Abstract
Human lung fibroblasts (HLFs) act as innate immune sentinel cells that amplify the inflammatory response to injurious stimuli. Here, we use targeted lipidomics to explore the hypothesis that HLFs also play an active role in the resolution of inflammation. We detected cyclooxygenase-2 (COX-2)-dependent production of both proinflammatory and proresolving prostaglandins (PGs) in conditioned culture medium from HLFs treated with a proinflammatory stimulus, IL-1β. Among the proresolving PGs in the HLF lipidome were several known ligands for peroxisome proliferator-activated receptor-γ (PPARγ), a transcription factor whose activation in the lung yields potent anti-inflammatory, antifibrotic, and proresolving effects. Next, we used a cell-based luciferase reporter to confirm the ability of HLF supernatants to activate PPARγ, demonstrating, for the first time, that primary HLFs activated with proinflammatory IL-1β or cigarette smoke extract produce functional PPARγ ligands; this phenomenon is temporally regulated, COX-2- and lipocalin-type PGD synthase-dependent, and enhanced by arachidonic acid supplementation. Finally, we used luciferase reporter assays to show that several of the PGs in the lipidome of activated HLFs independently activate PPARγ and/or inhibit NFκB. These results indicate that HLFs, as immune sentinels, regulate both proinflammatory and proresolving responses to injurious stimuli. This novel endogenous resolution pathway represents a new therapeutic target for globally important inflammatory diseases such as chronic obstructive pulmonary disease.
Keywords: primary human lung fibroblasts, peroxisome proliferator-activated receptor-γ, specialized proresolving mediators, lipidomics, NFκB
acute inflammation, the archetypal physiological response to injury, is intended to protect the host by orchestrating the entry of innate immune cells into injured tissue to mitigate damage and initiate repair. The inflammatory response must be carefully regulated, as uncontrolled acute inflammation or unresolved chronic inflammation can also cause tissue injury, loss of function, morbidity, and mortality. In the lung, unchecked acute inflammation can cause life-threatening impairment of gas exchange, while unresolved chronic inflammatory states contribute to important diseases, such as chronic obstructive pulmonary disease (COPD), asthma, and cancer (1, 44, 84). Despite the global importance of these chronic inflammatory lung diseases, there remains an urgent need for therapies that effectively curtail disease progression and reduce mortality. In the last decade, the quest to find such therapies has been radically transformed by the study of endogenous pathways that facilitate the resolution of inflammation. Long considered a passive process, resolution is now recognized as an active process mediated largely by specialized proresolving mediators derived from ω-3 and ω-6 polyunsaturated fatty acids (PUFAs) (6, 13, 70, 71). Studies on resolution indicate that injured innate immune cells initially respond with proinflammatory signals and later switch to proresolving signals (46).
Interstitial human lung fibroblasts (HLFs) are mesenchymal cells best known for secreting the extracellular matrix scaffolding that gives the lung its structural integrity and for their role in the pathogenesis of fibrosis. Notably, we previously demonstrated that HLFs also function as innate immune sentinels that respond to inhaled toxicants and pathogens by producing inflammatory mediators, amplifying the inflammatory response (38, 54). However, proresolving signaling by HLFs has not been reported. We hypothesized that, similar to other innate immune cells, HLFs would also produce proresolving lipid-derived mediators following activation. To test this hypothesis, we used a targeted lipidomics approach to generate the first reported lipid mediator profile (i.e., lipidome) of activated HLFs, presented here.
The most abundant lipid-derived mediator in the conditioned media of activated HLFs was prostaglandin (PG) E2, an eicosanoid with complex and context-dependent roles in the lung. More interestingly, the lipidome of activated HLFs also included several proresolving PGs, including known agonists of peroxisome proliferator-activated receptor (PPAR)-γ, a ligand-activated transcription factor that we have previously shown to exert potent anti-inflammatory and antifibrotic effects in the lung (26, 27, 34, 43). Similarly, a rapidly expanding body of evidence shows that PPARγ and its ligands play a vast array of proresolving roles in many organ systems (17). In this report we investigate the ability of HLFs to produce functional PPARγ ligands following exposure to activating injurious stimuli, uncovering a novel endogenous proresolving pathway with potential therapeutic implications for chronic inflammatory and/or fibrotic lung disease.
MATERIALS AND METHODS
Reagents.
Human embryonic kidney (HEK) 293FT cells (Invitrogen, Thermo Fisher Scientific, Waltham, MA) were maintained in DMEM (Life Technologies, Gaithersburg, MD) supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO), 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (Gibco, Carlsbad, CA). Recombinant human IL-1β (R & D Systems, Minneapolis, MN) was reconstituted to 5 μg/ml in 1× PBS + 0.1% BSA, divided into aliquots, stored at −80°C according to the manufacturer's specifications, and used at the concentrations indicated. The cyclooxygenase (COX)-2 inhibitors celecoxib, SC-58125, and NS-398; the lipocalin-type PGD synthase (L-PGDS) inhibitor AT-56; the hematopoietic PGD synthase (H-PDGS) inhibitor HQL-79; the NFκB inhibitors SC-514 and BAY 11-7082; and the PPARγ inhibitor GW-9662 (Cayman Chemical, Ann Arbor, MI) were prepared as stocks in DMSO, stored according to the manufacturers' instructions, and used in cell culture at the final concentrations indicated. The microsomal PGE synthase (mPGES)-1 inhibitor arzanol (Sigma-Aldrich) was diluted in DMSO and used at 0.5–2.5 μM final concentration in cell culture. Arachidonic acid (AA; Nu-Chek Prep, Elysian, MN) was prepared fresh for each experiment as a 10 mM stock solution in sterile ethanol and added to cell cultures to the final concentrations indicated. 13,14-Dihydro-15-keto-PGD2 (13,14dh-15k-PGD2), PGJ2, Δ12-PGJ2, 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2), PGA2, 13,14dh-15k-PGE2, and PGE2 (Cayman Chemical) were prepared as 10 mM stocks in DMSO and added to cell cultures to the final concentrations indicated.
Primary HLFs.
Primary HLF cultures were established from normal lung tissue of male and female donors as previously described (29) and as donated for research through the National Heart, Lung, and Blood Institute Molecular Atlas of Lung Development (LungMAP, RFA-HL-14-007) and maintained at 37°C in humidified 7% CO2 in MEM (Life Technologies) supplemented with 10% FBS (Sigma-Aldrich), 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (Gibco). Informed consent was obtained from all patient donors or next-of-kin when donation was made for research, under the approval of the University of Rochester Institutional Review Board. HLFs were used between passages 3 and 9 and were serum-starved for 24–48 h prior to treatment to reduce the effects of FBS components on induction of COX-2 and other inflammatory mediators. Cells were treated and harvested after 24–120 h. All experiments were performed in at least three different HLF strains; representative data from one experiment are shown in each figure.
Cigarette smoke extract.
Aqueous cigarette smoke extract (CSE) was freshly prepared for each experiment as previously described (36). Briefly, smoke from two research-grade reference cigarettes (1R3F, Kentucky Tobacco Research and Development Center, University of Kentucky, Lexington, KY) was bubbled through 20 ml of serum-free MEM at a rate of one cigarette per minute, and the pH of the resulting raw CSE was adjusted to 7.4 prior to sterile filtration through a 1.2-μm filter (25-mm Acrodisc, Pall, Ann Arbor, MI). Absorbance of the raw filtered CSE at 320 nm was then measured with a spectrophotometer, and CSE was diluted in serum-free MEM to the optical density at 320 nm absorbance units indicated for cell treatment.
Analysis of proinflammatory mediators.
For assessment of COX-2 protein induction by Western blotting, cells were lysed in 60 mM Tris, pH 6.8, and 2% SDS with 1× protease inhibitor cocktail (Sigma-Aldrich), and lysates were treated with 2× Laemmli sample loading buffer (Bio-Rad Laboratories, Hercules, CA) containing β-mercaptoethanol before use. Total protein (10 μg per lane) was subjected to SDS-PAGE, and protein gels were transferred to PVDF membranes (Millipore, Billerica, MA) and probed with antibodies specific for COX-2 (Cayman Chemical) and β-tubulin or GAPDH (Abcam, Cambridge, MA), which served as loading controls. Human IL-6 and IL-8 were measured in cell culture supernatants by commercial ELISA according to the manufacturer's instructions (R & D Systems). PGE2 was measured by competitive enzyme immunoassay (EIA) using commercially available reagents (Cayman Chemical) as previously described (42).
Sample preparation and liquid chromatography-mass spectrometry analysis of lipid mediators.
To generate samples for lipidomics analysis, HLFs were cultured in MEM without phenol red (Life Technologies, Gaithersburg, MD) and treated as described in the figure legends. In addition to conditioned medium, blank medium was analyzed for detection of background levels of lipids. Conditioned medium was harvested 72 h after treatment with IL-1β with or without the COX-2 inhibitor celecoxib and immediately stored at −80°C until analysis by liquid chromatography-mass spectrometry (LC-MS). Samples (500 μl) were spiked with deuterium-labeled standards that included 5 ng each of 15(S)-HETE-d8, 14(15)-EpETrE-d11, resolvin D2-d5, leukotriene B4-d4, and PGE1-d4 dissolved in methanol (adjusted to 15% in the sample) and mixed well before they were subjected to solid-phase extraction for PUFA metabolites on StrataX C18 cartridges (Phenomenex, Torrance, CA) as previously described (49, 50, 53). Briefly, the samples spiked with internal standard were applied to conditioned C18 cartridges, washed with 15% methanol in water followed by hexane, and dried under vacuum. The cartridges were then eluted with 0.5 ml of methanol, and the eluate was dried under a gentle stream of nitrogen. The residue was redissolved in 50 μl of a 1:1 mixture of methanol-25 mM aqueous ammonium acetate and subjected to LC-MS analysis.
