Neonatal epileptic seizures produce cerebrovascular disabilities that may contribute to neonatal encephalopathy. This study in newborn pigs provides evidence that selective head cooling during seizures can be used as an effective intervention that protects the neonatal brain by preventing cerebral vascular dysfunction.
Keywords: cerebral circulation, cerebral vascular injury, newborn pigs, epilepsy, mild hypothermia
Abstract
Epileptic seizures in neonates cause cerebrovascular injury and impairment of cerebral blood flow (CBF) regulation. In the bicuculline model of seizures in newborn pigs, we tested the hypothesis that selective head cooling prevents deleterious effects of seizures on cerebral vascular functions. Preventive or therapeutic ictal head cooling was achieved by placing two head ice packs during the preictal and/or ictal states, respectively, for the ∼2-h period of seizures. Head cooling lowered the brain and core temperatures to 25.6 ± 0.3 and 33.5 ± 0.1°C, respectively. Head cooling had no anticonvulsant effects, as it did not affect the bicuculline-evoked electroencephalogram parameters, including amplitude, duration, spectral power, and spike frequency distribution. Acute and long-term cerebral vascular effects of seizures in the normothermic and head-cooled groups were tested during the immediate (2–4 h) and delayed (48 h) postictal periods. Seizure-induced cerebral vascular injury during the immediate postictal period was detected as terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling-positive staining of cerebral arterioles and a surge of brain-derived circulating endothelial cells in peripheral blood in the normothermic group, but not in the head-cooled groups. During the delayed postictal period, endothelium-dependent cerebral vasodilator responses were greatly reduced in the normothermic group, indicating impaired CBF regulation. Preventive or therapeutic ictal head cooling mitigated the endothelial injury and greatly reduced loss of postictal cerebral vasodilator functions. Overall, head cooling during seizures is a clinically relevant approach to protecting the neonatal brain by preventing cerebrovascular injury and the loss of the endothelium-dependent control of CBF without reducing epileptiform activity.
NEW & NOTEWORTHY
Neonatal epileptic seizures produce cerebrovascular disabilities that may contribute to neonatal encephalopathy. This study in newborn pigs provides evidence that selective head cooling during seizures can be used as an effective intervention that protects the neonatal brain by preventing cerebral vascular dysfunction.
the immaturity of the newborn's brain makes neonates more susceptible to seizures (25, 45, 49, 51). Seizures are caused by an imbalance between inhibitory and excitatory neurotransmitters, producing synchronized epileptiform neuronal activation (51). The incidence rate of neonatal seizures is 1.8 to 3.5 per 1,000 live births annually for full-term neonates, whereas preterm infants may have a higher tendency for seizures (25). Neonatal seizures appear to be associated with a higher risk of neonatal mortality and neurological morbidity (14, 22, 25, 45, 49, 51). Recent improvement in neonatal intensive care has lowered the mortality rate of neonates who suffered seizures (42, 45). However, the improvement in survival rate does not reduce the long-term detrimental neurological outcome of neonatal seizures. It has long been recognized that prolonged seizures cause acute and delayed neuronal death due to glutamate neurotoxicity and inflammation (25, 50, 51). Epileptic seizures in the developing brain can produce lifelong developmental, cognitive, and motor deficits (1, 2, 11, 22, 25, 45, 47, 51). Serious alterations of neurological functions, referred to as the postictal state, can last from hours to days or even weeks and beyond after epileptic seizures (14, 22, 45).
The effects of seizures in the neonatal brain are not limited to direct neuronal loss. Our laboratory has demonstrated that neonatal seizures cause cerebral vascular injury, leading to sustained dysregulation of cerebral blood flow (CBF) (9, 31, 33, 34). Cerebral vascular endothelium is particularly vulnerable to seizure-induced injury by apoptosis. Apoptosis leads to sloughing of endothelium off the vascular wall, the appearance of injured brain-derived circulating endothelial cells (BCECs) in peripheral blood, and, finally, to prolonged impairment of endothelium-dependent regulation of CBF during the delayed postictal state (33–35). The presence of BCECs serves as an early systemic indicator of the severity of the endothelial damage that predicts the later occurrence of cerebral vascular dysfunction (35). Importantly, our pilot clinical studies in newborn babies with seizures of different etiologies suggest that the number of BCECs in peripheral blood can serve as an early predictor of adverse neurological outcome (39).
Preserving cerebral vascular functions is an important approach to neuroprotection, as the newborn brain is highly dependent on sustainable CBF regulation. We are searching for novel approaches to prevent cerebral vascular injury caused by neonatal epileptic seizures. Hypothermia achieved either by total body cooling or by selective head cooling has recently become the standard of practice for management of neonatal hypoxic-ischemic encephalopathy (HIE) in full-term and near-term babies in neonatal intensive care (5, 6, 13, 16, 18, 19, 21, 23, 24, 26, 37, 41, 42). Recent clinical trials have demonstrated that both methods of therapeutic hypothermia reduce mortality and improve neurodevelopmental outcome in infants with HIE (13, 16, 24, 42). However, the applicability of therapeutic cooling to treatment of seizures still remains obscure (7, 10, 16, 24, 29, 40, 41, 52, 54), although anticonvulsant and neuroprotective effects of hypothermia have been observed in some experimental models (6, 44, 55).
The present study, for the first time, investigates the effects of selective head cooling on cerebral vascular impairment caused by neonatal seizures in a translationally relevant large-animal model. The rationale for choosing selective head cooling is that the newborn infant's brain produces 70% of total body heat, and that the adverse effects of prolonged systemic cooling may be physiologically harmful to the sick neonate (18, 24). Here we address the hypothesis that selective head cooling during neonatal epileptic seizures protects the neonatal brain from cerebrovascular injury and preserves endothelial functions in CBF regulation.
METHODS
Vertebrate animals.
Newborn piglets purchased from a commercial breeder were used in all experiments (1–4 days old, 1.5–2.5 kg, either sex; total number of piglets, 80). The distribution of male and female piglets in the experimental groups was equal. Veterinary care was provided by the Department of Comparative Medicine, whose staff includes four full-time veterinarians in an American Association for Accreditation of Laboratory Animal Care-accredited program. All experimental protocols using animals were approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, in accordance with the National Institutes of Health guidelines for the care and use of animals in research.
Large-animal model of neonatal seizures.
Piglets were anesthetized with ketamine-xylazine (33:2.0 mg/kg im) and intubated with endotracheal tubes. Ketamine and xylazine are the preferred anesthetic substances in animal model experimentation due to their ability to facilitate rapid onset of anesthetic induction and their short-acting duration. To investigate long-term effects of seizures in the newborn cerebral circulation, all procedures were minimally invasive. Anesthetized pigs were intubated through the mouth and ventilated with 4% CO2, 21% O2, and 75% N2 to maintain physiological levels of blood gases, as our laboratory has established previously (9, 31, 32, 35). A butterfly needle was inserted into the ear vein for administration of pancuronium (0.2 mg/kg iv) before inducing seizures to block tonic-clonic motor activities. Additional injections of pancuronium (0.1 mg/kg) were given if visible evidence of seizures was seen. Seizures were induced by administration of bicuculline (3 mg/kg ip) (9, 31, 32, 35). Bicuculline induces generalized tonic-clonic seizures that last for ∼2 h by blocking inhibitory GABAA receptors on glutamatergic neurons, thus enhancing the excitatory neurotransmitter effects of glutamate. Heart rate and body temperature were recorded. After fully regaining consciousness from the seizures and anesthesia, piglets were extubated and transferred to the animal care facility. Piglets were placed in a well-controlled warm-temperature environment and fed ad libitum for 48 h. No major complications were noted in any experimental group during the 48-h postictal period.
To investigate acute effects of seizures, piglets were initially anesthetized with ketamine-xylazine (33:2 mg/kg im) and maintained by α-chloralose (50 mg/kg iv), as our laboratory has described previously (31, 35). Piglets were intubated via tracheotomy and ventilated with room air (9, 31, 32, 35). Catheters were surgically inserted into a femoral artery to monitor blood gases and systemic parameters, and into a femoral vein for blood sampling and administration of isotonic 0.9% NaCl/5% dextrose to maintain the blood volume (0.4 ml·kg−1·h−1). Piglets were paralyzed with pancuronium (0.2 mg/kg iv) before seizure induction (38).
Selective head cooling.
