Abstract
Acrolein is a major thiol-reactive component of cigarette smoke (CS) that is thought to contribute to increased asthma incidence associated with smoking. Here, we explored the effects of acute acrolein exposure on innate airway responses to two common airborne allergens, house dust mite and Alternaria alternata, and observed that acrolein exposure of C57BL/6 mice (5 ppm, 4 h) dramatically inhibited innate airway responses to subsequent allergen challenge, demonstrated by attenuated release of the epithelial-derived cytokines IL-33, IL-25, and IL-1α. Acrolein and other anti-inflammatory thiol-reactive electrophiles, cinnamaldehyde, curcumin, and sulforaphane, similarly inhibited allergen-induced production of these cytokines from human or murine airway epithelial cells in vitro. Based on our previous observations indicating the importance of Ca2+-dependent signaling, activation of the NADPH oxidase DUOX1, and Src/EGFR-dependent signaling in allergen-induced epithelial secretion of these cytokines, we explored the impact of acrolein on these pathways. Acrolein and other thiol-reactive electrophiles were found to dramatically prevent allergen-induced activation of DUOX1 as well as EGFR, and acrolein was capable of inhibiting EGFR tyrosine kinase activity via modification of C797. Biotin-labeling strategies indicated increased cysteine modification and carbonylation of Src, EGFR, as well as DUOX1, in response to acrolein exposure in vitro and in vivo, suggesting that direct alkylation of these proteins on accessible cysteine residues may be responsible for their inhibition. Collectively, our findings indicate a novel anti-inflammatory mechanism of CS-derived acrolein and other thiol-reactive electrophiles, by directly inhibiting DUOX1- and EGFR-mediated airway epithelial responses to airborne allergens.
Keywords: asthma, dual oxidase 1, IL-33, airway epithelium, cysteine oxidation, cigarette smoke
cigarette smoking is strongly linked with development of chronic lung diseases such as chronic obstructive pulmonary disease (COPD) and lung cancer (41, 49) and is also related to increased incidences of allergic diseases such as asthma (17, 39). However, the association between smoking and asthma is complex, and while several studies have demonstrated higher incidence of asthma in smokers, others did not find such association or in fact suggested an inverse association (9, 35, 49). Animal studies similarly highlight the complex interactions between cigarette smoke (CS) exposure and allergic asthma. While several studies indicate that CS has proinflammatory effects and can enhance allergic sensitization to innocuous antigens (28, 53), CS exposure can also inhibit features of allergic inflammation such as eosinophilia and mucus production due to its suppressive effects on the immune system (4, 52). Among the main CS components that are responsible for the adverse health effects of CS, especially nonmalignant diseases, are α,β-unsaturated electrophilic aldehydes such as acrolein (2,3-propenal), due to its ability to induce oxidative stress and its covalent interactions with cellular nucleophiles, such as protein cysteines, histidines, lysines, glutathione, and DNA bases (5, 23, 29, 31, 42, 51). Indeed, acrolein is a well-recognized environmental pollutant that has been associated with asthma incidence (3), but our recent studies with experimental models of allergic asthma also indicate similar diverse effects of acrolein exposure on allergic inflammation, being capable of enhancing allergen sensitization to otherwise innocuous antigens such as ovalbumin (35), but also markedly suppressing allergic responses to antigens in sensitized animals, due to its inhibitory effects on inflammatory signaling (48). These anti-inflammatory properties of acrolein have been attributed to its ability to activate Nrf2 and inhibit redox-sensitive transcription factors such as NF-κB and activator protein-1, due to alkylation of critical cysteines within these pathways (22, 23, 26, 48), and are shared by several dietary thiol-reactive electrophiles, e.g., curcumin (44), cinnamaldehyde (14), or sulforaphane (60), that have garnered considerable interest for their health-beneficial effects and ability to inhibit allergic airway inflammation in experimental models (1, 7, 8, 37).
The respiratory epithelium forms an interface between the external environment and the airways and plays a primary role in innate and adaptive immune responses to environmental pollutants and allergens that contribute to asthma pathogenesis (13, 19, 27). Recent studies have highlighted the critical importance of epithelial-derived cytokines such as IL-33, IL-1α, and IL-25 (IL-17E) in orchestrating type 2 inflammation and related pathological features of asthma largely via innate inflammatory mechanisms independent of adaptive immune responses (19, 33). Although some recent studies have explored the relationship between CS and IL-33 (30, 36, 40), the overall impact CS or acrolein on these innate epithelial response mechanisms is still poorly understood. Our recent studies demonstrated that allergen-induced epithelial release of IL-33 and IL-1α as alarmins in innate responses to airborne allergens such as house dust mite (HDM) or Alternaria alternata (ALT) depends strongly on the NADPH oxidase dual oxidase 1 (DUOX1) and redox-dependent activation of tyrosine kinase signaling via Src and EGFR (20). Since previous reports demonstrate that both CS as well as CS-derived acrolein are capable of activating Src (15, 59) and EGFR (11, 50), we hypothesized that acrolein exposure could impact on asthma biology by enhancing innate epithelial responses to airborne allergens. Strikingly, our present findings demonstrate that exposure to environmentally relevant concentrations of acrolein dramatically suppresses innate airway epithelial responses to subsequent challenge with HDM or ALT. We report that these inhibitory effects are shared with those of other anti-inflammatory (dietary) electrophiles and can be attributed to attenuated activation of DUOX1 and Src/EGFR signaling pathways due to direct covalent cysteine modifications within these proteins. Our findings offer further insight into the complex relationship between CS and allergic airway inflammation and highlight a novel anti-inflammatory mechanism by which acrolein and other related thiol-reactive electrophiles can suppress innate allergen-induced airway responses.
MATERIALS AND METHODS
Mouse studies.
C57BL/6 mice at age 8–10 wk (Charles River, Wilmington, MA) were placed in a small glass exposure chamber and exposed to acrolein vapor (5 ppm) for 1–4 h, as described previously (22, 48). At various times after acrolein exposure, mice were subjected to brief isoflurane anesthesia for oropharyngeal instillation of extracts of HDM (50 μg) (Greer Laboratories, Lenoir, NC) in 50 μl of PBS. At indicated times after allergen challenge, mice were euthanized and bronchoalveolar lavage (BAL) fluids and lung tissues were collected for the various analyses described below. All animal procedures were reviewed and approved by the Animal Care and Use Committee of the University of Vermont.
Cell culture and treatments.
Mouse tracheal epithelial (MTE) cells were obtained from C57BL/6 mice and cultured according to previously established protocols (2, 57). Immortalized human bronchial epithelial cells (HBE1) were cultured as previously described (55). Cells were grown to confluence in either 6-, 24-, or 96-well culture plates (Corning, Corning, NY) and starved in EGF-deficient media overnight prior to experimentation. Starved cells were treated with acrolein or other electrophiles at concentrations of 10–30 μM for 30 min, after which cells were stimulated with allergens (HDM or ALT) or ATP (100 μM). Effects on cell viability were determined using ATP TiterGlo assay reagent (Promega, Madison, WI) per manufacturer's instructions, and by analysis of LDH release (Pierce LDH Cytotoxicity Assay Kit; Thermo-Fisher, Waltham, MA). Conditioned culture medium was collected at various time points for analysis of cytokine secretion or extracellular H2O2 production, and cell extracts were prepared for biochemical assays and protein analyses by SDS-PAGE and Western blotting. All chemicals were obtained from Sigma Aldrich unless stated otherwise.