HPLC was performed on a Prominence XR system (Shimadzu) using a Luna C18 (3 μm, 2.1 × 150 mm) column. The mobile phase consisted of a gradient between 10:85:5 (vol/vol/vol) methanol-water-acetonitrile (A) and 90:5:5 (vol/vol/vol) methanol-water-acetonitrile (B), both containing 0.1% ammonium acetate. The gradient program with respect to the composition of B was as follows: 0–1 min, 50%; 1–8 min, 50–80%; 8–15 min, 80–95%; and 15–17 min, 95%. The flow rate was 0.2 ml/min. The HPLC eluate was directly introduced to the electrospray ionization source of a mass analyzer (model QTRAP5500, AB Sciex) in the negative-ion mode under the following conditions: curtain gas 35 psi, GS1 35 psi, GS2 65 psi, temperature 600°C, ion spray voltage −1,500 V, collision gas low, declustering potential −60 V, and entrance potential −7 V. The eluate was monitored by the multiple reaction monitoring (MRM) method to detect unique molecular ion-daughter ion combinations for each of the lipid mediators using a scheduled MRM around the expected retention time for each compound. Optimized collisional energies (18–35 eV) and collision cell exit potentials (7–10 V) were used for each MRM transition. Spectra of each peak detected in the scheduled MRM were recorded using enhanced product ion scan to confirm the structural identity.
The data were collected using Analyst 1.6.2 software, and the MRM transition chromatograms were quantitated by MultiQuant software (both from AB Sciex). The internal standard signals in each chromatogram were used for normalization, recovery, and relative quantitation of each analyte. Detection limits for lipid mediators were 1–5 pg on the column, and quantitation limits were 5–15 pg on the column (signal-to-noise ratio ≥3). For background subtraction, background lipid levels detected in three blank MEM samples [calculated as mean + (2 × SD)] were subtracted from the levels detected in each of three conditioned medium samples.
Luciferase reporter assays.
A PPAR response element (PPRE)-firefly luciferase reporter construct containing three copies of a PPRE (PPRE × 3 firefly luciferase), an SV40 Renilla luciferase construct (Promega, Madison, WI), and a PPARγ2 construct (28, 90) were introduced into HEK 293FT cells cultured in T25 flasks using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). As a negative control, a construct containing constitutively expressed firefly and Renilla luciferase (psiCHECK-2, Promega) was used instead of the PPRE and SV40 reporters. After 4–6 h, cells were washed, trypsinized, plated in 96-well plates, and treated with experimental supernatants or positive or negative controls (50–100 nM rosiglitazone or 0.1–0.2% DMSO vehicle). In some experiments, treatments were applied to reporter cells in the presence and absence of the PPARγ inhibitor GW-9662. After a further 12- to 16-h overnight incubation, firefly and Renilla luciferase activities were measured using the Dual-Glo luciferase assay (Promega) and read on a Varioskan Flash luminescent plate reader (Thermo Fisher Scientific). Firefly luciferase readings were normalized to Renilla luciferase readings, and the resulting ratios were normalized to control, vehicle-treated samples for statistical analysis.
To assess the effects of specific eicosanoids on NFκB activity, HEK 293FT cells were transfected as indicated above, with substitution of an NFκB-response element firefly luciferase construct (5) for the PPRE-luciferase construct. After they were plated in 96-well plates, NFκB reporter cells were treated with eicosanoids at the concentrations indicated or the known NFκB inhibitors SC-514 (20 μM) and BAY 11-7082 (1 μM) as controls. Treatments were applied in the presence and absence of IL-1β (10 pg/ml), and results were normalized to vehicle-treated cells as described above.
Statistical analysis.
Results are reported as means ± SD. Student's two-tailed t-tests were performed using GraphPad Prism (Graph-Pad Software, La Jolla, CA; www.graphpad.com). P < 0.05 was considered significant.
RESULTS
Primary HLFs upregulate COX-2 expression and produce PGE2, IL-6, and IL-8 in response to activation with IL-1β or CSE.
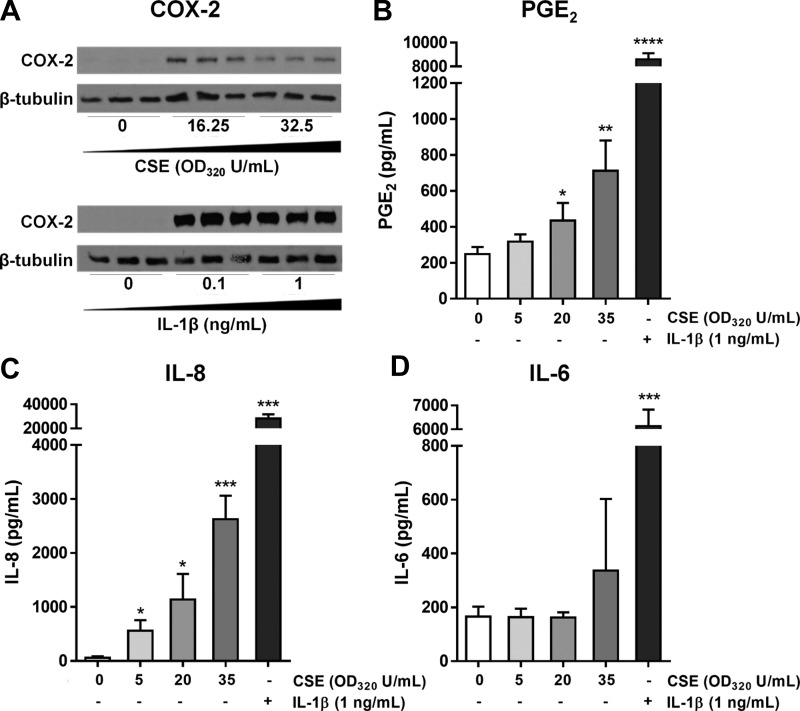
Interstitial HLFs act as “sentinels” that respond to inhaled toxicants and pathogens by producing inflammatory mediators (54). To establish a consistent and disease-relevant model of HLF activation, we exposed primary HLFs isolated from healthy lung tissue of human donors to one of two proinflammatory stimuli: CSE or IL-1β. CSE is widely used to model cigarette smoke exposure in submerged cultures, and we previously reported that HLFs activated with CSE produce IL-1β, upregulate COX-2 expression, and produce PGE2 (54). IL-1β is itself a strong HLF activator and is upregulated in acute lung inflammation, including during COPD exacerbations (24, 41, 67). Consistent with our previous findings, treatment with both CSE and IL-1β activated HLFs, as indicated by the induction of COX-2 expression (Fig. 1A), production of PGE2 (Fig. 1B), and secretion of the proinflammatory cytokines IL-6 and IL-8 (Fig. 1, C and D).
Fig. 1.
Primary human lung fibroblasts (HLFs) upregulate cyclooxygenase-2 (COX-2) expression and produce prostaglandin (PG) E2, IL-6, and IL-8 in response to activation with IL-1β or cigarette smoke extract (CSE). A: primary HLFs were treated with CSE [0, 16.25, or 32.5 optical density at 320 nm (OD320) U/ml] or IL-1β (0, 0.1, or 1 ng/ml), cells were harvested at 72 h after treatment, and COX-2 protein expression was analyzed by Western blotting. B–D: primary HLFs were treated for 24 h (B) or 48 h (C and D) with CSE or IL-1β, and supernatants were analyzed for PGE2 by enzyme immunoassay (EIA; B) and for IL-8 (C) and IL-6 (D) by ELISA. Values are means ± SD of 3 independent biological replicates per dose. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. untreated (by unpaired t-test).
IL-1β-activated primary HLFs produce both proinflammatory and proresolving eicosanoids in a COX-2-dependent manner.
Because they play a key role in the inflammatory response to inhaled injurious stimuli, we hypothesized that HLFs, similar to other innate immune cells, would produce proresolving signals following activation. To specifically investigate the production of mediators derived from membrane lipids, we subjected the conditioned medium of HLFs activated with IL-1β (with and without the COX-2 inhibitor celecoxib) to lipidomics analysis using LC-MS. Of the 136 lipids reported in the targeted analysis, 45 were detected in HLF supernatants at levels higher than background (Table 1), whereas the remaining 91 lipids were undetectable (Table 2). The levels of 17 lipids underwent statistically significant (P < 0.05) changes based on treatment; of these, 12 followed a common pattern, in that they were present at significantly higher levels in the supernatants of IL-1β- than vehicle-treated HLFs and were significantly reduced or undetectable in the supernatants of HLFs treated with IL-1β + celecoxib, indicating COX-2-dependent production of these mediators (Table 1).
Table 1.
