Abstract
Recent work from this laboratory showed that endoplasmic reticulum (ER) stress-induced apoptosis of alveolar epithelial cells (AECs) is regulated by the autocrine angiotensin (ANG)II/ANG1-7 system. The proteasome inhibitor MG132 or surfactant protein C (SP-C) BRICHOS domain mutation G100S induced apoptosis in human AECs by activating the proapoptotic cathepsin D and reducing antiapoptotic angiotensin converting enzyme-2 (ACE-2). This study tested the hypothesis that ER stress-induced apoptosis of human AECs might be mediated by influence of the unfolded protein response (UPR) on the autocrine ANGII/ANG1-7 system. A549 cells were challenged with MG132 or SP-C BRICHOS domain mutant G100S to induce ER stress and activation of UPR pathways. The results showed that either MG132 or G100S SP-C mutation activated all three canonical pathways of the UPR (IRE1/XBP1, ATF6, and PERK/eIF2α), which led to a significant increase in cathepsin D or in TACE (an ACE-2 ectodomain shedding enzyme) and eventually caused AEC apoptosis. However, ER stress-induced AEC apoptosis could be prevented by chemical chaperone or by UPR blockers. It is also suggested that ATF6 and IRE1 pathways might play important role in regulation of angiotensin system. These data demonstrate that ER stress induces apoptosis in human AECs through mediation of UPR pathways, which in turn regulate the autocrine ANGII/ANG1-7 system. They also demonstrated that ER stress-induced AEC apoptosis can be blocked by inhibition of UPR signaling pathways.
Keywords: lung injury, BRICHOS domain mutations, unfolded protein response, angiotensin system
apoptosis of alveolar epithelial cells (AECs) contributes to the pathogenesis of acute lung injury, pulmonary fibrosis, and chronic obstructive pulmonary disease (15). The concept that apoptosis is critical to lung disease pathogenesis is supported by numerous studies showing, for example, that caspase inhibitors (9, 35) or deletion of genes critical to apoptosis (1) could prevent experimental lung injury and fibrogenesis. Recent findings indicate that BRICHOS domain mutations in surfactant protein C (SP-C) induce endoplasmic reticulum (ER) stress and apoptosis in type II AECs and lead to subsequent lung fibrogenesis (32). Therefore, understanding the pathogenesis of lung injury and fibrosis requires understanding of the regulation of AEC apoptosis.
Endogenous and xenobiotic inducers of AEC apoptosis lead to the autocrine conversion of angiotensin II (ANGII) from its precursor angiotensinogen (AGT; Refs. 16, 34, 36). The apoptotic response to Fas ligand, TNF-α, or bleomycin requires the synthesis of ANGII, which is produced by AECs. Antisense oligonucleotides against AGT mRNA or neutralizing antibodies against ANGII were sufficient to block AEC apoptosis (16, 34, 36). Moreover, studies of lung biopsy specimens from patients with pulmonary fibrosis showed a dramatic increase in AGT mRNA and protein, which suggests that the generation of ANGII is important for human lung fibrosis (12).
The octapeptide ANGII is produced by the sequential cleavage of the decapeptide angiotensin I (ANGI) from the NH2-terminal end of AGT by cathepsin D, followed by a subsequent cleavage of two amino acids from COOH-terminal end of ANGI. This peptide is then further processed by removal of one final COOH-terminal amino acid by angiotensin converting enzyme 2 (ACE-2) to yield the heptapeptide angiotensin 1–7 (ANG1-7, 13). In concert with ANGII production, recent work in our laboratory showed that the ACE-2/ANG1-7/mas axis also plays an important role in regulating of AEC apoptosis (31). In a study of bleomycin-induced apoptosis of AECs, ACE-2 was shown as a protective factor by its abilities to 1) degrade the proapoptotic peptide ANGII, thus limiting its accumulation, and 2) generate the antiapoptotic peptide ANG1-7, which inhibits AEC apoptosis through the ANG1-7 receptor mas (31).
SP-C is the cleaved product of a precursor protein (proSP-C), which is synthesized by alveolar epithelial type II cells. Recent studies showed that the proSP-C contains a domain known as BRICHOS, which is thought to have chaperone-like properties that protect the peptide from aggregation. Mutations in the BRICHOS domain result in a product that cannot be processed normally in type II AECs, leading to accumulation of misfolded proSP-C in the ER. SP-C BRICHOS mutants (such as SP-CG100S, SP-CL188Q, and SP-CΔexon4) have been shown to induce the ER stress and activate the unfolded protein response (UPR), which lead to cell death and subsequent lung fibrosis (17, 22, 24).
The UPR is a signal transduction pathway that protects eukaryote cells from stress caused by the accumulation of unfolded or misfolded proteins in the ER (28). However, in the event of prolonged or severe ER stress that is not resolved, the UPR switches from protection of the cell to initiation of apoptosis (7). The UPR is comprised of signaling cascades that are governed by three ER transmembrane proteins: inositol-requiring element 1 (IRE1), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6), which remain inactive under nonstress conditions through the association with glucose-regulated protein 78/immunoglobulin heavy-chain-binding protein (GRP78/BiP; Ref. 27). On accumulation of misfolded or unfolded proteins, BiP dissociates from these three sensors, which leads to their activation and triggers the UPR (26).
This study examined the hypothesis that ER stress-induced apoptosis of human AECs might be mediated by UPR pathways that in turn modulate the autocrine ANGII/ANG1-7 system of these cells. The results reported herein demonstrate that ER stress induced by either chemical agent (MG132) or SP-C BRICHOS domain mutation (G100S) leads to the activations of all three UPR pathways, which results in an increase in cathepsin D and simultaneous activation of ACE-2 ectodomain shedding enzyme ADAM17/TACE, all of which can be prevented by the chemical chaperone sodium 4-phenylbutyrate (4-PBA).
MATERIALS AND METHODS
Reagents and materials.
Synthetic proteasome inhibitor MG132 (carboxybenzoxy-Leu-Leu-leucinal), chemical chaperone 4-PBA, IRE1 chemical inhibitor 4μ8C (8-formyl-7-hydroxy-4-methylcoumarin) and propidium iodide (PI) were purchased from Sigma Chemical (St. Louis, MO). PERK chemical inhibitor GSK2656157 was purchased from Santa Cruz Biotechnology (Dallas, TX). Antibodies for Western blotting were obtained as following: total IRE1, total PERK, total eukaryotic translational initiation factor 2 (eIF2α), cleaved caspase-3, activating transcription factor-4 (ATF4), ADAM17/TACE, CCAAT/enhancer-binding protein homologous protein (CHOP), β-actin (Cell Signaling, Beverly, MA); ACE-2, phospho-eIF2α, phospho-IRE1 (Abcam, Cambridge, MA); XBP1, phospho-PERK, ATF6, cathepsin D (Santa Cruz Biotechnology). All other reagents were purchased from Sigma Chemical or Bio-Rad (Melville, NY).
Cell culture.
The A549 human lung adenocarcinoma cell line was purchased from ATCC (Manassas, VA) and grown in 6- or 24-well chambers in Ham's F-12 medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin. The human primary alveolar epithelial cells were studied at day 2 of culture when they are morphologically and biochemically like type II cells (33). All cells were grown in 6-well or 24-well chambers and analyzed with inhibitors when they reached 70–80% confluency. For 4-PBA studies, cells were either incubated with 4-PBA (5 mM) for 1 h before treatment with MG132 (10 μM) for 24 h or transfected with SP-C plasmids for 8 h prior to treatment with 4-PBA for 48 h. For specific UPR inhibition, A549 cells when reached 70–80% confluent were exposed to 4μ8C (50 μM) or GSK2656157 (1 μM) for 1 h before treatment with MG132 (10 μM) for 24 h or transfected with SP-C plasmids for 48 h before challenge to 4μ8C or GSK2656157 for 24 h.
G100S mutant and wild-type SP-C plasmid transfection.
Human wild-type and G100S mutant SP-C DNA sequences carried in pIRES-dsRED plasmid were a kind gift from K. Morimoto of the Department of Clinical Medicine, Institute of Tropical Medicine, Nagasaki University (Nagasaki, Japan). Wild-type and G100S-containing plasmids were amplified using Plasmid Plus Maxi Kit (Qiagen, Valencia, CA) following the manufacturer's instructions. A549 cells grown to 70–80% confluence were transiently transfected with the indicated plasmid construct using Lipofectamine 2000 reagent (Invitrogen) as previously described (33).
Western blotting.
