We found that carotid baroreceptor unloading during muscle metaboreflex activation results in an additive interaction and causes vasoconstriction of all vascular beds, including ischemic active skeletal muscle. However, there is a larger vasoconstriction in other vascular beds causing redistribution of blood flow toward ischemic active skeletal muscle.
Keywords: exercise pressor reflex, ischemic active skeletal muscle, sympathetic vasoconstriction, carotid baroreceptor unloading, mild dynamic exercise
Abstract
The muscle metaboreflex and arterial baroreflex regulate arterial pressure through distinct mechanisms. During submaximal exercise muscle metaboreflex activation (MMA) elicits a pressor response virtually solely by increasing cardiac output (CO) while baroreceptor unloading increases mean arterial pressure (MAP) primarily through peripheral vasoconstriction. The interaction between the two reflexes when activated simultaneously has not been well established. We activated the muscle metaboreflex in chronically instrumented canines during dynamic exercise (via graded reductions in hindlimb blood flow; HLBF) followed by simultaneous baroreceptor unloading (via bilateral carotid occlusion; BCO). We hypothesized that simultaneous activation of both reflexes would result in an exacerbated pressor response owing to both an increase in CO and vasoconstriction. We observed that coactivation of muscle metaboreflex and arterial baroreflex resulted in additive interaction although the mechanisms for the pressor response were different. MMA increased MAP via increases in CO, heart rate (HR), and ventricular contractility whereas baroreflex unloading during MMA caused further increases in MAP via a large decrease in nonischemic vascular conductance (NIVC; conductance of all vascular beds except the hindlimb vasculature), indicating substantial peripheral vasoconstriction. Moreover, there was significant vasoconstriction within the ischemic muscle itself during coactivation of the two reflexes but the remaining vasculature vasoconstricted to a greater extent, thereby redirecting blood flow to the ischemic muscle. We conclude that baroreceptor unloading during MMA induces preferential peripheral vasoconstriction to improve blood flow to the ischemic active skeletal muscle.
NEW & NOTEWORTHY
We found that carotid baroreceptor unloading during muscle metaboreflex activation results in an additive interaction and causes vasoconstriction of all vascular beds, including ischemic active skeletal muscle. However, there is a larger vasoconstriction in other vascular beds causing redistribution of blood flow toward ischemic active skeletal muscle.
insufficient oxygen delivery to the active skeletal muscle during exercise causes accumulation of metabolites (e.g., hydrogen ions, lactic acid, etc.) which stimulate the group III and IV muscle afferents eliciting a reflex pressor response, termed the muscle metaboreflex (1, 4, 14, 19, 27, 32). Muscle metaboreflex activation during dynamic exercise increases mean arterial pressure (MAP) primarily by increases in cardiac output (CO) which occur via substantial tachycardia coupled with increased ventricular contractility and central blood volume mobilization which together maintains or slightly increases stroke volume (7, 11, 28, 29, 33, 37, 39). Although some regional vasoconstriction occurs with metaboreflex activation during submaximal exercise [e.g., coronary, renal, forelimbs (2, 3, 6, 20, 21)], total vascular conductance (TVC) is relatively unchanged (3, 8–10, 31).
At rest and during exercise the arterial baroreflex is the primary short-term negative feedback reflex which maintains arterial pressure on a beat-by-beat basis by regulating TVC and CO. The arterial baroreflex primarily modulates TVC as little change in CO occurs during steady state (5, 23, 34, 35, 38). Therefore, the muscle metaboreflex and the arterial baroreflex regulate arterial pressure via two distinct mechanisms. Muscle metaboreflex activation increases MAP primarily by increasing CO whereas arterial baroreflex activation increases MAP primarily through peripheral vasoconstriction. As workload increases, active skeletal muscle vasculature becomes an increasingly important target for baroreflex-mediated increases in MAP (5). We recently showed that muscle metaboreflex activation induces sympathetic vasoconstriction within the ischemic muscle itself thereby limiting the ability of the metaboreflex to improve muscle blood flow (15). Several studies have shown that the arterial baroreflex buffers the muscle metaboreflex-induced pressor response inasmuch as the metaboreflex pressor response is substantially larger after arterial baroreceptor denervation (18, 30). This buffering occurs primarily via baroreflex attenuation of metaboreflex-induced vasoconstriction (18). However, situations exist when these two powerful blood pressure-raising reflexes may be engaged concurrently in the same direction (e.g., exercise and hypovolemia or hemorrhage when both arterial pressure and skeletal muscle blood flow are lower). In this study, we quantified the contribution of CO and TVC to the pressor response elicited by carotid baroreceptor unloading (via bilateral carotid occlusion; BCO) at rest, during mild exercise, and during muscle metaboreflex activation. We investigated whether concurrent activation of the two reflexes undergoes an additive, occlusive, or facilitative interaction. Since carotid baroreceptor unloading induces peripheral vasoconstriction, to what extent the ischemic muscle vasoconstricts during concurrent activation of the baroreflex and the muscle metaboreflex is unknown. Previous studies hypothesized that increases in metabolic by-products in skeletal muscle during exercise reduces the efficacy of sympathetic nerves to elicit vasoconstriction (26, 36). We hypothesized that baroreceptor unloading during metaboreflex activation would still elicit substantial systemic peripheral vasoconstriction even within the ischemic hindlimb. However, if nonischemic beds are more susceptible to neurogenic vasoconstriction, then there may be redistribution of the available CO toward the ischemic muscle.
METHODS
Experimental subjects.
Eight adult mongrel canines (5 females, 3 males; 20–25 kg) were selected for the study. All animals were acclimatized to the laboratory surroundings and willing to run on a motor-driven treadmill. All the procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University and complied with the National Institutes of Health Guide to the Care and Use of Laboratory Animals. All animals exercised voluntarily during experimentation; no negative reinforcement techniques were utilized.
