Dysregulated ion transport is a hallmark of diarrhea. Therefore, present studies examined the alleviation of downregulation of the expression of NHE3 and DRA by the probiotic Lactobacillus acidophilus in a murine model of infection by Citrobacter rodentium, a diarrheal pathogen. Our studies demonstrate for the first time that counteracting C. rodentium-induced inhibition of NHE3 and DRA expression could contribute to antidiarrheal effects of L. acidophilus.
Keywords: infectious colitis, NaCl absorption, probiotics, EPEC
Abstract
Impaired absorption of electrolytes is a hallmark of diarrhea associated with inflammation or enteric infections. Intestinal epithelial luminal membrane NHE3 (Na+/H+ exchanger 3) and DRA (Down-Regulated in Adenoma; Cl−/HCO3− exchanger) play key roles in mediating electroneutral NaCl absorption. We have previously shown decreased NHE3 and DRA function in response to short-term infection with enteropathogenic E. coli (EPEC), a diarrheal pathogen. Recent studies have also shown substantial downregulation of DRA expression in a diarrheal model of infection with Citrobacter rodentium, the mouse counterpart of EPEC. Since our previous studies showed that the probiotic Lactobacillus acidophilus (LA) increased DRA and NHE3 function and expression and conferred protective effects in experimental colitis, we sought to evaluate the efficacy of LA in counteracting NHE3 and DRA inhibition and ameliorating diarrhea in a model of C. rodentium infection. FVB/N mice challenged with C. rodentium [1 × 109 colony-forming units (CFU)] with or without administration of live LA (3 × 109 CFU) were assessed for NHE3 and DRA mRNA and protein expression, mRNA levels of carbonic anhydrase, diarrheal phenotype (assessed by colonic weight-to-length ratio), myeloperoxidase activity, and proinflammatory cytokines. LA counteracted C. rodentium-induced inhibition of colonic DRA, NHE3, and carbonic anhydrase I and IV expression and attenuated diarrheal phenotype and MPO activity. Furthermore, LA completely blocked C. rodentium induction of IL-1β, IFN-γ, and CXCL1 mRNA and C. rodentium-induced STAT3 phosphorylation. In conclusion, our data provide mechanistic insights into antidiarrheal effects of LA in a model of infectious diarrhea and colitis.
NEW & NOTEWORTHY
Dysregulated ion transport is a hallmark of diarrhea. Therefore, present studies examined the alleviation of downregulation of the expression of NHE3 and DRA by the probiotic Lactobacillus acidophilus in a murine model of infection by Citrobacter rodentium, a diarrheal pathogen. Our studies demonstrate for the first time that counteracting C. rodentium-induced inhibition of NHE3 and DRA expression could contribute to antidiarrheal effects of L. acidophilus.
the model of infection with the murine pathogen Citrobacter rodentium (CR) has been used to complement in vitro studies of enteropathogenic and enterohemorrhagic Escherichia coli (EPEC and EHEC) infections of human intestinal epithelial cells (IECs) (11). EPEC, a food-borne noninvasive, attaching and effacing (A/E) pathogen, causes watery persistent diarrhea worldwide, more particularly in infants (24). EPEC does not produce toxins but instead attaches intimately to host intestinal epithelial cells (6). A pathogenicity locus in the EPEC genome encodes a number of virulence proteins including those of a type three secretion system (TTSS) through which bacteria directly deliver their virulence proteins into the host cells (12). CR, the only known murine A/E lesion-producing pathogen, expresses many of the same effectors found in typical EPEC strains. Therefore, CR infection of mice has become the “gold standard” small-animal model for investigating the virulence mechanisms of A/E pathogens including EPEC (11).
Following infection, CR intimately attaches to epithelial cells lining the cecum and colon (14). CR-induced pathologies may range from subclinical colonic epithelial hyperplasia to clinical diarrhea and colitis, depending on the age, genetic background, and health status of the hosts (2). Differential response to CR infection in different mouse strains could be, at least partly, due to difference in gut microbiota composition (16). Additionally, the ability of CR to modulate epithelial barrier function, mucosal healing, inflammation, and composition of commensal microbiota makes it a robust model to study the pathogenesis of human intestinal disorders including inflammatory bowel diseases (IBD), dysbiosis, and tumorigenesis (11).
Although early reports of CR outbreaks in mouse colonies were associated with diarrhea, until recently investigations into CR pathogenesis have focused primarily on epithelial hyperplasia, mucosal inflammation, and protective immunity. A growing number of laboratories, however, are recently focusing on the mechanisms by which CR infection causes diarrhea. Impairment of normal intestinal ion transport processes is a hallmark of infectious diarrhea (3, 21) as well as diarrhea associated with IBD (26). In this regard, our previous studies indicated that decreased absorption rather than an increased secretion could be primarily responsible for early onset of EPEC-induced diarrhea (18–20). EPEC infection decreased activities of both NHE3 and DRA, the key intestinal epithelial Na+/H+ and Cl−/HCO3− exchangers, respectively, and the effects were dependent upon the TTSS of EPEC (18, 20). Recent studies also showed that CR infection of susceptible FVB/N mice was accompanied by profound changes in expression of intestinal ion transporters including DRA and NHE3, accompanied by decreased serum levels of sodium and chloride ions (3).
Modulation of responses to CR infection by probiotics has been shown to reduce colonic hyperplasia, prevent bacterial attachment (10), or alleviate colitis (8, 9). However, there are no reports on beneficial effects of probiotics in counteracting CR-induced inhibition of ion transport and ameliorating diarrhea. Our previous studies demonstrated, for the first time, the efficacy of Lactobacillus acidophilus (LA) in enhancing NaCl absorption and elucidated the underlying mechanisms in both in vitro and in vivo models (4, 27, 34, 35). Our present studies demonstrate prophylactic effects of LA administration in FVB/N mice in alleviating CR-induced diarrhea via counteracting CR-induced inhibition of colonic NHE3 and DRA expression.
MATERIALS AND METHODS
Chemicals and antibodies.
RNeasy kits for RNA extraction were obtained from Qiagen (Valencia, CA), and real-time quantitative RT-PCR (qRT-PCR) kits were obtained from Stratagene (La Jolla, CA). DRA antibody was raised in rabbits against the COOH-terminal amino acid (745–764) sequence INTNGGLRNRVYEPVETKF of SLC26A3 (accession no. BC025671). Anti-NHE3 antibody was a kind gift from Dr. Eugene Chang of the University of Chicago. All other chemicals were of at least reagent grade and were obtained from Sigma (St. Louis, MO) or Fisher Scientific (San Jose, CA).
Bacterial culture.