Targeted lipidome of IL-1β-activated primary HLFs
| Lipid | HLFs + Vehicle | HLFs + IL-1β | P Value (vs. vehicle) | HLFs + IL-1β + Celecoxib | P Value (vs. IL-1β) |
|---|---|---|---|---|---|
| PGE2 | 69.93 ± 20.98 | 3,554.59 ± 264.11 | **** | 4.94 ± 9.22 | **** |
| PGA2 | nd | 332.38 ± 55.58 | *** | nd | *** |
| PGJ2 | 18.05 ± 3.53 | 181.76 ± 25.95 | *** | nd | *** |
| Δ12-PGJ2 | 13.03 ± 3.95 | 598.48 ± 74.84 | *** | 4.82 ± 8.35 | *** |
| PGF2α | nd | 39.58 ± 9.22 | *** | nd | *** |
| 15-keto PGF2α | 2.94 ± 5.10 | 204.92 ± 42.43 | ** | nd | ** |
| TXB2 | nd | 19.27 ± 3.85 | *** | nd | *** |
| 13,14dh-15k-PGD2 | 2.06 ± 3.56 | 19.70 ± 6.93 | * | nd | ** |
| 13,14dh-15k-PGE2 | nd | 174.11 ± 25.17 | *** | nd | *** |
| PGE1 | nd | 41.08 ± 12.41 | ** | nd | *** |
| 15(R)-PGE1 | 7.50 ± 1.54 | 50.40 ± 4.90 | *** | 2.57 ± 4.45 | *** |
| PGF1α | nd | 6.00 ± 0.84 | *** | nd | *** |
| 19(R)-OH PGF2α | 32.80 ± 2.68 | nd | **** | nd | |
| 15d-Δ12,14-PGJ2 | 11.60 ± 3.90 | nd | ** | 2.05 ± 3.54 | |
| 20-COOH LTB4 | 24.77 ± 7.36 | nd | ** | nd | |
| 14,15-diHETrE | 41.36 ± 11.87 | 25.61 ± 1.80 | nd | **** | |
| tetranor 12-HETE | 72.95 ± 9.96 | 34.41 ± 4.94 | ** | 12.38 ± 3.27 | ** |
| 8(S), 15(S)-diHETE | nd | 9.07 ± 15.71 | nd | ||
| 12(13)-diHOME | 34.54 ± 20.58 | 18.44 ± 16.67 | 9.63 ± 13.29 | ||
| 9(10)-epOME | nd | 3.02 ± 12.23 | nd | ||
| 14,15-epETE | 14.60 ± 12.77 | 2.93 ± 5.08 | nd | ||
| 17,18-epETE | 4.81 ± 8.33 | 5.25 ± 9.10 | nd | ||
| 8,9-epETrE | 4.88 ± 8.45 | nd | nd | ||
| 11,12-epETrE | nd | nd | 3.69 ± 6.38 | ||
| 14,15-epETrE | 6.76 ± 11.71 | 5.18 ± 8.97 | 5.48 ± 9.48 | ||
| iPF-VI | 4.66 ± 4.22 | nd | nd | ||
| 12-oxoLTB4 | nd | nd | 6.50 ± 11.25 | ||
| 9(S)-HOTrE | nd | 11.29 ± 59.74 | nd | ||
| 13(S)-HOTrE(g) | nd | 14.63 ± 25.35 | 3.85 ± 6.67 | ||
| 9-HODE | nd | 266.61 ± 934.23 | nd | ||
| 13-HODE | nd | 510.93 ± 1,656.12 | nd | ||
| 15(S)-HEPE | 6.00 ± 6.72 | 1.67 ± 11.01 | 2.39 ± 0.55 | ||
| 5-HETE | nd | 2.28 ± 3.96 | nd | ||
| 8-HETE | 5.40 ± 9.35 | nd | 4.24 ± 7.34 | ||
| 11-HETE | 2.32 ± 4.01 | 9.62 ± 3.17 | 3.40 ± 5.89 | ||
| 8(S)-HETrE | nd | 3.92 ± 6.79 | nd | ||
| 13-HDoHE | 11.90 ± 20.62 | nd | nd | ||
| 20-HDoHE | nd | 2.27 ± 3.94 | nd | ||
| 12-oxoETE | 5.44 ± 5.91 | nd | nd | ||
| 15d-Δ12,14-PGJ3 | nd | 1.65 ± 4.22 | nd | ||
| tetranor PGEM | 1.45 ± 2.51 | nd | 5.70 ± 9.88 | ||
| PGE3 | nd | 5.16 ± 8.94 | nd | ||
| 13,14dh-15k-PGF2α | nd | nd | 48.12 ± 83.35 | ||
| 19(R)-OH PGE1 | 13.62 ± 11.97 | nd | nd | ||
| RvD1 | 10.53 ± 23.10 | nd | 5.94 ± 19.26 |
Values (background-subtracted means ± SD) are presented as lipid concentration in conditioned media (pg/ml); n = 3 independent biological replicates per condition. Primary human lung fibroblasts (HLFs) were treated with IL-1β (0.1 ng/ml) with or without celecoxib (10 μM) for 72 h, and conditioned media were subjected to targeted lipidomics analysis using quantitative LC-MS. Blank medium (i.e., serum-free phenol red-free MEM, 3 replicates) was submitted and analyzed to detect background levels of lipids. Background subtraction was performed on each conditioned medium sample by subtraction of lipid levels of the blanks (mean + 2SD) from each sample measurement. Statistical analysis was performed using actual background-subtracted mediator concentrations; however, when background-subtracted concentrations were zero or negative, the result is listed as not detected (nd):
P < 0.05,
P < 0.01,
P < 0.001,
P < 0.0001 (by unpaired t-test).
Table 2.
Undetected lipids in conditioned medium of IL-1β-activated primary HLFs
| Eicosanoids | Docosanoids | Octadecanoids |
|---|---|---|
| Prostaglandins | 7(S)-maresin1 | 9,10-diHOME |
| 8-iso PGF2α | PD1 | 12(13)-epOME |
| 2,3-dinor PGE1 | 8-oxoRvD1 | 13(S)-HOTrE |
| Bicyclo PGE2 | 17-oxoRvD1 | 9-oxoOTrE |
| Bicyclo PGE1 | RvD2 | 13-oxoODE |
| 15-keto PGE2 | RvD3 | 9-oxoODE |
| PGD3 | RvD4 | |
| PGF3α | RvD5 | |
| 15-keto PGE1 | 10S,17S-diHDoHE | |
| D17-PGE1 | 4,17-diHDoHE | |
| PGD2 | 7,17-diHDoPE | |
| 11b-PGF2α | 19,20-diHDoPE | |
| 13,14dh-15k-PGE1 | 7(8)-epDPE | |
| 13,14dh-PGE1 | 10(11)-epDPE | |
| 6-keto PGE1 | 13(14)-epDPE | |
| Thromboxanes | 16(17)-epDPE | |
| 11dh-2,3-dinor TXB2 | 11-HDoHE | |
| 2,3-dinor TXB2 | 7-HDoHE | |
| 11dh-TXB3 | 10-HDoHE | |
| 11dh-TXB2 | 14-HDoHE | |
| Leukotrienes | 8-HDoHE | |
| LTB5 | 17-HDoHE | |
| LTB4 | 16-HDoHE | |
| 18-carboxy dinor LTB4 | ||
| 20-hydroxy LTB4 | ||
| Other eicosanoids | ||
| 11(R)-HEDE | ||
| 15(S)-HEDE | ||
| 15-oxo-EDE | ||
| 5,6-epETrE | ||
| Hydroxy/hydroperoxy-eicosatetraenoic acids | ||
| 5(S),6(S)-diHETE | ||
| 5(S),12(S)-diHETE | ||
| 5(S),15(S)-diHETE | ||
| 5,6-diHETE (EPA) | ||
| 8,9-epETE | ||
| 11,12-epETE | ||
| 9-HETE | ||
| 12-HETE | ||
| 15-HETE | ||
| 20-HETE | ||
| 5-oxoETE | ||
| Lipoxins | ||
| LXA4 | ||
| 15-epi LXA4 | ||
| 15-oxoLXA4 | ||
| LXA5 | ||
| LXB4 | ||
| Hydroxy/hydroperoxy-eicosatrienoic acids | ||
| 5,6-diHETrE | ||
| 8,9-diHETrE | ||
| 11,12-diHETrE | ||
| 5(S)-HETrE | ||
| 12(S)-HHTrE | ||
| Hydroxy/hydroperoxy-eicosapentaenoic acids | ||
| RvE1 | ||
| RvE3 | ||
| 5-HEPE | ||
| 9-HEPE | ||
| 8-HEPE | ||
| 11-HEPE | ||
| 12-HEPE | ||
| 18-HEPE |
Primary HLFs were treated with IL-1β (0.1 ng/ml) in the presence or absence of celecoxib (10 μM) for 72 h, and conditioned medium was subjected to targeted lipidomics analysis using quantitative LC-MS. Blank medium (i.e., serum-free phenol red-free MEM, 3 replicates) was submitted and analyzed to detect background levels of lipids. Lipids listed were nondetectable in conditioned media samples above background levels in blank medium. Background subtraction was performed on each conditioned medium sample by subtraction of lipid levels of the blanks (mean + 2SD) from each sample measurement.
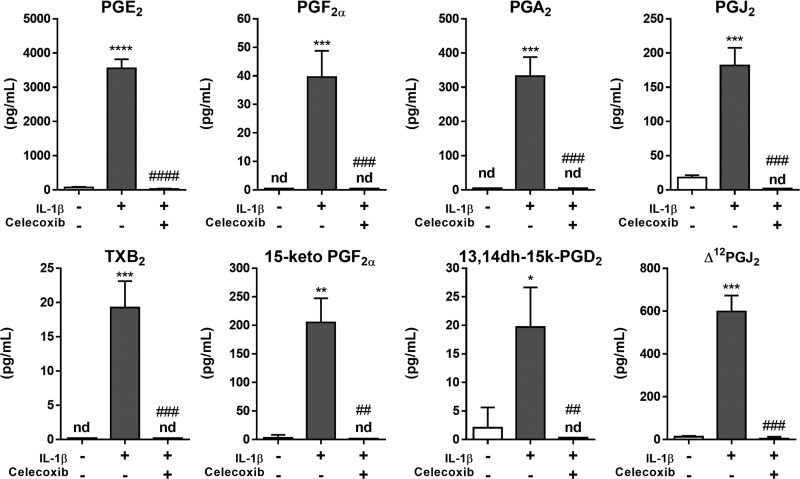
Several of the COX-2-dependent lipid mediators induced by IL-1β were classical proinflammatory eicosanoids, such as PGF2α and TXA2 (measured as TXB2) and their metabolites. PGE2 was also strongly upregulated, in agreement with our EIA data (Fig. 1B) and previously published results (54). However, the lipidome of activated HLFs also included additional PGs with known or predicted anti-inflammatory or proresolving function, including PGJ2, Δ12-PGJ2, and PGA2 (Fig. 2), which are known activators of PPARγ (89, 93). This is the first report that activated primary HLFs produce multiple endogenous PPARγ ligands with the potential to act as proresolving lipid mediators.
Fig. 2.
IL-1β-activated primary HLFs produce both proinflammatory and proresolving eicosanoids in a COX-2-dependent manner. Primary HLFs were treated with IL-1β (0.1 ng/ml) + celecoxib (10 μM) or vehicle (0.1% DMSO) for 72 h, and conditioned media were subjected to targeted lipidomics analysis using quantitative LC-MS (see materials and methods). Activated HLFs produced the pleiotropic PG PGE2 in greatest abundance; proinflammatory eicosanoids, PGF2α, 15-keto-PGF2α, and TXB2, as well as proresolving PGA2, PGJ2, Δ12-PGJ2, and PGD2 metabolites, were also detected. Values are means ± SD of 3 independent biological replicates per condition. nd, Not detected. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. vehicle alone; ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs. IL-1β + vehicle (by unpaired t-test).
Conditioned medium from IL-1β- or CSE-activated primary HLFs contains PPARγ ligands.
