New understanding of the directional flow of subarachnoid cerebrospinal fluid (CSF) through the Virchow-Robin space (VRS) to brain parenchyma, coupled with the demonstration here of rapid, insulin receptor-dependent trapping of plasma insulin by the brain microvasculature, underscores the direct role of insulin's blood-brain barrier transit to insulin delivery to the brain.
Keywords: blood-brain barrier, brain interstitial fluid, high-fat diet, insulin receptor, Virchow-Robin space
Abstract
Insulin affects multiple important central nervous system (CNS) functions including memory and appetite, yet the pathway(s) by which insulin reaches brain interstitial fluid (bISF) has not been clarified. Recent studies demonstrate that to reach bISF, subarachnoid cerebrospinal fluid (CSF) courses through the Virchow-Robin space (VRS) which sheaths penetrating pial vessels down to the capillary level. Whether insulin predominantly enters the VRS and bISF by local transport through the blood-brain barrier, or by being secreted into the CSF by the choroid plexus, is unknown. We injected 125I-TyrA14-insulin or regular insulin intravenously and compared the rates of insulin reaching subarachnoid CSF with its plasma clearance by brain tissue samples (an index of microvascular endothelial cell binding/uptake/transport). The latter process was more than 40-fold more rapid. We then showed that selective insulin receptor blockade or 4 wk of high-fat feeding each inhibited microvascular brain 125I-TyrA14-insulin clearance. We further confirmed that 125I-TyrA14-insulin was internalized by brain microvascular endothelial cells, indicating that the in vivo tissue association reflected cellular transport, not simply microvascular tracer binding.
NEW & NOTEWORTHY
New understanding of the directional flow of subarachnoid cerebrospinal fluid (CSF) through the Virchow-Robin space (VRS) to brain parenchyma, coupled with the demonstration here of rapid, insulin receptor-dependent trapping of plasma insulin by the brain microvasculature, underscores the direct role of insulin's blood-brain barrier transit to insulin delivery to the brain.
insulin regulates multiple neurophysiologic processes in the brain, including neuronal survival, synapse number, and dendrite plasticity (8, 23, 34). Together these affect appetite, behavior, and cognitive performance. Insulin administered into the cerebral ventricles (ICV) evokes a satiety signal (28), and loss of neuronal insulin signaling increases eating behavior (5). Brain glucose uptake is largely insulin independent; however, in rodent models, ICV insulin delivery improves liver and skeletal muscle glucose metabolism in insulin resistant models (11, 26) and suppresses lipolysis (30). While insulin resistance does not impair brain glucose uptake, it is associated with accelerated cognitive decline (33).
For plasma insulin to act on neurons or other cells throughout the brain, it must first reach brain interstitial fluid (bISF). There are two potential pathways: it could, like glucose, directly cross the brain microvascular endothelium in all brain regions or it could cross the blood-CSF barrier at the choroid plexus in the ventricles, circulate through the ventricular system and cisterna magna after which it enters the VRS, which courses with arteries and arterioles throughout the brain (13, 14), and from there cross the astrocyte foot processes to enter bISF. It is not currently known which pathway predominates for moving insulin from plasma to bISF. Early kinetic studies of insulin transfer to subarachnoid CSF in dogs reported that a “best fit” to kinetic measures of insulin transfer from plasma to CSF was obtained by a three-compartment model. The authors suggested that bISF might be an “intermediate compartment” through which insulin passed during transit from plasma to subarachnoid CSF (31). However, no direct evidence for this compartment assignment was provided, and quite recent work indicates a vectorial flow of CSF from the subarachnoid space through the Virchow-Robin paracellular channels, then to bISF, i.e., a direction opposite to that previously hypothesized (12–14).
There is evidence that insulin binds to brain microvasculature (35) and retention of intravenously injected radiolabeled insulin by the brain is a saturable process (1, 9). However, these studies did not define whether insulin was transported by or simply bound to the vascular cells. Insulin also traverses the endothelial-epithelial blood-CSF barrier at the choroid plexus via a saturable transport pathway (28), yet this process appears to be slow (32). Virtually all studies looking at insulin's CNS-mediated effects on appetite, metabolism, and insulin sensitivity have used ICV insulin administration, tacitly assuming the CSF circulation will replicate physiological insulin delivery. Likewise, studies examining the effect of pharmacological or dietary manipulations on insulin access to the brain based on earlier kinetic modeling have assumed insulin entering subarachnoid CSF reflects insulin that has passed through bISF (3, 4, 17). To our knowledge, no study has compared the rate of insulin's transfer to CSF vs. its removal by brain microvascular endothelium.
In the current study, we compared the initial rate (first 5 min) of brain 125I-TyrA14-insulin transfer to CSF with its retention by brain tissue from 3 separate brain regions. We found significant brain tissue insulin uptake preceded its appearance in CSF. Then, using CSF sampling during a 3-h insulin clamp we estimated the transfer of plasma insulin to CSF. From these measurements, we estimated that brain tissue removes insulin more than 40 times more rapidly than insulin's transfer to subarachnoid CSF. Employing the insulin receptor (IR) blocker S961, we confirmed that microvascular insulin uptake was IR-mediated. We then tested whether insulin was internalized by brain microvascular endothelial cells and the effect of IR blockade on internalization. We also tested whether a high-fat diet impacted insulin uptake by brain tissue.
METHODS
Animal Preparation
Four groups of male Sprague-Dawley rats were studied after an overnight fast. In the morning rats were anesthetized with thiobutabarbital (180 mg/kg; Sigma-Aldrich, St Louis, MO). Both jugular veins were cannulated with PE-50 tubing and used for either 125I-TyrA14-insulin or regular insulin infusion or blood sampling. Rats were allowed to stabilize for 30 min after surgery before beginning an infusion study. The rats were fixed in a stereotaxic frame to allow cisterna magna puncture for CSF sampling (groups 1–3) or for lateral ventricle CSF injection of 125I-TyrA14-insulin (group 4). The cisterna magna was punctured after flexing the head downward ∼110°, raising the body 3 cm, and introducing a needle at the coordinates anterior-posterior −1.5 mm, medial-lateral 0 mm, with the zero point being where the two ear bars meet. The dorsal-ventral coordinates were at the skin level. The needle was then inserted 6–7 mm under slight negative pressure from the syringe (21). As soon as the CSF started flowing, the needle advancement was stopped.