Analysis of cytokines by ELISA.
BAL fluids or cell-culture supernatants were analyzed for IL-33, IL-25 (IL-17E), and IL-1α using DuoSet ELISAs from R&D Systems (Minneapolis, MN).
Extracellular H2O2 production.
For analysis of extracellular H2O2 production, medium was replaced with HBSS before treatment with acrolein and other electrophiles (30 min) and allergen/ATP stimulation for 10 min. Collected HBSS was analyzed for H2O2 by reaction with dl-tyrosine (1 mM) and bovine lactoperoxidase (10 μg/ml) (Sigma) to generate dityrosine, which was determined by HPLC with fluorescence detection (46). Standard curves were prepared by similar analysis of reagent H2O2 (0.1–10 μM), in the presence or absence of electrophiles to ensure that they do not interfere with the assay.
Analysis of Ca2+ increases.
Cells were seeded in 96-well plates and preloaded with FluoForte AM Ca2+ detection dye (Enzo Life Sciences; 1 μM, 30 min) after which the cells were treated with 10–30 μM acrolein for 30 min. Baseline measurements were taken for 10 min in a Biotek Synergy fluorescence plate reader (excitation = 458 nm, emission = 528 nm), before stimulation with HDM, ALT, or ATP, after which fluorescence changes were monitored over 20 min.
Western blot analysis.
Treated cells were lysed in Western solubilization buffer and aliquots containing equal protein concentration (determined by the BCA method) were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies against phosphorylated and unphosphorylated forms of EGFR, Src, ERK, and STAT3 (Cell Signaling). Antibody binding was detected with HRP-conjugated secondary antibodies and visualized by chemiluminescence (Pierce, Rockford, IL) using a GE AI600 imaging system and analyzed via 1D gel analysis quantified using ImageQuant (GE Healthcare, Marlborough, MA).
Analysis of protein reduced thiol status.
Analysis of reduced thiol status in proteins was performed as previously described (20). Following acrolein treatments, HBE cells were lysed in Western solubilization buffer (deoxygenated) containing 100 μM EZ-Link Iodoacetyl-LC-Biotin and 20 U/ml catalase to avoid artificial cysteine oxidation. The cells with the modified lysis buffer were incubated at 37°C for 90 min. To remove excess biotin tag, the lysates were washed three times with 20 mM Tris·HCl (pH 7.4) utilizing Millipore 3000 MWCO centrifugal filtration devices (14,000 rpm, 15 min). Avidin chromatography was performed afterward to purify Iodoacetyl-LC-Biotin-tagged proteins as previously described (20). Eluted proteins and whole cells lysates (input controls) were analyzed with SDS-PAGE followed by Western blotting analysis utilizing α-DUOX1 [generously provided by Francoise Miot (10)], α-epidermal growth factor receptor, and α-Src antibodies (Cell Signaling).
Analysis of protein alkylation.
Treated cells were lysed in Western solubilization buffer, and lysates containing 300 μg protein were mixed with 5 mM (final concentration) of aldehyde-reactive probe (ARP) (Cayman Chemical) for 2 h at room temperature (54). Resulting biotin-tagged proteins were purified by avidin chromatography as described previously (20). Briefly, the lysates were washed 3 × with 300 μl of 20 mM Tris·HCl pH 7.4 on Millipore 3,000 MWCO centrifugal filtration devices (Millipore; 14,000 rpm, 15 min) to remove excess ARP. Biotinylated proteins were then purified by affinity chromatography by the addition of 50 μl (50% suspension) of high capacity neutravidin agarose resin (Thermo Scientific). The resin was washed successively with 1% SDS in H2O, 4 M urea in PBS (pH 7.4), 1 M NaCl in H2O, 100 μM ammonium bicarbonate with 10 mM 1,4-dithio-dl-threitol (DTT), and 100 μM ammonium bicarbonate, to remove nonspecific protein binding. Biotinylated proteins were then eluted from the resin with elution buffer (8.7 mM Tris, 70 mM SDS and 1 mM EDTA) at 90°C (10 min), and mixed with reducing sample buffer for separation by 10 or 18% SDS-PAGE and analyzed by Western blotting with antibodies against α-DUOX1 [generously provided by Francoise Miot (10)], α-epidermal growth factor receptor, and α-Src (Cell Signaling). Whole cell lysates were analyzed similarly for comparison as input controls.
Protein tyrosine kinase activity assay.
Recombinant active human EGFR kinase domain (SignalChem; sequence 695-end) was analyzed using the ADP-Glo assay (Promega) according to the protocols provided by the manufacturer, except that DTT was excluded from the kinase reaction buffer. EGFR (∼50 ng) was pretreated with varying concentrations of acrolein for 30 min before the addition of substrate (poly[4Glu:Tyr]; Sigma) and ATP (Promega) to analyze tyrosine kinase activity, which was measured by luminescence using a Biotek Synergy fluorescence plate reader. Tyrosine kinase activity was expressed relative to activity of untreated EGFR tyrosine kinase. Similar experiments were conducted using the C797S variant of the recombinant active human EGFR kinase domain (SignalChem).
Statistical analysis.
All quantitative data are represented as means and SD unless otherwise stated. Statistical significance between groups was determined by one-way ANOVA. Differences with P < 0.05 were considered statistically significant.
RESULTS
Acrolein inhalation suppresses acute HDM-induced cytokine production.