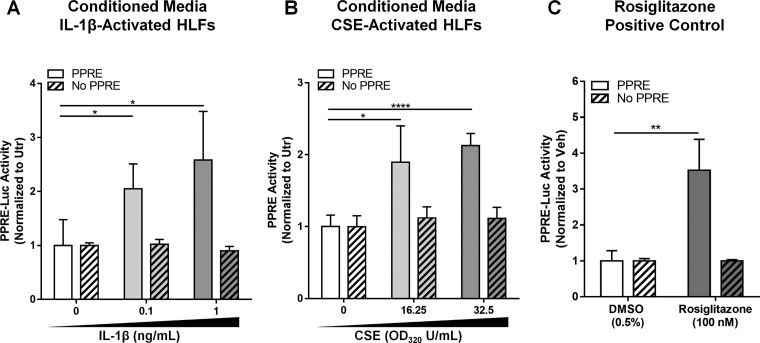
Analysis of the lipidome of activated HLFs revealed the COX-2-dependent production of several known ligands for PPARγ, a ligand-activated transcription factor that plays key roles in cellular activation and differentiation. We and others previously showed that PPARγ ligands have potent anti-inflammatory and antifibrotic effects in the lung (26, 27, 34, 43). To investigate whether HLFs produce functional PPARγ ligands, we tested supernatants of activated HLFs on a cell-based PPRE-luciferase reporter. Conditioned medium from HLFs treated with IL-1β or CSE, but not unstimulated HLFs, showed significantly elevated PPARγ activity in the reporter assay (Fig. 3), confirming for the first time the ability of HLFs to produce functional PPARγ ligands.
Fig. 3.
Conditioned medium from IL-1β- or CSE-activated primary HLFs contains peroxisome proliferator-activated receptor (PPAR)-γ (PPARγ) ligands. Primary HLFs were treated for 72 h with 0, 0.1, or 1 ng/ml IL-1β (A) or for 24 h with 0, 16.25, or 32.5 OD320 U/ml CSE (B) and then washed with 1× PBS and incubated in fresh medium for another 48 h before conditioned medium was harvested. Conditioned media were transferred to HEK 293FT cells transiently transfected with PPARγ with or without a PPAR response element (PPRE)-luciferase (Luc) reporter plasmid (see materials and methods). Conditioned media of HLFs activated with IL-1β (A) or CSE (B) activated the PPRE-luciferase reporter, indicating the presence of PPARγ ligands. Rosiglitazone (100 nM), a potent synthetic PPARγ agonist, was used as a positive control in all reporter assays (C). IL-1β alone did not activate the PPRE-luciferase reporter (data not shown). Values are means ± SD of 3 independent biological replicates per condition. *P < 0.05, **P < 0.01, ****P < 0.0001 (by unpaired t-test). Utr, untreated; Veh, vehicle.
PPARγ ligand production by IL-1β-activated HLFs is temporally regulated.
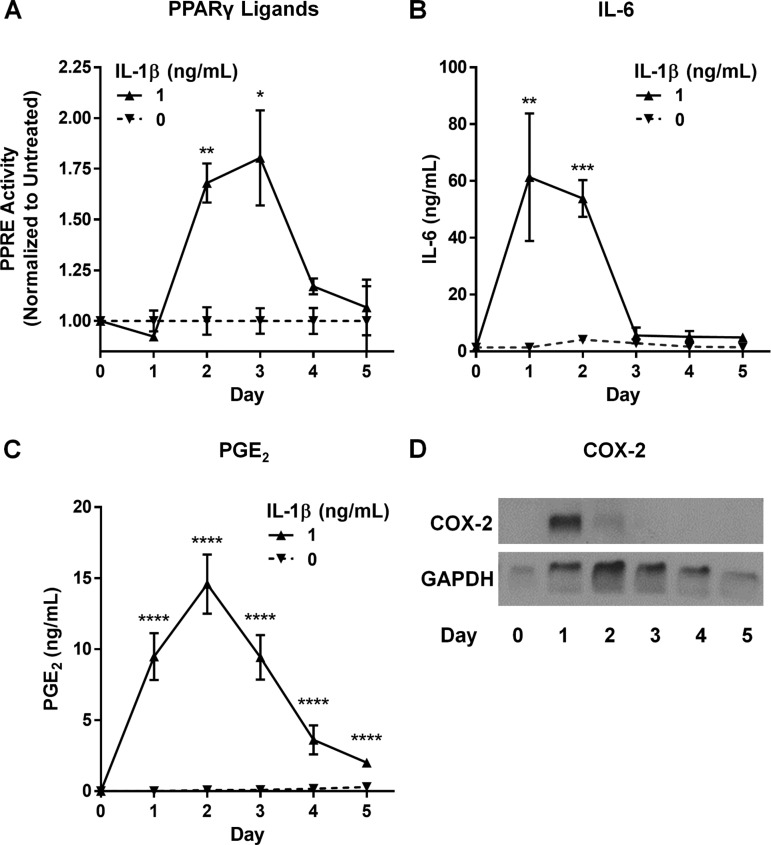
Our first experiments were performed using conditioned culture medium collected on day 3 after activation, which contained all the accumulated mediators produced over that time period. To specifically determine the kinetics of the pro- and anti-inflammatory response by activated HLFs, we treated HLFs with IL-1β and then replaced the medium with fresh medium every 24 h for 5 days. Cell lysates were also harvested every 24 h. Levels of PPARγ ligands, IL-6, and PGE2 were determined in HLF supernatants by reporter assay, ELISA, and EIA, respectively. COX-2 expression was determined in cell lysates by Western blotting. As shown in Fig. 4, IL-1β-induced IL-6 levels peak on day 1 and rapidly decline, returning to baseline by day 3. COX-2 expression also peaks on day 1, while PGE2 levels trail COX-2 induction, peaking on day 2 and then steadily declining. Production of PPARγ ligands lags COX-2 expression and peaks on day 3, 2 days after removal of the IL-1β stimulus, consistent with a potential role as promoters of resolution.
Fig. 4.
PPARγ ligand production by IL-1β-activated HLFs is temporally regulated. HLFs were treated with IL-1β (1 ng/ml) for 24 h, supernatants were removed, the cells were washed with 1× PBS, fresh medium was added; all medium was removed and replaced every 24 h thereafter. Supernatants were tested for PPARγ activity using a PPRE-luciferase reporter (A), IL-6 by ELISA (B), and PGE2 by EIA (C). In cell lysates, COX-2 protein levels were determined by Western blotting (D). PPARγ ligand production was evident by day 2 after IL-1β treatment, peaked on day 3, and returned to baseline by day 5. By contrast, IL-6 levels peaked on day 1 and returned to baseline by day 3. PGE2 levels trailed COX-2 induction, peaking on day 2 and then steadily declining. Values are means ± SD of 3–4 independent biological replicates per dose. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. untreated (by unpaired t-test).
PPARγ ligand production by IL-1β-activated HLFs is enhanced by AA supplementation.
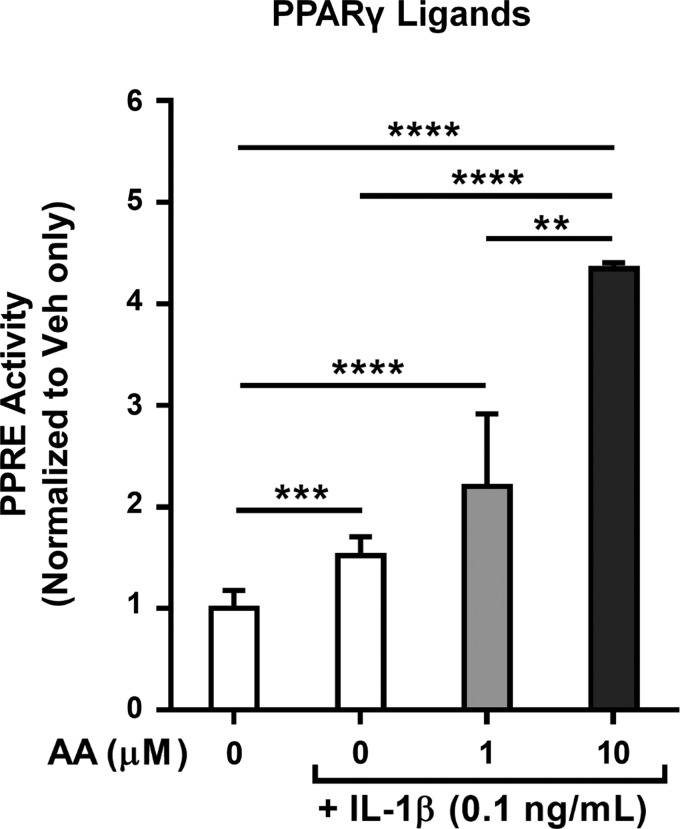
Because the known PPARγ ligands identified in the lipidome of activated HLFs are eicosanoids derived from the ω-6 PUFA AA, we anticipated that AA supplementation would enhance PPARγ ligand production by providing HLFs with additional substrate. Therefore, we next stimulated HLFs with IL-1β and varying concentrations of AA. As shown in Fig. 5, AA dramatically enhanced PPARγ ligand content in the supernatants of IL-1β-activated, but not nonactivated, HLFs. These data further support the concept of AA-derived eicosanoids acting as functional PPARγ ligands in the supernatants of activated HLFs.
Fig. 5.
PPARγ ligand production by IL-1β-activated HLFs is enhanced by arachidonic acid (AA) supplementation. Primary HLFs were treated for 72 h with 0.1 ng/ml IL-1β + vehicle (0.1% ethanol) or 0–10 μM AA; thereafter, supernatants were harvested and tested for PPARγ ligand content using a PPRE-luciferase reporter. AA dose-dependently enhanced IL-1β-induced PPARγ ligand production by HLFs. AA alone did not induce reporter activity (data not shown). Values are means ± SD of 3 independent biological replicates per condition. **P < 0.01, ***P < 0.001, ****P < 0.0001 (by unpaired t-test).