A549 cells were collected in ice-cold protein lysis buffer [50 nM Tris·HCl, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate and protease inhibitor cocktail (Complete Mini, Roche, Nutley, NJ)]. Cell suspensions were centrifuged at 13,200 rpm for 15 min at 4°C and supernatants were collected for protein detection. Protein samples were run on 7.5–12% SDS gradient polyacrylamide gels and transferred to PVDF membranes. Membranes were incubated in 5% nonfat dry milk buffer in 1 h at room temperature and incubated with primary antibodies at 4°C overnight then with secondary antibodies for 1 h at room temperature. Detection of the proteins was performed using Supersignal West Pico chemiluminescent substrate (GE Healthcare Biosciences, Pittsburgh, PA). β-Actin antibodies were used as loading control.
Knockdown of UPR pathway using siRNAs.
Antisense oligonucleotides against each human UPR sensors (ATF6, IRE1, PERK) were purchased from Santa Cruz Biotechnology. A549 cells were grown in six-well chambers until reaching 60–70% confluency then were transfected with antisense oligonucleotides against each target sensor (siATF6, siIRE1, siPERK) or with scrambled oligonucleotides (siCTL) by using Lipofectamine 2000 (Invitrogen). The oligonucleotide-to-Lipofectamine ratio was optimized by using FITC-labeled nucleotides (31) and transfection efficiencies were determined by Western blotting (data not shown). Human A549 cells were transfected with antisense oligonucleotides against UPR sensors for 24 h then treated with MG132 for 24 h or transfected with SP-C mutation for 48 h.
Detection of apoptosis.
After treatment with inhibitor, A549 cells were monitored for apoptosis by nuclear fragmentation assay using PI as described earlier (16, 33). Human AECs were fixed with 70% ethanol following a DNase-free RNase digestion in phosphate-buffered saline containing 5 μg/ml PI, and 24-well culture vessels were centrifuged to retain the detached cells during fixation with ethanol (16). Apoptotic cells were scored as cells with discrete nuclear fragments containing condensed chromatin, in a minimum of four different microscopic fields from at least three culture vessels per treatment group; in situ end labeling (ISEL) of fragmented DNA was examined for equating of apoptotic fragmented nuclei of AECs in the nuclear fragment assay (16). Caspase-3 activation, another marker of the apoptosis stage, was also measured by Western blot using antibodies specific for the active (cleaved) form of caspase-3 (31).
RESULTS
Recent work from our laboratory has shown that either the synthetic proteasome inhibitor MG132 or the G100S mutation of SP-C could upregulate BiP/GRP78 (32), which interacts with all three components of the UPR signaling pathways under cellular homeostatic conditions. Previous studies also demonstrated that 4-PBA, a well-known chemical chaperone, could rescue the mutant SP-C protein from trafficking and reduce ER stress in multiple cell types (25, 37). Therefore, we tested the role of 4-PBA in the regulation of UPR activation of AECs in response to proteasome inhibitor MG132 or to SP-C BRICHOS domain mutation G100S under similar conditions as previously reported (33).
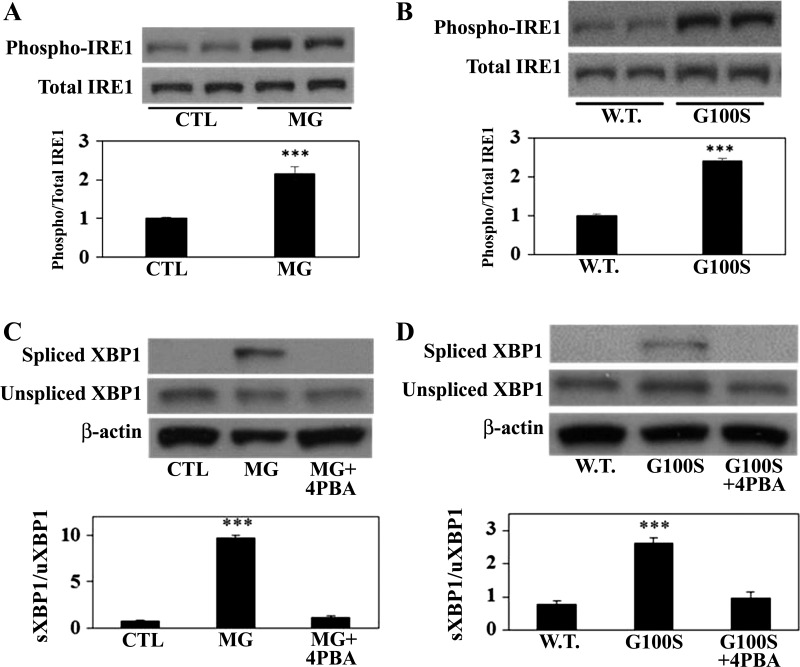
In response to ER stress, GRP78 dissociates from IRE1 which then undergoes dimerization and autophosphorylation. The data in Fig. 1 showed a significant increase of phospho-IRE1 protein in A549 cells treated with MG132 (Fig. 1A) or in A549 cells transfected with G100S SP-C mutant compared with the wild-type SP-C-expressing cells (Fig. 1B). Upon activation, phosphorylated IRE1 protein induces the splicing of X-box binding protein 1 (XBP1) mRNA (11). A549 cells exposed to MG132 or transfected with the mutant G100S showed an elevation in the protein level of spliced XBP1, but this splicing was prevented by the effect of chemical chaperone 4-PBA (Fig. 1, C and D). These results suggest that blockade of the UPR pathways by 4-PBA could inhibit the activation of ER stress-induced IRE1/XBP1 pathways.
Fig. 1.
Blocking of UPR pathways in AECs by 4-PBA can inhibit ER stress-induced activation of the IRE1 pathway. Human alveolar A549 cells either were incubated with MG132 (10 μM, A and C) for 24 h or were transfected with wild-type SP-C (W.T.) or G100S SP-C mutation plasmids (B and D) for 48 h in the presence or absence of 4-PBA (5 mM). Whole cell lysates were analyzed for phosphorylation of IRE1 (A and B) or splicing of XBP1 (C and D) by Western blotting. Bars represent means ± SE of at least 3 separate experiments. Significant differences were determined by ANOVA and Student-Newman-Keuls test; ***P < 0.001 (A and C) vs. control (CTL) or vs. W.T. (B and D).
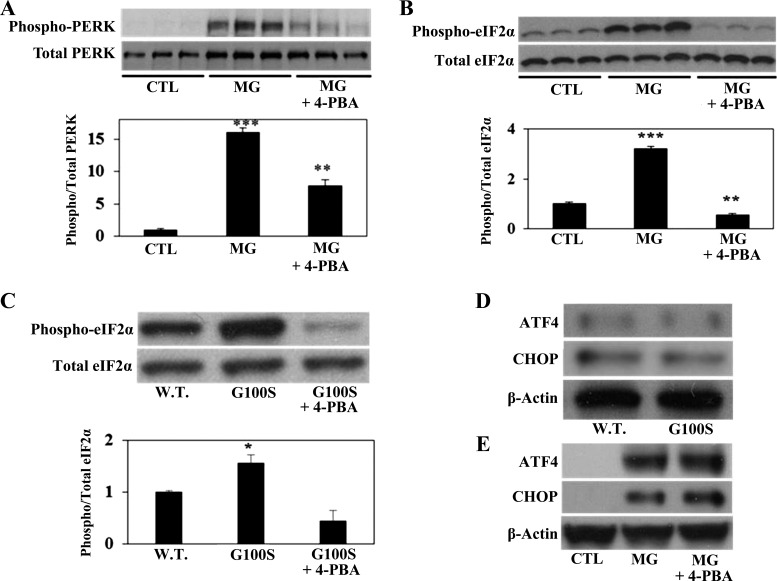
To identify the activity of the PERK pathway in response to ER stress, human alveolar A549 cells were challenged with synthetic ER stress inducer MG132 at the same concentration that activates the IRE1 pathway. As shown in Fig. 2A, the proteasome inhibitor MG132 strongly elevated the phosphorylation of PERK. This result is consistent with previous study by Ono and colleagues (22), who observed that A549 cells stably expressing the G100S mutation of SP-C, in the presence of proteasome inhibitor MG132, showed an increase in the level of phospho-PERK compared with the wild-type CP-C-expressing cells. Moreover and in agreement with the results shown above, the chemical chaperone 4-PBA significantly inhibited PERK phosphorylation in response to MG132 (Fig. 2A). Upon phosphorylation, the activated PERK phosphorylates the α-subunit of eIF2α. Here we demonstrate that either MG132 (Fig. 2B) or SP-C BRICHOS domain mutation (Fig. 2C) substantially upregulated the phosphorylation of eIF2α protein, which was strongly inhibited by protein chaperone 4-PBA.