Surgical procedures.
For each of the three surgical procedures, the animals were sedated with acepromazine (0.2–0.5 mg/kg im) and received preoperative analgesics {carprofen (4.4 mg/kg iv), buprenorphine (0.01–0.03 mg/kg im) and fentanyl [50–125 μg/h (72 h) transdermal delivery]}. Anesthesia was induced with ketamine and diazepam (5 mg/kg iv and 0.2–0.3 mg/kg iv, respectively) and maintained with isoflurane gas (1–3%). Cefazolin (antibiotic, 30 mg/kg iv) was administered pre- and postoperatively to avoid acute postoperative infections. Animals were closely monitored postoperatively and given buprenorphine (0.01–0.03 mg/kg im) and acepromazine (0.2–0.3 mg/kg iv) as needed. Cephalexin [antibiotic, 30 mg/kg po (BID)] was administered prophylactically for the entire term of the experimental protocol. All surgeries were performed using aseptic technique, and animals recovered for 2 wk after each surgery.
In the first surgical procedure, the thoracic cavity was opened via a left thoracotomy (3rd/4th intercostal space) approach and the pericardium was cut to expose the heart. A telemetry pressure transmitter (TA11 PA-D70, DSI) was tethered subcutaneously at the height of the left ventricular apex and two intercostal spaces caudal to the thoracotomy incision (n = 5). The tip of the pressure transducer catheter was inserted into the left ventricle and secured. A perivascular flow probe (20PAU, Transonic Systems) was placed around the ascending aorta to measure CO. Pacing wires were secured to the free wall of the right ventricle for studies unrelated to the present study. The pericardium was reapproximated and all wires were tunneled subcutaneously and exteriorized between the scapulae. The chest was closed in layers.
In the second surgical procedure, the abdominal aorta and left renal artery were exposed through a left retroperitoneal approach. Perivascular flow probes (Transonic Systems) were positioned around the terminal aorta (10PAU) and the left renal artery (4PSB) to measure hindlimb blood flow (HLBF) and renal blood flow (RBF), respectively. All side branches of the terminal aorta between the iliac arteries and the aortic flow probe were ligated and severed. Two hydraulic occluders (8–10 mm, DocXS Biomedical Products) were placed around the terminal aorta distal to the flow probe. A 19-gauge polyvinyl catheter (Tygon, S54-HL, Norton) was inserted into a side branch of the aorta cranial to the flow probe to measure systemic arterial pressure. A second catheter was inserted into a side branch of the aorta caudal to the occluders to measure arterial pressure below the occluders (femoral arterial pressure; FAP). When the catheterization of a caudal aortic branch was not possible, a side branch of the femoral artery was catheterized during the third surgical procedure. All instrumentation was tunneled subcutaneously and exteriorized between the scapulae, and the abdomen was closed in layers.
In the third surgical procedure, a hydraulic occluder (4–6 mm, DocXS Biomedical Products) was placed around each common carotid artery via a midline neck incision to perform bilateral carotid occlusion (BCO). The right jugular vein was catheterized (n = 3) for studies unrelated to the present study. Instrumentation was tunneled subcutaneously and exteriorized between the scapulae, and the neck was closed in layers.
Data acquisition.
Each animal was brought into the laboratory and allowed to roam freely and acclimate for ∼10–20 min, after which it was directed onto the treadmill. The CO, RBF, and HLBF flow probe cables were connected to flowmeters (TS420, Transonic Systems), and the left ventricular implant transmitter was turned on to collect data via telemetry (DSI). The arterial catheters were aspirated, flushed, and connected to pressure transducers (Transpac IV, ICU Medical). All hemodynamic variables, in addition to MAP (calculated) and HR (triggered by the CO signal), were monitored as real-time waveforms by a data-acquisition system (LabScribe, iWorx) and recorded for subsequent off-line analysis.
Experimental procedures.
All experiments were performed after the animals had fully recovered from surgery (i.e., were active and of good appetite). Each experiment began with the animal standing still on the treadmill until all resting hemodynamic data were observed to be stable (typically 5–10 min). Three different experimental protocols were used: BCO at rest, BCO during exercise, and BCO with muscle metaboreflex activation. In the first experimental protocol, steady-state data collection at rest was followed by BCO performed by inflating the carotid occluders for 2 min while the animals were standing on the treadmill. For BCO during exercise, after collecting resting steady-state data, the treadmill was turned on and gradually increased to a speed of 3.2 kph at 0% grade (mild exercise). The animals exercised for 5–10 min followed by BCO during the same workload. For BCO during metaboreflex activation, after collecting steady-state data during rest and mild exercise, muscle metaboreflex was engaged via graded reductions in HLBF (via partial inflation of terminal aortic occluders) during mild exercise. Each level of vascular occlusion was maintained until all parameters had reached steady state (typically 3–5 min). After the steady state for maximal metaboreflex activation, BCO was performed for 2 min. The large increase in MAP with baroreflex activation during metaboreflex activation would force more flow through the hindlimb occluders which would lessen metaboreflex activation; therefore the occluder resistance was increased to keep HLBF constant.
Data analysis.
MAP, FAP, CO, HR, RBF, left ventricular pressure (LVP), and HLBF were continuously recorded during each experimental procedure. Other hemodynamic parameters were calculated during off-line data analysis [e.g., total vascular conductance (TVC), renal vascular conductance (RVC), hindlimb vascular conductance (HVC) and conductance of all vascular beds except the hindlimbs (nonischemic vascular conductance; NIVC), maximal rate of rise in LVP (dP/dtmax), maximal rate of fall in LVP (dP/dtmin)]. TVC, RVC, HVC, and NIVC were calculated as CO/MAP, RBF/MAP, HLBF/FAP, and (CO-HLBF)/MAP, respectively. Due to technical issues with the left ventricular implant, dP/dtmax and dP/dtmin values were measured in only five animals. One-minute averages of all variables were taken during steady state at rest, free-flow exercise, muscle metaboreflex activation, and BCO. Mean values were averaged across all animals to obtain the sample mean of the study.
