This study features quantitative phenotyping of cardiac neural crest ablation-induced great vessel defects and valvular defects at four-chambered stages, as well as structural and functional abnormalities in cardiac cushions and blood flow at cardiac looping stages. The imaging tool used to make these measurements was optical coherence tomography.
Keywords: cardiac cushion, cardiac function, cardiac neural crest, cardiac valve, optical coherence tomography
Abstract
Cardiac neural crest cell (CNCC) ablation creates congenital heart defects (CHDs) that resemble those observed in many syndromes with craniofacial and cardiac consequences. The loss of CNCCs causes a variety of great vessel defects, including persistent truncus arteriosus and double-outlet right ventricle. However, because of the lack of quantitative volumetric measurements, less severe defects, such as great vessel size changes and valve defects, have not been assessed. Also poorly understood is the role of abnormal cardiac function in the progression of CNCC-related CHDs. CNCC ablation was previously reported to cause abnormal cardiac function in early cardiogenesis, before the CNCCs arrive in the outflow region of the heart. However, the affected functional parameters and how they correlate with the structural abnormalities were not fully characterized. In this study, using a CNCC-ablated quail model, we contribute quantitative phenotyping of CNCC ablation-related CHDs and investigate abnormal early cardiac function, which potentially contributes to late-stage CHDs. Optical coherence tomography was used to assay early- and late-stage embryos and hearts. In CNCC-ablated embryos at four-chambered heart stages, great vessel diameter and left atrioventricular valve leaflet volumes are reduced. Earlier, at cardiac looping stages, CNCC-ablated embryos exhibit abnormally twisted bodies, abnormal blood flow waveforms, increased retrograde flow percentage, and abnormal cardiac cushions. The phenotypes observed in this CNCC-ablation model were also strikingly similar to those found in an established avian fetal alcohol syndrome model, supporting the contribution of CNCC dysfunction to the development of alcohol-induced CHDs.
NEW & NOTEWORTHY
This study features quantitative phenotyping of cardiac neural crest ablation-induced great vessel defects and valvular defects at four-chambered stages, as well as structural and functional abnormalities in cardiac cushions and blood flow at cardiac looping stages. The imaging tool used to make these measurements was optical coherence tomography.
congenital heart defects (CHDs) are a major public health concern, since they affect ∼0.9% of newborns and account for about one-third of all birth defects worldwide (47, 66). The cause of CHDs is known to be multifactorial, but the steps leading to CHDs are not well understood. Many craniofacial syndromes include CHDs and are caused by genetic and/or environmental factors. Two such syndromes are DiGeorge syndrome, which is connected to a microdeletion in the 22q11 region of the chromosome, and fetal alcohol syndrome (FAS), which results from prenatal ethanol exposure. Both syndromes are recognized to be associated with disorders of the neural crest, a tissue composed of a transient, multipotent, migratory cell population involved in the development of many organs and systems (34, 60).
The role of neural crest cells (NCCs) in heart development has been illuminated through NCC ablation experiments in avian embryos (23, 38, 65). Deletion of NCCs between the mid-otic placode and somite 3 was found to consistently cause abnormalities in heart development; therefore, these cells are defined as cardiac NCCs (CNCCs) (35). Ablation of CNCCs leads to conotruncal defects such as persistent truncus arteriosus (PTA), double-outlet right ventricle, and many other types of great vessel defects, including absence, interruption, and incorrect branching (35).
Cardiac valve defects have not been investigated as extensively in CNCC-ablation models. Abnormal valve structure and function can lead to reduced cardiac output due to increased regurgitation (9) and result in severe heart dysfunction that can become life-threatening unless the valves are repaired or replaced (10, 11). Neural crest-derived cells are found in cardiac valves (50, 55), and valvular defects of various kinds have been occasionally reported in CNCC-ablation models (3, 25). However, detailed valve morphology and quantitative measurements have not been reported in previous studies, likely because of the lack of technology to image these small structures in three dimensions (3D).
Perturbations in early cardiac function may contribute to the progression of cardiac valve defects. For example, changes in retrograde flow during early cardiogenesis can affect klf2a expression and valve development (67). Hemodynamics have also been known to influence the development of cardiac cushions, which are valve progenitors (24, 48). Previous findings of abnormal cardiac function in CNCC-ablation models (40–42, 61) did not establish an association to valve development. The abnormalities assayed included increased blood flow velocity, decreased systolic and diastolic blood pressure, and depressed contractility (shortening fractions and ejection fractions). Parameters closely associated with valve development, such as retrograde flow and cardiac cushion development, were not as thoroughly investigated, perhaps because the available imaging technology restricted the measurements that could be made accurately or, until recently, because cardiac function was often overlooked as a contributing factor in the etiology of CHDs in favor of gene regulation.
Optical coherence tomography (OCT), which provides high-resolution (2–30 μm) 3D imaging at high speed, a field of view (1–3 mm), is well suited to embryonic imaging, including blood flow imaging (27, 32, 43, 64, 70). OCT is noninvasive and can image embryos cultured under near-physiological conditions. By treating ex vivo tissues with optical clearing methods, the field of view of OCT imaging can be significantly extended (39). Because of these features, OCT has been extremely useful in studies of avian heart development and CHD phenotyping (26, 30, 32).
In this study we aimed to phenotype and quantify defects in cardiac valves following CNCC ablation and to investigate the events that precede and could be early signs of the valve defects. We assayed abnormal hemodynamic parameters that are known to cause valve defects, as well as the structure and volume of cardiac cushion formation. Quail embryos, which offer access to the neural crest for easy and spatiotemporally specific manipulations, have been chosen for this study. They are also economical and highly accessible and can be cultured in ovo or in shell-less culture under physiological conditions, allowing imaging at specific embryonic stages without the requirement that the embryos be euthanized. OCT imaging was used to assay early and late developmental stages of quail embryonic hearts.
MATERIALS AND METHODS
Ethics statement.
Institutional Animal Care and Use Committee (IACUC) approval was not necessary for this study, because the embryos were euthanized prior to 3 days before hatching. The incubation period for quails is typically 17–18 days. We conducted experiments on incubation days 3 and 8, which were well before the stage requiring IACUC approval.
Embryo preparation and CNCC ablation.
Fertilized quail eggs (Coturnix coturnix; Boyd's Bird, Pullman, WA) were incubated in a humidified, forced-draft incubator (GQF Manufacturing, Savannah, GA) at 38°C until Hamburger and Hamilton (HH) stage 8–9 (∼30 h of incubation) (17, 38). The eggs were opened and placed in a shell-less culture in a sterilized 35-mm petri dish, and embryos were stained with neutral red (37). Similar to previous established methods, the bilateral neural folds from mid-otic placode to somite 3 were laser-ablated under observer guidance using a microscope (38). Continuous-wave, 35-mW infrared light at 1,465 nm (QPhotonics, Ann Arbor, MI) and visible pilot light were focused onto the embryo through a ready-to-use compact scan head (45). The laser spot size was estimated to be 200 μm on the surface of the embryo, resulting in irradiance of 1.1 × 106 W/cm2. The laser spot was scanned at 1 Hz for 20–25 s on each side of the neural crest. The embryos were returned to the incubator after ablation until the desired stages. To confirm the extent of the ablation area, a cohort of embryos was examined using propidium iodide staining to assess the specificity and efficacy of the laser ablation to the NCCs. Immediately after CNCC ablation, 20 μl of propidium iodide at a concentration of 10 μg/ml were applied to the embryo. After 5 min, fluorescence signals were imaged. Bright-field and fluorescence images were collected sequentially, and the regions that exhibited strong fluorescence were confirmed to be the regions of CNCCs. A group of sham control embryos were exposed to all other treatments except the scanning infrared laser exposure. A second control group of embryos that were not stained with neutral red were also included in the experiment to determine if the neutral red treatment affected the parameters that were assayed. There was no difference in any part of the results between the two control groups, so we present only results from the sham control group. Operations, assays, and postprocessing procedures are described below and summarized in Table 1.