Group 1.
Chow-fed rats were given a 20 nmol/kg iv bolus of the specific IR antagonist S961 (a gift from Novo-Nordisk, Copenhagen, Denmark) (n = 10) or saline (n = 10), 60 min before receiving a bolus (0.7 pmol) of carrier-free 125II-TyrA14-insulin (PerkinElmer, Waltham, MA) via the right jugular cannula. Subsequently, blood (0.1 ml/min) was withdrawn every minute through the left jugular cannula over 5 min, and 150 μl CSF was withdrawn at 5 min, as described above. The rat was then perfused with ice-cold saline (5 ml/min for 12 min) to clear labeled insulin from the vascular space and the brain was quickly excised, dissected, and frozen in liquid nitrogen.
Group 2.
Chow-fed rats (n = 7) underwent a 6 mU·min−1·kg−1 euglycemic insulin clamp (with Humulin R) as previously described (15) with sampling of blood and CSF (25 μl) at 0, 60, 120, and 180 min during the clamp.
Group 3.
Rats were either fed a high-fat diet (HFD, 60% of calories from saturated fat, n = 7) for 4 wk or were age-matched, chow-fed controls (n = 7). All animals received a bolus (0.7 pmol) of carrier-free 125I-TyrA14-insulin via the jugular vein and had blood, CSF, and tissues harvested as in group 1 (above).
Group 4.
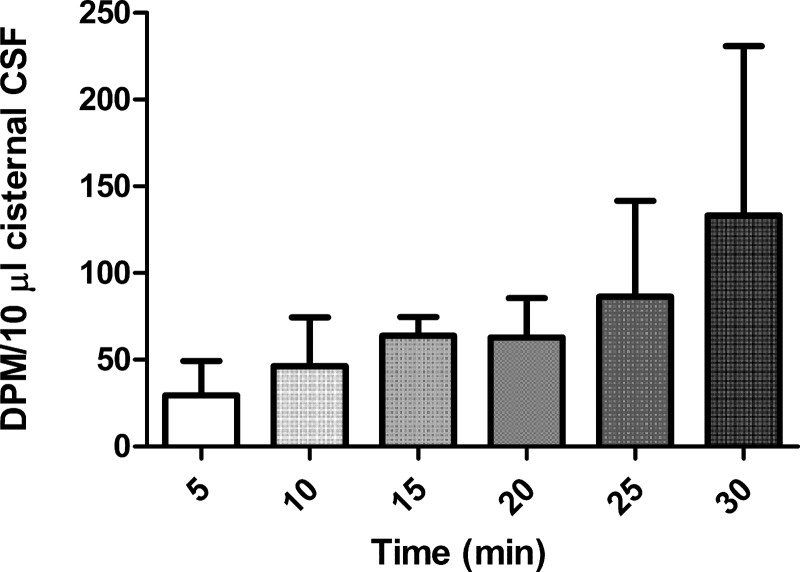
Rats (n = 5) received an intraventricular injection (lateral ventricle) of 125 I-TyrA14-insulin (10 μl) with serial sampling of 10 μl CSF over 30 min from the cisterna magna. This injectate volume is equivalent to the volume of CSF produced in 5 min in the adult rat (16). The CSF samples were stored in 0.5 ml Protein LoBind Tubes (Eppendorf, Hamburg, Germany) and frozen immediately after aspiration.
Insulin in the CSF and plasma was measured by an ultrasensitive rat insulin ELISA (Alpco, Salem, NH). This ELISA has a 2-fold greater sensitivity for Humulin R compared with rat insulin, so plasma concentrations measured during the clamp were divided by 2.
The study protocol was approved by the University of Virginia Animal Care and Use Committee and followed the Principles of Laboratory Animal Care (NIH publication no. 85-23, revised 1985).
125I-TyrA14-Insulin Administration and Analysis
As insulin receptor distribution in brain may differ between regions (18), the brain was divided into 3 sections: cortex, cerebellum, and basal nuclei in groups 1 and 3. Blood and brain samples were powdered, weighed, and mixed with 30% trichloroacetic acid (TCA) and centrifuged. After removing the supernatant, radioactivity in the tissue extracts was measured using a gamma counter (Packard, Cobra II, Canberra, Australia) and counts per minute (CPM) were corrected for counter efficiency to calculate disintegrations per minute (DPM). TCA precipitation of radiolabeled insulin yields estimates of intact insulin comparable to that obtained by immune precipitation (22, 38). Clearance of plasma radiolabeled insulin by brain tissues was estimated by dividing the DPM of insulin taken up over 5 min per gram of tissue by the average 125I-TyrA14-insulin radioactivity in 1.0 ml of TCA-precipitated plasma during that 5-min interval.