Fig. 2.
Inhibition of the UPR pathways by chemical chaperone can prevent the PERK/eIF2α but not ATF4/CHOP activation. Human A549 cells were incubated with MG132 (24 h for A and B; 12 h for E) or were transfected with plasmids containing wild-type SP-C or SP-C BRICHOS domain mutant G100S (48 h for C, 12 h for D) with or without chemical chaperone 4-PBA (5 mM). Whole cell lysates were then harvested for detection of phospho-PERK and total PERK (A), phospho-eIF2α and total eIF2α (B and C), and ATF4 and CHOP (D and E) by Western blotting. Anti-β-actin antibody was used as loading control. Bars are means + SE of at least 3 separate experiments. **P < 0.01 or ***P < 0.001 vs. CTL and *P < 0.05 vs. W.T. by ANOVA and Student-Newman-Keuls multiple-comparison test.
In response to ER stress, phosphorylated eIF2α induces the expression of ATF4, which in turn will induce the expression of another transcription factor, CHOP (27). However, in agreement with the results of Maguire and colleagues (17) who studied the Δexon-4 SP-C mutation, our previous work (33) showed that A549 cells transiently transfected with G100S SP-C mutation showed no significant increase in CHOP (33) or ATF4 protein (Fig. 2, C and D) compared with wild-type SP-C. Also, although recent study from our laboratory have found that MG132 increased the level of CHOP protein in A549 cells (33), it is suggested that chemical chaperone 4-PBA could not block the activation of either ATF4 or CHOP protein in A549 cells (Fig. 2E).
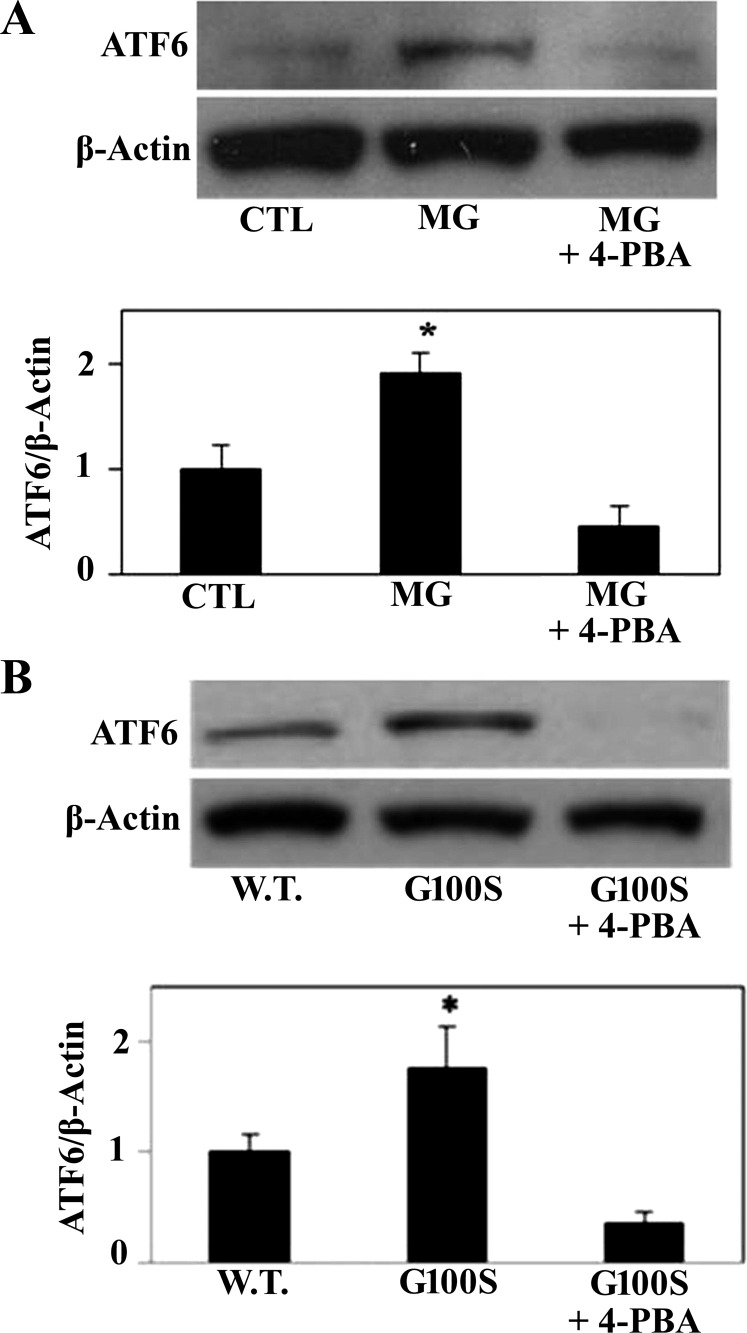
In similarity to IRE1 and PERK, ATF6 binds to GRP78 and remains inactive under homeostatic conditions (11). In response to prolonged or severe ER stress, however, ATF6 dissociates from BiP and is cleaved during the translocational process to generate the active form (11). Therefore, we next challenged A549 cells with ER stress inducers and assessed the level of ATF6 protein in response to ER stress in the presence or absence of 4-PBA. Figure 3 shows an increase of ATF6 protein in MG132-exposed A549 cells compared with vehicle-treated cells (Fig. 3A) or in mutant G100S-expressing cells compared with wild-type SP-C-expressing cells (Fig. 3B). However, this activation of ATF6 was prevented in the presence of sodium phenylbutyrate (Fig. 3).
Fig. 3.
Prevention of UPR pathways by 4-PBA can inhibit ER stress-induced ATF6 activation. A549 cells were treated with proteasome inhibitor MG132 for 24 h (A) or were transiently transfected with plasmids containing wild-type SP-C or SP-C BRICHOS domain mutation G100S for 48 h (B) in the presence or absence of 4-PBA. Cells lysates were harvested for immunoblotting of ATF6 protein and β-actin was used as the loading control. Bars are means ± SE of 3 independent experiments. *P < 0.05 vs. CTL and *P < 0.05 vs. W.T. by ANOVA and Student-Newman-Keuls multiple-comparison test.
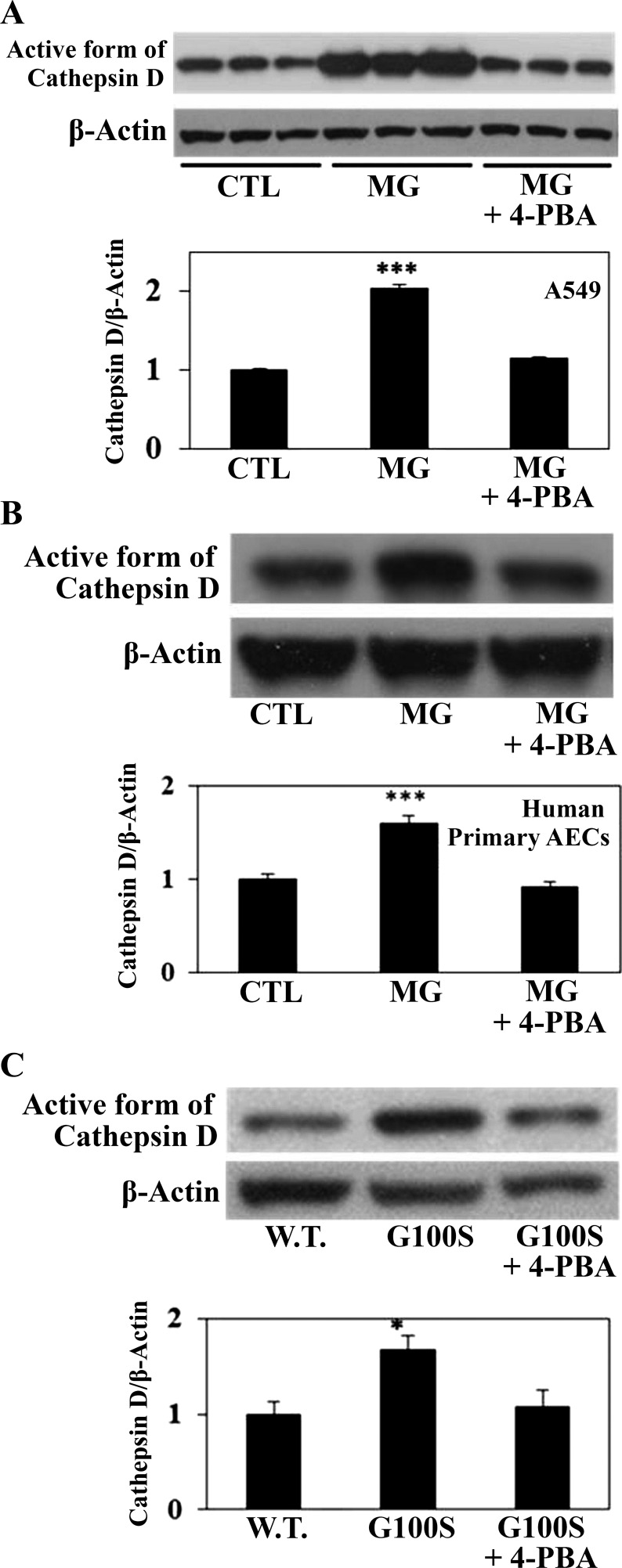
Earlier work from our laboratory has shown that cathepsin D, an aspartyl protease that enzymatically cleaves angiotensinogen to produce ANGII, is required for AEC apoptosis (14, 33). Recent studies from this laboratory have demonstrated that either fibrogenic agent bleomycin (13) or ER stress inducer MG132 (33) upregulated both the immunoreactive protein and enzymatic activity of cathepsin D. Therefore, the ability of UPR to regulate cathepsin D was tested by challenging AECs with ER stress inducers with or without 4-PBA. Figure 4 shows that MG132-induced cathepsin D activation was significantly reduced by 4-PBA either in A549 cell line (Fig. 4A) or in primary cultures of human lung alveolar epithelial cells (Fig. 4B). In agreement with that result, A549 cells transfected with G100S SP-C mutation show similar activation of cathepsin D by the G100S mutant but reduction of cathepsin D (active form) upon exposure to the UPR chemical chaperone 4-PBA (Fig. 4C).
