Focal and excessive neural inputs to the intrinsic cardiac nervous system increase activity and coherence among intrinsic cardiac neurons in association with an increased potential for atrial fibrillation; preemptive vagus nerve stimulation prevents such neurocardiac effects. The antiarrhythmic effects imparted by vagus nerve stimulation have memory.
Keywords: atrial arrhythmias, intrinsic cardiac nervous system, local circuit neurons, memory, stochastic neuronal interactivity, vagus
Abstract
Mediastinal nerve stimulation (MNS) reproducibly evokes atrial fibrillation (AF) by excessive and heterogeneous activation of intrinsic cardiac (IC) neurons. This study evaluated whether preemptive vagus nerve stimulation (VNS) impacts MNS-induced evoked changes in IC neural network activity to thereby alter susceptibility to AF. IC neuronal activity in the right atrial ganglionated plexus was directly recorded in anesthetized canines (n = 8) using a linear microelectrode array concomitant with right atrial electrical activity in response to: 1) epicardial touch or great vessel occlusion vs. 2) stellate or vagal stimulation. From these stressors, post hoc analysis (based on the Skellam distribution) defined IC neurons so recorded as afferent, efferent, or convergent (afferent and efferent inputs) local circuit neurons (LCN). The capacity of right-sided MNS to modify IC activity in the induction of AF was determined before and after preemptive right (RCV)- vs. left (LCV)-sided VNS (15 Hz, 500 μs; 1.2× bradycardia threshold). Neuronal (n = 89) activity at baseline (0.11 ± 0.29 Hz) increased during MNS-induced AF (0.51 ± 1.30 Hz; P < 0.001). Convergent LCNs were preferentially activated by MNS. Preemptive RCV reduced MNS-induced changes in LCN activity (by 70%) while mitigating MNS-induced AF (by 75%). Preemptive LCV reduced LCN activity by 60% while mitigating AF potential by 40%. IC neuronal synchrony increased during neurally induced AF, a local neural network response mitigated by preemptive VNS. These antiarrhythmic effects persisted post-VNS for, on average, 26 min. In conclusion, VNS preferentially targets convergent LCNs and their interactive coherence to mitigate the potential for neurally induced AF. The antiarrhythmic properties imposed by VNS exhibit memory.
NEW & NOTEWORTHY
Focal and excessive neural inputs to the intrinsic cardiac nervous system increase activity and coherence among intrinsic cardiac neurons in association with an increased potential for atrial fibrillation; preemptive vagus nerve stimulation prevents such neurocardiac effects. The antiarrhythmic effects imparted by vagus nerve stimulation have memory.
atrial fibrillation (AF) affects more than three million people a year in the United States, a prevalence that is projected to reach 5.6–12.1 million by 2050 (26, 47). Despite such prevalence, the underlying mechanisms of AF are not fully understood. Current treatments consist of pharmacological therapies that have been combined with localized atrial catheter-based or surgical ablation (18, 61). Ablation procedures are associated with complications such as the left atrial stiffness syndrome (24), microembolic episodes (58), and a risk of symptomatic or silent cerebral ischemia (21). Such drawbacks have increased the research focus on defining specific neural and cardiac substrate interactions underlying AF and with such information evolving novel nonpharmacological therapeutic options for its management (79). Bioelectric neuromodulation therapies for AF represent a novel approach to such management. Among these, vagus nerve stimulation (VNS) (43, 60, 62) and spinal cord stimulation (23, 66, 71) target various aspects of the cardiac neuronal hierarchy to reduce the arrhythmia potential.
The cardiac nervous system includes reflex networks located in the insular cortex, brain stem, spinal cord, intrathoracic sympathetic ganglia, and the intrinsic cardiac nervous system (ICNS) (3, 7, 81). It has been proposed that its ICNS component acts as the final coordinator of regional cardiac indexes, doing so under the influence of intrathoracic, spinal cord, and brain stem reflexes (7). Neural activity within the ICNS is influenced by afferent (mechanosensitive, chemosensitive, and ischemia-sensitive) and efferent neuronal inputs (7, 9, 80). These afferent and efferent inputs are processed by local circuit neurons (LCNs) in peripheral ganglia to modulate sympathetic and parasympathetic efferent postganglionic projections to all regions of the heart (3, 20, 33, 45). Neuronal imbalances within the ICNS can exert deleterious effects on cardiac function, including arrhythmia induction (8, 11, 57, 59). To date, which populations of neurons within the ICNS are so involved remains unresolved.
The model of atrial fibrillation used in this study is intermittent focal mediastinal nerve stimulation (MNS) (11). MNS elicits ICNS network hyperexcitability (23) that in turn deranges efferent neuronal outflows to atrial tissues, thereby causing heterogeneities in atrial electrical indexes (11, 56). Such heterogeneities in the atrial electrical substrate rapidly degenerate into self-limiting episodes of AF (11, 56). The MSN-induced AF episodes occur with a latency of ∼1 s from stimulation onset, have a duration of ∼30 s, and are reproducible over hours of experimentation (11, 23, 56). This model provides a reproducible experimental platform whereby antiarrhythmic therapies can be evaluated and optimized (11, 23, 40, 56). Prior work has demonstrated that MNS-induced AF can be eliminated by atropine (11), modified by timolol (11), and blunted by α-adrenoceptor blockade (56). Hexamethonium likewise reduces the number of AF responses to MNS stimulation from 90% baseline to 10% posttreatment (56). These data substantiate fundamental aspects of ICNS neural activity in relationship to regulation of the AF potential.
Vagus nerve stimulation modulates cardiac electrical indexes (42, 73) and, as such, has the potential to either increase or decrease the propensity to arrhythmias (18). Higher-intensity stimulations tend to increase atrial fibrillation inducibility (76, 78); lower-intensity vagal stimulation can stabilize atrial electrical function (17, 67). To understand the efficacy of VNS therapy with respect to atrial arrhythmia suppression, we first defined the response characteristics of functionally delineated subpopulations of intrinsic cardiac (IC) neurons to MNS-evoked AF and then the capacity of cervical VNS (right vs. left) to modify neural network and cardiac electrical responses to such destabilizing inputs. To this purpose, we directly recorded the activity of multiple neurons in the canine right atrial ganglionated plexuses (RAGP), an aggregate of IC neurons directly involved in control of chronotropic function (6, 46, 52). Data presented herein demonstrate mechanistically the pivotal role of LCNs in mediating AF secondary to neural imbalances and that these same neurons are the preferential target for bioelectric therapies to reduce the arrhythmogenic potential. We further demonstrate that these bioelectric interventions exhibit memory to extend the atrial antiarrhythmic effects well beyond the primary activity phase of VNS therapy.
METHODS
Animal preparation.
All experiments were performed in accordance with the guidelines for animal experimentation described in the Guide for the Care and Use of Laboratory Animals, Eighth Edition, National Academy Press, Washington DC, 2010. The Institutional Animal Care and Use Committee of the East Tennessee State University approved these experiments. Eleven mongrel dogs of either sex, weighing 18.6–26.9 kg, entered this study. Animals were sedated with propofol (3–8 mg/kg iv), followed by endotracheal intubation and mechanical ventilation. General anesthesia was maintained with isoflurane (1–2%, inhalation). Following completion of surgery, anesthesia was changed to α-chloralose (50 mg/kg iv bolus), with continuous infusion (8–12 mg·kg−1·h−1 iv) adjusted to effect throughout the duration of each study. The depth of anesthesia was assessed throughout the experiments by monitoring corneal reflexes, jaw tone, and hemodynamic indexes. Body temperature was maintained via a circulating water heating pad (Gaymar T/Pump; Gaymar Industries, Orchard Park, NY). At the completion of the experiments, animals were humanely killed under deep anesthesia and by inducing ventricular fibrillation via application of direct current stimulation and removing the heart.
Hemodynamic recording.
The left femoral artery was catheterized to record arterial blood pressure (Ao BP). The left femoral vein was catheterized to allow for fluid replacement, as well as anesthetic and pharmacological agent delivery. The right femoral artery was catheterized to monitor left ventricular chamber pressure (LVP) via placement in the left ventricular (LV) chamber of a Mikro-Tip Pressure Transducer Catheter (Millar Instruments, Houston, TX). Heart rate was monitored via a Lead II electrocardiogram (ECG). Pressures (Ao BP, LVP) and ECG were input to a Cambridge Electronics Design (model 1401) data acquisition system for continuous monitoring of hemodynamic status.