Partial reductions in HLBF by using the hydraulic occluders cause MAP to increase passively (MAPpassive) (3, 39). Thus the pressor response observed with metaboreflex activation in our experiments is a combination of a reflex increase in MAP (MAPactive) and the passive effect of the occluder. MAPpassive was calculated as COEX/(NIVCEX + HVCObserved), where the subscript EX indicates free-flow exercise levels. MAPactive, the increase in MAP due to reflex activation, was calculated by subtracting MAPpassive from the observed MAP values.
The percent contributions of CO and TVC to the baroreflex-induced pressor response were assessed by calculating the increase in MAP due to TVC (ΔMAPTVC) and the increase in MAP due to increases in CO (ΔMAPCO).
where the subscripts BCO and Pre indicate BCO and pre-BCO levels, respectively. Since all of the pressor response to BCO during muscle metaboreflex activation was due to peripheral vasoconstriction, the percent contribution of TVC was calculated as 100 − percent contribution of CO. BCO during MMA caused a large pressor response which led to an increase in HLBF which would in turn change the stimulus for muscle metaboreflex. Thus HLBF was kept constant by increasing the occluder resistance. We calculated the predicted rise in HLBF, if there was no change in occluder resistance, as MAP(MMA+BCO)/[occluder resistance(MMA) + hindlimb resistance(MMA+BCO)] where subscript MMA indicates values during muscle metaboreflex activation and MMA+BCO indicates values during coactivation of the two reflexes. Hindlimb resistance(MMA+BCO) was calculated as FAP during the coactivation of two reflexes divided by HLBF. Occluder resistance during metaboreflex was calculated as (MAP − FAP)/HLBF, all observed during metaboreflex activation.
Statistical analysis.
All hemodynamic data are reported as means ± SE. An α-level of P < 0.05 was used to determine statistical significance. Paired t-tests were performed to compare the responses between rest and BCO during rest. Average responses for each animal were analyzed with Systat software (Systat 11.0). A one-way ANOVA with repeated measures was used to compare hemodynamic data for time effect. In the event of a significant time effect, a C-matrix test for simple effects was performed.
RESULTS
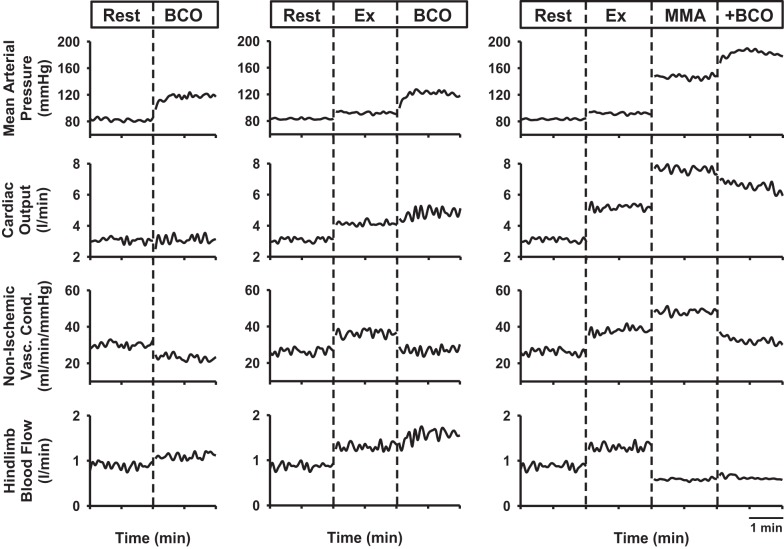
Figure 1 shows representative hemodynamic responses evoked by BCO during rest, mild exercise, and muscle metaboreflex activation in one animal. BCO at rest resulted in a large pressor response with little change in CO and a large decrease in NIVC, indicating that the pressor response was mediated predominately by peripheral vasoconstriction. From rest to exercise, there was moderate increase in CO, NIVC, and HLBF. BCO during mild exercise increased MAP, again predominately due to a large decrease in NIVC. Small increases in CO and HLBF also occurred. With metaboreflex activation during mild exercise, the large pressor response occurred solely via the large increase in CO as there was a modest increase in NIVC. Baroreceptor unloading during muscle metaboreflex activation caused a further large increase in MAP. This pressor response was mediated via peripheral vasoconstriction inasmuch as a large decrease in NIVC as well as a small decrease in CO occurred. To maintain the metaboreflex stimulus constant during concurrent activation of baroreflex and metaboreflex, HLBF was held constant by manually increasing the resistance of the hydraulic occluders on the terminal aorta.
Fig. 1.
Data from one animal showing mean arterial pressure, cardiac output, nonischemic vascular conductance, and hindlimb blood flow in response to bilateral carotid occlusion (BCO) during rest (left panel), mild exercise (Ex; middle panel), and muscle metaboreflex activation (MMA; right panel).