Fig. 4.
Blocking of UPR pathways in AECs can prevent ER stress-induced cathepsin D activation. In A and B, A549 cells (A) or primary culture of human alveolar epithelial cells (B) were treated with ER stress inducer MG132 (10 μM) for 24 h with or without 4-PBA (5 mM). In C, A549 cells were transfected with plasmids containing W.T. SP-C or SP-C BRICHOS domain mutant (G100S) for 48 h in the presence or absence of 4-PBA (5 mM). Whole cell lysates were harvested for detection of cathepsin D protein, active form (14). Anti-β-actin antibody was used as loading control. Results are means ± SE of at least 3 cell cultures. ***P < 0.001 vs. CTL and *P < 0.05 vs. W.T. by ANOVA and Student-Newman-Keuls multiple-comparison test.
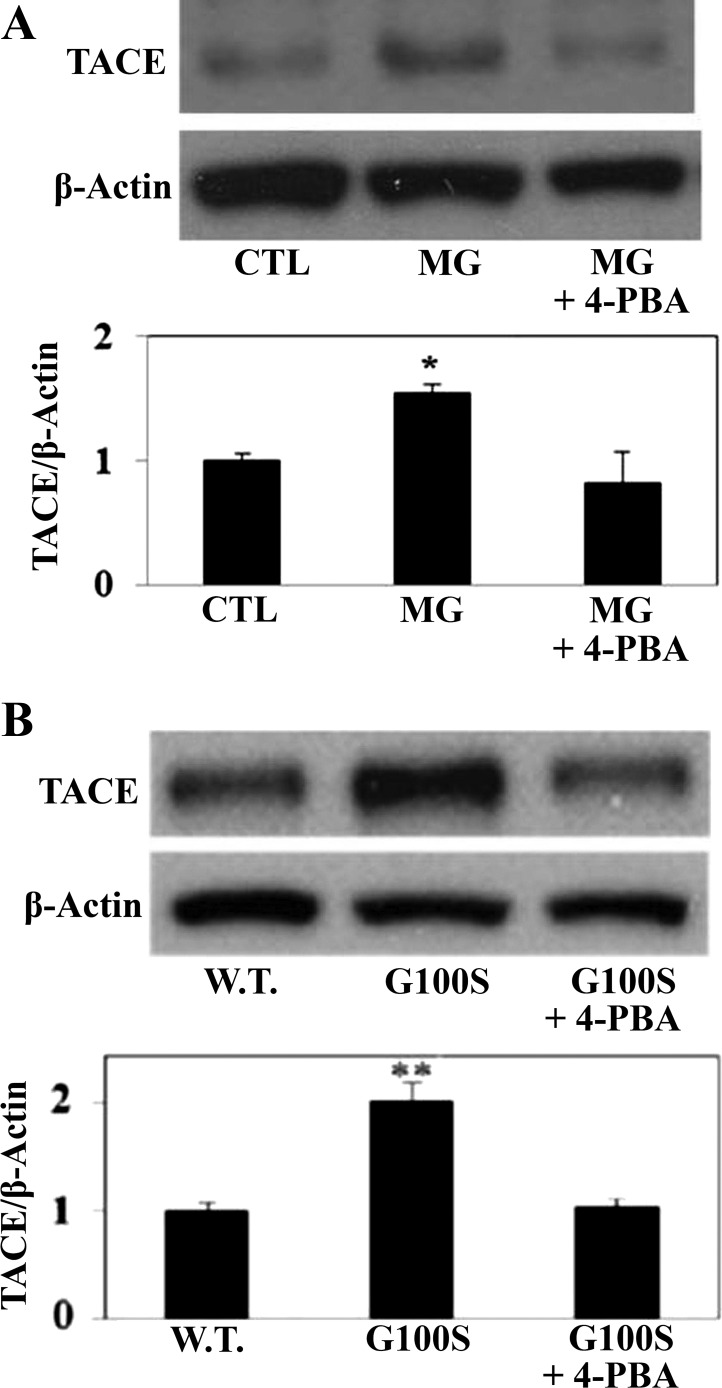
Our previous study proved that either MG132 or SP-C mutation decreased the activation of ACE-2 (33); thus we examined the level of ACE-2 in A549 cells challenged with ER stress inducers (MG132, G100S SP-C mutation) in the presence or absence of 4-PBA. Herein, we showed that ER stress-induced ACE-2 downregulation was potentially, but not significantly, prevented by chemical chaperone 4-PBA (data not shown). On the other hand, studies from other groups have reported that ADAM17/TACE, a TNF-α-converting enzyme, can cleave and release the ectodomain of ACE-2 in several tissues, including the lungs (5, 21). Recent studies from our laboratory demonstrated that ADAM17/TACE is involved in the modulation of the angiotensin system (21, 33). Figure 5 showed that ER stress in response to MG132 (Fig. 5A) or G100S SP-C BRICHOS domain mutation (Fig. 5B) elevated the level of ADAM17/TACE in A549 cells, and this activation can be prevented by the chemical chaperone 4-PBA.
Fig. 5.
Inhibition of the UPR pathways by chemical chaperone can inhibit the activation of ADAM17/TACE protein in AECs. Human A549 cells were exposed to MG132 (10 μM) for 24 h (A) or were transfected with plasmids containing wild-type SP-C or SP-C BRICHOS domain mutant G100S for 48 h (B) with or without 4-PBA (5 mM). Whole cell lysates were then harvested for detection of ADAM17/TACE and β-actin antibodies by Western blotting. Quantitation represents mean ± SE of at least 3 independent experiments. *P < 0.05 vs. CTL and **P < 0.01 vs. W.T. by ANOVA and Student-Newman-Keuls post hoc test.