CR (DBS100/255, ATCC) cultures were grown overnight with shaking in Luria-Bertani broth (LB) at 37°C, diluted (1:33) in serum-free and antibiotic-free tissue culture medium containing 0.5% mannose, and grown at 37°C with aeration to mid-log growth phase (5 × 108 cells/ml) (36). Bacteria were spun down and resuspended in 1 ml of sterile 1× PBS. LA (ATCC 4357) was grown overnight in MRS broth (Difco, Detroit, MI) at 37°C without shaking. Bacteria were spun down by centrifuging at 3,000 rpm for 10 min and resuspended in sterile 1× PBS (4, 27).
Mice.
FVB/N mice obtained from Jackson Laboratories were maintained in Jesse Brown Veterans Affairs Medical Center. In vivo studies performed in these mice were approved by the Animal Care Committee of the University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center. Treatment plan for the mice for the present study is outlined in Fig. 1. All mice were given antibiotic treatments (streptomycin, 5 g/l) in drinking water for 24 h, on day −2 (2 days before LA administration), followed by water alone for another 24 h (day −1). Aliquots (200 μl) containing 3 × 109 CFU of LA, as reported earlier from our laboratory (27), were gavaged per animal on days 0, 2, and 4. Aliquots (200 μl) of the resuspended CR cultures in PBS, as described above, (∼1 × 109 CFU of bacteria/mouse) were administered to susceptible FVB/N mice (4–6 wk old) on day 1 by oral gavage (36). Control mice received 200 μl of sterile 1× PBS. Mice were euthanized on 6th day post-CR infection. Intestines were resected, and mucosa was scraped for RNA extraction and lysate preparation. Sections (∼2 cm) of the different regions of intestine (ileum and colon) were immediately snap-frozen in optimal cutting temperature (OCT) embedding medium (Tissue-Tek OCT compound, Sakura) for immunofluorescence studies.
Fig. 1.
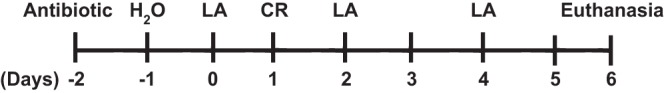
Plan of the animal experiment showing the days for antibiotic treatment, administration of L. acidophilus (LA) or C. rodentium (CR), and euthanasia.
Myeloperoxidase activity.
Myeloperoxidase (MPO) activity in the distal colon was assessed by the method of Krawisz et al. (22) with minor modifications. MPO activity was normalized to the amount of protein in supernatant as measured by Bradford's method and calculated as units per gram protein and expressed relative to control (considered as 100).
Histopathology.
For histopathological analysis, tissue sections were made from the OCT samples of representative region of the colon and viewed under the light microscope (×10–20) after hematoxylin and eosin (H&E) staining.
Real-time PCR.
RNA was extracted from mouse colonic mucosal samples using Qiagen RNeasy kits. RNA was reverse transcribed and amplified using a Brilliant SYBR Green qRT-PCR Master Mix kit (Stratagene). Mouse DRA or NHE3 was amplified with gene-specific primers as previously described by us (27, 35). Mouse GAPDH was amplified as an internal control. Mouse specific primers for IL-1β, CXCL1, IFN-γ, Na+/H+ exchanger regulatory factor (NHERF)1, 2, and 3, carbonic anhydrase I and IV, and GAPDH are presented in Table 1.
Table 1.
Gene-specific primers used for real-time PCR analysis of mRNA levels
| Gene | Primer Sequence (5′–3′) |
|---|---|
| Mouse IL-1β | F: GCAACTGTTCCTGAACTCAACT |
| R: ATCTTTTGGGGTCCGTCAACT | |
| Mouse CXCL1 | F: AAAGATGCTAAAAGGTGTCCCCA |
| R: AATTGTATAGTGTTGTCAGAAGCCA | |
| Mouse IFN-γ | F: ATGAACGCTACACACTGCATC |
| R: CCATCCTTTTGCCAGTTCCTC | |
| Mouse NHERF1 | F: CCAGTTTATCCGGTCTGGTAGAAC |
| R: CACCTCCACCAATCGGTCTC | |
| Mouse NHERF2 | F: GGCCAGTACATCCGCTCTG |
| R: CCTCCACGTTCTGCCCATTC | |
| Mouse NHERF3 | F: GAGGCCGCTCTGAATGATAAG |
| R: AGTACACACCCTTTTTACCTTGG | |
| Mouse carbonic anhydrase IV | F: CTCCTTCTTGCTCTGCTG |
| R: GACTGCTGATTCTCCTTA | |
| Mouse carbonic anhydrase I | F: TTGATGACAGTAGCAACC |
| R: CCAGTGAACTAAGTGAAG | |
| Mouse GAPDH | F: TGTGTCCGTCGTGGATCTGA |
| R: CCTGCTTCACCACCTCTTGAT |
F, forward primer; R, reverse primer.
Immunofluorescence staining in mouse colonic tissues.
Sections of colonic tissues from different mice groups were snap-frozen in OCT embedding medium. For immunostaining, 5-μm frozen sections were fixed with 1% paraformaldehyde in PBS for 10 min at room temperature. Fixed sections were washed in PBS, permeabilized with 5% Nonidet P-40 for 5 min, and blocked with 5% normal goat serum (NGS) for 30 min. Tissues were incubated with DRA or NHE3 antibody (1:100) in PBS with 1% NGS for 90 min at room temperature. After being washed, sections were incubated with Alexa Fluor 594-conjugated goat anti-rabbit IgG and Alexa Fluor 488-conjugated phalloidin (5 U/ml; Invitrogen) for 60 min. Sections were then washed and mounted under coverslips using Slowfade Gold antifade with DAPI reagent (Invitrogen). Sections were imaged using a Carl Zeiss LSM 510 laser-scanning confocal microscope equipped with ×20 water immersion objective. The quantitative assessment of immunofluorescence of NHE3 and DRA proteins was done by ImageJ software using villin as the internal control.
Statistical analysis.
Data are presented as means ± SE of 3–8 independent experiments. Differences between control vs. treated were analyzed using one-way ANOVA with Tukey's test. Differences were considered significant at P ≤ 0.05.
RESULTS
LA counteracts CR-induced decrease in NHE3 and DRA expression.