Endothelial Insulin Uptake In Vitro
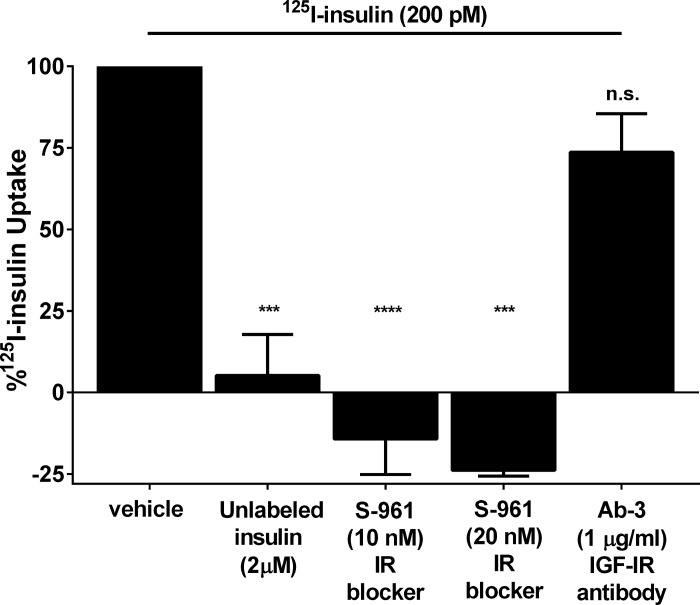
We tested whether labeled insulin could be taken up by cultured rat brain microvascular endothelial cells to ensure that the brain insulin uptake measured in vivo was not simply insulin bound to endothelial cell surfaces that was not removed by the vascular flushing procedure. Commercially available rat brain microvascular endothelial cells (RBMVECs, Cell Applications, San Diego, CA), passages 6–10, were grown to 90–95% confluence and serum-starved for 2 h before being incubated with 125I-TyrA14-insulin at a final concentration of 200 pM in HEPES binding buffer (HBB, 100 mM HEPES, 120 mM NaCl, 1.2 mM MgSO4, 5 mM KCl, 8 mM glucose, 1% BSA, pH 7.8) for 15 min. Uptake was stopped by placing cells on ice and washing twice with ice-cold HBB. Nonspecific binding was determined by adding ice-cold 125I-TyrA14-insulin (200 pM) to RBMVECs in HBB, immediately aspirating the solution, and washing with ice-cold HBB. RBMVECs were then incubated with an acid wash (0.5 M NaCl, 0.2 M acetic acid, pH 3) for 6 min and washed twice more with the acid wash to remove surface membrane-bound 125I-TyrA14-insulin. Cells were lysed with 1 M NaOH, and lysate radioactivity was quantified using the gamma counter and expressed as DPM normalized for protein content and corrected for nonspecific binding (10). In selected experiments we included either unlabeled insulin (2 μM) to test saturability of uptake or preincubated cells for 30 min with either S961 (10 nM or 20 nM) to test IR involvement or Ab-3 (1 μg/ml; an IGF-1 receptor antibody) before adding 125I-TyrA14-insulin to test for uptake via either IGF-1R or hybrid IGF-1R/IR receptors.
Statistical Analysis
Statistical comparisons between subgroups within groups 1 and 3 for plasma and for each tissue were made using unpaired Student's t-tests. A P value < 0.05 was considered significant.
RESULTS
Initial Clearance of 125I-TyrA14-Insulin from Plasma by Brain Tissue
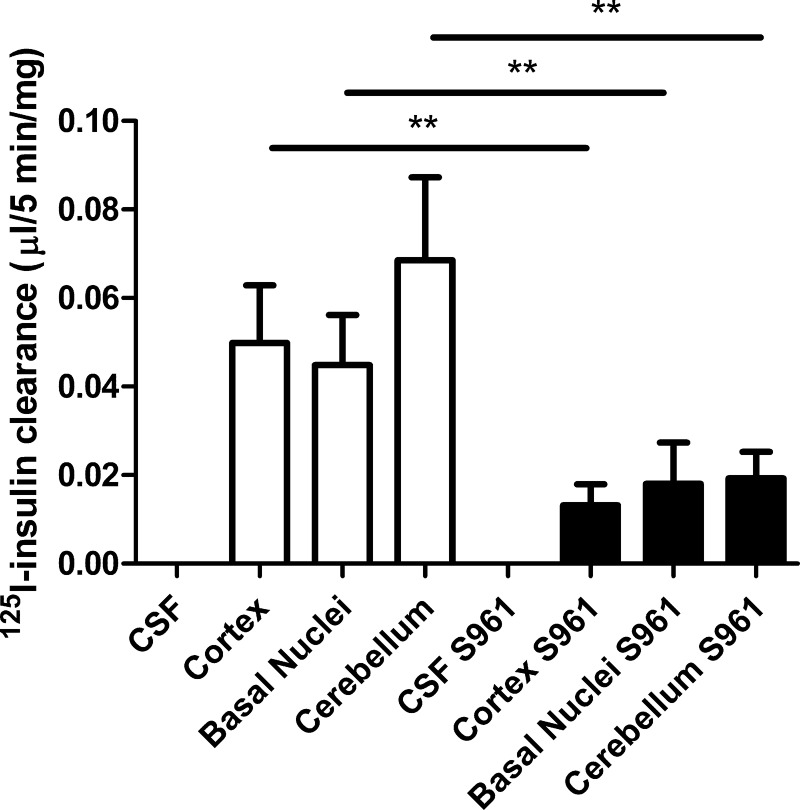
In group 1, rats weighed 273 ± 7 g at the time of study. Labeled insulin clearance was observed in the 3 brain regions sampled (cortex, basal nuclei, and cerebellum, expressed as microliters of plasma insulin cleared per 5 min per milligram tissue) within the first 5 min following its injection (Fig. 1). Clearance of 125I-TyrA14-insulin was similar in all 3 brain regions sampled (cortex 0.050 ± 0.013 μl·5 min−1·mg−1, basal nuclei 0.045 ± 0.011 μl·5 min−1·mg−1, cerebellum 0.068 ± 0.018 μl·5 min−1·mg−1). Pretreatment with the IR blocker S961 markedly slowed the clearance of 125I-TyrA14-insulin from plasma by each brain region compared with control rats (Fig. 1). The initial rates of plasma 125I-TyrA14-insulin clearance following S961 pretreatment were cortex 0.013 ± 0.004 μl·5 min−1·mg−1, P < 0.01; basal nuclei 0.018 ± 0.009 μl·5 min−1·mg−1, P < 0.001; cerebellum 0.019 ± 0.006 μl·5 min−1·mg−1; P < 0.01. 125I-TyrA14-insulin concentrations were undetectable in the CSF 5 min after intravenous administration in the presence or absence of S961 (Fig. 1). The finding that 125I-TyrA14-insulin was detected in brain samples (Fig. 1) before any tracer appeared in CSF supports the hypothesis that insulin transport into the brain may occur principally by microvascular endothelial insulin uptake across the blood-brain barrier. Moreover, the effect of S961 to blunt the brain's clearance of plasma insulin tracer implicates a role for the insulin receptor in this process.
