Abstract
Acute respiratory distress syndrome (ARDS) remains a leading cause of morbidity and mortality in both adult and pediatric intensive care units. A key event in the development of ARDS is neutrophil recruitment into the lungs leading to tissue damage and destruction. Interleukin-8 (IL-8) is the major human chemokine responsible for neutrophil recruitment into the lungs. Protein phosphatase 2A (PP2A) has been shown to be a key regulator of the mitogen-activated protein kinase (MAPK) cascades, which control the production of IL-8. Previously, our laboratory employed an in vitro model to show that inhibition of PP2A results in an increase in IL-8 production in human alveolar epithelial cells. The objective of this study was to determine whether PP2A regulated this response in vivo by investigating the impact of pharmacologic activation of PP2A on chemokine production and activation of the MAPK cascade and lung injury using endotoxin- and bacterial-challenge models of ARDS in mice. N6-cyclopentyladenosine (N6-CPA) increased PP2A activity and inhibited endotoxin-induced cytokine production in a murine alveolar macrophage cell line. N6-CPA pretreatment in mice challenged with intratracheal endotoxin decreased chemokine production, reduced neutrophil infiltration, and attenuated lung injury. Following initiation of lung injury with live Pseudomonas aeruginosa, mice that received N6-CPA 4 h following bacterial challenge showed attenuated chemokine production and reduced neutrophil infiltration compared with control mice. Pharmacologic PP2A activation both limited and prevented inflammation and tissue injury in two direct injury models of ARDS. These results suggest modulation of PP2A activity as a therapeutic target in ARDS.
Keywords: acute respiratory distress syndrome, protein phosphatase 2A, lung inflammation, neutrophil recruitment, murine acute lung injury model
since it was first described in 1967, acute respiratory distress syndrome (ARDS) continues to have a significant impact on public health with more than 200,000 cases of ARDS per year in the United States accounting for nearly 4,000,000 hospital days (1, 28). Both direct (e.g., pneumonia, aspiration) and indirect inflammatory stimuli (e.g., severe sepsis, trauma) are known to lead to the development of ARDS (38). The mortality rate of ARDS remains unacceptably high in both adults (>40%) and children (∼20%) despite recent insight as to mechanical ventilation and fluid management strategies that reduce mortality (10, 28, 36, 38, 41). Identification of an effective pharmacologic therapy to regulate the immunologic response contributing to the pathophysiology of ARDS may afford further improved outcomes.
A key component of the pathophysiology of ARDS is the recruitment of neutrophils into the lung from the intravascular space, which is mediated by proinflammatory chemokines (38). CXCL8/interleukin-8 (IL-8) is the major human proinflammatory chemokine that recruits neutrophils into the lungs, where release of proteinases, cationic polypeptides, and reactive oxygen species leads to the destruction of healthy lung tissue (13, 38). IL-8 concentrations correlate with the severity of lung injury in patients with ARDS and blocking IL-8 activity in animal models has been shown to alter the pathophysiology of ARDS and improve mortality (5, 8, 13, 15, 25, 26, 39). The use of antibodies to block IL-8 activity in human patients with ARDS raised concerns because of the association of anti-IL8:IL-8 complexes in bronchoalveolar lavage fluid (BALF) of patients with ARDS and the known role of immune complexes in triggering lung injury (30). Several studies have established that anti-IL8:IL-8 complex levels are correlated with progression of ARDS as well as with mortality (11, 17, 18). Thus alternative therapeutic approaches that attenuate IL-8 levels may afford improved outcomes by modulation of the inflammatory process during ARDS.
Chemokine production is a complex process involving the mitogen-activated protein kinase (MAPK) signaling pathways. The activation of MAPKs via phosphorylation is counterbalanced by a dephosphorylating system comprised of protein phosphatases thereby creating a dynamic regulatory process. The serine/threonine phosphatase protein phosphatase 2A (PP2A) regulates the MAPK pathways (16, 27, 31, 40). Our group showed that PP2A inhibition augmented IL-8 production from respiratory epithelial cells, implicating it as a key regulator of chemokine production during ARDS (6). These results suggest that activation of PP2A could have the opposite effect of attenuating cytokine production. Thus we hypothesized that PP2A activation would reduce chemokine production via increased dephosphorylation of MAPK to attenuate the degree of lung inflammation in vivo. Since treatment of myocardial and hippocampal cells with adenosine A1 receptor agonists increased PP2A activity (22–26), we investigated the role of PP2A activation on lung inflammation, using the small molecular adenosine A1 receptor agonist N6-cyclopentyladenosine (N6-CPA), and the sphingosine 1-phosphate receptor modulator FTY-720, which has also been shown to increase PP2A activity (7, 19, 20, 22, 32, 34).
MATERIALS AND METHODS
Serine/threonine phosphatase assay.
The murine alveolar macrophage cell line MH-S was purchased from American Type Culture Collection (ATCC, Manassas, VA). Cells were switched to low serum media, RPMI medium (GE Healthcare Hyclone, Logan, UT) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml; Life Technologies, GIBCO, Carlsbad, CA), and 0.5% heat inactivated fetal bovine serum (FBS; GE Healthcare Hyclone), overnight (12–18 h) before experiments. Cells were exposed to N6-cyclopentyladenosine (N6-CPA) (Sigma-Aldrich, St. Louis, MO) or FTY-720 (Sigma-Aldrich) in low serum media for the times and concentrations as indicated in results. Total cellular proteins were extracted in RIPA lysis and extraction buffer (Pierce Biotechnology, Rockford, IL), supplemented with Halt protease inhibitor cocktail (Pierce Biotechnology). Specific PP2A activity was quantified using purified PP2A protein (EMD Millipore, Temecula, CA) and measured using a commercial serine/threonine phosphatase assay (Life Technologies, Molecular Probes, Eugene, OR) following the manufacturers′ recommendations.
Cellular model of endotoxin-induced ARDS.