Selective head cooling was achieved by placing two cotton towel-wrapped ice packs on both sides of the intact scalp over the parietotemporal region. Piglets were kept on a servo-controlled heating pad during the experimentation. The steady-state reduction in brain (25.6 ± 0.3°C) and core body (33.5 ± 0.1°C; mild systemic hypothermia) temperatures was achieved within 30–60 min following the head ice pack placement. For preventive head cooling, head ice packs were placed 30 min before seizure induction and kept for the 2-h period of seizures. For therapeutic cooling, ice packs were placed 20 min after seizure induction and kept for the 2-h period of seizures. Ice packs were changed approximately every 30–40 min throughout application. The total duration of head cooling in all experiments was 1.5–2.5 h. After 2 h of sham control or seizures, head ice packs were removed, and piglets were rewarmed by heating pads. Core body temperature returned to normothermia level (37–38°C) in 2–3 h. In all experiments, the survival rate was 100%, and the recovery from head cooling and seizures was uneventful.
To evaluate the effects of selective head cooling on systemic and cerebrovascular parameters, piglets were randomly assigned into four experimental groups (N = 5–6 animals in each group): 1) group I, normothermic sham control (no seizures); 2) group II, normothermic seizure; 3) group III, preventive head cooling (head cooling was initiated 30 min before bicuculline administration and continued throughout 2 h of seizures; total duration of cooling, 2.5 h); and 4) group IV, therapeutic head cooling (head cooling was initiated 20 min after bicuculline administration and continued throughout 2 h of seizures; total duration of cooling, ∼1.5 h). Acute and long-term cerebral vascular outcomes of seizures in these groups were tested during immediate (2 and 4 h) and delayed (48 h) postictal periods by detecting cerebral vascular apoptosis and BCECs accumulation, and by evaluating cerebral vasodilator functions.
Body temperature measurements.
Brain and rectal temperatures were measured using the YSI tele-thermometer model 43TA (Yellow Spring Instrument, Yellow Springs, OH). Ear temperature was measured with the Brown ThermoScan ear thermometer (lower detection limit, 34°C). Core body temperature was continuously monitored in all experiments. Brain temperature was measured only in acute experiments, with or without placement of cranial window and electroencephalogram (EEG) electrodes. The brain thermocouple probe was inserted via a small hole drilled in the parietal bone in the left parietofrontal region. The probe was advanced to touch the intact dura mater and secured in place with bone wax. Following 30-min measurements of baseline control values of brain and core body temperatures, two ice packs were placed over the parietotemporal regions of the skull apart from the thermo-probe, cranial window, and electrodes, and the measurements were continuously taken for an additional 2-h period. Ice packs were changed approximately every 30–40 min throughout the experiment.
Detection of apoptosis in cerebral pial arterioles by TUNEL staining.
DNA degradation, a hallmark of apoptosis, was detected by fluorescein-based enzyme terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining (Roche Diagnostics, Indianapolis, IN), as described elsewhere (35). TUNEL-positive cells are detectable in cerebral vessels during the immediate postictal period (35). Pial arterioles were collected from normothermic and head-cooled pigs during baseline conditions and 4 h after seizure onset (N = 2 pigs in each group). Pial arterioles (50–70 μm, 3–5 arterioles/piglet) were excised from the cerebral cortex surface. Arterioles were air-dried on microscopic slides, fixed in 3.7% paraformaldehyde, and permeabilized in 0.1% Triton X-100 (35). The slides were viewed by an Olympus BX-50 fluorescence microscope and processed using cellSens software (Olympus Life Science) and Adobe Photoshop (Adobe Systems).
Immunodetection of BCECs.
BCECs were immunodetected in the fraction of peripheral blood mononuclear cells (PBMCs) from venous blood collected before the induction of seizures (baseline) and 2 and 4 h afterward, as described previously (35). Venous blood samples (3 ml) were collected in K3-EDTA-containing Vacutainer tubes (BD, Franklin Lakes, NJ). PBMCs were isolated by Histopaque 1077 density gradient centrifugation (35). PBMC smears on microscopic slides were air-dried, fixed in 3.7% paraformaldehyde, and permeabilized with 0.1% Triton X-100. BCECs were detected by the expression of blood-brain barrier-specific endothelial antigen glucose transporter type 1, using polyclonal antibodies from Chemicon Millipore (Temecula, CA). PBMC nuclei were visualized by counterstaining with the fluorescent dye 4,6-diamidino-2-phenylindole. The slides were viewed by an Olympus BX-50 fluorescence microscope and processed using cellSens software (Olympus Life Science) and Adobe Photoshop. The numbers of glucose transporter type 1-positive cells were normalized to the total number of nucleated 4,6-diamidino-2-phenylindole-labeled cells.
EEG.
We have compared cortical EEG recording in normothermic and head-cooled newborn pigs during the baseline and the ictal phase (N = 6 in each group). Two stainless steel screw electrodes were placed 2.5 cm apart and advanced ∼4 mm through the skull to the dura mater over the parietal cortex contralateral to the window placement. A reference electrode was clipped to the tongue of the piglet. EEG recordings were obtained using a differential low-noise amplifier (DAM 50; World Precision Instruments, Sarasota, FL), filtered between 0.1 and 25 Hz, and digitized at a rate of 250 Hz using a data-acquisition system (cDAQ-9172 chassis with NI 9215 input module; National Instruments, Austin, TX), and LabVIEW 2011 (National Instruments). To quantitatively compare the digitized EEG recordings, we analyzed EEG amplitude and power spectral density (PSD) in the δ- (1–3 Hz), θ- (4–7 Hz), α- (8–14 Hz), and β-bands (15–30 Hz), and the spike frequency distribution, as our laboratory has described in newborn pigs (32) and as conducted in newborns with seizures (43, 53, 54). The spectral analysis of EEG recordings was conducted using MATLAB R2011a (MathWorks, Natick, MA). PSD analysis was performed with a temporal resolution of 10 s and a frequency resolution of 0.1 Hz. Means from each experimental group were calculated for 5-min epochs. Frequency bands were examined by summing values of a specific frequency and its surrounding 1-Hz range, e.g., 1 ± 0.5 Hz, and were combined into the cognitive bandwidths from δ to β. For spike detection, EEG signals were parsed into 10-data point (0.04 s) windows, where the algorithm calculated mean, standard deviation, and maximum and minimum amplitudes. To detect seizure spikes, the standard deviation of the current 10-data point set was calculated and compared with a threshold value derived from the standard deviation of the respective recording's baseline activity. If the standard deviation surpassed threshold, spike count was incremented for the respective 5-min time block. As seizure spikes were often more than one window in length, it was necessary to retain maximums and minimums across multiple window segments to determine extrema for each seizure spike. After the initial instance of seizure spike, the signal was parsed window by window until the algorithm encountered a segment with a subthreshold standard deviation. At the end of each 5-min time block, spike count values were divided by the amount of elapsed time and recorded as average number of spikes per second.
Intravital microscopy of the cerebral vasculature.
Closed cranial window technique that allows observation of pial arterioles was used for testing cerebral vascular function during the delayed postictal period (48 h) (9, 31, 34, 35). Control and postictal piglets were anesthetized with ketamine-xylazine (33:2 mg/kg im) and maintained with α-chloralose (50 mg/kg iv). Core body temperature was kept at 37–38°C using a servo-controlled heating pad. A tracheostomy was performed with the placement of an uncuffed infant endotracheal tube. Piglets were ventilated with room air using a time-cycle pressure-limited infant mechanical ventilator. Femoral arterial and venous catheters were inserted for continuous monitoring of vital signs, blood sampling for analysis of gases, and for fluid administration. Closed cranial windows were placed at a left parietal cortex area via the craniotomy surgical procedure, as our laboratory has described before (9, 31, 35, 38). A closed space beneath the cranial window was filled with artificial cerebrospinal fluid bubbled with 6% CO2, 6% O2, and 88% N2 to achieve a physiological pH of 7.3–7.35. During the experimentations, all vital signs were constantly monitored. Mean arterial blood pressure was 50–80 mmHg, and heart rate remained in a range of 100–140 beats/min. Arterial blood gases were routinely checked, and ventilation was adjusted accordingly to maintain a normal physiological range for neonatal pigs (pH ≈ 7.4; arterial Pco2 ≈ 40 mmHg; arterial Po2 ≈ 80 mmHg).