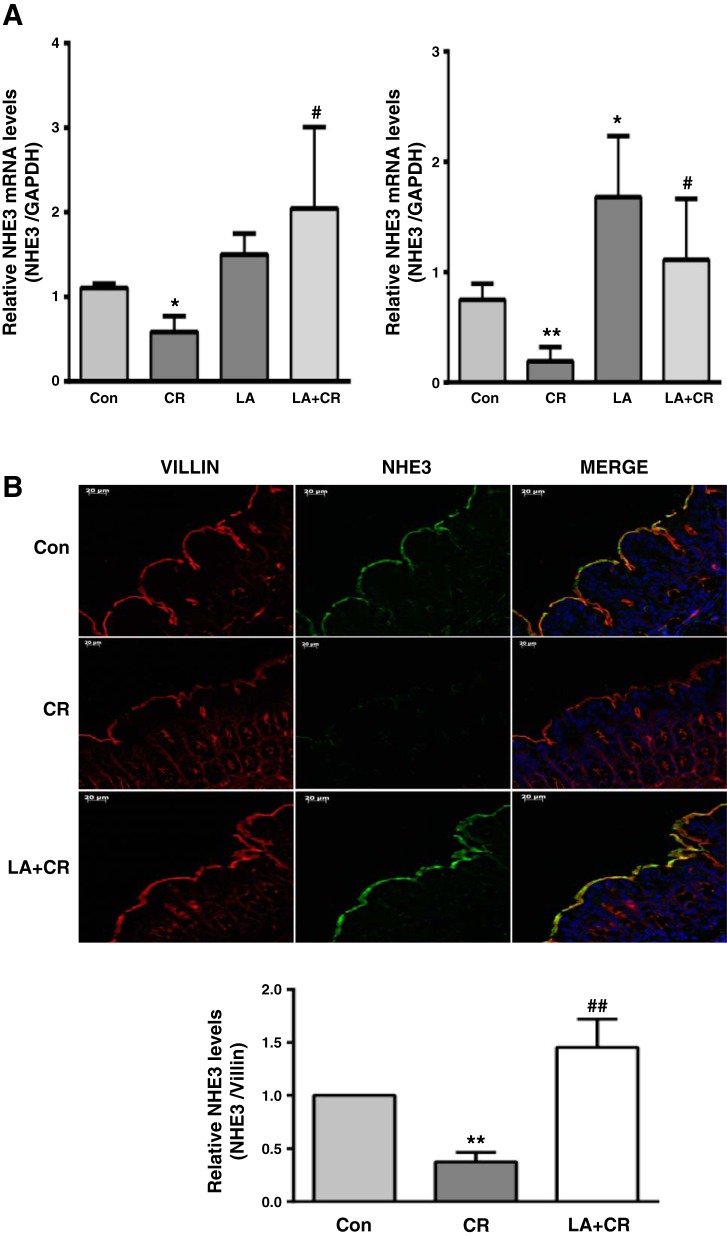
We have previously shown that short-term (30–60 min) infection of Caco-2 cells with EPEC, a human pathogen causing infantile diarrhea, inhibited NHE3 and DRA function via decreasing their apical cell surface levels (18, 20). Recent studies have also shown downregulation of NHE3 and DRA expression in murine diarrheal model of infection with CR, the mouse counterpart of human EPEC (3). In this regard, probiotics are known to ameliorate disease by counteracting pathogen infection via varied mechanisms (5). Our previous studies demonstrated the efficacy of the probiotic LA in enhancing NHE3 and DRA expression and function and also in counteracting inflammation-induced downregulation of DRA expression and function in both in vitro and in vivo models (4, 27, 34, 35). Therefore, we examined whether LA could also counteract CR-induced downregulation of NHE3 and DRA expression. As shown in Fig. 2A, colonic NHE3 mRNA was significantly decreased on day 6 following CR infection in both proximal and distal colon and this effect was significantly attenuated by oral administration of LA on days 0, 2, and 4. CR-induced downregulation of NHE3 mRNA and the counteracting effects of LA were more pronounced in the distal colon compared with proximal colon, presumably due to greater colonization of the pathogen in distal colon (14). However, we used mucosal samples of proximal colon to measure CR/LA effects on NHE3 protein as NHE3 expression is minimal in distal colon (38). Our results showed that LA also counteracted the decrease in NHE3 protein in proximal colon following CR infection (Fig. 2B).
Fig. 2.
LA counteracts CR-induced decrease in NHE3 mRNA and protein levels in the colon of FVB/N mice. A: relative mRNA abundance of NHE3 in total RNA samples extracted from proximal (left) and distal (right) colonic mucosa was determined by real-time RT-PCR using gene-specific primers. GAPDH was used as an internal control. Values are means ± SE of 4–8 separate experiments. *P < 0.05 vs. control, **P < 0.001 vs. control (Con), #P < 0.05 vs. CR. B, top: immunostaining of NHE3 (green) and villin (red) in proximal colonic mucosal sections. Representative image from 3 separate experiments. B, bottom: quantification of NHE3 intensity by ImageJ software and densitometric analysis relative to intensity of villin as the internal control. Values are means ± SE of 3 separate experiments. **P < 0.001 vs. control, ##P < 0.001 vs. CR.
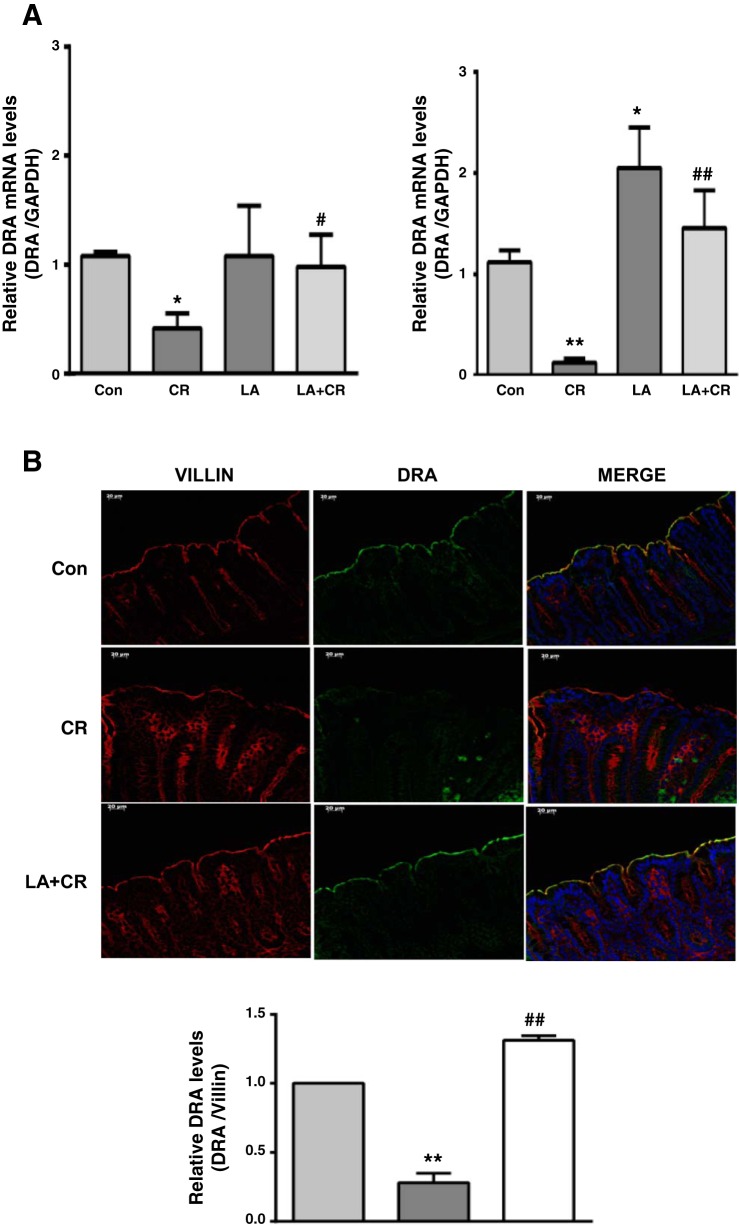
Recent studies have indicated that decreased expression of DRA is a key cause of CR-induced fatal diarrhea in mice (3). Therefore, we examined whether LA administration could also counteract CR-induced downregulation of DRA expression in mouse colon. As shown in Fig. 3A, CR substantially reduced DRA mRNA levels in mouse colon, with greater effects in the distal colon, whereas LA not only increased DRA mRNA but also significantly attenuated CR-induced downregulation of DRA mRNA. Similarly, as shown by immunofluorescence (Fig. 3B), mouse distal colonic DRA protein level was significantly decreased in response to CR infection and this decrease was blocked by LA.