Vagal stimulation (VNS).
Following a midline incision in the ventral neck, the right and left cervical vagi were exposed, and bipolar stimulation electrodes (PerrenialFlex, model 304, Cyberonics) were placed around each nerve. Cervical vagosympathetic trunks remained intact throughout each aspect of the protocol. Each lead was connected individually to a Grass S88 stimulator via separate PSIU6 constant current isolation units. Bradycardia thresholds for each nerve stimulated were identified using 20 Hz, 500-μs pulse width stimuli, as determined by progressive increases in current intensity until 10% bradycardia was evoked. With respect to right-sided VNS, this current was found to be, on average, 1.75 mA; for left-sided VNS it was 2.25 mA. VNS was applied to each vagus for 3-min periods (15 Hz; 500-μs pulse width) at a current intensity that was 1.2× bradycardia threshold.
Mediastinal nerve stimulation.
Following thoracotomy, an incision was made in the pericardial sac, and a pericardial cradle was formed. A bipolar electrode was affixed to the right atrium 1 cm dorsal to the sinoatrial node to record an atrial electrogram. Right-sided mediastinal nerves were identified visually coursing over the ventral and ventrolateral surface of the intrapericardial aspects of the superior vena cava. These mediastinal nerves represent aggregates of sympathetic and parasympathetic efferent axons, as well as interganglionic projections arising from local circuit neurons contained within the ICNS (11, 23, 27, 70).
Each nerve was stimulated individually using detailed published techniques (11, 23). Briefly, trains of five electrical stimuli (0.3–1.2 mA, 1 ms duration, 5 ms pulse interval) were delivered during individual atrial refractory periods to identified mediastinal sites for up to 20 s. Electrical stimuli were delivered to a mediastinal nerve via a roving bipolar probe electrode. Active nerve sites were identified by the immediate induction of atrial tachyarrhythmias (including atrial fibrillation) when first exposed to focal electrical stimuli. Each active mediastinal nerve site so identified was marked with India ink for repeated stimulation. By these means, two to four active nerve sites were identified in each animal. Contact between the bipolar electrodes and tissue was discontinued immediately after the onset of the atrial tachyarrhythmia to limit their durations (11, 56).
Neuronal recording.
Extracellular activity generated by intrinsic cardiac neurons in situ was recorded using a multichannel linear microelectrode array (MicroProbes, Gaithersburg, MD) that consisted of 16 platinum/iridium electrodes (25 μm-diameter electrode with an exposed tip of 2 mm; impedance 0.3–0.5 MΩ at 1 kHz). The linear microelectrode array was embedded in the right atrial fat that contained the right atrial ganglionated plexus (RAGP), as described previously (13). The connecting wires of the multichannel electrode, along with ground and reference wires, were attached to a 16-channel microelectrode amplifier with a headstage preamplifier (model 3600; A-M Systems, Carlsborg, WA). For each channel, filters were set to 300 Hz to 3 KHz and gain to 5 K. Another electrode was sewn to the atrial myocardium close to the RAGP to provide a reference right atrial electrogram that was used to determine atrial rate, duration and characterization of atrial arrhythmias, along with a timing index for subsequent identification of atrial electrical artifacts in IC neural recording data. The 16 microelectrode array signals, along with recorded cardiovascular indexes (ECG, right atrium electrogram, and hemodynamic data), were digitized via a Cambridge Electronics Design (model 1401) data acquisition system for off-line analysis. The sampling frequency for neuronal data was 5.26 kHz; it was six times lower (0.877 kHz) for all other recorded signals.
Identification of neuronal activity.
The extracellular activity generated by individual neuronal somata located within the RAGP was recorded. Identification of the activity generated by individual neurons via the 16-channel electrodes was performed off line using the Spike2 software program (Cambridge Electronic Design) in two steps: 1) artifact identification and blanking and 2) spike detection, waveform classification, and validation with principal component analysis as defined previously (13). With the use of these procedures, consistent waveforms derived from individual somata (not axons of passage) can be identified in situ for up to 8- to 10-h periods (13, 50, 69). Of the 11 animals, recordings with sufficient signal to noise were obtained in eight animals. As such, the remaining three were excluded from all subsequent analysis.
Statistical analysis of evoked changes in IC neuronal activity.
Using statistical approaches based on a Skellam distribution (63), the significance of changes in firing rates recorded before and during each intervention was computed post hoc for all identified IC neurons. We classified the behavior of identified neurons according to their activity characteristics in response to the following interventions: 1) touching the ventral LV and then right ventricle (conus vs. sinus); 2) 20 s descending aorta occlusion; 3) 20 s inferior vena cava occlusion; 4) stimulation (1 Hz for 1 min) of right vs. left cervical vagosympathetic trunk (RCV; LCV); and 5) stimulation (1 Hz for 1 min) of right vs. left stellate ganglia. By these means, each neuron was classified according to how it responded to each of those interventions by its change in firing rate, each serving at its own control. When a neuron responded solely to one or more of the afferent stressors (interventions 1–3 above), it was classified as an afferent LCN. Efferent LCNs were identified as those responding indirectly (variable latency) to one or more of the efferent (vagal vs. sympathetic; interventions 4 and 5 above) inputs. IC neurons that respond with a fixed latency to efferent inputs were classified at efferent IC neurons (7). IC neurons that responded indirectly to both afferent and efferent stressor were classified as convergent LCN (13). Identified neurons that did not respond to any of these stressors were classified as exhibiting unknown function.
The primary objective of this study was to assess the efficacy of preemptive VNS to alter the IC neural network response to MNS and thereby impact the atrial arrhythmogenic potential. Repeated-measure ANOVA was used to assess the effect of different factors on neuronal activity. The three-way ANOVA test was performed on RCV and LCV separately. It involved two within-subject factors (effect of MNS vs. baseline, and pre- vs. post-VNS response) and one between-subjects factor (neuron type). Huynd-Fedlt correction was applied to correct the violation of sphericity assumption. When significance was achieved overall for ANOVA (P < 0.05), post hoc test and all other paired-sample comparisons were done by paired t-test.
In each animal, a synchrony index (SI) was also calculated (44) to evaluate synchrony of activity generated among different populations of IC neurons. This index was estimated during: 1) baseline states compared with 2) during episodes of neurally induced atrial arrhythmias. The potential of VNS to alter IC synchrony was likewise assessed. There was a limitation of analysis imposed by the limited number of action potentials generated per neuron, especially when suppressed during VNS therapy; as such, synchrony analysis was not performed in those instances. The synchrony of activities displayed by different populations of identified neurons, as defined by Agmon (1), was performed by assessing the activity generated by pairs of identified neurons in each animal. To calculate such a SI, one neuron was defined as the reference and the other as the target neuron.
Different SI values were obtained that depended on which neuron was considered reference, thus making the SI a nonsymmetric measure. As such, calculation of this SI required the identification of coincidences of activities among differing neurons when reference and target neurons both generate activity within a time window of selected duration τ. We had previously defined the optimal value for τ with respect to intrinsic cardiac neuronal activities to be 40 ms (44). Given that some coincidences may be random in nature, the coincidence count was also estimated in surrogate data obtained by applying a random jitter to the reference spikes in each time window of duration 4τ (1). To obtain normalized SI values, the mean coincidence count in surrogate data was subtracted from the actual coincidence count identified. Thereafter, the resultant was divided by the number of reference spikes. Surrogate data also served to calculate a P value so that we could assess statistical significance of these data. When the number of neuron pairs demonstrating significant synchrony was so identified (P < 0.01 and SI >0.01), a Chi square test was performed to assign statistical significance to changes occurring in the number of synchronized pairs for each neuronal subtype combination studied (65).
AF characteristics.