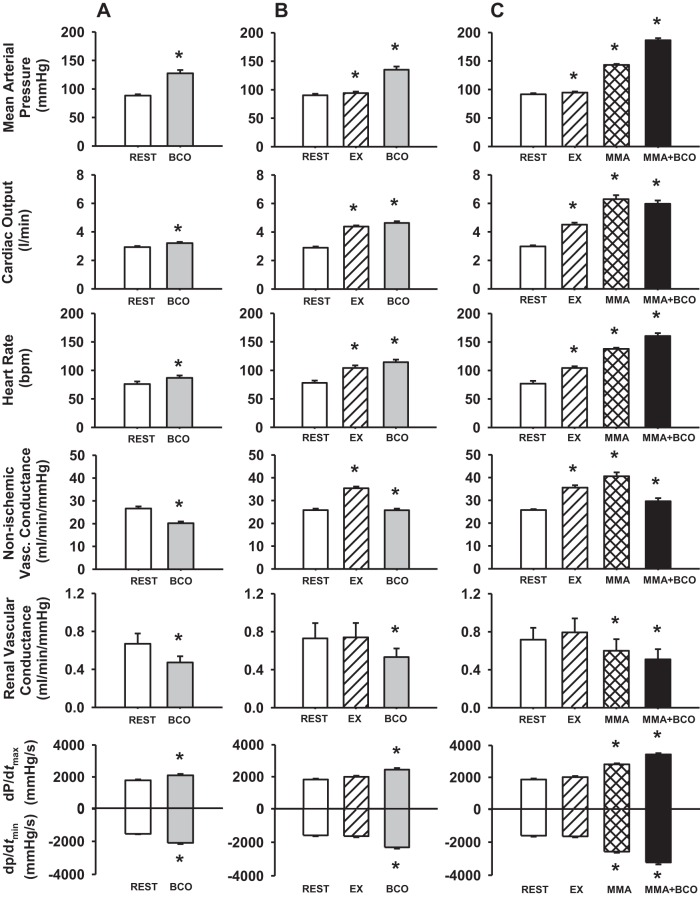
Figure 2 shows average values of MAP, CO, HR, NIVC, RVC, dP/dtmax, and dP/dtmin during BCO at rest, during mild exercise, and during metaboreflex activation.
Fig. 2.
Average hemodynamic data during rest (open bars) and bilateral carotid occlusion (BCO) at rest (gray bars) (A); rest, mild exercise (EX; hatched bars), and BCO during EX (gray bars) (B); and rest, EX, muscle metaboreflex activation (MMA; cross-hatched bars), and BCO during MMA (solid bars) (C). *P < 0.05 vs. previous setting.
BCO at rest.
Carotid baroreceptor unloading at rest resulted in a significant increase in MAP with small but significant increases in CO, HR, dP/dtmax, and dP/dtmin. Large significant decreases in NIVC and RVC occurred indicating that the pressor response was primarily caused by peripheral vasoconstriction.
BCO during exercise.
From rest to exercise, there were significant increases in MAP, CO, HR, and NIVC. BCO during exercise resulted in significant increases in MAP, CO, HR, dP/dtmax, and dP/dtmin and significant decreases in NIVC and RVC.
BCO during metaboreflex activation.
MAP, CO, HR, and NIVC increased significantly from rest to exercise. Muscle metaboreflex activation (via partial reduction in HLBF) caused significant increases in MAP, NIVC, CO, HR, dP/dtmax, and dP/dtmin while RVC decreased significantly. Coactivation of arterial baroreflex and muscle metaboreflex resulted in further significant increases in MAP, HR, dP/dtmax, and dP/dtmin and significant decreases in NIVC, CO, and RVC.
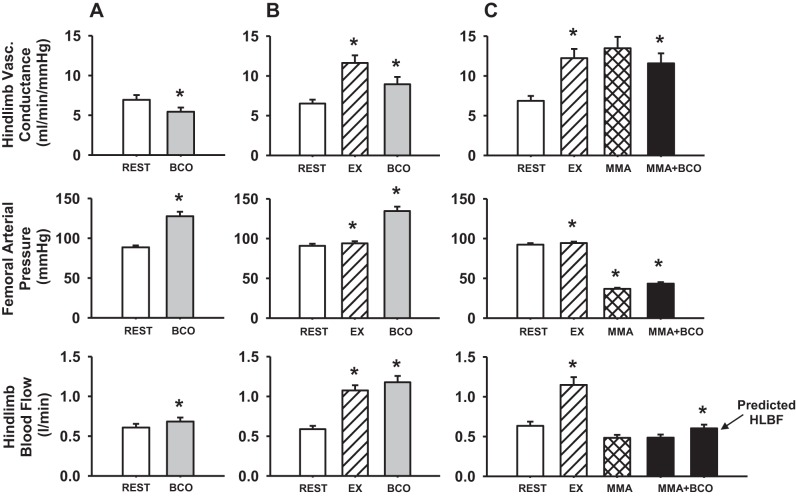
Figure 3 shows the hemodynamic changes in hindlimb vascular conductance, FAP, and HLBF during BCO at rest, during mild exercise and muscle metaboreflex activation. During BCO at rest, HVC significantly decreased indicating vasoconstriction in the hindlimb vasculature. HLBF increased despite the vasoconstriction as the large increase in arterial pressure forced more flow through the vasoconstricted hindlimbs. From rest to exercise, HVC, FAP, and HLBF increased significantly. In response to BCO during exercise, there was a large decrease in HVC accompanied by significant increases in FAP and HLBF. Muscle metaboreflex was activated during mild exercise by reducing HLBF to ∼40% of the free-flow exercising blood flow. We have recently shown that initial reductions in HLBF cause vasodilation in the hindlimb, but when the metaboreflex is engaged, vasoconstriction ensues which brings HVC back toward the levels observed prior to hindlimb ischemia (16). With BCO during muscle metaboreflex activation, there was a significant decrease in HVC indicating vasoconstriction of the ischemic active muscle with a significant increase in FAP. The HLBF during the coactivation of both reflexes was kept constant by increasing the hindlimb occluder resistance. If there was no change in the occluder resistance, HLBF would have increased significantly (predicted HLBF).
Fig. 3.
Average hindlimb vascular conductance, femoral arterial pressure, and hindlimb blood flow responses during rest (open bars) and bilateral carotid occlusion (BCO) at rest (gray bars) (A); rest, mild exercise (EX; hatched bars) and BCO during EX (gray bars) (B); and rest, EX, muscle metaboreflex activation (MMA; cross-hatched bars) and BCO during MMA (solid bars) (C). *P < 0.05 vs. previous setting.