Table 1.
A summary of operation, assay, and postprocessing of a cohort of embryos for day 3 analysis and a cohort of embryos for day 8 analysis
| Cohort 1: | Day 1 (HH stage 8–9) |
Day 8 (HH stage 34–35) |
Postprocessing | ||
|---|---|---|---|---|---|
| Operation | Assay | Purpose | Sample size (sham, experimental) | ||
| Sham/ablation | OCT volume imaging ex vivo | Great vessels | n = 8, 18 | Morphology analysis, diameter quantification | |
| Valves | n = 9, 24 | Segmentation, morphology analysis, quantification of leaflets | |||
| Cohort 2: | Day 1 (HH stage 8–9) |
Day 3 (HH stage 19–20) |
Postprocessing | ||
|---|---|---|---|---|---|
| Operation | Assay | Purpose | Sample size (sham, experimental) | ||
| Sham/Ablation | Pulsed Doppler OCT in vivo | Flow | n = 8, 26 | Waveform analysis and retrograde flow quantification | |
| OCT volume imaging in vivo | Body flexure | n = 8, 26 | Combining images | ||
| OCT volume imaging ex vivo | Cardiac cushions | n = 5, 10 | Morphology analysis and quantification | ||
HH, Hamburger and Hamilton; OCT, optical coherence tomography.
Imaging and analysis of late-stage septated hearts.
Cohorts of control and CNCC-ablated embryos were incubated in shell-less culture until HH stage 34–35, when the embryonic heart would normally have completed septation to become four-chambered. At these stages, the great vessels should have completed the major steps in morphogenesis and achieved their mature asymmetric branching pattern. The left atrioventricular (AV) valve leaflets should also be distinct, but not completely thinned. Hearts from control and experimental groups were excised, fixed in formalin, and optically cleared based on a ClearT protocol (30, 39). Optically cleared hearts were imaged with a linear-in-wave number spectral domain OCT system (21, 28, 30). The light source of the OCT system has a center wavelength at 1,310 nm and a full-width at half-maximum bandwidth of 75 nm. The axial and lateral resolutions of the system are ∼10 μm in air. The line rate of the OCT system is 47 kHz. 3D data sets acquired from OCT imaging were read into Amira software (Visualization Sciences Group, FEI, Burlington, MA) for 3D reconstruction, visualization, and segmentation. Great vessel outer diameters were manually measured on two-dimensional cross sections that were orthogonal to the vessels. Vessel inner diameters were not measured, because they often appeared to have irregular shapes that were difficult to quantify. Left AV valve leaflets were manually segmented, and the volume was computed.
Structural and functional imaging of embryos at the cardiac looping stage.
Control and CNCC-ablated embryos in shell-less culture were moved from the incubator into a customized environmentally controlled OCT imaging chamber (28) at HH stage 19–20 (late cardiac looping stage) to maintain physiological conditions. First, whole embryos were imaged with OCT to visualize body flexure and accurately stage the embryos. Three 4 × 4 mm regions were imaged for each embryo to cover the entire body area. In postprocessing, projection images were generated from the OCT volumes by volume rendering in Amira software. These projection images were stitched together to generate a single image of the entire embryo. Staging of the embryos was primarily based on limb development (2). Other features, such as body/head curvatures, can be significantly altered in the CNCC-ablated embryos and are not reliable features for staging. After whole body imaging, pulsed Doppler traces were acquired from the right vitelline artery of each embryo by OCT M-mode Doppler imaging. The flow patterns of the vitelline arteries have been reported to reflect cardiac function (16, 31). The percentage of retrograde flow was calculated as the ratio of negative to positive flow area (16, 31). Morphology phenotypes were determined from the flow traces by three blinded experts. Finally, after OCT M-mode Doppler imaging, the whole embryo was dissected from the yolk and fixed in formalin, and the hearts were imaged with OCT. The hearts were dissected to preserve the attachments to the body to maintain their shape and orientation during imaging. Segmentation and analysis of the cardiac cushions were performed using Amira software.
Statistical analysis.
For data acquired from day 8, four-chambered hearts, Student's t-test was used for great vessel and valve volume measurements (P < 0.05). For data from day 3, cardiac looping-stage embryos, Student's t-test was used to compare percentage of retrograde flow with cushion volumes (P < 0.05). Correlation coefficients were calculated using Matlab (MathWorks, Natick, MA). Cushion morphology was compared and cushion and flow phenotypes were correlated using Fisher's exact test.
RESULTS
CNCC-ablated embryos developed neural crest-related CHDs, such as great vessel defects.
Cohorts of experimental and control embryos were allowed to develop until HH stage 34–35 (day 8), after normal completion of cardiac septation, great vessel branching, and valve leaflet differentiation. The survival rates of the sham control and experimental groups were 55% (11 of 20) and 41% (24 of 58), respectively. Gross body defects were identified during dissection. Heart defects were diagnosed using two-dimensional sections and 3D volume reconstructions acquired from OCT imaging. Embryo phenotypes are summarized in Table 2. When the hearts were excised, the great vessels were cut ∼2 mm from the heart. Therefore, defects in the distal portion of the great vessels are not identified here. Examples of 3D volume reconstruction of great vessel defects are shown in Fig. 1, A–D. Control embryos at this stage have five great vessels: right brachiocephalic artery (RBA), left brachiocephalic artery (LBA), aortic arch (AA), right pulmonary artery, and left pulmonary artery. The RBA, LBA, and AA branch off the aortic trunk from the left ventricle, and the right and left pulmonary arteries branch off the pulmonary trunk from the right ventricle. Both trunks are located in the middle of the base of the heart. In PTA, the pulmonary and aortic vessels branch from a single trunk, and a ventricular septal defect (VSD) is present. No other VSDs, except PTA and double-outlet right ventricle hearts, were observed, although it is possible that small VSDs cannot be identified without flow data. Missing one or more vessels is categorized as “absent great vessel defects,” although, in some cases, the absent vessels may branch at locations beyond the imaging field of view (high take-off) and, thus, were not truly “missing.” Other defects were great vessel stenosis and incorrect branching, which sometimes coexisted in the same heart. For example, both PTA and absent great vessel defect were present in a few cases.
Table 2.
Day 8 (HH stage 34) embryo phenotypes
| Parameter | Group |
|
|---|---|---|
| Sham | CNCC-ablated | |
| No. of survivors | 11 | 24 |
| Survival rate | 55% | 41% |
| Normal body | 10 (91%) | 18 (75%) |
| Unfused chest wall | 1 (9%) | 5 (21%) |
| Twisted body | 0 (0%) | 1 (4%) |
| Heart with obvious defects | 0 (0%) | 11 (46%) |
| PTA | 0 (0%) | 6 (25%) |
| DORV | 0 (0%) | 2 (8%) |
| Absent great vessel | 0 (0%) | 4 (17%) |
| Great vessel stenosis | 0 (0%) | 3 (13%) |
| Incorrect great vessel branchinga | 0 (0%) | 1 (4%) |
CNCC, cardiac neural crest cell; PTA, persistent truncus arteriosus; DORV, double-outlet right ventricle.