Atrial electrograms were recorded from the ventral right atrial free wall and referenced to a Wilson Central terminal. From these atrial electrograms, the following response characteristics were determined during the atrial tachyarrhythmia: 1) latency (defined as the interval from the first applied stimulus to tachyarrhythmia initiation); 2) duration of the AF (defined as time from onset to self-termination of AF); and 3) dominant frequency of atrial activity during induced AF episode. When AF was not initiated by MNS, AF duration was by definition set to zero. The duration of AF episodes recorded before and after VNS were compared by reference to the duration of each, as obtained from one or more AF episodes induced before and after full recovery from the VNS protocols. The effects of VNS therapy were separated into four categories, using MNS as the constant defined stressor: 1) AF prevention (AF initiation failed); 2) AF mitigation (AF duration reduced by 20% or more); 3) AF prolongation (AF duration increased by at least 20%); and 4) having no effect. Results were considered to be not significant (no effect) when occurring within the 20% range.
Time dependence of VNS effect.
Kaplan-Meier survival analysis was performed to estimate how long the effect of VNS lasted as represented by the varied number (up to 7) of successive AF initiation attempts (at 5- or 10-min intervals after the first, if needed). When a mediastinal nerve stimulus evoked an AF episode as long as the reference (control state) episode, sequential MNS trials were terminated. Accordingly, VNS efficacy at time t was defined as the percentage of experiments for which the latest unsuccessful AF attempt (if any) occurred after time t. A second survival curve was also created based on the percentage of experiments in which the latest mitigated AF episode (if any) occurred after time t to determine how long VNS effectiveness lasted.
RESULTS
Functional response characteristics of identified right atrial neurons.
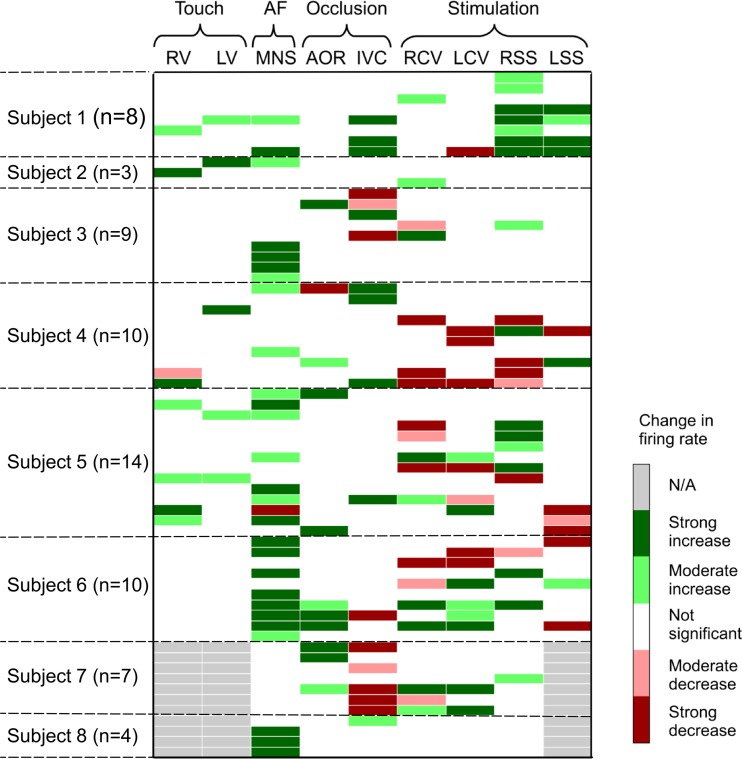
A total of 89 neurons were identified in the 8 animals studied (11.1 ± 3.5 neurons/dog). The response characteristics of individual neurons differed with respect to the stressor tested, which could be reflected as either an increase or decrease in activity (Fig. 1). Of the 89 identified right atrial neurons (those that generated spontaneous activity), 65 neurons were functionally classified as being 1) afferent (n = 15; 17%), 2) efferent (n = 20; 22%), or 3) convergent local circuit neurons (n = 30; 34%). The rest (n = 24; 27%) did not respond to any of these imposed stressors; as such, their function was labeled as being unknown.
Fig. 1.
Intrinsic cardiac (IC) neurons classified based on their functional responses to afferent stressor [touch of right (RV) or left (LV) ventricle; occlusion of descending aorta (AOR) or inferior vena cava (IVC)] vs. efferent stressor {right (RCV) or left (LCV) cervical vagus or stellate ganglia [right (RSS); left (LSS)] electrical stimulation} interventions. In response to stressors, IC firing could either increase (green bars) or decrease (red bars), with that individual response being stable over time. Gray bars mean a specific test was not done for that neuron (designated N/A). Afferent-related IC neurons were defined as those that responded differentially to at least one of the following stressors: RV, LV, AOR, or IVC. Efferent-related IC neurons responded to cervical vagal and/or stellate ganglion stimulation. Convergent IC neurons were modulated by both afferent and efferent inputs. Note that 11 IC neurons (∼13%) responded solely to the mediastinal nerve stimulation (MNS) stressor. Approximately 27% of spontaneously identified IC neurons were unaffected by any stressors tested; as such, they are defined as unknown and are not shown in this panel.
Effects of right-sided mediastinal nerve stimulation on cardioneural activity.
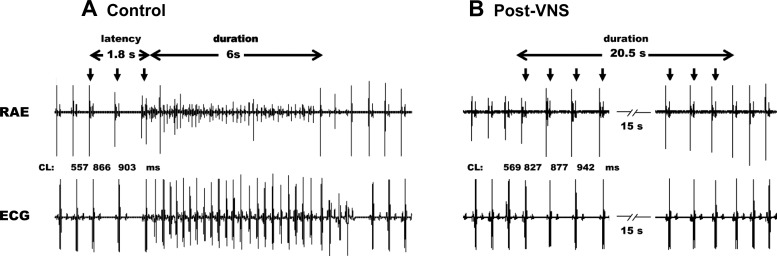
Figure 2 illustrates a representative atrial arrhythmic response elicited by brief periods of MNS stimulation before (control; A) and following (post-VNS; B) preemptive VNS. In the control state, MNS on average induced transient periods of AF with a latency to onset of 2.68 ± 2.32 s, a duration of 11.1 ± 1.2 s, and a dominant frequency of 7.1 ± 0.1 Hz during AF. Note that bradycardia usually preceded the onset of atrial tachycardia/AF (Fig. 2A) and that this onset transient bradycardia was maintained following VNS (Fig. 2B). In this same animal, VNS pretreatment prevented the tachyarrhythmias induced by MNS (Fig. 2B), even when applied for up to 20 s. The hemodynamic response to VNS is summarized in Table 1. The evoked changes in chronotropic and left ventricular inotropic function, with suppression during the active VNS phase followed by a rebound phase (1 min duration) following stimulation, are consistent with the 1.2× threshold intensity used herein. By onset of MNS stressors post-VNS, hemodynamics had returned to baseline values (data not shown).
Fig. 2.
Vagus nerve stimulation (VNS) effects on MNS-induced atrial fibrillation (AF). Atrial electrical activity recorded from a unipolar electrode on the ventral right atrial free wall along with lead II ECG. Bursts of electrical stimuli applied to a caudal right-sided mediastinal nerve during the atrial refractory period (downward arrows) elicited arrhythmias before (A) but not after (B) preemptive right-sided VNS.
Table 1.
Hemodynamic response to preemptive VNS
| HR, beats/min | LVSP | LVEDP | LV +dp/dt | |
|---|---|---|---|---|
| Baseline | 83.8 ± 4.8 | 122.1 ± 5.1 | 2.2 ± 0.6 | 1,913.9 ± 125.5 |
| VNS | 57.5 ± 3.7* | 120.3 ± 5.6 | 2.8 ± 0.6 | 1,935.4 ± 154.2 |
| Post-VNS | 95.0 ± 4.4# | 127.8 ± 5.5# | 2.2 ± 0.8 | 2,308.8 ± 181.9# |
Data reflect means ± SE for heart rate (HR), left ventricular systolic pressure (LVSP), left ventricular diastolic pressure (LVEDP), and first derivative of left ventricular (LV) pressure (dp/dt) before (baseline), during, and for 1 min following vagus nerve stimulation (VNS). P < 0.01 from baseline (
) and VNS (#).