Fig. 3.
LA counteracts CR-induced decrease in DRA mRNA and protein levels in the distal colon of FVB/N mice. A: relative mRNA abundance of DRA in total RNA samples extracted from proximal (left) and distal (right) colonic mucosa was determined by real-time RT-PCR using gene-specific primers. GAPDH was used as an internal control. Values are means ± SE of 4–8 separate experiments. *P < 0.05 vs. control, **P < 0.001 vs. control, #P < 0.05 vs. CR, ##P < 0.001 vs. CR. B, top: immunostaining of DRA (green) and villin (red) in distal colonic mucosal sections. Representative image from 3 separate experiments. B, bottom: quantification of DRA intensity by ImageJ software and densitometric analysis relative to intensity of villin as the internal control. Values are means ± SE of 3 separate experiments. **P < 0.001 vs. control, ##P < 0.001 vs. CR.
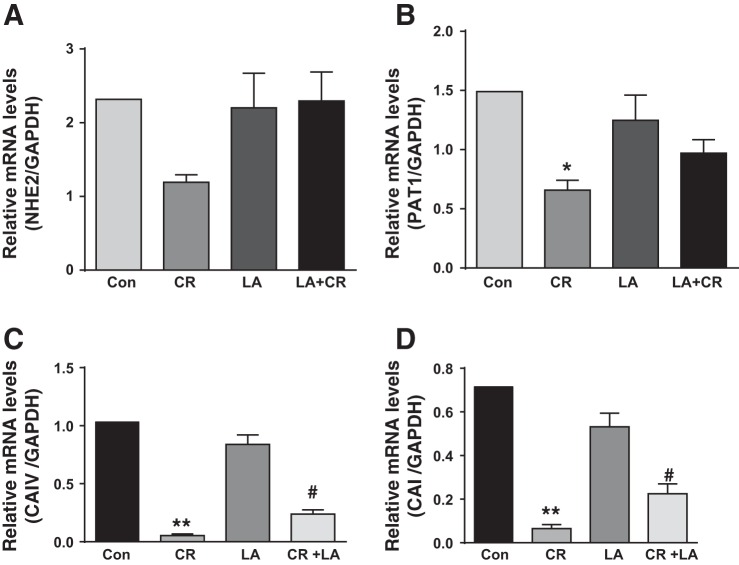
LA had no effect on normal or CR-induced alterations in NHE2 and PAT1 mRNA levels.
We also measured mRNA levels of NHE2, another member of NHE gene family expressed in luminal membrane, and PAT1, another member of the SLC26 gene family speculated as apical membrane Cl−/HCO3− exchanger, in response to CR infection and/or LA. Although previous studies showed marginal but significant decrease in NHE2 following CR infection for 9 days (1), the present studies showed marginal decrease in NHE2 after 6 days of exposure to CR was not statistically significant (Fig. 4A). Furthermore, LA had no significant effect on basal NHE2 mRNA, similar to our earlier observations (35). Similarly, CR significantly decreased PAT1 mRNA, whereas LA alone had no effect and did not counteract the inhibitory effects of CR (Fig. 4B). These results suggest that LA effects in counteracting inhibitory effects of CR were specific to NHE3 and DRA.
Fig. 4.
LA had no significant effect on normal or CR-induced alterations in NHE2 and PAT1 mRNA levels but counteracts CR-induced decrease in carbonic anhydrase (CA) isoforms: relative mRNA abundance of NHE2 (A), PAT1 (B), carbonic anhydrase IV (CAIV; C), and carbonic anhydrase I (CAI; D) in total RNA samples extracted from distal colonic mucosa was determined by real-time RT-PCR using gene-specific primers. GAPDH was used as an internal control. Values are means ± SE of 4 separate experiments. *P < 0.05 vs. control, **P < 0.001 vs. control, #P < 0.05 vs. CR.
LA counteracts CR-induced decrease in carbonic anhydrase isoforms.
The pool of bicarbonate required for DRA function (Cl−/HCO3− exchange activity) is maintained by carbonic anhydrases (CAs) (7). Previous studies showed CR-induced downregulation of CAI and CAIV, 9 days postinfection in FVB/N mice, which, along with decreased DRA, was attributed to disturbed chloride homeostasis resulting in fatal diarrhea (1, 3). Parallel to LA effects on DRA and NHE3, therefore, we sought to examine whether LA also counteracts CR-induced decrease in expression of CAI and CAIV. As shown in Fig. 4, 6th day postinfection with CR, colonic CAI (C) and CAIV (D) mRNA levels were significantly reduced, and LA alone had no effect but significantly attenuated CR-induced decrease in CAI and CAIV mRNA.
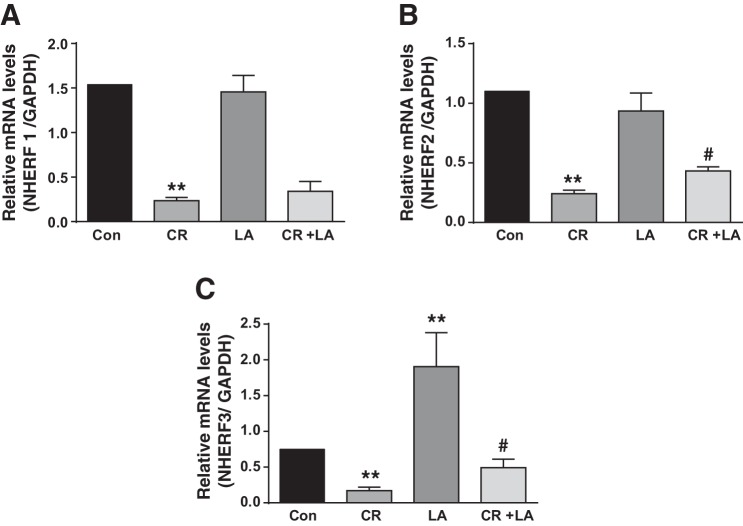
LA effects on CR-induced downregulation of NHERF protein expression.