Incorrect great vessel branching other than the types listed above.
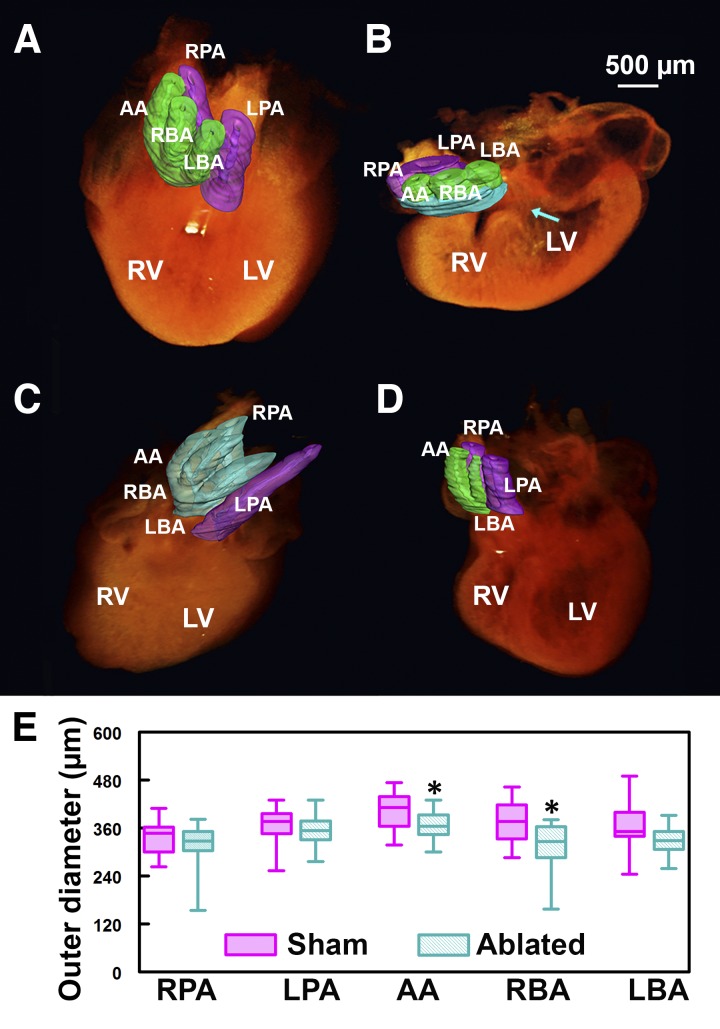
Fig. 1.
Cardiac neural crest cell (CNCC)-related great vessel defects. A–D: 3-dimensional (3D) optical coherence tomography (OCT) volume reconstructions and great vessel segmentation. Green, aortic trunk vessels; purple, pulmonary trunk vessels; cyan, branching errors. A: control embryonic heart. B: an example of persistent truncus arteriosus (PTA). Cyan-colored region at the great vessels represents common trunk; arrow points to ventricular septal defect. C: example of incorrect branching. Aortic trunk branches into 4, instead of 3, vessels, and pulmonary trunk branches into 1, instead of 2, vessels. D: absent great vessel. E: box plot of great vessel outer diameter measurements. Box represents 25–75%; whiskers represent range. Aortic arch (AA) and right brachiocephalic artery (RBA) are significantly smaller in CNCC-ablated than control group. *P < 0.05. LBA, left brachiocephalic artery; RPA, right pulmonary artery; LPA, left pulmonary artery; LV, left ventricle; RV, right ventricle.
We also made quantitative measurements of hearts with all five great vessels, in addition to qualitative assessment of the great vessel defects (Fig. 1E). The outer diameters of the AA and RBA are significantly smaller (by 11% and 16%, respectively) in the CNCC-ablated than control embryos. The diameter of the LBA has a decreasing trend without reaching statistical significance, possibly because of large biological variances.
CNCC-ablated embryos developed left AV valvular defects.
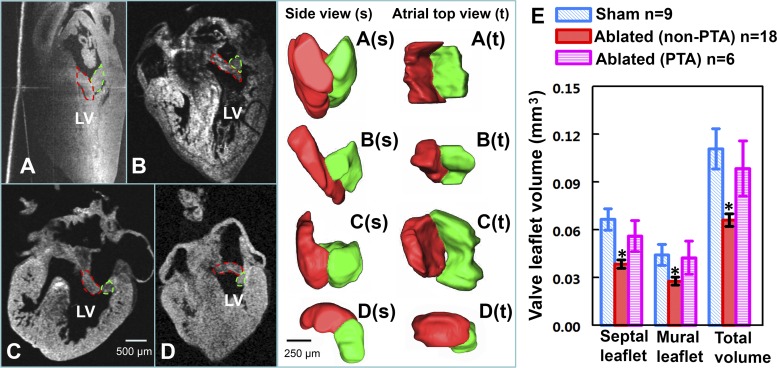
Left AV valvular defects were consistently observed in CNCC-ablated embryos. At HH stage 34–35, after optical clearing, the internal structures of the heart, including the septum, trabeculae, and valves, can be clearly identified in OCT cross sections. Normally developed left AV valve leaflets at this stage are relatively thick and consist of a longer septal leaflet and a short and thick mural leaflet. Together, these two leaflets form a V-shaped structure, which results in a relatively large contact region for the surfaces of the valve leaflets (Fig. 2A). This orientation of the two leaflets ensures unobstructed blood flow from the atrium to the dilating ventricle and impedes regurgitant flow when the ventricle contracts. Cardiac function can be aberrant if the leaflets are malformed or the positions of the two leaflets are abnormal. One obvious potential consequence is regurgitation, which, in the long term, may lead to more severe defects. Almost all embryos from the CNCC-ablated group presented some degree of left AV valvular abnormalities (Fig. 2). One heart (Fig. 2B) had very thin septal and mural leaflets. Another heart had PTA, with the two valve leaflets forming a bowl shape, rather than the normal V shape (Fig. 2C). This resulted in very little contact of the two valve leaflets along their sides. A small gap between the two valve leaflets was detected from a rotated view of valve leaflet segmentation. This specific phenotype was detected in five of six PTA hearts, with gaps of various sizes. In another example, the relative position of the valve leaflets was at a reversed V-shaped orientation (Fig. 2D). Overall, leaflet volumes (septal, mural, and total) were significantly smaller in the CNCC-ablated than control group. Interestingly, the leaflet volumes of PTA hearts (6 hearts) were quite similar to the controls, so they were considered separately. PTA and non-PTA experimental groups were each tested against the sham control group, and the results are shown in Fig. 2E. The non-PTA hearts from the ablation group showed significantly decreased septal leaflet volume (43% decrease, P < 0.05), mural leaflet volume (38% decrease, P < 0.05), and total volume (40% decrease, P < 0.05) compared with the sham controls. Valve leaflet volume in the PTA group was not significantly different from that in the control group.
Fig. 2.
CNCC-ablated embryos exhibit left atrioventricular (AV) valve defects. A–D: 2-dimensional OCT cross sections of 4-chambered hearts from a sham control embryo (A) and 3 CNCC-ablated embryos (B–D). Left AV valve leaflets are outlined in red (septal valve leaflets) and green (mural valve leaflets) dashed lines. A(s)–D(s): side views of 3D reconstructions of the left AV valve leaflets that correspond to A–D. A(t)–D(t): views of the atrial side. E: volume of left AV valve leaflets for 9 sham control embryos, 18 CNCC-ablated embryos that do not have PTA, and 6 PTA hearts from the CNCC-ablated embryos. Error bars, SE. *P < 0.05.