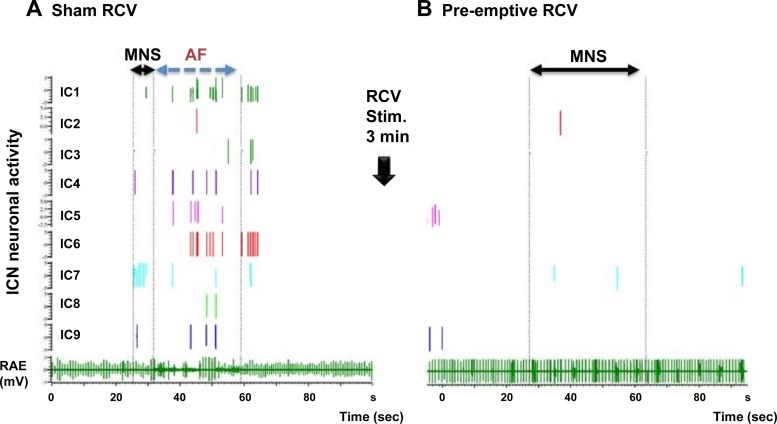
MNS stimulation triggered changes in IC activity leading to atrial arrhythmias, with residual effects continuing even postconversion to sinus rhythm. In the representative animal depicted in Fig. 3, in the control state bursting of activity was elicited among nine identified right atrial neurons by MNS (Fig. 3A, MNS: sham RCV). Neural activity enhancement occurred immediately before the induction of the transient atrial arrhythmia (cf., AF). Activity persisted in five of these nine neurons for a brief period of time even after spontaneous conversion to sinus rhythm. Average neuronal activity recorded among all classified IC neurons across all animals was 0.11 ± 0.29 Hz in control states, increasing to 0.51 ± 1.30 Hz (P < 0.001) during the MNS-induced atrial tachyarrhythmia. From subset analysis, IC activity increased preferentially among convergent LCNs (0.13 ± 0.3 to 0.88 ± 1.73 Hz, P < 0.001) in response to MNS, with afferent LCNs responding to a lesser degree (0.07 ± 0.3 to 0.14 ± 0.43 Hz, P < 0.032). No changes were identified in identified efferent LCN populations (0.11 ± 0.3 to 0.21 ± 0.74 Hz, P = 0.24).
Fig. 3.
Representative responses to MNS before (A) and after (B) RCV VNS. A right atrial electrogram (RAE, bottom) is displayed with concomitant activities generated by 9 identified IC neurons. A: control state where AF was induced by right-sided MNS. B: response when the same MNS site was stimulated 1 min following 3 min of preemptive right-sided VNS. Horizontal solid arrows delimit time of MNS nerve stimulations. Broken vertical lines (A) indicate duration of AF induced by MNS. Note that following RCV (B), MNS failed to induce AF, even when applied for a longer time period (20 s). IC activity correspondingly remained unchanged during and following MNS stimulation.
Effects of ipsilateral vagus nerve stimulation on right-sided atrial neuronal activity and the potential for neurally induced atrial arrhythmias.
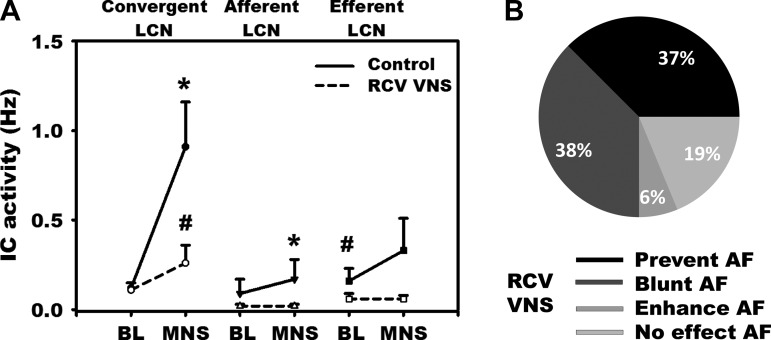
Preemptive right-sided VNS mitigated IC neural responses to MNS (Figs. 3B and 4A). It blunted or prevented the potential for neurally induced AF by 75% (Fig. 4B), with no significant changes in onset latency or dominant frequency in residual arrhythmias. Before VNS, MNS increased the activity among both afferent and convergent LCN subpopulations (Fig. 4A). Following preemptive right-sided VNS, basal activity was differentially decreased among efferent LCNs (0.16 ± 0.4 vs. 0.06 ± 0.19 Hz, P < 0.01). Post-VNS, MNS-induced excitation of convergent LCNs was blunted (0.91 ± 1.73 vs. 0.26 ± 0.73 Hz; P < 0.002), being totally eliminated among afferent LCN populations (Fig. 4A).
Fig. 4.
A: response of IC neurons to MNS before (solid line) vs. following (broken line: RCV VNS) preemptive right-sided (ipsilateral) bioelectric therapy. IC neurons were subclassified as convergent, afferent, or efferent LCNs (cf., Fig. 1). Convergent LCNs were the predominant population of neurons activated by MNS and the primary target for preemptive RCV neuromodulation therapy. B: impact of ipsilateral (right-sided) VNS therapy on the atrial arrhythmogenic potential to MNS, classified according to whether it prevented, blunted, or enhanced AF or exerted no effects. P < 0.05 from baseline (*) and control (sham VNS; #).
Effects of contralateral vagus nerve stimulation on right-sided atrial neuronal activity and the potential for neurally induced atrial arrhythmias.
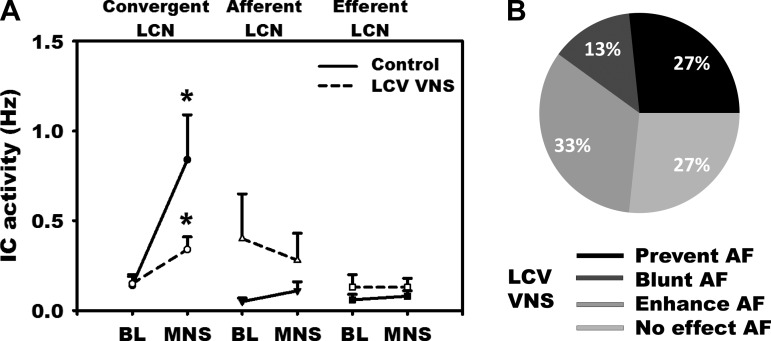
In contrast to ipsilateral VNS, left-sided vagus stimulation exerted no significant change in basal IC neuronal activity (Fig. 5A). However, as with right-sided VNS, LCV differentially mitigated the MNS-induced increase in convergent LCN activity (0.84 ± 1.74 vs. 0.34 ± 0.49 Hz, P = 0.057). In contradistinction to ipsilateral-mediated effects, though blunted, the neural activity in convergent neurons still increased significantly above baseline during MNS following the LCV VNS. The potential for MNS-induced AF was prevented or blunted 40% by LCV VNS and without effect in 27% of cases. Preemptive left-sided VNS enhanced AF induced from 33% of right-sided MNS sites evaluated (Fig. 5B).
Fig. 5.
A: response of IC neurons to MNS before (solid line) vs. after (broken line: LCV VNS) preemptive left-sided (contralateral) bioelectric therapy. IC neurons were subclassified as convergent, afferent, or efferent LCNs, as defined in Fig. 1. Convergent LCNs were the predominant population activated by MNS and the primary target for preemptive LCV therapy. B: impact of contralateral VNS therapy on the atrial arrhythmogenic potential to MNS. Whereas LCV VNS mitigated the AF potential for 40% of MNS sites tested, in contradistinction to RCV VNS it enhanced that potential in 1/3 of MNS sites tested. *P < 0.05 from baseline.
IC network characteristics: neuronal synchrony.