The members of the NHERF family of PDZ-adaptor proteins are known to interact with NHE3 and DRA and modulate their trafficking, membrane retention and function (32). Since previous reports (1, 3) and our present studies showed significant downregulation of NHE3 and DRA in response to CR infection, we sought to examine whether expression of these regulatory proteins that govern NHE3 and DRA activity is also altered. As shown in Fig. 5, CR infection significantly downregulated NHERF 1 (A), 2 (B), and 3 (C) expression at day 6 in distal colon. LA alone had no effect on NHERF 1 and 2 but significantly increased NHERF3 mRNA level, whereas in the LA+CR group LA counteracted CR-induced downregulation of NHERF 2 and 3, but not NHERF1.
Fig. 5.
LA effects on CR-induced downregulation of NHERF mRNA expression. Relative mRNA abundance of NHERF1 (A), 2 (B), and 3 (C) in total RNA samples extracted from distal colonic mucosa was determined by real-time RT-PCR using gene-specific primers. GAPDH was used as an internal control. Values are means ± SE of 4 separate experiments. **P < 0.001 vs. control, #P < 0.05 vs. CR.
LA attenuates CR-induced diarrheal phenotype.
Colon weight-to-length ratio that gives an indication of fluid accumulation was used to measure diarrheal phenotype. Colon weight and length were recorded for each mouse immediately after death (6th day of CR inoculation). A significant increase in the colon weight-to-length ratio, an indication of fluid accumulation, was observed in the CR group compared with control. LA alone had no effect on the ratio; however, LA significantly attenuated CR-induced increase in colon weight-to-length ratio (Fig. 6).
Fig. 6.
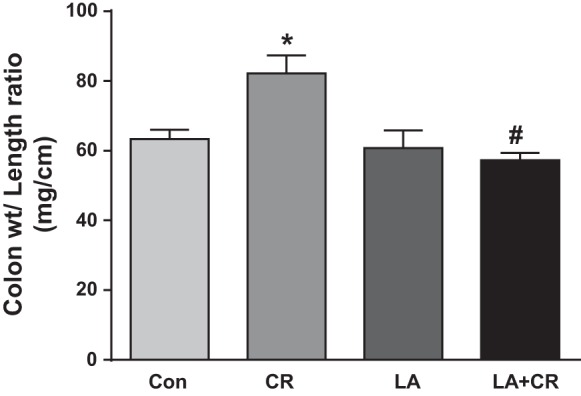
LA reverses CR-induced increase in colon weight-to-length ratio. Colon weight and length were recorded for each mouse immediately after death (6th day of CR inoculation). Data were expressed as means ± SE of the ratio of weight/length in each group (n = 4). *P < 0.05 vs. control, #P < 0.05 vs. CR.
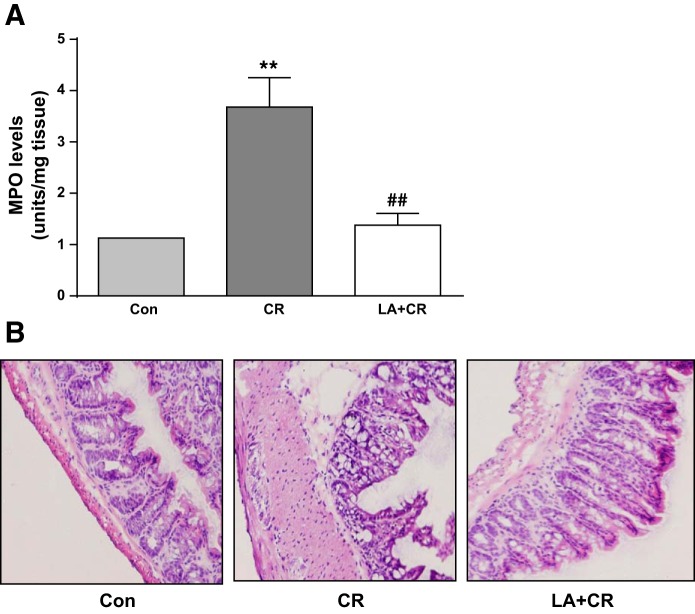
LA reduces MPO activity and mucosal damage in CR-infected mouse colon.
Because the CR-induced colitis mostly affects the distal part of the colon, a part (2 cm) of distal colon was used for determination of MPO activity. Colonic MPO level, an index of neutrophil accumulation, was significantly increased in CR-infected mice compared with control (Fig. 7A). This increase in MPO level was significantly attenuated by LA treatment in the LA + CR group. These results suggested that LA exerts its protective effects in the colon of mice with colitis partly by decreasing inflammation caused by CR infection. H&E staining of colonic mucosa also showed protective effects of LA as evident from restoration of CR-induced mucosal damage and attenuation of infiltration of immune cells (Fig. 7B).
Fig. 7.
LA reduces CR-induced MPO activity and mucosal damage in mouse distal colon. A: MPO activity, measured as described in materials and methods and calculated as units/g protein are shown as % of control (n = 4). **P < 0.001 vs. control, ##P < 0.001 vs. CR. B: H&E staining of colonic mucosa showed CR-induced mucosal damage and submucosal infiltration of immune cells that were attenuated in the LA+CR group. Representative image of 3 separate experiments.
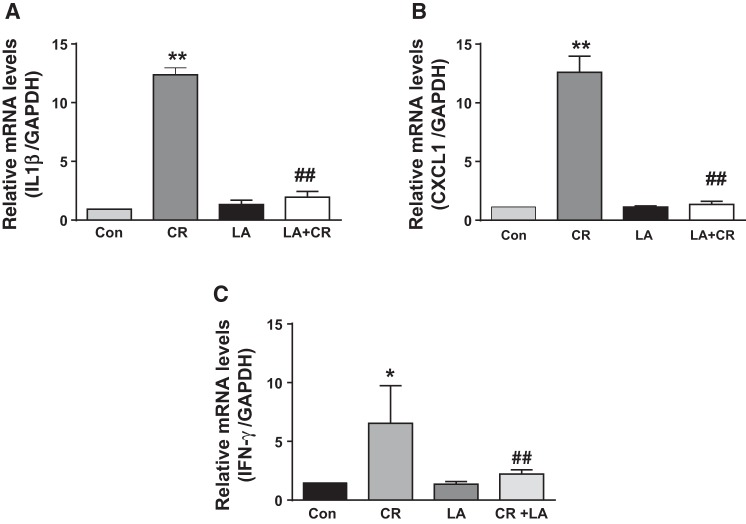
LA attenuates CR-induced increase in proinflammatory cytokines.