CNCC-ablated embryos developed body flexure abnormalities at early stages.
At HH stage 19–20, the survival rate for sham control and ablated embryos was 83% and 68%, respectively.
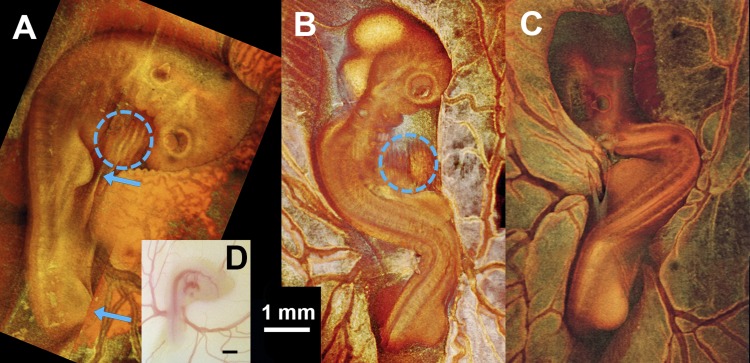
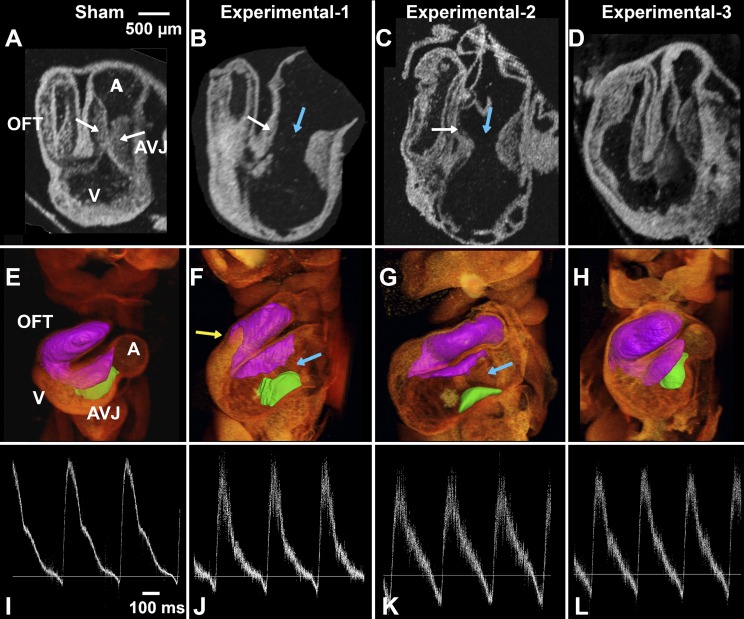
Body flexure abnormalities at late looping stages (HH stage 18–20) were mentioned in previous CNCC-ablation studies (46, 65), yet they were neither described nor presented. At this stage, control embryos on the yolk (Fig. 3A) usually have a relatively straight trunk, with the right side of the head exposed and the head curving smoothly, with the looped heart easily observable on the right side of the body tucked under the head (blue dashed-line circles in Fig. 3). The right wing and leg buds are located at the middle and end of the trunk, both pointing forward (blue arrows in Fig. 3A). CNCC-ablated embryos (16 of 26), in contrast, displayed a different degree of body twisting and rotation (Fig. 4, B and C). In a substantial number of cases (8 of 26), the head and/or the heart was buried under the body (Fig. 3C).
Fig. 3.
Whole embryo images demonstrating body flexure [Hamburger and Hamilton (HH) stage 19–20]. A–C: projection images of a sham control embryo (A) and 2 CNCC-ablated embryos (B and C) from 3D OCT volume rendering. D: microscope photo of embryo shown in A. Blue arrows point to limb buds that were used for staging embryos. Blue dashed-line circles, embryonic hearts (with artifacts due to the beating motion). Heart in C is not visible. Limb morphology indicates that all 3 embryos are at HH stage 19–20, closer to HH stage 20.
Fig. 4.
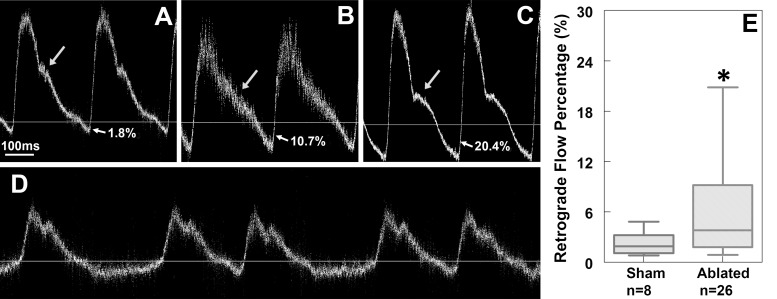
CNCC-ablated embryos have abnormal flow profiles at HH stage 19–20. A–D: pulsed Doppler traces from 1 sham control embryo and 3 CNCC-ablated embryos. A pulse Doppler trace from a control embryo typically has a large positive peak (forward flow) with a prominent shoulder (arrow in A) and a very small negative peak (retrograde flow). Retrograde flow percentage (indicated by arrows and percentages in A–C) is calculated as the ratio of negative to positive peak area. In some CNCC-ablated embryos, the prominent shoulders (arrows in A and C) disappear (arrow in B). Retrograde flow percentages of the 2 examples are 10.7% and 20.4% for B and C, respectively. D: pulse Doppler trace from another experimental embryo that exhibited an arrhythmia. E: box plot of retrograde flow percentage for control and experimental groups. Box represents 25–75%; whiskers represent range. *P < 0.05.
CNCC-ablated embryos exhibited abnormal flow at cardiac looping stages.
At HH stage 19–20, M-mode Doppler OCT images were obtained from the right vitelline artery of each embryo. These particular stages were chosen for live imaging assays, because embryos at these stages are undergoing important cardiogenesis events, such as endothelial-to-mesenchymal transition in the endocardial cushions and conduction repatterning (7, 49). As shown in the control embryos and previously reported healthy embryos, a pulsed Doppler trace recorded from a normally developing embryo consists of a positive peak, which is forward flow, and a small, but clearly detectable, negative peak, which is retrograde flow (Fig. 4A) (16, 31). Quantitative measurements showed that the retrograde flow percentage (ratio of the area of the negative peak to the area of the positive peak) in normal embryos was consistently low (∼3%). The CNCC-ablated embryos, in contrast, were found to have significantly increased percentages of retrograde flow with a large variability in that flow (Fig. 4, B, C, and E). Also, control embryos usually had a shoulder at around the middle of the falling slope of forward flow (arrow in Fig. 4A). In contrast, this shoulder was sometimes absent, reduced, or at a different position in the CNCC-ablated group (Fig. 4, B and C). We also observed one CNCC-ablated embryo with arrhythmia (Fig. 4D). There was no difference between the control and ablated groups in heart rate (216 ± 27 and 223 ± 14 beats/min, respectively, P = 0.37). No correlation was found between body flexure abnormalities and retrograde flow measurements.
CNCC-ablated embryos developed abnormal cardiac cushions at cardiac looping stages.