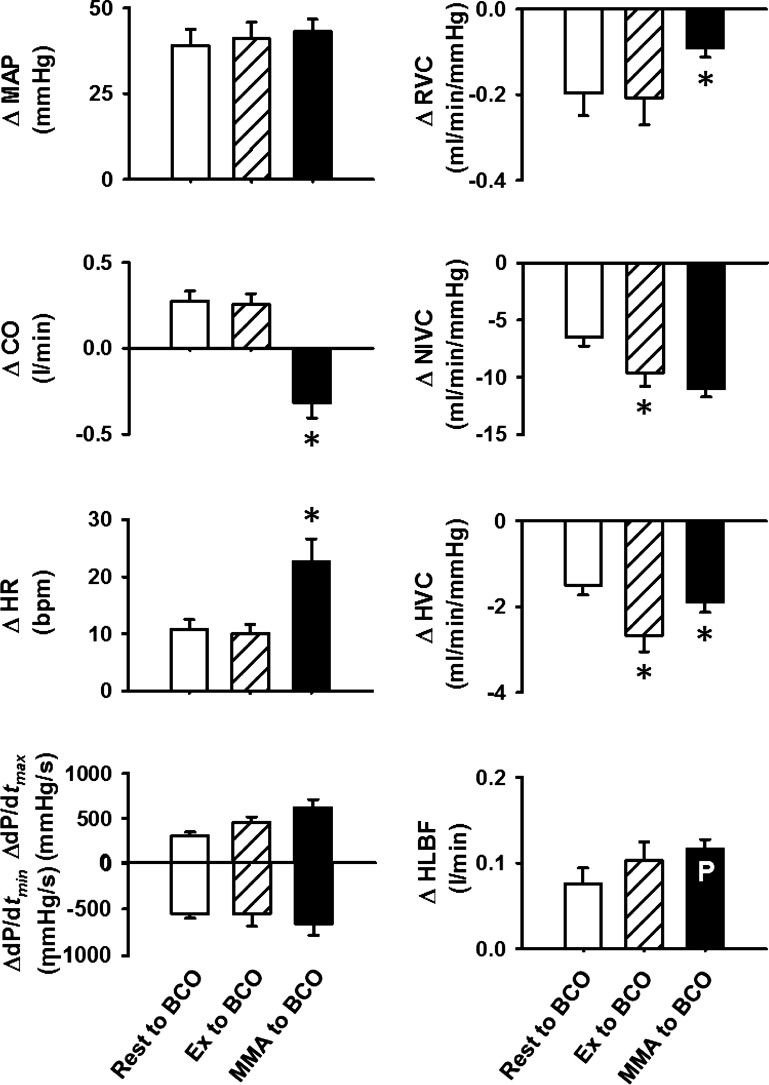
Figure 4 shows the absolute changes in the cardiovascular parameters evoked by BCO at rest, during mild exercise, and during metaboreflex activation in eight normal animals. The pressor responses evoked by BCO at rest, during exercise, and during metaboreflex activation were not different from each other. Changes in CO, HR, dP/dtmax, dP/dtmin, RVC, and HLBF were similar during BCO at rest and BCO during mild exercise. However, decreases in NIVC and HVC were significantly larger with BCO during exercise than BCO at rest. With coactivation of baroreflex and metaboreflex, there was a further increase in HR with further improvement in contractility and lusitropy (as shown in Fig. 2); however, CO decreased slightly. While the vasoconstriction of nonischemic vascular beds in response to coactivation of reflexes was similar to that during BCO with exercise, the reduction in RVC and HVC was significantly smaller than with BCO during exercise. To maintain the stimulus for muscle metaboreflex, occluder resistance was adjusted to keep HLBF constant. However, the predicted increase in HLBF during coactivation of reflexes was similar to the increase in HLBF seen in response to BCO during mild exercise.
Fig. 4.
The absolute changes (Δ) in hemodynamic parameters from rest to bilateral carotid occlusion (BCO) during rest (open bars), from exercise (Ex) to BCO during Ex (hatched bars), and from muscle metaboreflex activation (MMA) to BCO during MMA (filled bars). The bar inscribed with P indicates the predicted HLBF during coactivation of two reflexes. MAP, mean arterial pressure; CO, cardiac output; HR, heart rate; dP/dtmax, maximal rate of change in left ventricular pressure; dP/dtmin, minimal rate of change in left ventricular pressure; RVC, renal vascular conductance; NIVC, nonischemic vascular conductance; HVC, hindlimb vascular conductance; HLBF, hindlimb blood flow. *P < 0.05 vs. previous setting.
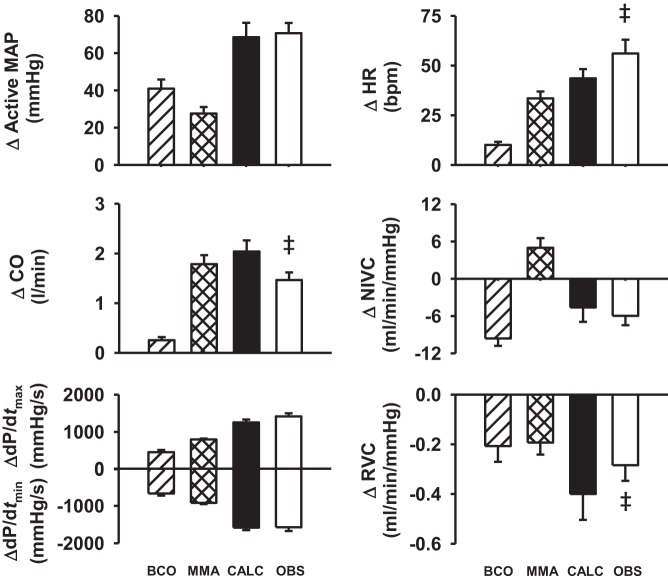
To investigate the mode of interaction between arterial baroreflex and muscle metaboreflex (i.e., additive, facilitative, or occlusive interaction), the observed responses during coactivation of baroreflex and metaboreflex activation were compared with the sum of metaboreflex-induced and BCO-induced responses during exercise. Figure 5 shows absolute changes in hemodynamic parameters in response to BCO during exercise, muscle metaboreflex activation, the arithmetic sum of muscle metaboreflex-induced and BCO-induced responses during exercise, and the observed hemodynamic changes in response to coactivation of baroreflex and metaboreflex. The calculated arithmetic sum of the individual responses and actual observed responses were not different for MAP, NIVC, and ventricular function, indicating an additive interaction. However, the observed responses were significantly higher than calculated summation of the individual responses for HR, indicating facilitative interaction. The summation of the individual responses was greater than the observed responses for CO and RVC, indicating that for these parameters the arterial baroreflex and muscle metaboreflex summate as occlusive interaction.