We also measured mRNA levels of few proinflammatory cytokines following CR infection and modulation of their levels in response to LA administration. Our results showed that there was substantial increase in mRNA levels of IL-1β, CXCL1, and IFN-γ in the colonic mucosa of CR-infected mice. LA alone showed no effect on the cytokine levels, but significantly blocked the CR-induced increase in their mRNA levels in the CR+LA group (Fig. 8).
Fig. 8.
LA attenuates CR-induced increase in proinflammatory cytokines. Relative mRNA abundance of IL-1β (A), CXCL1 (B), and IFN-γ (C) in total RNA samples extracted from distal colonic mucosa was determined by real-time RT-PCR using gene-specific primers. GAPDH was used as an internal control. Values are means ± SE of 4 separate experiments. *P < 0.05 vs. control, **P < 0.001 vs. control, ##P < 0.001 vs. CR.
LA blocks CR-induced STAT3 phosphorylation.
Our earlier studies showed involvement of JAK-STAT pathway in IFN-γ-induced downregulation of DRA gene transcription (28). Since present studies showed CR induction of IFN-γ mRNA and its reversal by LA, we also examined STAT phosphorylation in mouse colonic mucosa in response to CR and/or LA. Results showed significantly increased STAT3 phosphorylation in the colon of CR group that was blocked in the CR+LA group (Fig. 9).
Fig. 9.
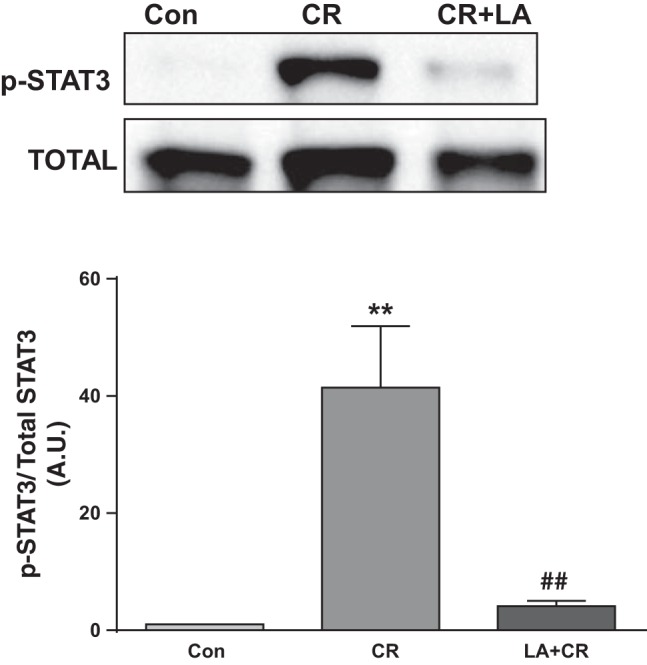
LA blocks CR-induced STAT3 phosphorylation. Top: lysates prepared from mucosal scrappings of mice in different groups were subjected to SDS-PAGE and probed with phospho-STAT3 antibody in immunoblotting. After stripping with 0.2 N NaOH, blots were reprobed with STAT-3 antibody. Representative blot of 4 separate experiments. Bottom: densitometric analysis of band intensities of phospho-STAT3/total STAT3 in different groups (n = 4). **P < 0.001 vs. control, ##P < 0.001 vs. CR.
DISCUSSION
Na+/H+ exchanger isoform 3 (NHE3) and DRA (Down-Regulated in Adenoma, the apical membrane Cl−/HCO3− exchanger) play major roles in mediating coupled electroneutral NaCl absorption in the mammalian intestine (13, 17). Impaired expression and/or function of NHE3 and DRA have been shown to cause diarrhea in experimental models (3, 30, 34). In this regard, EPEC, a food-borne human enteric pathogen, is known to cause watery persistent diarrhea particularly in infants. Our previous studies have implicated impaired NaCl absorption as a major contributing factor for diarrhea caused by early EPEC infection (18, 20). Our studies demonstrated that short-term (30–60 min) infection of Caco-2 cells with EPEC inhibits NHE3 and DRA function via decreasing their apical cell surface levels (18, 20). On the other hand, recent studies have shown substantial downregulation of NHE3 and DRA expression in murine diarrheal model of infection with CR, the mouse counterpart of human EPEC (1, 3).
Mice are generally considered poor models for studying watery diarrhea caused by pathogenic agents that infect humans (3). In this regard, CR is the only known murine A/E-producing pathogen and, therefore, can serve as a small animal model for EPEC infection (9, 11). Typically, this organism causes severe, but self-limiting, epithelial hyperplasia with a variable degree of inflammation in the distal colon of most inbred and outbred lines of laboratory mice (1). Therefore, CR infection of mice has been utilized to model epithelial hyperplasia, mucosal inflammation and immunity and also several important human intestinal disorders, including Crohn's disease, ulcerative colitis, and, more recently, colon tumorigenesis (11). However, the use of this model for studying diarrheal diseases to date has been limited, as CR colonization of mice with different genetic background causes inflammation, but typically may cause profuse, little, or no diarrhea depending on the mouse strain used (3). In this regard, recent studies have shown that mortality in CR-infected susceptible inbred mouse strain FVB/N was primarily due to fatal diarrhea that caused disturbances in intestinal ion transport, massive fluid loss, and dehydration associated with a remarkable downregulation of expression of DRA, the intestinal apical membrane Cl−/HCO3− exchanger (3). Therefore, we used FVB/N mice in the present studies to test our hypothesis that the probiotic LA can alleviate CR-induced diarrhea via counteracting the inhibitory effects of the pathogen in downregulating NHE3 and DRA expression.