MH-S cells were treated with 10 μM N6-CPA for 1 h before endotoxin stimulation. Endotoxin stimulation was initiated by incubating cells in 0.5% FBS medium containing LPS from Escherichia coli O55:B5 (Sigma-Aldridge) at a concentration of 100 ng/ml for the times indicated in the results section. Cell culture supernatants were collected to determine CXCL1 production using a commercially available ELISA kit (R&D Systems, Minneapolis, MN). To determine MAPK cascade activation cells were harvested and lysed using RIPA lysis and extraction buffer, supplemented with Halt protease and phosphatase inhibitors (Pierce Biotechnology). Samples were separated using SDS-PAGE and transferred to a PVDF membrane that were probed with either rabbit monoclonal antibodies against phosphorylated and total SAPK-JNK (Thr183/Tyr185), p42/44 MAPK (ERK1/2) (Thr202/Tyr204), and p38 (Thr180/Tyr182) (Cell Signaling Technology, Danvers, MA), or mouse monoclonal antibodies directed against GAPDH (EMD Millipore, Temecula, CA) according to the manufacturers' recommendations. A species appropriate horseradish peroxidase-conjugated secondary antibody (GE Healthcare) and ECL Prime detection solution (GE Healthcare) were used to analyze protein expression. Semiquantitative densitometry analysis was conducted on three independent experiments as previously described using the open-source ImageJ analysis package (National Institutes of Health, Bethesda, MD) (29).
Primary alveolar epithelial cell culture.
Primary murine alveolar type II epithelial cells were isolated as previously described (24). Approximately 94% purity of isolated cells was confirmed via fluorescence microscopy (data not shown).
Animal care and husbandry.
All animal studies described herein were approved by the University of Michigan University Committee on Use and Care of Animals and conducted in accordance with National Institutes of Health guidelines for ethical animal treatment. Animals were housed in individually ventilated cages with ad libitum access to food and water.
Murine model of direct intratracheal endotoxin-induced lung injury.
Aged and sex-matched C57BL/6 strain mice (Jackson Laboratories, Bar Harbor, ME) were intratracheally pretreated with N6-CPA (0.1 ml of 100 μM) for the time described in results followed by an intratracheal LPS challenge (5 mg/kg), using the technique previously described by Su et al. (33). Briefly, mice were anesthetized using a blend of isoflurane and oxygen and then suspended at an angle of ∼60°. A sterile, 22-gauge catheter (BD, Sandy, UT) was gently advanced posterior of the tongue into the trachea. Intratracheal positioning of the catheter was confirmed by palpation of the abdomen and thorax while the catheter was connected to a small animal ventilator (Harvard Apparatus, Holliston, MA). Concentrations of LPS were calculated and diluted with PBS so that all mice received an intratracheal injection with a total volume of 0.1 ml. Lungs were fixed in vivo following the current American Thoracic Society guidelines for lung histology, 4 and 24 h postendotoxin challenge (14), BALF was collected as described below 24 h postendotoxin challenge.
Murine model of Pseudomonas aeruginosa-induced lung injury.
Age and sex matched C57BL/6 mice were intratracheally challenged with 1 × 108 colony-forming units of P. aeruginosa diluted to a total volume of 0.1 ml with PBS. Four hours postinfection N6-CPA (0.1 ml of 100 μM) was intratracheally instilled. Bronchial alveolar lavage fluid was collected a total of 28 h postinfection.
BALF differential cell counts.
BALF was collected by intratracheal instillation and aspiration of 1 ml of PBS twice, and then repeated with another one ml of PBS. The BALF was then centrifuged at 500 g for 5 min. BALF was collected and frozen at −80°C for chemokine quantification as described below. The cell pellet was then resuspended in 800 μl of PBS. The cell suspension was then prepared on glass slides using a cytospin and fixed with a Camco Stain Pak (Cambridge Diagnostic Products, Fort Lauderdale, FL). Total BALF protein concentrations were determined by bicinchoninic acid assay (BCA) according to the manufacturer's recommendations (Thermo Fisher Scientific). Personnel conducting differential counts were blinded to the experimental conditions.
Chemokine determination (ELISA).
Immunoreactive CXCL1 and CXCL2 concentrations were determined using commercially available mouse CXCL1 (KC; R&D Systems; Minneapolis, MN) or mouse CXCL2 (MIP-2; Life Technologies) ELISA kits. Immunoreactive IL-8 concentration was determined by a commercially available human IL-8 ELISA cytoset (Life Technologies). All procedures were performed according to the manufacturers' protocols.
Statistical analysis.
The results reported herein consist of at least three independent experiments conducted on multiple days. Results are reported as means ± SE. All statistical significance was determined using an unpaired two-tailed Student's t-test, one-way ANOVA with Bonferroni's correction for multiple comparisons, or two-way ANOVA using Sidak's correction for multiple comparisons where appropriate. Statistical tests were conducted using Prism GraphPad for Windows (GraphPad Software, La Jolla, CA).
RESULTS
N6-cyclopentyladenosine increases ex vivo PP2A activity in both MH-S cell line and primary alveolar epithelial cells.