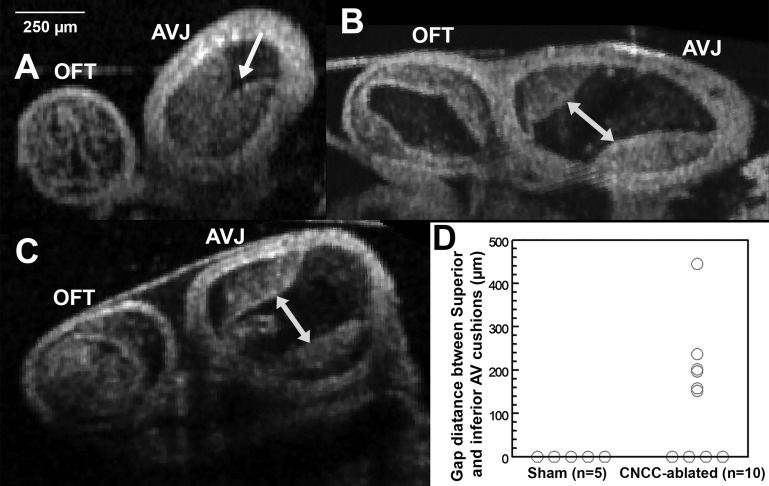
Abnormal flow waveforms and increased levels of percent retrograde flow in CNCC-ablated embryos suggest anomalies in cardiac cushion function and morphology. After the functional pulsed Doppler assay, each embryo was dissected, fixed, and prepared for OCT structural imaging (Fig. 5). Cardiac cushions from volumetric images of 5 control and 10 CNCC-ablated embryos were manually segmented in Amira software. Cardiac cushions at the AV junction were segmented into two regions: the inferior AV cushion, which is a clearly delineated region, and the superior AV cushion, which is connected to the cushions in the outflow tract. The inferior and superior AV cushions in control embryos are thick and usually contact each other, resulting in a largely occluded lumen (Fig. 6A). The outflow tract cushions of control embryos (5 of 5) are usually thick and cover the length of the outflow tract. In the experimental group, 6 of 10 embryos clearly showed significant gaps between the inferior and superior AV cushions and much reduced lumen coverage, as shown in 2-dimensional and 3D images (Fig. 5, B, C, F, G, J, and K) and cross-sectional images (Fig. 6). AV gap measurements are shown in Fig. 6D. Furthermore, 6 of 10 experimental embryos showed reduced luminal coverage of the outflow tract cushions. Fisher's exact test suggested that the gap phenotype and the reduced outflow tract cushion coverage phenotype of the experimental group are significantly different from controls (Table 3). Four of six hearts showed both AV cushion gap phenotype and reduced outflow tract cushion coverage, yet these two types of defects did not correlate with each other (correlation coefficient = 0.58, P = 0.10). Although the cushions appeared to be quite different in structure, calculated cushion volumes were not significantly different between the two groups (P = 0.13). One explanation is that the CNCC-ablated group developed dilated hearts (cf. Fig. 6, B and C, with Fig. 6A) and much larger lumen areas (cf. Fig. 6, B and C, with Fig. 6A). Therefore, a similar cardiac cushion volume was spread over a much larger region and failed to occlude the lumen.
Fig. 5.
CNCC-ablated embryos have abnormal endocardial cushions at HH stage 19–20. A–D: 2-dimensional OCT sections of HH stage 19–20 embryonic hearts. E–H: 3D OCT volume rendering and cushion segmentations [inferior AV cushion (green) and fusion of superior AV cushion and outflow tract cushions (purple)] of HH stage 19–20 embryonic hearts. I–L: flow traces recorded in vivo. Panels in each column show data from the same embryo in vivo (pulse Doppler trace) and ex vivo (3D heart structural imaging) as labeled on the top of each column. A, E, and I: sham control embryo; B–D, F–H, and J–L: 3 experimental embryos. White arrows, superior and inferior cushions (thick cushions that touch each other in the control embryo and thin cushions in ablated embryos); blue arrows, large gaps between superior and inferior AV cushions in the ablated embryos (B, C, F, and G); yellow arrow, reduced cushion coverage in an ablated embryo. D: CNCC-ablated embryo that appeared to have relatively normal cushions. A, atrium; AVJ, AV junction; V, ventricle; OFT, outflow tract.
Fig. 6.
OCT cross-sectional images of AV cushions. A: control embryonic heart. B and C: CNCC-ablated embryonic hearts. Arrow in A, inferior and superior AV cushions contact each other and fill most of the lumen; double-headed arrows in B and C, gaps between inferior and superior AV cushions in ablated hearts. D: gap distance measurements. All 3 images share the same scale bar on the top left corner.
Table 3.
Analysis of cardiac cushion phenotypes, including the gap phenotype between cushions at the AV junction and reduced outflow tract cushion coverage phenotype of the luminal surface
| Cushion Morphology Analysis |
|||
|---|---|---|---|
| Inferior and superior AV cushion |
P value (2-tailed) | ||
| No gap | Gap | ||
| Sham | 5 | 0 | 0.044 |
| Ablation | 4 | 6 | |
| Outflow tract cushion | |||
|---|---|---|---|
| Full coverage | Reduced coverage | P value (2-tailed) | |
| Sham | 5 | 0 | 0.044 |
| Ablation | 4 | 6 | |
AV, atrioventricular.
Potential links between flow waveform and cushion development at late cardiac looping stages.
Because the same embryos provided both pulsed Doppler flow measurements and cushion measurements, these two data sets can be compared and correlated. Phenotypes and measurements considered here are shoulder phenotypes (normal or abnormal) and retrograde flow percentage from the flow trace, correlated with AV cushion phenotypes (gap or no gap in the AV cushions and full or reduced coverage in the outflow tract cushions) and cushion volumes from the cardiac cushion analysis. Among 15 embryos, including sham and CNCC-ablated embryos, for which cushion volumes were measured, in 2 embryos, flow traces were too noisy to allow us to confidently distinguish the presence of the shoulder feature and, therefore, were excluded from this analysis. All control embryos had low levels of percent retrograde flow, no or negligible gap between inferior and superior AV cushions, and complete outflow tract cushion coverage. Among the embryos that had significant gaps between inferior and superior AV cushions, a shoulder was absent in pulsed Doppler flow traces from four of five embryos. All eight embryos that had no gaps between AV cushions had normal shoulders in their pulse Doppler traces. Fisher's exact test suggested a strong correlation between the cushion gap phenotype and the shoulder feature in pulsed Doppler traces (see examples in Fig. 5 and summary of correlation in Table 4). This indicates that the normal AV cushion morphology while the heart is beating might be responsible for the shoulder feature in the flow pattern. No correlation was found between the AV cushion gap and increased levels of percent retrograde flow. No correlation was observed between outflow tract cushion coverage (P = 0.27) and percent retrograde flow, nor was retrograde flow percentage significantly correlated with total cushion volume (P = 0.14). The cause of increased retrograde flow percentage might be more complicated than that determined here by a single parameter.
Table 4.
Correlation between AV cushion gap and flow shoulder phenotype
| AV Cushion/Flow | Shoulder |
Correlation Coeff | P Value | |
|---|---|---|---|---|
| Normal | Abnormal | |||
| Gap | 1 | 4 | 0.843 | 0.007 |
| No gap | 8 | 0 | ||
DISCUSSION
Late-stage heart defects in our CNCC-ablation quail model were consistent with documented CHDs in NCC-related clinical syndromes, as well as previous CNCC-ablation models in other species such as chicken or mouse (6, 23). These defects involve a spectrum of great vessel abnormalities (23). Previous studies have mostly focused on patterning defects of the great vessels and the associated changes in molecular expression (14, 36, 51, 62). More detailed structural alterations were largely ignored. Changes in great vessel dimensions may be important indicators or predictors of more severe diseases such as compromised cardiac function. We have quantitatively measured great vessel diameters and detected significantly decreased great vessel sizes in the CNCC-ablation model. CNCCs play an important role in repatterning the AA arteries into the mature great arteries as well as providing smooth muscle cells for the great arteries themselves (22, 35). This decrease in vessel diameter may be due to fewer CNCCs reaching and populating the AA mesenchyme. These “minor” alterations may change the relative flows through each branch, which may lead to more severe consequences in the future, although further research is needed to test this hypothesis.