For intravital microscopy, several medium pial arterioles (50–100 μm) were selected for observation in each piglet. Arteriolar diameter was measured using a digital videomicrometer connected to a Wild Heerbrugg M3B Type-S intravital microscope. We tested cerebral vascular responses to topical vasodilators applied to the brain surface under the cranial window. We used the following: 1) the endothelium-dependent vasodilator bradykinin (10−6 M); 2) the endothelium- and astrocyte-dependent vasodilators glutamate (10−4 M) and hemin (10−5 M); and 3) the vascular smooth muscle-dependent vasodilators sodium nitroprusside (10−5 M) and isoproterenol (10−5 M) that act independently of endothelial and/or astrocytic influences (9, 31, 34, 35).
Statistical analysis.
Values are presented as means ± SE of absolute values or percentage of control. Statistical comparison among groups was accomplished by determining the degree of significance in the difference of the mean values between groups using repeated-measures ANOVA, followed by Fisher's protected least significant difference test to isolate differences between groups. A level of P < 0.05 was considered significant in all statistical tests.
RESULTS
Effects of head cooling on body temperature, cerebral arterioles, and systemic parameters.
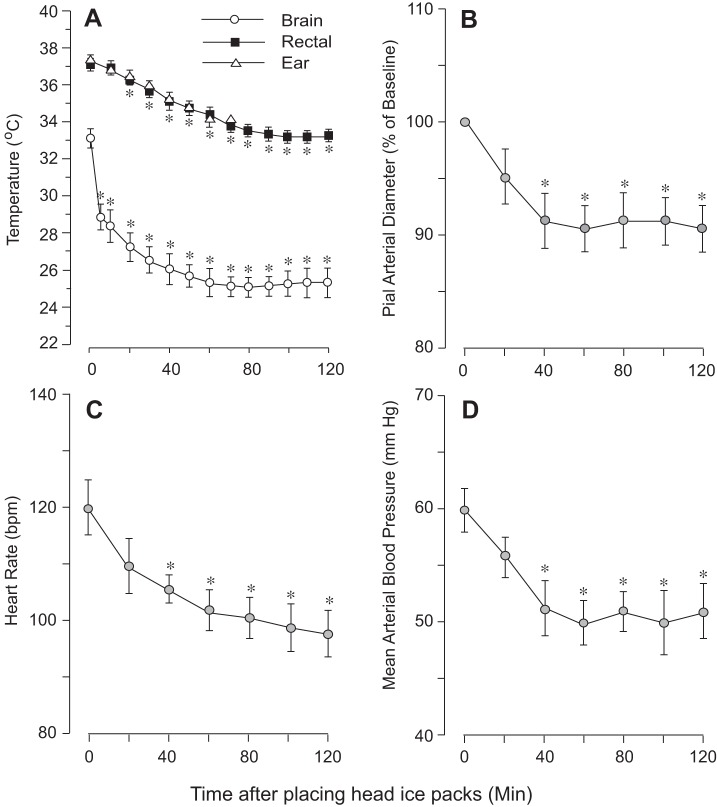
To detect the dynamics of brain and core temperatures during selective head cooling, the experiments were conducted without cranial window placement (N = 5). Piglets were kept on a servo-controlled heating pad throughout the experiment. During a 30-min baseline period, brain temperature was significantly lower than rectal temperature (33.0 ± 0.5°C and 37.3 ± 0.2°C, respectively, P < 0.05), whereas no differences were detected between tympanic and rectal temperatures (37.5 ± 0.4°C and 37.3 ± 0.3°C, respectively, P > 0.05). Selective head cooling by ice packs initiated a rapid (10–20 min) reduction in brain, rectal, and ear temperatures that reached steady-state levels in 30–60 min (Fig. 1A). The most dramatic temperature reduction was observed in the brain (25.6 ± 0.3°C) vs. rectal temperature (33.5 ± 0.1°C). Head cooling also reduced heart rate (Fig. 1C) and mean arterial blood pressure (Fig. 1D), which reached steady levels after 30–40 min of ice pack placement. In the experiments involving cranial window placement (N = 6), selective head cooling reduced the brain and rectal temperature to 25.2 ± 0.4°C and 33.5 ± 0.2°C, respectively, and had a moderate vasoconstrictor effect on pial arterioles (Fig. 1B). Arterial blood gases during a 2-h period of head cooling remained at a physiological range of pH, 7.40 ± 0.04; arterial Pco2, 38 ± 5 mmHg; and arterial Po2, 85 ± 5 mmHg.
Fig. 1.
Effects of head cooling during baseline conditions on brain and core temperature (A), diameter of pial cerebral arterioles (B), heart rate (C), and blood pressure (D). Two ice packs were placed on each side of the head, whereas the piglet's body was warmed with a servo-controlled heating pad. A: the dynamics of brain, rectal, and tympanic temperatures (N = 5). B–D: cerebrovascular and systemic effects of head cooling (N = 6). Values are means ± SE. *P < 0.05, compared with the basal normothermic values. bpm, Beats/min.
Effects of head cooling during seizures on cerebral hyperemia and systemic vascular parameters.
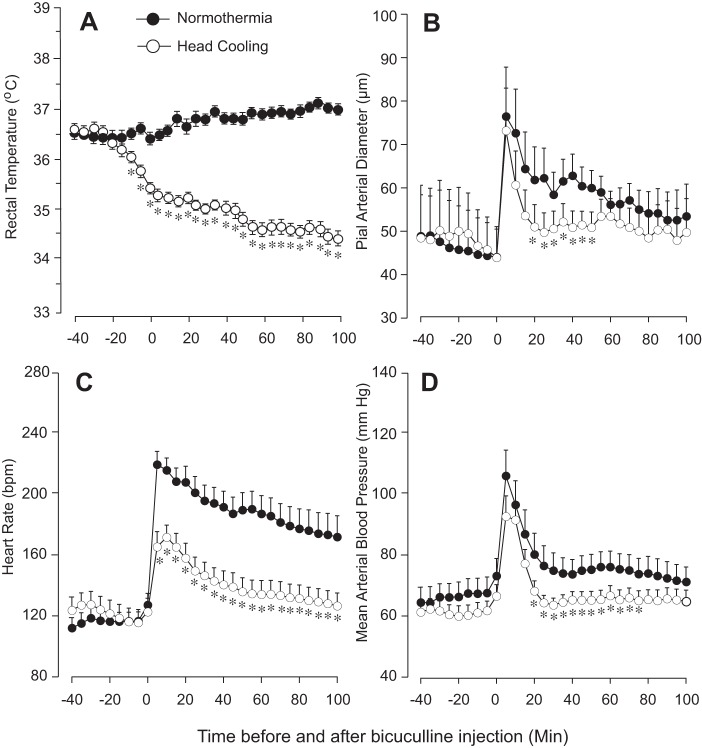
Preventive head cooling was initiated 30 min before seizures to achieve mild hypothermia (core temperature, 34–35°C; Fig. 2A). This temperature range was chosen based on guidelines for selective head cooling by the Olympic Cool-Cap System in neonatal clinical practice for babies with hypoxic-ischemic brain injury (5, 16, 18, 42).
Fig. 2.
Effects of seizures on core temperature (A), diameter of pial arterioles (B), heart rate (C), and blood pressure (D) in normothermic and head-cooled newborn pigs (N = 6 in each group). For preventive head cooling, head ice packs were placed 30 min before bicuculline administration and kept for the 2-h duration of seizures. A: rectal temperature in normothermic and head-cooled groups. B–D: cerebrovascular and systemic effects of seizures in normothermic and head-cooled groups. Values are means ± SE. *P < 0.05, compared with the normothermic values.
In both normothermic and head-cooled groups (N = 6 pigs in each group), seizures caused an immediate vasodilation of pial arterioles (Fig. 2B) as indicative of cerebral hyperemia response to neuronal activation. In both groups, maximal ictal vasodilation (70–80% over the baseline diameter) was achieved in 5 min after bicuculline injection (Fig. 2B). However, we observed certain differences in the dynamics of the time-dependent cerebral hyperemia response in the normothermic and head-cooled groups. In the normothermic group, cerebral hyperemia persisted for a >100-min ictal period, whereas, in the head-cooled group, the duration of the maximal cerebral hyperemia response was largely reduced to 20–40 min (Fig. 2B). During the late ictal period (60–100 min), we observed no significant differences in cerebral vasodilation (22 ± 5 and 14 ± 4% in normothermic and head-cooled groups, respectively, P > 0.05, Fig. 2B).