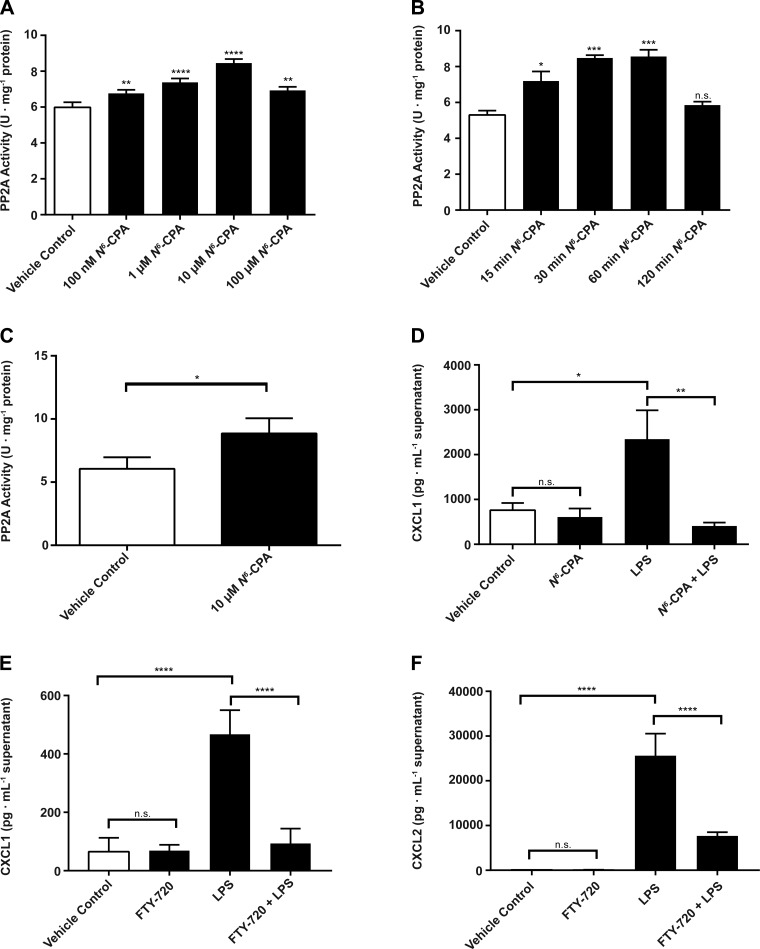
We first aimed to establish the effect of PP2A activation by N6-CPA using a murine alveolar macrophage cell line (MH-S). N6-CPA treatment increased PP2A activity in a dose-dependent manner in MH-S cells. PP2A activity in cells treated with 10 μM N6-CPA for 30 min was increased by 41% (Fig. 1A) compared with cells that did not receive N6-CPA treatment (5.98 ± 0.28 vs. 8.46 ± 0.22; P < 0.0001). MH-S cells exposed to 10 μM N6-CPA treatment showed a time-dependent increase in PP2A activation (Fig. 1B). One hour of N6-CPA treatment resulted in a 61% increase in PP2A activity compared with cells that did not receive N6-CPA treatment (5.31 ± 0.42 vs. 8.56 ± 0.64; P < 0.001). Note, at this dose no increase in cellular apoptosis following N6-CPA treatment was observed (data not shown). We also show that primary alveolar epithelial cells treated for 1 h with 10 μM N6-CPA increases PP2A activity by ∼60% (Fig. 1C) compared with untreated cells (6.04 ± 0.36 vs. 9.67 ± 1.13; P = 0.0374).
Fig. 1.
N6-cyclopentyladenosine (N6-CPA) increases protein phosphatase 2A (PP2A) activity and decreases endotoxin-induced chemokine production in MH-S cells. A: MH-S cells were treated with increasing concentrations (1–100 μM) of N6-CPA for 30 min. B: MH-S cells were exposed to 10 μM N6-CPA for between 15 and 120 min. Specific PP2A activity was calculated from a standard curve of purified PP2A protein. C: cultured primary alveolar epithelial cells were treated with 10 μM N6-CPA for 1 h. D: MH-S cells were pretreated with 10 μM N6-CPA for 1 h before 6 h LPS (100 ng/ml) stimulation. CXCL1 production in cell supernatants was determined via ELISA. E: MH-S cells were pretreated with 10 μM FTY-720 for 1 h before 6 h LPS stimulation. CXCL1 production or CXCL2 production (F) in cell supernatants was determined via ELISA. Statistical analysis is summarized inset as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; statistical significance is compared with vehicle control in A and B using one-way ANOVA with Bonferroni's correction and as shown in C using a two-tailed student's t-test.
PP2A activation reduces chemokine production in murine alveolar macrophage cells.
We next examined the effect of PP2A activation on CXCL1 and CXCL2 protein expression, chemokines, which play a key role in ARDS and the recruitment of neutrophils into the lung space. Congruent with our previous results that showed increased IL-8 production following PP2A inhibition, activation of PP2A before endotoxin stimulation decreased production of CXCL1 in MH-S cells. CXCL1 production was decreased by 82% (Fig. 1D) in cells that received N6-CPA pretreatment before LPS stimulation for 4 h compared with vehicle-treated cells (2,344 ± 646 vs. 407 ± 79; P < 0.01). These data suggested that N6-CPA pretreatment reduced in vitro production of CXCL1 and provided timing and doses to be utilized in in vivo experiments. In addition, we show that pretreatment of MH-S cells with FTY-720, which has previously been shown to result in PP2A activation, results in an 80% decrease in CXCL1 (468 ± 34 vs. 93 ± 21; P < 0.0001) and a 70% decrease in CXCL2 (25,633 ± 2,005 vs. 7,688 ± 336) production compared with cells stimulated with endotoxin alone (Fig. 1, E and F, respectively).
PP2A activation blunts JNK phosphorylation following endotoxin stimulation.