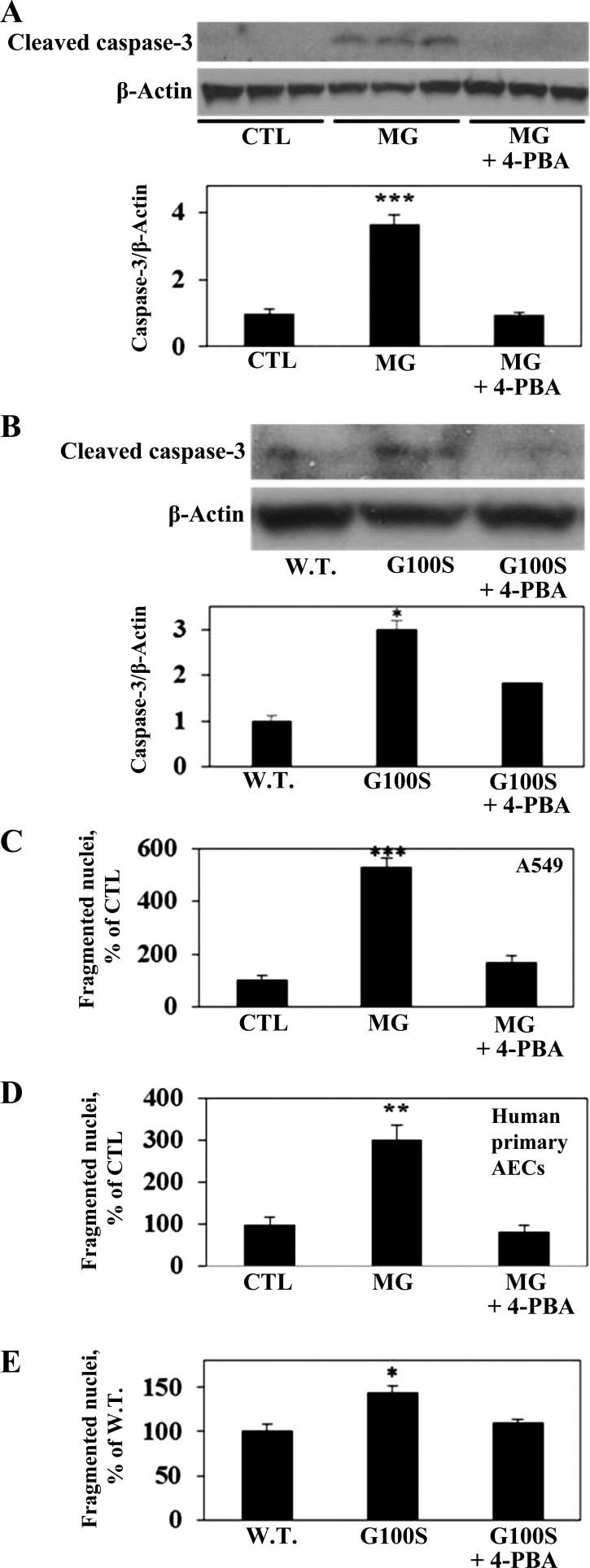
To test the role of 4-PBA in its effect on ER stress-induced apoptosis of AECs, A549 cells were exposed to ER stress inducers, with or without 4-PBA, and were then assessed for caspase-3 activation. Figure 6 shows that either MG132 (Fig. 6A) or G100S SP-C mutation (Fig. 6B) strongly activated the cleavage of caspase-3, and this activation was significantly inhibited by chaperone 4-PBA. More importantly, human alveolar epithelial A549 cells were measured for nuclear fragmentation, a marker of the final stage of apoptosis. As shown in Fig. 6, both MG132 and the G100S mutant significantly increased the nuclear fragmentation of A549 cells or human primary AECs, in agreement with our earlier results (33). More importantly, the UPR inhibitor 4-PBA significantly inhibited nuclear fragmentation induced by either MG132 (Fig. 6, C and D) or the SP-C BRICHOS domain mutation G100S (Fig. 6E).
Fig. 6.
The chemical chaperone 4-PBA prevents ER stress-induced caspase-3 activation and nuclear fragmentation in AECs. A549 cells were challenged with MG132 (10 μM, A and C) for 24 h or were transiently transfected with plasmids containing wild-type SP-C or SP-C BRICHOS domain mutant G100S (B and E) for 48 h in the presence or absence of 4-PBA (5 mM). Human primary cells were treated with MG132 (10 μM, D) with or without 4-PBA (5 mM). Cells were then harvested for Western blot detection of cleaved (active form) of caspase-3 (A and B) or measured of fragmented nuclei (C–E). Bars represent means ± SE of at least 3 cell cultures. ***P < 0.001 vs. CTL (A, C, D); ***P < 0.001 vs. W.T. (B), and *P < 0.05 vs. W.T. (B and E), all by ANOVA and Student-Newman-Keuls multiple-comparison test.
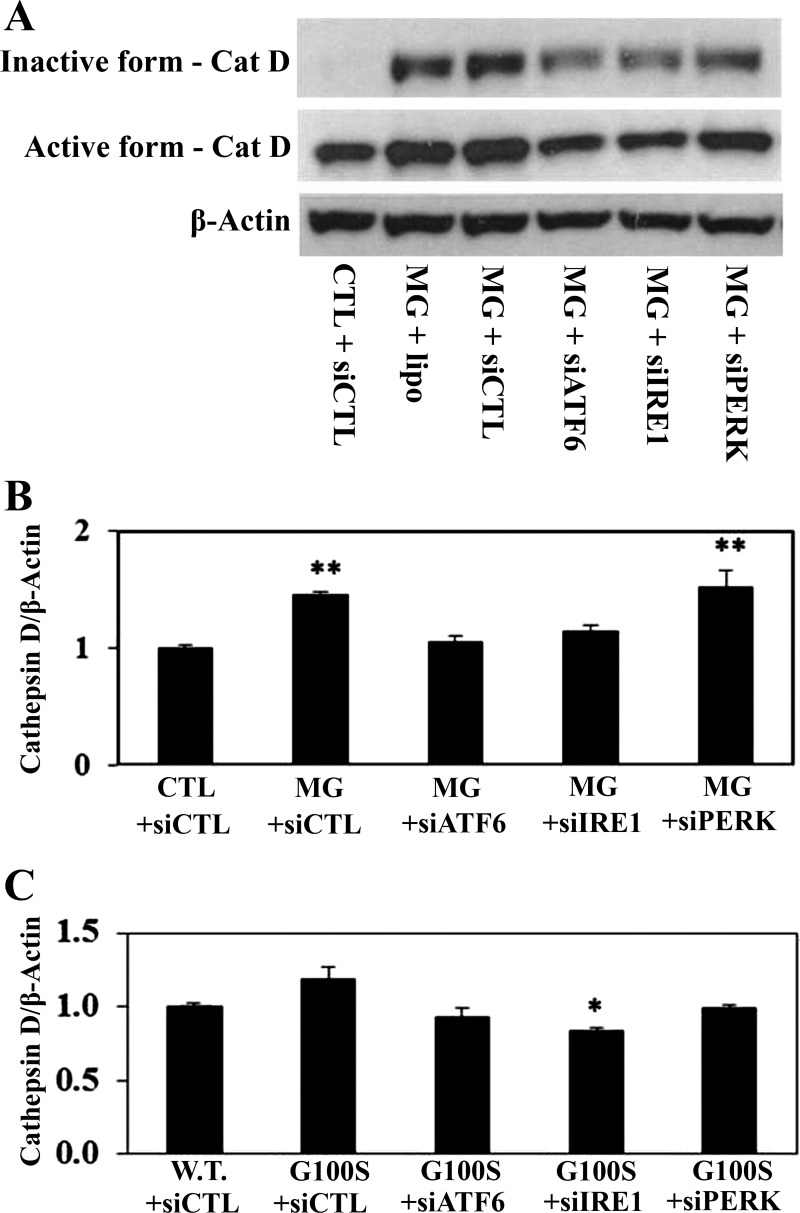
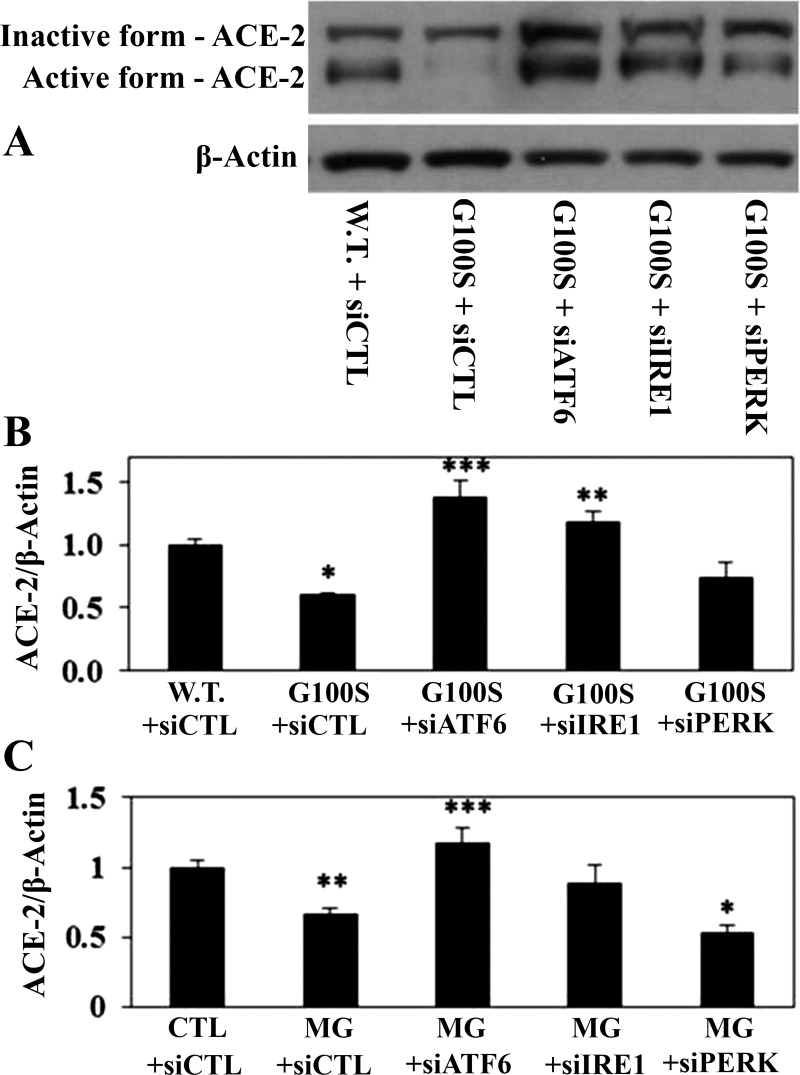
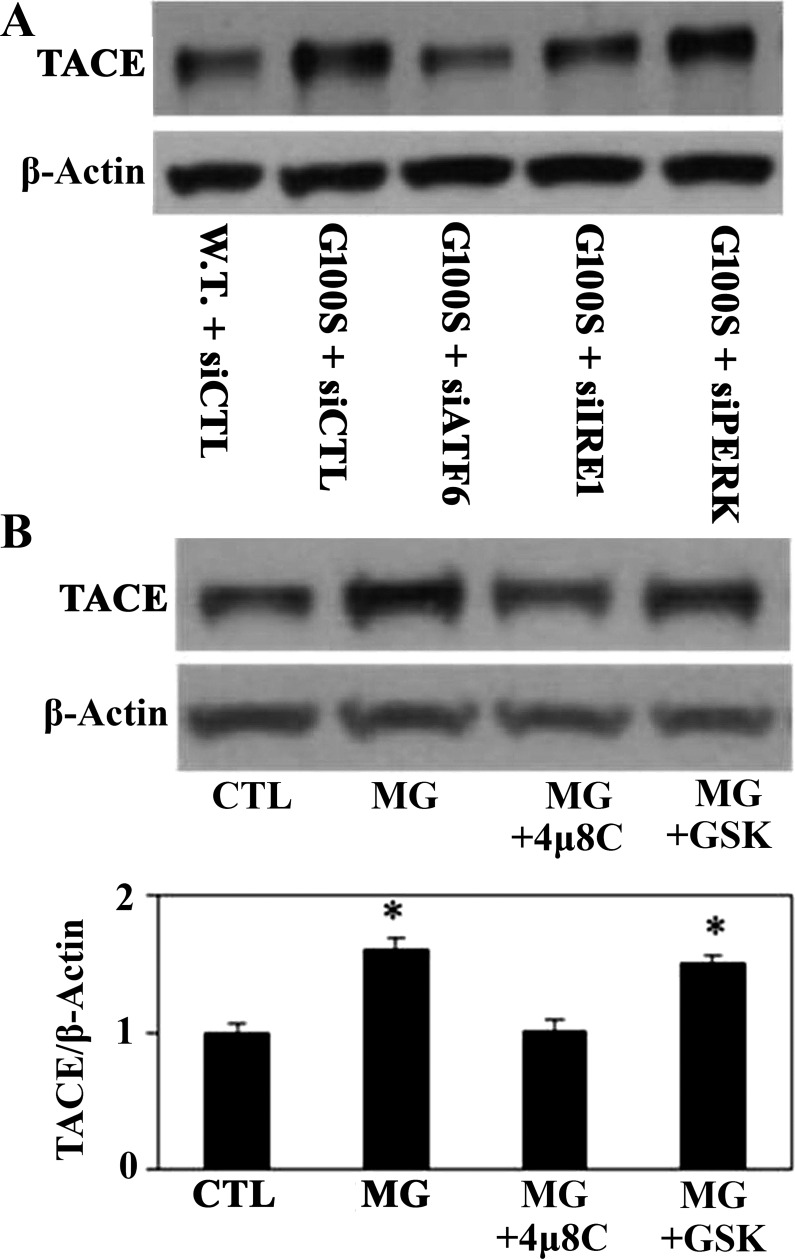
To further investigate which UPR pathway is involved in regulation of the angiotensin system, we used the siRNAs against each target sensor to block the activity of each pathway. Figure 7 demonstrated that inhibition of ATF6 or IRE1 pathway by knockdown of ATF6 or IRE1 pathway using antisense oligonucleotides showed a potential to prevent MG132- or SP-C mutation-induced cathepsin D activation of A549 cells. In addition, knockdown of ATF6 or IRE1 pathway using antisense oligonucleotides showed significant effect on preventing of ER stress-induced ACE-2 downregulation (Fig. 8). On the other hand, blocking of PERK pathway by antisense nucleotides did not show effect on diminishing of ER stress-induced cathepsin D activation or ACE-2 downregulation (Figs. 7 and 8). Consistent with these data, knockdown of ATF6 or IRE1 pathway using antisense oligonucleotides, compared with PERK pathway, appeared to have more effect on preventing of SP-C mutation-induced TACE activation in A549 cells (Fig. 9A).
Fig. 7.