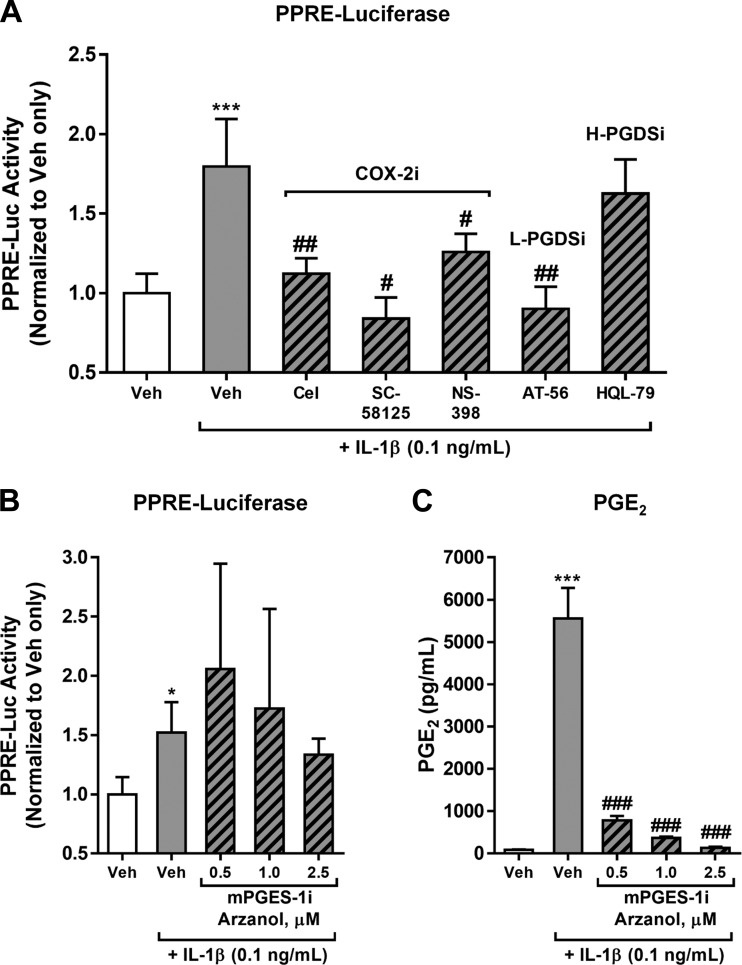
PPARγ ligand production by IL-1β-activated HLFs is dependent on COX-2 and L-PGDS, but not mPGES-1 or H-PGDS.
Many of the lipid mediators identified by LC-MS in supernatants of IL-1β-stimulated HLFs were COX-2-dependent (Table 1). To confirm that the PPARγ ligand production was also COX-2-dependent, we treated HLFs with IL-1β + one of three COX-2 inhibitors chosen to determine effectiveness across a range of selectivity for COX-2. The most selective, celecoxib, has a selectivity index (SI = ratio of COX-1 IC50 to COX-2 IC50) of ∼500; SC-58125, with intermediate selectivity has a SI of ∼150, and NS-398, with the least selectivity, has a SI of 42. Using a PPRE-luciferase reporter, we then analyzed supernatants for PPARγ ligand content. Indeed, all three COX-2 inhibitors abolished IL-1β-induced PPARγ ligand production by HLFs, confirming COX-2 dependence (Fig. 6A).
Fig. 6.
PPARγ ligand production by IL-1β-activated HLFs is dependent on COX-2 and lipocalin-type PGD synthase (L-PGDS), but not microsomal PGE synthase (mPGES-1) or hematopoietic PGDS (H-PDGS). Primary HLFs were treated for 72 h with 0.1 ng/ml IL-1β + vehicle (0.1% DMSO) or 1 of 3 selective COX-2 inhibitors (COX-2i), celecoxib (Cel) at 1 μM, SC-58125 at 1 μM, or NS-398 at 10 μM; the L-PGDS inhibitor (L-PGDSi) AT-56 at 100 μM; or the H-PGDS inhibitor (H-PGDSi) HQL-79 at 25 μM (A) or the mPGES-1 inhibitor (mPGESi) arzanol (Arz) at 0.5–2.5 μM (B). Conditioned medium was then harvested and tested for PPARγ ligand content using a PPRE-luciferase reporter (A and B) or PGE2 content was determined by EIA (C). Values are means ± SD of 3–6 independent biological replicates per condition. *P < 0.05, ***P < 0.001 vs. vehicle alone; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. IL-1β + vehicle (by unpaired t-test).
Because the known PPARγ ligands we identified in the lipidome of activated HLFs included metabolites of both PGE2 (i.e., PGA2) and PGD2 (i.e., PGJ2 and Δ12-PGJ2), we also used pharmacological inhibition to evaluate the relative contributions of these families of PGs to overall PPARγ activity. As shown in Fig. 6, inhibition of L-PGDS using AT-56 completely abrogated IL-1β-induced PPARγ ligand production by HLFs. On the other hand, inhibitors of H-PGDS (HQL-79) and mPGES-1 (arzanol) did not dampen IL-1β-induced PPARγ ligand production, suggesting that products of H-PGDS constitute the majority of the net PPARγ ligand content in HLF supernatants.
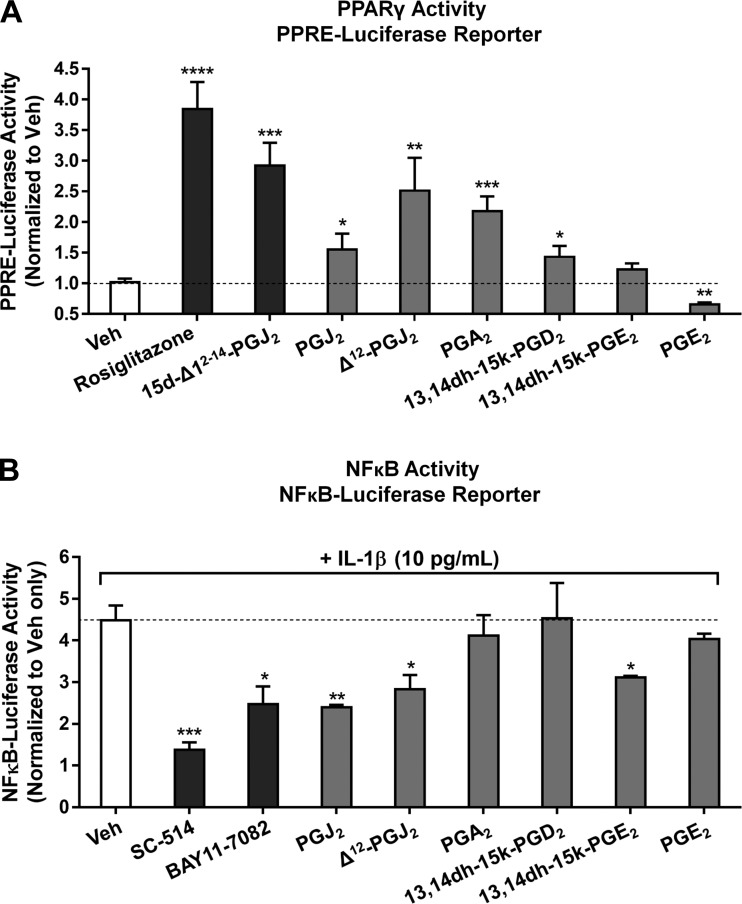
Activated HLF-derived PGs and PG metabolites activate PPARγ and inhibit NFκB.
Several of the mediators we identified in the lipidome of activated HLFs have previously been reported to have PPARγ ligand activity. We next wanted to determine whether these mediators contributed to functional activation of the PPRE-luciferase reporter by conditioned HLF medium. Therefore, we evaluated several key compounds to determine if they could directly activate the PPRE reporter. Four PGs (PGJ2, Δ12-PGJ2, 13,14dh-15k-PGD2, and PGA2) significantly induced PPRE-luciferase reporter activity, indicating activation of PPARγ. Interestingly, PGE2 significantly inhibited baseline PPRE-luciferase activity, whereas its metabolite, 13,14dh-15k-PGE2, had no significant effect on reporter activity.
In addition to activating PPREs, many PPARγ ligands have been reported to exert anti-inflammatory effects via PPARγ-dependent transrepression of NFκB (82). To identify HLF-derived PGs with inhibitory effects on NFκB, we used a cell-based NFκB response element-luciferase reporter treated with IL-1β in the presence of known NFκB inhibitors as positive controls or purified HLF-derived PGs and PG metabolites. Three HLF-derived PGs, PGJ2, Δ12-PGJ2, and 13,14dh-15k-PGE2, significantly inhibited IL-1β-induced NFκB activity in the reporter, whereas PGA2, PGE2, and 13,14dh-15k-PGE2 did not (Fig. 7B). Together, these results establish for the first time that primary HLFs activated with a proinflammatory stimulus produce proresolving PGs with PPARγ-activating and NFκB-inhibiting activities.
Fig. 7.
HLF-derived PGs and PG metabolites activate PPARγ and inhibit NFκB. A: PGs and PG metabolites identified in the lipidome of activated HLFs [or vehicle (0.1% DMSO)] were applied at 5 μM final concentration to a PPRE-luciferase reporter in the presence of the PPARγ inhibitor GW-9662 (10 μM) or vehicle (0.1% DMSO); rosiglitazone (50 nM) and 15d-PGJ2 (1 μM) were used as positive controls. After 16 h of incubation, the ratio of firefly to Renilla luciferase activity was calculated in the presence and absence of GW-9662 and normalized as described in materials and methods. B: PGs and PG metabolites identified in the lipidome of activated HLFs [or vehicle (0.1% DMSO)] were applied at 5 μM final concentration to a NFκB-response element-luciferase reporter for 1 h prior to addition of IL-1β (10 pg/ml) or vehicle (PBS), and reporter cells were incubated for an additional 16 h prior to determination of firefly and Renilla luciferase levels as described in materials and methods. The NFκB inhibitors SC-514 (20 μM) and BAY 11-7082 (1 μM) were used as controls. Values are means ± SD of 3 independent biological replicates per condition. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. vehicle alone (by unpaired t-test).
DISCUSSION
Interstitial lung fibroblasts are mesenchymal cells best known for their roles in the healing, repair, and pathogenesis of fibrotic disease. By producing extracellular matrix, HLFs provide the lung with structural integrity and form the scaffolding on which other lung cells rest. Moreover, we and others have reported that HLFs play an important role as innate immune sentinel cells that respond to inhaled toxicants and pathogens by expressing inducible COX-2 and producing eicosanoids, such as PGE2, and proinflammatory cytokines, such as IL-6 and IL-8, amplifying the acute inflammatory response (19, 38, 54). Acute inflammation, the prototypical physiological response to injury, is intended to protect the host. On the other hand, excessive inflammation can cause tissue damage and impair gas exchange. Moreover, failure to resolve inflammation can lead to a chronic inflammatory state that underlies important lung diseases, such as COPD, asthma, and cancer (1, 44, 84). It is thus critical that both the magnitude and duration of pulmonary inflammation be precisely controlled. Detailed studies of experimental and clinical exudates, followed by mechanistic studies in several organ systems, have shown that innate immune cells respond to injurious stimuli first with proinflammatory signals and then with an active switch to proresolving signals (46). This discovery has buttressed a paradigm shift in the understanding of resolution: more than a mere passive waning of proinflammatory signals, resolution is mediated largely by active proresolving signals, and investigators increasingly appreciate the therapeutic potential of harnessing these signals in the lung for treatment of inflammatory pulmonary diseases (13, 47, 48, 70–72).