Fig. 1.
The three left bars designate the clearance of insulin by three brain regions in the control animals. The three right bars indicate insulin clearance in animals pretreated with S961 60 min prior to radioisotope injection. No bars are shown for cerebrospinal fluid (CSF) as there was no radioactivity present in the CSF samples in either condition. **P < 0.01.
Time Course for Insulin's Transfer from Plasma into CSF
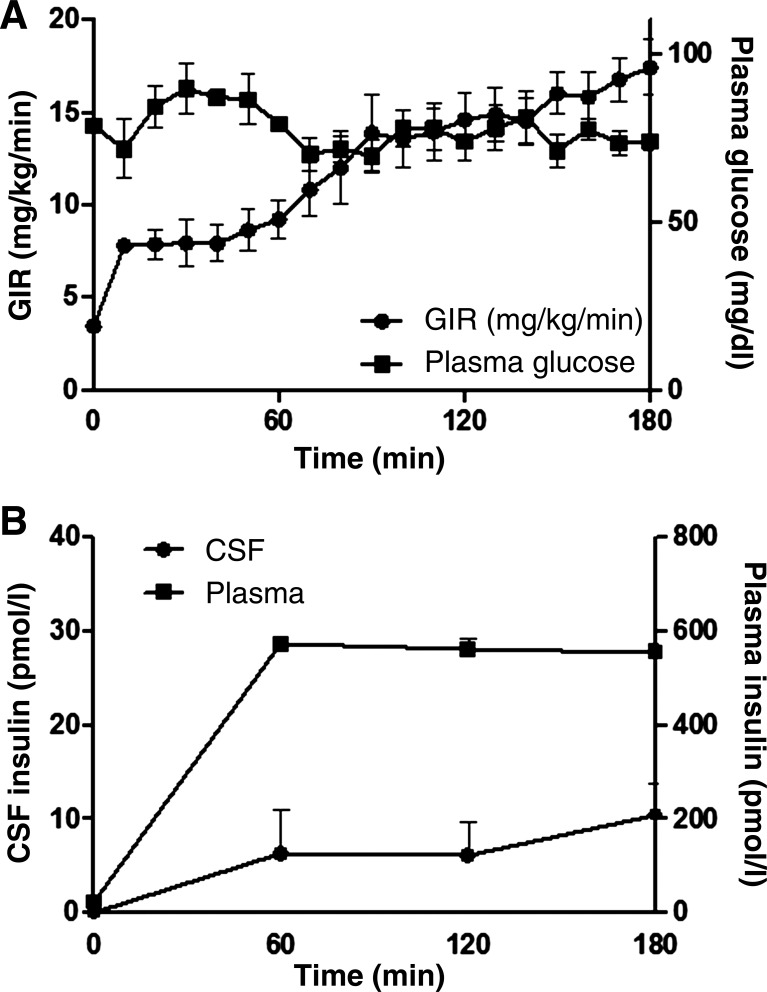
Group 2 rats weighed 278 ± 10 g and had average fasting glucose concentrations of 4.3 ± 0.2 mmol/l and fasting plasma insulin concentrations of 49 ± 6 pmol/l. Fasting insulin concentrations in CSF were below the detection limit of the ELISA kit (<3.44 pmol/l) for all rats. During the clamp, plasma insulin levels rose to 573 ± 12 pmol/l at 60 min and remained stable (556 ± 19 pmol/l at 180 min, P < 0.001 vs. baseline). By contrast, CSF insulin concentrations slowly rose to 10 ± 3 pmol/l at 180 min, P < 0.05 vs. baseline (Fig. 2). The plasma to CSF insulin gradient at 180 min was >50-fold. Both the fasting CSF insulin concentrations and the time course for the rate of increase in insulin concentrations during the clamp are strongly reminiscent of those previously seen in canine studies (32).
Fig. 2.
A: the plasma glucose concentration and the glucose infusion rate required to maintain euglycemia. B: time course for changes in insulin concentration in CSF and plasma during the 180-min euglycemic insulin clamp.
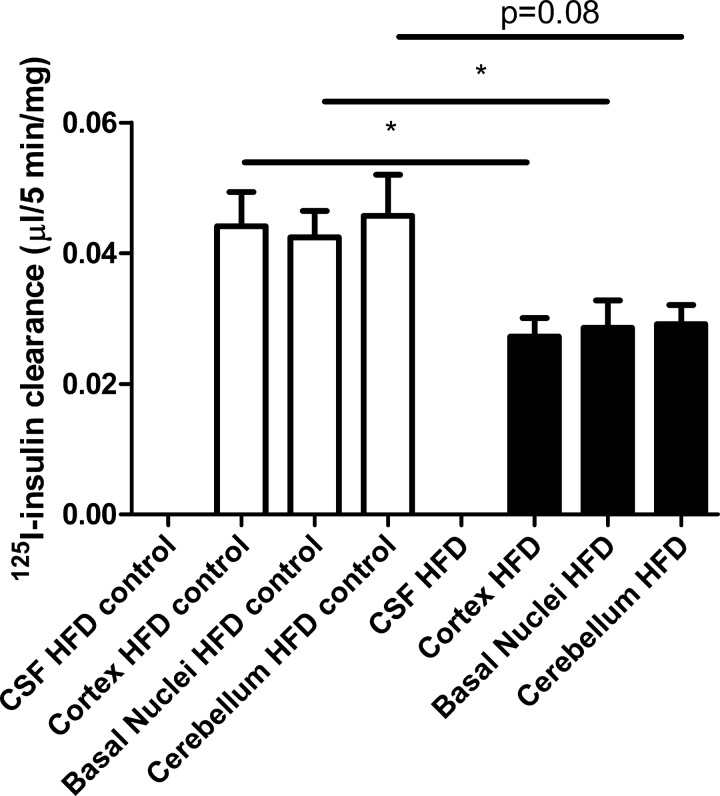
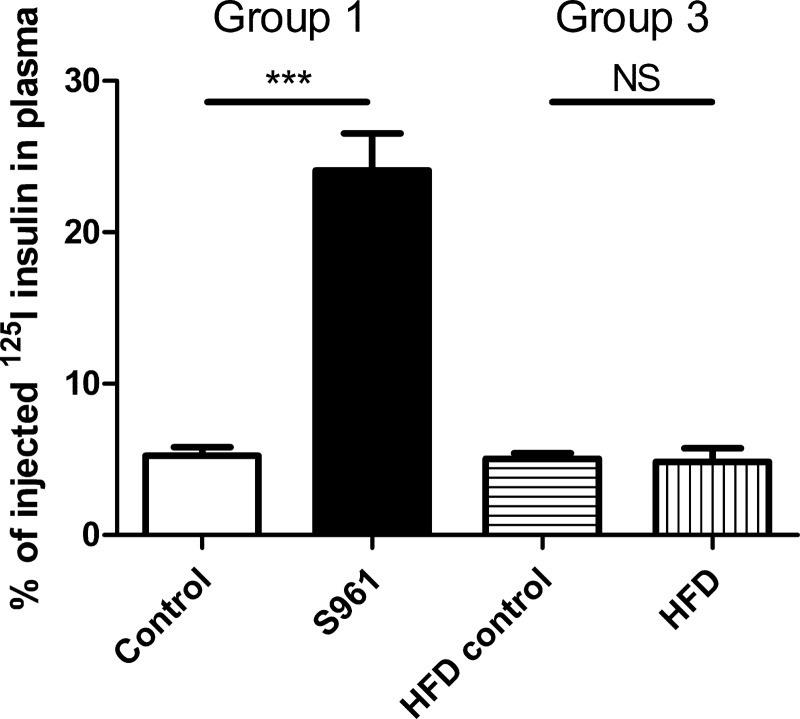
In group 3, HFD rats weighed more than their age-matched controls (444 ± 8 vs. 400 ± 6 g, P < 0.001). The plasma insulin concentration trended nonsignificantly higher in HFD animals (1.3 ± 0.2 vs. 0.9 ± 0.2 ng/ml, P = 0.09). Compared with the age-matched controls in group 3, 125I-TyrA14-insulin clearance was significantly reduced (Fig. 3) in cortex (0.027 ± 0.003 vs. 0.044 ± 0.005 μl·5 min−1·mg−1, P < 0.05) and the basal nuclei (0.029 ± 0.004 vs. 0.42 ± 0.004 μl·5 min−1·mg−1, P < 0.05) and trended lower in the cerebellum (0.029 ± 0.003 vs. 0.046 ± 0.006 μl·5 min−1·mg−1, P = 0.08). The plasma radiolabeled insulin concentration at 5 min following the insulin tracer bolus did not differ between HFD and chow-fed rats (Fig. 4). These results indicate that HFD feeding decreases insulin clearance by the brain microvasculature.
Fig. 3.
Three left bars indicate insulin clearance by three brain regions of rats matched for age with high-fat diet-fed (HFD) animals (right three bars). No bars are shown for CSF as there was no radioactivity present in the CSF samples in either condition. *P < 0.05.
Fig. 4.
Bars indicate the percent of injected 125I-TyrA14-insulin remaining in plasma at 5 min post iv injection in group 1 (left, with and without treatment with S961) and group 3 (right, with and without HFD) animals. ***P < 0.001.