Knockdown of UPR pathways using antisense oligonucleotides can prevent the ER stress-induced cathepsin D (Cat D) upregulation. A549 cells were challenged with either siRNAs against UPR target sensors (siATF6, siIRE1, siPERK) or scramble siRNAs (siCTL) for 24 h then were treated with MG132 (10 μM, A and B) for 24 h or were transfected with plasmids containing wild-type SP-C or SP-C BRICHOS domain mutant G100S for 48 h (C). Western blotting was used to detect cathepsin D protein (active form) from whole cell lysates. β-Actin was used as loading control. **P < 0.01 vs. CTL + siCTL by ANOVA and Student-Newman-Keuls multiple-comparison test, and *P < 0.05 vs. G100S+siCTL by Student's t-test.
Fig. 8.
Blockade of UPR pathways by knockdown of UPR sensor antisense oligonucleotides can prevent the ER stress-induced decrease of ACE-2 protein. A549 cells were challenged with either siRNAs against UPR target sensors (siATF6, siIRE1, siPERK) or scramble siRNAs (siCTL) for 24 h and then were transfected with plasmids containing wild-type SP-C or SP-C BRICHOS domain mutant G100S (A and B) for 48 h or were incubated with MG132 (10 μM, C) for 24 h. Whole cell lysates were harvested for detection of ACE-2 protein (inactive and active forms) by Western blotting. Anti-β-actin antibody was used as loading control. *P < 0.05, **P < 0.01 or ***P < 0.001 vs. W.T. + siCTL (B) or vs. CTL + siCTL (C), all by ANOVA and Student-Newman-Keuls multiple-comparison test.
Fig. 9.
Inhibition of UPR pathways can prevent ER stress-induced ADAM17/TACE activation of AECs. In A, human A549 cell line was challenged with either siRNAs against UPR target sensors (siATF6, siIRE1, siPERK) or scramble siRNAs (siCTL) for 24 h then was transfected with plasmids containing wild-type SP-C or SP-C BRICHOS domain mutant G100S for 48 h. In B, A549 cells were incubated with IRE1 blocker (4μ8C, 50 μM) or PERK blocker (GSK2656157, 1 μM) for 1 h before treatment with MG132 (10 μM) for 24 h. Whole cell lysates were harvested for detection of ADAM17/TACE protein by Western blotting. β-Actin was used as loading control. *P < 0.05 vs. CTL (B) by ANOVA and Student-Newman-Keuls multiple-comparison test.
Recent studies have reported the findings of various potent and selective inhibitors for IRE1 and PERK (1, 3). Among those is 4μ8C, which has been demonstrated to be a selective blocker of IRE1 that can inactivate the Xbp1 splicing and IRE1-mediated mRNA degradation in MEF and HEK-293T cells (3). Furthermore, Atkins and colleagues (1) have identified GSK2656157, an ATP-competitive inhibitor, as a potent blocker of PERK that has been shown the ability to prevent ER stress-induced PERK and eIF2α phosphorylation in human pancreatic adenocarcinoma BxPC3 cells. Figure 9B shows that 4μ8C can prevent the TACE upregulation in response to MG-induced ER stress while GSK2656157 did not show a similar effect.
