi Z, Luo J, Wu X, Wang Y, Schwartz RJ, Liu M. SMYD1, the myogenic activator, is a direct target of serum response factor and myogenin. Nucleic Acids Res 37: 7059–7071, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Zhong Y, Wang Z, Gao J, Xu J, Chu W, Zhang J, Fang S, Du SJ. Smyd1b is required for skeletal and cardiac muscle function in zebrafish. Mol Biol Cell 24: 3511–3521, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu G, Ding W, Neiman J, Mulder KM. Requirement of Smad3 and CREB-1 in mediating transforming growth factor-beta (TGF beta) induction of TGF beta 3 secretion. J Biol Chem 281: 29479–29490, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Maeda T, Hobbs RM, Merghoub T, Guernah I, Zelent A, Cordon-Cardo C, Teruya-Feldstein J, Pandolfi PP. Role of the proto-oncogene Pokemon in cellular transformation and ARF repression. Nature 433: 278–285, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Margulies KB, Matiwala S, Cornejo C, Olsen H, Craven WA, Bednarik D. Mixed messages: Transcription patterns in failing and recovering human myocardium. Circ Res 96: 592–599, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell-Jordan S, Chen H, Franklin S, Stefani E, Bentolila LA, Vondriska TM. Features of endogenous cardiomyocyte chromatin revealed by super-resolution STED microscopy. J Mol Cell Cardiol 53: 552–558, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell-Jordan SA, Holopainen T, Ren S, Wang S, Warburton S, Zhang MJ, Alitalo K, Wang Y, Vondriska TM. Loss of Bmx nonreceptor tyrosine kinase prevents pressure overload-induced cardiac hypertrophy. Circ Res 103: 1359–1362, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monte E, Chen H, Kolmakova M, Parvatiyar M, Vondriska TM, Franklin S. Quantitative analysis of chromatin proteomes in disease. J Vis Exp 70: 4294, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monte E, Mouillesseaux K, Chen H, Kimball T, Ren S, Wang Y, Chen JN, Vondriska TM, Franklin S. Systems proteomics of cardiac chromatin identifies nucleolin as a regulator of growth and cellular plasticity in cardiomyocytes. Am J Physiol Heart Circ Physiol 305: H1624–H1638, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagandla H, Lopez S, Yu W, Rasmussen TL, Tucker HO, Schwartz RJ, Stewart MD. Defective myogenesis in the absence of the muscle-specific lysine methyltransferase SMYD1. Dev Biol 410: 86–97, 2016. [DOI] [PubMed] [Google Scholar]
- 36.Olson EN, Backs J, McKinsey TA. Control of cardiac hypertrophy and heart failure by histone acetylation/deacetylation. Novartis Found Symp 274: 3–12; discussion 13–19, 152–155, 272-156, 2006. [PubMed] [Google Scholar]
- 37.Olson EN, Schneider MD. Sizing up the heart: Development redux in disease. Genes Dev 17: 1937–1956, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Paige SL, Thomas S, Stoick-Cooper CL, Wang H, Maves L, Sandstrom R, Pabon L, Reinecke H, Pratt G, Keller G, Moon RT, Stamatoyannopoulos J, Murry CE. A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell 151: 221–232, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res 90: 1044–1054, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev 12: 331–343, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446: 444–448, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res 89: 20–25, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature 407: 221–226, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Stewart MD, Lopez S, Nagandla H, Soibam B, Benham A, Nguyen J, Valenzuela N, Wu HJ, Burns AR, Rasmussen TL, Tucker HO, Schwartz RJ. Mouse myofibers lacking the SMYD1 methyltransferase are susceptible to atrophy, internalization of nuclei and myofibrillar disarray. Dis Model Mech 9: 347–359, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan X, Rotllant J, Li H, De Deyne P, Du SJ. SmyD1, a histone methyltransferase, is required for myofibril organization and muscle contraction in zebrafish embryos. Proc Natl Acad Sci USA 103: 2713–2718, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest 123: 37–45, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voelkel T, Andresen C, Unger A, Just S, Rottbauer W, Linke WA. Lysine methyltransferase Smyd2 regulates Hsp90-mediated protection of the sarcomeric titin springs and cardiac function. Biochim Biophys Acta 1833: 812–822, 2013. [DOI] [PubMed] [Google Scholar]
- 49.Wamstad JA, Alexander JM, Truty RM, Shrikumar A, Li F, Eilertson KE, Ding H, Wylie JN, Pico AR, Capra JA, Erwin G, Kattman SJ, Keller GM, Srivastava D, Levine SS, Pollard KS, Holloway AK, Boyer LA, Bruneau BG. Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell 151: 206–220, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei JQ, Shehadeh LA, Mitrani JM, Pessanha M, Slepak TI, Webster KA, Bishopric NH. Quantitative control of adaptive cardiac hypertrophy by acetyltransferase p300. Circulation 118: 934–946, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wohlschlaeger J, Schmitz KJ, Schmid C, Schmid KW, Keul P, Takeda A, Weis S, Levkau B, Baba HA. Reverse remodeling following insertion of left ventricular assist devices (LVAD): A review of the morphological and molecular changes. Cardiovasc Res 68: 376–386, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110: 479–488, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang QJ, Chen HZ, Wang L, Liu DP, Hill JA, Liu ZP. The histone trimethyl lysine demethylase JMJD2A promotes cardiac hypertrophy in response to hypertrophic stimuli in mice. J Clin Invest 121: 2447–2456, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]