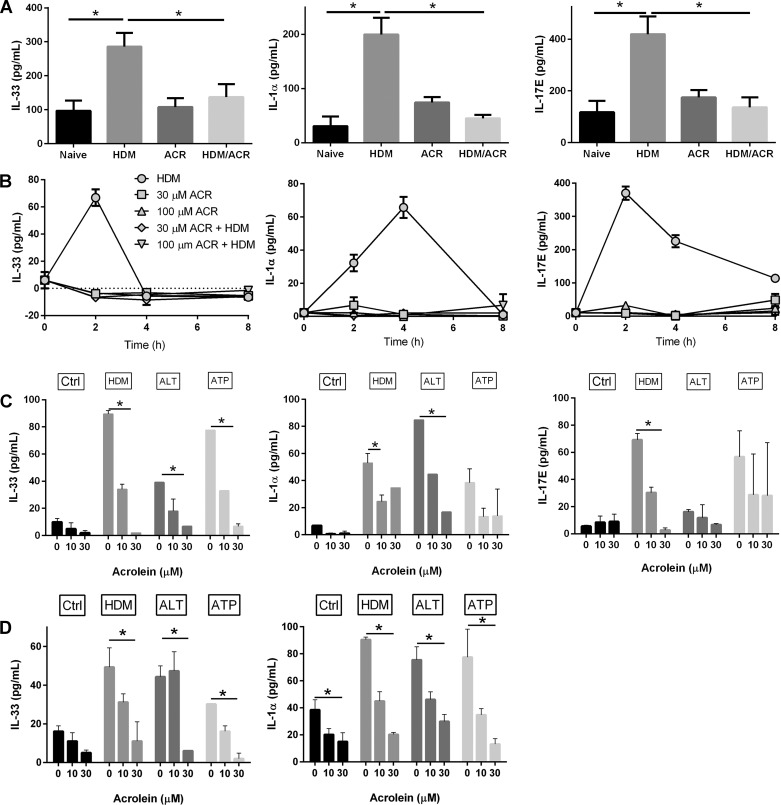
To evaluate the impact of acrolein exposure on innate airway responses to inhaled allergens, C57BL/6 mice were exposed to acrolein vapor (5 ppm) for up to 4 h and subsequently challenged with HDM. BAL fluids were collected 1 h after HDM challenge to analyze for production of epithelia-derived cytokines, IL-33, IL-25 (IL-17E), and IL-1α, critical early mediators of airway inflammatory responses to allergens (19). As expected (20), HDM challenge induced rapid increases in IL-33 and IL-17E, as well as IL-1α (Fig. 1A). Acrolein exposure for 4 h did not significantly enhance release of these cytokines, but acrolein exposure dramatically inhibited the release of these cytokines in response to subsequent HDM challenge (Fig. 1A). Similar inhibitory effects were observed using acrolein preexposure of 1 h instead of 4 h, although effects were less dramatic and not always statistically significant (results not shown). To determine whether the observed inhibitory effects reflect attenuated cytokine production by the respiratory epithelium, similar studies were performed using in vitro stimulation of cultured MTE cells (Fig. 1B). As shown, exposure of MTE cells to HDM induced rapid and time-dependent release of IL-33, IL-25, and IL-1α, consistent with previous findings (20), and these responses were markedly attenuated after pretreatment with acrolein (30–100 μM). To extend these findings, effects of acrolein preexposure were evaluated on cytokine production by a different allergen, ALT, or by ATP, a critical mediator of allergen-induced epithelial cytokine production (20, 24), demonstrating that acrolein pretreatment also significantly attenuated secretion of IL-33, IL-1α, and IL-17 in response to these other stimuli, measured 2 h after allergen challenge (Fig. 1C). Similar findings were also obtained in comparable experiments using human bronchial epithelial HBE1 cells (Fig. 1D). Together, these findings indicate that the anti-inflammatory actions of acrolein (23, 48) also extend to inhibitory actions on innate epithelial responses to allergens that contribute to development of allergic asthma.
Fig. 1.
Acrolein (ACR) inhalation suppresses innate airway allergen responses. A: C57BL/6 mice, naive or ACR (5 ppm) exposed for 4 h, were challenged with HDM, and BAL levels of IL-33, IL-1α, and IL-25/IL-17E were measured by ELISA. Mean values ± SE from 3 independent experiments are shown. Naive n = 6, HDM n = 12, ACR n = 6, HDM/ACR n = 16; *P < 0.05. B: MTE cells were exposed to ACR for 30 min followed by exposure to HDM. At indicated time points, conditioned media were analyzed for IL-33, IL-1α, or IL-17E. C: MTE cells were exposed to ACR (30 min) and challenged with HDM, ALT, or ATP for 2 h, and production of IL-33, IL-1α, and IL-17E was measured. *P < 0.05 by 1-way ANOVA. D: HBE1 cells were pretreated with ACR (30 min) prior to stimulation with HDM, ALT, or ATP for 2 h. Production of IL-33 or IL-1α was measured after 2 h. Data represent average of 2 experiments with 2 replicates each. *P < 0.05.
Allergen-induced epithelial IL-33 secretion is inhibited by thiol-reactive electrophiles.
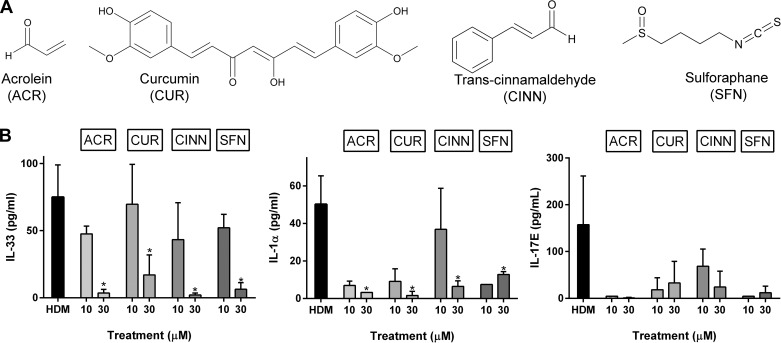
The anti-inflammatory actions of acrolein are largely mediated by its thiol reactivity and interaction of proteins with redox-sensitive cysteine residues (5, 23, 31, 48) and are analogous to anti-inflammatory properties of biological or dietary electrophiles, such as curcumin, cinnamaldehyde, or sulforaphane (14, 44, 60). We therefore determined whether these other electrophiles could similarly suppress allergen-induced secretion of IL-33, IL-17E, or IL-1α. Indeed, as shown in Fig. 2, each of these electrophiles dose dependently inhibited HDM-induced release of IL-33, IL-17E, and IL-1α from MTE cells, suggesting that these inhibitory actions are mediated by a common mechanism related to protein thiol modification in target proteins. Neither electrophile induced significant acute cytotoxicity at concentrations up to 30 μM, based on two independent assays of cell viability (results not shown), indicating that their inhibitory effects were not due to loss of cell viability. Moreover, the acute nature of their inhibitory effects suggests that they are most likely unrelated to transcriptional regulation of anti-inflammatory mechanisms due to, e.g., Nrf2 activation (22, 23, 26) and instead involve direct modification of critical cysteine residues involved in the signaling cascade that mediates allergen-induced epithelial secretion of IL-33 or IL-1α (20).
Fig. 2.
Different thiol-reactive electrophiles inhibit allergen-induced cytokine production by MTE cells. MTE cells were treated with either ACR, curcumin (CUR), cinnemaldehyde (CINN), or sulforaphane (SFN) for 30 min and subsequently stimulated with HDM for 2 h. A: molecular structures of different electrophiles. B: ELISA analysis of cytokine production in conditioned media. *P < 0.05.
Acrolein exposure does not affect allergen-induced Ca2+ increases but prevents EGFR activation.
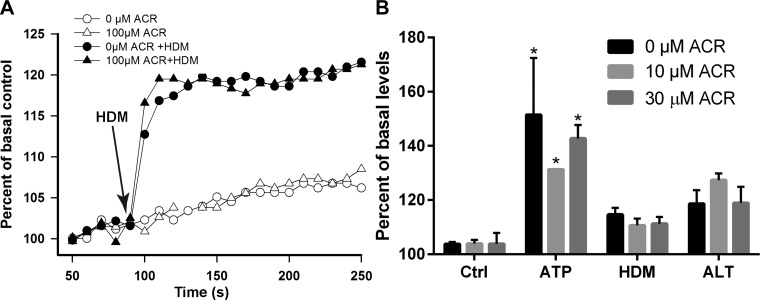
Epithelial production of IL-33, IL-25, and IL-1α in response to allergens such as HDM or ALT was previously shown to involve ATP-dependent Ca2+ signaling and activation of the NADPH oxidase DUOX1 and subsequent oxidant-dependent transactivation of EGFR signaling (20, 46). We therefore first tested the impact of acrolein on allergen-induced Ca2+ signaling, by preloading HBE1 cells with FluoForte AM Ca2+ detection dye prior to acrolein treatment and allergen challenge. As shown in Fig. 3A, HDM challenge resulted in rapid increases in intracellular Ca2+, which was not significantly affected after acrolein pretreatment. Similarly, acrolein exposure did not affect Ca2+ increase in response to other allergens or ATP (Fig. 3B), indicating that acrolein exposure did not affect allergen-induced Ca2+ mobilization from the endoplasmic reticulum or Ca2+ influx.