Fig. 5.
Absolute changes (Δ) in cardiovascular responses evoked by bilateral carotid occlusion (BCO) during mild exercise (hatched bars), muscle metaboreflex activation (MMA; cross-hatched bars), calculated MMA + BCO during exercise (CALC; filled bars), and observed combined effect of the MMA and BCO during MMA (OBS; open bars). ‡P < 0.05 vs. calculated sum of reflex-induced response during MMA and BCO during exercise.
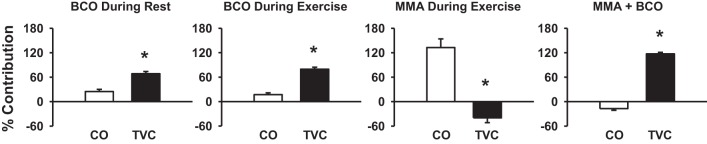
Figure 6 shows the relative contribution of CO and TVC in mediating the rise in MAP in response to BCO during rest, BCO during exercise, muscle metaboreflex activation, and BCO during metaboreflex activation. Pressor response to BCO during rest and exercise was primarily due to peripheral vasoconstriction as there is only a small contribution of CO. Muscle metaboreflex-induced pressor response was virtually solely driven by the increase in CO as there is negative contribution of TVC. Pressor response with BCO during metaboreflex activation was solely due to the increase in peripheral vasoconstriction.
Fig. 6.
Percent contribution of cardiac output (CO; open bars) and total vascular conductance (TVC; filled bars) to the pressor response evoked by BCO at rest, BCO during exercise, muscle metaboreflex activation (MMA), and BCO during MMA. *P < 0.05 vs. CO contribution.
DISCUSSION
This is the first study to investigate the strength and mechanisms of the muscle metaboreflex and arterial baroreflex when activated separately and concurrently during dynamic exercise, and the effect of this interaction on ventricular function, cardiac output, and the distribution of total peripheral blood flow. We found that the pressor responses to baroreceptor unloading and metaboreflex activation were additive; however, the mechanisms mediating each pressor response were diametrically opposite: arterial baroreflex activation primarily induces peripheral vasoconstriction whereas metaboreflex activation primarily raises CO. When activated concurrently, profound pressor responses occur due to substantial vasoconstriction coupled with increased CO. Further, we found that baroreceptor unloading during metaboreflex activation causes vasoconstriction even within the ischemic active skeletal muscle. However, the vasoconstriction in all other vascular beds combined is greater than that in the ischemic muscle which thereby causes redistribution of cardiac output toward the ischemic beds. This shift in total systemic blood flow toward the ischemic muscle during combined baroreflex and metaboreflex activation attenuates the level of metaboreflex stimulation.
Muscle metaboreflex and arterial baroreflex interaction.
Reflexes interact in three different ways: additive interaction, where the coactivation responses equal the sum of reflex responses when activated individually; facilitative interaction, where responses during coactivation of two reflexes are larger than the sum of individual reflex responses; and occlusive interaction, where the coactivation responses are smaller than the sum of individual reflex responses. We observed that the pressor response evoked by BCO during metaboreflex activation was similar to the sum of metaboreflex-induced and BCO-induced pressor response during exercise, indicating additive interaction between the two reflexes. The same conclusion holds for ventricular function and NIVC responses. However, CO and RVC responses exhibit occlusive interaction and HR responses undergo facilitative interaction upon coactivation of the two reflexes. Therefore, the interaction between arterial baroreflex and muscle metaboreflex when activated simultaneously is intimately dependent on the hemodynamic parameter being investigated. Why differential interaction mechanisms exist for different hemodynamic parameters is unclear. CO responses may be limited by the large increases in afterload with simultaneous baroreflex and metaboreflex activation. Limitations in CO responses could obviously affect the changes in MAP. There may also be differential activation of regional sympathetic activity. Previous studies in humans showed that the pressor response (12, 13, 25), leg vasoconstriction (12), and rise in muscle sympathetic nerve activity (13) in response to brief (seconds) carotid baroreceptor unloading was greater when performed during muscle metaboreflex activation induced by post-exercise muscle ischemia (PEMI) whereas the HR responses were similar (12, 13, 25). Metaboreflex activation via PEMI engages the reflex during the recovery from exercise, a setting with different baseline levels of autonomic activity, HR and CO than when the reflex is engaged during dynamic exercise. Nevertheless, these studies indicate that species as well as the mode of metaboreflex activation may affect the interaction between these reflexes.
Regulation of skeletal muscle blood flow.