Insulin Transit through the Ventricular System
As experiments conducted in group 2 had indicated slow arrival of intravenously administered insulin in cisternal CSF and given that in groups 1 and 3 there was no radiolabeled insulin in cisternal CSF 5 min after intravenous 125I-TyrA14-insulin injection, we questioned whether the very slow insulin appearance in cisternal CSF reflected its slow entry into CSF at the choroid plexus or slow flow through the ventricular system. Figure 5 shows the time course for the appearance of radiolabeled insulin in cisternal CSF when it is directly injected into the (right) lateral ventricle. Tracer was detectable in the cisternal CSF within 5 min of injection and rose slowly and quite variably over the course of 30 min, suggesting that while a slow CSF circulation in part contributes to the slow rate of appearance of intravenously injected insulin in cisternal CSF, transfer into CSF at the choroid plexus may be an even greater contributor.
Fig. 5.
Time course for the appearance of radiolabeled insulin in the cisterna magna CSF following ICV injection.
Insulin Is Internalized by Endothelial Cells
Serum-starved RBMVECs were incubated with 200 pM 125I-TyrA14-insulin. Cellular insulin uptake reached a steady state within ∼ 15 min (data not shown). Adding 2 μM unlabeled regular insulin decreased 125I-TyrA14-insulin uptake by 95% compared with 125I-TyrA14-insulin alone (P < 0.001, Fig. 6). S961 at either 10 or 20 nM fully blocked endothelial cell 125I-TyrA14-insulin uptake (P < 0.0001 vs. control and P < 0.001, respectively, Fig. 6), indicating an insulin-receptor-dependent effect. On the other hand, Ab-3, which blocks access to the exofacial domain of the IGF-1 receptor, had little effect on 125I-TyrA14-insulin uptake (74 ± 12% vs. control, P = NS). This suggests that when physiological concentrations of insulin are used, insulin uptake by the brain endothelial cell is dependent upon the IR but not the IGF-1 receptor. As extracellular binding of insulin cannot account for the insulin associated with these cultured endothelial cells and as blocking the IR blocks endothelial cell insulin uptake entirely, it is likely that the in vivo uptake by brain tissue is likewise not simply binding of insulin to sites on the microvasculature but includes internalization of insulin by the endothelial cell.
Fig. 6.
The uptake of 125I-TyrA14-insulin (200 pM) by RBMVECs after 15 min of incubation by control cells compared with cells also exposed to either a high concentration of unlabeled insulin, S961 at two concentrations, or the IGF-1 receptor antibody Ab-3 (1 mg/l). ***P < 0.001 vs. vehicle; ****P < 0.001 vs. vehicle.