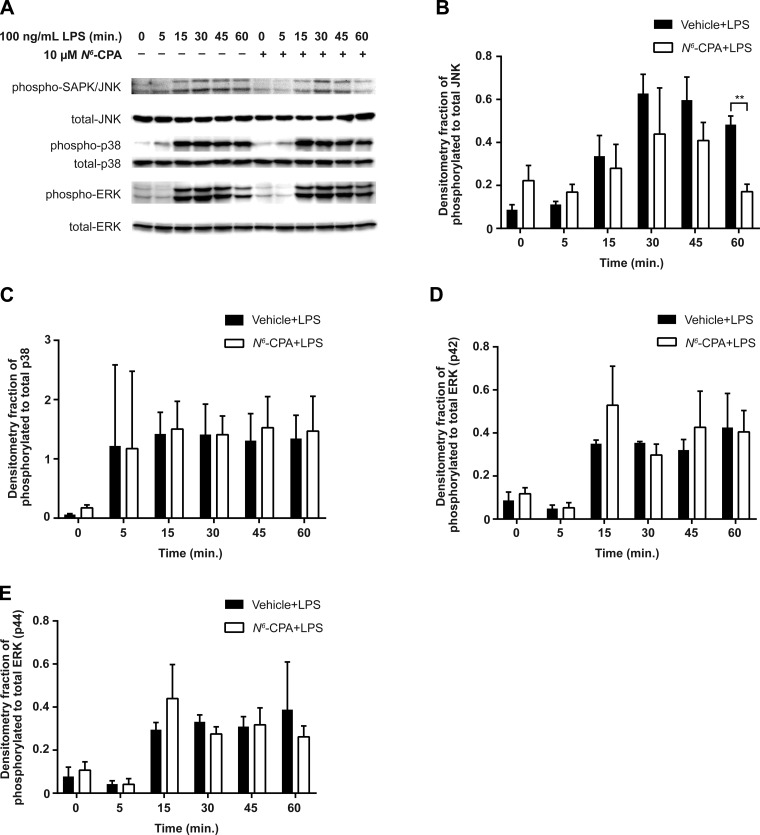
As PP2A is known to attenuate MAPK activation via dephosphorylation of serine and threonine residues, we determined the effect of N6-CPA treatment on MAPK activation by LPS (Fig. 2A). MH-S cells pretreated with 10 μM N6-CPA for 1 h resulted in decreased JNK phosphorylation at 60 min following LPS stimulation (Fig. 2B) compared with cells which did not receive N6-CPA pretreatment before LPS stimulation. No differences in phosphorylation of p38 (Fig. 2C) or ERK (Fig. 2, D and E) were observed between cells that received N6-CPA pretreatment compared with those stimulated with LPS alone. These data suggest that PP2A activation decreases JNK phosphorylation resulting in attenuated proinflammatory CXCL1 production.
Fig. 2.
N6-CPA pretreatment decreases phospho-JNK signaling following endotoxin stimulation in MH-S cells. A: MH-S cells were pretreated with 10 μM N6-CPA for 1 h and then stimulated with LPS (100 ng/ml) for the indicated time points, pictured blots are representative of 3 independent experiments. Phosphorylation of the terminal MAPKs JNK (B), p38 (C), ERK (p42; D), and ERK (p44; E) were assessed by semiquantitative densitometry. Statistical analysis was performed using two-way ANOVA test with Sidak's correction for multiple comparisons. **P < 0.01.
PP2A activation attenuates inflammatory response and decreases tissue inflammation in a murine model of endotoxin-induced ARDS.
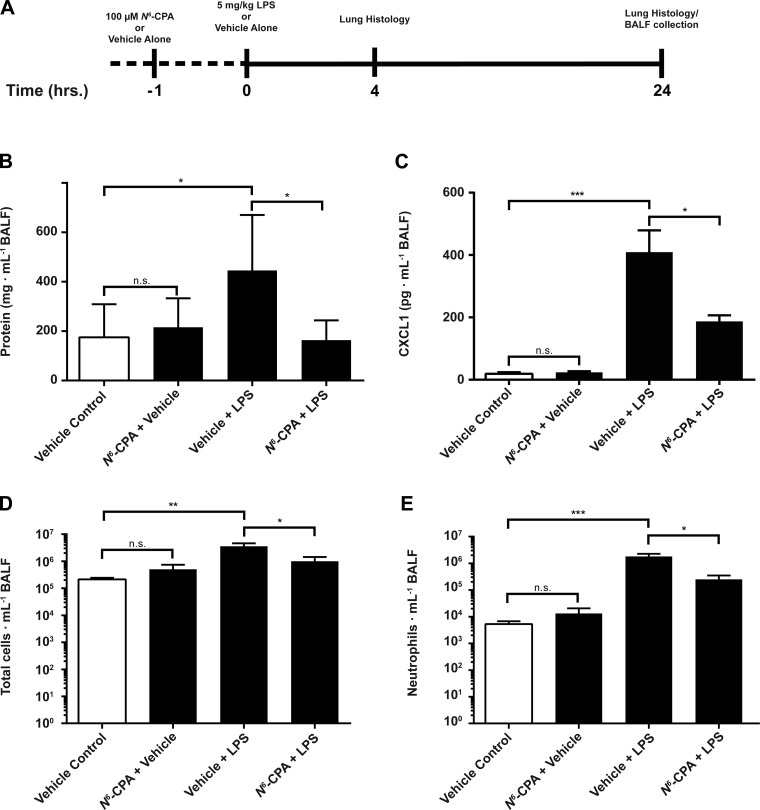
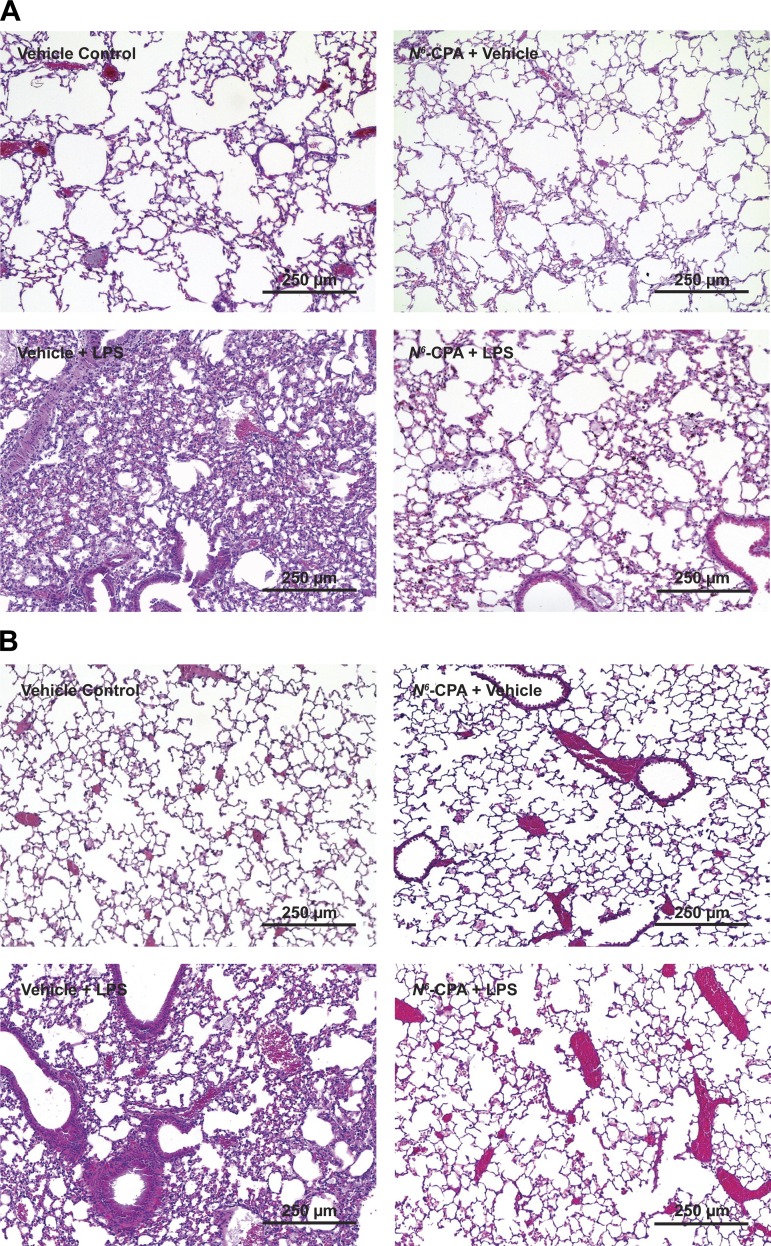
Following our experiments using a cellular model of endotoxin-induced inflammation, we examined the effect of PP2A activation in the context of an in vivo model of direct endotoxin-induced ARDS (Fig. 3A). Mice that received intratracheal N6-CPA pretreatment showed a 63% decrease (Fig. 3B) of protein in BALF (445.9 ± 224.6 vs. 163.9 ± 80.1; P < 0.05) and a 54% decrease in CXCL1 production at 24 h after LPS (Fig. 3C) compared with mice receiving endotoxin alone (409 ± 70 vs. 187 ± 20; P < 0.05). This attenuation of chemokine production had a significant effect on consequent cellular recruitment to the lungs. Total cellular infiltrates present in the BALF of mice pretreated with N6-CPA was decreased by 72% (Fig. 3D; 3.6 × 106 ± 