Normothermic piglets responded to seizures by increasing heart rate from 100–120 to 180–220 beats/min for the duration of neuronal activation (Fig. 2C) and by a transient (1–10 min) elevation of mean arterial blood pressure (Fig. 2D). In the head-cooled group, the tachycardia and hypertensive responses to seizures were reduced in both amplitude and duration (<40 min) compared with those in the normothermic group (Fig. 2, C and D).
Effects of head cooling on bicuculline-evoked epileptiform activity.
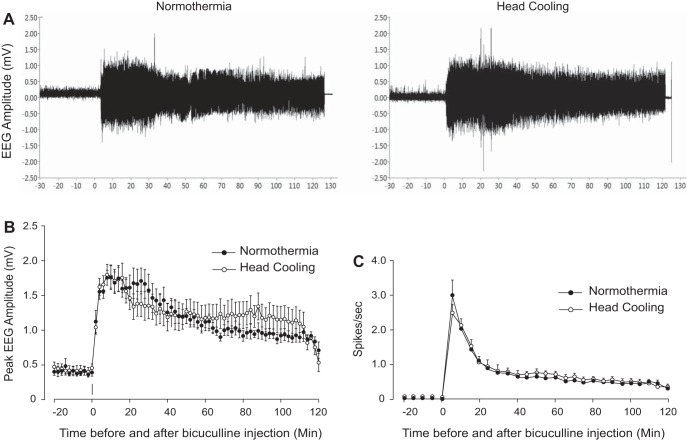
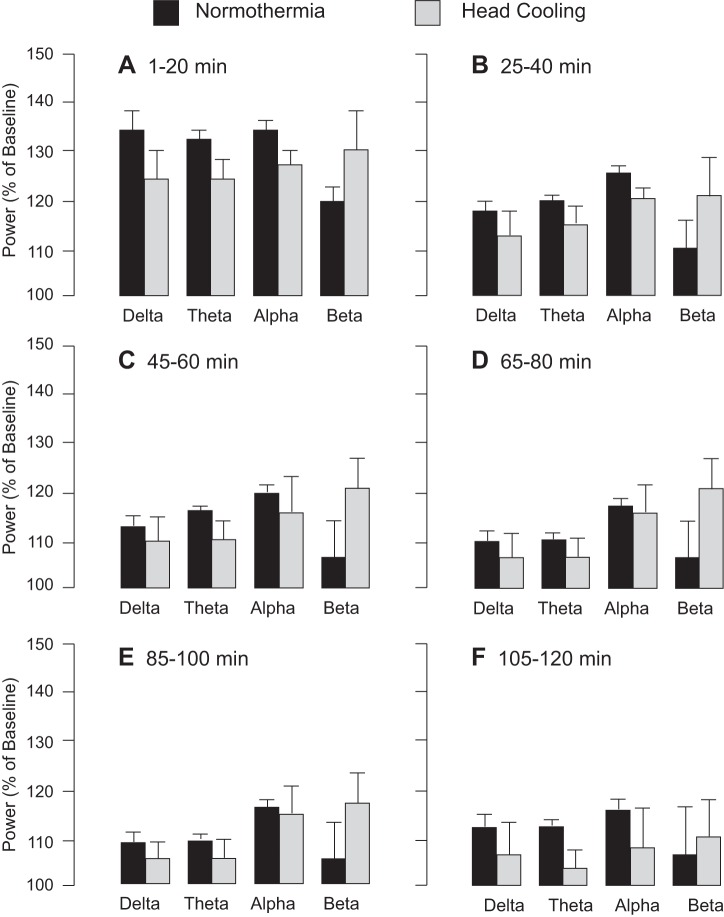
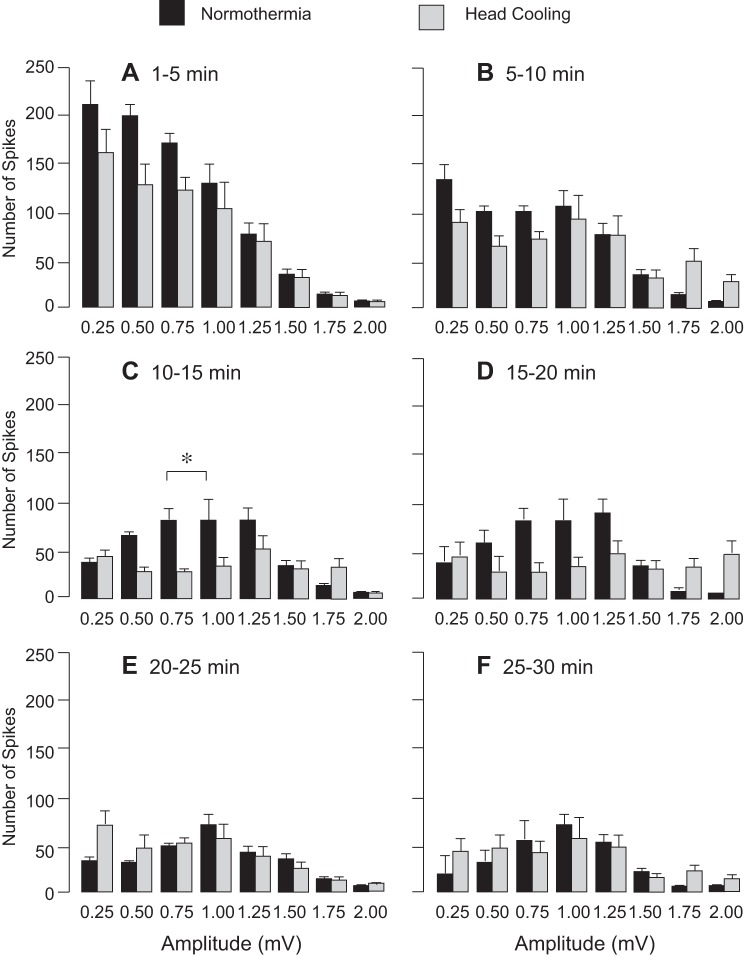
Preventive head cooling was initiated 30 min before seizure onset to achieve the brain temperature of 25.2 ± 0.4°C and core temperature of 33.5 ± 0.2°C. We analyzed EEG amplitude, spike frequency, and PSD distribution during a 30-min baseline period and a 2-h period after bicuculline administration in control and head-cooled piglets (N = 6 pigs in each group). Preictal head cooling produced no significant changes in the baseline EEG parameters, including amplitude (Fig. 3, A and B), spike frequency (Fig. 3C), and PSD values in the 1- to 30-Hz range (P > 0.1). Bicuculline (3 mg/kg ip) evoked epileptiform neuronal discharges characterized by an elevation in EEG amplitude (Fig. 3, A and B) spike frequency (Fig. 3C), and PSD in the δ- (<4 Hz), θ- (7.5 Hz), α- (8–13 Hz), and β-frequency bands (>13 Hz) (Fig. 4) sustained for a 2-h period in both normothermic and head-cooled pigs. In the head-cooled group, the bicuculline-evoked EEG amplitude (Fig. 3B), spike frequency (Fig. 3C), and PSD values (Fig. 4) were not reduced compared with those of the normothermic group. A trend of increased power in the β-frequency band in the hypothermia group was notable (Fig. 4), but not significantly different compared with the normothermic group (P > 0.2). The analysis of the number of spikes over the wide amplitude range (0.25–2.00 mV) showed only few transient differences between normothermic and head-cooled groups (Fig. 5). Overall, head cooling did not cause sustained suppression of bicuculline-induced epileptiform discharges and, therefore, did not produce anticonvulsant effects in newborn piglets.
Fig. 3.
Bicuculline-evoked EEG parameters in normothermic and head-cooled newborn pigs (N = 6 in each group). For preventive head cooling, head ice packs were placed 30 min before bicuculline administration and kept for the 2-h duration of seizures. A: representative EEG recordings in control and head-cooled pigs. B and C: mean peak EEG amplitudes (B) and the spike frequencies (C) in control and head-cooled groups. Values are means ± SE.
Fig. 4.
Power spectral density distribution during seizures in normothermic and head-cooled newborn pigs (N = 6 in each group). For preventive head cooling, head ice packs were placed 30 min before bicuculline administration and kept for the duration of seizures. Power in δ- (<4 Hz), θ- (7.5 Hz), α- (8–13 Hz), and β-frequency bands (>13 Hz) was calculated for 1–20 min (A), 25–40 min (B), 45–60 min (C), 65–80 min (D), 85–100 min (E), and 105–120 min (F) after bicuculline administration. Values are means ± SE.
Fig. 5.