DISCUSSION
The primary focus of these studies was to ascertain whether the predominant path for insulin delivery from circulating plasma to brain tissue involved the brain microvascular endothelial cell or the CSF circulation. We recognize that our tissue sampling does not identify whether tracer insulin is associated with the endothelium, astroglia, neurons, or bISF. Microdissection studies would also not address this as >100 mg of tissue is required for detection of radioactivity despite using carrier-free radiolabeled insulin in the injection. Previous, in vivo autoradiographic studies likewise lack the spatial resolution needed to identify the cellular origin of the insulin tracer (9). It is also true that the autoradiographic method does not allow differentiation of intact labeled insulin from labeled degradation products. For studies with 125I-TyrA14-insulin, we intentionally measured insulin clearance over a brief 5-min interval, both to assess the initial step of insulin clearance which, in tissues with a continuous endothelium (e.g., brain, muscle and adipose), involves principally the endothelial barrier, and to minimize the contribution of 125I-TyrA14-insulin degradation products. The findings with group 1 studies indicate that insulin transfer to brain is more rapid than transfer to CSF, but did not allow quantitation of the latter. Use of the insulin clamp in group 2 allows measurement of the rate of insulin transfer to CSF, albeit with several assumptions: 1) the average CSF volume in the adult rat is ∼250 μl (2); 2) the adult rat makes CSF at a rate of ∼2 μl/min (16); and 3) the average adult rat brain weighs ∼1.5 g. The change in CSF insulin concentration during the insulin clamp (group 2) was ∼8 pmol·l−1·180 min−1. Assuming that the entire amount of CSF made during that interval(∼ 360 μl) contained insulin at a concentration of 10 pmol/l, this would be equivalent to 3,600 × 10−6 pmol/180 min transferred from blood to CSF. That is, 20 × 10−6 pmol of insulin/min transferred from blood to the whole brain CSF. Considering the data from the radiolabeled insulin injections, we can estimate the insulin clearance from blood by brain tissue to be ∼55 μl·g−1 (the average of the three tissue samples)·5 min−1 or 11 μl·g−1·min−1. With a plasma insulin concentration of 50 pmol/l, this amounts to 550 × 10−6 pmol·g−1·min−1. For a 1.5-g adult rat brain this would amount to 825 × 10−6 pmol of insulin/min transferred from plasma to brain tissue. This suggests that the insulin transfer rate across the microvascular endothelial cell is ∼41 times greater than that of insulin transfer to newly formed CSF. It is important to note that the low transfer rate into cisternal CSF occurred despite the fact that plasma insulin concentrations during the clamp (Fig. 2) were more than 10-fold higher than during the radioisotope clearance measurements (Figs. 1 and 3). If insulin uptake by the blood-brain barrier microvascular endothelial cell increases linearly with plasma insulin concentration, then the plasma to brain tissue transfer across the blood-brain barrier (BBB) would be more than 400-fold greater than transfer to CSF. These findings suggest that subarachnoid CSF is unlikely to provide an important route for insulin entry to brain. Interestingly, the transfer of glucose from plasma to brain in humans (estimated from the rate of brain glucose utilization of 70 mg/min for a 70-kg person) exceeds by ∼200-fold the transfer of glucose from plasma to subarachnoid CSF (450 μl CSF made/min × 70 mg/dl CSF glucose concentration). Thus the microvascular pathway appears to be important for both small nutrients and insulin. These findings strongly indicate the brain microvascular endothelium as potentially the major site for insulin's delivery to bISF. Beyond that, our findings identify that this transport is mediated by the insulin receptor when insulin is present at physiological concentrations. We note that the peptide inhibitor S961, unlike large doses of unlabeled insulin, does not significantly interfere with insulin binding to the IGF-1 receptor (or presumably hybrid insulin/IGF-I receptors) (29). This may be particularly important as it is estimated that much of insulin receptor protein in brain may be present in hybrid receptors (18). Indeed, the demonstrated effect of S961 to acutely decrease overall plasma radiolabeled insulin clearance underscores the IR's importance to body insulin kinetics. Beyond identifying insulin receptor-mediated transfer as the major pathway for brain microvascular insulin uptake, we also find that exposure of animals to a high-fat diet decreases brain radiolabeled insulin clearance. However, the plasma insulin was higher in the HFD rats and this might also limit receptor-mediated brain insulin clearance.
CSF sampling has been regularly used as a surrogate measure for insulin penetration into the CNS (28). It has been demonstrated that factors which provoke peripheral insulin resistance [high-fat diet (4), obesity (17), glucocorticoid administration (3)] each can interfere with insulin appearance in the CSF. These findings suggest a potential physiological/pathological role for regulation of insulin transfer to subarachnoid CSF. However, the slow rate of insulin transfer to CSF indicated by the current findings argues against a physiological role for subarachnoid CSF as a source for delivery of insulin to brain. However, subarachnoid CSF may in some manner reflect bISF [insulin] if insulin that passes through bISF subsequently mixes completely with subarachnoid CSF [a pathway consistent with the kinetic model proposed by Schwartz and colleagues (31)]. Little is known of insulin's clearance from bISF. Potential pathways for bISF insulin clearance include uptake and degradation by neurons or glia; return to the perivenous VRS and clearance through either the brain's venous or its recently described lymphatic system (19); or migration to subarachnoid CSF. The quantitative role of each in brain insulin clearance is unknown.
A second factor that mitigates against subarachnoid CSF as a significant source of bISF insulin is the very low fasting CSF insulin concentrations and minimal changes that occur during peripheral hyperinsulinemia observed in humans (36) and dogs (32) as well as in rats (Fig. 2). The basal fasting CSF insulin concentration was below the detectable limit of a sensitive assay and even after 3 h of steady-state hyperinsulinemia, CSF insulin was only <1/3 of the basal plasma insulin concentration and <1/50th the ambient plasma insulin concentration. At these very low insulin concentrations (unless CNS insulin receptors are much more insulin sensitive than peripheral insulin receptors) it would seem doubtful that CSF insulin could exert significant biological actions on the brain. It is worth noting that insulin transfer into CSF has been demonstrated to be a saturable process based on the effect of adding unlabeled insulin (31). Whether this transfer utilizes the insulin receptor, as appears to be the case for the blood-brain barrier, or whether it may involve the more promiscuous megalin transfer pathway, which is thought to be involved in proximal tubule insulin reabsorption in the kidney (27), and in IGF-1 transfer to CSF in the choroid plexus (7), is unknown.