During progressive dynamic exercise the skeletal muscle vasculature becomes an increasingly important target vascular bed mediating the rise in arterial pressure in response to baroreceptor unloading (5, 23). We recently showed that muscle metaboreflex activation induces sympathetic vasoconstriction within ischemic active muscle, thereby limiting the ability of the metaboreflex to improve muscle blood flow (15). BCO during metaboreflex activation causes further vasoconstriction within the ischemic hindlimb vasculature, although the decrease in HVC during coactivation of two reflexes is significantly smaller than that observed in response to BCO during normal exercise. One of the plausible causes for this smaller vasoconstriction in the hindlimb vasculature could be functional sympatholysis, a phenomenon described as attenuated sympathetic vasoconstriction of the active skeletal muscle due to accumulation of metabolites in the muscle (26, 36). The large increase in MAP in response to BCO during metaboreflex activation increases HLBF despite the vasoconstriction in hindlimb vasculature. Therefore, to maintain the same metaboreflex stimulus during coactivation of the reflexes, HLBF was kept constant by increasing the occluder resistance in these experiments. If there was no change in the occluder resistance, the HLBF (predicted HLBF) would have increased significantly with the rise in arterial pressure driving more flow through the occluder resistance as well as the hindlimb vascular resistance. With a decrease in CO and vasoconstriction in the hindlimb vasculature during coactivation of the reflexes, the only way HLBF could have increased is by a larger vasoconstriction of the nonischemic vasculature than the ischemic hindlimb vasculature. This preferential vasoconstriction in the nonischemic vasculature redirects blood flow to the hindlimbs which thereby increases HLBF. This rise in HLBF would act to actually attenuate the metaboreflex activation. Interestingly, we calculated that the predicted HLBF during coactivation of the two reflexes would have virtually restored HLBF almost back to the metaboreflex threshold level of HLBF (Fig. 7).
Fig. 7.
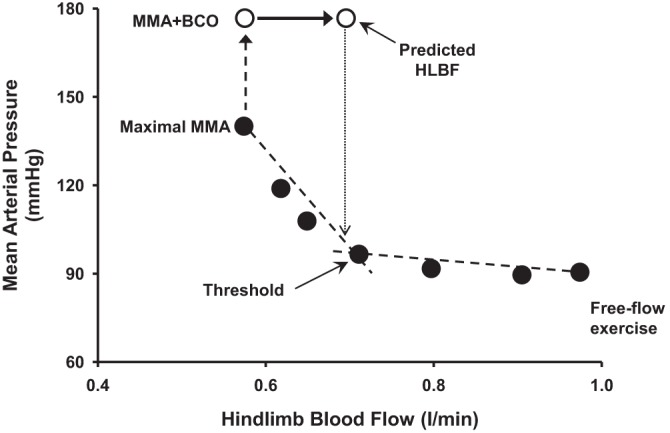
Mean arterial pressure and hindlimb blood flow during free-flow mild exercise and graded reductions in HLBF eliciting muscle metaboreflex activation (MMA; filled circles) followed by bilateral carotid occlusion (BCO) during MMA (open circles) in an experiment.
Where does vasoconstriction occur?
Previous studies have shown that active skeletal muscle becomes the primary target bed for baroreflex-induced peripheral vasoconstriction with increasing exercise intensity (5). We observed that although BCO during metaboreflex activation caused vasoconstriction within ischemic active muscle, blood flow to the ischemic muscle would still have improved (given that occluder resistance was not changed) due to a larger vasoconstriction in other vascular beds. Since active skeletal muscle constitutes the largest proportion of TVC during exercise, the preferential vasoconstriction during coactivation of reflexes most likely occurs in active skeletal muscle other than the hindlimb. Although there is some vasoconstriction of inactive beds such as renal vasculature during coactivation of baroreflex and muscle metaboreflex, inactive beds do not have much potential to increase MAP or redirect blood flow to other vasculatures (5, 22). In the present study, the RVC responses underwent occlusive interaction upon coactivation of baroreflex and muscle metaboreflex.
Perspectives and Significance
In normal individuals, metaboreflex activation causes large increases in CO and MAP which in turn increase blood flow and oxygen delivery to the active vascular beds. The arterial baroreflex buffers the metaboreflex pressor response by about 50% by attenuating peripheral vasoconstriction (18). In conditions such as heart failure, where increases in CO become limited, the only mechanism to increase MAP is via peripheral vasoconstriction. Inasmuch as the strength of arterial baroreflex is impaired in heart failure (17, 24), this would lessen the ability of arterial baroreflex to attenuate peripheral vasoconstriction induced by metaboreflex activation, leading to markedly large increases in vascular sympathetic tone, particularly in the active ischemic muscle. Moreover, baroreceptor unloading during metaboreflex activation leads to larger vasoconstriction of the nonischemic vasculature causing improvement in blood flow to the ischemic muscle. In heart failure, this increase in HLBF would be attenuated, abolished, or possibly even reversed into functional vasoconstriction which could elicit a rampant positive feedback spiral where baroreflex-mediated vasoconstriction in muscle further engages the metaboreflex leading to a veritable sympathetic storm. It is unknown whether this interaction contributes to the often massive peripheral vasoconstriction and exercise intolerance observed during exercise in patients with heart failure.
GRANTS
This study was supported by National Heart, Lung and, Blood Institute Grants HL-55473 and HL-126706.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.K., D.S., M.D.S., and D.S.O’L. conception and design of research; J.K., A.A., H.W.H., A.C.K., T.M.M., Y.H.A., M.D.D., and D.S.O’L. performed experiments; J.K., A.C.K., T.M.M., A.T.L., and D.S.O’L. analyzed data; J.K., D.S., M.D.D., and D.S.O’L. interpreted results of experiments; J.K., A.T.L., and D.S.O’L. prepared figures; J.K., M.D.S., and D.S.O’L. drafted manuscript; J.K., A.A., H.W.H., A.C.K., D.S., T.M.M., Y.H.A., A.T.L., M.D.D., M.D.S., and D.S.O’L. edited and revised manuscript; J.K., A.A., H.W.H., A.C.K., D.S., T.M.M., Y.H.A., A.T.L., M.D.D., M.D.S., and D.S.O’L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank J. Helme-Day and A. Nelson for expert technical assistance and animal care.