Probiotics are live microorganisms that, when given in sufficient quantities, confer health benefits to hosts. There are strong indications from clinical trials that some probiotics including Lactobacilli could be promising complementary prophylactic and/or therapeutic agents for treating various forms of diarrheal disorders (15, 25). The mechanisms of probiotic action include blocking production of microbial toxins, competitive exclusion of pathogen adhesion, modulation of the immune system, and improvement of gut barrier function (15). However, the mechanisms of antidiarrheal effects of probiotics are not fully understood (21). A hallmark of diarrhea is the dysregulation of normal intestinal ion transport processes (29, 31). We and others have reported disturbances in electrolyte absorption in models of infectious diarrhea as well as diarrhea associated with inflammatory bowel diseases (18, 26, 31). Similar to earlier reports (1, 3), our present studies also showed substantial downregulation of NHE3 and DRA mRNA and protein levels on 6th day following CR challenge. The effects were more pronounced in the distal colon compared with proximal colon likely due to greater colonization of the pathogen in distal colon. In this regard, our previous studies have shown that LA increases NHE3 and DRA mRNA and protein levels in vitro and in vivo in C57BL/6 mice and also counteracts downregulation of DRA in experimental models of inflammation (27, 34, 35). Previous studies have shown the efficacy of various Lactobacilli, including LA, in modulating CR-induced infectious colitis and colonic hyperplasia (8–10), although their effects in ameliorating diarrhea were not known. Our present studies for the first time demonstrated LA amelioration of infectious diarrhea, as assessed by colon weight-to-length ratio, which could at least partly be via blocking CR-induced downregulation of NHE3 and DRA expression. Importantly, LA effects were found to be specific for NHE3 and DRA as there was no effect on the expression of NHE2, another isoform of apically expressed NHE, and PAT1, another candidate transporter mediating apical membrane Cl−/HCO3− exchange in mouse small intestine (39). The pool of bicarbonate required for DRA function (Cl−/HCO3− exchange activity) maintains CAs abundantly expressed and localized in the apical membrane (i.e., CAIV) and the cytoplasm (i.e., CAI and CAII) of colonocytes catalyze reversible hydration/dehydration of CO2 and water, thereby producing bicarbonates required for DRA function (Cl−/HCO3− exchange activity) (7). The administration of CA inhibitors results in a significant reduction of colonic chloride absorption, confirming the functional relationship of CAs and DRA in intestinal ion homeostasis (37). Previous studies showed CR-induced downregulation of CAI and CAIV 9 days postinfection in FVB/N mice, which, along with decreased DRA, was attributed to disturbed chloride homeostasis and resulting fatal diarrheal conditions (3). Our results showed that LA also counteracted CR-induced decrease in the expression of CA I and IV. Therefore, LA seems to promote DRA activity not only via increasing its expression, but also by maintaining the pool of bicarbonate required for DRA function (Cl−/HCO3− exchange activity).
The members of the NHERF family of PDZ-adaptor proteins interact with the COOH-terminal cytoplasmic domain of transporters and channels such as NHE3, DRA, PAT1, and CFTR, form multimolecular assemblies, and modulate their trafficking, membrane retention, and associated signaling events and functions (32). Earlier studies reported modulation of NHERF1 expression/activity following EPEC or CR infection (1, 33). Therefore, it was logical to examine the effects of CR infection on the expression levels of NHERF isoforms and their alterations in response to LA that could indirectly affect NaCl absorption via modulation of NHE3 and DRA activity. Our results showed downregulation of NHERF1, 2, and 3 following CR infection. However, LA could block the inhibitory effects of CR on NHERF2 and 3 expression, but not on NHERF1 expression. Although the relative role of various NHERF isoforms in modulating NHE3 and DRA function is not fully defined, recent studies showed that DRA activity depends primarily on its PDZ interactions with NHERF2 and 3 (23). Overall, our results suggest that LA-mediated blocking of the inhibitory effects of CR infection on NHERF2 and 3 expression could be important in alleviating CR-induced diarrhea.
Earlier studies on LA-mediated amelioration of CR-induced inflammation and colitis were performed in C57BL/6 or BALB/c mice (8–10). However, CR infection in adult mice of these strains is self-limiting and does not cause appreciable diarrhea (3). In this regard, recent studies showed diarrhea as a cause of mortality following CR infection of FVB/N mice, a susceptible inbred mouse strain (1). Therefore, we extended our studies to assess the efficacy of LA in alleviating CR-induced inflammatory responses in FVB/N mice that exhibit both colitis and profound diarrheal phenotype. Similar to earlier reports on BALB/c mice (9), our present studies showed that LA inhibited CR-induced elevation of MPO activity, a measure of neutrophil accumulation in the distal colonic mucosa. LA-mediated decrease in immune cells recruitment to submucosa was also evident in H&E staining. Furthermore, LA inhibited CR-induced increase in proinflammatory cytokines IL-1β, IFN-γ, and CXCL1. To what extent CR-induced inflammatory responses are linked to associated diarrhea in FVB/N mice is not fully understood. However, earlier studies reported that reduced expression of DRA or CAIV following CR infection was independent of the inflammatory status and did not correlate with the expression levels of proinflammatory cytokines (3). Therefore, further mechanistic studies will be of critical importance to dissect the role of infection and/or infection-induced inflammatory responses in modulating the expression of NHE3 and DRA. In this regard, our previous studies have shown the involvement of JAK-STAT pathway in cytokine-mediated downregulation of DRA expression (28). Interestingly, our present studies showed that CR infection substantially increases STAT-3 phosphorylation in colon, consistent with that shown in a recent report (40), which is completely blocked in the CR + LA group, suggesting the involvement of JAK-STAT pathway in modulating DRA expression by CR and LA. These results indicate that at least part of the effects of CR on DRA expression could be secondary to inflammation. However, further mechanistic studies will be needed to define the role of CR-induced STAT3 phosphorylation in mediating the effects of the pathogen on DRA expression and to understand the mechanism of downregulation of DRA in response to CR infection.
In summary, our investigation of a prophylactic approach for amelioration of CR-induced diarrhea and inflammation in mice via inoculation of live LA has shown that probiotic inoculation resulted in an attenuation of CR-induced inhibition of NHE3 and DRA expression that also likely resulted in alleviation of diarrhea. Our results demonstrated the suitability of FVB/N mice as an appropriate in vivo diarrheal model for mechanistic studies on EPEC-induced dysregulation of intestinal NaCl absorption. Furthermore, besides contributing to our understanding of the pathogenesis of CR-induced diarrhea, our studies also suggested novel strategies for utilizing antidiarrheal potential of LA for the prevention and treatment of diarrhea associated with intestinal bacterial infections.
GRANTS
These studies were supported by National Institute of Digestive and Kidney Diseases (NIDDK) Grants DK-54016, DK-81858, DK-92441 (P. K. Dudeja), and DK-71596 (W. A. Alrefai); Department of Veteran Affairs Grants BX 002011 (P. K. Dudeja) and BX 000152 (W. A. Alrefai); NIDDK Grants DK-098170 (R. K. Gill) and DK-96245 (S. Saksena); and Bill & Melinda Gates Foundation Grant OPP1058288 (A. Borthakur).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.K., A.N.A., H.P.C., I.C., S.P., and T.G. performed experiments; A.K., A.N.A., H.P.C., I.C., S.P., T.G., and A.B. analyzed data; A.K., A.N.A., H.P.C., I.C., S.P., S.S., R.K.G., W.A.A., and A.B. interpreted results of experiments; A.K., A.N.A., and H.P.C. prepared figures; S.S., R.K.G., W.A.A., A.B., and P.K.D. conception and design of research; S.S., R.K.G., W.A.A., A.B., and P.K.D. edited and revised manuscript; A.B. drafted manuscript; A.B. and P.K.D. approved final version of manuscript.