1.0 × 106 vs. 1.0 × 106 ± 4.4 × 105; P < 0.05) and neutrophil infiltration was reduced by 86% (Fig. 3E; 1.8 × 106 ± 1.0 × 105 vs. 2.5 × 105 ± 1.0 × 105; P < 0.05) compared with mice that did not receive N6-CPA pretreatment before endotoxin challenge. Lung histology prepared from mice that received N6-CPA pretreatment compared with mice challenged with endotoxin alone show decreased intra-alveolar neutrophil infiltration, interstitial edema, and deposition of proteinaceous hyaline membranes characteristic of ARDS pathology at both four (Fig. 4A) and 24 h postendotoxin challenge (Fig. 4B).
Fig. 3.
N6-CPA pretreatment attenuates inflammatory response in direct intratracheal endotoxin murine model of acute respiratory distress syndrome (ARDS). Mice received 0.1 ml of 100 μM N6-CPA intratracheally 1 h before endotoxin (5 mg/kg) challenge. A: samples from mice were harvested at 4 and 24 h following endotoxin challenge for lung histology and at 24 h for bronchoalveolar lavage fluid (BALF) collection. B: 100 μM N6-CPA pretreatment 1 h before endotoxin challenge decreased BALF protein concentration by 63% compared with mice which did not receive N6-CPA treatment. C: 100 μM N6-CPA pretreatment 1 h before endotoxin challenge attenuated CXCL1 cytokine production by 54% compared with mice which did not receive N6-CPA pretreatment. Total cellular infiltrates (D) and neutrophil infiltration (E) were reduced by 72 and 86, respectively. Students two-tailed t-test for statistical significance is summarized inset as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Mice were randomized to experimental conditions, consisting of at least 6 mice per group.
Fig. 4.
N6-CPA pretreatment attenuates inflammation in a direct intratracheal endotoxin-induced murine model of ARDS. Mice received 0.1 ml of 100 μM N6-CPA intratracheally 1 h before endotoxin (5 mg/kg) challenge. Lungs were harvested en bloc at 4 (A) and 24 (B) h following endotoxin challenge and then fixed, processed, and stained with hematoxylin and eosin. Photomicrographs are representative images from at least 3 animals per group.
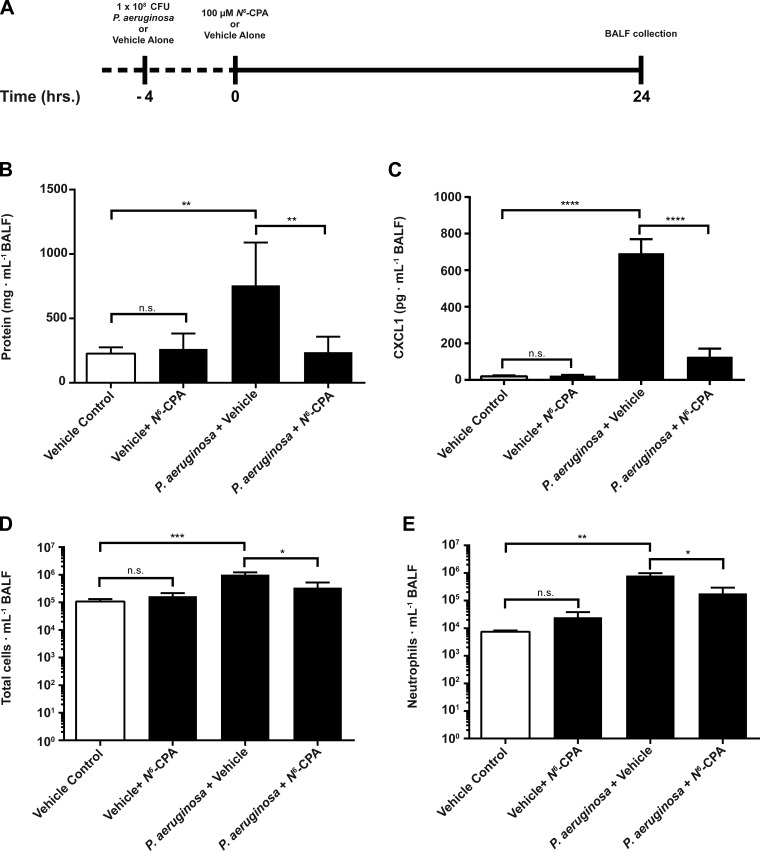
PP2A activation after direct live bacterial challenge reduces lung inflammation in an in vivo murine model of ARDS.