Fig. 3.
ACR inhibition of allergen-induced cytokine production is independent of Ca2+ signaling. HBE1 cells were preloaded with FluoForte AM and pretreated with ACR before stimulation with HDM for analysis of intracellular Ca2+ increase. A: representative kinetic increases in Ca2+-induced fluorescence. B: quantitative increase in Ca2+ fluorescence measured 10 min after HDM challenge. *P < 0.05 compared with corresponding control.
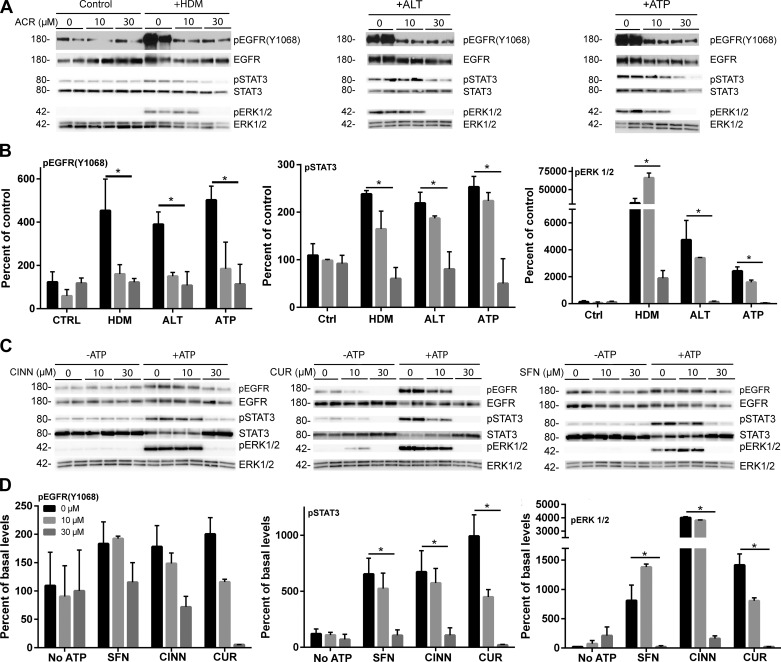
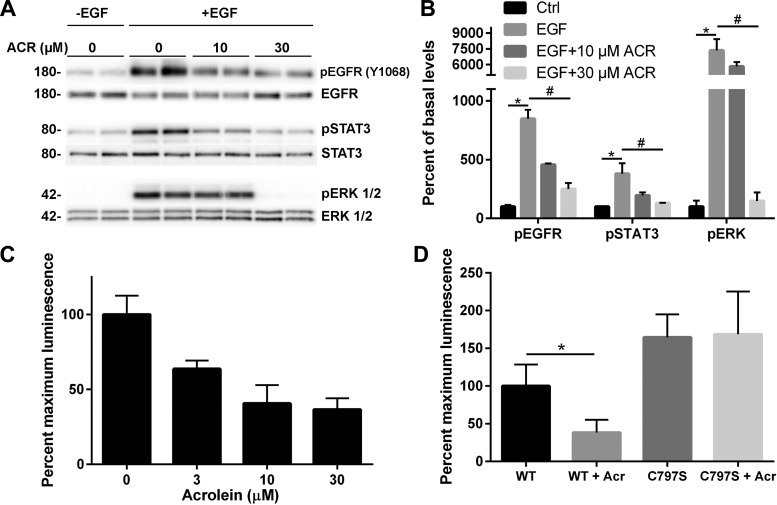
We recently demonstrated that allergen-induced epithelial IL-33 secretion is mediated by activation of Src and EGFR through oxidative modification of cysteine residues within these kinases (20). Therefore, acrolein could conceivably inhibit these kinases by covalent modification of their cysteine residues. We therefore analyzed the effect of acrolein exposure on HDM-induced activation of EGFR and downstream signaling pathways, by Western blot analysis of EGFR autophosphorylation and phosphorylation of STAT3 and ERK1/2, downstream targets of activated Src and EGFR (20). While acrolein exposure alone did not significantly affect EGFR phosphorylation or activation of STAT3 or ERK1/2, it dramatically prevented allergen-induced activation of each of these pathways in a dose-dependent manner (Fig. 4, A and B). Moreover, similar inhibitory effects on ATP-dependent activation of EGFR, STAT3, and ERK1/2 were also observed after pretreatment with other thiol-reactive electrophiles, sulforaphane, cinnamaldehyde, or curcumin (Fig. 4, C and D). These findings suggest that these electrophiles can inhibit allergen-induced EGFR activation, potentially by direct modification of reactive cysteine residues within EGFR or Src (16, 38, 45), or by interfering with upstream redox-sensitive events that mediate EGFR activation. To ascertain the relevance of such a direct inhibitory mechanism, we determined the ability of acrolein exposure to inhibit EGFR activation by direct stimulation with EGF ligand. Indeed, as shown in Fig. 5, acrolein pretreatment markedly inhibited phosphorylation of EGFR, as well as STAT3 and ERK1/2, in response to exogenous EGF. To evaluate whether acrolein can directly reduce EGFR tyrosine kinase activity, recombinant EGFR tyrosine kinase was incubated directly with acrolein, which was found to result in dose-dependent loss of tyrosine kinase activity (Fig. 5C). Moreover, this inhibitory action was not observed with the C797S mutant EGFR kinase under similar conditions (Fig. 5D), suggesting that it was due to alkylation of C797 within the ATP-binding domain of EGFR, which is also the target of clinically used covalent EGFR inhibitors (21, 45). Collectively, these findings indicate that acrolein and other thiol-reactive electrophiles can inhibit allergen-induced epithelial cytokine responses by direct targeting of EGFR, a critical mediator of these cytokine responses, although interference of these electrophiles with other upstream targets in this signaling pathway may contribute as well to these inhibitory actions.
Fig. 4.
ACR and related thiol-reactive electrophiles inhibit allergen-induced activation of EGFR, ERK1/2, and STAT3. A and B: HBE1 cells were pretreated with ACR (30 min) prior to stimulation with HDM or ATP for 15 min and pEGFR (Y1068), pERK1/2, or pSTAT3, as well as their unphosphorylated forms, were analyzed by Western blot. A: representative Western blots. B: densitometry analysis of pEGFR (Y1068), pERK1/2, or pSTAT3, normalized to unphosphorylated proteins, from 3 experiments in duplicate. *P < 0.05. C and D: HBE1 cells were pretreated with ACR, CUR, CINN, or SFN (30 min) prior to stimulation with ATP for 15 min and pEGFR (Y1068), pERK1/2, or pSTAT3 was determined by Western blot. C: representative Western blots. D: densitometry analysis of pEGFR (Y1068), pERK1/2, or pSTAT3. *P < 0.05.
Fig. 5.
ACR directly inhibits EGFR activation by modification of C797. A and B: HBE1 cells were exposed to ACR (30 min) and stimulated with EGF (100 ng/ml) for 10 min for analysis of activation of EGFR, STAT3, and ERK 1/2 by Western blot as in Fig. 4. Representative blots (A) and densitometry data from 2 independent experiments (B) are shown. *P < 0.05 compared with untreated control; #P < 0.05 compared with control EGF treatment. C: effect of ACR treatment (30 min) of recombinant EGFR kinase domain on tyrosine kinase activity. D: kinase activity analysis of wild-type and C797S variant form of EGFR. The kinase activity of wild-type enzyme was significantly reduced due to the effect of ACR while C797S variant's activity remained unchanged. *P < 0.05.