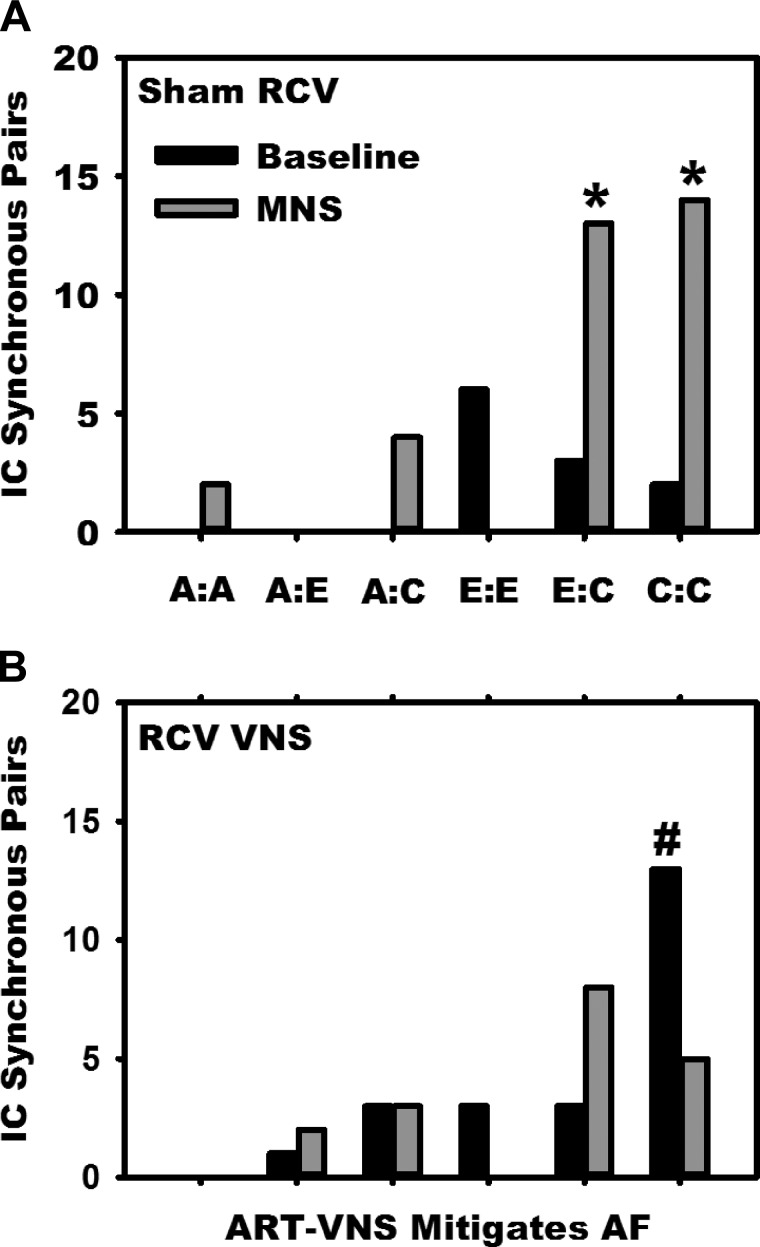
The MNS-induced increases in IC activity are reflective of common shared inputs and/or IC network interconnections mediated by LCNs (7). Figure 6 evaluates this short-term interactive potential by determining synchrony among the specific pairs of IC neurons identified within the RAGP during baseline conditions, as well as during MNS-induced changes 1) before (top) and following (bottom) preemptive right-sided VNS. In the sham (unstimulated) treatment state, note that, while there was minimal coherence of activity among the various subpopulations of IC neurons identified, in response to MNS there was a preferential increase in IC synchrony among convergent LCNs, as well as between convergent and efferent LCN subpopulations. Following right-sided VNS, while there was a differential increase in synchrony during baseline states among convergent LCNs (Fig. 6, bottom), any MNS-induced change in IC synchrony was extinguished.
Fig. 6.
MNS-induced changes in IC network synchrony. The synchronized activities generated by identified pairs of IC neurons [synchrony index (SI) >0.01 and P < 0.01] were determined and classified post hoc, according to comparing concomitant activities generated by: afferent LCNs (A); efferent LCNs (E); and convergent LCNs (C). Vertical columns represent the degree of synchrony (number of synchronous pairs) between the 6 combinations of neuron pairings elicited during: 1) baseline (black bars) vs. 2) MNS-induced (gray bars) arrhythmias. These relationships are depicted in untreated (sham VNS, A) states and following preemptive bioelectric therapy (RCV VNS, B). Note that MNS induced differential increases in synchrony between efferent-to-convergent IC pairs (E:C), as well as convergent-to-convergent neuronal pairings (C:C) (top). Whereas at baseline preemptive RCV differentially increased synchrony between convergent LCNs, it eliminated the increase in synchrony across all other neuronal subclass pairings during MNS (bottom). *P < 0.02 from baseline and #P < 0.01 sham to RCV VNS state.
IC network characteristics: memory.
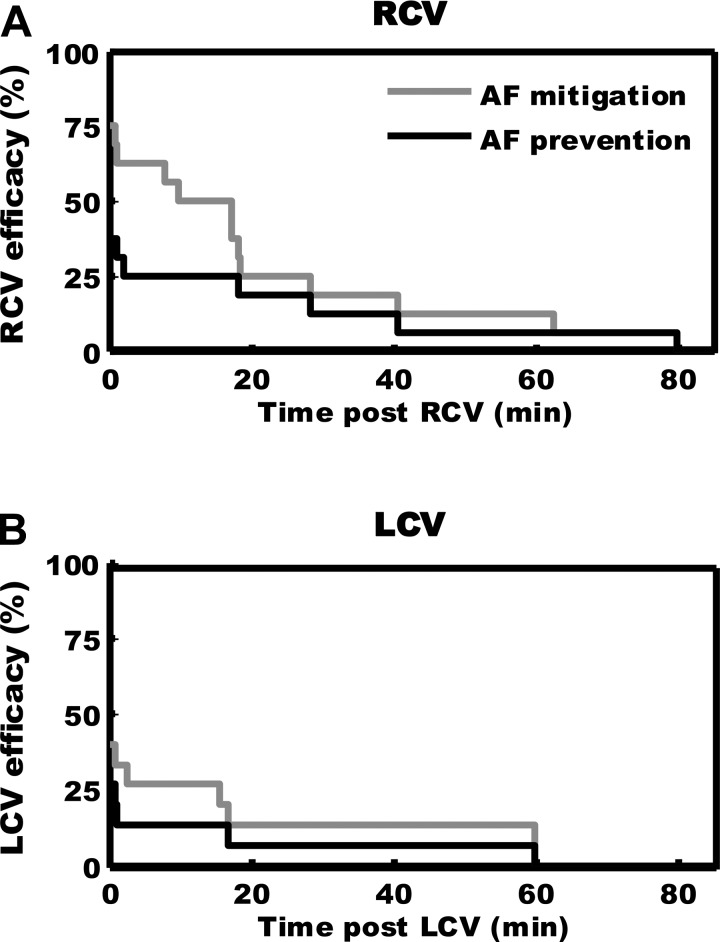
The efficacy of VNS therapy in terms of shortening/preventing MNS-induced arrhythmias (post-VNS) was assessed via Kaplan-Meier survival analysis (Fig. 7). Following right-sided VNS, antiarrhythmic effects against repeated MNS-induced arrhythmias were attenuated for 20 min after VNS therapy (top) and extinguished by ∼40 min post-VNS [fitting exponential function resulted in a time constant of 26 ± 2 min (95% confidence interval); Fig. 7A]. While the overall antiarrhythmic efficacy of contralateral VNS was reduced (Fig. 7, bottom), the time constants derived from RCV vs. LCV responses were not significantly different (log-rank test). For corresponding MNS-induced changes in IC activity, the pre-VNS-induced change in convergent activity (0.11 to 1.57 Hz, P = 0.023) was suppressed immediately after VNS (0.04 to 0.38 Hz, P = 0.17) and recovered ∼30 min post-VNS (0.07 to 1.28 Hz, P = 0.016). Following recovery, characteristics of MNS-induced AF (latency, duration, and dominant frequency) were similar to sham VNS control (data not shown).
Fig. 7.
VNS-induced antiarrhythmic effects exhibit memory. A: evolution of effects induced by right-sided VNS therapy on the capacity of MNS to induce AF (%efficacy), as a function of time posttherapy. Light gray curve represents the percentage of cases (Kaplan-Meier survival curve) in which AF duration was mitigated (shortened or prevented); dark curve indicates time effects of preemptive RCV in preventing MNS-induced AF. B: similar data derived with respect to AF potential when left-sided (LCV) therapy was applied preemptively.