We also qualitatively and quantitatively phenotyped valvular defects. Changes in valve morphology and/or the relative position of leaflets are important indicators for impaired cardiac function (19). Any failure in valve closure can cause leakage and increase retrograde flow, reducing cardiac output and, potentially, causing heart failure (54). Abnormal morphology, as well as decreased volume, of the left AV valves in the CNCC-ablated embryos can be an indication of disrupted cardiac function. Such abnormalities at this stage may result in more severe problems in further development of the valves, the heart, and even the rest of the body.
CHDs can be initiated by genetic or functional abnormalities at very early stages (12, 31). In this study we investigated early onset of cardiac abnormalities with regard to early cardiac hemodynamic function and cardiac cushions. With Doppler OCT, we were able to detect blood flow profiles, measure retrograde flow percentage, and correlate the flow profile to cardiac cushion structure at cardiac looping stages. We consistently observed the prominent shoulder feature in pulse Doppler flow traces from normally developing looping-stage embryos (16, 31) and the absent shoulder phenotype in CNCC-ablated embryos. On the basis of the shape and timing, the shoulder in the flow profile is thought to be caused by AV cushion closure. This is a concept similar to the dicrotic notch in aortic pressure graphs of mature heart cycles. Because it was difficult to directly confirm the timing and shoulder feature and AV cushion closure in HH stage 19 hearts because of the motion of the heart and lack of imaging depth in the presence of blood, the AV cushion morphology of dissected hearts was examined as an alternative. Our results showed that the absence of prominent shoulders was correlated with the AV cushion gap phenotype. This suggested that, in CNCC-ablated embryos, the AV cushions could fail to appose and close the lumen properly during the heartbeat, leading to abnormal flow in the AV junction and a pattern change in the overall flow profile (5). We did not detect a significant decrease in cardiac cushion volume. The gaps are more likely caused by abnormal cushion distribution or changes in the overall shape and size of the AV canal, rather than simply the volume of the cushions. Heart dilation was reported to be a compensatory mechanism for decreased heart function (41). In our experiment, CNCC-ablated embryos also suffered from dilated heart tubes, where normal volume of the cardiac cushion may be inadequate to close the lumen properly (Fig. 6). Our results are consistent with those of Nomura-Kitabayashi et al. (52), who used ultrasound and micro-CT to measure flow and cushion thickness at embryonic day 11.5 (comparable to an avian developmental stage later than HH stage 19) in a NCC-deficient mouse model. They found that NCC-deficient mice exhibited retrograde flow at the dorsal aorta and thinner cardiac cushions than the wild-type mice.
Early hemodynamics and cardiac cushions are critical factors for outflow tract and cardiac valve development. Although this study does not directly provide causal associations between the early abnormalities and late-stage defects, numerous other studies have investigated the important role of early flow/cushion abnormalities in late-stage defects. For example, flow patterns and biomechanical forces can affect molecular signaling or gene expression and cardiogenesis (15, 18, 20, 56, 67). During cardiac development, immature valves and valve progenitors perform functions similar to mature valves in the adult heart (5, 52) and abnormal cardiac cushion formation/endothelial-to-mesenchymal transition eventually leads to defective valves (7). Overall, the interplay among cardiac morphology, cardiac functions, and gene expression is critical in cardiogenesis (58, 67).
It is unclear whether cardiac function or morphology initiated the abnormalities that we observed at HH stage 19–20 in the CNCC-ablation model. Cardiac function and morphology are known to influence each other (20, 58). For example, alterations of retrograde flow in the zebrafish led to arrested valve growth and affected the normal expression of klf2a, a transcription factor that is crucial for normal valve development (67). On the other hand, it has been shown that cardiac cushion size/thickness by itself can impact signaling, gene expression, and electrical events in the early embryonic heart (4). In the avian embryo, CNCCs start to populate the pharyngeal arches at HH stage 13 and do not migrate to the heart until HH stage 25 (33). Which process occurs earlier in this CNCC-ablation model? One possibility is that abnormal blood flow leads to defective cardiac cushions. Blood flow occurs well before HH stage 19. The absence of CNCCs around pharyngeal arches may contribute to abnormal flow by altering resistance or compliance at earlier stages and, subsequently, cardiac cushion malformation at HH stage 19–20. Another possibility is that cushion morphology is altered before hemodynamics. It was suggested that altered fibroblast growth factor 8 signaling might be responsible for the early myocardial dysfunction (23). Abnormal cardiac jelly formation at HH stage 14 (68) could cause abnormal flow. In the future, assaying cardiac hemodynamic function and imaging cardiac jelly formation at even earlier stages may potentially resolve these two different possible causes.
In this study we also observed that CNCC-ablated embryos exhibited body flexure abnormalities at cardiac looping stages, which were previously mentioned (46, 65) but were not investigated in detail. In most studies the embryos with these deformities were not analyzed, because their hearts were usually under the body and difficult to visualize using conventional stereomicroscopes. However, the body twist can be a very important feature, as Männer et al. reported a significantly lower survival rate for these embryos than for CNCC-ablated embryos without a body flexure defect (46). Similar abnormal embryo body flexure was observed in other heart disease models, including zebrafish (59) and quail (31). In our FAS quail model (31), ethanol-exposed embryos exhibited body twisting very similar to that observed in this study, as well as similar abnormalities in hemodynamics. Ford et al. showed that optically pacing the looping-stage embryonic heart at a higher-than-intrinsic rate for 5 min at HH stage 14 (which caused ∼1 h of abnormal heart function, including high regurgitant flow) led to body flexure abnormalities at HH stage 19 and CHDs at late stages (13). This suggested that twisting of the body axis may be a secondary effect resulting from earlier abnormal cardiac functions or abnormal cardiac looping. This evidence of early body phenotypes in heart disease models is consistent with the clinical finding of the correlation of spinal abnormalities to cardiac defects (1a, 44).
Besides the body twisting, many early- and late-stage phenotypes of the CNCC-ablation model strikingly resemble our FAS quail model (30, 31). At HH stage 34–35, defects such as abnormal great vessels and incorrect great vessel alignment were observed in both models. Measurements of the outer diameters showed 6–10% and 11–16% decreases in the FAS and CNCC-ablation models, respectively, for the aortic branches, where the smooth muscle cells are mainly derived from CNCCs. Another late-stage phenotype identified in both models was an abnormal left AV valve. Besides morphological similarities, the total volume of the valve leaflets of the FAS and CNCC-ablation models was decreased by 45% and 40%, respectively, as measured by 3D OCT imaging. This may be a direct effect of CNCC disorder or an indirect influence of abnormal early cardiac morphology and function. These early cardiac abnormalities include the lack of a prominent shoulder in the flow waveform, increased percent retrograde blood flow, and defective cardiac cushion formation. It was noticed many years ago that DiGeorge syndrome and FAS share common characteristics, including abnormalities of the face, cardiovascular system, central nervous system, and immune system (1, 63). Numerous studies have demonstrated the influence of alcohol exposure on the NCCs, which results in increased NCC apoptosis or impeded NCC migration signaling (8, 60, 69). The similarities described here between the quail FAS and CNCC-ablation models further support the theory that the reduction of NCCs may at least be partially involved in ethanol-induced birth defects, including CHDs (60).