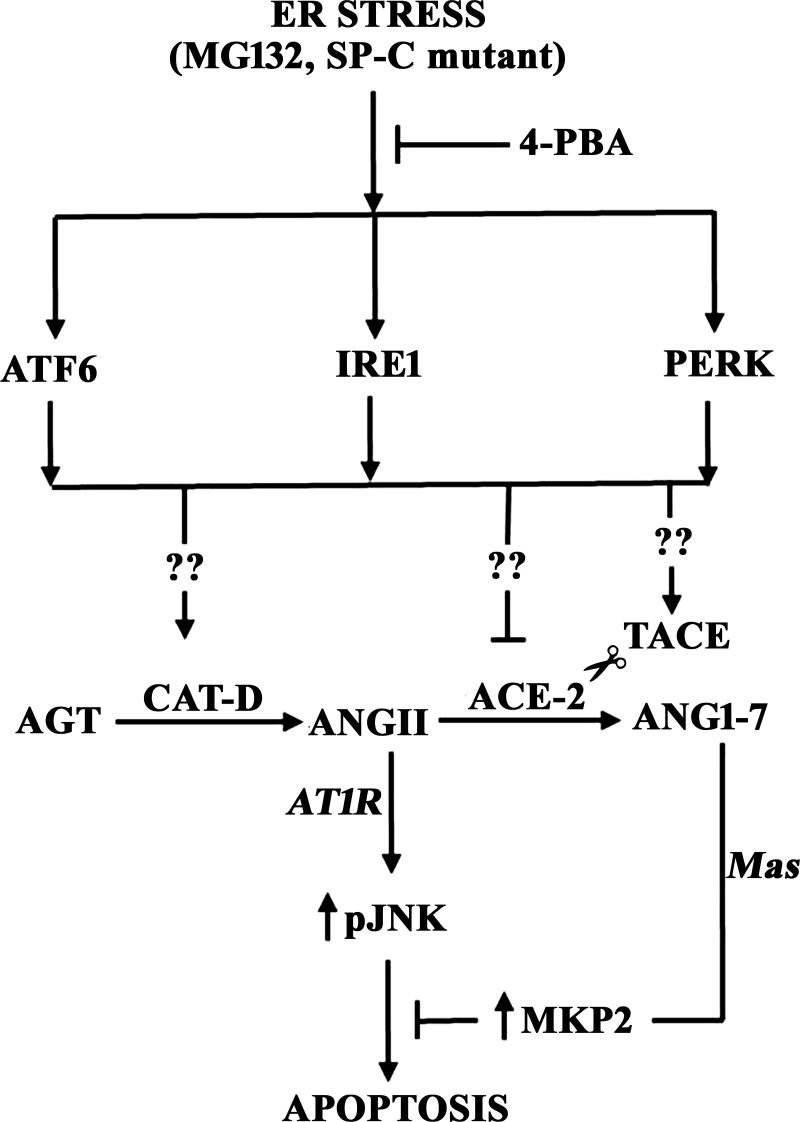
Figure 10 presents a summary of the effect of ER stress and UPR activation on apoptosis of AECs through modulation of the autocrine angiotensin system.
Fig. 10.
UPR activation regulates ER stress-induced apoptosis of alveolar epithelial cells by modulating the autocrine angiotensin system. ER stress in response to either MG132 or SP-C mutation activates all three UPR arms: the PERK/eIF2α, IRE1/XBP1, and ATF6 pathways. Induction of the UPR upregulates cathepsin D, an aspartyl protease that is required for AEC apoptosis through its production of ANGII from angiotensinogen (33), or downregulates ACE-2 by TACE/ADAM17-mediated ectodomain shedding, each by unknown mechanisms (??) yet to be identified. In turn, cathepsin D activation and ACE-2 downregulation control the ratio of the proapoptotic ANGII and the antiapoptotic ANG1-7, which inhibits ANGII-induced JNK phosphorylation and apoptosis through mas-mediated upregulation of MAP kinase phosphatase-2 (MKP-2, see Ref. 4).
DISCUSSION
A variety of studies have documented roles for apoptosis of AECs in acute lung injury, COPD, and pulmonary fibrosis (15). Recent studies have reported that mutations in the BRICHOS domain of the SP-C, which is exclusively synthesized by alveolar epithelial type II cells, can result in protein misfolding, accumulation, induction of ER stress, and apoptosis of AECs (8, 20). However, chronic ER stress and UPR activation have also been observed in both sporadic and familial IPF, despite the absences of SP-C mutations (8). Taken together, multiple reports implicated that ER stress and UPR activation have a prominent role in the pathogenesis of IPF (27). It is for these reasons that the ER stress inducers MG132 and the fibrogenic SP-C mutant G100S (33) were chosen for study in this report.
Several lines of evidence have established that the angiotensin system plays important role in the pathogenesis of lung fibrosis, including the apoptosis of lung AECs (29). Alveolar cells treated with apoptosis inducers such as bleomycin or endogenous toxins (Fas ligand, TNF-α) showed an increase in AGT mRNA, AGT protein, and the derived enzyme octapeptide ANGII (34, 36). ANGII is known as proapoptotic and is required for the apoptosis of epithelial cells in many organs, including the liver, pancreas, and lungs (30). An earlier study from our laboratory reported that bleomycin-induced apoptosis of AECs requires the conversion of AGT to ANGII, a process that requires the proteolytic activity of cathepsin D (14). Additionally, our recently published work showed that the ER stress inducer MG132 upregulated cathepsin D activation in AECs (33). The findings herein showed an increase in the level of cathepsin D in response to ER stress can be reduced by the chemical chaperone 4-PBA, or by knockdown of the ATF6 or IRE1 pathway. The resulting reduction of cathepsin D (or an increase in ACE-2) would reduce apoptosis by reducing the ANGII/ANG1-7 ratio as discussed earlier (33). The mechanisms by which ATF6 or IRE1 regulates cathepsin D activation will be an interesting topic for future investigation.
Recent reports from our laboratory demonstrated that ACE-2 is a protective protein that inhibits apoptosis through its abilities of balancing the proapoptotic ANGII and its antiapoptotic degradation product ANG1-7 (31). In this study, we suggest that chemical chaperone 4-PBA could not significantly prevent the decrease of ACE-2 in challenged to MG132 or G100S SP-C mutation, but ATF6 or IRE1 has the potential to inhibit this downregulation of ACE-2. Moreover, studies of cultured human airway epithelial cells have suggested that shedding of ACE-2 ectodomain plays important role in SARS infection or inflammation (21, 23, 33). Our previous study has reported that ACE-2 ectodomain shedding enzyme ADAM17/TACE plays a role in the G100S-induced loss of ACE-2 (33). In addition, it was also demonstrated in our study that TAPI2, an ADAM17/TACE inhibitor, could prevent MG132- or clastocystin-induced cathepsin D activation of A549 cells (33). The findings herein demonstrate that either MG132 or G100S SP-C mutation upregulated the activation of ADAM17/TACE protein, and chemical chaperone 4-PBA could prevent the G100S-induced TACE activation of alveolar epithelial A549 cells. It is also suggested that blocking of ATF6 or IRE1 pathway by knockdown of antisense oligonucleotides or by chemical inhibitor (4μ8C) showed potential to prevent the ER stress-induced TACE activation. These results suggest that the ER stress and UPR activation might regulate the apoptosis of AECs via angiotensin signaling mechanisms that involve the ADAM17/TACE induction, and either ATF6- or IRE1-induced TACE activation has the potential to explain the induction of apoptosis of AECs by ER stress.
Previous work from our laboratory has demonstrated that the autocrine ANGII/ANG1-7 system regulates apoptosis of AECs in response to ER stress induced by either chemical proteasome inhibitor (MG132) or by SP-C BRICHOS domain mutation (G100S, 33). In that study, we reported that either the protective antiapoptotic peptide ANG1-7 or the nonselective ANGII receptor blocker saralasin could significantly prevent the ER stress-induced apoptosis of alveolar epithelial cells by inhibiting the caspase activation and nuclear fragmentation. The present study showed that UPR blockers inhibited the ER stress-induced apoptosis of AECs. Taken together, our results support the concept of UPR-modulated changes in the autocrine ANGII/ANG1-7 system in the regulation of ER stress-induced apoptosis of alveolar epithelial cells.