Mean seizure spike amplitude distribution among head-cooled and control groups 1–30 min after seizure onset (N = 6 in each group). The number of spikes in the 0.25–2.00 amplitude ranges were analyzed for 1- to 5-min (A), 5- to 10-min (B), 10- to 15-min (C), 15- to 20-min (D), 20- to 25-min (E), and 25- to 30-min (F) segments after seizure onset. Values are means ± SE. *P < 0.05 compared with the normothermic values.
Head cooling during seizures prevents acute cerebral vascular injury.
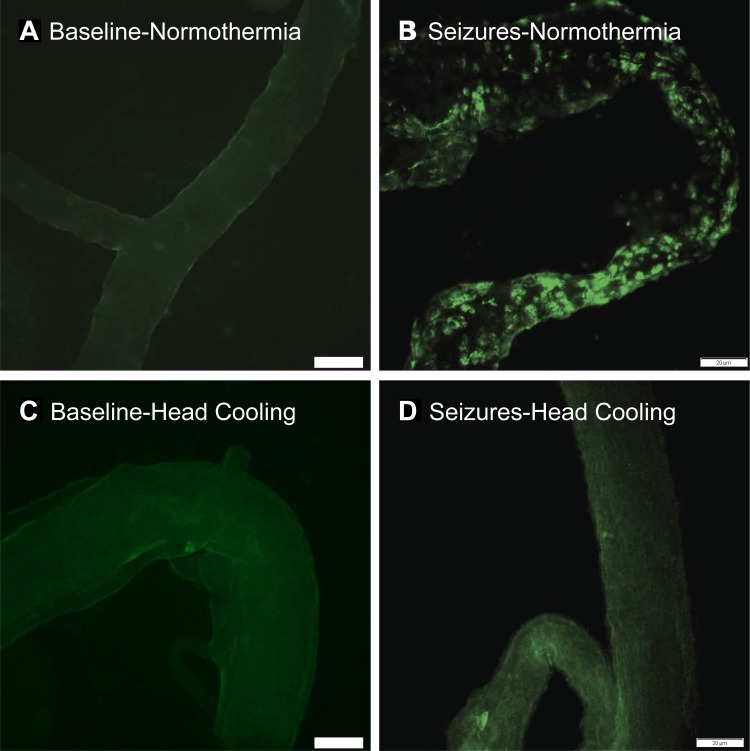
Early indicators of cerebral vascular endothelial injury include appearance of apoptotic TUNEL-positive cells in pial arterioles and endothelial sloughing from cerebral vessels, leading to the rise of BCECs in the peripheral blood 2–4 h after seizure onset (35). Consistent with these findings, we detected numerous apoptotic TUNEL-positive cells in cerebral arterioles in the normothermic group (Fig. 6), accompanied by a three- to fivefold increase in BCECs (Fig. 7). In contrast, when head ice packs were placed 30 min before seizures (preventive head cooling) and kept for an additional 2 h during seizures, the appearance of TUNEL-positive vascular cells and BCECs during the immediate postictal period was completely prevented (Figs. 6 and 7). Importantly, when head cooling was initiated during the advanced ictal period (therapeutic head cooling), we also observed a significant reduction of BCECs (Fig. 7). These observations provide strong evidence that head cooling, used in either preventive or therapeutic protocols, alleviates cerebral vascular injury caused by seizures.
Fig. 6.
Effects of head cooling on TUNEL staining of pial cerebral arterioles (representative images). For preventive head cooling, head ice packs were placed 30 min before bicuculline administration. Pial arterioles were excised from normothermic (A and B) and head-cooled piglets (C and D) during baseline (A and C) and 4 h after bicuculline (B and D). We analyzed TUNEL staining in 3–5 pial arterioles from each animal (N = 2 pigs in each group), and representative images were selected for presentation. Bar, 20 μm.
Fig. 7.
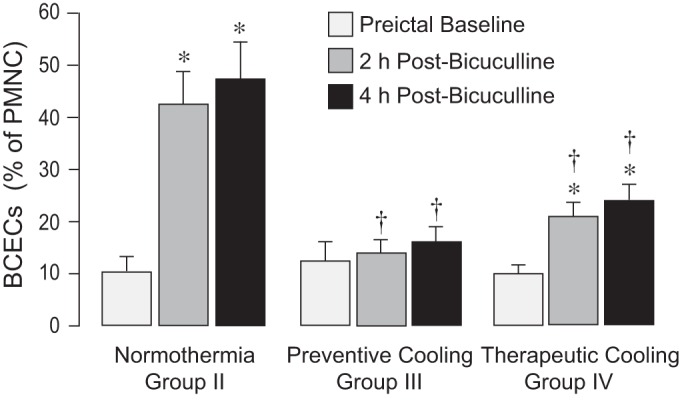
Immunodetection of brain-derived circulating endothelial cells (BCECs) in peripheral blood. BCECs were detected in the fraction of peripheral blood mononuclear cells (PBMCs) by expression of the blood-brain barrier-specific endothelial antigen, glucose transporter GLUT-1. Blood samples were collected from normothermic (group II) and head-cooled piglets (preventive and therapeutic cooling, groups III and IV, respectively) during baseline and 2 and 4 h after seizure onset. The numbers of BCECs were expressed as percentage of the total number of nucleated peripheral mononuclear cells visualized by 4′,6-diamidino-2-phenylindole. Values are means ± SE; N = 5 piglets for each data point. *P < 0.05 compared with the corresponding baseline values. †P < 0.05 compared with the corresponding normothermic values.
Effects of preventive and therapeutic ictal head cooling on postictal cerebral vasodilator functions.
The long-term effects of seizures on cerebral vasodilator functions were tested in the normothermic and head-cooled groups during the delayed postictal period (48 h after seizures). In the normothermic group (N = 6), postictal cerebral vascular responses to the endothelium-dependent vasodilator bradykinin and the endothelium/astrocyte-dependent vasodilators glutamate and hemin were reduced by 50–70% compared with the values in intact control piglets (Fig. 8). In contrast, no reduction of postictal vascular reactivity to all tested vasodilators was observed in the two head-cooled groups (Fig. 8). Prevention of long-term impairment of endothelium-mediated vasodilator functions was observed when head cooling was initiated either before seizures (preventive cooling protocol) or during the advanced ictal period (therapeutic cooling protocol) (Fig. 8). The postictal responses of pial arterioles to endothelium-independent vasodilators isoproterenol and sodium nitroprusside were not impaired in the normothermic group or in either of the head cooled groups (Fig. 9). These data confirm our laboratory's previous observations that neonatal epileptic seizures do not produce sustained cerebral vascular smooth muscle dysfunction (9, 31). Moreover, they demonstrate that ∼2-h of head cooling, followed by a 3-h rewarming and a 48-h recovery period, has no harmful effects on cerebral vascular smooth muscle.
Fig. 8.
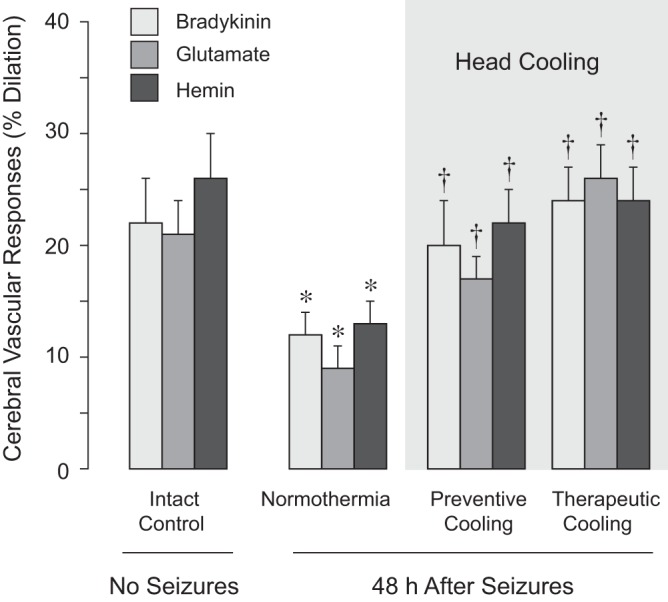
Detection of endothelium-dependent cerebral vasodilator functions during delayed postictal period. Cerebral vascular responses to endothelium-dependent vasodilators bradykinin (10−6 M), glutamate (10−4 M), and hemin (10−5 M) were tested in the intact control group (no seizures, N = 8) and in three postictal groups (48 h after seizures). The postictal groups included the normothermia group II (N = 6), the preventive head-cooled group III (head ice packs placed 30 min before seizure onset, N = 6), and the therapeutic head-cooled group IV (head ice packs placed 20 min after seizure onset, N = 6). Values are means ± SE. *P < 0.05 compared with the corresponding intact control values. †P < 0.05 compared with the corresponding normothermic values.