Much of the published literature on HLFs emphasizes their roles in fibrosis or as proinflammatory innate immune sentinels; their contribution to the resolution of inflammation has not been thoroughly investigated. However, studies in other organ systems have begun to unveil key functions for fibroblasts in regulating inflammation. For instance, fibroblasts exert a pivotal immunomodulatory role in the spleen, where they choreograph the differentiation of regulatory dendritic cells (83, 95). Moreover, fibroblast-derived chemokines and growth factors direct the trafficking, retention, and egress of leukocytes recruited to inflamed tissue, and dysfunctional fibroblast-leukocyte interactions contribute to a variety of chronic inflammatory diseases (reviewed in Ref. 58). Given this information and the well-established role of HLFs as sentinels, we hypothesized that, similar to bona fide innate immune cells, HLFs might produce proresolving mediators following injury or activation. We found that activated primary HLFs indeed produce both pro- and anti-inflammatory lipid mediators and that some of these mediators have PPARγ ligand activity. Furthermore, the production of anti-inflammatory mediators is temporally regulated. Overall, our results show that HLFs produce proinflammatory mediators immediately after stimulation and then transition to an anti-inflammatory and proresolving profile over the succeeding days, consistent with the proresolving pattern that has been recently described in other immune cells.
Using targeted lipidomics, we generated the first reported lipidome of activated primary HLFs, which revealed the COX-2-dependent production of several classical proinflammatory eicosanoids, such as PGF2α and TXA2 and their metabolites. The most abundant bioactive lipid detected in the supernatants of IL-1β-activated HLFs was PGE2, classically considered a proinflammatory eicosanoid because of its roles in inflammatory pain (39) and fever (66). We previously reported that proinflammatory stimuli, including IL-1β, CSE, Pseudomonas aeruginosa autoinducer N-(3-oxododecanoyl) homoserine lactone, and crystalline silica, increase PGE2 production by HLFs (5, 36, 54, 57, 79, 96). However, PGE2 is also reported to have anti-inflammatory or proresolving effects in the lung. For instance, PGE2 suppresses allergic inflammation (23, 94), and alveolar epithelium-derived PGE2 was recently shown to promote the release of microparticles laden with suppressor of cytokine signaling-3 by alveolar macrophages, which in turn dampen inflammatory signaling by alveolar epithelium (81). PGE2 impairs phagocytosis and NETosis and reduces bacterial killing by innate immune cells in vivo and in vitro (2–4, 21, 22, 65, 69). On the other hand, deletion of mPGES-1 paradoxically results in impaired bacterial killing in mouse models of pneumococcal pneumonia (20) and tuberculosis (16), underscoring the complex and seemingly disparate roles of PGE2 in the lung.
Most notably, PGA2 and several metabolites of proresolving PGD2 (i.e., 13,14dh-15k-PGD2, PGJ2, and Δ12-PGJ2) were also increased several days after stimulus, supporting our hypothesis that primary HLFs are indeed capable of producing proresolving lipid mediators following activation. A similar result was previously reported in the HFL-1 cell line and in rat lung fibroblasts treated with lipopolysaccharide (LPS) (91). PGA2 and PGJ2 are the dehydration products of PGE2 and PGD2, respectively; both of these cyclopentenones have antitumor and antiviral effects (30, 68), and PGA2 is a robust anti-inflammatory mediator in the brain, where it has been shown to inhibit the activation of murine microglia and astrocytes (68). Collectively, PGD2 and its metabolites are potent agonists of the resolution of acute inflammation (56, 60, 85).
Interestingly, among the IL-1β-induced mediators we detected in the HLF lipidome, several (i.e., PGJ2, Δ12-PGJ2, and PGA2) shared the ability to activate PPARγ. Moreover, we used a cell-based PPRE-luciferase reporter assay to confirm that HLFs activated with IL-1β or CSE produce functional PPARγ ligands (Fig. 3). While PPARγ is best known for its proadipogenic, insulin-sensitizing effects, a growing body of evidence indicates that, in addition to these well-studied functions, PPARγ and its ligands can promote many aspects of the resolution of inflammation, as we recently reviewed (17). In the lung particularly, activation of PPARγ in several cell types promotes the resolution of inflammation, making ligands attractive therapeutic candidates for chronic inflammatory and fibrotic diseases (7–10, 32, 64, 75). For instance, epithelial cell PPARγ activation promotes normal development and maturation, enhances repair following acute injury, and modulates the inflammatory response to cigarette smoke exposure (76–78, 80). In alveolar macrophages (AMs), PPARγ activation dampens cigarette smoke-induced signaling through Toll-like receptor types 2 and 4, suppresses production of proinflammatory leukotriene B4 and IL-8, and ameliorates oxidative burst in response to phorbol myristate acetate and LPS (61, 92). AMs from healthy patients constitutively express high levels of PPARγ, whereas deficient PPARγ expression has been detected in the AMs of patients with pulmonary alveolar proteinosis (12), and PPARγ deletion in murine AMs exacerbates the production of Th1 cytokines following cigarette smoke exposure (52). Activation of PPARγ also reduces LPS-induced airway neutrophilia (11, 74), promotes a phagocytic M2 macrophage phenotype and efferocytosis of apoptotic neutrophils (15, 45), and attenuates hyperoxia-induced neonatal lung injury (18, 62, 63). PPARγ ligands attenuate the metalloproteinase-antimetalloproteinase imbalance and reverse the emphysema phenotype in cigarette smoke-exposed mice. (35, 73).
In addition to having paracrine effects on other lung cell types, HLF-derived PPARγ ligands may exert autocrine proresolving effects on lung fibroblasts themselves. We previously showed that PPARγ ligands induce the expression by primary HLFs of the key antioxidant enzyme heme oxygenase-1 in a PPARγ-dependent manner (27) and that, in primary HLFs activated with IL-1β or crystalline silica, PPARγ ligands inhibit production of IL-6, monocyte chemoattractant protein-1, COX-2, and PGE2 in a PPARγ-independent manner (34). Moreover, we and others previously demonstrated that exogenously administered PPARγ ligands exert potent antifibrotic effects in the lung (14, 26, 31, 43, 51, 55, 57). Notably, PGE2 is also well known to have antifibrotic effects in the lung (25, 37, 59, 87) and might act additively or synergistically with these PPARγ ligands to inhibit pulmonary fibrosis. Nevertheless, our discovery of an endogenous source of PPARγ ligands discloses a novel proresolving pathway with potential therapeutic implications in chronic inflammatory or fibrotic lung disease.
We tested individual purified putative PPARγ ligands in our PPRE-luciferase reporter to determine whether they could independently activate PPARγ, and we found that PGJ2, Δ12-PGJ2, and PGA2 acted as PPARγ ligands, consistent with previous reports. Additionally, we found modest PPARγ ligand activity by the product of enzymatic metabolism of PGD2 by PG-15-dehydrogenase (PG-15-dh) and 13,14-reductase, 13,14dh-15k-PGD2; this is, to our knowledge, the first report of PPARγ ligand activity by this metabolite. Additionally, we found a significant inhibitory effect of PGE2 on PPRE-luciferase activity in our system. While there is abundant research to show that PPARγ activation negatively regulates PGE2 production, the inhibition of PPARγ by PGE2 is, to our knowledge, an original finding. This finding suggests that PPARγ and PGE2 may exert negative feedback on one another; such a relationship has been suggested in lung cancer (33). Interestingly, the mPGES-1 inhibitor arzanol did not dampen the overall capacity of HLF supernatants to activate PPARγ, although at least one PGE2 metabolite (i.e., PGA2) is a known PPARγ ligand. This lack of inhibition of PPRE activity by arzanol may reflect the absence of PGE2, which apparently exerts inhibitory effects on the PPRE reporter as noted above. Moreover, inhibition of PGE2 synthesis may spare the common substrate PGH2 for the production of stronger PPARγ ligands derived from PGD2.
In addition to PPRE-dependent effects, PPARγ ligands exert anti-inflammatory effects independent of PPARγ binding PPREs; some of these ligands can directly inhibit NFκB and/or activate PPARγ and permit it to bind to and transrepress NFκB (82). As NFκB is a master mediator of inflammation, its inhibition is the basis for many reported anti-inflammatory actions of PPARγ ligands (34, 92). Three of the PGs produced by stimulated HLFs significantly inhibited IL-1β-induced NFκB activation. Of these, two (i.e., PGJ2 and Δ12-PGJ2) also activated PPARγ. The third, the product of enzymatic metabolism of PGE2 by PG-15-dh and 13,14-reductase, 13-14dh-15k-PGE2, weakly inhibited NFκB, although it did not show ligand activity in the PPRE-luciferase reporter. This may indicate inhibition of NFκB independent of PPARγ activation. Since we used equimolar concentrations of purified PGs, it remains possible that 13-14dh-15k-PGE2 may activate PPARγ at higher or lower concentrations. These reporter experiments using purified PGs provide proof of principle that the PGs produced by activated HLFs can indeed activate PPARγ and inhibit NFκB.
In summary, lung fibroblasts play a key role in maintaining normal lung homeostasis. Until now, the contribution of fibroblasts to normal resolution and the importance of fibroblasts in dysregulated resolution leading to chronic disease have been undervalued. Our findings suggest that more than mere sentinels and amplifiers of inflammatory signals, HLFs may synchronize the complex process of resolution, initially mounting a defensive inflammatory response to injury, but later acting as negative regulators to keep the response in check. Accordingly, defective downregulation of inflammation by fibroblasts might contribute to acute and chronic inflammatory lung diseases, such as acute respiratory distress syndrome, asthma, and COPD. Importantly, the production of PPARγ ligands by IL-1β-activated HLFs is COX-2-dependent, underscoring the key role of this enzyme in mediating the production of not only proinflammatory, but potentially proresolving and anti-fibrotic, eicosanoids. In fact, several investigators studying idiopathic pulmonary fibrosis have found deficient COX-2 activity and resulting diminished PGE2 production by lung fibroblasts grown from fibrotic lung tissue compared with those isolated from normal lung tissue (40, 86, 88). Our findings thus have potential implications for idiopathic pulmonary fibrosis, where the deficient production of antifibrotic PPARγ ligands may also result from COX-2 deficiency. Overall, our findings open new avenues of research into how lung fibroblasts might coordinate with lung resident and transient inflammatory cells to promote resolution and maintain homeostasis.