In conclusion, we have quantitatively phenotyped the great vessels and left AV valvular defects in a CNCC-ablation model. Abnormal hemodynamic flow patterns and cardiac cushion formation were investigated as a potential mechanism for the valve defects observed at later stages. Similar defective phenotypes were observed in avian models of alcohol exposure and CNCC ablation, measured at looping and four-chambered stages. This supports the hypothesis that CNCCs play a role in alcohol-induced CHDs, with abnormal early cardiac hemodynamics and cardiac cushion development as a potential mechanism. While this study provides a correlation between structural and flow abnormalities at early and late stages, a causal link would require further studies. In future studies to investigate the association between early abnormalities and late-stage defects, we will use noninvasive OCT imaging to longitudinally follow hemodynamic parameters and structure of CNCC-ablated embryos. Better optical clearing techniques will facilitate the imaging of even older hearts and allow measurements on more mature valves and great vessels. It may become possible to image the heart intact to diagnose distal great vessel defects such as coarctation of the aorta and measure the dimensions of the arteries and branching. We will also investigate even earlier, more detailed aspects of cardiac function such as absolute blood flow and shear stress at the pharyngeal arches, which are specific CNCC migration sites (27, 53). These early functions may serve as reliable predictors of later developmental defects, potentially enabling rapid screening methods for studies of rescue/prevention interventions.
GRANTS
This project was supported by National Heart, Lung, and Blood Institute Grants R01 HL-083048, R01 HL-095717, R01 HL-126747, and R21 HL-115373. P. Ma was partially supported by a Chinese Government Scholarship. G. H. Karunamuni is supported by a postdoctoral fellowship from the American Heart Association.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.M., M.W., and A.M.R. developed the concept and designed the research; P.M. and S.G. performed the experiments; P.M. and S.G. analyzed the data; P.M., S.G., G.H.K., M.W.J., M.W., and A.M.R. interpreted the results of the experiments; P.M. prepared the figures; P.M. drafted the manuscript; P.M., S.G., G.H.K., M.W.J., M.W., and A.M.R. edited and revised the manuscript; P.M., S.G., G.H.K., M.W.J., M.W., and A.M.R. approved the final version of the manuscript.
REFERENCES
- 1.Ammann AJ, Wara DW, Cowan MJ, Barrett DJ, Stiehm E. The DiGeorge syndrome and the fetal alcohol syndrome. Am J Dis Child 136: 906–908, 1982. [DOI] [PubMed] [Google Scholar]
- 1a.Basu PS, Elsebaie H, Noordeen M. Congenital spinal deformity: a comprehensive assessment at presentation. Spine 27: 2255–2259, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Bellairs R, Osmond M. Atlas of Chick Development. New York: Academic, 2005. [Google Scholar]
- 3.Besson WT, Kirby ML, Van Mierop LH, Teabeaut JR. Effects of the size of lesions of the cardiac neural crest at various embryonic ages on incidence and type of cardiac defects. Circulation 73: 360–364, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Bressan M, Yang PB, Louie JD, Navetta AM, Garriock RJ, Mikawa T. Reciprocal myocardial-endocardial interactions pattern the delay in atrioventricular junction conduction. Development 141: 4149–4157, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butcher JT, McQuinn TC, Sedmera D, Turner D, Markwald RR. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res 100: 1503–1511, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 125: 813–824, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res 105: 408–421, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czarnobaj J, Bagnall KM, Bamforth JS, Milos NC. The different effects on cranial and trunk neural crest cell behaviour following exposure to a low concentration of alcohol in vitro. Arch Oral Biol 59: 500–512, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Dal-Bianco JP, Beaudoin J, Handschumacher MD, Levine RA. Basic mechanisms of mitral regurgitation. Can J Cardiol 30: 971–981, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral regurgitation: a multivariate analysis. Circulation 91: 1022–1028, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Enriquez-Sarano M, Tajik AJ, Schaff HV, Orszulak TA, Bailey KR, Frye RL. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation 90: 830–837, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Fishman MC, Chien KR. Fashioning the vertebrate heart: earliest embryonic decisions. Development 124: 2099–2117, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Ford SM, McPheeters MT, Wang YT, Gu S, Doughman YQ, Strainic JP, Rollins AM, Watanabe M, Jenkins MW. Pacing-induced congenital heart defects assessed by OCT (Abstract). Proc SPIE 2016, p. 9697. [Google Scholar]
- 14.Franz T. Persistent truncus arteriosus in the Splotch mutant mouse. Anat Embryol (Berl) 180: 457–464, 1989. [DOI] [PubMed] [Google Scholar]
- 15.Groenendijk BC, Hierck BP, Vrolijk J, Baiker M, Pourquie MJ, Gittenberger-de Groot AC, Poelmann RE. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ Res 96: 1291–1298, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Gu S, Jenkins MW, Peterson LM, Doughman YQ, Rollins AM, Watanabe M. Optical coherence tomography captures rapid hemodynamic responses to acute hypoxia in the cardiovascular system of early embryos. Dev Dyn 241: 534–544, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamburger V, Hamilton LH. A series of normal stages in the development of the chick embryo. Dev Dyn 195: 231–272, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Heckel E, Boselli F, Roth S, Krudewig A, Belting HG, Charvin G, Vermot J. Oscillatory flow modulates mechanosensitive klf2a expression through trpv4 and trpp2 during heart valve development. Curr Biol 25: 1354–1361, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol 73: 29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hove JR, Köster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421: 172–177, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hu Z, Rollins AM. Fourier domain optical coherence tomography with a linear-in-wave number spectrometer. Optics Lett 32: 3525–3527, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Hutson MR, Kirby ML. Model systems for the study of heart development and disease: cardiac neural crest and conotruncal malformations. In: Seminars in Cell and Developmental Biology. New York: Elsevier, 2007, p. 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutson MR, Kirby ML. Neural crest and cardiovascular development: a 20-year perspective. Birth Defects Res C Embryo Today 69: 2–13, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Icardo JM. Endocardial cell arrangement: role of hemodynamics. Anat Rec 225: 150–155, 1989. [DOI] [PubMed] [Google Scholar]
- 25.Jain R, Engleka KA, Rentschler SL, Manderfield LJ, Li L, Yuan L, Epstein JA. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J Clin Invest 121: 422, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkins MW, Patel P, Deng H, Montano MM, Watanabe M, Rollins AM. Phenotyping transgenic embryonic murine hearts using optical coherence tomography. Appl Optics 46: 1776–1781, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins MW, Peterson L, Watanabe M, Rollins AM, Gu S, Gargesha M, Wilson DL. Measuring hemodynamics in the developing heart tube with four-dimensional gated Doppler optical coherence tomography. J Biomed Optics 15: 066022–066024, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins MW, Watanabe M, Rollins AM. Longitudinal imaging of heart development with optical coherence tomography. IEEE J 18: 1166–1175, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karunamuni G, Gu S, Doughman YQ, Noonan AI, Rollins AM, Jenkins MW, Watanabe M. Using optical coherence tomography to rapidly phenotype and quantify congenital heart defects associated with prenatal alcohol exposure. Dev Dyn 244: 607–618, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karunamuni G, Gu S, Doughman YQ, Peterson L, Mai K, McHale Q, Jenkins MW, Linask KK, Rollins AM, Watanabe M. Ethanol exposure alters early cardiac function in the looping heart: a mechanism for congenital heart defects? Am J Physiol Heart Circ Physiol 306: H414–H421, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karunamuni GH, Gu S, Ford MR, Peterson LM, Ma P, Wang YT, Rollins AM, Jenkins MW, Watanabe M. Capturing structure and function in an embryonic heart with biophotonic tools. Front Physiol 5: 351, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karunamuni GH, Ma P, Gu S, Rollins AM, Jenkins MW, Watanabe M. Connecting teratogen-induced congenital heart defects to neural crest cells and their effect on cardiac function. Birth Defects Res C Embryo Today 102: 227–250, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keyte A, Hutson MR. The neural crest in cardiac congenital anomalies. Differentiation 84: 25–40, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirby ML. Cardiac Development. New York: Oxford University Press, 2007. [Google Scholar]