Fig. 9.
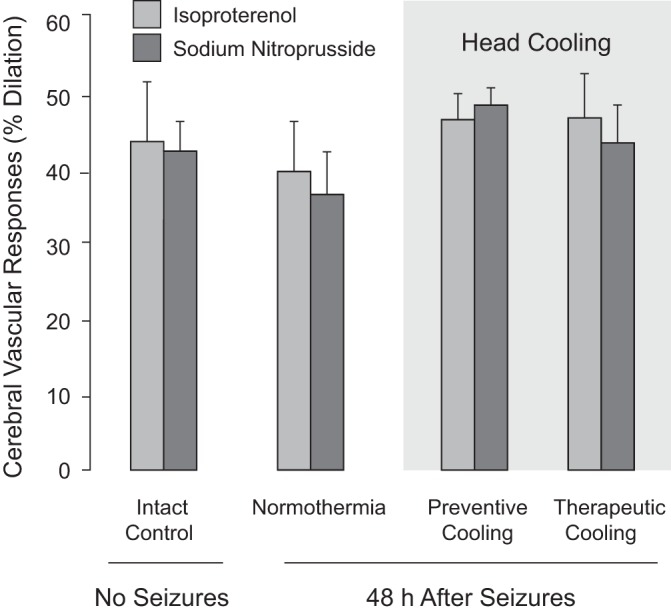
Detection of endothelium-independent cerebral vasodilator functions during delayed postictal period. Cerebral vascular responses to endothelium-independent vascular smooth muscle-dependent vasodilators isoproterenol (10−5 M) and sodium nitroprusside (10−5 M) were tested in the intact control group (no seizures, N = 8) and in three postictal groups (48 h after seizures). The postictal groups included the normothermia group II (N = 6), the preventive head-cooled group III (head ice packs placed 30 min before seizure onset, N = 6), and the therapeutic head-cooled group IV (head ice packs placed 20 min after seizure onset, N = 6). Values are means ± SE.
DISCUSSION
The major novel finding of the present study is that selective head cooling is an effective method for vascular neuroprotection of the neonatal brain that prevents long-term detrimental effects of seizures on endothelial regulation of CBF. In normothermic newborn pigs, epileptic seizures cause cerebral vascular endothelial injury and sustained postictal cerebral vascular dysfunction. Continuous selective head cooling during status epilepticus, initiated before or after seizure onset, 1) does not reduce epileptiform activity, 2) prevents apoptosis in cerebral vessels, 3) reduces the number of BCECs as selective peripheral markers of brain endothelium injury, and 4) prevents the long-term loss of the endothelium-dependent regulation of CBF during the delayed postictal period.
Epileptic seizures and neonatal brain.
Epileptic seizures lead to neuronal damage in the neonatal brain and produce subsequent life-long developmental, cognitive, and motor deficits (1, 2, 6, 10, 11, 14, 22, 47). The effects of seizures in the brain are not limited to neuronal loss. Previously, our laboratory has demonstrated that neonatal seizures cause cerebrovascular injury and long-term impairment of CBF regulation (9, 31, 33–35). Cerebral circulatory disorders in newborns are the leading cause of neurological disabilities that represent a serious healthcare problem. The importance of an adequate blood supply to the neonatal brain is particularly important because of the rapid development of neurons and high susceptibility to inflammation (1, 2, 14). Protecting the cerebral vasculature from seizure-induced injury and CBF dysregulation ultimately translates to neuroprotection.
The present study addressed the hypothesis that selective head cooling during neonatal epileptic seizures protects the neonatal brain by preventing cerebrovascular injury. Mild hypothermia achieved by total body cooling or by head cooling is currently used in neonatal critical care as a therapeutic option for neuroprotection in infants with hypoxic-ischemic brain injury, total brain ischemia due to cardiac arrest, and traumatic brain injury (5, 6, 16, 18, 23, 24, 26, 37, 41–43). To date, no experimental studies have been conducted on the effects of hypothermia on the cerebral vascular outcome of seizures. Our study is the first to investigate whether selective head cooling during status epilepticus prevents cerebral vascular damage and CBF impairment during the delayed postictal period.
Experimental model of neonatal seizures and selective head cooling.
Numerous causes of neonatal seizures include perinatal hypoxia-ischemia, severe intraventricular hemorrhage, infections, metabolic disorders, vascular and developmental defects, etc. (1–3, 10, 14, 22, 25, 42, 47, 51–53). Glutamate excitotoxicity is a primary mechanism in seizure-induced brain injury (10, 14, 25, 50, 51, 53). Bicuculline, a competitive GABAA receptor antagonist, produces sustained generalized tonic-clonic seizures that reproduce glutamate excitotoxicity (4, 9, 34, 38). Similar to bicuculline-induced seizures in newborn pigs, seizures in human neonates are also associated with restriction of GABAA-mediated inhibition and enhancement of glutamatergic excitation (51). Overall, the bicuculline large-animal model allows investigating the consequences of neonatal epileptic seizures, independently of seizures etiology.
Therapeutic systemic mild hypothermia in neonates can be produced by whole body cooling or by selective head cooling. Recent clinical trials show that both methods of therapeutic hypothermia have reduced mortality and produced favorable neuro-developmental outcome in neonates with HIE (13, 16, 24, 42). The rationale for choosing selective head cooling over total body cooling in our experiments is as follows: 1) the newborn infant's brain produces 70% of total body heat; 2) systemic hypothermia may be physiologically harmful to the sick neonate; and 3) selective head cooling produces a deeper reduction in brain vs. core temperature (18, 20, 24).
Selective head cooling with ice packs placed on intact scalp is an effective and easy-to-use approach to achieve a mild systemic hypothermia. The overall duration of selective head cooling in our experiments was ∼2 h. Head ice packs allowed deeper reduction of brain vs. core body temperature. In 30–60 min of head cooling, the brain temperature reduced to 25–26°C, whereas the core body temperature reached a mild hypothermic level (33–35°C), despite continuous warming of the body with servo-controlled heating pads. Other investigators also reported that selective head cooling supplemented with body heating reduced brain and the core temperatures in newborn pigs (36, 46). Head cooling had a mild vasoconstrictor effect on pial arterioles, reflective of the decreased baseline CBF (8), and caused a moderate reduction in heart rate and systemic blood pressure. The dynamics of the cerebrovascular and systemic parameters reached reduced steady-state levels within 30–60 min following stabilization of the brain and core body temperatures. After an ∼2-h period of head cooling, normothermia was achieved in 2–3 h of reheating using servo-controlled heating pads. The survival rate in all experiments was 100%, and the recovery was uneventful, suggesting that the reheating protocol was successful.
Selective head cooling and neonatal electrographic seizures.
Current evidence for the efficacy of mild hypothermia in the treatment of neonatal seizures remains obscure due to the scarcity and contradictory nature of experimental and clinical studies. Due to lack of solid experimental and clinical evidence on anticonvulsant effects of selective head or total body cooling, mild hypothermia is not accepted as a part of antiepileptic therapy in neonatal care. Recent clinical reports in patients with hypoxic-ischemic brain damage indicate that mild therapeutic hypothermia did not prevent the incidence of seizures and had little or no effect in ongoing status epilepticus in neonates or adults (7, 10, 16, 24, 29, 40, 41, 52, 54). However, favorable effects of head cooling on reducing the occurrence of seizures in newborns with HIE also have been described (15). Anticonvulsant and neuroprotective effects of hypothermia have been reported in animal models of neonatal seizures (6, 44, 55). In preterm fetal sheep, head cooling suppressed early epileptiform events caused by severe hypoxia, although the incidence of delayed seizures was not reduced (6). Therefore, current clinical and experimental data do not provide a uniform answer as to whether mild therapeutic hypothermia suppresses epileptiform activity. In contrast, severe hypothermia (10–20°C) achieved by direct brain cooling or by total body cooling appears to reduce the intensity and duration of epileptic discharges in adult and pediatric rat models of seizures (27, 29, 30).
First, we addressed the question of whether head cooling has anticonvulsant effects. In our model of bicuculline-induced epileptic seizures in newborn pigs, selective head cooling and resulting mild hypothermia had no anticonvulsant effects. The analysis of EEG amplitude, spectral power within a 1- to 15-Hz frequency range, and the frequency content of the EEG demonstrate that mild hypothermia achieved by selective head cooling initiated preictally and maintained for the 2-h duration of the ictal period did not suppress bicuculline-evoked epileptiform activity and did not reduce seizure duration.