To investigate the potential utility of pharmacologic activation of PP2A in a clinical setting in which patients commonly present with ongoing inflammatory processes triggered by bacterial pathogens, we explored the use of N6-CPA following initiation of lung inflammation using live P. aeruginosa. Mice that received intratracheal N6-CPA 4 h following instillation of live P. aeruginosa (Fig. 5A) showed ∼69% decrease (Fig. 5B) of protein concentration in BALF (757.4 ± 331.7 vs. 238.0 ± 119.6; P < 0.01). In this model, CXCL1 production was attenuated by ∼82% (Fig. 5C) in mice that received intratracheal N6-CPA 4 h following instillation of live P. aeruginosa compared with mice challenged with bacteria alone (693 ± 77 vs. 128 ± 44; P < 0.0001). Total cellular infiltrates in BALF was reduced by more than 66% (Fig. 5D; 1.0 × 106 ± 2.1 × 105 vs. 3.4 × 105 ± 1.8 × 105; P > 0.05), while neutrophil infiltration was reduced by 77% (Fig. 5E; 8.0 × 105 ± 1.9 × 105 vs. 1.8 × 105 ± 1.1 × 105; P < 05) in mice that received N6-CPA treatment following live bacteria challenge compared with mice that did not receive N6-CPA treatment.
Fig. 5.
N6-CPA treatment attenuates inflammation in a direct intratracheal Pseudomonas aeruginosa instillation murine model of ARDS. A: mice were intratracheally challenged with 1 × 108 colony-forming units P. aeruginosa and then received 100 μM N6-CPA or vehicle alone 4 h postinfection. B: 100 μM N6-CPA treatment 4 h postinfection decreased BALF protein concentration by ∼69% compared with mice which did not receive N6-CPA treatment. C: 100 μM N6-CPA treatment 4 h postinfection attenuated CXCL1 cytokine production by 82% compared with mice which did not receive N6-CPA treatment. Total cellular infiltrates (D) and neutrophil infiltration (E) were reduced by 66 and 77%, respectively. Students two-tailed t-test for statistical significance is summarized inset as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Mice were randomized to experimental conditions, consisting of at least 6 mice per group.
DISCUSSION
Despite almost half a century of intense research into the pathophysiology and clinical management of ARDS, it remains a significant cause of morbidity and mortality in both adult and pediatric intensive care units with limited pharmacologic therapies identified to reduce the morbidity and mortality. The hallmark of ARDS is neutrophil infiltration into the alveolar space resulting in destruction of lung tissue, breakdown of the alveolar capillary membrane, parenchymal edema, and decreased gas exchange. Our group has previously shown that PP2A inhibition augments IL-8 production in human bronchial alveolar epithelial cells suggesting that modulation of PP2A activity could affect consequent chemokine production via MAPK regulation (6). Herein we provide data that demonstrate pharmacologic activation of PP2A using N6-CPA decreased the production of the primary murine neutrophil chemotactic chemokine CXCL1, reduced neutrophil infiltration, and attenuated lung inflammation in direct endotoxin-induced and live bacteria murine models of ARDS.
N6-CPA, a selective adenosine A1 receptor agonist, has previously been shown to result in PP2A activation in both rat myocardial and hippocampal cells (2, 19, 20). Murine alveolar macrophages are the primary resident immune cells responsible for the initial production of proinflammatory cytokines in response inflammatory insult. Activation of A1 receptors using A1-specific agonists and the subsequent effects on PP2A's regulation of the inflammatory response in direct models of acute lung injury has not previously been studied to our knowledge. Pretreatment with the adenosine A1 receptor-specific agonist 2-chloro-N6-cyclopentyladenosine (CCPA) before ischemia-reperfusion injury has been shown to be protective against pulmonary edema, neutrophil infiltration and cytokine levels in BALF (9). Treatment with either adenosine A1, A2A, or A3 receptor agonists during the reperfusion phase of an ischemia-reperfusion model of lung injury have also shown similar anti-inflammatory effects (12). We used an alveolar macrophage cell line to model this process and showed that N6-CPA pretreatment resulted in increased PP2A activity in a time- and dose-dependent manner (Fig. 1). Desensitization of adenosine A1 receptors through both G-protein receptor uncoupling and receptor internalization acts as a homeostatic negative feedback loop and has been previously reported to occur following adenosine A1 receptor stimulation with pharmacologic agonists (3, 4). Here we observed a similar phenomenon whereby PP2A activity in cells that received more than 1 h of 10 μM N6-CPA treatment returned to basal levels suggesting receptor desensitization (Fig. 1). This result may have important consequences on the treatment frequency employing this therapeutic target. In addition, we show that N6-CPA treatment increases the PP2A activity of cultured primary alveolar epithelial cells (Fig. 1C). These data suggest that intratracheal administration of PP2A activators has the potential to broadly modulate PP2A activity in additional cell types beyond alveolar macrophages and could help to explain the significant attenuation of inflammation we observe in our murine models of ARDS. Lastly, we also demonstrate pretreatment of MH-S cells with FTY-720, a sphingosine 1-phosphate-1 receptor modulator that has previously been shown to increase PP2A activity through multiple mechanisms, also results in attenuated production of both CXCL1 and CXCL2 following (Fig. 1, E and F) endotoxin challenge in MH-S cells (7).