GRANTS
This work was supported by National Institutes of Health Grants P30 ES-001247, R01 HL-120908, T32 HL-066988, U01 HL-122700, and S10 RR-027926.
DISCLAIMERS
S. H. Lacy is funded in part by the US Army Medical Department. The views expressed herein are those of the author and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
S.H.L., C.F.W., T.H.T., P.J.S., and R.P.P. developed the concept and designed the research; S.H.L. and K.R.M. performed the experiments; S.H.L., C.F.W., T.H.T., K.R.M., K.V.H., P.J.S., and R.P.P. analyzed the data; S.H.L., C.F.W., T.H.T., K.V.H., P.J.S., and R.P.P. interpreted the results of the experiments; S.H.L. prepared the figures; S.H.L. drafted the manuscript; S.H.L., C.F.W., T.H.T., K.R.M., P.J.S., and R.P.P. edited and revised the manuscript; S.H.L., C.F.W., T.H.T., K.R.M., K.V.H., P.J.S., and R.P.P. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge Drs. Gloria Pryhuber and Ravi Misra (LungMAP Human Tissue Core) for providing HLFs. We especially thank the tissue donors and their families for valuable contributions to this research.
REFERENCES
- 1.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev 18: 3–10, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Aronoff DM, Bergin IL, Lewis C, Goel D, O'Brien E, Peters-Golden M, Mancuso P. E-prostanoid 2 receptor signaling suppresses lung innate immunity against Streptococcus pneumoniae. Prostaglandins Other Lipid Mediat 98: 23–30, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol 173: 559–565, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Aronoff DM, Lewis C, Serezani CH, Eaton KA, Goel D, Phipps JC, Peters-Golden M, Mancuso P. E-prostanoid 3 receptor deletion improves pulmonary host defense and protects mice from death in severe Streptococcus pneumoniae infection. J Immunol 183: 2642–2649, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baglole CJ, Maggirwar SB, Gasiewicz TA, Thatcher TH, Phipps RP, Sime PJ. The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-κB family member RelB. J Biol Chem 283: 28944–28957, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol 16: 51–67, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker J, Delayre-Orthez C, Frossard N, Pons F. Regulation of inflammation by PPARs: a future approach to treat lung inflammatory diseases? Fundam Clin Pharmacol 20: 429–447, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Belvisi MG, Hele DJ. Peroxisome proliferator-activated receptors as novel targets in lung disease. Chest 134: 152–157, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Belvisi MG, Hele DJ, Birrell MA. Peroxisome proliferator-activated receptor-γ agonists as therapy for chronic airway inflammation. Eur J Pharmacol 533: 101–109, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Belvisi MG, Mitchell JA. Targeting PPAR receptors in the airway for the treatment of inflammatory lung disease. Br J Pharmacol 158: 994–1003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birrell MA, Patel HJ, McCluskie K, Wong S, Leonard T, Yacoub MH, Belvisi MG. PPAR-γ agonists as therapy for diseases involving airway neutrophilia. Eur Respir J 24: 18–23, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Bonfield TL, Farver CF, Barna BP, Malur A, Abraham S, Raychaudhuri B, Kavuru MS, Thomassen MJ. Peroxisome proliferator-activated receptor-γ is deficient in alveolar macrophages from patients with alveolar proteinosis. Am J Respir Cell Mol Biol 29: 677–682, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40: 315–327, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L1146–L1153, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Caito S, Yang SR, Kode A, Edirisinghe I, Rajendrasozhan S, Phipps RP, Rahman I. Rosiglitazone and 15-deoxy-Δ12,14-prostaglandin J2, PPARγ agonists, differentially regulate cigarette smoke-mediated pro-inflammatory cytokine release in monocytes/macrophages. Antioxidants Redox Signal 10: 253–260, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, Serhan CN, Behar SM, Remold HG. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med 205: 2791–2801, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croasdell A, Duffney PF, Kim N, Lacy SH, Sime PJ, Phipps RP. PPARγ and the innate immune system mediate the resolution of inflammation. PPAR Res 2015: 549691, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rehan VK. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-β and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol 296: L1031–L1041, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz A, Chepenik KP, Korn JH, Reginato AM, Jimenez SA. Differential regulation of cyclooxygenases 1 and 2 by interleukin-1β, tumor necrosis factor-α, and transforming growth factor-β1 in human lung fibroblasts. Exp Cell Res 241: 222–229, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Dolan JM, Weinberg JB, O'Brien E, Abashian A, Procario MC, Aronoff DM, Crofford LJ, Peters-Golden M, Ward L, Mancuso P. Increased lethality and defective pulmonary clearance of Streptococcus pneumoniae in microsomal prostaglandin E synthase-1-knockout mice. Am J Physiol Lung Cell Mol Physiol 310: L1111–L1120, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domingo-Gonzalez R, Katz S, Serezani CH, Moore TA, Levine AM, Moore BB. Prostaglandin E2-induced changes in alveolar macrophage scavenger receptor profiles differentially alter phagocytosis of Pseudomonas aeruginosa and Staphylococcus aureus post-bone marrow transplant. J Immunol 190: 5809–5817, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domingo-Gonzalez R, Martinez-Colon GJ, Smith AJ, Smith CK, Ballinger MN, Xia M, Murray S, Kaplan MJ, Yanik GA, Moore BB. Inhibition of neutrophil extracellular trap formation after stem cell transplant by prostaglandin E2. Am J Respir Crit Care Med 193: 186–197, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Draijer C, Boorsma CE, Reker-Smit C, Post E, Poelstra K, Melgert BN. PGE2-treated macrophages inhibit development of allergic lung inflammation in mice. J Leukoc Biol 100: 95–102, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endo T, Ogushi F, Sone S, Ogura T, Taketani Y, Hayashi Y, Ueda N, Yamamoto S. Induction of cyclooxygenase-2 is responsible for interleukin-1β-dependent prostaglandin E2 synthesis by human lung fibroblasts. Am J Respir Cell Mol Biol 12: 358–365, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Epa AP, Thatcher TH, Pollock SJ, Wahl LA, Lyda E, Kottmann RM, Phipps RP, Sime PJ. Normal human lung epithelial cells inhibit transforming growth factor-β induced myofibroblast differentiation via prostaglandin E2. PLos One 10: e0135266, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson HE, Kulkarni A, Lehmann GM, Garcia-Bates TM, Thatcher TH, Huxlin KR, Phipps RP, Sime PJ. Electrophilic peroxisome proliferator-activated receptor-γ ligands have potent antifibrotic effects in human lung fibroblasts. Am J Respir Cell Mol Biol 41: 722–730, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferguson HE, Thatcher TH, Olsen KC, Garcia-Bates TM, Baglole CJ, Kottmann RM, Strong ER, Phipps RP, Sime PJ. Peroxisome proliferator-activated receptor-γ ligands induce heme oxygenase-1 in lung fibroblasts by a PPARγ-independent, glutathione-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 297: L912–L919, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 83: 803–812, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Fries KM, Sempowski GD, Gaspari AA, Blieden T, Looney RJ, Phipps RP. CD40 expression by human fibroblasts. Clin Immunol Immunopathol 77: 42–51, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Fukushima M, Kato T, Narumiya S, Mizushima Y, Sasaki H, Terashima Y, Nishiyama Y, Santoro MG. Prostaglandin A and J: antitumor and antiviral prostaglandins. Adv Prostaglandin Thromboxane Leukot Res 19: 415–418, 1989. [PubMed] [Google Scholar]
- 31.Genovese T, Cuzzocrea S, Di Paola R, Mazzon E, Mastruzzo C, Catalano P, Sortino M, Crimi N, Caputi AP, Thiemermann C, Vancheri C. Effect of rosiglitazone and 15-deoxy-Δ12,14-prostaglandin J2 on bleomycin-induced lung injury. Eur Respir J 25: 225–234, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Gross NJ. Novel antiinflammatory therapies for COPD. Chest 142: 1300–1307, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Hazra S, Peebles KA, Sharma S, Mao JT, Dubinett SM. The role of PPAR in the cyclooxygenase pathway in lung cancer. PPAR Res 2008: 790568, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogan CM, Thatcher TH, Sapinoro RE, Gurell MN, Ferguson HE, Pollock SJ, Jones C, Phipps RP, Sime PJ. Electrophilic PPARγ ligands attenuate IL-1β and silica-induced inflammatory mediator production in human lung fibroblasts via a PPARγ-independent mechanism. PPAR Res 2011: 318134, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou G, Yin Y, Han D, Wang QY, Kang J. Rosiglitazone attenuates the metalloprotease/anti-metalloprotease imbalance in emphysema induced by cigarette smoke: involvement of extracellular signal-regulated kinase and NFκB signaling. Int J Chron Obstruct Pulm Dis 10: 715–724, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLos One 8: e58258, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E2 inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol 292: L405–L413, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman J, Graf BA, Leung EC, Pollock SJ, Koumas L, Reddy SY, Blieden TM, Smith TJ, Phipps RP. Fibroblasts as sentinel cells: role of the CDcd40-CDcd40 ligand system in fibroblast activation and lung inflammation and fibrosis. Chest 120: 53s–55s, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Kawabata A. Prostaglandin E2 and pain—an update. Biol Pharm Bull 34: 1170–1173, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Keerthisingam CB, Jenkins RG, Harrison NK, Hernandez-Rodriguez NA, Booth H, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-β in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 158: 1411–1422, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim KS, Jung H, Shin IK, Choi BR, Kim DH. Induction of interleukin-1β (IL-1β) is a critical component of lung inflammation during influenza A (H1N1) virus infection. J Med Virol 87: 1104–1112, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1− subpopulations exhibit distinct phenotypes. Eur J Immunol 32: 477–485, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Kulkarni AA, Thatcher TH, Olsen KC, Maggirwar SB, Phipps RP, Sime PJ. PPAR-γ ligands repress TGFβ-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: implications for therapy of fibrosis. PLos One 6: e15909, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lane N, Robins RA, Corne J, Fairclough L. Regulation in chronic obstructive pulmonary disease: the role of regulatory T-cells and Th17 cells. Clin Sci (Lond) 119: 75–86, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Lea S, Plumb J, Metcalfe H, Spicer D, Woodman P, Fox JC, Singh D. The effect of peroxisome proliferator-activated receptor-γ ligands on in vitro and in vivo models of COPD. Eur Respir J 43: 409–420, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol 2: 612–619, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol 76: 467–492, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy BD, Vachier I, Serhan CN. Resolution of inflammation in asthma. Clin Chest Med 33: 559–570, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maddipati KR, Romero R, Chaiworapongsa T, Zhou SL, Xu Z, Tarca AL, Kusanovic JP, Munoz H, Honn KV. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J 28: 4835–4846, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maddipati KR, Zhou SL. Stability and analysis of eicosanoids and docosanoids in tissue culture media. Prostaglandins Other Lipid Mediat 94: 59–72, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Malekinejad H, Mehrabi M, Khoramjouy M, Rezaei-Golmisheh A. Antifibrotic effect of atorvastatin on paraquat-induced pulmonary fibrosis: role of PPARγ receptors. Eur J Pharmacol 720: 294–302, 2013. [DOI] [PubMed] [Google Scholar]