Acrolein and other thiol-reactive electrophiles inhibit activation of DUOX1.
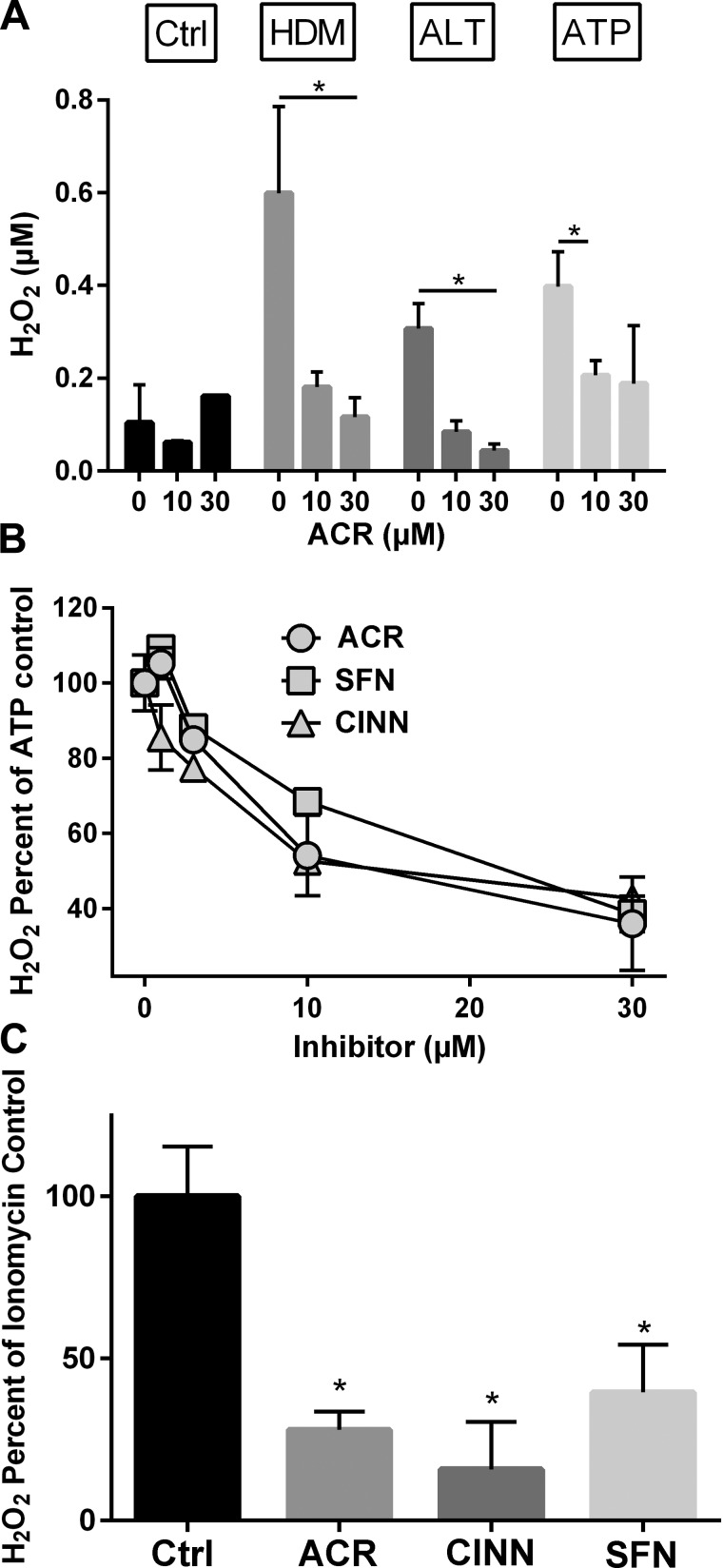
Activation of airway epithelial EGFR by allergens and ATP is mediated by a transactivation mechanism involving intermediate Ca2+-dependent activation of the NADPH oxidase DUOX1 (20). We therefore determined the effects of acrolein on allergen-induced activation of DUOX1, measured by extracellular H2O2 production (20). While HDM, ALT, and ATP each resulted in significant increases in extracellular H2O2 as expected, these responses were surprisingly inhibited by acrolein pretreatment (Fig. 6A), at doses similar to those capable of suppressing cytokine release (Fig. 1). Similar inhibitory effects were observed after pretreatment with other thiol-reactive electrophiles, such as sulforaphane or cinnamaldehyde (Fig. 6B), and these electrophiles were also found to inhibit receptor-independent DUOX1 activation induced by the Ca2+ ionophore ionomycin (Fig. 6C). Hence, these electrophiles likely inhibit DUOX1 by a direct targeting mechanism rather than by indirect actions on upstream mechanisms involved in receptor-dependent DUOX1 activation. Moreover, these findings indicate that, in addition to targeting EGFR, acrolein and other electrophiles may also suppress allergen-induced epithelial responses by direct targeting of DUOX1.
Fig. 6.
ACR and other thiol-reactive electrophiles inhibit allergen-induced H2O2 production by HBE cells. A: HBE1 cells were pretreated with ACR (30 min) prior to stimulation with HDM, ALT, or ATP, and production of extracellular H2O2 in conditioned medium was measured after 15 min as described in materials and methods. B: dose-dependent inhibition of ATP-induced H2O2 production by ACR, SFN, or CINN. Data were expressed relative to ATP-stimulated H2O2 production in the absence of electrophiles and corrected for basal H2O2 production in the absence of ATP. C: inhibition of ionomycin-stimulated H2O2 production by ACR, SFN, or CINN (30 μM). *P < 0.05.
Acrolein induces cysteine modification and carbonylation of DUOX1, Src, and EGFR.
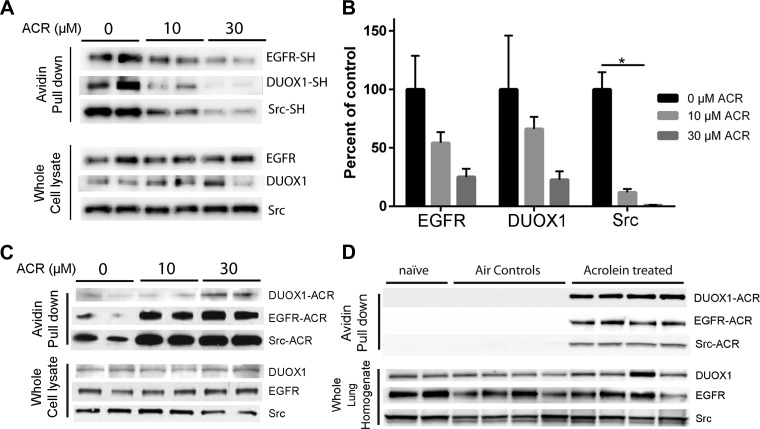
Our findings indicate that acrolein and other thiol-reactive electrophiles inhibit allergen-induced activation of DUOX1, Src, and EGFR. Since each of these proteins contains conserved cysteine residues that are critical in regulating their activity (16, 20, 38), we considered that acrolein or other electrophiles might inhibit these proteins by direct modification of their cysteine residues by Michael addition. To test this, we determined whether acrolein treatment induces cysteine modification of these proteins, by evaluating cysteine thiol content within these proteins using Iodoacetyl-LC-Biotin labeling and analysis of avidin-purified proteins by Western blot. Indeed, acrolein exposure showed a dose-dependent decrease in Iodoacetyl-LC-Biotin labeling of DUOX1, Src, as well as EGFR (Fig. 7, A and B), indicating modification of one or more of their cysteine residues. Cysteine modification by acrolein and similar electrophiles is primarily mediated by Michael addition, which in case of acrolein results in a protein-bound carbonyl adduct (5, 31). To determine whether such Michael addition occurs in DUOX1, Src, or EGFR, lysates from untreated or acrolein-treated cells derivatized with ARP, which binds to protein-associated carbonyl moieties providing them with a biotin tag. Indeed, avidin-purified proteins from acrolein-treated cells contained increased levels of DUOX1, Src, and EGFR (Fig. 7C), indicating that each of these proteins was carbonylated due to Michael addition. Similar analysis of lung tissue homogenates from acrolein-exposed mice (5 ppm; 4 h) also revealed significant increases in carbonylated forms of Src, EGFR, and DUOX1 compared with lungs from air-exposed control mice (Fig. 7D), indicating that the inhibition of allergen-induced lung responses after in vivo acrolein inhalation is related to carbonylation and inactivation of these proteins.