Previously, our group showed that PP2A was a key regulator of the production of IL-8 in human alveolar epithelial cells (6). PP2A regulates the activation of terminal MAPKs, largely through the dephosphorylation of upstream c-Jun NH2-terminal kinases (JNKs) (40). Our data indicated that in MH-S cells increased PP2A activity results in accelerated dephosphorylation kinetics of the terminal MAPK JNK. JNK has previously been shown to play a role in regulating the production of both IL-8 and CXCL1 through the transcriptional activator AP-1 (21). Our data clearly show that PP2A activation blunted terminal MAPK JNK inflammatory signaling that occurred following MH-S cell incubation with endotoxin and ultimately limited the production of the proinflammatory chemotactic chemokines CXCL1 (Fig. 1, D and E) and CXCL2 (Fig. 1F).
Having established that PP2A activation inhibited production of CXCL1 in a murine alveolar macrophage cell line, we sought to determine if pharmacologic activation of PP2A could block inflammation in an in vivo model of ARDS. Direct intratracheal instillation of endotoxin is an established small animal model of ARDS that accurately reproduces the profound neutrophil infiltration characteristic of the pathology of human ARDS (23, 35). Our data show that PP2A activation inhibited production of proinflammatory CXCL1 and reduced neutrophil infiltration into the interalveolar space (Fig. 2). Histological examination of lung sections showed pharmacologic PP2A activation before endotoxin challenge resulted in decreased hyaline membrane deposition, attenuated neutrophil infiltration, and preserved normal lung architecture (Fig. 3). Together, these data extend our observations from the in vitro, cell culture model to further show that PP2A activation attenuates endotoxin-induced inflammation in an in vivo murine model of ARDS.
While PP2A activation before inflammation in both a cell culture model and small animal model certainly serves to provide mechanistic insight into its role in the regulation of lung inflammation, the majority of patients succumbing to ARDS are unlikely to benefit from prophylactic treatment of an impending respiratory crisis. Instead, therapeutic treatment for early and evolving ARDS remains an important goal. We therefore addressed the more clinically relevant question as to the impact of pharmacologic PP2A activation on propagation of pulmonary inflammation following lung infection. We showed that PP2A activation, 4 h following initiation of direct lung injury using live P. aeruginosa bacteria, attenuated CXCL1 production and prevented neutrophil infiltration (Fig. 4). PP2A makes up as much as 1% of total cellular proteins, and as such it is reasonable to conclude that even marginal increases in PP2A activity could have a significant impact on intracellular signaling pathways (37). These data show that PP2A activation could be an effective strategy to modulate the inflammatory response of patients with ARDS.
A limitation of these studies is the potential for fundamental differences in the immune response between mice and humans in the context of pulmonary inflammation. ARDS is a complex process resultant of a dysfunctional immune response in response to both direct and indirect causes of lung injury. While we use both endotoxin and live bacteria murine models of direct lung injury, both primarily act through Toll-like receptor 4 (TLR4) signaling. Further studies are needed to better understand the role of PP2A in regulating the innate inflammatory response to stimuli that act through other signaling pathways such as gram-positive bacteria, viruses, and damage-associated molecular pattern molecules (DAMPs). Additionally, we will need to investigate the effect of PP2A activation on bacterial clearance or animal survival and the long-term impact of PP2A activation on lung remodeling, as well as the mechanism by which N6-CPA activates PP2A in alveolar macrophages. These studies were beyond the scope of this manuscript and are the subject of ongoing studies in our laboratory. Here we have provided evidence that PP2A activation reduces CXCL1 production by murine alveolar macrophages suggesting that PP2A activation can both prevent and rescue pulmonary inflammation in both a direct endotoxin and live bacterial in vivo model of ARDS.
GRANTS
This work was supported by a National Institutes of Health Grants R01-GM-066839 (to T. P. Shanely) and K12-HD-047349 and K08-HD-062142 (to T. T. Cornell).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
W.M.M., T.P.S., and T.T.C. conception and design of research; W.M.M., W.W.R., A.J.F., P.E.R., S.P.R., and L.S. performed experiments; W.M.M., W.W.R., A.J.F., P.E.R., S.P.R., L.S., T.P.S., and T.T.C. analyzed data; W.M.M., W.W.R., A.J.F., P.E.R., S.P.R., L.S., T.P.S., and T.T.C. interpreted results of experiments; W.M.M., W.W.R., A.J.F., and S.P.R. prepared figures; W.M.M. and A.J.F. drafted manuscript; W.M.M., T.P.S., and T.T.C. edited and revised manuscript; W.M.M. and T.T.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ann Marie Levine for generously providing the clinical P. aeruginosa isolates used in these studies. We also thank Dr. Nicholas Lukacs and Susan Morris for providing technical assistance and reagents to isolate primary alveolar epithelial cells used in these studies.