- 52.Malur A, McCoy AJ, Arce S, Barna BP, Kavuru MS, Malur AG, Thomassen MJ. Deletion of PPARγ in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J Immunol 182: 5816–5822, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Markworth JF, Vella L, Lingard BS, Tull DL, Rupasinghe TW, Sinclair AJ, Maddipati KR, Cameron-Smith D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol 305: R1281–R1296, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martey CA, Pollock SJ, Turner CK, O'Reilly KM, Baglole CJ, Phipps RP, Sime PJ. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: implications for lung inflammation and cancer. Am J Physiol Lung Cell Mol Physiol 287: L981–L991, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Milam JE, Keshamouni VG, Phan SH, Hu B, Gangireddy SR, Hogaboam CM, Standiford TJ, Thannickal VJ, Reddy RC. PPAR-γ agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L891–L901, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson SM, Shay AE, James JL, Carlson BA, Urban JF Jr, Prabhu KS. Selenoprotein expression in macrophages is critical for optimal clearance of parasitic helminth Nippostrongylus brasiliensis. J Biol Chem 291: 2787–2798, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Reilly KM, Phipps RP, Thatcher TH, Graf BA, Van Kirk J, Sime PJ. Crystalline and amorphous silica differentially regulate the cyclooxygenase-prostaglandin pathway in pulmonary fibroblasts: implications for pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L1010–L1016, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, Salmon M, Buckley CD. A stromal address code defined by fibroblasts. Trends Immunol 26: 150–156, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Penke LR, Huang SK, White ES, Peters-Golden M. Prostaglandin E2 inhibits α-smooth muscle actin transcription during myofibroblast differentiation via distinct mechanisms of modulation of serum response factor and myocardin-related transcription factor-A. J Biol Chem 289: 17151–17162, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxy-Δ12,14-PGJ2. Proc Natl Acad Sci USA 104: 20979–20984, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reddy RC, Keshamouni VG, Jaigirdar SH, Zeng X, Leff T, Thannickal VJ, Standiford TJ. Deactivation of murine alveolar macrophages by peroxisome proliferator-activated receptor-γ ligands. Am J Physiol Lung Cell Mol Physiol 286: L613–L619, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Rehan VK, Sakurai R, Corral J, Krebs M, Ibe B, Ihida-Stansbury K, Torday JS. Antenatally administered PPAR-γ agonist rosiglitazone prevents hyperoxia-induced neonatal rat lung injury. Am J Physiol Lung Cell Mol Physiol 299: L672–L680, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rehan VK, Wang Y, Patel S, Santos J, Torday JS. Rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr Pulmonol 41: 558–569, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Remels AH, Gosker HR, Schrauwen P, Langen RC, Schols AM. Peroxisome proliferator-activated receptors: a therapeutic target in COPD? Eur Respir J 31: 502–508, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Rogers LM, Thelen T, Fordyce K, Bourdonnay E, Lewis C, Yu H, Zhang J, Xie J, Serezani CH, Peters-Golden M, Aronoff DM. EP4 and EP2 receptor activation of protein kinase A by prostaglandin E2 impairs macrophage phagocytosis of Clostridium sordellii. Am J Reprod Immunol 71: 34–43, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 10: 2193–2216, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Rotta Detto Loria J, Rohmann K, Droemann D, Kujath P, Rupp J, Goldmann T, Dalhoff K. Haemophilus influenzae infection upregulates the NLRP3 inflammasome and leads to caspase-1-dependent secretion of interleukin-1—a possible pathway of exacerbations in COPD. PLos One 8: e66818, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sasaguri T, Masuda J, Shimokado K, Yokota T, Kosaka C, Fujishima M, Ogata J. Prostaglandins A and J arrest the cell cycle of cultured vascular smooth muscle cells without suppression of c-myc expression. Exp Cell Res 200: 351–357, 1992. [DOI] [PubMed] [Google Scholar]
- 69.Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol 37: 562–570, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol 27: 200–215, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harbor Perspect Biol 7: a016311, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 6: 1191–1197, 2005. [DOI] [PubMed] [Google Scholar]
- 73.Shan M, You R, Yuan X, Frazier MV, Porter P, Seryshev A, Hong JS, Song LZ, Zhang Y, Hilsenbeck S, Whitehead L, Zarinkamar N, Perusich S, Corry DB, Kheradmand F. Agonistic induction of PPARγ reverses cigarette smoke-induced emphysema. J Clin Invest 124: 1371–1381, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma R, Kaundal RK, Sharma SS. Amelioration of pulmonary dysfunction and neutrophilic inflammation by PPARγ agonist in LPS-exposed guinea pigs. Pulm Pharmacol Ther 22: 183–189, 2009. [DOI] [PubMed] [Google Scholar]
- 75.Sime PJ. The antifibrogenic potential of PPARγ ligands in pulmonary fibrosis. J Investig Med 56: 534–538, 2008. [DOI] [PubMed] [Google Scholar]
- 76.Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Andalcio T, Shapiro SD, Mariani TJ. Epithelial cell PPARγ is an endogenous regulator of normal lung maturation and maintenance. Proc Am Thorac Soc 3: 510–511, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Tsai LW, Ingenito EP, Gonzalez F, Shapiro SD, Mariani TJ. Epithelial cell PPARγ contributes to normal lung maturation. FASEB J 20: 1507–1509, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Simon DM, Mariani TJ. Role of PPARs and retinoid X receptors in the regulation of lung maturation and development. PPAR Res 2007: 91240, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith RS, Kelly R, Iglewski BH, Phipps RP. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J Immunol 169: 2636–2642, 2002. [DOI] [PubMed] [Google Scholar]
- 80.Solleti SK, Simon DM, Srisuma S, Arikan MC, Bhattacharya S, Rangasamy T, Bijli KM, Rahman A, Crossno JT Jr, Shapiro SD, Mariani TJ. Airway epithelial cell PPARγ modulates cigarette smoke-induced chemokine expression and emphysema susceptibility in mice. Am J Physiol Lung Cell Mol Physiol 309: L293–L304, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Speth JM, Bourdonnay E, Penke LR, Mancuso P, Moore BB, Weinberg JB, Peters-Golden M. Alveolar epithelial cell-derived prostaglandin E2 serves as a request signal for macrophage secretion of suppressor of cytokine signaling 3 during innate inflammation. J Immunol 196: 5112–5120, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. 15-Deoxy-Δ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc Natl Acad Sci USA 97: 4844–4849, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Svensson M, Maroof A, Ato M, Kaye PM. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity 21: 805–816, 2004. [DOI] [PubMed] [Google Scholar]
- 84.Trevor JL, Deshane JS. Refractory asthma: mechanisms, targets, therapy. Allergy 69: 817–827, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trivedi SG, Newson J, Rajakariar R, Jacques TS, Hannon R, Kanaoka Y, Eguchi N, Colville-Nash P, Gilroy DW. Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity. Proc Natl Acad Sci USA 103: 5179–5184, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vancheri C, Sortino MA, Tomaselli V, Mastruzzo C, Condorelli F, Bellistri G, Pistorio MP, Canonico PL, Crimi N. Different expression of TNF-α receptors and prostaglandin E2 production in normal and fibrotic lung fibroblasts: potential implications for the evolution of the inflammatory process. Am J Respir Cell Mol Biol 22: 628–634, 2000. [DOI] [PubMed] [Google Scholar]
- 87.White ES, Atrasz RG, Dickie EG, Aronoff DM, Stambolic V, Mak TW, Moore BB, Peters-Golden M. Prostaglandin E2 inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol 32: 135–141, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest 95: 1861–1868, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Willson TM, Lehmann JM, Kliewer SA. Discovery of ligands for the nuclear peroxisome proliferator-activated receptors. Ann NY Acad Sci 804: 276–283, 1996. [DOI] [PubMed] [Google Scholar]
- 90.Woeller CF, O'Loughlin CW, Pollock SJ, Thatcher TH, Feldon SE, Phipps RP. Thy1 (CD90) controls adipogenesis by regulating activity of the Src family kinase, Fyn. FASEB J 29: 920–931, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu D, Zheng S, Li W, Yang L, Liu Y, Zheng X, Yang Y, Yang L, Wang Q, Smith FG, Jin S. Novel biphasic role of resolvin D1 on expression of cyclooxygenase-2 in lipopolysaccharide-stimulated lung fibroblasts is partly through PI3K/AKT and ERK2 pathways. Mediat Inflamm 2013: 964012, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yin Y, Hou G, Li E, Wang Q, Kang J. PPARγ agonists regulate tobacco smoke-induced Toll like receptor 4 expression in alveolar macrophages. Respir Res 15: 28, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M, Lazar MA. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem 270: 23975–23983, 1995. [DOI] [PubMed] [Google Scholar]
- 94.Zaslona Z, Okunishi K, Bourdonnay E, Domingo-Gonzalez R, Moore BB, Lukacs NW, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses allergic sensitization and lung inflammation by targeting the E prostanoid 2 receptor on T cells. J Allergy Clin Immunol 133: 379–387, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, Guo J, Huang X, Chen T, Wang J, Cao X. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol 5: 1124–1133, 2004. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y, Cao HJ, Graf B, Meekins H, Smith TJ, Phipps RP. CD40 engagement up-regulates cyclooxygenase-2 expression and prostaglandin E2 production in human lung fibroblasts. J Immunol 160: 1053–1057, 1998. [PubMed] [Google Scholar]