Our recent studies found that antiapoptotic ANG1-7 could inhibit JNK phosphorylation in AECs challenged with either apoptotic agent bleomycin or ANGII (31). Additionally, inhibition of ANG1-7 receptor mas by A779 or antisense nucleotides against mas mRNA showed an increase in the level of phosphorylated JNK and cleaved caspase-3, thus enhancing bleomycin-induced apoptosis of AECs (31, 33). On the other hand, ANGII-induced apoptosis of AECs is mediated by ANG receptor, which also could be blocked by AT1-selective ANG blockers (losartan) or an ANG-nonselective blocker (saralasin). In the light of our recent study showing that the autocrine ANGII/ANG1-7 system is involved in the ER stress-induced apoptosis of AECs (33), it is suggested here that ER stress and UPR activation might potentially modulate the activity of both angiotensin axes through the mechanisms that are yet to be elucidated.
In summary, this study demonstrates that ER stress induced either by proteasome inhibitor MG132 or by SP-C BRICHOS domain mutation G100S could lead to the activation of all three canonical pathways of the UPR. Blockade of UPR pathways could lead to a decrease in the ANGII-producing pathway cathepsin D and ACE-2 ectodomain shedding protein ADAM17/TACE or an increase in ANGII-degrading pathway ACE-2. We show that ER stress-induced apoptosis of human AECs is mediated by UPR pathways that modulate the autocrine ANGII/ANG1-7 system of these cells and suggest that understanding the mechanisms by which ER stress and UPR regulate the angiotensin system will support understanding of the apoptosis of AECs, thus providing potential treatments of fibrotic lung disease.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-45136.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.N. and B.D.U. conception and design of research; H.N. performed experiments; H.N. and B.D.U. analyzed data; H.N. and B.D.U. interpreted results of experiments; H.N. and B.D.U. prepared figures; H.N. drafted manuscript; H.N. and B.D.U. approved final version of manuscript; B.D.U. edited and revised manuscript.
REFERENCES
- 1.Atkins C, Liu Q, Minthorn E, Zhang S, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, Choudhry AE, Alsaid H, Jucker BM, Axten JM, Kumar R. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res 73: 1993–2002, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Budinger GR, Mutlu GM, Eisenbart J, Fuller AC, Bellmeyer AA, Baker CM, Wilson M, Ridge K, Barrett TA, Lee VY, Chandel NS. Proapoptotic Bid is required for pulmonary fibrosis. Proc Natl Acad Sci USA 103: 4604–4609, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D, Harding HP. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci USA 109: E869–E878, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopallawa I, Uhal BD. Angiotensin 1–7/mas inhibits apoptosis of alveolar epithelial cells through upregulation of MAP kinase phosphatase-2. Am J Physiol Lung Cell Mol Physiol 310: L240–L248, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haga S, Nagata N, Okamura T, Yamamoto N, Sata T, Yamamoto N, Sasazuki T, Ishizaka Y. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS CoV are candidate antiviral compounds. Antiviral Res 85: 551–555, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haschek WM, Witschi HP. Pulmonary fibrosis: a possible mechanism. Toxicol Appl Pharmacol 51: 475–487, 1979. [DOI] [PubMed] [Google Scholar]
- 7.Healy SJ, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol 625: 234–246, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathicpulmonary fibrosis. Am J Respir Crit Care Med 178: 838–846, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwano K, Kunitake R, Maeyama T, Hagimoto N, Kawasaki M, Matsuba T, Yoshimi M, Inoshima I, Yoshida K, Hara N. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol 280: L316–L325, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, Morrisey EE, Beers MF, Yull FE, Blackwell TS. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA 108: 10562–10567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenna S, Trojanowska M. The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr Opin Rheumatol 24: 663–668, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Molina-Molina M, Abdul-Hafez A, Ramirez J, Serrano-Mollar A, Xaubet A, Uhal BD. Extravascular sources of lung angiotensin peptide synthesis in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 291: L887–L895, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Molina-Molina M, Abdul-Hafez A, Xaubet A, Uhal BD. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol 295: L178–L185, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Rayford H, Shu R, Zhuang J, Uhal BD. Essential role for cathepsin D in bleomycin-induced apoptosis of alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L46–L51, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Shu R, Filippatos G, Uhal BD. Apoptosis in lung injury and remodeling. J Appl Physiol 97: 1535–42, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Zhang H, Soledad-Conrad V, Zhuang J, Uhal BD. Bleomycin induced apoptosis of alveolar epithelial cells requires angiotensin synthesis de novo. Am J Physiol Lung Cell Mol Physiol 284: L501–L507, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Maguire JA, Mulugeta S, Beers MF. Multiple ways to die: delineation of the unfolded protein response and apoptosis induced by surfactant protein C BRICHOS mutants. Int J Biochem Cell Biol 44: 101–112, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maly DJ, Papa FR. Druggable sensors of the unfolded protein response. Nat Chem Biol 10: 892–901, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed TL, Nguyen HT, Abdul-Hafez A, Dang VX, Dang MT, Gewolb IH, Uhal BD. Prior hypoxia prevents downregulation of ACE-2 by hyperoxia in fetal human lung fibroblasts. Exp Lung Res 42: 121–130, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulugeta S, Nureki S, Beers MF. Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 309: L507–L525, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oarhe CI, Dang V, Dang M, Nguyen H, Gopallawa I, Gewolb IH, Uhal BD. Hyperoxia downregulates angiotensin-converting enzyme-2 in human fetal lung fibroblasts. Pediatr Res 77: 656–662, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono S, Tanaka T, Ishida M, Kinoshita A, Fukuoka J, Takaki M, Sakamoto N, Ishimatsu Y, Kohno S, Hayashi T, Senba M, Yasunami M, Kubo Y, Yoshida LM, Kubo H, Ariyoshi K, Yoshiura K, Morimoto K. Surfactant protein C G100S mutation causes familial pulmonary fibrosis in Japanese kindred. Eur Respir J 38: 861–869, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Rzymski T, Petry A, Kračun D, Rieß F, Pike L, Harris AL, Görlach A. The unfolded protein response controls induction and activation of ADAM17/TACE by severe hypoxia and ER stress. Oncogene 31: 3621–3634, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Pulido L, Devos D, Valencia A. BRICHOS: a conserved domain in proteins associated with dementia, respiratory distress and cancer. Trends Biochem Sci 27: 329–332, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Stewart GA, Ridsdale R, Martin EP, Na CL, Xu Y, Mandapaka K, Weaver TE. 4-Phenylbutyric acid treatment rescues trafficking and processing of a mutant surfactant protein-C. Am J Respir Cell Mol Biol 47: 324–331, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7: 880–885, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanjore H, Lawson W, Blackwell TS. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochim Biophys Acta 1832: 940–947, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tirasophon W, Lee K, Callaghan B. The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev 14: 2725–2736, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uhal BD, Dang M, Dang V, Llatos R, Cano E, Abdul-Hafez A, Markey J, Piasecki CC, Molina-Molina M. Cell cycle-dependence of ACE-2 explains downregulation in Idiopathic pulmonary fibrosis. Eur Respir J 42: 198–210, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Uhal BD, Kim JK, Li X, Molina-Molina M. Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages. Curr Pharm Des 13: 1247–1256, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Uhal BD, Li X, Xue A, Gao X, Abdul-Hafez A. Regulation of alveolar epithelial cell survival by the ACE-2/angiotensin 1–7/Mas axis. Am J Physiol Lung Cell Mol Physiol 301: L269–L274, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhal BD, Nguyen H. The Witschi Hypothesis revisited after 35 years: genetic proof from SP-C BRICHOS domain mutations. Am J Physiol Lung Cell Mol Physiol 305: L906–L911, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhal BD, Nguyen H, Dang M, Gopallawa I, Jiang J, Dang V, Ono S, Morimoto K. Abrogation of ER stress-induced apoptosis of alveolar epithelial cells by angiotensin 1–7. Am J Physiol Lung Cell Mol Physiol 305: L33–L41, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R, Alam G, Zagariya A, Gidea C, Pinillos H, Lalude O, Choudhary G, Uhal BD. Apoptosis of lung epithelial cells in response to TNF-α requires angiotensin II generation de novo. J Cell Physiol 185: 253–259, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Wang R, Ibarra-Sunga O, Pick R, Uhal BD. Abrogation of bleomycin induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol 279: L143–L151, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, Zagariya A, Ang E, Ibarra-Sunga O, Uhal BD. Fas-induced apoptosis of alveolar epithelial cells requires angiotensin II generation and receptor interaction. Am J Physiol Lung Cell Mol Physiol 277: L1245–L1250, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Zhong Q, Zhou B, Ann DK, Minoo P, Liu Y, Banfalvi A, Krishnaveni MS, Dubourd M, Demaio L, Willis BC, Kim KJ, duBois RM, Crandall ED, Beers MF, Borok Z. Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am J Respir Cell Mol Biol 45: 498–509, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]