There has been controversy regarding whether insulin is produced within the brain and whether that source of insulin might be more relevant to insulin action within the CNS compared with contributions from pancreatic insulin secretion. We did not attempt to address this potential source of insulin. Nevertheless, we are intrigued by the recent demonstration that some neurogliaform cells increase insulin mRNA (as measured by single cell PCR) in response to local glucose concentration and that these cells can signal postsynaptically to nearby cells, and blockade of the insulin receptor locally with S961 decreases this signaling (25). However, there is no real quantitation of gross insulin secretion within the CNS and much needs to be done to address the significance of this finding to overall CNS action of insulin.
The IR is a crucial mediator of insulin-stimulated glucose uptake by muscle and fat and its blockade (29) or genetic absence (6) produces hyperglycemia, hyperinsulinemia, and impaired insulin sensitivity. In Fig. 1, we show that pharmacological inhibition of the IR with S961 markedly reduces insulin clearance from plasma by each brain region studied indicating clearance is IR-mediated at each site. Interestingly, the initial rate of total plasma insulin clearance (Fig. 4) was not affected by 4 wk of HFD. However, HFD did decrease brain insulin clearance. The basis for this effect is not clear. It is perhaps reminiscent of the previously reported effect of HFD to reduce insulin entry into CSF (4), which also involves brain microvascular endothelium as well as epithelial cells at the choroid plexus.
It is interesting to consider the “efficiency” of insulin clearance by brain microvasculature. Compared with skeletal muscle which has a resting blood flow of ∼3 ml·100 ml−1·min−1, the brain has relatively luxuriant blood flow on the order of 60 ml·100 ml tissue−1·min−1. We observed that insulin clearance (per g of tissue) is similar for skeletal muscle (data not shown) and brain. This suggests that the fractional extraction of insulin by the brain is only ∼5% as active as for skeletal muscle. This observation emphasizes the restrictive nature of the BBB. Nevertheless, it is clear that insulin transits endothelial cell of the BBB much more rapidly than the blood-CSF barrier.
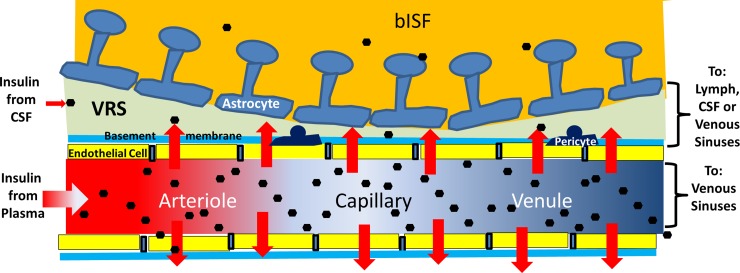
We emphasize that insulin being cleared from plasma by the brain microvasculature may not be equivalent to its entry into bISF. Figure 7 illustrates the pathways available for insulin to enter and leave bISF. Insulin taken up by the brain endothelial cell must transit the cell to access to the VRS. Then it still must pass the astroglial foot processes to reach brain parenchyma (14). This barrier appears substantially less restrictive than the microvascular endothelial cell. It does however restrict passage of larger molecules (14). For example, a 3-kDa labeled dextran enters brain parenchyma more slowly than a 759-Dalton fluorophore, and large macromolecules are excluded (14). Whether and to what degree this barrier restricts insulin's passage to brain parenchyma has not been characterized.
Fig. 7.
Schematic of insulin (black hexagons) delivery to and removal from the brain. Insulin in the microcirculation enters endothelial cells through an insulin receptor-dependent process and presumably crosses to the VRS. Insulin also enters the VRS via flow of subarachnoid CSF along penetrating pial arteries. Insulin that is not removed from plasma returns to the systemic venous circulation. Insulin entering the VRS might act on astrocytes, or pass-through clefts between astrocytes foot processes to enter bISF and act on neurons. Potential pathways for bISF clearance include uptake and degradation by neurons or glia; return to the perivenous VRS and clearance through either the brain's venous or lymphatic drainage; or migration to subarachnoid CSF.
In the current study we demonstrated using RBMVECs (Fig. 5) that insulin not only binds to its receptor but is also internalized by brain endothelial cells, confirming prior work by Miller et al. (24) with bovine brain microvascular cells. In the periphery, insulin is transported across the endothelial layer after it has been taken up by the endothelial cell (28). We therefore assume that this holds true in the brain microvascular endothelium as well. We have previously reported that bovine aortic endothelial cell uptake of 50 nM FITC-insulin is significantly inhibited by Ab-3 antibody to the IGF-I receptor (37). We have not tested the effect of Ab-3 on insulin uptake by RBMVEC using pharmacological insulin concentrations.
A limitation to the current work relates to whether insulin might be degraded either during passage through the microvascular endothelium or in the VRS. Insulin degrading enzyme (IDE) is abundantly present in the brain, and recent data suggest it is present within the brain microvascular endothelial cell, including both the apical and basolateral membrane (20). As sampling from the VRS is not feasible, we could not demonstrate the actual transfer of insulin beyond the endothelial cell.
In conclusion, insulin is cleared by brain microvasculature by an insulin receptor-mediated process. For the brain, the microvascular BBB, not the blood-CSF barrier, is the relevant site for insulin transfer. Thus subarachnoid CSF sampled from ventricular, cisternal, or lumbar sites may not provide a meaningful surrogate for assessing insulin transfer to the brain. Four weeks of HFD significantly blunts brain insulin; the pathophysiological significance of this effect warrants further study.