Cerebral hyperemia, a physiological response to match CBF to neuronal activation, closely follows the timing, intensity, and dynamics of epileptic discharges in newborn pigs (24). In our model of neonatal seizures, preventive head cooling did not reduce the cerebral hyperemia response to seizures. This is consistent with its lack of effects on bicuculline-evoked neuronal discharges. In both normothermic and head-cooled piglets, dilation of pial resistance arterioles was sustained for the 2-h duration of the ictal period, suggesting that mild hypothermia induced by head cooling did not reduce the cerebral hyperemia response to seizures. Severe tachycardia is a common feature of seizures, and heart rate-based monitoring has been used for detection of seizures in newborn pigs and in patients (34, 48). However, in head-cooled ictal piglets, we observed a reduction in the extent and duration of the tachycardic response during the ictal state, despite sustained neuronal activation. This important observation on disconnection between heart rate and continuous epileptiform activity suggests that the tachycardic response may no longer be used as an indicator of ongoing seizures in head-cooled subjects.
Selective head cooling and cerebrovascular protection during neonatal seizures.
We present experimental evidence that 1.5- to 2.5-h head cooling, initiated either before or during the ictal period, greatly improves the long-term outcome of neonatal seizures on cerebral vascular functions. In normothermic newborn pigs, epileptic seizures have debilitating effects on endothelium-dependent CBF regulation during the delayed postictal period (9, 31, 35). Our data clearly demonstrate that head cooling during seizures preserves the integrity of the endothelial lining of cerebral vessels and prevents loss of endothelium-mediated influences on CBF regulation. In head-cooled newborn pigs, the vasodilator function of pial arterioles, the major resistance vessels in the neonatal brain, is completely preserved during the delayed postictal period. In contrast, in the normothermic group the endothelium- and endothelium/astrocyte-mediated cerebral vasodilator responses remain greatly impaired 48 h after seizures. The cerebroprotective effects were observed when continuous head cooling was initiated not only preventively, but also during the advanced ictal period. This finding has an important therapeutic implication by providing an opportunity to implement this efficient cerebroprotective treatment during the 20-min therapeutic window in neonates with seizures. By preventing cerebral vascular injury and long-term loss of endothelium-dependent CBF regulation, head cooling protects the neonatal brain and may reduce the adverse neurological outcome of seizures.
Peripheral cell markers of cerebral vascular endothelial injury and selective head cooling.
Endothelial cells of brain origin termed brain-derived endothelial cells (BCECs) appear in peripheral blood as a result of endothelial injury by apoptosis, leading to endothelium dislodging from cerebral vessels (35). Evidence in the newborn pig model of epileptic seizures suggests that BCECs may serve as early markers of cerebral vascular dysfunction in the neonatal cerebral circulation (35). Our clinical findings support these experimental observations. We observed abnormally high numbers of BCECs in newborn babies with seizures due to perinatal asphyxia and severe intraventricular hemorrhage (III/IV); these infants also had poor neurological outcome (39). Overall, our experimental and clinical findings suggest that BCEC levels in peripheral blood offer a proper biological indicator that reflects cerebrovascular insult and recovery.
In normothermic pigs, we observed a surge of BCECs in blood and appearance of TUNEL-positive cells in pial vessels during the immediate postictal period (2–4 h) that was followed by the impairment of endothelium-dependent cerebral vasodilator functions during the delayed postictal period (48 h). In contrast, selective head cooling during epileptic seizures prevented the surge in BCECs and TUNEL-positive vascular cells concomitantly with complete protection of postictal cerebral vascular functions. Importantly, both preventive and therapeutic protocols of selective head cooling (total duration, ∼2 h) were effective in preventing cerebral vascular injury and postictal cerebral vascular dysfunction.
The mechanism of cerebroprotective effects of head cooling.
The mechanism by which head cooling prevents cerebral vascular injury caused by seizures is yet to be uncovered. In our large-animal model of neonatal seizures, selective head cooling and the resultant mild hypothermia had no anticonvulsant effects. Clearly, other mechanisms account for the cerebroprotective effects of head cooling on the outcome of neonatal seizures. The neuroprotective mechanisms of hypothermia may include reduction of metabolic rate, prevention of neuronal apoptosis, reduction of brain edema and intracranial pressure, prevention of oxidative stress and reperfusion injury, etc. (4, 8, 14, 17, 19, 20, 26, 56). Our laboratory and other investigators have reported that neonatal seizures induce oxidant generation (4, 34). Our laboratory also provided evidence that oxidative stress is the major contributor to seizure-induced cerebral vascular injury (33, 34). Therefore, reduction of oxidative stress during seizures may account for the cerebroprotective effects of head cooling. NADPH oxidase and the mitochondrial respiratory chain, the major sources of reactive oxygen species in the neonatal brain during seizures, are linked to postictal cerebral vascular endothelial injury (33, 34). In this aspect, reducing the ictal activation of NADPH oxidase and the mitochondrial respiratory chain by mild cooling can contribute to improved endothelial survival and function.
The limitations of the study.
We acknowledge that our findings on cerebroprotective effects of head cooling cannot be universally extrapolated to all models of vascular injury in the neonatal brain. Thus selective head and total body cooling in newborn pigs failed to prevent the acute loss of glutamatergic vasodilation of cerebral arterioles caused by brain ischemia (36). The different findings can be explained by substantial experimental differences between this and our studies, including distinct models of cerebral vascular injury (ischemia vs. seizures), relatively short duration of hypothermia (30 min vs. ∼2 h in our studies), and acute postischemic (1 h) vs. delayed postictal (48 h, our studies) periods of testing cerebral vascular functions.
Our study has not addressed potential sex-related differences in the cerebral vascular outcome of neonatal seizures. We used newborn pigs of both sexes to establish the major key findings on cerebroprotective effects of selective head cooling. Overall, there are only a few studies that have specifically addressed seizure outcomes in newborns as a function of sex and gender. Very limited data in human neonates indicate that gender-related differences are, at best, minimal and that they appear to involve a slightly higher incidence of seizures in boys (12). Clinical studies do not provide a strong case for the contribution of sex-related variables to the incidence and outcome of seizures. In clinical trials on therapeutic cooling in infants with HIE, no gender relevance to incidence and neurological outcome of HIE has been revealed (13, 18, 42). However, recent reports have indicated that early-life seizures may produce different histopathological and neurological outcomes in immature girls and boys (3). Future studies are needed to address the important issue of potential sex-related differences in the model of neonatal seizure-induced cerebral vascular injury.
In conclusion, continuous selective head cooling for ∼2-h period during neonatal epileptic seizures prevents cerebral vascular injury and provides long-lasting protection of endothelium-dependent regulation of CBF during the postictal period. As a translational implication of these findings, we propose that selective head cooling has an advantage over whole body cooling because it allows a deeper reduction of brain vs. core body temperature to minimize potential harmful effects of systemic hypothermia to the sick neonate. By protecting CBF regulation in the neonatal brain, selective head cooling may reduce neurological sequelae and improve the long-term neurodevelopmental outcome of seizures.
GRANTS
The work was supported by the National Institutes of Health Grants HL-99655, NS-63936, and HL-34059 (to H. Parfenova and C. W. Leffler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
M.H., M.P., A.d.J.C., C.W.L., and H.P. conception and design of research; M.H., E.J.J., A.d.J.C., A.L.F., J.L., S.B., and D.Z. performed experiments; M.H., E.J.J., A.d.J.C., J.L., D.Z., and H.P. analyzed data; M.H., M.P., A.d.J.C., S.B., D.Z., C.W.L., and H.P. interpreted results of experiments; M.H., E.J.J., and H.P. drafted manuscript; M.H., M.P., E.J.J., A.d.J.C., A.L.F., J.L., S.B., D.Z., C.W.L., and H.P. approved final version of manuscript; M.P., A.d.J.C., C.W.L., and H.P. edited and revised manuscript; E.J.J., S.B., D.Z., and H.P. prepared figures.