Fig. 7.
ACR exposure induces cysteine modification and carbonylation within DUOX1, EGFR, and Src. A and B: untreated or ACR-treated HBE1 cells (30 min) were lysed in the presence of the thiol-specific reagent EZ-Link Iodoacetyl-LC-Biotin, and avidin-purified proteins as well as whole cell lysates were analyzed by Western blot for DUOX1, EGFR, and Src. A: representative blots. B: densitometry analysis of relative thiol content of DUOX1, EGFR, or Src from 2 experiments in duplicate. *P < 0.05. C: untreated or ACR-treated HBE1 cells (30 min) were derivatized with ARP, and avidin-purified proteins or whole cell lysates (input controls) were evaluated by Western blot for DUOX1, EGFR, or Src. D: mice were exposed to ACR (5 ppm; 4 h) or filtered air, and lung homogenates were derivatized with biotin hydrazide for similar analysis of carbonylated proteins. Representative blots from 2–3 separate experiments are shown.
DISCUSSION
The primary objective of these studies was to address the mechanisms by which CS exposure may impact the development of allergic asthma. While previous studies have indicated that CS and its major electrophile acrolein are capable of enhancing allergic sensitization to innocuous antigens (35, 43) and suppress antigen-induced inflammation in sensitized animals due to its immunosuppressive properties (43, 48), recent evidence has highlighted the critical importance of epithelial-derived cytokines such as IL-33 in type 2 inflammation and related features of allergic asthma, which largely involve innate responses independent of adaptive immune pathways (19, 33). The impact of CS exposure on expression or activation of IL-33 is complex and incompletely understood (30, 36, 40). We hypothesized that acrolein exposure might enhance innate epithelial responses to airborne allergens, due to its adverse effects on epithelial integrity or barrier function and enhanced release of alarmins such as IL-33 and IL-1α. However, acute exposure to acrolein was surprisingly found to dramatically suppress epithelial secretion of IL-33 as well as IL-1α and IL-17E in response to allergen challenge, and similar inhibitory actions were observed by several diet-derived thiol-reactive electrophiles that have been attributed strong health benefits due to their anti-inflammatory properties. The inhibitory effects of acrolein on IL-33 secretion is consistent with a recent report indicating that CS exposure can suppress epithelial secretion of IL-33 even though it promotes epithelial IL-33 mRNA expression (36). Therefore, while several recent reports highlight the involvement of IL-33 in CS-induced inflammation and development of COPD, our studies indicate that electrophiles in CS (of which acrolein is the most prominent example) can paradoxically suppress certain actions of IL-33 by inhibiting its secretion from epithelia in response to allergic stimuli.
Our present studies highlight a previously unrecognized mechanism by which acrolein and other electrophiles can suppress innate epithelial cytokine production, which is independent of Nrf2-mediated induction of anti-inflammatory genes and associated with inhibition of allergen-induced activation of DUOX1, and activation of Src and EGFR, critical events in allergen-induced secretion of IL-33 and IL-1α from the airway epithelium (20). From the perspective of the potential importance of epithelial IL-33 secretion in epithelial host defense mechanisms against allergens or parasites (33, 34), these inhibitory effects of CS-derived acrolein could potentially be detrimental; however, they may also help prevent activation of type 2 inflammatory responses in the context of allergic disease. Therefore, the inhibitory mechanisms described in this work likely contribute to the widely reported beneficial and anti-inflammatory effects of various thiol-reactive electrophiles, including acrolein.
Although acrolein and the various other electrophiles used in this study vary widely with respect to their molecular structure, they share a common feature that they are soft electrophiles that are highly reactive with nucleophilic protein cysteine residues. Thus their common ability to inhibit DUOX1-dependent H2O2 production and activation of Src and EGFR is likely related to their ability to target susceptible cysteine residues, although alternative mechanisms cannot be ruled out (58). Indeed, both EGFR and Src are known to contain conserved cysteine residues, and their reversible oxidation has been implicated in regulation of tyrosine kinase activation (16, 38, 45). Moreover, both Src and EGFR possess noncatalytic cysteines within or near the ATP pocket (C277 in Src and C797 in EGFR) that have been exploited as targets for selective covalent inhibitors of these kinases to improve their anticancer activities (12, 25, 45). Therefore, covalent modification of these cysteines by acrolein or other small electrophilic compounds could conceivably contribute to inhibition of their kinase activity. Consistent with this notion, while acrolein readily inhibits EGFR kinase activity in the wild-type enzyme, no such inhibition was observed with the C797S mutant, indirectly demonstrating the critical role of C797 in its inhibitory action. Although not tested in this study, inhibition of Src activation by these electrophiles might also be related to direct covalent modifications of Cys residues within Src, as implied by the loss of thiol content and increased carbonylation of Src.
In addition to the above mentioned direct inhibitory actions on Src and EGFR, an intriguing and somewhat unexpected finding was that acrolein, as well as other electrophiles, were also capable of inhibiting DUOX1 activation, as measured by ATP- or ionomycin-dependent H2O2 production. Moreover, this occurred at concentrations that were comparable to those needed to inhibit allergen-induced IL-33 secretion, which would suggest that inhibition of DUOX1 may be the primary and most proximal mechanism by which these electrophiles inhibit this allergen response. Importantly, DUOX1 contains a number of conserved cysteines within its extracellular peroxidase homology domain (PHD), which are believed to be highly solvent exposed (32) and are thus susceptible to modification by exogenous thiol-reactive agents such as acrolein. Furthermore, mutation studies have indicated that Cys residues within the DUOX PHDs are involved in DUOX maturation and full activation (6, 32). Recent studies by Dupuy and coworkers (6) indicated that Cys582 and Cys568 within the PHD of the DUOX homolog DUOX2 promote interactions with its maturation factor DUOXA2, by engaging in intramolecular disulfide bridges between DUOX2 and DUOXA2. Although it is presently unclear whether analogous cysteines within DUOX1 are critical for interactions with DUOXA1, modification of these cysteines by acrolein could conceivably inhibit DUOX1, either by preventing its interaction with DUOXA1 or by other mechanisms yet to be defined. Consistent with this notion, Iodoacetyl-LC-Biotin labeling indicated significant modification of accessible Cys residues by acrolein, although we have not been capable of identifying the precise Cys target(s) by mass spectrometry due to difficulties with purification of sufficient quantities of DUOX1 protein for such analysis. Identification of the precise Cys residues involved in inhibition of DUOX1 by these electrophiles would be highly useful in designing selective pharmacological approaches to inhibit DUOX1, based on covalent targeting approaches similar to those used to inhibit Src or EGFR.