REFERENCES
- 1.Ashbaugh D, Boyd Bigelow D, Petty T, Levine B. Acute repiratory distress in adults. Lancet 2: 319–323, 1967. [DOI] [PubMed] [Google Scholar]
- 2.Brust TB, Cayabyab FS, Zhou N, MacVicar BA. p38 Mitogen-activated protein kinase contributes to adenosine A1 receptor-mediated synaptic depression in area CA1 of the rat hippocampus. J Neurosci 26: 12427–12438, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandrasekera PC, Wan TC, Gizewski ET, Auchampach JA, Lasley RD. Adenosine A1 receptors heterodimerize with β1- and β2-adrenergic receptors creating novel receptor complexes with altered G protein coupling and signaling. Cell Signal 25: 736–742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y, Tao Y, Sun J, Wang Y, Xu X, Chen J, Chi ZQ, Liu JG. Adenosine A1 receptor agonist N6-cyclohexyl-adenosine induced phosphorylation of delta opioid receptor and desensitization of its signaling. Acta Pharmacol Sin 31: 784–790, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA. High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome. Infect Immun 61: 4553–4559, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornell TT, Hinkovska-Galcheva V, Sun L, Cai Q, Hershenson MB, Vanway S, Shanley TP. Ceramide-dependent PP2A regulation of TNFα-induced IL-8 production in respiratory epithelial cells. Am J Physiol Lung Cell Mol Physiol 296: L849–L856, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristóbal I, Madoz-Gúrpide J, Manso R, González-Alonso P, Rojo F, García-Foncillas J. Potential anti-tumor effects of FTY720 associated with PP2A activation: a brief review. Curr Med Res Opin 32: 1137–1141, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly SC, Haslett C, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, Pollok AJ, Grant IS. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 341: 643–647, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez LG, Sharma AK, LaPar DJ, Kron IL, Laubach VE. Adenosine A1 receptor activation attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg 145: 1654–1659, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury. Am J Respir Crit Care Med 171: 995–1001, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Fudala R, Krupa A, Stankowska D, Allen Timothy C, Kurdowska AK. Anti-interleukin-8 autoantibody:interleukin-8 immune complexes in acute lung injury/acute respiratory distress syndrome. Clin Sci 114: 403–412, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Gazoni LM, Walters DM, Unger EB, Linden J, Kron IL, Laubach VE. Activation of A1, A2A, or A3 adenosine receptors attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg 140: 440–446, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med 17: 293–307, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181: 394–418, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorens PG, Van Damme J, De Backer W, Bossaert L, De Jongh RF, Herman AG, Rampart M. Interleukin 8 (IL-8) in the bronchoalveolar lavage fluid from patients with the adult respiratory distress syndrome (ARDS) and patients at risk for ARDS. Cytokine 4: 592–597, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol 12: 186–192, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Kurdowska A, Noble JM, Grant IS, Robertson CR, Haslett C, Donnelly SC. Anti-interleukin-8 autoantibodies in patients at risk for acute respiratory distress syndrome. Crit Care Med 30: 2335–2337, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Kurdowska A, Noble JM, Steinberg KP, Ruzinski JT, Hudson LD, Martin TR. Anti-interleukin 8 autoantibody: interleukin 8 complexes in the acute respiratory distress syndrome. Relationship between the complexes and clinical disease activity. Am J Respir Crit Care Med 163: 463–468, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Hofmann PA. Antiadrenergic effects of adenosine A1 receptor-mediated protein phosphatase 2a activation in the heart. Am J Physiol Heart Circ Physiol 283: H1314–H1321, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Hofmann PA. Modulation of protein phosphatase 2A by adenosine A1 receptors in cardiomyocytes: role for p38 MAPK. Am J Physiol Heart Circ Physiol 285: H97–H103, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Lo HM, Shieh JM, Chen CL, Tsou CJ, Wu WB. Vascular endothelial growth factor induces CXCL1 chemokine release via JNK and PI-3K-dependent pathways in human lung carcinoma epithelial cells. Int J Mol Sci 14: 10090–10106, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall M, Anilkumar N, Layland J, Walker SJ, Kentish JC, Shah AM, Cave AC. Protein phosphatase 2A contributes to the cardiac dysfunction induced by endotoxemia. Cardiovasc Res 82: 67–76, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messier EM, Mason RJ, Kosmider B. Efficient and rapid isolation and purification of mouse alveolar type II epithelial cells. Exp Lung Res 38: 363–373, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Miller EJ, Cohen AB, Matthay MA. Increased interleukin-8 concentrations in the pulmonary edema fluid of patients with acute respiratory distress syndrome from sepsis. Crit Care Med 24: 1448–1454, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Miller EJ, Cohen AB, Nagao S, Griffith D, Maunder RJ, Martin TR, Weiner-Kronish JP, Sticherling M, Christophers E, Matthay MA. Elevated levels of NAP-1/interleukin-8 are present in the airspaces of patients with the adult respiratory distress syndrome and are associated with increased mortality. Am Rev Respir Dis 146: 427–432, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Reyes JG, Robayna IG, Delgado PS, Gonzalez IH, Aguiar JQ, Rosas FE, Fanjul LF, Galarreta CM. c-Jun is a downstream target for ceramide-activated protein phosphatase in A431 cells. J Biol Chem 271: 21375–21380, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanley TP, Schmal H, Warner RL, Schmid E, Friedl HP, Ward PA. Requirement for C-X-C chemokines (macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant) in IgG immune complex-induced lung injury. J Immunol 158: 3439–3448, 1997. [PubMed] [Google Scholar]
- 31.Shanley TP, Vasi N, Denenberg A, Wong HR. The serine/threonine phosphatase, PP2A: endogenous regulator of inflammatory cell signaling. J Immunol 166: 966–972, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Snabaitis AK, D'Mello R, Dashnyam S, Avkiran M. A novel role for protein phosphatase 2A in receptor-mediated regulation of the cardiac sarcolemmal Na+/H+ exchanger NHE1. J Biol Chem 281: 20252–20262, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Su X, Looney M, Robriquet L, Fang X, Matthay MA. Direct visual instillation as a method for efficient delivery of fluid into the distal airspaces of anesthetized mice. Exp Lung Res 30: 479–493, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Tikh EI, Fenton RA, Chen JF, Schwarzschild MA, Dobson JG Jr. Adenosine A1 and A2A receptor regulation of protein phosphatase 2A in the murine heart. J Cell Physiol 216: 83–90, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Ulich TR, Watson LR, Yin SM, Guo KZ, Wang P, Thang H, del Castillo J. The intratracheal administration of endotoxin and cytokines I Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am J Pathol 138: 1485–1496, 1991. [PMC free article] [PubMed] [Google Scholar]
- 36.Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care 20: 3–9, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Virshup DM. Protein phosphatase 2A: a panoply of enzymes. Curr Opin Cell Biol 12: 180–185, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Yokoi K, Mukaida N, Harada A, Watanabe Y, Matsushima K. Prevention of endotoxemia-induced acute respiratory distress syndrome-like lung injury in rabbits by a monoclonal antibody to IL-8. Lab Invest 76: 375–384, 1997. [PubMed] [Google Scholar]
- 40.Zhao B, Sun L, Haas M, Denenberg AG, Wong HR, Shanley TP. PP2A regulates upstream members of the c-jun N-terminal kinase mitogen-activated protein kinase signaling pathway. Shock 29: 181–188, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics 124: 87–95, 2009. [DOI] [PubMed] [Google Scholar]