DISCUSSION
The major findings of this study are: 1) enhanced activity on convergent LCNs underlies neurally induced AF; 2) VNS therapy attenuates AF via its effects on select intrinsic cardiac neuronal populations, namely convergent LCNs; 3) disruptive neural inputs to the ICNS increase coherence of activity among IC neurons, and preemptive VNS prevents such effects; 4) ipsilateral VNS imparts a greater impact on IC neural function and the ability to stabilize the ICNS against neural imbalance; and 5) the antiarrhythmic effects imparted by VNS have memory.
ICN modulation of cardiac function.
The ICNS is composed of heterogeneous populations of neurons loosely organized in multiple ganglionated plexi located within atrial and ventricular tissues (7, 13, 75). These IC neurons can be functionally stratified by their in situ behavior based on their responses to different stressors according to whether they belong to either afferent, efferent, or convergent LCN subtypes (7, 13). Structure is intricately intertwined to function (13, 16, 50). The convergent LCNs are responsible for primary reflex integration within the ICNS (7), coordinating atrial and ventricular tissues via its efferent outputs. With respect to central autonomic efferent preganglionic axons, they project directly on intrinsic cardiac efferent postganglionic (intrinsic cardiac parasympathetic and sympathetic) neurons and convergent LCNs (13, 46, 52). These IC network interactions are critical to mediating sympathetic/parasympathetic cardiomotor outflow to control regional cardiac function (46, 51).
ICN processing and atrial arrhythmias.
Asymmetric neural inputs to the IC network increase the potential for atrial tachycardia/AF (11, 18). Stochastic processing within that network underlies the instability that can occur within the ICNS to initiate arrhythmias (37, 38). The resultant “hyperstochasticity” displayed among its convergent LCNs in response to MNS appears to be fundamental to any enhancement of an arrhythmia potential (23). Our study shows that any such enhancement of activity among IC LCNs is associated with increases in their coherence to effect local efferent neuronal outflows (27, 46). Such coherence, or lack thereof, is ultimately dependent on intraganglionic interconnections (34, 69). Our data indicate that IC network interactions can be targeted therapeutically to modify atrial arrhythmia induction.
VNS therapy not only impacts excitability among select populations of intrinsic cardiac neurons but also the coherence of function displayed among its varied neuronal populations (36). Before VNS, MNS increased functional connectivity within convergent-to-convergent neuron pairs and between convergent and efferent IC neuron populations. These data suggests that excessive inputs can cascade through the local neural networks with the potential to overwhelm local feedback mechanisms leading to excessive efferent outputs to disparate regions of the heart. This neural signature can be tempered by VNS, primarily via its suppression of convergent IC neural activity. By dampening intrinsic cardiac neural circuits the potential for atrial arrhythmias is reduced.
Unilateral VNS can exert bilateral influences on IC neural function (13, 50) and on control of regional cardiac function (5, 42, 72). Previous studies have demonstrated that aggregates of the intrinsic ganglionic plexus neurons exert preferential spheres of influence on cardiac indexes, manifested by their direct and indirect projections to cardiomyocytes (6, 74, 75). With respect to the RAGP, although it exerts preferential control of sinoatrial nodal pacemaker activity, some of its neurons also influence distant atrial and ventricular electrical and contractile indexes (6, 74). Medullary derived parasympathetic efferent preganglionic neurons likewise have spheres of influence (22, 28), reflecting their projections onto specific populations of intrinsic cardiac neurons as well as their interactions mediated by interganglionic projections (7, 28, 45, 54). Our data show that ipsilateral VNS exerts substantially greater antiarrhythmic effects when targeting right atrial neuronal networks than contralateral preganglionic projections to such ganglia (Fig. 7). Presumably, this reflects insufficient preganglionic efferent innervation of respective (contralateral vs. ipsilateral) aggregates of IC neurons (51, 53, 54). This anatomical-functional heterogeneity likely underlies any increased AF potential that right-sided ICNS neural imbalance elicits in the presence of left-sided VNS therapy.
VNS and memory.
Regardless of VNS site of delivery, its antiarrhythmic effects exhibit memory. For this study, 3 min of VNS conferred protection for up to 26 min. First and foremost, memory is neural and not myocyte dependent (4, 10, 12). It likely involves in the short term local release of neuromodulators and plasticity within local neural network processing (30, 32, 37, 39, 48) and in the longer term changes in synaptic efficacy (14, 29). While the precise structure/function mechanisms underlying short- to longer-term effects of VNS on neural function and the nerve/myocyte interface remain poorly defined, future studies should consider potential contributions by muscarinic (11, 56, 64), angiotensin (30, 41), and adrenergic (31, 56) receptor mechanisms.
Limitations.
Among the limitations of this study are 1) the effects of anesthesia on cardiovascular reflexes initiated by stimulating the cervical vagosympathetic nerve trunk and its associated afferent projections (5, 72); 2) the use of a single stimulation protocol for VNS; 3) the small sampling size of the neurons identified within the intrinsic cardiac nervous system compared with the complexity of the protocol employed; and 4) neural imbalances evaluated against a normal atrial electrophysiological substrate. It is to be expected that the addition of an altered atrial electrical substrate, as with chronic rapid pace, the neural-myocyte interface will remodel and the efficacy of bioelectric therapies impacted (18, 68). The stimulation protocol used herein was chosen to engage central (afferent-mediated) and peripheral (efferent-mediated) aspects of the cardiac nervous system (5) but at a level here the local circuit neurons of the intrinsic cardiac nervous system still exert dominant control (36). Because of the dispersed nature of the intrinsic cardiac nervous system (75), even with microarray technology, the yield of recorded IC neurons is low (13, 50). However, functional characteristics of the recorded neurons remain stable in time (13, 23, 50). Finally, long-term VNS has documented efficacy in management of arrhythmias (35, 60) and contractile dysfunction (15, 19, 49) in states of progressive cardiac pathology.
Perspectives and significance.
What is clear from recent studies is that there is asymmetry in neural remodeling with progressive cardiac disease and that this neural process is a major determinant of adverse outcomes, including the potential for arrhythmias (2, 18, 20). The adaptations in neuronal remodeling must likewise be evaluated in terms of the alterations in the cardiac electrophysiological substrate (18, 25). In contradistinction to ablation approaches, a major advantage of electrical neuromodulation when applied at more rostral sites in the cardiac neuraxis is that single point therapy can moderate reflex function in the disparate ganglia within the ICNS (7, 54, 55, 77). As demonstrated herein, this form of bioelectric therapy is readily reversible, has a rapid therapeutic onset, and exhibits memory (induces effects that outlast application). For the first time, we have also defined the pivotal role of local circuit neurons in mediating neurally involved arrhythmias and as the primary target for bioelectric medicine.
GRANTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (RO1-HL-71830 to J. L. Ardell) and the Natural Sciences and Engineering Research Council of Canada (No. 386647-2010 to V. Jacquemet).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S., E.B., J.-P.L., J.A.A., A.V., V.J., and J.L.A. analyzed data; S.S., E.B., J.-P.L., J.A.A., A.V., V.J., K.S., and J.L.A. interpreted results of experiments; S.S., J.A.A., A.V., V.J., and J.L.A. prepared figures; S.S., E.B., J.-P.L., J.A.A., A.V., V.J., K.S., and J.L.A. drafted manuscript; S.S., E.B., J.-P.L., J.A.A., A.V., V.J., K.S., and J.L.A. edited and revised manuscript; S.S., E.B., J.-P.L., J.A.A., A.V., V.J., K.S., and J.L.A. approved final version of manuscript; E.B., J.A.A., and J.L.A. performed experiments; J.A.A. and J.L.A. conception and design of research.