Our present studies highlight common actions of acrolein and other electrophiles on activation of DUOX1, Src, or EGFR, which are based on their thiol reactivity. However, it is important to point out that these electrophiles are highly promiscuous and are not selective for specific protein targets (47). Indeed, their effects on overall allergen responses and downstream signaling mechanisms (e.g., ERK1/2, STAT3) are undoubtedly more complex since these electrophiles are capable of reacting with many other redox-sensitive targets as well that could affect these pathways. Moreover, even though the various electrophiles used in this study can be classified as “soft” electrophiles, meaning that they react primarily with soft nucleophiles such as (protein) cysteine residues, their reactivity toward cysteines and other nucleophiles (e.g., lysine, histidine) also depends on their relative electrophilicity and softness (31), as well as other structural determinants that would affect solubility and cellular distribution, and steric factors that affect (noncovalent) interactions with proteins. Nevertheless, their shared ability to suppress allergen-induced activation of DUOX1 and EGFR, which represent the most proximal steps in this overall pathway, suggests that these inhibitory effects are due to a common mechanism and that direct cysteine modification within these proteins likely contributes to these inhibitory effects.
In summary, our findings show that acrolein and similar thiol-reactive electrophiles can inhibit innate epithelial responses to common allergens implicated in asthma development and reveal a previously unrecognized anti-inflammatory mechanism involving inhibition of DUOX1 and Src/EGFR signaling as key mediators of epithelial release of IL-33, IL-25, and IL-1α, which contribute importantly to asthma pathology. Indeed, recent observations indicating enhanced DUOX1 expression (20) and EGFR activation (18) in the airways of asthmatic subjects, which conceivably contribute to disease pathology, suggest that the anti-inflammatory actions of acrolein and other electrophiles in attenuating allergic inflammation may in part be related to their inhibitory actions on DUOX1 and/or EGFR. While inhibitors of EGFR have been demonstrated to be capable of inhibiting allergic asthma symptoms in animal models, even though the clinical application is questionable (18, 56), electrophile-based approaches to selectively inhibit DUOX1 may present an attractive new opportunity for therapeutic management of allergic asthma in cases when conventional therapies are insufficient.
GRANTS
This work was supported by research grants from the NIH to A. van der Vliet (R01 ES021476 and HL085646) and fellowship support to A. C. Little (T32 HL076122) and D. E. Heppner (F32 HL129706).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.D., W.d.J., E.O., R.A.B., M.H., and A.H. performed experiments; K.D., W.d.J., D.E.H., A.L., and A.v.d.V. analyzed data; K.D., W.d.J., D.E.H., A.L., and A.v.d.V. interpreted results of experiments; K.D. prepared figures; K.D. drafted manuscript; K.D. and A.v.d.V. edited and revised manuscript; K.D., W.d.J., and A.v.d.V. approved final version of manuscript; E.O. and A.v.d.V. conception and design of research.
REFERENCES
- 1.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 41: 40–59, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcorn JF, Guala AS, Velden van der J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YMW. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-β1. J Cell Sci 121: 1036–1045, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bein K, Leikauf GD. Acrolein — a pulmonary hazard. Mol Nutr Food Res 55: 1342–1360, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Botelho FM, Llop-Guevara A, Trimble NJ, Nikota JK, Bauer CMT, Lambert KN, Kianpour S, Jordana M, Stämpfli MR. Cigarette smoke differentially affects eosinophilia and remodeling in a model of house dust mite asthma. Am J Respir Cell Mol Biol 45: 753–760, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Cai J, Bhatnagar A, Pierce WM. Protein modification by acrolein: formation and stability of cysteine adducts. Chem Res Toxicol 22: 708–716, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carré A, Louzada RAN, Fortunato RS, Ameziane-El-Hassani R, Morand S, Ogryzko V, de Carvalho DP, Grasberger H, Leto TL, Dupuy C. When an intramolecular disulfide bridge governs the interaction of DUOX2 with its partner DUOXA2. Antioxid Redox Signal 23: 724–733, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao LK, Hua KF, Hsu HY, Cheng SS, Lin IF, Chen CJ, Chen ST, Chang ST. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem Toxicol 46: 220–231, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan PS, Subhashini Dash D, Singh R. Intranasal curcumin attenuates airway remodeling in murine model of chronic asthma. Int Immunopharmacol 21: 63–75, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Coogan PF, Castro-Webb N, Yu J, O'Connor GT, Palmer JR, Rosenberg L. Active and passive smoking and the incidence of asthma in the Black Women's Health Study. Am J Respir Crit Care Med 191: 168–176, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275: 23227–23233, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Deshmukh HS, Case LM, Wesselkamper SC, Borchers MT, Martin LD, Shertzer HG, Nadel JA, Leikauf GD. Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am J Respir Crit Care Med 171: 305–314, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Engel J, Richters A, Getlik M, Tomassi S, Keul M, Termathe M, Lategahn J, Becker C, Mayer-Wrangowski S, Grütter C, Uhlenbrock N, Krüll J, Schaumann N, Eppmann S, Kibies P, Hoffgaard F, Heil J, Menninger S, Ortiz-Cuaran S, Heuckmann JM, Tinnefeld V, Zahedi RP, Sos ML, Schultz-Fademrecht C, Thomas RK, Kast SM, Rauh D. Targeting drug resistance in EGFR with covalent inhibitors: a structure-based design approach. J Med Chem 58: 6844–6863, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol 205: 621–631, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang SH, Rao YK, Tzeng YM. Cytotoxic effect of trans-cinnamaldehyde from cinnamomum osmophloeum leaves on human cancer cell lines. Int J Appl Sci Eng 2: 136–147, 2004. [Google Scholar]
- 15.Geraghty P, Hardigan A, Foronjy RF. Cigarette smoke activates the proto-oncogene c-src to promote airway inflammation and lung tissue destruction. Am J Respir Cell Mol Biol 50: 559–570, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannoni E, Chiarugi P. Redox circuitries driving Src regulation. Antioxid Redox Signal 20: 2011–2025, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Gilliland FD, Islam T, Berhane K, Gauderman WJ, McConnell R, Avol E, Peters JM. Regular smoking and asthma incidence in adolescents. Am J Respir Crit Care Med 174: 1094–1100, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gras D, Chanez P, Vachier I, Petit A, Bourdin A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol Ther 140: 290–305, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Holtzman MJ, Byers DE, Alexander-Brett J, Wang X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat Rev Immunol 14: 686–698, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hristova M, Habibovic A, Veith C, Janssen-Heininger YMW, Dixon AE, Geiszt M, van der Vliet A. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J Allergy Clin Immunol 137: 1545–1556.e11, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia Y, Yun CH, Park E, Ercan D, Manuia M, Juarez J, Xu C, Rhee K, Chen T, Zhang H, Palakurthi S, Jang J, Lelais G, DiDonato M, Bursulaya B, Michellys PY, Epple R, Marsilje TH, McNeill M, Lu W, Harris J, Bender S, Wong KK, Jänne PA, Eck MJ. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 534: 129–132, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasahara DI, Poynter ME, Othman Z, Hemenway D, van der Vliet A. Acrolein inhalation suppresses lipopolysaccharide-induced inflammatory cytokine production but does not affect acute airways neutrophilia. J Immunol 181: 736–745, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci 57: 6–15, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol 186: 4375–4387, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwarcinski FE, Fox CC, Steffey ME, Soellner MB. Irreversible inhibitors of c-Src kinase that target a nonconserved cysteine. ACS Chem Biol 7: 1910–1917, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert C, Li J, Jonscher K, Yang TC, Reigan P, Quintana M, Harvey J, Freed BM. Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-κB1 DNA binding domain. J Biol Chem 282: 19666–19675, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol 134: 499–507, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Lanckacker EA, Tournoy KG, Hammad H, Holtappels G, Lambrecht BN, Joos GF, Maes T. Short cigarette smoke exposure facilitates sensitisation and asthma development in mice. Eur Respir J 41: 1189–1199, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Holian A. Acrolein: a respiratory toxin that suppresses pulmonary host defense. Rev Environ Health 13: 99–108, 1997. [PubMed] [Google Scholar]
- 30.Liew FY. Cigarette smoke resets the alarmin IL-33 in COPD. Immunity 42: 401–403, 2015. [DOI] [PubMed] [Google Scholar]
- 31.LoPachin RM, Gavin T. Molecular mechanisms of aldehyde toxicity: a chemical perspective. Chem Res Toxicol 27: 1081–1091, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meitzler JL, Hinde S, Bánfi B, Nauseef WM, Ortiz de Montellano PR. Conserved cysteine residues provide a protein-protein interaction surface in dual oxidase (DUOX) proteins. J Biol Chem 288: 7147–7157, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 42: 1005–1019, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DMW, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci USA 112: 10762–10767, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Brien E, Spiess PC, Habibovic A, Hristova M, Bauer RA, Randall MJ, Poynter ME, van der Vliet A. Inhalation of the reactive aldehyde acrolein promotes antigen sensitization to ovalbumin and enhances neutrophilic inflammation. J Immunotoxicol 13: 191–197, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pace E, Di Sano C, Sciarrino S, Scafidi V, Ferraro M, Chiappara G, Siena L, Gangemi S, Vitulo P, Giarratano A, Gjomarkaj M. Cigarette smoke alters IL-33 expression and release in airway epithelial cells. Biochim Biophys Acta 1842: 1630–1637, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Park JH, Kim JW, Lee CM, Kim YD, Chung SW, Jung ID, Noh KT, Park JW, Heo DR, Shin YK, Seo JK, Park YM. Sulforaphane inhibits the Th2 immune response in ovalbumin-induced asthma. BMB Rep 45: 311–316, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Paulsen CE, Truong TH, Garcia FJ, Homann A, Gupta V, Leonard SE, Carroll KS. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat Chem Biol 8: 57–64, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polosa R, Thomson NC. Smoking and asthma: dangerous liaisons. Eur Respir J 41: 716–726, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Qiu C, Li Y, Li M, Li M, Liu X, McSharry C, Xu D. Anti-interleukin-33 inhibits cigarette smoke-induced lung inflammation in mice. Immunology 138: 76–82, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahman I, MacNee W. Oxidant/antioxidant imbalance in smokers and chronic obstructive pulmonary disease. Thorax 51: 348–350, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randall MJ, Hristova M, van der Vliet A. Protein alkylation by the α,β-unsaturated aldehyde acrolein. A reversible mechanism of electrophile signaling? FEBS Lett 587: 3808–3814, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbins CS, Pouladi MA, Fattouh R, Dawe DE, Vujicic N, Richards CD, Jordana M, Inman MD, Stampfli MR. Mainstream cigarette smoke exposure attenuates airway immune inflammatory responses to surrogate and common environmental allergens in mice, despite evidence of increased systemic sensitization. J Immunol 175: 2834–2842, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Ruby AJ, Kuttan G, Dinesh Babu K, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett 94: 79–83, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz PA, Kuzmic P, Solowiej J, Bergqvist S, Bolanos B, Almaden C, Nagata A, Ryan K, Feng J, Dalvie D, Kath JC, Xu M, Wani R, Murray BW. Covalent EGFR inhibitor analysis reveals importance of reversible interactions to potency and mechanisms of drug resistance. Proc Natl Acad Sci USA 111: 173–178, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sham D, Wesley UV, Hristova M, van der Vliet A. ATP-mediated transactivation of the epidermal growth factor receptor in airway epithelial cells involves DUOX1-dependent oxidation of Src and ADAM17. PLoS One 8: e54391, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spiess PC, Deng B, Hondal RJ, Matthews DE, van der Vliet A. Proteomic profiling of acrolein adducts in human lung epithelial cells. J Proteomics 74: 2380–2394, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spiess PC, Kasahara D, Habibovic A, Hristova M, Randall MJ, Poynter ME, van der Vliet A. Acrolein exposure suppresses antigen-induced pulmonary inflammation. Respir Res 14: 107, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 9: 377–384, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi K, Kato M, Suzuki H, Akhand AA, Wu J, Hossain K, Miyata T, Matsumoto Y, Nimura Y, Nakashima I. Acrolein induces activation of the epidermal growth factor receptor of human keratinocytes for cell death. J Cell Biochem 81: 679–688, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Tang M, Wang H, Hu Y, Chen WS, Akao M, Feng Z, Hu W. Acrolein induced DNA damage, mutagenicity and effect on DNA repair. Mol Nutr Food Res 55: 1291–1300, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thatcher TH, Benson RP, Phipps RP, Sime PJ. High dose but not low dose mainstream cigarette smoke suppresses allergic airway inflammation by inhibiting T cell function. Am J Physiol Lung Cell Mol Physiol 295: L412–L421, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trimble NJ, Botelho FM, Bauer CMT, Fattouh R, Stämpfli MR. Adjuvant and anti-inflammatory properties of cigarette smoke in murine allergic airway inflammation. Am J Respir Cell Mol Biol 40: 38–46, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Vasil'ev YV, Tzeng SC, Huang L, Maier CS. Protein modifications by electrophilic lipoxidation products: adduct formation, chemical strategies and tandem mass spectrometry for their detection and identification. Mass Spectrom Rev 33: 157–182, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wesley UV, Bove PF, Hristova M, McCarthy S, van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem 282: 3213–3220, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Woodruff PG, Wolff M, Hohlfeld JM, Krug N, Dransfield MT, Sutherland ER, Criner GJ, Kim V, Prasse A, Nivens MC, Tetzlaff K, Heilker R, Fahy JV. Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181: 438–445, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Wu R, Smith D. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro 18: 800–812, 1982. [DOI] [PubMed] [Google Scholar]
- 58.Yadav IS, Nandekar PP, Shrivastava S, Sangamwar A, Chaudhury A, Agarwal SM. Ensemble docking and molecular dynamics identify knoevenagel curcumin derivatives with potent anti-EGFR activity. Gene 539: 82–90, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Liu H, Borok Z, Davies KJA, Ursini F, Forman HJ. Cigarette smoke extract stimulates epithelial-mesenchymal transition through Src activation. Free Radic Biol Med 52: 1437–1442, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA 91: 3147–3150, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]