GRANTS
This work was supported by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-057878 and DK-073059) and American Diabetes Association (11-BS6) to E. J. Barrett, and the National Heart, Lung, and Blood Institute (5-T32-HL-007284) and American Heart Association (14PRE20100048) to S. M. Gray.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.I.M., S.G., and E.J.B. conception and design of research; R.I.M., S.G., and K.A. performed experiments; R.I.M., S.G., K.A., and E.J.B. analyzed data; R.I.M. and E.J.B. interpreted results of experiments; R.I.M., S.G., and E.J.B. prepared figures; R.I.M. drafted manuscript; R.I.M., S.G., and E.J.B. edited and revised manuscript; R.I.M., S.G., K.A., and E.J.B. approved final version of manuscript.
REFERENCES
- 1.Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19: 883, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Bass NH, Lundborg P. Postnatal development of bulk flow in the cerebrospinal fluid system of the albino rat: clearance of carboxyl-[14C]inulin after intrathecal infusion. Brain Res 52: 323–332, 1973. [DOI] [PubMed] [Google Scholar]
- 3.Baura GD, Foster DM, Kaiyala K, Porte D Jr, Kahn SE, Schwartz MW. Insulin transport from plasma into the central nervous system is inhibited by dexamethasone in dogs. Diabetes 45: 86–90, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Begg DP, Mul JD, Liu M, Reedy BM, D'Alessio DA, Seeley RJ, Woods SC. Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats. Endocrinology 154: 1047–1054, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction [see comments]. Science 289: 2122–2125, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559–569, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci 25: 10884–10893, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 58: 708–719, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy KR, Pardridge WM. Blood-brain barrier transcytosis of insulin in developing rabbits. Brain Res 420: 32–38, 1987. [DOI] [PubMed] [Google Scholar]
- 10.Genders AJ, Frison V, Abramson SR, Barrett EJ. Endothelial cells actively concentrate insulin during its transendothelial transport. Microcirculation 20: 434–439, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heni M, Wagner R, Kullmann S, Veit R, Mat Husin H, Linder K, Benkendorff C, Peter A, Stefan N, Haring HU, Preissl H, Fritsche A. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes 63: 4083–4088, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Iliff JJ, Goldman SA, Nedergaard M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol 14: 977, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123: 1299–1309, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4: 147ra111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inyard AC, Clerk LH, Vincent MA, Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes 56: 2194–2000, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Johanson CE, Woodbury DM. Changes in CSF flow and extracellular space in the developing rat. In: Drugs and the Developing Brain. Springer, 1974, p. 281–287. [Google Scholar]
- 17.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49: 1525–1533, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63: 2232–2243, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch JA, George AM, Eisenhauer PB, Conn K, Gao W, Carreras I, Wells JM, McKee A, Ullman MD, Fine RE. Insulin degrading enzyme is localized predominantly at the cell surface of polarized and unpolarized human cerebrovascular endothelial cell cultures. J Neurosci Res 83: 1262–1270, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Mahat MY, Fakrudeen Ali Ahamed N, Chandrasekaran S, Rajagopal S, Narayanan S, Surendran N. An improved method of transcutaneous cisterna magna puncture for cerebrospinal fluid sampling in rats. J Neurosci Methods 211: 272–279, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Majumdar S, Genders AJ, Inyard AC, Frison V, Barrett EJ. Insulin entry into muscle involves a saturable process in the vascular endothelium. Diabetologia 55: 450–456, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNay EC, Recknagel AK. Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem 96: 432–442, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller DW, Keller BT, Borchardt RT. Identification and distribution of insulin receptors on cultured bovine brain microvessel endothelial cells: possible function in insulin processing in the blood-brain barrier. J Cell Physiol 161: 333–341, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Molnar G, Farago N, Kocsis AK, Rozsa M, Lovas S, Boldog E, Baldi R, Csajbok E, Gardi J, Puskas LG, Tamas G. GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J Neurosci 34: 1133–1137, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5: 566–572, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Orlando RA, Rader K, Authier F, Yamazaki H, Posner BI, Bergeron JJ, Farquhar MG. Megalin is an endocytic receptor for insulin. J Am Soc Nephrol 9: 1759–1766, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Porte D Jr, Baskin DG, Schwartz MW. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes 54: 1264–1276, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Schaffer L, Brand CL, Hansen BF, Ribel U, Shaw AC, Slaaby R, Sturis J. A novel high-affinity peptide antagonist to the insulin receptor. Biochem Biophys Res Commun 376: 380–383, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Scherer T, O'Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S, Milsom T, Puchowicz M, Scheja L, Zechner R, Fisher SJ, Previs SF, Buettner C. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab 13: 183–194, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz MW, Bergman RN, Kahn SE, Taborsky GJ Jr, Fisher LD, Sipols AJ, Woods SC, Steil GM, Porte D Jr. Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. J Clin Invest 88: 1272–1281, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz MW, Sipols A, Kahn SE, Lattemann DF, Taborsky GJ Jr, Bergman RN, Woods SC, Porte D Jr. Kinetics and specificity of insulin uptake from plasma into cerebrospinal fluid. Am J Physiol Endocrinol Metab 259: E378–E383, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122: 1316–1338, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem 94: 1158–1166, 2005. [DOI] [PubMed] [Google Scholar]
- 35.van Houten M, Posner BI. Insulin binds to brain blood vessels in vivo. Nature 282: 623–625, 1979. [DOI] [PubMed] [Google Scholar]
- 36.Wallum BJ, Taborsky GJ, Porte D, Figlewicz DP, Jacobson L, Beard JC, Ward WK, Dorsa D. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab 64: 190–194, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Liu Z, Li G, Barrett EJ. The vascular endothelial cell mediates insulin transport into skeletal muscle. Am J Physiol Endocrinol Metab 291: E323–E332, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Zeleznik AJ, Roth J. Demonstration of the insulin receptor in vivo in rabbits and its possible role as a reservoir for the plasma hormone. J Clin Invest 61: 1363–1374, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]