REFERENCES
- 1.Abend NS, Wusthoff CJ. Neonatal seizures and status epilepticus. J Clin Neurophysiol 29: 441–448, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarval M, Fox SM. Pediatric seizures. Emerg Med Clin North Am 31: 733–754, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Akman O, Moshé SL, Galanopoulou AS. Sex-specific consequences of early life seizures. Neurobiol Dis 72: 153–166, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstead WM, Mirro R, Leffler CW, Busija DW. Cerebral superoxide anion generation during seizures in newborn pigs. J Cereb Blood Flow Metab 9: 175–179, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, Edwards AD TOBY Study Group. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med 371: 140–149, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Bennet L, Dean JM, Wassink G, Gunn AJ. Differential effects of hypothermia on early and late epileptiform events after severe hypoxia in preterm fetal sheep. J Neurophysiol 97: 572–578, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Boylan GB, Kharoshankaya L, Wusthoff CJ. Seizures and hypothermia: importance of electroencephalographic monitoring and considerations for treatment. Semin Fetal Neonatal Med 20: 103–108, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Busija DW, Leffler CW. Hypothermia reduces cerebral metabolic rate and cerebral blood flow in newborn pigs. Am J Physiol Heart Circ Physiol 253: H869–H873, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Carratu P, Pourcyrous M, Fedinec AL, Leffler CW, Parfenova H. Endogenous heme oxygenase prevents impairment of cerebral vascular functions caused by seizures. Am J Physiol Heart Circ Physiol 285: H1148–H1157, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Chapman KE, Raol YH, Brooks-Kayal A. Neonatal seizures: controversies and challenges in translating new therapies from the lab to the isolette. Eur J Neurosci 35: 1857–1865, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clancy RR. Summary proceedings from the neurology group on neonatal seizures. Pediatrics 117: S23–S27, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SS, Stonestreet BS. Sex differences in behavioral outcome following neonatal hypoxia ischemia: Insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic injury. Exp Neurol 256: 70–73, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 mo of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340: c363, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferriero DM. Neonatal brain injury. N Engl J Med 351: 1985–1995, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Glass HC, Nash KB, Bonifacio SL, Barkovich AJ, Ferriero DM, Sullivan JE, Cilio MR. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr 159: 731–735, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicenter randomized trial. Lancet 365: 663–670, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez FF, Ferriero DM. Neuroprotection in the newborn infant. Clin Perinatol 36: 859–880, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillet R, Edwards AD, Thoresen M, Ferriero DM, Gluckman PD, Whitelaw A, Gunn AJ; CoolCap Trial Group. Seven- to eight-year follow-up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatr Res 71: 205–209, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics 102: 885–892, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Gunn AJ, Thoresen M. Hypothermic neuroprotection. NeuroRx 3: 154–169, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris MN, Carey WA, Ellsworth MA, Haas LR, Hartman TK, Lang TR, Colby CE. Perceptions and practices of therapeutic hypothermia in American neonatal intensive care units. Am J Perinatol 31: 15–20, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol 36: 901–914, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Holzer M. Therapeutic hypothermia following cardiac arrest. Best Pract Res Clin Anaesthesiol 27: 335–346, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis RG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 1: CD003311, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol 36: 881–900, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karnatovskaia LV, Wartenberg KE, Freeman WD. Therapeutic hypothermia for neuroprotection: history, mechanism, risks and clinical applications. Neurohospitalist 4: 153–163, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowski AB, Kanaan H, Schmitt FC, Holtkamp M. Deep hypothermia terminates status epilepticus-an experimental study. Brain Res 1446: 119–126, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Monin P, Stonestreet BS, Oh W. Hyperventilation restores autoregulation of cerebral blood flow in postictal piglets. Pediatr Res 30: 294–298, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Motamedi GK, Lesser RP, Vicini S. Therapeutic brain hypothermia, its mechanisms of action, and its prospects as a treatment for epilepsy. Epilepsia 54: 959–970, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Niquet J, Gezalian M, Baldwin R, Wasterlain CG. Neuroprotective effects of deep hypothermia in refractory status epilepticus. Ann Clin Transl Neurol 2: 1105–1115, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parfenova H, Carratu P, Tcheranova D, Fedinec A, Pourcyrous M, Leffler CW. Epileptic seizures cause extended postictal cerebral vascular dysfunction that is prevented by HO-1 overexpression. Am J Physiol Heart Circ Physiol 288: H2843–H2850, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Parfenova H, Daley ML, Carratu P, Leffler CW. Heme oxygenase inhibition reduces neuronal activation evoked by bicuculline in newborn pigs. Brain Res 1014: 87–96, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Parfenova H, Leffler CW. Cerebroprotective functions of HO-2. Curr Pharm Des 14: 443–53, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parfenova H, Leffler CW, Basuroy S, Liu J, Fedinec AL. Antioxidant roles of heme oxygenase, carbon monoxide, and bilirubin in cerebral circulation during seizures. J Cereb Blood Flow Metab 32: 1024–1034, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parfenova H, Leffler CW, Tcheranova D, Basuroy S, Zimmermann A. Epileptic seizures increase circulating endothelial cells in peripheral blood as early indicators of cerebral vascular damage. Am J Physiol Heart Circ Physiol 298: H1687–H1698, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perciaccante JV, Domoki F, Puskar M, Busija DW. Effects of hypothermia on neuronal-vascular function after cerebral ischemia in piglets. Am J Physiol Regul Integr Comp Physiol 283: R1362–R1367, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Pietrini D, Piastra M, Luca E, Mancino A, Conti G, Cavaliere F, De Luca D. Neuroprotection and hypothermia in infants and children. Curr Drug Targets 13: 925–935, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Pourcyrous M, Leffler CW, Bada HS, Korones SB, Stidham GL, Busija DW. Effects of pancuronium bromide on cerebral blood flow changes during seizures in newborn pigs. Pediatr Res 31: 636–639, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Pourcyrous M, Basuroy S, Tcheranova D, Arheart KL, Leffler CW, Parfenova H. Brain-derived circulating endothelial cells in peripheral blood of newborn infants with seizures: a potential biomarker for cerebrovascular injury. Physiol Rep 3: e12345, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossetti AO. What is the value of hypothermia in acute neurologic diseases and status epilepticus? Epilepsia 52, Suppl 8: 64–66, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Sarkar S, Donn SM, Bapuraj JR, Bhagat I, Barks JD. Distribution and severity of hypoxic-ischaemic lesions on brain MRI following therapeutic cooling: selective head versus whole body cooling. Arch Dis Child Fetal Neonatal Ed 97: F335–F339, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, Higgins RD; Eunice Kennedy Shriver NICHD Neonatal Research Network. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 36: 2085–2092, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr 163: 465–470, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Takei Y, Nishikawa Y, Tachibana M, Takami T, Miyajima T, Hoshika A, Takashima S. Hypothermia during kainic acid-induced seizures reduces hippocampal lesions and cerebral nitric oxide production in immature rabbits. Brain Dev 26: 176–183, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, Volpe J, Bourgeois B, du Plessis A. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics 177: 1270–1280, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Thoresen M, Simmons M, Satas S, Tooley J, Silver IA. Effective selective head cooling during post-hypoxic hypothermia in newborn piglets. Pediatr Res 49: 594–599, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Uria-Avellanal C, Marlow N, Rennie JM. Outcome following neonatal seizures. Semin Fetal Neonatal Med 18: 224–232, 2013. [DOI] [PubMed] [Google Scholar]
- 48.van Elmpt WJ, Nijsen TM, Griep PA, Arends JB. A model of heart rate changes to detect seizures in severe epilepsy. Seizure 15: 366–375, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Van Rooij LG, Hellström-Westas L, de Vries LS. Treatment of neonatal seizures. Semin Fetal Neonatal Med 18: 209–215, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Vezzani A, Fujinami RS, White HS, Preux PM, Blümcke I, Sander JW, Löscher W. Infections, inflammation and epilepsy. Acta Neuropathol (Berl) 131: 211–234, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volpe JJ. Neurology of the Newborn (5th Ed). Philadelphia, PA: Saunders, 2008. [Google Scholar]
- 52.Wheless JW. Treatment of refractory convulsive status epilepticus in children: other therapies. Semin Pediatr Neurol 17: 190–194, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Wusthoff CJ. Diagnosing neonatal seizures and status epilepticus. J Clin Neurophysiol 30: 115–121, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, Wang A, Cook N, Donnelly M, Clancy R, Abend NS. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol 26: 724–728, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang XF, Chang JH, Rothman SM. Long-lasting anticonvulsant effect of focal cooling on experimental neocortical seizures. Epilepsia 44: 1500–1505, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 13: 267–278, 2012. [DOI] [PubMed] [Google Scholar]