REFERENCES
- 1.Agmon A. A novel, jitter-based method for detecting and measuring spike synchrony and quantifying temporal firing precision. Neural Syst Circuits 2: 5, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajijola OA, Yagishita D, Reddy NK, Yamakawa K, Vaseghi M, Downs AM, Hoover DB, Ardell JL, Shivkumar K. Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: neuropeptide and morphologic changes. Heart Rhythm 12: 1027–1035, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardell JL, Andresen MC, Armour JA, Billman GE, Chen PS, Foreman RD, Herring N, O'Leary DS, Sabbah HN, Schultz HD, Sunagawa K, Zucker IH. Translational neurocardiology: preclinical models and cardioneural integrative aspects. J Physiol 594: 3877–3909, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ardell JL, Cardinal R, Vermeulen M, Armour JA. Dorsal spinal cord stimulation obtunds the capacity of intrathoracic extracardiac neurons to transduce myocardial ischemia. Am J Physiol Regul Integr Comp Physiol 297: R470–R477, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardell JL, Rajendran PS, Nier HA, KenKnight BH, Armour JA. Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. Am J Physiol Heart Circ Physiol 309: H1740–H1752, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol Heart Circ Physiol 251: H764–H773, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Armour JA. Potential clinical relevance of the “little brain” on the mammalian heart. Exp Physiol 93: 165–176, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Armour JA, Hageman GR, Randall WC. Arrhythmias induced by local cardiac nerve stimulation. Am J Physiol 223: 1068–1075, 1972. [DOI] [PubMed] [Google Scholar]
- 9.Armour JA, Kember G. Cardiac sensory neurons. In: Basic and Clinical Neurocardiology, edited by Armour JA, Ardell JL. New York, NY: Oxford Univ Press, 2004, p. 79–117. [Google Scholar]
- 10.Armour JA, Linderoth B, Arora RC, DeJongste MJ, Ardell JL, Kingma JG Jr, Hill M, Foreman RD. Long-term modulation of the intrinsic cardiac nervous system by spinal cord neurons in normal and ischaemic hearts. Auton Neurosci 95: 71–79, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Armour JA, Richer LP, Page P, Vinet A, Kus T, Vermeulen M, Nadeau R, Cardinal R. Origin and pharmacological response of atrial tachyarrhythmias induced by activation of mediastinal nerves in canines. Auton Neurosci 118: 68–78, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Baddeley A. Working memory: theories, models, controversies. Annu Rev Psychol 63: 1–29, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591: 4515–4533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaumont E, Southerland EM, Hardwick JC, Wright GL, Ryan S, Li Y, KenKnight BH, Armour JA, Ardell JL. Vagus nerve stimulation mitigates intrinsic cardiac neuronal and adverse myocyte remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol 309: H1198–H1206, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley U, Shivkumar K, Ardell JL. Autonomic regulation therapy in heart failure. Curr Heart Fail Rep 12: 284–293, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardinal R, Page P, Vermeulen M, Ardell JL, Armour JA. Spatially divergent cardiac responses to nicotinic stimulation of ganglionated plexus neurons in the canine heart. Auton Neurosci 145: 55–62, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Yu L, Zhou X, Liu Q, Jiang H, Zhou S. Low-level vagus nerve stimulation: an important therapeutic option for atrial fibrillation treatment via modulating cardiac autonomic tone. Int J Cardiol 199: 437–438, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 114: 1500–1515, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Ferrari GM. Vagal stimulation in heart failure. J Cardiovasc Transl Res 7: 310–320, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res 116: 2005–2019, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaita F, Caponi D, Pianelli M, Scaglione M, Toso E, Cesarani F, Boffano C, Gandini G, Valentini MC, De Ponti R, Halimi F, Leclercq JF. Radiofrequency catheter ablation of atrial fibrillation: a cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation 122: 1667–1673, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Geis GS, Wurster RD. Cardiac responses during stimulation of the dorsal motor nucleus and nucleus ambiguus in the cat. Circ Res 46: 606–611, 1980. [DOI] [PubMed] [Google Scholar]
- 23.Gibbons DD, Southerland EM, Hoover DB, Beaumont E, Armour JA, Ardell JL. Neuromodulation targets intrinsic cardiac neurons to attenuate neuronally mediated atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol 302: R357–R364, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson DN, Di Biase L, Mohanty P, Patel JD, Bai R, Sanchez J, Burkhardt JD, Heywood JT, Johnson AD, Rubenson DS, Horton R, Gallinghouse GJ, Beheiry S, Curtis GP, Cohen DN, Lee MY, Smith MR, Gopinath D, Lewis WR, Natale A. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm 8: 1364–1371, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Gloschat CR, Koppel AC, Aras KK, Brennan JA, Holzem KM, Efimov IR. Arrhythmogenic and metabolic remodeling of failing human heart. J Physiol 594: 3963–3980, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. J Am Med Assoc 285: 2370–2375, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Gray AL, Johnson TA, Ardell JL, Massari VJ. Parasympathetic control of the heart. II. A novel interganglionic intrinsic cardiac circuit mediates neural control of heart rate. J Appl Physiol (1985) 96: 2273–2278, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Gray AL, Johnson TA, Lauenstein JM, Newton SS, Ardell JL, Massari VJ. Parasympathetic control of the heart. III. Neuropeptide Y-immunoreactive nerve terminals synapse on three populations of negative chronotropic vagal preganglionic neurons. J Appl Physiol (1985) 96: 2279–2287, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Hardwick JC, Ryan SE, Beaumont E, Ardell JL, Southerland EM. Dynamic remodeling of the guinea pig intrinsic cardiac plexus induced by chronic myocardial infarction. Auton Neurosci 181: 4–12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardwick JC, Ryan SE, Powers EN, Southerland EM, Ardell JL. Angiotensin receptors alter myocardial infarction-induced remodeling of the guinea pig cardiac plexus. Am J Physiol Regul Integr Comp Physiol 309: R179–R188, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardwick JC, Southerland EM, Girasole AE, Ryan SE, Negrotto S, Ardell JL. Remodeling of intrinsic cardiac neurons: effects of beta-adrenergic receptor blockade in guinea pig models of chronic heart disease. Am J Physiol Regul Integr Comp Physiol 303: R950–R958, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herring N. Autonomic control of the heart: going beyond the classical neurotransmitters. Exp Physiol 100: 354–358, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herring N, Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp Physiol 94: 46–53, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Hoover DB, Isaacs ER, Jacques F, Hoard JL, Page P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience 164: 1170–1179, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Qian J, Yao W, Wang N, Zhang Z, Cao C, Song B, Zhang Z. Vagus nerve stimulation reverses ventricular electrophysiological changes induced by hypersympathetic nerve activity. Exp Physiol 100: 239–248, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Kember G, Ardell JL, Armour JA, Zamir M. Vagal nerve stimulation therapy: what is being stimulated? PLoS One 9: e114498, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kember G, Armour JA, Zamir M. Dynamic neural networking as a basis for plasticity in the control of heart rate. J Theor Biol 317: 39–46, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Kember G, Armour JA, Zamir M. Neural control hierarchy of the heart has not evolved to deal with myocardial ischemia. Physiol Genomics 45: 638–644, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Kember G, Armour JA, Zamir M. Neural control of heart rate: the role of neuronal networking. J Theor Biol 277: 41–47, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Leiria TL, Glavinovic T, Armour JA, Cardinal R, de Lima GG, Kus T. Longterm effects of cardiac mediastinal nerve cryoablation on neural inducibility of atrial fibrillation in canines. Auton Neurosci 161: 68–74, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Levett JM, Murphy DA, McGuirt AS, Ardell JL, Armour JA. Cardiac augmentation can be maintained by continuous exposure of intrinsic cardiac neurons to a beta-adrenergic agonist or angiotensin II. J Surg Res 66: 167–173, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Levy MN, Martin PJ. Neural control of the heart. In: Handbook of Physiology. The Cardiovascular System. The Heart. Bethesda, MD: Am Physiol Soc, 1979, vol. 1, sect. 2, p. 581–620. [Google Scholar]