REFERENCES
- 1.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansorge EJ, Shah SH, Augustyniak R, Rossi NF, Collins HL, O'Leary DS. Muscle metaboreflex control of coronary blood flow. Am J Physiol Heart Circ Physiol 283: H526–H532, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O'Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Boushel R, Madsen P, Nielsen HB, Quistorff B, Secher NH. Contribution of pH, diprotonated phosphate and potassium for the reflex increase in blood pressure during handgrip. Acta Physiol Scand 164: 269–275, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Collins HL, Augustyniak RA, Ansorge EJ, O'Leary DS. Carotid baroreflex pressor responses at rest and during exercise: cardiac output vs. regional vasoconstriction. Am J Physiol Heart Circ Physiol 280: H642–H648, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O'Leary DS. Muscle metaboreflex-induced coronary vasoconstriction functionally limits increases in ventricular contractility. J Appl Physiol 109: 271–278, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJS, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc 35: 221–228, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Crisafulli A, Piras F, Filippi M, Piredda C, Chiappori P, Melis F, Milia R, Tocco F, Concu A. Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. J Physiol Sci 61: 385–394, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Ichinose MJ, Sala-Mercado JA, Coutsos M, Li Z, Ichinose TK, Dawe E, O'Leary DS. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol Heart Circ Physiol 298: H245–H250, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichinose M, Nishiyasu T. Muscle metaboreflex modulates the arterial baroreflex dynamic effects on peripheral vascular conductance in humans. Am J Physiol Heart Circ Physiol 288: H1532–H1538, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during muscle metaboreflex activation in humans. J Physiol 544: 939–948, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Kaur J, Machado TM, Alvarez A, Krishnan AC, Hanna HW, Altamimi YH, Senador D, Spranger MD, O'Leary DS. Muscle metaboreflex activation during dynamic exercise vasoconstricts ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 309: H2145–H2151, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur J, Spranger MD, Hammond RL, Krishnan AC, Alvarez A, Augustyniak RA, O'Leary DS. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in beta2-mediated vasodilation. Am J Physiol Heart Circ Physiol 308: H524–H529, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JK, Augustyniak RA, Sala-Mercado JA, Hammond RL, Ansorge EJ, O'Leary DS. Heart failure alters the strength and mechanisms of arterial baroreflex pressor responses during dynamic exercise. Am J Physiol Heart Circ Physiol 287: H1682–H1688, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O'Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol 288: H1374–H1380, 2005. [DOI] [PubMed] [Google Scholar]
- 19.McCloskey DL, Mitchell JH. Reflex cardiovascular and respiratory responses originating exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittelstadt SW, Bell LB, O'Hagan KP, Clifford PS. Muscle chemoreflex alters vascular conductance in nonischemic exercising skeletal muscle. J Appl Physiol 77: 2761–2766, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Mittelstadt SW, Bell LB, O'Hagan KP, Sulentic JE, Clifford PS. Muscle chemoreflex causes renal vascular constriction. Am J Physiol Heart Circ Physiol 270: H951–H956, 1996. [DOI] [PubMed] [Google Scholar]
- 22.O'Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol Heart Circ Physiol 260: H632–H637, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivier NB, Stephenson RB. Characterization of baroreflex impairment in conscious dogs with pacing-induced heart failure. Am J Physiol Regul Integr Comp Physiol 265: R1132–R1140, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Papelier Y, Escourrou P, Helloco F, Rowell LB. Muscle chemoreflex alters carotid sinus baroreflex response in humans. J Appl Physiol 82: 577–583, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962. [DOI] [PubMed] [Google Scholar]
- 27.Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988. [DOI] [PubMed] [Google Scholar]
- 28.Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O'Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Sheriff DD, Augustyniak RA, O'Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Sheriff DD, O'Leary DS, Scher AM, Rowell LB. Baroreflex attenuates pressor response to graded muscle ischemia in exercising dogs. Am J Physiol Heart Circ Physiol 258: H305–H310, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P, Hughson RL. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J Appl Physiol 103: 228–233, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Sinoway LI, Wroblewski KJ, Prophet SA, Ettinger SM, Gray KS, Whisler SK, Miller G, Moore RL. Glycogen depletion-induced lactate reductions attenuate reflex responses in exercising humans. Am J Physiol Heart Circ Physiol 263: H1499–H1505, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Spranger MD, Sala-Mercado JA, Coutsos M, Kaur J, Stayer D, Augustyniak RA, O'Leary DS. Role of cardiac output versus peripheral vasoconstriction in mediating muscle metaboreflex pressor responses: dynamic exercise versus postexercise muscle ischemia. Am J Physiol Regul Integr Comp Physiol 304: R657–R663, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephenson RB, Donald DE. Reflexes from isolated carotid sinuses of intact and vagotomized conscious dogs. Am J Physiol Heart Circ Physiol 238: H815–H822, 1980. [DOI] [PubMed] [Google Scholar]
- 35.Stephenson RB, Donald DE. Reversible vascular isolation of carotid sinuses in conscious dogs. Am J Physiol Heart Circ Physiol 238: H809–H814, 1980. [DOI] [PubMed] [Google Scholar]
- 36.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol (1985) 97: 731–738, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Victor RG, Seals DR. Reflex stimulation of sympathetic outflow during rhythmic exercise in humans. Am J Physiol Heart Circ Physiol 257: H2017–H2024, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Walgenbach SC, Donald DE. Inhibition by carotid baroreflex of exercise-induced increases in arterial pressure. Circ Res 52: 253–262, 1983. [DOI] [PubMed] [Google Scholar]
- 39.Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983. [DOI] [PubMed] [Google Scholar]