- 36.Kirby ML. Cellular and molecular contributions of the cardiac neural crest to cardiovascular development. Trends Cardiovasc Med 3: 18–23, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Kirby ML. Nodose placode contributes autonomic neurons to the heart in the absence of cardiac neural crest. J Neurosci 8: 1089–1095, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirby ML, Waldo KL. Role of neural crest in congenital heart disease. Circulation 82: 332–340, 1990. [DOI] [PubMed] [Google Scholar]
- 39.Kuwajima T, Sitko AA, Bhansali P, Jurgens C, Guido W, Mason C. ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue. Development 140: 1364–1368, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leatherbury L, Braden DS, Tomita H, Gauldin HE, Jackson WF. Hemodynamic changes: wall stresses and pressure gradients in neural crest-ablated chick embryos. Ann NY Acad Sci 588: 305–313, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Leatherbury L, Connuck DM, Gauldin HE, Kirby ML. Hemodynamic changes and compensatory mechanisms during early cardiogenesis after neural crest ablation in chick embryos. Pediatr Res 30: 509–512, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Leatherbury L, Gauldin HE, Waldo K, Kirby ML. Microcinephotography of the developing heart in neural crest-ablated chick embryos. Circulation 81: 1047–1057, 1990. [DOI] [PubMed] [Google Scholar]
- 43.Li P, Yin X, Shi L, Rugonyi S, Wang RK. In vivo functional imaging of blood flow and wall strain rate in outflow tract of embryonic chick heart using ultrafast spectral domain optical coherence tomography. J Biomed Optics 17: 0960061–0960068, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu YT, Guo LL, Tian Z, Zhu WL, Yu B, Zhang SY, Qiu GX. A retrospective study of congenital scoliosis and associated cardiac and intraspinal abnormities in a Chinese population. Eur Spine J 20: 2111–2114, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma P, Wang YT, Gu S, Watanabe M, Jenkins MW, Rollins AM. Three-dimensional correction of conduction velocity in the embryonic heart using integrated optical mapping and optical coherence tomography. J Biomed Optics 19: 076004, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Männer J, Seidl W, Steding G. Experimental study on the significance of abnormal cardiac looping for the development of cardiovascular anomalies in neural crest-ablated chick embryos. Anat Embryol (Berl) 194: 289–300, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 130: 749–756, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Menon V, Eberth JF, Goodwin RL, Potts JD. Altered hemodynamics in the embryonic heart affects outflow valve development. J Cardiovasc Dev Dis 2: 108–124, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mikawa T, Hurtado R. Development of the cardiac conduction system. Semin Cell Dev Biol 18: 90–100, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res 98: 1547–1554, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Nishibatake M, Kirby ML, Van Mierop LH. Pathogenesis of persistent truncus arteriosus and dextroposed aorta in the chick embryo after neural crest ablation. Circulation 75: 255–264, 1987. [DOI] [PubMed] [Google Scholar]
- 52.Nomura-Kitabayashi A, Phoon CK, Kishigami S, Rosenthal J, Yamauchi Y, Abe K, Yamamura Ki Samtani R, Lo CW, Mishina Y. Outflow tract cushions perform a critical valve-like function in the early embryonic heart requiring BMPRIA-mediated signaling in cardiac neural crest. Am J Physiol Heart Circ Physiol 297: H1617–H1628, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson LM, Jenkins MW, Gu S, Barwick L, Watanabe M, Rollins AM. 4D shear stress maps of the developing heart using Doppler optical coherence tomography. Biomed Optics Express 3: 3022–3032, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phoon CK, Ji RP, Aristizábal O, Worrad DM, Zhou B, Baldwin HS, Turnbull DH. Embryonic heart failure in NFATc1−/− mice: novel mechanistic insights from in utero ultrasound biomicroscopy. Circ Res 95: 92–99, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Plein A, Fantin A, Ruhrberg C. Neural crest cells in cardiovascular development. In: Current Topics in Developmental Biology, edited by Paul AT. New York: Academic, 2015, chapt. 6, p. 183–200. [DOI] [PubMed] [Google Scholar]
- 56.Reckova M, Rosengarten C, deAlmeida A, Stanley CP, Wessels A, Gourdie RG, Thompson RP, Sedmera D. Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ Res 93: 77–85, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Scherz PJ, Huisken J, Sahai-Hernandez P, Stainier DY. High-speed imaging of developing heart valves reveals interplay of morphogenesis and function. Development 135: 1179–1187, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Shu X, Cheng K, Patel N, Chen F, Joseph E, Tsai HJ, Chen JN. Na,K-ATPase is essential for embryonic heart development in the zebrafish. Development 130: 6165–6173, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Smith SM, Garic A, Flentke GR, Berres ME. Neural crest development in fetal alcohol syndrome. Birth Defects Res C Embryo Today 102: 210–220, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart DE, Kirby ML, Sulik KK. Hemodynamic changes in chick embryos precede heart defects after cardiac neural crest ablation. Circ Res 59: 545–550, 1986. [DOI] [PubMed] [Google Scholar]
- 62.Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development 131: 2205–2218, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sulik KK, Johnston MC, Daft PA, Russell WE, Dehart DB, Opitz JM, Reynolds JF. Fetal alcohol syndrome and DiGeorge anomaly. Critical ethanol exposure periods for craniofacial malformations as illustrated in an animal model. Am J Med Genet 25: 97–112, 1986. [DOI] [PubMed] [Google Scholar]
- 64.Syed SH, Larin KV, Dickinson ME, Larina IV. Optical coherence tomography for high-resolution imaging of mouse development in utero. J Biomed Optics 16: 046004–046006, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomita H, Connuck DM, Leatherbury L, Kirby ML. Relation of early hemodynamic changes to final cardiac phenotype and survival after neural crest ablation in chick embryos. Circulation 84: 1289–1295, 1991. [DOI] [PubMed] [Google Scholar]
- 66.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 58: 2241–2247, 2011. [DOI] [PubMed] [Google Scholar]
- 67.Vermot J, Forouhar AS, Liebling M, Wu D, Plummer D, Gharib M, Fraser SE. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol 7: e1000246, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waldo K, Zdanowicz M, Burch J, Kumiski DH, Stadt HA, Godt RE, Creazzo TL, Kirby ML. A novel role for cardiac neural crest in heart development. J Clin Invest 103: 1499–1507, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang G, Bieberich E. Prenatal alcohol exposure triggers ceramide-induced apoptosis in neural crest-derived tissues concurrent with defective cranial development. Cell Death Dis 1: e46, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yelbuz TM, Choma MA, Thrane L, Kirby ML, Izatt JA. Optical coherence tomography: a new high-resolution imaging technology to study cardiac development in chick embryos. Circulation 106: 2771–2774, 2002. [DOI] [PubMed] [Google Scholar]