- 43.Lin Y, Bian N, Li H, Chen J, Xing H, Li H, Huang D, Lan X, Gong B, Zhou L, Liu R, Guan M, Zhang D, Du G, Huang Z, Chen X, Zhang T, Feng J, Wu S, Wang L, Zhang A, Li Z. Effects of low-level autonomic stimulation on prevention of atrial fibrillation induced by acute electrical remodeling. Sci World J 2013: 781084, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longpre JP, Salavatian S, Beaumont E, Armour JA, Ardell JL, Jacquemet V. Measure of synchrony in the activity of intrinsic cardiac neurons. Physiol Meas 35: 549–566, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAllen RM, Salo LM, Paton JF, Pickering AE. Processing of central and reflex vagal drives by rat cardiac ganglion neurones: an intracellular analysis. J Physiol 589: 5801–5818, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGuirt AS, Schmacht DC, Ardell JL. Autonomic interactions for control of atrial rate are maintained after SA nodal parasympathectomy. Am J Physiol Heart Circ Physiol 272: H2525–H2533, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 104: 1534–1539, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Parsons RL. Mammalian cardiac ganglia as local integration centers: histochemical and electrophysiological evidence. In: Neural Mechanisms in Cardiovascular Regulation, edited by Dun NJ, Machado BH, Pilowsky PM. Boston, MA: Kluwer, 2004, p. 335–356. [Google Scholar]
- 49.Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH, Anand IS. Extended follow-up of patients with heart failure receiving autonomic regulation therapy in the ANTHEM-HF study. J Card Fail 22: 639–642, 2016. [DOI] [PubMed] [Google Scholar]
- 50.Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, Shivkumar K. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol 594: 321–341, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Randall DC, Brown DR, Li SG, Olmstead ME, Kilgore JM, Sprinkle AG, Randall WC, Ardell JL. Ablation of posterior atrial ganglionated plexus potentiates sympathetic tachycardia to behavioral stress. Am J Physiol Regul Integr Comp Physiol 275: R779–R787, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Randall DC, Brown DR, McGuirt AS, Thompson GW, Armour JA, Ardell JL. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol 285: R1066–R1075, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Randall WC, Ardell JL. Selective parasympathectomy of automatic and conductile tissues of the canine heart. Am J Physiol Heart Circ Physiol 248: H61–H68, 1985. [DOI] [PubMed] [Google Scholar]
- 54.Randall WC, Ardell JL, O'Toole MF, Wurster RD. Differential autonomic control of SAN and AVN regions of the canine heart: structure and function. Prog Clin Biol Res 275: 15–31, 1988. [PubMed] [Google Scholar]
- 55.Randall WC, Milosavljevic M, Wurster RD, Geis GS, Ardell JL. Selective vagal innervation of the heart. Ann Clin Lab Sci 16: 198–208, 1986. [PubMed] [Google Scholar]
- 56.Richer LP, Vinet A, Kus T, Cardinal R, Ardell JL, Armour JA. α-Adrenoceptor blockade modifies neurally induced atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol 295: R1175–R1180, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scherlag BJ, Nakagawa H, Jackman WM, Lazzara R, Po SS. Non-pharmacological, non-ablative approaches for the treatment of atrial fibrillation: experimental evidence and potential clinical implications. J Cardiovasc Transl Res 4: 35–41, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Schwarz N, Kuniss M, Nedelmann M, Kaps M, Bachmann G, Neumann T, Pitschner HF, Gerriets T. Neuropsychological decline after catheter ablation of atrial fibrillation. Heart Rhythm 7: 1761–1767, 2010. [DOI] [PubMed] [Google Scholar]
- 59.Shen MJ, Choi EK, Tan AY, Lin SF, Fishbein MC, Chen LS, Chen PS. Neural mechanisms of atrial arrhythmias. Nat Rev Cardiol 9: 30–39, 2012. [DOI] [PubMed] [Google Scholar]
- 60.Shen MJ, Shinohara T, Park HW, Frick K, Ice DS, Choi EK, Han S, Maruyama M, Sharma R, Shen C, Fishbein MC, Chen LS, Lopshire JC, Zipes DP, Lin SF, Chen PS. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation 123: 2204–2212, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 114: 1004–1021, 2014. [DOI] [PubMed] [Google Scholar]
- 62.Sheng X, Scherlag BJ, Yu L, Li S, Ali R, Zhang Y, Fu G, Nakagawa H, Jackman WM, Lazzara R, Po SS. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol 57: 563–571, 2011. [DOI] [PubMed] [Google Scholar]
- 63.Shin HC, Aggarwal V, Acharya S, Schieber MH, Thakor NV. Neural decoding of finger movements using Skellam-based maximum-likelihood decoding. IEEE Trans Biomed Eng 57: 754–760, 2010. [DOI] [PubMed] [Google Scholar]
- 64.Smith FM, McGuirt AS, Leger J, Armour JA, Ardell JL. Effects of chronic cardiac decentralization on functional properties of canine intracardiac neurons in vitro. Am J Physiol Regul Integr Comp Physiol 281: R1474–R1482, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Snedocor G, Cochrane W. Statistical Methods. Ames, IA: Iowa State Univ Press, 1980. [Google Scholar]
- 66.Southerland EM, Gibbons DD, Smith SB, Sipe A, Williams CA, Beaumont E, Armour JA, Foreman RD, Ardell JL. Activated cranial cervical cord neurons affect left ventricular infarct size and the potential for sudden cardiac death. Auton Neurosci 169: 34–42, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol 65: 867–875, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stavrakis S, Nakagawa H, Po SS, Scherlag BJ, Lazzara R, Jackman WM. The role of the autonomic ganglia in atrial fibrillation. JACC Clin Electrophysiol 1: 1–13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson GW, Collier K, Ardell JL, Kember G, Armour JA. Functional interdependence of neurons in a single canine intrinsic cardiac ganglionated plexus. J Physiol 528: 561–571, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waldmann M, Thompson GW, Kember GC, Ardell JL, Armour JA. Stochastic behavior of atrial and ventricular intrinsic cardiac neurons. J Appl Physiol 101: 413–419, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Wang S, Zhou X, Huang B, Wang Z, Liao K, Saren G, Lu Z, Chen M, Yu L, Jiang H. Spinal cord stimulation protects against ventricular arrhythmias by suppressing left stellate ganglion neural activity in an acute myocardial infarction canine model. Heart Rhythm 12: 1628–1635, 2015. [DOI] [PubMed] [Google Scholar]
- 72.Yamakawa K, Rajendran PS, Takamiya T, Yagishita D, So EL, Mahajan A, Shivkumar K, Vaseghi M. Vagal nerve stimulation activates vagal afferent fibers that reduce cardiac efferent parasympathetic effects. Am J Physiol Heart Circ Physiol 309: H1579–H1590, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamakawa K, So EL, Rajendran PS, Hoang JD, Makkar N, Mahajan A, Shivkumar K, Vaseghi M. Electrophysiological effects of right and left vagal nerve stimulation on the ventricular myocardium. Am J Physiol Heart Circ Physiol 307: H722–H731, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuan BX, Ardell JL, Hopkins DA, Armour JA. Differential cardiac responses induced by nicotine sensitive canine atrial and ventricular neurones. Cardiovasc Res 27: 760–769, 1993. [DOI] [PubMed] [Google Scholar]
- 75.Yuan BX, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec 239: 75–87, 1994. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Ilsar I, Sabbah HN, Ben David T, Mazgalev TN. Relationship between right cervical vagus nerve stimulation and atrial fibrillation inducibility: therapeutic intensities do not increase arrhythmogenesis. Heart Rhythm 6: 244–250, 2009. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Mazgalev TN. Arrhythmias and vagus nerve stimulation. Heart Fail Rev 16: 147–161, 2011. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Scherlag BJ, Lu Z, Niu GD, Yamanashi WS, Hogan C, Fields J, Ghias M, Lazzara R, Jackman WM, Po S. Comparison of atrial fibrillation inducibility by electrical stimulation of either the extrinsic or the intrinsic autonomic nervous systems. J Interv Card Electrophysiol 24: 5–10, 2009. [DOI] [PubMed] [Google Scholar]
- 79.Zipes DP. Antiarrhythmic therapy in 2014: contemporary approaches to treating arrhythmias. Nat Rev Cardiol 12: 68–69, 2015. [DOI] [PubMed] [Google Scholar]
- 80.Zucker IH, Gilmore JP. Reflex Control of the Circulation. Boca Raton, FL: CRC, 1991. [Google Scholar]
- 81.Zucker IH, Patel KP, Schultz HD. Neurohumoral stimulation. Heart Fail Clin 8: 87–99, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]