REFERENCES
- 1.Borenshtein D, Fry RC, Groff EB, Nambiar PR, Carey VJ, Fox JG, Schauer DB. Diarrhea as a cause of mortality in a mouse model of infectious colitis. Genome Biol 9: R122, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borenshtein D, McBee ME, Schauer DB. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr Opin Gastroenterol 24: 32–37, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Borenshtein D, Schlieper KA, Rickman BH, Chapman JM, Schweinfest CW, Fox JG, Schauer DB. Decreased expression of colonic Slc26a3 and carbonic anhydrase IV as a cause of fatal infectious diarrhea in mice. Infect Immun 77: 3639–3650, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138: 1355–1359, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britton RA, Versalovic J. Probiotics and gastrointestinal infections. Interdiscip Perspect Infect Dis 2008: 290769, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celli J, Deng W, Finlay BB. Enteropathogenic Escherichia coli (EPEC) attachment to epithelial cells: exploiting the host cell cytoskeleton from the outside. Cell Microbiol 2: 1–9, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Charney AN, Dagher PC. Acid-base effects on colonic electrolyte transport revisited. Gastroenterology 111: 1358–1368, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Chen CC, Chiu CH, Lin TY, Shi HN, Walker WA. Effect of probiotics Lactobacillus acidophilus on Citrobacter rodentium colitis: the role of dendritic cells. Pediatr Res 65: 169–175, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CC, Louie S, Shi HN, Walker WA. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr Res 58: 1185–1191, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Collins JW, Chervaux C, Raymond B, Derrien M, Brazeilles R, Kosta A, Chambaud I, Crepin VF, Frankel G. Fermented dairy products modulate Citrobacter rodentium-induced colonic hyperplasia. J Infect Dis 210: 1029–1041, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins JW, Keeney KM, Crepin VF, Rathinam VA, Fitzgerald KA, Finlay BB, Frankel G. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol 12: 612–623, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Dean P, Kenny B. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr Opin Microbiol 12: 101–109, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudeja P, Gill RK, Ramaswamy K. Absorption-secretion and epithelial cell function. In: Colonic Diseases, edited by Koch TR. Totowa, NJ: Humana, 2003, p. 3–24. [Google Scholar]
- 14.Eckmann L. Animal models of inflammatory bowel disease: lessons from enteric infections. Ann NY Acad Sci 1072: 28–38, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 7: 503–514, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh S, Dai C, Brown K, Rajendiran E, Makarenko S, Baker J, Ma C, Halder S, Montero M, Ionescu VA, Klegeris A, Vallance BA, Gibson DL. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am J Physiol Gastrointest Liver Physiol 301: G39–G49, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms and regulation of NaCl absorption in the human intestine. In: Recent Research Developments in Physiology (vol. 1). Trivandrum, India: Research Signpost, 2003, p. 643–677.
- 18.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht G, Koutsouris A. Enteropathogenic E. coli attenuates secretagogue-induced net intestinal ion transport but not Cl− secretion. Am J Physiol Gastrointest Liver Physiol 276: G781–G788, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Hodges K, Alto NM, Ramaswamy K, Dudeja PK, Hecht G. The enteropathogenic Escherichia coli effector protein EspF decreases sodium hydrogen exchanger 3 activity. Cell Microbiol 10: 1735–1745, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoque KM, Chakraborty S, Sheikh IA, Woodward OM. New advances in the pathophysiology of intestinal ion transport and barrier function in diarrhea and the impact on therapy. Expert Rev Anti Infect Ther 10: 687–699, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87: 1344–1350, 1984. [PubMed] [Google Scholar]
- 23.Lissner S, Hsieh CJ, Nold L, Bannert K, Bodammer P, Sultan A, Seidler U, Graeve L, Lamprecht G. The PDZ-interaction of the intestinal anion exchanger downregulated in adenoma (DRA; SLC26A3) facilitates its movement into Rab11a-positive recycling endosomes. Am J Physiol Gastrointest Liver Physiol 304: G980–G990, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Ochoa TJ, Contreras CA. Enteropathogenic Escherichia coli infection in children. Curr Opin Infect Dis 24: 478–483, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preidis GA, Hill C, Guerrant RL, Ramakrishna BS, Tannock GW, Versalovic J. Probiotics, enteric and diarrheal diseases, and global health. Gastroenterology 140: 8–14, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priyamvada S, Gomes R, Gill RK, Saksena S, Alrefai WA, Dudeja PK. Mechanisms underlying dysregulation of electrolyte absorption in inflammatory bowel disease-associated diarrhea. Inflamm Bowel Dis 21: 2926–2935, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298: G395–G401, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saksena S, Singla A, Goyal S, Katyal S, Bansal N, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-γ. Am J Physiol Gastrointest Liver Physiol 298: G159–G166, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandle GI. Infective and inflammatory diarrhoea: mechanisms and opportunities for novel therapies. Curr Opin Pharmacol 11: 634–639, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Seidler U, Lenzen H, Cinar A, Tessema T, Bleich A, Riederer B. Molecular mechanisms of disturbed electrolyte transport in intestinal inflammation. Ann NY Acad Sci 1072: 262–275, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Seidler U, Singh AK, Cinar A, Chen M, Hillesheim J, Hogema B, Riederer B. The role of the NHERF family of PDZ scaffolding proteins in the regulation of salt and water transport. Ann NY Acad Sci 1165: 249–260, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Simpson N, Shaw R, Crepin VF, Mundy R, FitzGerald AJ, Cummings N, Straatman-Iwanowska A, Connerton I, Knutton S, Frankel G. The enteropathogenic Escherichia coli type III secretion system effector Map binds EBP50/NHERF1: implication for cell signalling and diarrhoea. Mol Microbiol 60: 349–363, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Singh V, Kumar A, Raheja G, Anbazhagan AN, Priyamvada S, Saksena S, Jhandier MN, Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Am J Physiol Gastrointest Liver Physiol 307: G623–G631, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh V, Raheja G, Borthakur A, Kumar A, Gill RK, Alakkam A, Malakooti J, Dudeja PK. Lactobacillus acidophilus upregulates intestinal NHE3 expression and function. Am J Physiol Gastrointest Liver Physiol 303: G1393–G1401, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snoeks L, Weber CR, Turner JR, Bhattacharyya M, Wasland K, Savkovic SD. Tumor suppressor Foxo3a is involved in the regulation of lipopolysaccharide-induced interleukin-8 in intestinal HT-29 cells. Infect Immun 76: 4677–4685, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sterling D, Alvarez BV, Casey JR. The extracellular component of a transport metabolon. Extracellular loop 4 of the human AE1 Cl−/HCO3− exchanger binds carbonic anhydrase IV. J Biol Chem 277: 25239–25246, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Talbot C, Lytle C. Segregation of Na/H exchanger-3 and Cl/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. Am J Physiol Gastrointest Liver Physiol 299: G358–G367, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Wittkopf N, Pickert G, Billmeier U, Mahapatro M, Wirtz S, Martini E, Leppkes M, Neurath MF, Becker C. Activation of intestinal epithelial Stat3 orchestrates tissue defense during gastrointestinal infection. PLoS One 10: e0118401, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]